Abstract
Medications that elicit an alternate pathway for nitrogen excretion such as oral sodium phenylbutyrate (NaPBA) and glycerol phenylbutyrate (GPB) and intravenous sodium phenylacetate (NaPAA) are important for the management of urea cycle disorders (UCDs). Plasma concentrations of their primary metabolite, phenylacetate (PAA), as well as the ratio of PAA to phenylacetylglutamine (PAGN) are useful for guiding dosing and detecting toxicity. However, the frequency of toxic elevations of metabolites and associated clinical covariates is relatively unknown. A retrospective analysis was conducted on 1255 plasma phenylbutyrate metabolite measurements from 387 individuals. An additional analysis was also conducted on a subset of 68 individuals in whom detailed clinical information was available. In the course of these analyses, abnormally elevated plasma PAA and PAA:PAGN were identified in 39 individuals (4.15% of samples) and 42 individuals (4.30% of samples), respectively. Abnormally elevated PAA and PAA:PAGN values were more likely to occur in younger individuals and associate positively with dose of NAPBA and negatively with plasma glutamine and glycine levels. These results demonstrate that during routine clinical management, the majority of patients have PAA levels that are deemed safe. As age is negatively associated with PAA levels however, children undergoing treatment with NaPBA may need close monitoring of their phenylbutyrate metabolite levels.
Keywords: Glycerol phenylbutyrate, nitrogen-scavengers, phenylbutyrate, phenylacetate, PAA:PAGN, urea cycle disorders
1. Introduction
Urea cycle disorders (UCDs) are inborn errors of metabolism characterized by an inability to dispose of waste-nitrogen resulting in the accumulation of ammonia, a toxic nitrogenous compound. Deficiency of one of the enzymes or transporters required for urea synthesis results in a primary UCD: N-acetylglutamate synthase deficiency (NAGSD, MIM# 237310), carbamoyl phosphate synthetase 1 deficiency (CPS1D, MIM# 311250), ornithine transcarbamylase deficiency (OTCD, MIM# 311250), argininosuccinate synthetase 1 deficiency (ASS1D, MIM# 215700), argininosuccinate lyase deficiency (ASLD, MIM# 207900), arginase 1 deficiency (ARG1D, MIM# 207800), citrin deficiency (CITRD, MIM# 605814), and hyperornithinemia-hyperammonemia-homocitrullinuria syndrome (HHHS, MIM# 238970). Prevention of hyperammonemia is a central focus of the long-term management of UCDs; this typically entails a combination of a protein-restricted diet, amino acid supplementation, and use of nitrogen-scavenging medications. Sodium benzoate (NaB), sodium phenylacetate (NaPAA), sodium phenylbutyrate (NaPBA), and glycerol phenylbutyrate (GPB) are nitrogen-scavenging medications which elicit alternate pathways for excretion of waste-nitrogen.[1–3] Benzoate combines with glycine to form hippuric acid, which can be excreted in urine. GPB and NaPBA are converted to phenylacetic acid (PAA) which when conjugated with glutamine forms phenylacetylglutamine (PAGN) that can also be excreted in urine (Figure 1).[1, 4] The use of an intravenous formulation of NaB and NaPAA for the treatment of acute hyperammonemia and oral formulations of NaB, NaPBA, and GPB for prevention of hyperammonemia have become standard-of-care for treatment of UCDs.[5] Glutamine has two nitrogen atoms compared with one in glycine; thus, theoretically, on a equimolar basis, nitrogen-scavenging efficacy of phenylbutyrate is twice that of benzoate. This theoretical advantage of NaPBA over NaB is likely to be one of many potential reasons for the preferential use of NaPBA and GPB for the long-term management of UCDs in the United States.[6]
Figure 1. Alternate pathway of nitrogen excretion by phenylacetate.
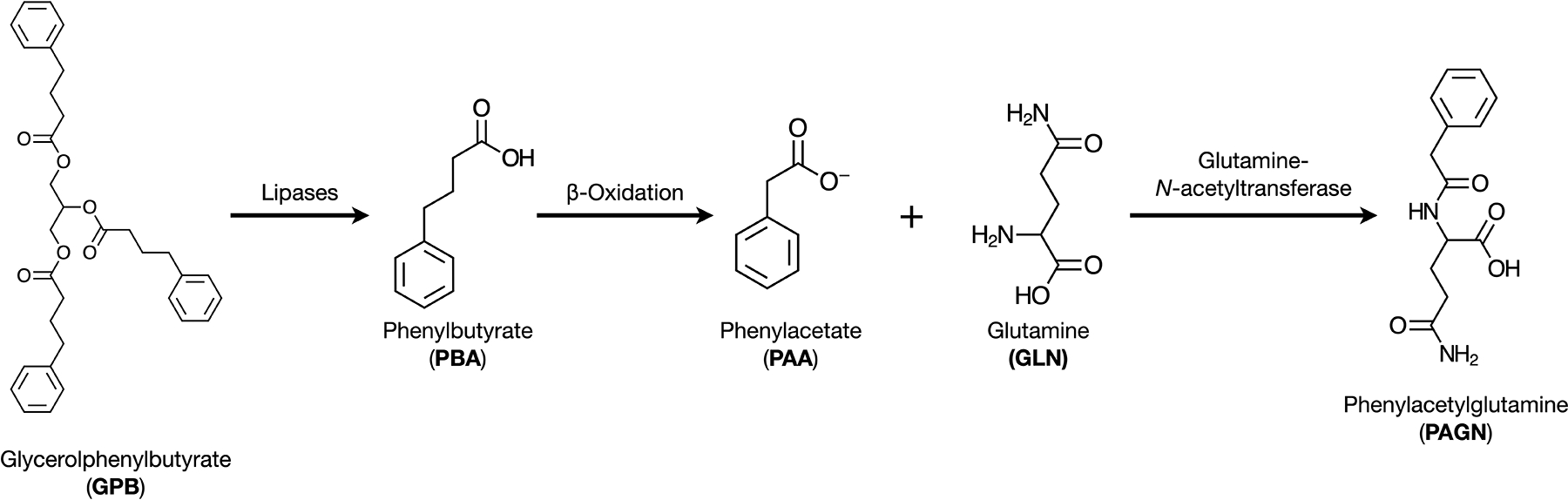
GPB is converted to PBA via the action of pancreatic lipases. PBA, through β-oxidation is converted into its active form PAA. PAA is conjugated to glutamine (GLN) by the enzyme glutamine-N-acetyltransferase to form phenylacetylglutamine (PAGN).
The ongoing adjustment and monitoring of dietary as well as alternate pathway therapy with NaPBA and GPB must account for growth, severity of underlying UCD, prior history of hyperammonemia, and intercurrent illnesses. Whereas plasma ammonia and amino acid concentrations including glutamine are the primary biochemical biomarkers that are utilized to help guide therapy, over the recent years, phenylbutyrate (PBA) metabolite analyses are also being increasingly used to evaluate the safety and efficacy of therapy.[7–9] Ultra-performance liquid chromatography/tandem mass spectroscopy-based methods (UPLC-MS/MS) have been developed for quantifying PAA, PBA, and PAGN concentrations as well as ratios between these analytes in plasma and urine.[7–9] In a recent survey-based analysis of clinicians (n=52) providing care for individuals with UCDs, 58% reported that they had ordered PBA metabolite testing.[10] Such testing may be of utility in assessing the efficacy of conjugation of PAA-based medications, in identifying individuals with elevated levels of PAA, and in monitoring of treatment compliance. Whereas previous studies have suggested that plasma PAA concentrations above 500 μg/mL are toxic, neurological adverse events in humans have been observed at much lower levels of PAA.[11, 12] One population pharmacokinetic modeling study has shown that the Km for glutamine-N-acetyltransferase which conjugates PAA to glutamine is approximately 190 μM for PAA.[13] Furthermore, using clinical trial data, it has been shown that the inflection points for increasing PAA concentrations occur at PAA and PAA:PAGN ratio levels greater than 200 μg/mL and 2.5, respectively.[12] Thus while these levels are now the more commonly used clinical cut offs, they actually may be more conservative when monitoring of safety.[9]
While PBA metabolite analyses are being utilized widely,[10] currently, there is a paucity of data on the true incidence of elevated PAA levels and the clinical and biochemical covariates that associate with such elevated levels. Additionally, in a survey-based study, Ficicioglu et al. have shown that many practitioners who ordered such testing did not have a clear understanding of the targeted range of analytes, validity of these assays, and their interpretation.[10] In this study, we ascertained the prevalence of elevated plasma PAA levels from a large dataset of plasma PBA metabolite assays conducted by a single referral clinical laboratory in the U.S. over a seven-year period. Additionally, to understand the various clinical or biochemical factors that can associate with the levels of the plasma PBA metabolite analytes, we reviewed the clinical and laboratory data from a subset of patients that were treated at a single tertiary care hospital. Our study is an important step to enhance our understanding of safety and efficacy of PAA-based medications in the real-world scenario as a means of improving overall patient safety and compliance.
2. Materials and Methods
2.1. PBA metabolite testing
Plasma samples included in this study were sent for clinical testing at Baylor Genetics, Houston, TX, U.S. This laboratory, certified by the College of American Pathologists and in compliance with the Clinical Laboratory Improvement Amendments, is a referral laboratory wherein the PBA metabolite analyses are performed on samples received from across the U.S. Samples were processed and analyzed utilizing previously described protocols.[9] Note that the clinical laboratory uses the LLoQ (lower limit of quantitation) as its cutoff for reliable determination for reporting. The LLoQ thresholds in this test are 0.04 μg/ml for PAA, 2 μg/ml for PBA, and 2.6 μg/ml for PAGN. The study protocol was approved by the Institutional Review Board (IRB) of Baylor College of Medicine. For this study, data from samples submitted in the period from July 2013 to April 2020 were analyzed retrospectively. All PBA metabolite tests included in the study were ordered by referring health care providers. For analyses, all samples during the study period were included with no testing eligibility criteria or exclusions. Information regarding age at the time of analysis, sex assigned at birth, and diagnosis (when available) was extracted from a centralized database housed on a secure server of the laboratory. This information was made available to the laboratory via requisition forms that were submitted along with the plasma samples by the referring provider.
2.2. Clinical and laboratory data from patients at a single tertiary referral center
Samples submitted to Baylor Genetics did not have accompanying clinical information other than age, sex assigned at birth, and in a subset of samples, type of UCD. Thus, analyses aimed at understanding the association of analyte levels with various clinical and laboratory covariates could not be conducted on the entire dataset. To overcome this limitation, in a subset of samples that were submitted for analyses from Texas Children’s Hospital, the primary pediatric hospital affiliated with Baylor College of Medicine, Houston, TX, we retrospectively extracted relevant clinical and laboratory data. The study protocol was approved by the IRB of Baylor College of Medicine. All individuals who had ever had a plasma PBA metabolite assay performed for their care between July 2013 to April 2020 were included in the analyses. The following clinical data were extracted from the time of sample submission for PBA metabolite analysis: age, sex assigned at birth, weight (in kg), body surface area (BSA), UCD type, type of nitrogen-scavenging medication (oral formulations of NaB vs. NaPBA vs. GPB vs combinatorial therapy vs. intravenous NaPAA/NaB), dose of medications (in g/m2/day), amino acid supplementation (yes vs. no and arginine vs. citrulline), dose of amino acid supplement (in g/kg body weight/day), and recommended daily protein allowance. The following laboratory data were collected if obtained within 24 hours of the plasma PBA metabolite assay: plasma ammonia, glutamine, glycine, and citrulline and serum alanine transaminase (ALT), aspartate transaminase (AST), gamma-glutamyl transferase (GGT), and creatinine (Cr). The laboratory samples were analyzed in the clinical diagnostic laboratories at Texas Children’s Hospital or an outside diagnostic laboratory as part of routine clinical care.
2.2. Statistical Analysis:
Demographic and baseline data for the entire set of assays and patients were summarized by means with standard deviations and frequencies with percentages. Separate simple linear regression models estimated the association between age at first visit (years) and each outcome measure on plasma PBA metabolite analysis (i.e., PBA, PAA, PAGN, PBA:PAA, PAA:PAGN, and PBA:PAGN); these outcome measures were log-transformed for regression analysis. Change in response over time was estimated using general linear mixed models with random intercept and slope. A separate model was fit for each outcome. Statistical significance was assessed at the 0.05 level (two-sided).
Categorical variables were summarized by frequencies and percentages while continuous measures were summarized by means with standard deviations. The association between each clinical or laboratory covariate with each PBA analyte concentration (i.e., PBA, PAA, PAGN, PBA:PAA, PAA:PAGN, and PBA:PAGN) was tested using unadjusted linear regression models. Separate multiple linear regression models were then constructed for each analyte measure. Predictor variables for each analyte were selected from the results for the unadjusted linear regression models. If the unadjusted P-value from the unadjusted linear regression model was <0.05, then the variable was included in the multiple linear models. All analyte outcome measures were log-transformed for analysis.
3. Results:
3.1. Characteristics of the study population
During the study period, a total of 1255 plasma PBA assays were performed. These samples represented 387 unique individuals (Table 1). The median age of individuals at the time of the first testing was 10.6 years (range 4 days to 68 years) (Table S1). Approximately 61% (n=235) had more than one assay collected during the study period with 16% of individuals having had more than 5 assays during the study period. At the time of their first assay, 30% (n=117) of individuals were adults (age >18 years) and 70% (n=270) were children. Whereas information on the age and sex assigned at birth was available on all, clinical diagnoses were only available for approximately 40% (n=154) of individuals. The most common diagnosis was OTCD (n=80, 52%) followed by ASS1D (n=29; 19%), ARG1D (n=19; 12%), and ASLD (n=13, 8%). Approximately 5% (n=8) were diagnosed with “other” conditions. This group included diagnoses of “hyperammonemia”, lysinuric protein intolerance, and HHH syndrome.
Table 1:
Characteristics of the study population that underwent PBA metabolite testing (*percentages rounded to the nearest whole number).
| Characteristic | No. (%)* |
|---|---|
| Age at First Assay | |
| <2 years | 88 (23) |
| 2–18 years | 182 (47) |
| >18 years | 117 (30) |
| Diagnoses | |
| Diagnosis information not available | 233 (60) |
| Diagnosis information available | 154 (40) |
| OTCD | 80 (52) |
| ASS1D | 29 (19) |
| ARG1D | 19 (12) |
| ASLD | 13 (8) |
| CPS1D | 5 (3) |
| Other | 8 (5) |
| Total Individuals | 387 |
| Number of assays per Individual | |
| Individuals with 1 assay | 152 (39) |
| Individuals with 2 assays | 71 (18) |
| Individuals with 3–5 assays | 104 (27) |
| Individuals with >5 assays | 60 (16) |
| Total number of assays | 1255 |
3.2. Plasma PAA and PAA:PAGN levels in samples collected during routine clinical management
Plasma PAA concentration greater than 200 μg/mL and/or PAA:PAGN ratio greater than 2.5 were used as cut off values for upper limits of normal.[9, 12] Overall, 52 of 1254 (4.1%) samples were identified with elevated plasma PAA concentrations (n=38 individuals). The mean/median (SD; range) age of individuals at the time these assays were obtained was 8/5 years (8 years; 9 days to 42 years). Elevated PAA:PAGN ratios were identified in 54 of 1254 (4.3%) assays (n=42 individuals). The mean/median (SD; range) age of individuals was 8/4 years (9 years; 4 days to 40 years) at the time of assay. A total of 33 assays (2.6%) were found to have both PAA and PAA:PAGN elevated (n=27 individuals); the mean/median (SD; range) age in this subset of individuals was 7/3 years (9 years; 9 days to 40 years). These results demonstrate that in the vast majority of individuals who underwent testing, plasma PAA levels were within ranges that are considered safe.
3.3. PAA and PAA:PAGN show a negative association with age in samples collected during routine clinical management
Maximum likelihood methods were used to generate a simple linear regression for age and analyte concentration during the first assay for each individual (Figure 2). Statistically significant relationships were found between: (1) age and PAA concentration, (2) age and PAA:PAGN and (3) age and PBA:PAA. On average, the concentration of plasma PAA decreased by 2.3% (95% CI: −3.7, −0.8; P=0.002) and PAA:PAGN decreased by 1.9% (95% CI: −2.7, −1.0; P<0.001) per year increase in age at first assay (Table S2). Plasma PBA:PAA increased by 1.9% (95% CI: 0.5, 3.2) per year increase in age at first measurement (P=0.007). No significant associations were observed between concentrations of PBA, PAGN, and PBA:PAGN and age at first assay. When assays were analyzed over time, statistically significant associations were found only between (1) age and PBA:PAGN and (2) age and PAA:PAGN (Table S3). On average, PBA:PAGN increased by 9.7% (95% CI: 2.7, 17.1; P=0.006) per year while PAA:PAGN increased by 6.1% (95% CI: 1.1, 11.4; P=0.017) per year.
Figure 2. Association between PBA metabolic assay analytes and age.
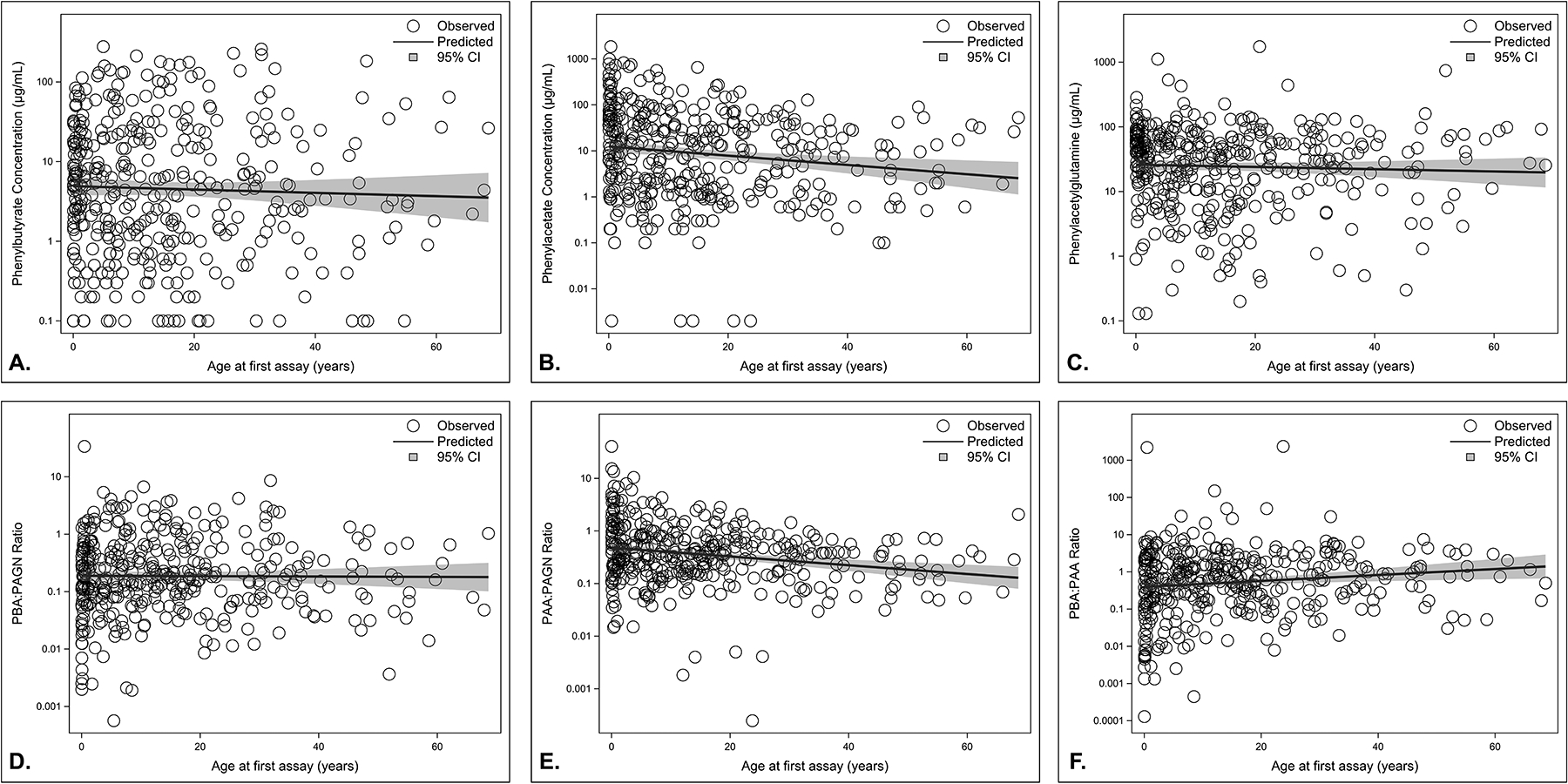
Likelihood function for age and analyte concentration at the time of the first assay received at Baylor Genetics show that PAA and PAA:PAGN have a negative association with age; whereas, PBA:PAA shows a positive association with age. Sample size = 387. Circles indicate individual observes values. The line and shaded bands represent the predicted mean change with age and upper and lower bounds of the prediction, respectively. A: Phenylbutyrate (PBA) concentration plotted against age. B: Phenylacetate (PAA) concentration plotted against age. C: Phenylacetylglutamine (PAGN) plotted against age. D: PBA:PAGN ratio plotted against age. E: PAA:PAGN ratio plotted against age. F: PBA:PAA ratio plotted against age.
3.4. Association of clinical covariates with drug metabolite concentrations
To examine the association between clinical and diagnostic covariates and analyte levels on plasma PBA metabolic levels, we analyzed data from 68 individuals at the time of their first PBA level testing (n = 68 assays). All patients were being treated at Texas Children’s Hospital, Houston, TX, USA (Table 2) and had a diagnosis of a UCD or underlying metabolic disorder. OTCD was the most common UCD, followed by ASS1D, ARG1D, ASLD, and CPS1D. In this cohort, 41 individuals were being treated with GPB while 27 were receiving NaPBA; all individuals were receiving doses that were within the recommended dose ranges. Thirteen individuals were also being treated with NaB in addition to therapy with GPB or NaPBA. All except one individual had been prescribed a diet that was limited to the recommended, age-appropriate dietary allowance (RDA) of protein.
Table 2:
Association between clinical covariates, PBA metabolite concentrations, and PBA metabolite ratios. All statistically significant associations have been marked in bold font.
| Variable | No. Observations | Value | Unadjusted Linear Regression Result (p-value) | ||||
|---|---|---|---|---|---|---|---|
| logPAA | logPBA | logPAGN | log(PAA:PAGN) | ||||
| Mean (SD) age at first assay (years) | 68 | 14.7 (14.8) | 0.0212 | 0.4088 | 0.1064 | 0.0404 | |
| Mean (SD) body surface area m2 | 68 | 1.1 (0.6) | 0.0032 | 0.6787 | 0.0799 | 0.0036 | |
| Diagnosis N (%) | 68 | CPS1D | 4 (5.9) | 0.0616 | 0.3435 | 0.1404 | 0.0599 |
| OTCD | 32 (47.1) | ||||||
| ASS1D | 15 (22.1) | ||||||
| ASLD | 4 (5.9) | ||||||
| ARG1D | 10 (14.7) | ||||||
| Others | 3 (4.4) | ||||||
| Mean (SD) GPB dose in g/m2/day | 68 | 4.9 (4.6) | 0.1243 | 0.4966 | 0.4639 | 0.0867 | |
| Mean (SD) NaPBA dose in g/m2/day | 68 | 3.4 (4.8) | 0.0019 | 0.3315 | 0.0401 | 0.0042 | |
| Mean (SD) Ammonia μmol/L | 46 | 34.4 (74.2) | 0.7397 | 0.4809 | 0.2871 | 0.6929 | |
| Mean (SD) AST U/L | 43 | 53.5 (40.8) | 0.7901 | 0.8684 | 0.3973 | 0.6485 | |
| Mean (SD) ALT U/L | 43 | 93.4 (147.1) | 0.3319 | 0.4976 | 0.4519 | 0.0179 | |
| Mean (SD) GGT U/L | 7 | 49.7 (47.0) | 0.0883 | 0.5375 | 0.3547 | 0.0144 | |
| Mean (SD) Glutamine μmol/L | 62 | 610.4 (261.2) | 0.0019 | 0.9903 | 0.0428 | 0.0013 | |
| Mean (SD) Glycine μmol/L | 62 | 249.0 (110.4) | 0.0042 | 0.3528 | 0.0652 | 0.0026 | |
Unadjusted linear regression modeling demonstrated that both log transformed PAA and PAA:PAGN showed an association with age, dose of NaPBA (but not GPB), plasma glycine, and plasma glutamine concentrations (Table 2, Table S4). There was an inverse relationship between PAA and age, plasma glutamine, and plasma glycine levels (i.e., higher values for each covariate was associated with lower PAA). Similar relationships were observed between PAA:PAGN ratios. Furthermore, PAA:PAGN also showed a negative association with plasma ALT and GGT levels (i.e., higher AST or GGT was associated with lower PAA:PAGN).
A multiple linear regression model was constructed for each PBA drug analyte utilizing the statistically significant co-variates identified. The model for logPAA, for example, consisted of age, NaPBA dose, plasma glycine, and plasma glutamine (Table S5). Based on this type of model, the relationships between logPAA, NaPBA dose, plasma glycine, and plasma glutamine reached statistical significance. On average, an increase in one g/m2/day dose of NaPBA was associated with a 13.2% (95% CI: 4.2%, 22.6%) increase in PAA (P=0.004) by one unit. Increase in plasma glutamine by 1 μmol/ml was associated with decrease in PAA of 0.246% (95% CI: −0.42%, −0.07%; P=0.006). Similarly, for logPAGN levels, statistically significant associations were observed with NaPBA dose and plasma glutamine levels (Table S6).
The multiple linear regression model for logPAA:PAGN was constructed using age, NaPBA dose, serum ALT, plasma glutamine, and plasma glycine levels (serum GGT levels were excluded due to multiple missing values) (Table S7). After adjusting for the other clinical variables, PAA:PAGN was found to be significantly associated with NaPBA dose and plasma glutamine only. On average, an increase in one g/m2/ day dose of NaPBA was associated with a 7.25% (95% CI: 2.3%, 12.2%; P=0.005) increase in PAA:PAGN. Similarly, a 0.124% (95% CI: −0.227%, −0.021%) decrease in PAA:PAGN decreases was associated with a unit increase in plasma glutamine (P=0.0192).
4. Discussion:
Medications that promote nitrogen excretion via the conjugation of PAA with glutamine are a key component in the management of patients with UCDs. An overarching goal for adjusting the dose of these medications is to optimize the nitrogen excretion to reduce the risk of hyperammonemia while preventing the accumulation of unconjugated PAA that can cause adverse reactions. Elevated PAA levels have been associated with nausea, headache, fatigue, weakness, somnolence, confusion and altered mental status[14–16]; some of these adverse effects overlap with symptoms of hyperammonemia. Neurological adverse events have typically been associated with intravenous formulations of NaPAA, and previous work has estimated that toxic levels of NaPAA are greater than 400 μg/ml.[14] However, such adverse events have also been observed with oral formulations of PBA and at PAA levels that are well below the 400 μg/ml. For example, in one study in healthy adult volunteers, neurological adverse events were noted with increasing frequency with elevated doses of GPB and these events occurred even when the mean PAA levels were below 100 μg/ml.[17] Analyses of PBA metabolite data from samples obtained in the course of clinical trials in adults and children (i.e., healthy volunteers, individuals with UCDs, and individuals with hepatic encephalopathy) have shown that there is a curvilinear relationship of PAA concentrations and PAA:PAGN.[13, 18] At PAA concentrations of 200 μg/ml and PAA:PAGN of 2.5, there is a sharp upward inflection and individuals with PAA:PAGN ≥ 2.5 tended to have higher PAA levels than those with a ratio ≤ 2.5.[12] Additionally, in that study, PAA:PAGN ratios ≥ 2.5 conferred a 20 times higher probability of being associated with PAA levels in the toxic ranges. Thus, PAA concentration of ≥ 200 μg/ml and PAA:PAGN ratio of ≥ 2.5 would be reasonable and conservative estimates for “safe levels”.
In healthy adults, we have previously demonstrated that conjugation efficacy for NaPBA is ~65%, and that the conjugation efficacy was superior at a lower dose of NaPBA (7.15 g/m2/day) as compared a higher dose of the medication (3.575 g/m2/day).[6] Thus, increases in dose of medication may not translate to a linear increase in efficacy. Monitoring therapy with PBA metabolite analyses may help in understanding the prevalence of high PAA or high PAA:PAGN in individuals with UCDs. Such analyses have been performed in the setting of controlled clinical trials wherein there is more oversight on the prescription of medications and participant adherence to the prescribed dose. These analyses have, however, rarely been performed in the context of routine clinical care. In this study, we not only estimated the frequency of finding PAA and PAGN levels above the proposed safe ranges but also analyzed the potential clinical and biochemical covariates that are associated with drug metabolite concentrations.
Overall, less than 5% of all clinical plasma samples submitted for analyses had PAA concentration ≥ 200 μg/ml or PAA:PAGN ≥2.5; only 2.6% samples had both parameters above the safe range. These results show that in a real-world scenario, treatment with medications that elicit nitrogen excretion via the conjugation of PAA with glutamine are not associated with PAA levels that would be considered unsafe. These results are reassuringly consistent with data from carefully controlled clinical trials.[10, 12] PAA levels are significantly affected by the time of collection of the sample and can fluctuate widely based on the relationship between the timing of the dose and the time of blood draw. However, PAA:PAGN has shown to be relatively stable and unaffected by the timing of sample collection. Interestingly, we found elevated PAA:PAGN ratios even in individuals in whom PAA levels were well below 200 μg/ml. In one metabolite analysis for example, a PAA:PAGN ratio of 3.0 was found in the setting of significantly low plasma PAA (0.30 μg/ml) and PAGN (0.10 μg/ml) concentrations. Such levels may indicate either sub-therapeutic dosing or poor adherence and, in addition, such low concentrations are difficult to quantify accurately making ratio calculations highly imprecise. Thus, we propose that evaluation of the PAA:PAGN ratio alone is likely to overestimate the risk of toxic PAA levels, in some cases, and that both PAA and PAA:PAGN be considered when interpreting these tests.
At the level of the entire cohort, we found a negative association between age at which the first sample was collected and PAA and PAA:PAGN. The lack of detailed clinical information including the context of use, formulation and dose of medication, and severity of UCD being treated make it difficult to identify the reasons that drive such an association. However, some potential explanations include the following: 1) children with more metabolically unstable UCDs during the periods of infancy and early childhood may have received a higher dose per body surface area of the medication, 2) samples from children may overrepresent samples drawn during intravenous therapy with NaPAA, 3) the ability of pancreatic lipases to hydrolyze GPB in infants, especially those younger than 2 months of age, and the conjugation capacity of the pediatric liver may lead to less than optimal drug conjugation. Regardless of the cause, the findings of higher levels of PAA and PAA:PAGN in younger children is an important indicator that such therapy should be followed more closely in the pediatric population.
Our analyses identified potential clinical covariates including age, ALT, AST, GGT, glutamine, glycine, and dose of NaPBA that associate with PAA or PAA:PAGN. However, on multiple linear regression analysis, only the dose of NaPBA and plasma glutamine levels associate with PAA or PAA:PAGN. Intuitively, a higher dose of NaPBA may be associated with higher PAA levels, as conjugation efficacy may not increase linearly with dose, and a larger plasma glutamine pool may lead to higher PAGN levels.[13, 17–20] Interesting such associations were not found with GPB. It is possible that the better pharmacokinetics of GPB compared to NaPBA could be responsible for this difference.
These results must be interpreted in the context of the strengths and limitations of the dataset. The samples were analyzed in a single referral laboratory using established standard operating procedures, thus reducing the analytical variability. However, at the level of the entire cohort of samples, we did not have access to detailed clinical information like severity of UCD, formulations or dose of medication, relationship between timing of sample collection and administration of medications, symptoms at the time of sample collection, frequently of hyperammonemia and absence or presence of liver disease. These limitations precluded finding associations in this large sample size. The sample sizes for some of the rarer forms of UCDs were limited thus limiting the analyses in a disorder-specific manner. To address the limitation of clinical information at the level of the entire cohort, we extracted and analyzed clinical data from a small cohort of individuals followed at a large UCD referral center. These analyses allowed us to test associations between clinical covariates and the drug metabolite levels. Furthermore, these samples were not collected on all patients receiving care in a standardized manner but rather based on the clinical practice preference of individual providers. Despite these limitations, our study is the most comprehensive examination of PBA metabolite assays and highlights facets of UCD care that have previously remained unexplored.
In summary, in patients undergoing PBA metabolite testing during routine clinical management, the vast majority of individuals have levels that are deemed safe. The levels of PAA tend to be higher in younger individuals and thus, children may have to be monitored more closely. Increased dosage of NaPBA is associated with increased PAA levels and thus, clinicians may want to consider getting PBA metabolite testing while increasing dosing of these medications to minimize the risk of toxicity.
Supplementary Material
Funding Statement:
This work was funded by a Urea Cycle Disorders Consortium training fellowship grant (K.E.G.). The Urea Cycle Disorders Consortium [UCDC; U54HD061221] is part of the National Institutes of Health (NIH) Rare Disease Clinical Research Network (RDCRN), supported through a collaboration between the Office of Rare Diseases Research (ORDR), the National Center for Advancing Translational Science (NCATS), the Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), and the National Institute of Diabetes and Digestive and Kidney Diseases. The Urea Cycle Disorders Consortium is also supported by the O’Malley Foundation and the Kettering Fund. This study was supported by the Baylor College of Medicine Intellectual and Developmental Disability Research Centers [P50 HD103555] from the Eunice Kennedy Shriver National Institute of Child Health and Human Development. The content is solely the responsibility of the authors and does not necessarily represent the official views of the Eunice Kennedy Shriver National Institute of Child Health and Human Development or the National Institutes of Health. L.C.B. holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund
Footnotes
Ethics Declaration: This study was approved with a waiver of consent by the Baylor College of Medicine Institutional Review Board.
Conflict of Interest: K.E.G., N.L., Q.S., S.H.E., L.C.B., and S.C.S.N. are employees of the Baylor College of Medicine, which generates genetic testing revenue in a partnership with Baylor Genetics.
Data Availability:
All patient data has been de-identified and is available here and in the Supplementary Materials.
References
- [1].Brusilow S, Valle D, and Batshaw M, New Pathways of Nitrogen Excretion in Inborn Errors of Urea Synthesis. The Lancet, 1979. 314(8140): p. 452–454. 10.1016/s0140-6736(79)91503-4 [DOI] [PubMed] [Google Scholar]
- [2].Maestri NE, Clissold DB, and Brusilow SW, Long-term survival of patients with argininosuccinate synthetase deficiency. J Pediatr, 1995. 127(6): p. 929–35. 10.1016/s0022-3476(95)70030-7 [DOI] [PubMed] [Google Scholar]
- [3].Diaz GA, Krivitzky LS, Mokhtarani M, Rhead W, et al. , Ammonia control and neurocognitive outcome among urea cycle disorder patients treated with glycerol phenylbutyrate. Hepatology, 2013. 57(6): p. 2171–9. 10.1002/hep.26058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Batshaw ML, MacArthur RB, and Tuchman M, Alternative pathway therapy for urea cycle disorders: twenty years later. J Pediatr, 2001. 138(1 Suppl): p. S46–54; discussion S54–5. 10.1067/mpd.2001.111836 [DOI] [PubMed] [Google Scholar]
- [5].Haberle J, Burlina A, Chakrapani A, Dixon M, et al. , Suggested guidelines for the diagnosis and management of urea cycle disorders: First revision. J Inherit Metab Dis, 2019. 42(6): p. 1192–1230. 10.1002/jimd.12100 [DOI] [PubMed] [Google Scholar]
- [6].Nagamani SCS, Agarwal U, Tam A, Azamian M, et al. , A randomized trial to study the comparative efficacy of phenylbutyrate and benzoate on nitrogen excretion and ureagenesis in healthy volunteers. Genet Med, 2018. 20(7): p. 708–716. 10.1038/gim.2017.167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Mokhtarani M, Diaz GA, Rhead W, Lichter-Konecki U, et al. , Urinary phenylacetylglutamine as dosing biomarker for patients with urea cycle disorders. Mol Genet Metab, 2012. 107(3): p. 308–14. 10.1016/j.ymgme.2012.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Mokhtarani M, Diaz GA, Lichter-Konecki U, Berry SA, et al. , Urinary phenylacetylglutamine (U-PAGN) concentration as biomarker for adherence in patients with urea cycle disorders (UCD) treated with glycerol phenylbutyrate. Mol Genet Metab Rep, 2015. 5: p. 12–14. 10.1016/j.ymgmr.2015.09.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jiang Y, Almannai M, Sutton VR, Sun Q, et al. , Quantitation of phenylbutyrate metabolites by UPLC-MS/MS demonstrates inverse correlation of phenylacetate:phenylacetylglutamine ratio with plasma glutamine levels. Mol Genet Metab, 2017. 122(3): p. 39–45. 10.1016/j.ymgme.2017.08.011 [DOI] [PubMed] [Google Scholar]
- [10].Ficicioglu C, Liu N, Sun Q, Burdett A, et al. , Perceptions and use of phenylbutyrate metabolite testing in urea cycle disorders: Results of a clinician survey and analysis of a centralized testing database. Mol Genet Metab, 2021. 10.1016/j.ymgme.2021.12.007 [DOI] [PubMed] [Google Scholar]
- [11].Simell O, Sipila I, Rajantie J, Valle DL, et al. , Waste nitrogen excretion via amino acid acylation: benzoate and phenylacetate in lysinuric protein intolerance. Pediatr Res, 1986. 20(11): p. 1117–21. 10.1203/00006450-198611000-00011 [DOI] [PubMed] [Google Scholar]
- [12].Mokhtarani M, Diaz GA, Rhead W, Berry SA, et al. , Elevated phenylacetic acid levels do not correlate with adverse events in patients with urea cycle disorders or hepatic encephalopathy and can be predicted based on the plasma PAA to PAGN ratio. Mol Genet Metab, 2013. 110(4): p. 446–53. 10.1016/j.ymgme.2013.09.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Monteleone JP, Mokhtarani M, Diaz GA, Rhead W, et al. , Population pharmacokinetic modeling and dosing simulations of nitrogen-scavenging compounds: disposition of glycerol phenylbutyrate and sodium phenylbutyrate in adult and pediatric patients with urea cycle disorders. J Clin Pharmacol, 2013. 53(7): p. 699–710. 10.1002/jcph.92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Thibault A, Cooper MR, Figg WD, Venzon DJ, et al. , A phase I and pharmacokinetic study of intravenous phenylacetate in patients with cancer. Cancer research, 1994. 54(7): p. 1690–1694. [PubMed] [Google Scholar]
- [15].Thibault A, Samid D, Cooper MR, Figg WD, et al. , Phase I study of phenylacetate administered twice daily to patients with cancer. Cancer, 1995. 75(12): p. 2932–2938. [DOI] [PubMed] [Google Scholar]
- [16].Praphanproj V, Boyadjiev S, Waber L, Brusilow S, et al. , Three cases of intravenous sodium benzoate and sodium phenylacetate toxicity occurring in the treatment of acute hyperammonaemia. Journal of inherited metabolic disease, 2000. 23(2): p. 129–136. [DOI] [PubMed] [Google Scholar]
- [17].McGuire BM, Zupanets IA, Lowe ME, Xiao X, et al. , Pharmacology and safety of glycerol phenylbutyrate in healthy adults and adults with cirrhosis. Hepatology, 2010. 51(6): p. 2077–85. 10.1002/hep.23589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Berry SA, Vockley J, Vinks AA, Dong M, et al. , Pharmacokinetics of glycerol phenylbutyrate in pediatric patients 2months to 2years of age with urea cycle disorders. Mol Genet Metab, 2018. 125(3): p. 251–257. 10.1016/j.ymgme.2018.09.001 [DOI] [PubMed] [Google Scholar]
- [19].Marini JC, Lanpher BC, Scaglia F, O’Brien WE, et al. , Phenylbutyrate improves nitrogen disposal via an alternative pathway without eliciting an increase in protein breakdown and catabolism in control and ornithine transcarbamylase-deficient patients. Am J Clin Nutr, 2011. 93(6): p. 1248–54. 10.3945/ajcn.110.009043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].MacLeod EL, Hall KD, and McGuire PJ, Computational modeling to predict nitrogen balance during acute metabolic decompensation in patients with urea cycle disorders. J Inherit Metab Dis, 2016. 39(1): p. 17–24. 10.1007/s10545-015-9882-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All patient data has been de-identified and is available here and in the Supplementary Materials.


