Abstract
Composed of obesity and lipid parameters, the cardiometabolic index (CMI) has emerged as a novel diagnostic tool. Originally developed for diabetes diagnosis, its application has expanded to identifying patients with cardiovascular diseases, such as atherosclerosis and hypertension. However, the relationship between CMI and non-alcoholic fatty liver disease (NAFLD) and liver fibrosis in the US population remains unclear. This cross-sectional study analyzed data from the National Health and Nutrition Examination Survey (NHANES) spanning 2017–2020, involving 2996 participants aged 20 years or older. Vibration controlled transient elastography using a FibroScan® system (model 502, V2 Touch) with controlled attenuation parameter measurements identified NAFLD at a threshold of ≥ 274 dB/m, while liver stiffness measurement (LSM) results (median, ≥ 8.2 kPa) indicated fibrosis. A multifactorial logistic regression model explored the relationship between CMI and NAFLD and fibrosis. The effectiveness of CMI in detecting NAFLD and liver fibrosis was assessed through receiver operating characteristic curve analysis. Controlling for potential confounders, CMI showed a significant positive association with NAFLD (adjusted OR = 1.44, 95% CI 1.44–1.45) and liver fibrosis (adjusted OR = 1.84, 95% CI 1.84–1.85). The Areas Under the Curve for predicting NAFLD and fibrosis were 0.762 (95% CI 0.745 ~ 0.779) and 0.664(95% CI 0.633 ~ 0.696), respectively, with optimal cut-off values of 0.462 and 0.527. There is a positive correlation between CMI and NAFLD and fibrosis, which is a suitable and simple predictor of NAFLD and fibrosis.
Keywords: NAFLD, Hepatic steatosis, Liver fibrosis, NHANES, Cardiometabolic index
Subject terms: Endocrinology, Medical research
Introduction
Nonalcoholic fatty liver disease (NAFLD) is a primary cause of cirrhosis and hepatocellular carcinoma and has significant socioeconomic impacts1. NAFLD predominantly manifests as hepatic steatosis, lobular inflammation, hepatocyte ballooning, and fibrosis2. The recently published multisociety Delphi consensus statement on the new fatty liver disease nomenclature, which replaces NAFLD with metabolic dysfunction-associated steatotic liver disease (MASLD), clearly reveals the etiology of the disease and the risk factors3. It is considered the liver’s expression of metabolic syndrome1,4,5.
Even though liver biopsy stands as the gold standard for NAFLD diagnosis, its invasive characteristics frequently limit its acceptance. As a result, transient elastography, a non-invasive technique, has gained substantial attention due to its clinical utility6. This technique concurrently assesses both the median liver stiffness measurement (LSM) and controlled attenuation parameter (CAP), shedding light on hepatic steatosis and fibrosis6.
First introduced in 2015, the cardiometabolic index (CMI) is derived from the product of the waist-to-height ratio (WHtR) and the triglyceride (TG) to high-density lipoprotein cholesterol (HDL-C) ratio. It has been proposed as a predictive tool for assessing risks associated with type 2 diabetes and cardiovascular diseases7. Studies have identified correlations between the CMI and conditions such as hyperuricaemia, obstructive sleep apnoea, and stroke8–10. Given the promising diagnostic capability of the CMI for various metabolic disorders, its potential role in NAFLD diagnosis warrants further investigation. This hypothesis has been verified among Asian populations, yet comprehensive research remains limited in US populations11. Moreover, no studies have yet explored the correlation between the CMI and liver fibrosis.
In this study, data from the National Health and Nutrition Examination Survey (NHANES) was utilized to examine the correlation between the CMI and NAFLD and fibrosis.
Methods
Study population
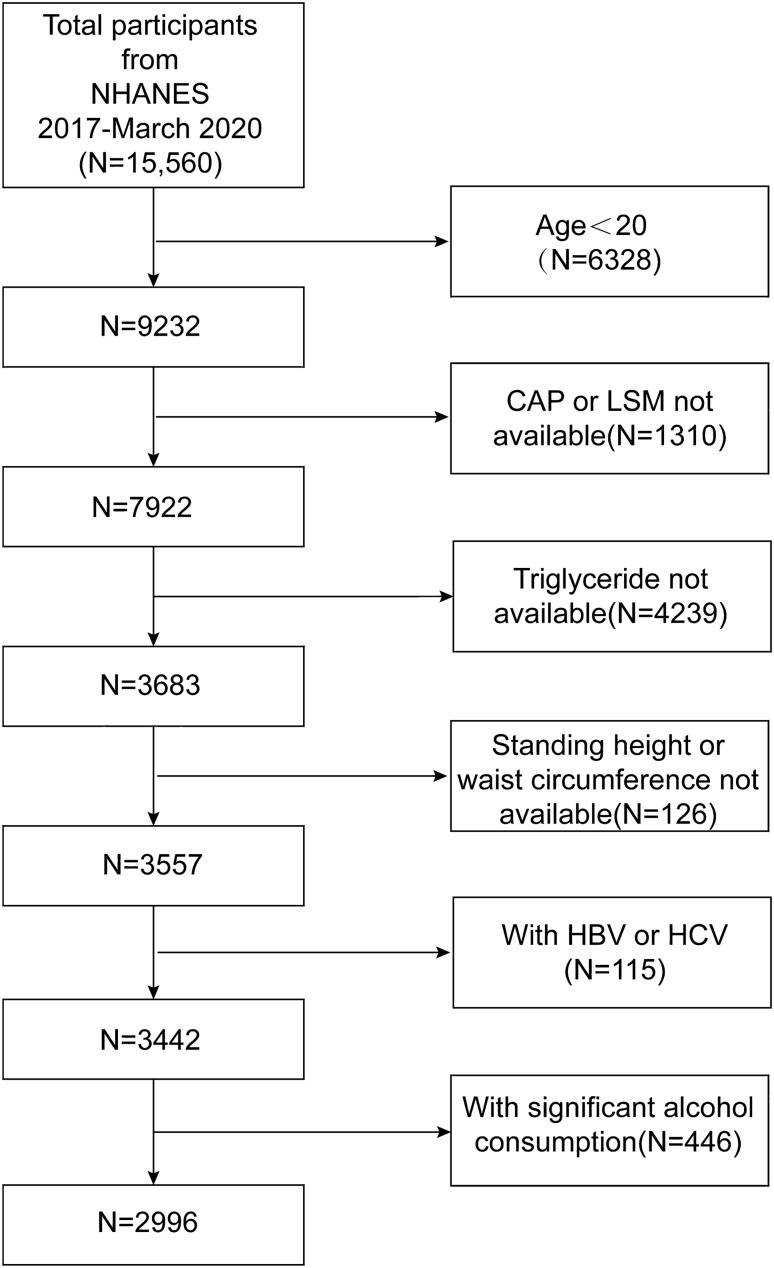
The NHANES serves as a nationally representative database, capturing the health and nutritional status of U.S. children and adults. Analysis of NHANES datasets from 2017 to 2020 revealed data for 15,560 participants. The analytical sample was narrowed down to 2996 subjects after applying the following exclusion criteria: individuals below the age of 20 years, individuals with a history of heavy alcohol consumption (defined as the daily intake of 4 or 5 or more drinks), subjects diagnosed with hepatitis B or C, and participants with incomplete laboratory or examination data. The inclusion and exclusion criteria are depicted in Fig. 1.
Figure 1.
Flowchart of participant selection.
Data collection
The outcome variables of the study were NAFLD and liver fibrosis, with the CMI value serving as the independent variable. The CMI value was determined using the following formula: TG/HDL-C × WHtR. Utilizing a FibroScan Model 502 V2 Touch system, trained NHANES staff acquired participants’ LSM and CAP values through vibration-controlled transient elastography (VCTE) assessments. A CAP value ≥ 274 dB/m was indicative of hepatic steatosis, whereas an LSM ≥ 8.2 kPa was indicative of fibrosis12.
Additional variables were collected based on previous research findings and clinical expertise13,14. They encompassed the following demographic, health, and biochemical markers: Age, sex, race, body mass index (BMI), smoking status, diabetes, hypertension, total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), alanine aminotransferase (ALT), albumin (ALB), gamma-glutamyl transferase (GGT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), creatinine (Cr), Total bilirubin (TB), uric acid (UA).
Statistical analysis
To account for the complex sampling design of the NHANES, we incorporated weights (WTSAFPRP) into our analysis, in accordance with recommendations from the NHANES official website. Data management and statistical analyses were performed using SAS 9.4(version 9.4 for Windows, SAS Institute, Inc., Cary, NC, USA). Qualitative data were described using n (%), while quantitative data that did not follow a normal distribution were described using Median (P25, P75). Comparisons between groups were conducted using the rank-sum test. A binary logistic regression analysis was utilized to examine the relationship between CMI and NAFLD and fibrosis. In the multivariate analysis, considering the intercorrelation among variables, the Variance Inflation Factor (VIF) was employed to assess multicollinearity. To control for potential confounding factors that might affect the results, a series of models were established: Model 1, which did not adjust for any confounding factors; Model 2, which adjusted for gender, age, BMI, and race based on Model 1; Model 3, which further adjusted for smoking, diabetes, and hypertension based on Model 2; Model 4, which incorporates adjustments for all non-collinear variables including TC, AST, GGT, ALB, BUN, TB, and UA, building upon the adjustments made in Model 3. A P-value of ≤ 0.05 was considered statistically significant. The diagnostic efficacy of CMI for detecting NAFLD and fibrosis was assessed through receiver operating characteristic (ROC) curve analysis.
Ethical statement
The research involving human participants underwent a thorough review and received approval from the Research Ethics Review Board of the NCHS. All patients or participants gave their written informed consent to be part of this study.
Subject characteristics
A total of 2996 adults, identified via the established inclusion and exclusion criteria, constituted the study cohort. The cohort’s demographics and key clinical characteristics are as follows. The mean age was 49.98 ± 17.33 years. Of the participants, 45.23% were males and 54.77% were females. A total of 13.05% of the participants were Mexican American, 10.35% were Other Hispanic, 33.14% were Non-Hispanic White, 24.67% were Non-Hispanic Black, 18.79% were from other racial groups. NAFLD was diagnosed in 1311 individuals and liver fibrosis in 275, constituting 43.76% and 9.18% of the study population, respectively.
Compared to non-NAFLD individuals, those with NAFLD were more likely to be older, male, and of Mexican American ethnicity. They also exhibited higher rates of smoking, diabetes, and hypertension, along with significantly elevated levels of BMI, TG, TC, LDL-C, GGT, ALT, AST, BUN, UA, Cr, and TB. In contrast, their ALB and HDL-C levels were significantly lower. CMI values, on average, were significantly lower in individuals without NAFLD compared to those with the condition (0.32 vs 0.71, P < 0.001). Additionally, a greater proportion of NAFLD patients were found in the highest CMI quartiles (Q3 and Q4), a significant difference compared to the non-NAFLD group (P < 0.001). These findings are detailed in Table 1.
Table 1.
Clinical and biochemical characteristics of the study subjects with or without NAFLD.
| Non-NAFLD | NAFLD | |||
|---|---|---|---|---|
| Total | CAP < 274 dB/m (n = 1685) | CAP ≥ 274 dB/m (n = 1311) | P | |
| CMI [M (P25, P75)] | 0.46 (0.25,0.81) | 0.32 (0.20,0.54) | 0.71 (0.43,1.11) | < 0.001# |
| CMI quartile | ||||
| Q1 (≤ 0.25) | 749 (25.00) | 634 (37.63) | 115 (8.77) | < 0.001 |
| Q2 (0.25 ~ 0.46) | 749 (25.00) | 504 (29.91) | 245 (18.69) | |
| Q3 (0.46 ~ 0.81) | 749 (25.00) | 340 (20.18) | 409 (31.20) | |
| Q4 (> 0.81) | 749 (25.00) | 207 (12.28) | 542 (41.34) | |
| Sex | ||||
| Men | 1355 (45.23) | 711 (42.20) | 644 (49.12) | < 0.001 |
| Women | 1641 (54.77) | 974 (57.80) | 667 (50.88) | |
| Age [M (P25, P75)] | 51.0 (35.0,64.0) | 46.0 (32.0,62.0) | 54.0 (41.0,65.0) | < 0.001# |
| BMI [M (P25, P75)] | 28.6 (24.7,33.5) | 26.0 (23.0,29.9) | 32.0 (28.2,37.1) | < 0.001# |
| Race | ||||
| Mexican American | 391 (13.05) | 166 (9.85) | 225 (17.16) | < 0.001 |
| Other Hispanic | 310 (10.35) | 173 (10.27) | 137 (10.45) | |
| Non-Hispanic white | 993 (33.14) | 534 (31.69) | 459 (35.01) | |
| Non-Hispanic black | 739 (24.67) | 474 (28.13) | 265 (20.21) | |
| Other race—Including multi-racial | 563 (18.79) | 338 (20.06) | 225 (17.16) | |
| Smoke | ||||
| Yes | 1121 (37.44) | 599 (35.59) | 522 (39.82) | < 0.001 |
| No | 1873 (62.56) | 1084 (64.41) | 789 (60.18) | |
| Diabetes | ||||
| Yes | 448 (14.96) | 150 (8.91) | 298 (22.73) | < 0.001 |
| No | 2546 (85.04) | 1533 (91.09) | 1013 (77.27) | |
| Hypertension | ||||
| Yes | 1099 (36.74) | 475 (28.26) | 624 (47.63) | < 0.001 |
| No | 1892 (63.26) | 1206 (71.74) | 686 (52.37) | |
| TG (mmol/L) [M (P25, P75)] | 1.01 (0.68,1.50) | 0.81 (0.60,1.21) | 1.30 (0.90,1.84) | < 0.001# |
| TC (mmol/L) [M (P25, P75)] | 4.68 (4.06,5.40) | 4.63 (4.01,5.33) | 4.76 (4.14,5.51) | < 0.001# |
| HDL-C (mmol/L) [M (P25, P75)] | 1.32 (1.09,1.60) | 1.42 (1.19,1.71) | 1.19 (1.03,1.45) | < 0.001# |
| LDL-C (mmol/L) [M (P25, P75)] | 2.77 (2.20,3.36) | 2.69 (2.17,3.31) | 2.85 (2.25,3.44) | < 0.001# |
| AST(IU/L) [M (P25, P75)] | 19.0 (15.0,23.0) | 18.0 (15.0,22.0) | 19.0 (16.0,25.0) | < 0.001# |
| ALT(IU/L) [M (P25, P75)] | 17.0 (13.0,25.0) | 15.0 (11.0,21.0) | 21.0 (15.0,30.0) | < 0.001# |
| GGT(IU/L) [M (P25, P75)] | 21.0 (15.0,30.0) | 18.0 (13.0,25.0) | 24.0 (18.0,37.0) | < 0.001# |
| ALB(g/L) [M (P25, P75)] | 40.0 (38.0,42.0) | 41.0 (39.0,43.0) | 40.0 (38.0,42.0) | < 0.001# |
| BUN (mmol/L) [M (P25, P75)] | 5.0 (3.9,6.1) | 4.6 (3.9,6.1) | 5.0 (3.9,6.1) | < 0.001# |
| Cr(mmol/L) [M (P25, P75)] | 73.4 (61.9,86.6) | 73.4 (61.9,87.5) | 74.4 (61.0,88.8) | < 0.001# |
| TB (umol/L) [M (P25, P75)] | 6.8 (5.1,10.3) | 6.8 (5.1,10.3) | 7.1 (5.1,10.4) | < 0.001# |
| UA (umol/L) [M (P25, P75)] | 315.2 (261.7, 374.7) | 297.4 (249.8, 350.9) | 339.0 (285.5, 398.5) | < 0.001# |
Values are n (%) or mean (standard deviation) or median (P25, P75).
NAFLD, nonalcoholic fatty liver disease; TC, total cholesterol; HDL-C, high-density lipoprotein cholesterol; TG, triglyceride; LDL-C, low-density lipoprotein cholesterol; ALT, alanine aminotransferase; ALB, albumin; GGT, gamma-glutamyl transferase; AST, aspartate aminotransferase; BUN, blood urea nitrogen; Cr, creatinine; TB, total bilirubin; UA, uric acid; LSM, liver stiffness measurement; CMI, cardiometabolic index.
"#" indicates that the rank-sum test was used.
Cirrhosis patients, more likely to be older, Mexican American, and smokers, exhibited higher rates of diabetes and hypertension, along with elevated levels of BMI, TG, ALT, AST, GGT, BUN, Cr, TB, and UA. Conversely, their LDL-C, HDL-C, ALB, and TC levels were lower. The CMI was significantly higher in the hepatic fibrosis group than in individuals without hepatic fibrosis (0.73 vs 0.44, P < 0.001), with a larger proportion found in the highest CMI quartiles (Q3, Q4, P < 0.001), as detailed in Table 2.
Table 2.
Clinical and biochemical characteristics of the study subjects with or without liver fibrosis.
| Normal | Liver fibrosis | |||
|---|---|---|---|---|
| Total | < 8.2kPa (n = 2721) | ≥ 8.2kPa (n = 275) | P | |
| CMI [M (P25, P75)] | 0.46 (0.25,0.81) | 0.44 (0.24,0.77) | 0.73 (0.43,1.11) | < 0.001# |
| CMI quartile | ||||
| Q1 (≤ 0.25) | 749 (25.00) | 724 (26.61) | 25 (9.09) | < 0.001 |
| Q2 (0.25 ~ 0.46) | 749 (25.00) | 698 (25.65) | 51 (18.55) | |
| Q3 (0.46 ~ 0.81) | 749 (25.00) | 676 (24.84) | 73 (26.55) | |
| Q4 (> 0.81) | 749 (25.00) | 623 (22.90) | 126 (45.82) | |
| Sex | ||||
| Men | 1355 (45.23) | 1209 (44.43) | 146 (53.09) | < 0.001 |
| Women | 1641 (54.77) | 1512 (55.57) | 129 (46.91) | |
| Age [M (P25, P75)] | 51.0 (35.0,64.0) | 50.0 (34.0,63.0) | 58.0 (43.0,67.0) | < 0.001# |
| BMI [M (P25, P75)] | 28.6 (24.7,33.5) | 28.1 (24.5,32.6) | 35.8 (29.3,42.0) | < 0.001# |
| Race | ||||
| Mexican American | 391 (13.05) | 346 (12.72) | 45 (16.36) | < 0.001 |
| Other Hispanic | 310 (10.35) | 283 (10.40) | 27 (9.82) | |
| Non-Hispanic white | 993 (33.14) | 892 (32.78) | 101 (36.73) | |
| Non-Hispanic black | 739 (24.67) | 676 (24.84) | 63 (22.91) | |
| Other race—Including multi-racial | 563 (18.79) | 524 (19.26) | 39 (14.18) | |
| Smoke | ||||
| Yes | 1121 (37.44) | 1004 (36.93) | 117 (42.55) | < 0.001 |
| No | 1873 (62.56) | 1715 (63.07) | 158 (57.45) | |
| Diabetes | ||||
| Yes | 448 (14.96) | 337 (12.39) | 111 (40.36) | < 0.001 |
| No | 2546 (85.04) | 2382 (87.61) | 164 (59.64) | |
| Hypertension | ||||
| Yes | 1099 (36.74) | 941 (34.65) | 158 (57.45) | < 0.001 |
| No | 1892 (63.26) | 1775 (65.35) | 117 (42.55) | |
| TG (mmol/L) [M (P25, P75)] | 1.01 (0.68,1.50) | 0.98 (0.67,1.49) | 1.22 (0.85,1.75) | < 0.001# |
| TC (mmol/L) [M (P25, P75)] | 4.68 (4.06,5.40) | 4.71 (4.11,5.43) | 4.37 (3.78,5.15) | < 0.001# |
| HDL (mmol/L) [M (P25, P75)] | 1.32 (1.09,1.60) | 1.34 (1.11,1.63) | 1.16 (1.03,1.40) | < 0.001# |
| LDL (mmol/L) [M (P25, P75)] | 2.77 (2.20,3.36) | 2.77 (2.22,3.39) | 2.48 (1.94,3.23) | < 0.001# |
| AST(IU/L) [M (P25, P75)] | 19.0 (15.0,23.0) | 19.0 (15.0,23.0) | 21.0 (17.0,29.0) | < 0.001# |
| ALT (IU/L) [M (P25, P75)] | 17.0 (13.0,25.0) | 17.0 (12.0,24.0) | 23.0 (15.0,35.0) | < 0.001# |
| GGT(IU/L) [M (P25, P75)] | 21.0 (15.0,30.0) | 20.0 (14.0,29.0) | 30.0 (20.0,54.0) | < 0.001# |
| ALB(g/L) [M (P25, P75)] | 40.0 (38.0,42.0) | 40.0 (38.0,42.0) | 39.0 (37.0,42.0) | < 0.001# |
| BUN (mmol/L) [M (P25, P75)] | 5.0 (3.9,6.1) | 5.0 (3.9,6.1) | 5.4 (4.3,6.8) | < 0.001# |
| Cr(mmol/L) [M (P25, P75)] | 73.4 (61.9,86.6) | 73.4 (61.9,86.6) | 75.1 (62.8,90.2) | < 0.001# |
| TB (umol/L) [M (P25, P75)] | 6.8 (5.1,10.3) | 6.8 (5.1,10.3) | 8.6 (5.1,10.3) | < 0.001# |
| UA (umol/L) (®x ± s) | 321.9 ± 85.3 | 318.6 ± 84.6 | 354.2 ± 84.6 | < 0.001 |
Values are n (%) or mean (standard deviation) or median (P25, P75).
"#" indicates that the rank-sum test was used.
Association between CMI and NAFLD
In the multivariate logistic regression model, CMI was significantly positively associated with NAFLD, and this association remained consistent across various models: the unadjusted model (Model 1), the minimally adjusted model (Model 2), the partially adjusted model (Model 3), and the fully adjusted model for all non-collinear variables (Model 4). In the fully adjusted model, an increase of one unit in CMI was associated with a 44% increase in the risk of NAFLD (adjusted OR: 1.44; 95% CI 1.44, 1.45). Furthermore, compared to the first quartile of CMI, the risk of NAFLD for subjects in the second, third, and fourth quartiles increased by 2.53, 6.92, and 14.48 times, respectively, with these results remaining robust after stepwise adjustment for confounding factors (Table 3).
Table 3.
Association between CMI and NAFLD.
| Modle1 | Modle2 | Modle3 | Modle4 | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |
| CMI | 8.73 (8.72 ~ 8.73) | < 0.001 | 3.47 (3.46 ~ 3.47) | < 0.001 | 3.34 (3.33 ~ 3.34) | < 0.001 | 1.44 (1.44 ~ 1.45) | < 0.001 |
| CMI quartile | ||||||||
| Q1 (≤ 0.25) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||
| Q2 (0.25 ~ 0.46) | 2.53 (2.53 ~ 2.54) | < 0.001 | 1.27 (1.27 ~ 1.28) | < 0.001 | 1.24 (1.24 ~ 1.25) | < 0.001 | 0.90 (0.90 ~ 0.91) | < 0.001 |
| Q3 (0.46 ~ 0.81) | 6.92 (6.92 ~ 6.93) | < 0.001 | 2.90 (2.90 ~ 2.91) | < 0.001 | 2.75 (2.74 ~ 2.75) | < 0.001 | 1.37 (1.37 ~ 1.38) | < 0.001 |
| Q4 (> 0.81) | 14.48 (14.46 ~ 14.49) | < 0.001 | 4.36 (4.36 ~ 4.37) | < 0.001 | 4.09 (4.09 ~ 4.10) | < 0.001 | 0.97 (0.97 ~ 0.98) | < 0.001 |
Model 1 is unadjusted. Model 2 is adjusted for gender, age, race, and BMI. Model 3 is further adjusted for smoking, diabetes, and hypertension based on Model 2. Model 4 is additionally adjusted for TC, AST, GGT, ALB, BUN, TB, and UA based on Model 3.
Association between CMI and liver fibrosis
The relationship between higher CMI levels and increased liver fibrosis risk was notably strong and positive, maintaining significance even after adjusting for all non-collinear covariates (adjusted OR: 1.84; 95% CI 1.84, 1.85). Analysis by CMI quartiles revealed a progressive increase in liver fibrosis risk with higher quartiles: individuals in Q2, Q3, and Q4 experienced a 2.23, 3.32, and 6.10 times greater risk, respectively, compared to those in Q1. This pattern of association persisted even after comprehensive stepwise adjustment for potential confounders, as elaborated in Table 4.
Table 4.
Association between CMI and hepatic fibrosis.
| Modle1 | Modle2 | Modle3 | Modle4 | |||||
|---|---|---|---|---|---|---|---|---|
| OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | OR (95%CI) | P | |
| CMI | 1.37 (1.37 ~ 1.37) | < 0.001 | 1.08 (1.08 ~ 1.08) | < 0.001 | 1.05 (1.05 ~ 1.05) | < 0.001 | 1.84 (1.84 ~ 1.85) | < 0.001 |
| CMI quartile | ||||||||
| Q1 (≤ 0.25) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | 1.00 (ref) | ||||
| Q2 (0.25 ~ 0.46) | 2.23 (2.22 ~ 2.23) | < 0.001 | 1.19 (1.19 ~ 1.20) | < 0.001 | 1.08 (1.08 ~ 1.09) | < 0.001 | 1.18 (1.18 ~ 1.19) | < 0.001 |
| Q3 (0.46 ~ 0.81) | 3.32 (3.31 ~ 3.33) | < 0.001 | 1.27 (1.27 ~ 1.28) | < 0.001 | 1.04 (1.04 ~ 1.05) | < 0.001 | 1.22 (1.22 ~ 1.23) | < 0.001 |
| Q4 (> 0.81) | 6.10 (6.09 ~ 6.11) | < 0.001 | 1.65 (1.64 ~ 1.65) | < 0.001 | 1.28 (1.27 ~ 1.28) | < 0.001 | 1.74 (1.74 ~ 1.75) | < 0.001 |
Model 1 is unadjusted. Model 2 is adjusted for gender, age, race, and BMI. Model 3 is further adjusted for smoking, diabetes, and hypertension based on Model 2. Model 4 is additionally adjusted for TC, AST, GGT, ALB, BUN, TB, and UA based on Model 3.
The ability of CMI to detect NAFLD and liver fibrosis
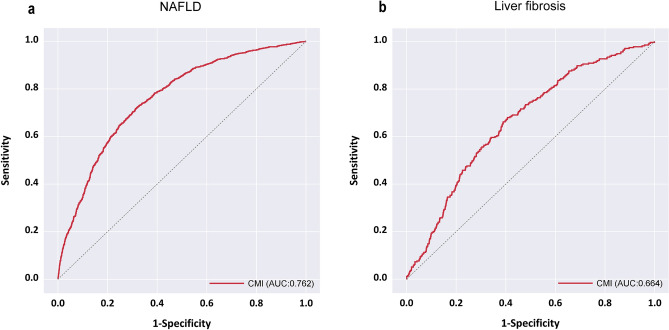
To evaluate the predictive accuracy of the CMI for NAFLD and liver fibrosis, ROC curve analysis was performed. The Areas Under the Curve (AUC) for predicting NAFLD using CMI, depicted in Fig. 2a, was 0.762(95% CI 0.745 ~ 0.779). For liver fibrosis prediction with CMI, shown in Fig. 2b, the AUC was 0.664(95% CI 0.633 ~ 0.696). Detailed analyses, including optimal cutoff values and their corresponding sensitivity and specificity, are presented in Table 5. Specifically, the optimal cutoff for NAFLD prediction was identified as 0.462, yielding a sensitivity of 72.2% and a specificity of 68.1%. For liver fibrosis, the cutoff was established at 0.527, resulting in a sensitivity of 68.0% and a specificity of 59.3%.
Figure 2.
ROC Curves for CMI in Diagnosing NAFLD and liver fibrosis (a) NAFLD (b) liver fibrosis.
Table 5.
CMI performance metrics for NAFLD and fibrosis screening.
| AUC | Z | P | Cut Off | Sen | Spe | Youden | PPV | NPV | |
|---|---|---|---|---|---|---|---|---|---|
| NAFLD | 0.762 (0.745 ~ 0.779) | 30.02 | < 0.001 | 0.462 | 0.722 | 0.681 | 0.404 | 0.638 | 0.759 |
| Liver fibrosis | 0.664 (0.633 ~ 0.696) | 10.12 | < 0.001 | 0.527 | 0.680 | 0.593 | 0.273 | 0.144 | 0.948 |
Sen, sensitivity; Spe, specificity; Youden, Youden’s Index; PPV, positive predictive value; NPV, negative predictive value.
Discussion
To our knowledge, this cross-sectional analysis represents the first extensive clinical investigation into the association between the CMI and both NAFLD and liver fibrosis in the U.S. population, involving 2996 participants. We discovered a significant positive correlation between CMI and both NAFLD and liver fibrosis, persisting even after adjustment for potential confounders through multivariate logistic regression. With AUC of 0.762 for NAFLD and 0.664 for liver fibrosis, our results suggest that CMI serves as an effective predictive marker for these conditions, indicating good diagnostic performance.
Introduced in 2015, the CMI is a novel marker derived from obesity and lipid profiles. Initially used in diabetes diagnosis, the CMI showed a robust correlation with hyperglycaemia and diabetes in both sexes, with notable sex-specific differences7. Another prospective study in middle-aged and older Chinese adults showed the same results: A positive association was observed between the CMI and the risk of new-onset type 2 diabetes in middle-aged and older Chinese adults, with a high CMI value recognized as a contributing factor to the development of type 2 diabetes15. In an analysis of 174,698 adults, there was a notable correlation between the CMI and hyperuricaemia. This association proved to be more robust than connections with other indices, such as body fat percentage, BMI, the body roundness index, and the visceral fat index16. The new MASLD definition emphasizes the significant impact of cardiometabolic risk factors (overweight or obesity, elevated blood glucose, low high-density lipoprotein cholesterol, hypertension, and hypertriglyceridemia) on the development and progression of fatty liver disease. It is inferred that the CMI can also serve as a predictive marker for NAFLD. Recently, a correlation between NAFLD and the CMI was found in a Chinese cohort study. After adjusting for potential confounding factors, a higher CMI value was independently associated with NAFLD. For every standard deviation increase in the CMI value, the risk of non-alcoholic fatty liver disease increases by 28%11. In another study of 943 Chinese participants, similar findings were demonstrated. Further subgroup analyses showed significant interactions between the CMI and the risk of MAFLD in terms of sex, age, and BMI17. Previous research has investigated the relationship between CMI and the incidence of NAFLD in Asian populations. However, it remains uncertain whether this correlation exists in other ethnic groups. Moreover, while NAFLD is prevalent in the general population, only a limited subset progresses to advanced liver fibrosis. The precise identification of this subset is crucial from a clinical standpoint. Existing literature underlines that fibrosis staging is the primary predictor of both overall and liver-specific mortality in NAFLD patients18. Previous indicators used to assess hepatic steatosis, such as the fatty liver index (FLI) and hepatic steatosis index (HSI), along with the NAFLD Fibrosis Score used for measuring fibrosis, are relatively complex to calculate and less suitable for clinical application. There is a need for simpler indicators to screen the target population for further examination.
There is a strong association between obesity and NAFLD progression19, with central obesity posing a greater risk than peripheral obesity20–22. Visceral fat accumulation plays a partial role in causing hepatic steatosis in overweight and obese individuals, with females being particularly affected23. The severity of hepatic steatosis correlates positively with visceral and subcutaneous abdominal adiposity24. This relationship is evident not only in hepatic steatosis but also in the progression of hepatic fibrosis. A longitudinal study indicated that abdominal adiposity was the primary risk factor associated with changes in LSM values and the progression of moderate to advanced liver fibrosis in the cohort25. Gastric weight-loss surgery has been shown to significantly alleviate hepatic steatosis and fibrosis26. Visceral adipose tissue, characterized by heightened lipolysis and insulin resistance, supplies the liver with free fatty acids (FFAs) that are subsequently esterified into TG27. Furthermore, this tissue releases pro-inflammatory mediators such as tumor necrosis factor-α (TNF-α) and Interleukin-6 (IL-6), fostering insulin resistance. Such inflammatory mediators initiate macrophage infiltration, activate Kupffer cells, and stimulate hepatic stellate cells, leading to the secretion of extracellular matrix proteins and subsequent fibrotic progression28,29. For the assessment of abdominal obesity, the WHtR is recognized as a robust measure. The WHtR is based on waist circumference, and its sensitivity is not affected by height, offering easy computation and consistency across populations. Compared to traditional metrics such as BMI, the WHtR provides a more precise gauge of abdominal obesity30,31. Strong associations have been identified between the WHtR and fatty liver manifestations in paediatric and adolescent cohorts32.
In epidemiology, hepatic steatosis has been associated with insulin resistance33,34. Hepatic steatosis results in insulin resistance, and the converse is also true. Steatotic livers further the worsening of insulin resistance by hindering the removal of insulin from portal blood, thereby maintaining a continuous cycle of deterioration. Insulin resistance also stands out as a pivotal factor in the pathogenesis and natural progression of NAFLD35. An imbalance in the production of TNF-α, IL-6, leptin, free fatty acids, and adiponectin leads to insulin resistance and inflammation, which are the primary pathophysiologies for liver fibrosis in patients with fatty liver36. Among type 2 diabetes patients, insulin resistance is identified as an independent risk factor associated with liver fibrosis37. A study by Ercin CN on 215 biopsy-confirmed NAFLD male patients suggests that insulin resistance values, rather than visceral adiposity index values, are independently correlated with liver fibrosis38. The ratio of TG to HDL-C serves as an indicative tool for insulin resistance39–42. The association between insulin resistance and TGs as well as the TG/HDL-C ratio is more significant in women than in men43. Multiple studies have highlighted the efficacy of the TG/HDL-C ratio in predicting NAFLD44–46, a fact further corroborated by Fan et al.’s cross-sectional analysis, which emphasized the significant correlation of the TG/HDL-C ratio with NAFLD risk in healthy subjects47.
Study strengths and limitations
The study has several strengths that enhance the credibility and validity of the results: the large sample size reinforces the dependability of the research outcomes. Employing weighting mitigates biases stemming from oversampling. The consistent results across the main and sensitivity analyses suggest robustness in the findings. Analysing distinct subgroups enhanced data utilization and augmented the reliability of the conclusions.
Limitations
The cross-sectional nature of the study underscores correlations but does not establish causality; thus, prospective research is imperative for validating causative relationships. The potential influence of unaccounted confounding factors cannot be entirely negated. While VCTE offers insights into liver steatosis and hepatic fibrosis, it is not the gold standard. A liver biopsy remains indispensable for a definitive diagnosis.
Conclusion
The research demonstrates a positive correlation between the CMI and both NAFLD and liver fibrosis in the U.S. population. Given that CMI is a reproducible and easily measurable indicator, it holds considerable value in screening for NAFLD and fibrosis in adults.
Author contributions
L.S.Y. and S.Y.Z. contributed to the study design. L.S.Y. and X.Y.H. were responsible for conducting the study and collecting data. Both L.S.Y. and S.S.W. took part in data analysis and the interpretation of results. L.S.Y. and C.C. took the lead in writing and revising the manuscript, as well as preparing tables and figures. All authors made significant contributions to the article and approved the final version for submission. There is no conflict of interests regarding the publication of this article.
Funding
This research did not receive any specific grant from any funding agency in the public, commercial or not-for-profit sector.
Data availability
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes.
Competing interests
The authors confirm that there were no commercial or financial affiliations that could be perceived as potential conflicts of interest during the research process.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Younossi ZM. Non-alcoholic fatty liver disease—A global public health perspective. J. Hepatol. 2019;70(3):531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 2.Oeda S, Tanaka K, Oshima A, Matsumoto Y, Sueoka E, Takahashi H. Diagnostic accuracy of FibroScan and factors affecting measurements. Diagnostics. 2020;10(11):940. doi: 10.3390/diagnostics10110940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multisociety Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78(6):1966–1986. doi: 10.1097/HEP.0000000000000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Araújo AR, Rosso N, Bedogni G, Tiribelli C, Bellentani S. Global epidemiology of non-alcoholic fatty liver disease/non-alcoholic steatohepatitis: What we need in the future. Liver Int. 2018;38:47–51. doi: 10.1111/liv.13643. [DOI] [PubMed] [Google Scholar]
- 5.Lazarus JV, Mark HE, Anstee QM, Arab JP, Batterham RL, Castera L, et al. Advancing the global public health agenda for NAFLD: A consensus statement. Nat. Rev. Gastroenterol. Hepatol. 2022;19(1):60–78. doi: 10.1038/s41575-021-00523-4. [DOI] [PubMed] [Google Scholar]
- 6.Hashemi S-A, Alavian S-M, Gholami-Fesharaki M. Assessment of transient elastography (FibroScan) for diagnosis of fibrosis in non-alcoholic fatty liver disease: A systematic review and meta-analysis. Casp. J. Intern. Med. 2016;7(4):242. [PMC free article] [PubMed] [Google Scholar]
- 7.Wakabayashi I, Daimon T. The, “cardiometabolic index” as a new marker determined by adiposity and blood lipids for discrimination of diabetes mellitus. Clin. Chim. Acta. 2015;438:274–278. doi: 10.1016/j.cca.2014.08.042. [DOI] [PubMed] [Google Scholar]
- 8.Cai X, Hu J, Wen W, Wang J, Wang M, Liu S, et al. Associations of the cardiometabolic index with the risk of cardiovascular disease in patients with hypertension and obstructive sleep apnea: Results of a longitudinal cohort study. Oxid. Med. Cell. Longev. 2022;2022:1–15. doi: 10.1155/2022/4914791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang H, Sun Y, Wang S, Qian H, Jia P, Chen Y, et al. Body adiposity index, lipid accumulation product, and cardiometabolic index reveal the contribution of adiposity phenotypes in the risk of hyperuricemia among Chinese rural population. Clin. Rheumatol. 2018;37:2221–2231. doi: 10.1007/s10067-018-4143-x. [DOI] [PubMed] [Google Scholar]
- 10.Li F-E, Luo Y, Zhang F-L, Zhang P, Liu D, Ta S, et al. Association between cardiometabolic index and stroke: A population-based cross-sectional study. Curr. Neurovasc. Res. 2021;18(3):324–332. doi: 10.2174/1567202618666211013123557. [DOI] [PubMed] [Google Scholar]
- 11.Zou J, Xiong H, Zhang H, Hu C, Lu S, Zou Y. Association between the cardiometabolic index and non-alcoholic fatty liver disease: Insights from a general population. BMC Gastroenterol. 2022;22(1):1–10. doi: 10.1186/s12876-022-02099-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eddowes PJ, Sasso M, Allison M, Tsochatzis E, Anstee QM, Sheridan D, et al. Accuracy of FibroScan controlled attenuation parameter and liver stiffness measurement in assessing steatosis and fibrosis in patients with nonalcoholic fatty liver disease. Gastroenterology. 2019;156(6):1717–1730. doi: 10.1053/j.gastro.2019.01.042. [DOI] [PubMed] [Google Scholar]
- 13.Niu Y, Zhang W, Zhang H, Li X, Lin N, Su W, et al. Serum creatinine levels and risk of nonalcohol fatty liver disease in a middle-aged and older Chinese population: A cross-sectional analysis. Diabetes/Metab. Res. Rev. 2022;38(2):e3489. doi: 10.1002/dmrr.3489. [DOI] [PubMed] [Google Scholar]
- 14.Xie R, Xiao M, Li L, Ma N, Liu M, Huang X, et al. Association between SII and hepatic steatosis and liver fibrosis: A population-based study. Front. Immunol. 2022;13:925690. doi: 10.3389/fimmu.2022.925690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qiu Y, Yi Q, Li S, Sun W, Ren Z, Shen Y, et al. Transition of cardiometabolic status and the risk of type 2 diabetes mellitus among middle-aged and older Chinese: A national cohort study. J. Diabetes Investig. 2022;13(8):1426–1437. doi: 10.1111/jdi.13805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu XZ, Li HH, Huang S, Zhao DB. Association between hyperuricemia and nontraditional adiposity indices. Clin. Rheumatol. 2019;38:1055–1062. doi: 10.1007/s10067-018-4374-x. [DOI] [PubMed] [Google Scholar]
- 17.Duan S, Yang D, Xia H, Ren Z, Chen J, Yao S. Cardiometabolic index: A new predictor for metabolic associated fatty liver disease in Chinese adults. Front. Endocrinol. 2022;13:1004855. doi: 10.3389/fendo.2022.1004855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chan W-K. Comparison between obese and non-obese nonalcoholic fatty liver disease. Clin. Mol. Hepatol. 2023;29(Suppl):S58. doi: 10.3350/cmh.2022.0350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dao AD, Nguyen VH, Ito T, Cheung R, Nguyen MH. Prevalence, characteristics, and mortality outcomes of obese and nonobese MAFLD in the United States. Hepatol. Int. 2023;17(1):225–236. doi: 10.1007/s12072-022-10436-2. [DOI] [PubMed] [Google Scholar]
- 20.Huh Y, Cho YJ, Nam GE. Recent epidemiology and risk factors of nonalcoholic fatty liver disease. J. Obes. Metab. Syndr. 2022;31(1):17–27. doi: 10.7570/jomes22021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Angulo P. NAFLD, obesity, and bariatric surgery. Gastroenterology. 2006;130(6):1848–1852. doi: 10.1053/j.gastro.2006.03.041. [DOI] [PubMed] [Google Scholar]
- 22.Kim D, Chung GE, Kwak M-S, Seo HB, Kang JH, Kim W, et al. Body fat distribution and risk of incident and regressed nonalcoholic fatty liver disease. Clin. Gastroenterol. Hepatol. 2016;14(1):132–138.e4. doi: 10.1016/j.cgh.2015.07.024. [DOI] [PubMed] [Google Scholar]
- 23.Liu F, Chen S, Li X, Li S, Xiao Y, Han J, et al. Obesity-induced hepatic steatosis is partly mediated by visceral fat accumulation in subjects with overweight/obesity: A cross-sectional study. Obes. Facts. 2023;16(2):164–172. doi: 10.1159/000527595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ko H-J, Woo S, Han J, Kim YM, Lim HJ, Kim M-J, et al. Which obesity index is the most useful marker for predicting hepatic steatosis in children and adolescents with obesity? A cross-sectional study using quantitative magnetic resonance imaging. Obes. Res. Clin. Pract. 2023;17(4):335–342. doi: 10.1016/j.orcp.2023.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Julián MT, Ballesta S, Pera G, Pérez-Montes de Oca A, Soldevila B, Caballería L, et al. Abdominal obesity and dsyglycemia are risk factors for liver fibrosis progression in NAFLD subjects: A population-based study. Front. Endocrinol. 2023;13:1051958. doi: 10.3389/fendo.2022.1051958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norouzian Ostad A, Rajabzadeh F, Jamialahmadi T, Goshayeshi L, Ranjbar G, Rezvani R, et al. Impact of gastric bypass surgery on the liver fibrosis of patients with extreme obesity and nonalcoholic fatty liver disease in 30-month follow-up. Updates Surg. 2023;75(3):659–669. doi: 10.1007/s13304-022-01421-3. [DOI] [PubMed] [Google Scholar]
- 27.Friedman SL, Neuschwander-Tetri BA, Rinella M, Sanyal AJ. Mechanisms of NAFLD development and therapeutic strategies. Nat. Med. 2018;24(7):908–922. doi: 10.1038/s41591-018-0104-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vancells Lujan P, Viñas Esmel E, Sacanella ME. Overview of non-alcoholic fatty liver disease (NAFLD) and the role of sugary food consumption and other dietary components in its development. Nutrients. 2021;13(5):1442. doi: 10.3390/nu13051442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kumar V, Xin X, Ma J, Tan C, Osna N, Mahato RI. Therapeutic targets, novel drugs, and delivery systems for diabetes associated NAFLD and liver fibrosis. Adv. Drug Deliv. Rev. 2021;176:113888. doi: 10.1016/j.addr.2021.113888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ross R, Neeland IJ, Yamashita S, Shai I, Seidell J, Magni P, et al. Waist circumference as a vital sign in clinical practice: A consensus statement from the IAS and ICCR working group on visceral obesity. Nat. Rev. Endocrinol. 2020;16(3):177–189. doi: 10.1038/s41574-019-0310-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin M-S, Lin T-H, Guo S-E, Tsai M-H, Chiang M-S, Huang T-J, et al. Waist-to-height ratio is a useful index for nonalcoholic fatty liver disease in children and adolescents: A secondary data analysis. BMC Public Health. 2017;17(1):1–8. doi: 10.1186/s12889-017-4868-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Al-Attas OS, Al-Daghri NM, Alokail MS, Alkharfy KM, Draz H, Yakout S, et al. Association of body mass index, sagittal abdominal diameter and waist-hip ratio with cardiometabolic risk factors and adipocytokines in Arab children and adolescents. BMC Pediatr. 2012;12(1):119. doi: 10.1186/1471-2431-12-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dongiovanni P, Stender S, Pietrelli A, Mancina R, Cespiati A, Petta S, et al. Causal relationship of hepatic fat with liver damage and insulin resistance in nonalcoholic fatty liver. J. Intern. Med. 2018;283(4):356–370. doi: 10.1111/joim.12719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Watt MJ, Miotto PM, De Nardo W, Montgomery MK. The liver as an endocrine organ—Linking NAFLD and insulin resistance. Endocr. Rev. 2019;40(5):1367–1393. doi: 10.1210/er.2019-00034. [DOI] [PubMed] [Google Scholar]
- 35.Lonardo A, Lombardini S, Ricchi M, Scaglioni F, Loria P. Hepatic steatosis and insulin resistance. Aliment. Pharmacol. Ther. 2005;22:64–70. doi: 10.1111/j.1365-2036.2005.02600.x. [DOI] [PubMed] [Google Scholar]
- 36.Bugianesi E, McCullough AJ, Marchesini G. Insulin resistance: A metabolic pathway to chronic liver disease. Hepatology. 2005;42(5):987–1000. doi: 10.1002/hep.20920. [DOI] [PubMed] [Google Scholar]
- 37.Aller R, Sigüenza R, Pina M, Laserna C, Antolín B, Burgueño B, et al. Insulin resistance is related with liver fibrosis in type 2 diabetic patients with non-alcoholic fatty liver disease proven biopsy and Mediterranean diet pattern as a protective factor. Endocrine. 2020;68:557–563. doi: 10.1007/s12020-020-02268-7. [DOI] [PubMed] [Google Scholar]
- 38.Ercin CN, Dogru T, Genc H, Celebi G, Aslan F, Gurel H, et al. Insulin resistance but not visceral adiposity index is associated with liver fibrosis in nondiabetic subjects with nonalcoholic fatty liver disease. Metab. Syndr. Relat. Disord. 2015;13(7):319–325. doi: 10.1089/met.2015.0018. [DOI] [PubMed] [Google Scholar]
- 39.Ren X, Za C, Zheng S, Han T, Li Y, Liu W, et al. Association between triglyceride to HDL-C ratio (TG/HDL-C) and insulin resistance in Chinese patients with newly diagnosed type 2 diabetes mellitus. PloS One. 2016;11(4):e0154345. doi: 10.1371/journal.pone.0154345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou M, Zhu L, Cui X, Feng L, Zhao X, He S, et al. The triglyceride to high-density lipoprotein cholesterol (TG/HDL-C) ratio as a predictor of insulin resistance but not of β cell function in a Chinese population with different glucose tolerance status. Lipids Health Dis. 2016;15(1):1–9. doi: 10.1186/s12944-016-0270-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gong R, Luo G, Wang M, Ma L, Sun S, Wei X. Associations between TG/HDL ratio and insulin resistance in the US population: A cross-sectional study. Endocr. Connect. 2021;10(11):1502–1512. doi: 10.1530/EC-21-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Giannini C, Santoro N, Caprio S, Kim G, Lartaud D, Shaw M, et al. The triglyceride-to-HDL cholesterol ratio: Association with insulin resistance in obese youths of different ethnic backgrounds. Diabetes Care. 2011;34(8):1869–1874. doi: 10.2337/dc10-2234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.He J, He S, Liu K, Wang Y, Shi D, Chen X. The TG/HDL-C ratio might be a surrogate for insulin resistance in Chinese nonobese women. Int. J. Endocrinol. 2014;2014:105168. doi: 10.1155/2014/105168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Godoy-Matos AF, Silva Júnior WS, Valerio CM. NAFLD as a continuum: From obesity to metabolic syndrome and diabetes. Diabetol. Metab. Syndr. 2020;12(1):1–20. doi: 10.1186/s13098-020-00570-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wu K-T, Kuo P-L, Su S-B, Chen Y-Y, Yeh M-L, Huang C-I, et al. Nonalcoholic fatty liver disease severity is associated with the ratios of total cholesterol and triglycerides to high-density lipoprotein cholesterol. J. Clin. Lipidol. 2016;10(2):420–425. doi: 10.1016/j.jacl.2015.12.026. [DOI] [PubMed] [Google Scholar]
- 46.Fukuda Y, Hashimoto Y, Hamaguchi M, Fukuda T, Nakamura N, Ohbora A, et al. Triglycerides to high-density lipoprotein cholesterol ratio is an independent predictor of incident fatty liver; A population-based cohort study. Liver Int. 2016;36(5):713–720. doi: 10.1111/liv.12977. [DOI] [PubMed] [Google Scholar]
- 47.Fan N, Peng L, Xia Z, Zhang L, Song Z, Wang Y, et al. Triglycerides to high-density lipoprotein cholesterol ratio as a surrogate for nonalcoholic fatty liver disease: A cross-sectional study. Lipids Health Dis. 2019;18:1–6. doi: 10.1186/s12944-019-0986-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Publicly available datasets were analyzed in this study. This data can be found here: https://www.cdc.gov/nchs/nhanes.