Abstract
We have found differences among the populations of hepatitis C virus sequences in serum, peripheral blood mononuclear cells (PBMCs), and various tissues in patients with chronic hepatitis C. These results are compatible with the existence of independent viral compartments in the infected host. Our results also suggest that PBMCs, and probably various tissues, can selectively adsorb viral subpopulations differing in the E2 region.
The issue of extrahepatic replication of hepatitis C virus (HCV) remains controversial. Although several groups of researchers have reported the presence of HCV negative-strand RNA, which is a replicative intermediate, in peripheral blood mononuclear cells (PBMCs) (11–13), it has been shown that the reverse transcription (RT)-PCR used for detection of the viral negative strand lacks strand specificity (6), and subsequent studies employing assays optimized for strand specificity failed to demonstrate the presence of the viral negative strand in PBMCs (5, 10).
We have recently reported that HCV negative-strand RNA can be detected in various extrahepatic tissues from AIDS patients (8) and that the presence of extrahepatic HCV replication may correlate with changes in the 5′ untranslated region (5′UTR) quasispecies composition at the involved sites (7). However, as these studies were conducted on severely immunocompromised subjects, it is unclear whether the results are applicable to immunocompetent patients. In addition, appropriate control studies necessary to exclude the possibility that various tissues adsorb a subset of the circulating HCV population could not be performed.
In the current study, we analyzed HCV in autopsy tissues from three human immunodeficiency virus (HIV)-negative subjects who died of terminal HCV-related liver insufficiency and in PBMCs from five other patients with chronic hepatitis C. In addition to strand-specific detection of HCV negative-strand RNA, we compared the viral quasispecies composition at various sites assuming that in the presence of independent viral compartments, it could differ, much like that of HIV (1, 4). Two different viral regions were analyzed: the highly conserved 5′UTR and the highly variable E2 envelope region.
Biological samples.
Tissue samples were collected from three HIV-negative patients with end-stage HCV-related disease who died of liver insufficiency. Tissue samples were obtained during routine autopsies conducted within 48 h of death and stored at −80°C until analysis. Samples of the following tissues were collected: liver, bone marrow, mediastinal lymph node, pancreas, thyroid, adrenal gland, kidney, lung, spleen, and skin. RNA was extracted as described previously (8); 1 μg of total RNA (as determined by spectrophotometry) was routinely used for RT-PCR.
PBMCs were collected from five HIV-negative, HCV-positive patients with chronic hepatitis. PBMCs were isolated by standard density gradient centrifugation. RNA was extracted from 5 × 106 to 1 × 107 cells (7) and dissolved in 30 μl of water. Ten microliters of this RNA solution was used for RT-PCR.
Strand-specific RT-PCR with Tth.
Tth-based RT-PCR detection of the HCV RNA negative strand was performed as described elsewhere (8). This assay was capable of detecting about 100 genomic equivalent molecules of the correct strand while unspecifically detecting ≥108 equivalents of the incorrect strand.
Analysis of HCV quasispecies.
Nested or heminested protocols were used to maximize the yield of the PCR product. Amplification of the 5′UTR was conducted with RT-PCR as previously described (7, 8). The internal primers used were 5′-ACTGTCTTCACGCAGAAAGCGTC-3′ (nucleotides [nt] 57 to 79) and 5′-CAAGCACCCTATCAGGCAGTACC-3′ (nt 307 to 285).
The E2 region, including the hypervariable region, was amplified by using primers 5′-ASGGGGCTGGGA/GGTGAAA/GCAATAT/CAC-3′ (nt 1882 to 1857) and 5′-CATGGGATATGATGATGAAC/TTGGT-3′ (nt 1297 to 1320) for the first round and 5′-AGGCTATCATTGCAACCAG-3′ (nt 1639 to 1620) and 5′-GCTGCTCCGGATCCCACA-3′ (nt 1349 to 1366) for the nested round. Occasionally, other primers were used (2).
HCV quasispecies were compared by the single-strand conformation polymorphism (SSCP) assay as previously described (9). The HCV genotypes were determined by direct sequencing of the NS5 region (9).
Detection of the HCV RNA negative strand.
The presence of the HCV RNA negative strand was documented in all three liver samples and in pancreas tissue from subject A by using a Tth-based strand-specific assay. When serum-PBMC pairs were analyzed, the HCV RNA negative strand was not detected in any of the samples.
Analysis of 5′UTR sequences in tissue samples.
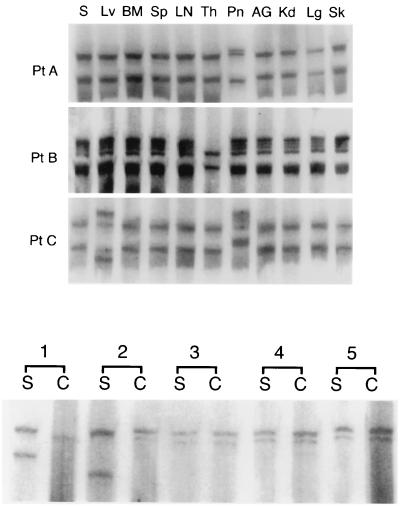
5′UTR sequences of HCV were amplified from all tissue samples in each of the three autopsy cases (subjects A to C). SSCP analysis revealed the presence of identical band patterns in the majority of tissues from a given patient (Fig. 1). However, sequences recovered from the pancreases of patients A and C and sequences recovered from the thyroid of patient B were different from all of the other samples from the same patients. These three disparate PCR products were further analyzed by sequencing and compared to the sequences recovered from the serum, the liver, and other tissues. As shown in Fig. 2, viral sequences recovered from the pancreatic tissue of patients A and C and sequences recovered from the thyroid of patient B differed from the “master” sequence recovered from the serum, the liver, and other tissues by 2 to 16 nt.
FIG. 1.
(Top) Analysis by SSCP assay of 5′UTR HCV sequences amplified from serum (S) and various autopsy tissues from subjects A to C. The tissues examined included liver (Lv), bone marrow (BM), spleen (Sp), lymph node (LN), thyroid (Th), pancreas (Pn), adrenal gland (AG), kidney (Kd), lung (Lg), and skin (Sk). (Bottom) Analysis by SSCP assay of 5′UTR HCV sequences amplified from PBMCs and serum from patients 1 to 5. Lanes S and C represent viral sequences recovered from serum and PBMCs, respectively.
FIG. 2.
Nucleotide sequence alignment of the 5′UTR fragment of HCV recovered from autopsy tissues (patients [Pt] A to C) and serum-PBMC pairs (patients 1 and 2). The sequences are compared to the prototype HCV-1 sequence published by Choo et al. (3), which is shown on the top line. Periods indicate sequence identity with HCV-1; dashes indicate gaps introduced to preserve alignment. S, serum; P, pancreas; Th, thyroid; C, PBMCs.
Analysis of 5′UTR sequences in PBMCs.
SSCP analysis of the 5′UTR revealed the presence of indistinguishable band patterns from PBMCs and serum in three of five patients studied while the band patterns of patients 1 and 2 were different (Fig. 1). The presence of identical and different viral sequences in PBMCs and sera was subsequently verified by direct sequencing of PCR products. In both cases, where SSCP band patterns from serum and PBMCs were dissimilar, the PBMC- and serum-derived HCV master sequences differed by 3 and 5 nt (Fig. 2).
Analysis of the 5′UTR of adsorbed virions.
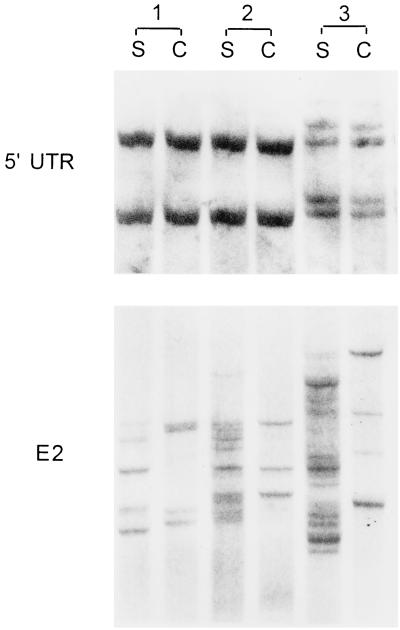
The following experiments have been carried out to exclude the possibility that human PBMCs and/or various tissues can concentrate a subset of the circulating HCV population differing in the 5′UTR. PBMCs were collected from a single HCV-negative donor and washed three times in phosphate-buffered saline (PBS), after which 5 × 106 of the cells were incubated in 2 ml of serum from patient 2 and two other chronic hepatitis C patients. After 1 h of shaking incubation at 37°C, the PBMCs were separated from the serum, washed four times in PBS, extracted, and amplified as described earlier. Subsequent SSCP analysis did not reveal any differences between serum- and PBMC-derived 5′UTR sequences (Fig. 3). Even after the incubation was extended to 4 h, no differences were observed.
FIG. 3.
SSCP analysis of adsorption of HCV on PBMCs. Cells (5 × 106) collected from an HCV-negative donor were incubated in 2 ml of serum (S) from patient 2 (lanes 1) and two other chronic hepatitis C patients (lanes 2 and 3). After 1 h of shaking incubation at 37°C, the PBMCs (C) were separated from the serum, washed four times in PBS, extracted, and amplified as described in the text.
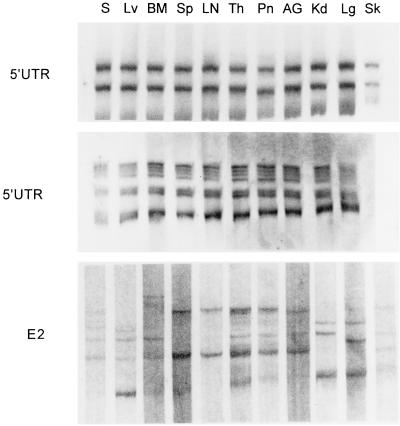
A similar experiment was conducted to check whether various tissues could selectively adsorb subpopulations of virions differing in the 5′UTR. Tissue samples were obtained from an HCV-negative patient who died of cryptogenic cirrhosis. Samples (4 to 5 mg) of various tissues were incubated in the presence of RNase inhibitor (Rnasin; Promega) in 400 μl of serum from subject B and from one other chronic hepatitis C patient. After 1 h of shaking incubation at 37°C, the samples were separated from the serum by centrifugation and washed several times in PBS. Subsequently, RNA was extracted and amplified. As seen in Fig. 4, tissue- and serum-derived 5′UTRs remained identical. However, unlike in the PBMC adsorption experiments, the analysis could not be repeated by using a longer incubation time as this would eventually have led to degradation of the RNA.
FIG. 4.
SSCP analysis of adsorption of HCV on various tissues. Tissue samples were obtained from an HCV-negative patient who died of cryptogenic cirrhosis. Samples (4 to 5 mg) of various tissues were incubated in the presence of an RNase inhibitor in 400 μl of serum from patient B and HCV-positive serum from a chronic hepatitis patient (see text). For definitions of the abbreviations, see the legend to Fig. 1.
E2 quasispecies analysis.
The E2 region is typically highly divergent, resulting in the presence of multiple viral quasispecies. Since it encodes envelope proteins, it is the primary candidate for artifactual polymorphism related to adsorption, as different variants could hypothetically bind with various affinities to human cells. Accordingly, experiments identical to those for the 5′UTR were conducted in which HCV-positive sera were incubated with HCV-negative human tissues and PBMCs. As seen in Fig. 4, even after a short 1-h incubation, E2 quasispecies amplified from a number of tissues differed from those present in the serum with which those tissues were incubated. However, since the tissues were not preserved intact, it cannot be excluded that these apparent differences were related to adsorption of virions to intracellular components. In this respect, incubation of HCV-positive sera with PBMCs, the integrity of which remained largely intact throughout the experiment, is more likely to provide clear-cut evidence of selective E2 quasispecies adsorption on the outer cell membrane. As seen in Fig. 3, after 1 h of incubation, serum- and PBMC-derived E2 sequences became different. The observed band pattern was reproducible. Thus, as human PBMCs and possibly various tissues apparently selectively adsorb viral subpopulations differing in the E2 region, the results of E2 quasispecies comparisons should be interpreted with caution. However, it is unclear whether sequences derived from adsorbed HCV would actually affect viral sequences already present in the tissue. Moreover, it cannot be excluded, although it seems unlikely, that some of the observed bands represent unspecific amplification products.
When the E2 region was amplified from tissue samples and PBMC-serum pairs, multiple differences were evident (data not shown). However, in the light of the above-described adsorption studies, it is unclear whether these differences were related to the existence of independent viral compartments.
Distribution of PBMC- and tissue-specific sequences.
Several sets of specific primers have been designed with mismatches at the 3′ end with respect to one or the other viral sequence. After optimization, the strain-specific assays developed were found to be capable of detecting the minor sequence in the PCR product even when it was present at a concentration of 1:106 relative to the other sequence. However, the 5′UTR sequences present in the pancreas of patient C were not different enough from serum-derived 5′UTR sequences to allow an efficient and specific PCR assay to be developed.
The pancreas-derived sequences of patient A were detected in lymph node, thyroid, and adrenal gland samples, while the thyroid-derived sequences of patient B were detected in lymph node, bone marrow, skin, and adrenal gland samples. By analysis of serial dilutions of the PCR products, it was determined that their titer was 2 to 3 logs lower than the titer of the major serum-derived strain.
The PBMC-derived sequences of patients 1 and 2 were not detected in serum. However, the serum-derived HCV RNA sequence was detected in PBMCs at a ratio of 1:102 to 1:103 with respect to the PBMC-derived sequence. The latter ratio likely represents the approximate ratio of cell-adsorbed to cell-derived HCV RNA and suggests that, at least for PBMCs, the majority of the viral RNA is of cellular origin.
In summary, by studying PBMCs and a wide range of autopsy tissues from HCV-infected immunocompetent subjects, we found that a number of samples contained 5′UTR quasispecies differing from those found in the liver or circulating in the serum. The presence of these tissue-unique sequences is compatible with independent viral replication at extrahepatic sites, although the lineage of the infected cells is unclear. Our results also suggest that PBMCs, and probably various tissues, can selectively adsorb viral subpopulations differing in the E2 region.
REFERENCES
- 1.Ait-Khaled M, McLaughlin J E, Johnson M A, Emery V C. Distinct HIV-1 long terminal repeat quasispecies present in nervous tissues compared to that in lung, blood and lymphoid tissues of an AIDS patient. AIDS. 1995;9:675–683. doi: 10.1097/00002030-199507000-00002. [DOI] [PubMed] [Google Scholar]
- 2.Cabot B, Esteban J I, Martell M, Genescà J, Vargas V, Esteban R, Guardia J, Gómez J. Structure of replicating hepatitis C virus (HCV) quasispecies in the liver may not be reflected by analysis of circulating HCV virions. J Virol. 1997;71:1732–1734. doi: 10.1128/jvi.71.2.1732-1734.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Choo Q L, Richman K H, Han J H, Berger K, Lee C, Dong C, Gallegos C, Coit D, Medina-Selby R, Barr P J, et al. Genetic organization and diversity of the hepatitis C virus. Proc Natl Acad Sci USA. 1991;88:2451–2455. doi: 10.1073/pnas.88.6.2451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein L G, Kuiken C, Blumberg B M, Hartman S, Sharer L R, Clement M, Goudsmit J. HIV-1 V3 domain variation in brain and spleen of children with AIDS: tissue-specific evolution within host-determined quasispecies. Virology. 1991;180:583–590. doi: 10.1016/0042-6822(91)90072-j. [DOI] [PubMed] [Google Scholar]
- 5.Lanford R E, Chavez D, Chisari F V, Sureau C. Lack of detection of negative-strand hepatitis C virus RNA in peripheral blood mononuclear cells and other extrahepatic tissues by the highly strand-specific rTth reverse transcriptase PCR. J Virol. 1995;69:8079–8083. doi: 10.1128/jvi.69.12.8079-8083.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanford R E, Sureau C, Jacob J R, White R, Fuerst T R. Demonstration of in vitro infection of chimpanzee hepatocytes with hepatitis C virus using strand-specific RT/PCR. Virology. 1994;202:606–614. doi: 10.1006/viro.1994.1381. [DOI] [PubMed] [Google Scholar]
- 7.Laskus T, Radkowski M, Wang L F, Jang S J, Vargas H, Rakela J. Hepatitis C virus quasispecies in patients infected with HIV-1: correlation with extrahepatic viral replication. Virology. 1998;248:164–171. doi: 10.1006/viro.1998.9269. [DOI] [PubMed] [Google Scholar]
- 8.Laskus T, Radkowski M, Wang L F, Vargas H, Rakela J. Search for hepatitis C virus extrahepatic replication sites in patients with acquired immunodeficiency syndrome: specific detection of negative-strand viral RNA in various tissues. Hepatology. 1998;28:1398–1401. doi: 10.1002/hep.510280531. [DOI] [PubMed] [Google Scholar]
- 9.Laskus T, Wang L F, Rakela J, Vargas H, Pinna A D, Tsamandas A C, Demetris A J, Fung J. Dynamic behavior of hepatitis C virus in chronically infected patients receiving liver graft from infected donors. Virology. 1996;220:171–176. doi: 10.1006/viro.1996.0297. [DOI] [PubMed] [Google Scholar]
- 10.Mellor J, Haydon G, Blair C, Livingstone W, Simmonds P. Low level or absent in vivo replication of hepatitis C virus and hepatitis G virus/GB virus C in peripheral blood mononuclear cells. J Gen Virol. 1998;79:705–714. doi: 10.1099/0022-1317-79-4-705. [DOI] [PubMed] [Google Scholar]
- 11.Muller H M, Pfaff E, Goeser T, Kallinowski B, Solbach C, Theilmann L. Peripheral blood leukocytes serve as a possible extrahepatic site for hepatitis C virus replication. J Gen Virol. 1993;74:669–676. doi: 10.1099/0022-1317-74-4-669. [DOI] [PubMed] [Google Scholar]
- 12.Saleh M G, Tibbs C J, Koskinas J, Pereira L M, Bomford A B, Portmann B C, McFarlane I G, Williams R. Hepatic and extrahepatic hepatitis C virus replication in relation to response to interferon therapy. Hepatology. 1994;20:1399–1404. doi: 10.1002/hep.1840200604. [DOI] [PubMed] [Google Scholar]
- 13.Wang J T, Sheu J C, Lin J T, Wang T H, Chen D S. Detection of replicative form of hepatitis C virus RNA in peripheral blood mononuclear cells. J Infect Dis. 1992;166:1167–1169. doi: 10.1093/infdis/166.5.1167. [DOI] [PubMed] [Google Scholar]