Abstract
CD4+ T cells are thought to be critical in the maintenance of virus-specific CD8+ cytotoxic T-cell (CTL) responses. In human immunodeficiency virus type 1 (HIV-1) infection, a selective decline in HIV-1-specific CTL as the CD4+ T-cell count decreases has been reported. Using HLA-peptide tetrameric complexes, we show the presence at high frequency of HIV-1- and cytomegalovirus-specific CD8+ T cells when the peripheral CD4+ T-cell count was low or zero in three HIV-1-infected patients. No direct virus-specific CD8+-mediated effector activity was seen in these subjects, suggesting antigen unresponsiveness, although tetramer-sorted cells could be expanded in vitro in the presence of interleukin-2 into responsive effector cells. Thus, virus-specific CD8+ T cells can be maintained in the peripheral circulation at high frequency in the absence of circulating peripheral CD4+ T cells, but these cells may lack direct effector activity. Strategies designed to overcome this antigen unresponsiveness may be of value in therapies for the treatment of AIDS.
Human immunodeficiency virus type-1 (HIV-1)-specific cytotoxic T cells (CTL) play a central role in the control of HIV-1 infection. The magnitude of the HIV-1-specific CTL response is associated with control of viremia, with a positive correlation between high CTL response and low viral load (16, 28, 29). If virus-specific CTL are temporarily reduced, as with simian immunodeficiency virus-infected monkeys infused with an anti-CD8 antibody, the plasma viral load increases (19, 26, 37). Individuals with long-term nonprogressive disease usually have strong HIV-1-specific CTL responses, while those with active HIV-1 disease have much weaker responses (5, 10, 16, 23, 34, 35).
With progression of HIV-1 disease in untreated individuals, there is a selective decline in HIV-1-specific CTL, while CTL responses to other viruses, such as Epstein-Barr virus or cytomegalovirus (CMV), appear to remain intact much longer (7, 13, 22, 31). CD4+ T cells are critical in providing support for the maintenance of virus-specific CTL responses (1, 6, 17, 27, 32, 40), and the decline in HIV-1-specific CTL numbers with disease progression has been partially attributed to a decrease in HIV-1-specific T-cell help (31, 36). In individuals with low CD4+ T-cell counts, it would be expected that insufficient help would be provided to maintain a high frequency of HIV-1-specific CTL, and at late stage, other virus-specific CTL would also decrease in frequency. There are reports of some HIV-1-infected individuals who remain AIDS free for several years with low CD4+ T-cell counts (low CD4+ T-cell count long-term survivors) (20, 21), but the mechanisms by which they control viral replication are unknown. We speculated that these individuals might be able to bypass the requirement for CD4+ T-cell help to maintain effector CTL at high frequency.
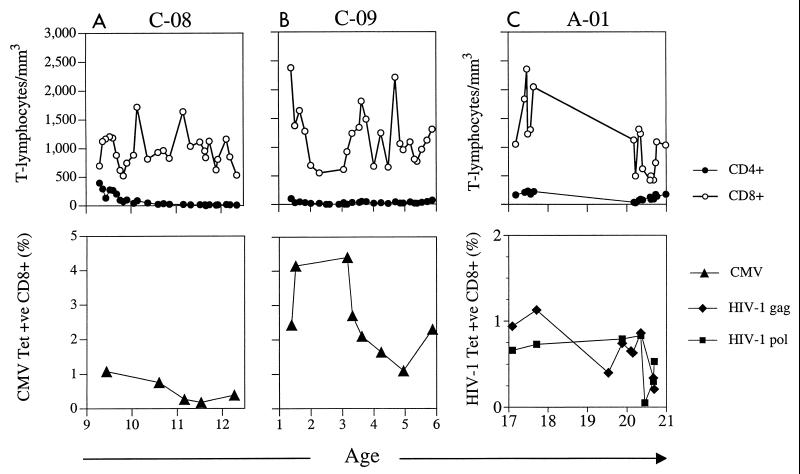
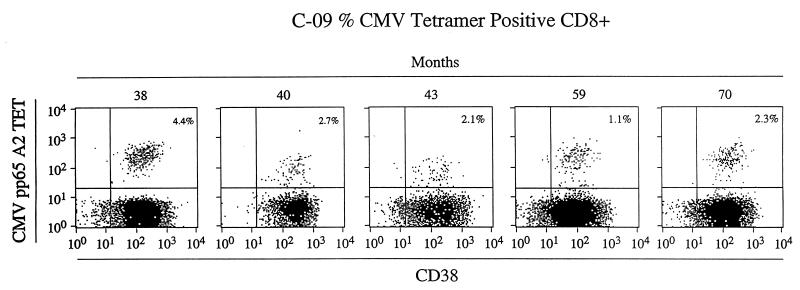
We quantified virus-specific CD8+ T-cell responses by using major histocompatibility complex peptide tetrameric complexes in three HIV-1-infected individuals who had low or absent CD4+ T cell counts for periods of up to 5 years. HLA class I haplotype was determined by molecular HLA typing (39), and all three subjects had HLA-A*0201. All patients had been treated with antiretroviral therapy. To determine the quantity of HIV-1-specific CD8+ T-cell responses, cryopreserved peripheral blood mononuclear cells (PBMC) were thawed and stained with HLA-A*0201 HIV-1 Gag amino acids 77 to 85 (SLYNTVATL), and Pol amino acids 476 to 484 (ILKEPVHGV) tetrameric complexes (3, 14, 15, 29, 30). Tetrameric peptide complexes were tested on known HLA-A*0201-positive HIV-1 or on CMV CD8+ T-cell lines as positive controls and on HLA-A*0201-negative PBMC as negative controls (data not shown). Staining of PBMC from the first two patients, C-08 and C-09, showed no HLA-A*0201 HIV-1 Gag or Pol peptide-specific CD8+ T-cell responses at three time points (data not shown), but HLA-A*0201-restricted CMV pp65 amino acids 495 to 503 (NLVPMVATV) specific CD8+ T-cell responses were detected with a CMV pp65 peptide tetrameric complex (41). A very high frequency of HLA-A*0201 CMV pp65-specific CD8+ T-cell responses (mean, 2.59% CD8+ T cells, standard deviation [SD], ± 1.15%; range, 1.1 to 4.4%; median, 2.31%) were detected in child C-09 over this 5-year period, including a CMV CD8+ T-cell level of 4.4% when the absolute CD4+ T-cell count was zero (Fig. 1). In order to address the question of whether there was continued cell activation of virus-specific CTL during the study period, we analyzed the cell surface phenotype of the tetramer-positive cells in patient C-09. Figure 2 shows the maintenance of CD38 intensity on CMV-tetrameric-complex-staining cells over the study period. The presence of CD38 expression argues for the continued activation and proliferation of CMV-specific CTL, probably due to viral replication, although no symptomatic CMV disease was noted in patients C-09 or C-08 and levels of plasma CMV DNA were undetectable (data not shown). Moderate levels of HLA-A*0201 CMV pp65-specific CD8+ T-cell responses (mean, 0.4%, SD ± 0.25; range, 0.18 to 0.76%; median, 0.33%) were also detected in child C-08 at multiple time points when the frequency of CD4+ T cells was <5% (Fig. 1).
FIG. 1.
A and B illustrate the changes in HLA-A*0201 CMV pp65 CTL frequency from patients C-08 and C-09 over time. C illustrates the changes in HLA-A*0201 HIV-1 Gag and Pol CTL frequency from patient A-01 over time. Absolute CD4+ and CD8+ T-cell counts are shown in the top panels. The percentage of CD8+ T cells staining with the HLA-A*0201 pp65 CMV (A and B) or HLA-A*0201 HIV-1 Gag or Pol (C) tetramers are shown in the bottom panels. The x axis shows age in years.
FIG. 2.
Serial flow cytometry profiles from patient C-09 over time. The CD8+ T-cell population is illustrated with CD38 expression along the x axes and the HLA-A*0201 pp65 CMV tetramer staining along the y axes. The percentage of CD8+ T cells staining with tetramer is documented in the top right hand corner of each panel.
Priming of the CD8+ CTL response is usually dependent upon help mediated by CD4+ T cells. Antigen-stimulated CD4+ T helper cells express CD40L which interact with, and trigger, CD40, a surface receptor that can activate dendritic cells (DC) (4, 11, 33, 38). Without CD4+ T cells, we hypothesized that an alternative mechanism for DC activation and triggering of CTL could be mediated by the interaction of CD40 on the DC with CD40L on CTL themselves. DC would then produce interleukin-12 (IL-12) to promote CTL responses. A fraction of CD8+ T cell clones have been shown to express CD40L (18). No CD40L expression on CMV-specific CTL clones derived from subject C-09 was detectable by fluorescence-activated cell sorter staining (data not shown). Alternative explanations for the maintenance of virus-specific CD8+ T-cell responses at high frequencies include help mediated by residual CD4+ T cells in lymph nodes or peripheral lymphoid tissues or by direct stimulation from DC which have taken up HIV-1-infected apoptosed cells (2).
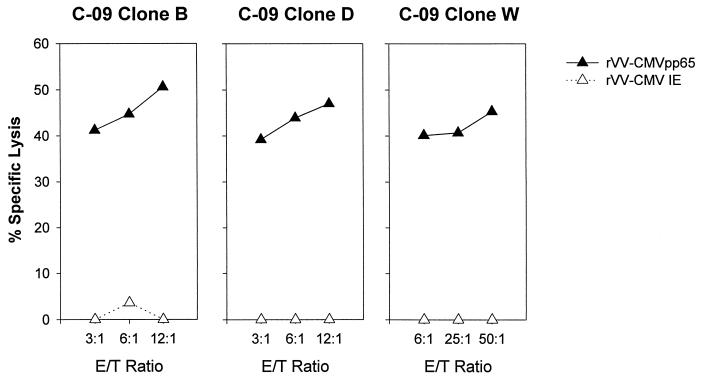
As the tetramer-positive CTL population might be an indicator of circulating CTL, but without effector function, we sorted HLA-A*0201 pp65 CMV-specific tetramer-positive CTL from patient C-09 into 96-well U-bottom plates (9) and cloned these at a concentration of 1 cell/well in the presence of IL-2 with irradiated allogeneic feeders. After 14 days of culture, wells were tested for CTL activity on autologous BCL targets with or without pp65 peptide. Three pp65 CMV-specific CD8+ T-cell clones were generated. These were expanded in vitro and were tested for effector lytic activity against targets infected with recombinant vaccinia viruses expressing CMV-pp65 or control (Fig. 3). The HLA-A*0201-restricted CMV-specific CD8+ T-cell clones were highly active in recognizing peptide to nanomolar concentrations, and all clones were HLA-A*0201 restricted (data not shown). This data demonstrates that CMV-specific CTL could be cloned from PBMC and had effector potential at a time when no peripheral CD4+ T cells were present.
FIG. 3.
CTL activity of the CMV-specific CTL clones from subject C-09. CTL clones were tested on autologous BCL-infected with rVV CMV-pp65 or control (rVV CMV IE). The percentages of specific lysis at three effector-target (E/T) ratios is shown.
No HLA-A*0201 CMV pp65-specific CTL were detected in a third patient, A-01. We analyzed HLA-A*0201 HIV-1 Gag- or Pol-peptide-specific CTL, and staining of PBMC at 17 years of age showed high levels of HLA-A*0201 HIV-1 Gag- (0.94%) and Pol- (0.66%) specific CTL. Longitudinal analysis of PBMC over the subsequent 2 years showed fluctuating levels of Gag CTL (mean, 0.6%, SD, ± 0.17; range, 0.4 to 0.86%; median, 0.65%) and Pol CTL (mean, 0.81%, SD, ± 0.03; range, 0.79 to 0.83%; median, 0.81%) (Fig. 1). In order to test the direct effector function of the HIV-1-specific CD8+ T cells of subject A-01, their ability to secrete gamma interferon (IFN-γ) in response to stimulation with a recombinant vaccinia virus expressing HIV-1 Pol was assessed at two time points (24). No IFN-γ-secreting cells were detected (data not shown).
These observations indicate that high frequencies of virus-specific CD8+ T cells can be maintained in the absence of circulating peripheral CD4+ T cells, although we cannot rule out residual CD4+-mediated T-cell help from CD4+ T cells sequestered in secondary lymphoid tissues, such as the gut-associated lymphoid tissue.
Do these tetramer-positive CD8+ T cells mount effector functions? Two recent reports have identified CD8+ effector cells by using major histocompatibility complex-peptide tetrameric complexes which were found to be functionally unresponsive (25, 42). In the first report, lymphocytic choriomeningitis virus-specific CD8+ T cells in mice expressed activation markers and proliferated in vivo, but they were unable to elaborate any antiviral effector functions (42). This unresponsive phenotype was more pronounced under conditions of CD4+ T-cell deficiency. In the presence of CD4+ T-cell help, adequate CD8+ effector activity was maintained, and the chronic viral infection eventually resolved. In the second report, circulating CD8+ T-cell populations specific for tumor-associated antigens were functionally unresponsive, unable to directly lyse melanoma target cells or produce cytokines in response to mitogens (25). The authors speculate that this may explain why such cells are unable to control tumor growth.
In this study, we found that CMV-specific CD8+ T cells sorted by tetramer at time of low CD4+ T-cell count could be cloned in vitro in the presence of IL-2 with feeders. These CMV-specific CTL clones killed pp65 CMV peptide-labeled targets in a 51Cr release assay and secreted IFN-γ in response to CMV-specific antigenic stimulation. This clearly demonstrates that the CMV-tetramer-positive CD8+ T-cell population has the capacity to expand and proliferate into functioning effector cells with appropriate costimulation and cytokine support. However, the ability of the CMV-specific CD8+ T cells to mount direct effector functions appears compromised, as only low numbers of IFN-γ-secreting T cells were observed in a direct ELISPOT assay. The fact that a low-CD4+ T-cell count (<50 per μl) is associated with an increased risk of CMV end-organ disease (12) supports the hypothesis that under conditions of low CD4+ T-cell count, CMV-specific CD8+ T cells are functionally unresponsive, although no apparent CMV-related disease was seen in these patients. However, our observation that in vitro culture can promote expansion of this apparently unresponsive population would favor therapeutic strategies designed to give exogenous cytokines, such as the administration of recombinant IL-2 (8). It will be important to further determine the capacity of HIV-1 and CMV-specific CD8+ T cells to mount effector functions and the conditions in which antigenic unresponsiveness can be reversed.
Acknowledgments
We thank the patients involved in this study and G. Mazzara, Therion, for HIV-1 recombinant vaccinia viruses; S. Riddell, Fred Hutchinson Cancer Research Center, Seattle, Wash., for CMV recombinant vaccinia viruses; M. Gately, Hoffman-La-Roche, for recombinant IL-2; Murli Perswani for CMV quantification; and Wendy Chen and Maryanne Small for administrative assistance.
This study was supported by NIH grants R01-HD34336-01 (W.B.) and R01 AI44595 (D.F.N.) and by the Medical Research Council, United Kingdom (A.J.M.). Hans Spiegel is a scholar of the Elisabeth Glaser Pediatric AIDS Foundation.
REFERENCES
- 1.Ahmed R, Butler L D, Bhatti L. T4+ T helper cell function in vivo: differential requirement for induction of antiviral cytotoxic T-cell and antibody responses. J Virol. 1988;62:2102–2106. doi: 10.1128/jvi.62.6.2102-2106.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Albert M, Sauter B, Bhardwaj N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature. 1998;392:86–89. doi: 10.1038/32183. [DOI] [PubMed] [Google Scholar]
- 3.Altman J D, Moss P A H, Golder P J R, Barouch D H, McHeyzer-Williams M G, Bell J I, McMichael A J, Davis M M. Phenotypic analysis of antigen-specific T lymphocytes. Science. 1996;274:94–96. [PubMed] [Google Scholar]
- 4.Bennett S R, Carbone F R, Karamalis F, Miller J F, Heath W R. Induction of a CD8+ cytotoxic T lymphocyte response by cross-priming requires cognate CD4+ T cell help. J Exp Med. 1997;186:65–70. doi: 10.1084/jem.186.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao Y, Qin L, Zhang L, Safrit J, Ho D D. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Eng J Med. 1995;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 6.Cardin R D, Brooks J W, Sarawar S R, Doherty P C. Progressive loss of CD8+ T cell-mediated control of a gamma-herpesvirus in the absence of CD4+ T cells. J Exp Med. 1996;184:863–871. doi: 10.1084/jem.184.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmichael A, Jin X, Sissons P, Borysiewicz L. Quantitative analysis of the human immunodeficiency virus type 1 (HIV-1)-specific cytotoxic T lymphocyte (CTL) response at different stages of HIV-1 infection: differential CTL responses to HIV-1 and Epstein Barr virus in late disease. J Exp Med. 1993;177:249–256. doi: 10.1084/jem.177.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davey R T, Jr, Chaitt D G, Albert J M, Piscitelli S C, Kovacs J A, Walker R E, Falloon J, Polis M A, Metcalf J A, Masur H, Dewar R, Baseler M, Fyfe G, Giedlin M A, Lane H C. A randomized trial of high- versus low-dose subcutaneous interleukin-2 outpatient therapy for early human immunodeficiency virus type 1 infection. J Infect Dis. 1999;179:849–858. doi: 10.1086/314678. [DOI] [PubMed] [Google Scholar]
- 9.Dunbar P R, Ogg G S, Chen J, Rust N, van der Bruggen P, Cerundolo V. Direct isolation, phenotyping and cloning of low-frequency antigen-specific cytotoxic T lymphocytes from peripheral blood. Curr Biol. 1998;8:413–416. doi: 10.1016/s0960-9822(98)70161-7. [DOI] [PubMed] [Google Scholar]
- 10.Dyer W B, Ogg G S, Demoitie M A, Jin X, Geczy A F, Rowland-Jones S L, McMichael A J, Nixon D F, Sullivan J S. Strong human immunodeficiency virus (HIV)-specific cytotoxic T-lymphocyte activity in Sydney Blood Bank Cohort patients infected with nef-defective HIV type 1. J Virol. 1999;73:436–443. doi: 10.1128/jvi.73.1.436-443.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.French R R, Chan H T, Tutt A L, Glennie M J. CD40 antibody evokes a cytotoxic T-cell response that eradicates lymphoma and bypasses T-cell help. Nat Med. 1999;5:548–553. doi: 10.1038/8426. [DOI] [PubMed] [Google Scholar]
- 12.Gerard L, Leport C, Flandre P, Houhou N, Salmon-Ceron D, Pepin J M, Mandet C, Brun-Vezinet F, Vilde J L. Cytomegalovirus (CMV) viremia and the CD4+ lymphocyte count as predictors of CMV disease in patients infected with human immunodeficiency virus. Clin Infect Dis. 1997;24:836–840. doi: 10.1093/clinids/24.5.836. [DOI] [PubMed] [Google Scholar]
- 13.Geretti A M, Dings M E, van Els C A, van Baalen C A, Wijnholds F J, Borleffs J C, Osterhaus A D. Human immunodeficiency virus type 1 (HIV-1)- and Epstein-Barr virus-specific cytotoxic T lymphocyte precursors exhibit different kinetics in HIV-1-infected persons. J Infect Dis. 1996;174:34–45. doi: 10.1093/infdis/174.1.34. [DOI] [PubMed] [Google Scholar]
- 14.Goulder P J R, Sewell A K, Lalloo D G, Price D A, Whelan J A, Evans J, Taylor G P, Luzzi G, Giangrande P, Phillips R E, McMichael A J. Patterns of immunodominance in HIV-1-specific cytotoxic T lymphocyte responses in two human histocompatibility leukocyte antigens (HLA)-identical siblings with HLA-A*0201 are influenced by epitope mutation. J Exp Med. 1997;185:1423–1433. doi: 10.1084/jem.185.8.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gray C M, Lawrence J, Schapiro J M, Altman J D, Winters M A, Crompton M, Loi M, Kundu S K, Davis M M, Merigan T C. Frequency of class I HLA-restricted anti-HIV CD8+ T cells in individuals receiving highly active antiretroviral therapy (HAART) J Immunol. 1999;162:1780–1788. [PubMed] [Google Scholar]
- 16.Greenough T C, Brettler D B, Somasundaran M, Panicali D L, Sullivan J L. Human immunodeficiency virus type 1-specific cytotoxic T lymphocytes (CTL), virus load, and CD4 T cell loss: evidence supporting a protective role for CTL in vivo. J Infect Dis. 1997;176:118–125. doi: 10.1086/514013. [DOI] [PubMed] [Google Scholar]
- 17.Hasenkrug K J, Brooks D M, Dittmer U. Critical role for CD4(+) T cells in controlling retrovirus replication and spread in persistently infected mice. J Virol. 1998;72:6559–6564. doi: 10.1128/jvi.72.8.6559-6564.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hermann P, Van-Kooten C, Gaillard C, Banchereau J, Blanchard D. CD40 ligand-positive CD8+ T cell clones allow B cell growth and differentiation. Eur J Immunol. 1995;25:2972–2977. doi: 10.1002/eji.1830251039. [DOI] [PubMed] [Google Scholar]
- 19.Jin X, Bauer D, Tuttleton S, Lewin S, Gettie A, Blanchard J, Irwin C, Safrit J, Mittler J, Weinberger L, Kostrikis L, Zhang L, Perelson A, Ho D. Dramatic rise in plasma viremia after CD8(+) T cell depletion in simian immunodeficiency virus-infected Macaques. J Exp Med. 1999;189:991–998. doi: 10.1084/jem.189.6.991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keet I P, Janssen M, Veugelers P J, Miedema F, Klein M R, Goudsmit J, Coutinho R A, de Wolf F. Longitudinal analysis of CD4 T cell counts, T cell reactivity, and human immunodeficiency virus type 1 RNA levels in persons remaining AIDS-free despite CD4 cell counts <200 for >5 years. J Infect Dis. 1997;176:665–671. doi: 10.1086/514088. [DOI] [PubMed] [Google Scholar]
- 21.Keet I P, Krol A, Klein M R, Veugelers P, de Wit J, Roos M, Koot M, Goudsmit J, Miedema F, Coutinho R A. Characteristics of long-term asymptomatic infection with human immunodeficiency virus type 1 in men with normal and low CD4+ cell counts. J Infect Dis. 1994;169:1236–1243. doi: 10.1093/infdis/169.6.1236. [DOI] [PubMed] [Google Scholar]
- 22.Kersten M J, Klein M R, Holwerda A M, Miedema F, van Oers M H. Epstein-Barr virus-specific cytotoxic T cell responses in HIV-1 infection: different kinetics in patients progressing to opportunistic infection or non-Hodgkin's lymphoma. J Clin Investig. 1997;99:1525–1533. doi: 10.1172/JCI119315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Klein M R, van Baalen C A, Holwerda A M, Kerkhof Garde S R, Bende R J, Keet I P M, Eeftinck-Schattenkerk J-K M, Osterhaus A D M E, Schuitemaker H, Miedema F. Kinetics of gag-specific cytotoxic T lymphocyte responses during the clinical course of HIV-1 infection: a longitudinal analysis of rapid progressors and long-term asymptomatics. J Exp Med. 1995;181:1365–1372. doi: 10.1084/jem.181.4.1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Larsson M, Jin X, Ramratnam B, Ogg G S, Engelmayer J, Demoitie M A, McMichael A J, Cox W I, Steinman R M, Nixon D, Bhardwaj N. A recombinant vaccinia virus based ELISPOT assay detects high frequencies of Pol-specific CD8 T cells in HIV-1-positive individuals. AIDS. 1999;13:767–777. doi: 10.1097/00002030-199905070-00005. [DOI] [PubMed] [Google Scholar]
- 25.Lee P P, Yee C, Savage P A, Fong L, Brockstedt D, Weber J S, Johnson D, Swetter S, Thompson J, Greenberg P D, Roederer M, Davis M M. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat Med. 1999;5:677–685. doi: 10.1038/9525. [DOI] [PubMed] [Google Scholar]
- 26.Matano T, Shibata R, Siemon C, Connors M, Lane H C, Martin M A. Administration of an anti-CD8 monoclonal antibody interferes with the clearance of chimeric simian/human immunodeficiency virus during primary infections of rhesus macaques. J Virol. 1998;72:164–169. doi: 10.1128/jvi.72.1.164-169.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Matloubian M, Concepcion R, Ahmed R. CD4+ T cells are required to sustain CD8+ cytotoxic T-cell responses during chronic viral infection. J Virol. 1994;68:8056–8063. doi: 10.1128/jvi.68.12.8056-8063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Musey L, Hughes J, Schacker T, Shea T, Corey L, McElrath M J. Cytotoxic-T-cell responses, viral load, and disease progression in early human immunodeficiency virus type 1 infection. N Engl J Med. 1997;337:1267–1274. doi: 10.1056/NEJM199710303371803. [DOI] [PubMed] [Google Scholar]
- 29.Ogg G S, Jin X, Bonhoeffer S, Dunbar P R, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Cerundolo V, Hurley A, Markowitz M, Ho D D, Nixon D F, McMichael A J. Quantitation of HIV-1-specific cytotoxic T lymphocytes and plasma load of viral RNA. Science. 1998;279:2103–2106. doi: 10.1126/science.279.5359.2103. [DOI] [PubMed] [Google Scholar]
- 30.Ogg G S, Jin X, Bonhoeffer S, Moss P, Nowak M A, Monard S, Segal J P, Cao Y, Rowland-Jones S L, Hurley A, Markowitz M, Ho D D, McMichael A J, Nixon D F. Decay kinetics of human immunodeficiency virus-specific effector cytotoxic T lymphocytes after combination antiretroviral therapy. J Virol. 1999;73:797–800. doi: 10.1128/jvi.73.1.797-800.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pitcher C J, Quittner C, Peterson D M, Connors M, Koup R A, Maino V C, Picker L J. HIV-1-specific CD4+ T cells are detectable in most individuals with active HIV-1 infection, but decline with prolonged viral suppression. Nat Med. 1999;5:518–525. doi: 10.1038/8400. [DOI] [PubMed] [Google Scholar]
- 32.Rahemtulla A, Fung-Leung W P, Schilham M W, Kundig T M, Sambhara S R, Narendran A, Arabian A, Wakeham A, Paige C J, Zinkernagel R M, Miller R G, Mak T W. Normal development and function of CD8+ cells but markedly decreased helper cell activity in mice lacking CD4. Nature. 1991;353:180–184. doi: 10.1038/353180a0. [DOI] [PubMed] [Google Scholar]
- 33.Ridge J P, Di Rosa F, Matzinger P. A conditioned dendritic cell can be a temporal bridge between a CD4+ T-helper and a T-killer cell. Nature. 1998;393:474–478. doi: 10.1038/30989. [DOI] [PubMed] [Google Scholar]
- 34.Rinaldo C, Huang X L, Fan Z F, Ding M, Beltz L, Logar A, Panicali D, Mazzara G, Liebmann J, Cottrill M, Gupta P. High levels of anti-human immunodeficiency virus type 1 (HIV-1) memory cytotoxic T-lymphocyte activity and low viral load are associated with lack of disease in HIV-1-infected long-term nonprogressors. J Virol. 1995;69:5838–5842. doi: 10.1128/jvi.69.9.5838-5842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Riviere Y, McChesney M B, Porrot F, Tanneau-Salvadori F, Sansonetti P, Lopez O, Pialoux G, Feuillie V, Mollereau M, Chamaret S. Gag-specific cytotoxic responses to HIV type 1 are associated with a decreased risk of progression to AIDS-related complex or AIDS. AIDS Res Hum Retrovir. 1995;11:903–907. doi: 10.1089/aid.1995.11.903. [DOI] [PubMed] [Google Scholar]
- 36.Rosenberg E S, Billingsley J M, Caliendo A M, Boswell S L, Sax P E, Kalams S A, Walker B D. Vigorous HIV-1-specific CD4+ T cell responses associated with control of viremia. Science. 1997;278:1447–1450. doi: 10.1126/science.278.5342.1447. [DOI] [PubMed] [Google Scholar]
- 37.Schmitz J, Kuroda M, Santra S, Sasseville V, Simon M, Lifton M, Racz P, Tenner-Racz K, Dalesandro M, Scallon B, Ghrayeb J, Forman M, Montefiori D, Rieber E, Letvin N, Reimann K. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science. 1999;283:857–860. doi: 10.1126/science.283.5403.857. [DOI] [PubMed] [Google Scholar]
- 38.Schoenberger S P, Toes R E, van der Voort E I, Offringa R, Melief C J. T-cell help for cytotoxic T lymphocytes is mediated by CD40-CD40L interactions. Nature. 1998;393:480–483. doi: 10.1038/31002. [DOI] [PubMed] [Google Scholar]
- 39.Tonks S, Marsh S G, Bunce M, Bodmer J G. Molecular typing for HLA class I using ARMS-PCR: further developments following the 12th International Histocompatibility Workshop. Tissue Antigens. 1999;53:175–183. doi: 10.1034/j.1399-0039.1999.530208.x. [DOI] [PubMed] [Google Scholar]
- 40.Von Herrath M G, Coon B, Oldstone M B. Low-affinity cytotoxic T-lymphocytes require IFN-gamma to clear an acute viral infection. Virology. 1997;229:349–359. doi: 10.1006/viro.1997.8442. [DOI] [PubMed] [Google Scholar]
- 41.Wills M R, Carmichael A J, Mynard K, Jin X, Weekes M P, Plachter B, Sissons J G P. The human cytotoxic T-lymphocyte (CTL) response to cytomegalovirus is dominated by structural protein pp65: frequency, specificity, and T-cell receptor usage of pp65-specific CTL. J Virol. 1996;70:7569–7579. doi: 10.1128/jvi.70.11.7569-7579.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zajac A J, Blattman J N, Murali-Krishna K, Sourdive D J, Suresh M, Altman J D, Ahmed R. Viral immune evasion due to persistence of activated T cells without effector function. J Exp Med. 1998;188:2205–2213. doi: 10.1084/jem.188.12.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]