Abstract
Background:
Drosophila melanogaster flies are smooth, low upkeep and safe model organisms, they can be effortlessly used in different fields of life sciences like genomics, biotechnology, genetics, disease model, and Wolbachia-based approaches to fight vectors and the pathogens they transmit.
Methods:
Fruit fly specimens were collected in 25 districts (14 provinces) of Iran and their morphological recognition was proven by molecular analysis based on sequence homology of mitochondrial COI barcode region. Essential information and specific requirements were provided for laboratory rearing of D. melanogaster.
Results:
Drosophila melanogaster colonies were found in 23 out of 25 districts. Also, five related species coincident with D. melanogaster were reported in this study including D. ananassae/D. parapallidosa, D. hydei, D. repleta, Zaprionus indianus (Diptera: Drosophilidae), and Megaselia scalaris (Diptera: Phoridae). The Iranian D. melanogaster molecular signature and their rearing techniques have been described here. The complete life cycle, from (egg to adult), takes approximately 8 days at 25 °C. Some biological points have been presented with highlighting capturing, rearing, culturing, and embryo collection along with primitive recognition and segregation between females and males have been presented. A recipe for culture media and the quantity of various ingredients have been provided.
Conclusion:
This is the first report on the D. repleta and D. ananassae/D. parapallidosa species for the country. Results of this study provide efficient and effective rearing procedures which are requirement for both small-scale for facilitating entomological research and large-scale use in justifiable vector control management such as disease model or Dengue control.
Keywords: Fruit fly, Mass rearing, Wolbachia, Arboviruses, Iran
Introduction
The fruit fly Drosophila melanogaster (Meigen), a miniature insect nearby three mm long, was used broadly as a model organism biology through the last era and for genetics research (1). Fruit flies act for a model investigative device for exploring genetics and biology (2). Morgan received 1933 Nobel Prize for a successions of genetic studies of Drosophila that directed them to make the chromosome model of heredity (1).
Drosophila melanogaster fruit flies are cosmopolitan in distribution, easy to capture, easy to produce and maintenance, low-cost, have a little life length (15–20 days), are relaxed to sex, and need very little area or special tools (3, 4). Drosophila melanogaster is utilized as a typical organism to study fields varying from essential genetics and disease models to the improvement of tissues and organs. There are about sixty percent homology between Drosophila genome and that of humans. Drosophila genome has few redundant region, and approximately more than 70 percent of the genes accountable for human diseases or disorders have similar gene in fruit flies (5). These traits, together with a short production time, small maintenance costs, and the readiness of powerful genetic apparatuses, permit the fruit fly to be desirable to search difficult pathways associated in biomedical research, containing cancer (6). The full genome of D. melanogaster was sequenced in 2000 (7). The likelihood to subordinate the supremacy of traditional genetics with an extensive variation of molecular and cellular methods has fascinated many scientists to study on Drosophila in different disciplines, including cell biology, neurobiology, regulation of aging, behavior, gene expression, development, and the physiopathology of human disorders or diseases (1).
Wolbachia bacteria are best known for their capability to guard their hosts against and to inhibit the transmission of pathogens. Wolbachia protection against pathogens was first reported in Drosophila, where host flies containing Wolbachia had higher persistence contrasted to uninfected flies when confronted with RNA viruses (8–10) or the entomopathogenic fungus Beauveria bassiana (11). In mosquitoes, Wolbachia inhibits or interferes the reproduction and spread of human pathogens particularly arboviruses (e.g., dengue virus) and insect-specific viruses (12), rodent and human malaria parasites (13–16), and non-human animal pathogens containing the filarial nematode Brugia pahangi (17, 18). The model organism D. melanogaster naturally harboring the wMel Wolbachia strain which is well recognized for cytoplasmic incompatibility (CI)-induction and preventing the dengue transmission. Syncytial embryo microinjection has been employed broadly for transferring Wolbachia in different insect classes (19). A Wolbachia strain separated from D. melanogaster (wMel) and artificially introduced in the mosquito vector Aedes aegypti, is currently being used as a bio-agent to control the transmission of mosquito-borne diseases such as Zika, Dengue, and Chikungunya. wMel and wMelPop strains strongly reduce Dengue virus transmission from infected mosquitoes (20). Literature indicates that several mechanisms might have been involved in the suppression of pathogens within vector body including struggle for a inadequate resource such as cholesterol, production of reactive oxygen species (ROS), instruction of immune genes, and subsidiary host gene regulation over other cellular machinery (sfRNA, RNAi) (21).
Recently, the discovery of Ae. aegypti in the south of Iran caused great concern to prevent its expansion in that area and probable transmission of the diseases for which it is a vector. Approaches using Wolbachia for the prevention of diseases transmitted by normal and un-infected insects depend on the effective founding of constant Wolbachia infections, typically by embryonic microinjection of Wolbachia-infected cytoplasm or purified Wolbachia from infected insect hosts, including D. melanogaster (22). The success of embryonic microinjection operation requires continuous purification of Wolbachia from donor D. melanogaster and inject purified Wolbachia into Ae. aegypti recipient embryos to determine the optimal number of Wolbachia-infected individuals that need to be introduced into the natural population.
Transfection techniques normally employed embryonic microinjection to relocate Wolbachia-infected embryo homogenate or embryo cytoplasm. The embryo cytoplasm or homogenate ought to be infused at 25 °C in less than five hours (23) and it is unbearable to use embryo cytoplasm or homogenate in following injection. Therefore, laboratory rearing of D. melanogaster flies to take permanent access to many Drosophila embryos (early embryos will collected every 30 min) for the purification of Wolbachia bacteria or direct transfer of Wolbachia-infected embryo cytoplasm in Aedes embryonic microinjection operation is highly needed.
For these reasons, providing laboratory rearing of fruit flies of D. melanogaster makes a perfect model organism for rearing in our research laboratory. This study was conducted to provide basic biological information and rearing in laboratory conditions associated D. melanogaster flies from Iran.
Materials and Methods
Study area: Field collections of Drosophila
We have tried to collect fruit fly specimens from a wide range of geographical regions (25 districts, 14 provinces) throughout the country. Adult fruit flies were collected from different habitats: human dwellings, jungle, and riverside, using the methods described in Hoffmann et al. (24).
Fruit flies were collected by retaining containers with banana in the stands and netting the adults afterwards a few hours, or by collecting adult fruit flies immediately from fallen fruit. The collected specimens were transferred to the Insect Molecular Biology Laboratory in the School of Public Health, Tehran University of Medical Sciences, Tehran.
Species Identification
Adult fruit fly specimens were recognized to species level using a standard morphological key (25). Moreover, PCR-directed sequencing of mitochondrial DNA cytochrome c oxidase subunit 1 (barcoding region) was used to confirm morphological identification for a subset of specimens, including the unknown or suspected specimens that had lost crucial identifying morphological characteristics. Collins et al. (1987) method was used to extract genomic DNA (26). A 710 bp barcoding region of the mtDNA-COI gene was amplified by polymerase chain reaction (PCR), using the following primers: LCO 1490 (5′-GGTCAACAAATCA TAAAGATATTGG-3′) and HCO 2198 (5′-AAACTTCAGGGTGACCAAAAAATCA-3′) (27). The PCR reaction was performed in a overall volume of 25 μL using the Taq DNA Polymerase 2xMaster Mix RED, Ampliqon (Denmark), with the following reagents: 12.5 μL of Master mix, 1 μL of each primer (10 mM), 10–20 ng (1–2 μL) of DNA extract, and 8.5–9.5 μL of double distilled water. The PCR thermal program was 5 minutes at 94 °C, followed by 30 cycles of 45s at 94 °C, 30s at 50 °C, and 1 minute at 72 °C, with a final extension at 72 °C for 10 minutes. PCR amplicons were assessed by 1% agarose gel electrophoresis, followed by GreenViewer staining and visualizing using a UV transilluminator. Sharp and cleaned amplicons were sequenced bidirectionally by the Genetic Codon Company, Tehran, Iran, using the same primers as for PCR amplification. The quality of raw sequences was enhanced using the Chromas 2.6.5 program, by deleting regions with poor quality at both ends of the sequences. The consensus of sure sequences was inspected using the NCBI (nucleotide collection) database package (28).
Life Cycle and some bionomic characters of D. melanogaster
Drosophila belongs to dipteran insects with holometabolous metamorphosis and has distinct stages of development including egg, larva, pupa and adult. Duration of these stages can be affected by the temperature (2). Drosophila melanogaster flies were reared in 500- and 1000 mL plastic culture bottles with cotton plugs. They were maintained at room temperature of about 25 °C during the entire experiment. Flies were monitored for development events, and average durations of different stages in their life.
Sexing of fruit fly specimens at pupation and adult stages was performed using morphological characters (29). The criteria for identification virgin specimens were explained according to the meconium and or the time passed after adult emergence.
Culture medium preparation:
The materials and recipe to prepare culture medium for fruit flies was provided in this study. Generally, agar powder, wheat flour, sugar, yeast (dried), and propionic acid are necessary for the medium.
Results
Drosophila melanogaster distribution in Iran
In total, 1273 fruit flies were collected from the study areas (25 districts of the country) and identified to species level using morphological characteristics and or COI barcoding sequences, through comparison with Genbank published sequences. Most of the flies found in this study belonged to D. melanogaster (21 out of 25 colonies) (Table 1). In addition to D. melanogaster, five other drosophilae or related species including D. ananassae (Doleschall) or D. parapallidosa (Tobari), D. hydei (Sturtevant), D. repleta (Wollaston), Zaprionus indianus (Gupta), and Megaselia scalaris (Loew) were found respectively from Chalous-Pakdasht, Tabriz, Chabahar, Kerman, Mashhad, and Ramsar locations (Fig. 1). This is the first report of the species D. repleta and D. ananassae (or D. parapallidosa) in the country.
Table 1.
The distribution of Drosophila spp. and related species in Iran, 2021
| No | Origin, location | Direction | Fly species | GenBank ID Number | Closest Entry in Genbank, (Country, Similarity rate) | Reference |
|---|---|---|---|---|---|---|
| 1 | Amol | North | D. melanogaster | NA | NA | NA |
| 2 | Chalus | North |
D. melanogaster
D. hydei |
NA OR077700 |
NA KJ463782 (USA, 100) KJ671603 (New Zealand, 100) |
NA DS DS |
| 3 | Ramsar | North |
D. ananassae or D. parapallidosa |
OR077701 |
MK659805 (NI, 99.85) MK659836 (NI, 99.85) |
DS DS |
| 4 | Salmanshahr | North | D. melanogaster | NA | NA | NA |
| 5 | Qaemshahr | North | D. melanogaster | NA | NA | NA |
| 6 | Tehran | Center | D. melanogaster | NA | NA | NA |
| 7 | karaj | Center | D. melanogaster | NA | NA | NA |
| 8 | Pakdasht | Center |
D. melanogaster
D. hydei |
NA OR077699 |
NA KJ671603 (New Zealand 100) KJ463782 (USA, 100) |
NA (30) (31) |
| 9 | Isfahan | Center | D. melanogaster | NA | NA | NA |
| 10 | Kashan | Center | D. melanogaster | NA | NA | NA |
| 11 | Shahrekourd | Center | D. melanogaster | NA | NA | NA |
| 12 | Kerman | Center | Zaprionus indianus | OR077697 |
EF632366 (India, 99.69) EF632355 (Benin, 99.69) |
DS DS |
| 13 | Bojnurd | Northeast | D. melanogaster | NA | NA | NA |
| 14 | Mashhad | Northeast |
D. melanogaster Megaselia scalaris (KM88* ) Megaselia scalaris (PM33* ) |
NA OR077703 OR077704 |
NA MT396285 (S.Korea, 99.55) AB907181(India, 99.39) KU949579 (Iran, 99.18) JQ941745 (China, 99.70) MT396354 (S.Korea, 99.54) |
NA (32) DS (32) DS (32) |
| 15 | Urmia | Northwest | D. melanogaster | NA | NA | NA |
| 16 | Darreh-ye Qasemlu | Northwest | D. melanogaster | NA | NA | NA |
| 17 | Miandoab | Northwest | D. melanogaster | NA | NA | NA |
| 18 | Tabriz | Northwest | D. repleta | OR077702 |
MG605140 (USA, 98.78) MG605139 (USA, 98.78) |
DS DS |
| 19 | Sanandaj | West | D. melanogaster | NA | NA | NA |
| 20 | lorestan | West | D. melanogaster | NA | NA | NA |
| 21 | Shiraz | South | D. melanogaster | NA | NA | NA |
| 22 | Ahvaz | Southwest | D. melanogaster | NA | NA | NA |
| 23 | Dezful | Southwest | D. melanogaster | OR077696 |
KY559390 (Finland, 99.69) KJ767245 (Uzbekistan, 99.69) |
DS DS |
| 24 | Zahedan | Southeast | D. melanogaster | OR077695 |
KY559390 (Finland, 100) KJ767245 (Uzbekistan, 100) |
DS DS |
| 25 | Chabahar | Southeast | Zaprionus indianus | OR077698 |
EF632366 (India, 99.54) EF632355 (Benin, 99.54) |
DS DS |
A: not indicated, DS: direct submission,
*: Voucher specimen
Fig. 1.
Adult specimens of five fruit flies or related species to Drosophila melanogaster found in the study, Iran, 2021 a: Zaprionus indianus, b: Drosophila ananassae, c: D. heydei, d: D. repleta, e: Megaselia scalaris (Original photos)
D. melanogaster sexing
Sexing according to the adult feature
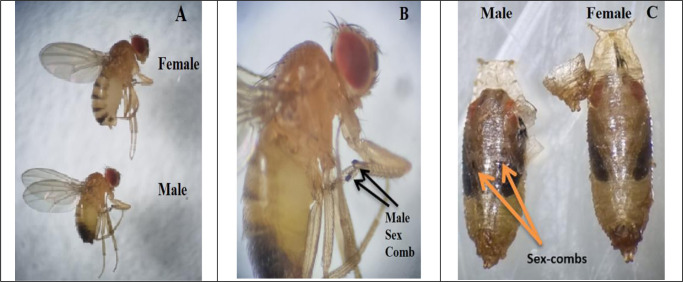
Drosophila melanogaster is sexually dimorphous and the sexes can be differentiated by the following morphological characters as already explained by (29): males are slightly smaller than females. Sexing of fruit flies is performed based on the variations in the magnitude of the females and males (Fig. 2A). In females, tip of the abdomen is pointed whereas it is somewhat rounded in males (Fig. 2A). There are eight segments in females’ abdomen, with seven being obviously noticeable whereas the final three abdominal segments are merged in males, so that only five segments are visibly detectible (Fig. 2A). In D. melanogaster fruit fly, and numerous other Drosophila species, the males’ abdominal tip is opaquer than the ones in females (Fig. 2A). However, the variation in abdominal pigmentation is not true in the newly emerged flies. The female and male genitalia, evident on the ventral face of abdominal tip, are clearly different, with the male genital plate showing darker and harder. The males possess a sex comb (Fig. 2B) as a row of around ten black, stable bristles on the first tarsal segment of front legs. In disparity, female specimens have rather pointed posterior ends with no sex combs. Moreover, females and males can also be recognized in the pupal stage by inspecting their forelegs. In the last pupal phase, male pupae can be recognized by the sex-comb which are black hairy tufts exhibit on the first tarsal segment of foreleg and obvious over the pupal case (Fig. 2C).
Fig. 2.
Sexual dimorphism in Drosophila melanogaster adults in size, shape, segments, and color of abdomen (A), presence of sex comb in adult male prolegs (B) and pupal stage (C) (Original photos)
D. melanogaster Virginity
Virginity according to adult eclosion time
In fruit fly experiments particularly genetic studies, it is important that female be a virgin to determine genetic background of progeny. A female Drosophila can store and use the sperm from a single insemination (33). Thus, it is necessary to select virgin females for genetic crosses (34). Female fruit fly does not couple within the first 8–12 h after eclosion from the pupae (1). In this study female flies mate 4–6 h after eclosion at 25 °C. Therefore, we suggest females to be separated from the males before 4 h post emerging and kept separately to use once needed.
Virginity according to meconium
In the initial hours after adult emergence, young virgin adults are distinguishing from older adults as their bodies will be very light in coloration and they are very light with a unique dark greenish spot obvious underneath the abdomen cuticle. This spot is known as meconium, which is the remnants of their last food prior to pupating. The adult flies expel meconium from the body in a few hours after eclosion. Adults having meconium may be consistently considered as virgins yet in a collection that has not been separated within the 6 h gap (Fig. 3). We found the freshly emerged adult fruit flies, sooner less than 6 h post eclosion.
Fig. 3.

Meconium on virgin adult Drosophila melanogaster (Original photo)
Selecting virgin in pupal stage
In addition to the methods mentioned above, researcher can separate males and females in pupal stage. For this purpose, we used a fine brush to select and separate mature and darkened pupae. Mature pupae are reserved on a glass slide followed by examining their ventral side under a stereo-binocular microscope. Their legs can be seen via semi-transparent pupal case. Female’s pupa does not have the sex comb on their front legs (Fig. 2C) and can be transferred to a separate food vial.
Culture media for fruit fly maintenance in laboratory
Rearing media preparation
An exclusive recipe for preparation of fly food has been prepared in our laboratory. The necessary materials include agar powder 40–60 gr, wheat flour 300 gr, sugar 300 gr, yeast (dried) 200 g, propionic acid 25 mL, and distilled water 5 liter. Firstly, the yeast, sugar and wheat flour were mixed in cold water and then boiled on heater, then slowly added the agar powder to the medium till agar is completely dissolved. The mixture was then cooked on heater for 7–10 minutes. Once the medium temperature reaches close to 50 degrees centigrade, propionic acid is added and mixed well. Throughout the preparation, the culture should be continually blended. The cooked food was poured in bottles (around 30–40 mL each) and allowed it to harden as it cools down. Afterwards 4–5 h, any damp deposited on the bottle’s walls wiped dry with fiber and plugged with cotton. The food is ready to use after one day. The bottles could furthermore be kept in a refrigerator for 1–2 weeks for forthcoming use.
Media for Drosophila embryo collection
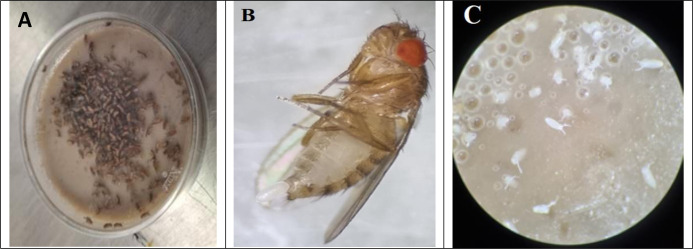
Wolbachia-infected D. melanogaster is being used in transfection studies for Dengue control program. Flies will kept at 25 °C using normal Drosophila rearing settings (35). Microinjection experiments (Transfection of Wolbachia bacteria to Aedes embryos), require collection of relatively large number of D. melanogaster (donor of Wolbachia) early embryos in a limited time (every 30 min) (23). In general, embryos are gathered in Petri dishes encompassing Drosophila culture, because of the assist with which they can be collected from the food surface. For this reason, the media has been poured (10 mL) in Petri dishes, then about 150 adult flies that already were numbed in freezer for few minutes were released in Petri dish (Fig. 4A). The lid of Petri dishes was sealed with adhesive tape to avoid escaping flies. After adults’ egg laying (Fig. 4B), Drosophila embryo (Fig. 4C).
Fig. 4.
Drosophila melanogaster culturing in Petri dish (A), female’ egg laying (B) and D. melanogaster’s eggs (embryos) in media culture (Original photos)
Drosophila melanogaster Life Cycle
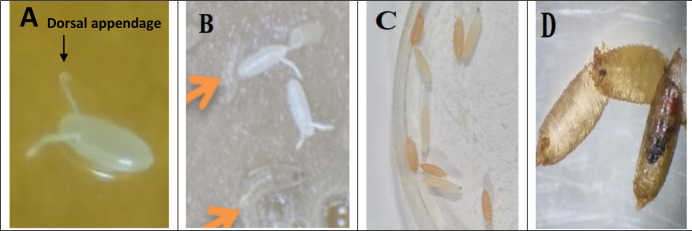
Four discrete stages exist in the life cycle of D. melanogaster fruit fly: egg (Fig. 5A), larva (Fig. 5B), pupa (Fig. 5C) and adult (Fig. 5D). Eggs of D. melanogaster are white in color and are approximately 0.5 mm in length with two dorsal appendages (Fig. 5A). This two egg threads or dorsal projections develops from its anterior end to avoid egg from sinking into soft breeding places. A fresh culture of D. melanogaster can produce new adults in 9 days at 25 °C: 5 days in the egg and larval phases, and 4 days in the pupal period. Principally it should be noted that higher temperature resulted in a shorter life cycle. In this study condition at 25 °C, eggs hatched after 24 h, and the 1st tiny instar larva appeared. The larval phases comprise of three mobile steps or instar larvae, the first (L1), second (L2) and third (L3) instars. After 24 h, the 2nd instar larvae appeared and turned into 3rd instar larvae after 24 hours. The first, second and initial third instar larvae mostly persist inside the food media, aggressively feed, and raise their body mass. Late third instar larvae get out of food and scramble in seek of a dry position for pupation, which is commonly the wall bottle or the upper cover of Petri dish, where they are sustained in laboratory.
Fig. 5.
Drosophila melanogaster egg with dorsal appendages (A), first instar larva (B), third instar larva and brown pupal stage (C) and white, brown and late pupa (D) (Original photos)
Late third instar larvae stick at dry positions using the adhesive protein produced by their salivary glands. For four days pupa completed their development cycle from white pupa (which in the commencement is white and soft), brown pupa (harder cuticle and duskier in color because of sclerotization and chitinization), mid pupa and late pupa with eye pigments which they experience the final molt to begin the pupal phase.
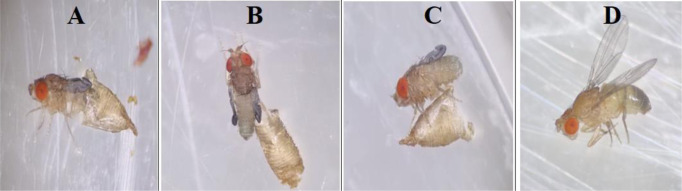
At the end of pupal stage, the imago opens by driving its way over the anterior end of the pupal case (Fig. 6A). The newly emerged D. melanogaster fly is lengthened, igniter in color, and with folded wings with visible meconium (Fig 6B and 6C). In the next 2–3 h, the wings open out; the body blackens and reaches the typical adult body form while the meconium pass away (Fig. 6D). Female flies get ready to couple in 4–6 h after emergence.
Fig. 6.
Drosophila melanogaster adult eclosion stages: pupal case (imago) ecloses by imposing its way over the anterior end of the imago (A) freshly emerged adult, with light color and folded wings (B and C), adult with expanded wings, darken body, and attained the typical adult body shape (D) (Original photos)
Discussion
Despite the extensive use of Drosophila in biological and genetic research, the fruit fly fauna of Iran is inadequately studied. The family Drosophilidae is an extremely diverse, universal family of flies that contains fruit flies and pomace or vinegar flies (36). This is the first survey to investigate fruit fly specimens’ distribution from a wide range of geographical regions (25 districts, 14 provinces) throughout the country.
Previous research on Drosophila species in the province of West Azarbaijan recorded ten drosophilid species (37), which were all new to the west of Iran (except for D. melanogaster). Interestingly, one drosophilid genus Lordiphosa Basden and three species Scaptodrosophila lebanonensis (Wheeler), Lordiphosa andalusiaca (Strobl), and D. hydei (Sturtevant), were reported for the first time in Iran. In addition, the species Zaprionus indianus (Gupta), D. simulans Sturtevant, D. phalerata Meigen, D. subobscura Collin, Scaptomyza flava (Fallén), and S. pallida (Zetterstedt), were recorded in the study area.
In the current study most of the fruit flies were D. melanogaster (21 colonies), while D. ananassae (or D. parapallidosa) specimens were found in only one location, Ramsar in the North of the country. Although we also found specimens belonging to species like M. scalaris, D. hydei, D. repleta, and Z. indianus (Fig. 1) respectively from Mashhad, Chalous-Pakdasht, Tabriz, Chabahar-Kerman. In 2015 the first report of urogenital myiasis due to M. scalaris in Iran was published (38). Also, this species was found on human cadavers in Tehran District (33).
In this study we used the mtDNA-COI gene for species identification of fruit flies. This gene has been displayed to be a powerful molecular marker to identify different insects at species or even population level. Several studies have assessed the effectiveness of COI gene and proved to be successful for many groups of in vertebrates, such as, flies, mosquitoes, cockroaches, and black flies (33, 39–47).
This is the first report on the described D. repleta (Patterson) and D. ananassae (or D. parapallidosa) species for the country. Drosophila repleta, frequently referred to as dark-eyed fruit fly or dark-eyed vinegar fly, is a synanthropic species of Nearctic-Neotropical origin (48). The larvae of this species forage on yeast in putrefying and fermenting organic matter (49). Because of this ecological niche, these fruit flies have turned into pests in various settings where food debris exists. In the agricultural setting, D. repleta fruit fly can be an annoyance pest in animal and poultry facilities (50). Drosophila repleta has been shown to scatter from pit toilets into nearby residences from around 305 m away (51). Spreading of flies from animal facilities to local houses may be of some trouble because fruit fly species can transfer pathogens (52). Black et al. (2018) showed that D. repleta is a carrier of foodborne pathogens containing Salmonella Saint Paul, Escherichia coli, and Listeria innocua onto human food (53).
Drosophila hydei belongs to subgroup of the repleta species group (54). Strangely, this fruit fly is known to have a long sperm proximately 23 mm, ten times the size of the male's body (55). Drosophila hydei are frequently observed on dung piles globally and are one of the more usual flies used as feeders in the domestic animal market. Flightless forms of this species are reported (56).
The COI sequence analysis of fruit fly specimens in Ramsar, northern Iran, revealed a similar sequence homology (99.85%) to either D. ananassae or D. parapallidosa species. These two sister taxa are new for Iran, belong to the subgenus Sophophora, melanogaster group, ananassae subgroup, and the D. ananassae species complex. Like D. melanogaster, D. ananassae is a useful model organism for genetic studies and is among the 12 fruit fly species that its genome was sequenced (57). Drosophila ananassae is a human-commensal species scattered in subtropical and tropical regions worldwide, and it contains a number of sibling or cryptic species in Asia-Oceania (58–61). This is suggested that D. parapallidosa has recently moved northward from a tropical (hot and humid) to a subtropical region and has happened to sympatric with D. ananassae there. It is proposed that D. parapallidosa is constantly sympatric with D. ananassae, but not the other way around (62).
Zaprionus indianus was another fruit fly species reported in this study. Zaprionus indianus was reported for the first time from western Iran (Zirtang-e Siab in Lorestan Province) (63) according to BLAST analysis of COI sequences. This species is known as an invasive species, also recognized as the striped vinegar fly in the United State of America and the African fig fly in South America. This is a fig pest in the new- and old-world countries, and it could cause economic lose in the areas they are present (64). The distribution and its possible stable establishing in Iran especially in fig farms should thus be monitored in the future to evaluate its damages.
Drosophila fruit flies are holometabolous insects and four development stages: egg, larva, pupa, and adult can be seen in their life cycle. In laboratories, D. melanogaster is usually maintained at 25 or 18 °C (the lower temperature is used principally for keeping stocks) (1); we prepare 25 °C for all the timing. The generation time in 25 °C took roughly 9 days from fertilized egg to freshly emerge adult. At 25 °C a new and fresh culture of D. melanogaster produced new adults in nine days: five days in the egg plus larval phases, and four days in the pupal stage. Flagg reported that at 21°C a fresh culture of D. melanogaster could create new adults in two weeks; eight days in the prepupal stages, and six days in the pupal phase (34). It is known that development time of immature stages of insects is affected by the temperature (65–67).
Maintenance of D. melanogaster fly colonies in laboratory needs permanent supervision and attention to guarantee that flies are fit and there is an optimal condition inside the culture. Density is one of the important biotic factors in laboratory rearing of insects. Over-crowding is a frequent trouble as fruit flies continue laying eggs and increase their amounts within the restrained area and resources accessible in the bottle/vial (29), younger larvae consume aggressively and mainly stay interior the diet until they prime for pupation. Nevertheless, overpopulation imposes much the young larvae (1st and 2nd instar larvae) to scramble out of the food stuff. The D. melanogaster flies must be relocated repeatedly to fresh bottles/vials with diet because rotting food and fly remains charm infecting pathogens like mites, fungi, and bacteria. In the existing work we relocated D. melanogaster flies repeatedly to new containers within 25–30 days. Establishing a new medium with around 10–20 flies in containers and less than fifty fruit flies in jugs or bottles prevents overcrowding and asserts a vigorous culture.
To date, the ectoparasitic mite Macrocheles subbadius and Allantonematid nematodes linked with mycophagous fruit flies are known as parasite interaction with drosophilid colonies (68). We have maintained a D. melanogaster colony in the laboratory since 2017. Mites are a main trouble in any insect laboratory colonies. These tiny animals can be simply noticed in aged colonies as mites walk surrounding blank pupal cases. They put a pearly feature of 10–20 eggs near pupae. Mites cause a significant risk to the developing colonies. In addition to feeding lifeless flies, mites similarly damage vital pupae and embryos, causing devastation of the fly colony. During these few years, except for some cases of nematode contamination we didn’t have any other kinds of major infections in Drosophila cultures. The source of contamination was discarded in those cases.
Historically, fruit fly colonies in laboratories have been kept on bananas. However, since banana converts very dim and hard to process, agar-based culturing medium using wheat cream or maize powder, has suited universal. Several culture media have been introduced, all built on simple ingredients such as sucrose, propionic acid, yeast (not yeast extract), and agar (1). Some media encompassing a diverse additives comprising rice, raisins, oat hulls, pears, and molasses have been recorded (4). A few of the procedures applied for grounding of fruit fly diet in diverse laboratories are (28): 1: Agar-Maize powder-sugar food, 2: Corn meal-Agar medium, 3: Wheat Cream-Jaggery Medium, 4: Corn flour-Jaggery Medium, 5: Banana- Barley-jaggery medium. In current study culture media based on Agar powder, wheat flour, sugar, yeast (dried), propionic acid, and distilled water were used for maintenance of flies in laboratory. All fly cultures in the food media which are prepared in this way can be stored in the freezer for up to 3 months in sterilized bottles.
Conclusion
This is the first report of the species D. repleta and D. ananassae/D. parapallidosa in the country. Further monitoring and investigation is suggested for the fruit fly species that can carry pathogens, food-borne diseases, or act as agricultural pests. Results of this study provide effective and efficient rearing information and recipe which are important for both minor entomological investigation and large-scale exploitation in maintainable vector control management such as disease model or Dengue control.
Acknowledgements
We are grateful to Mr Ghasemi for helping us in insectarium in the Department of Vector Biology and Control of Diseases, SPH, TUMS. Research was supported by Elite Researcher Grant Committee under award number 963441 from the National Institutes for Medical Research Development (NIMAD), Tehran, Iran. Also, this study was supported by the Tehran University of Medical Sciences, Iran, Grant number 46186.
Footnotes
Ethical consideration
The protocols were conducted in this study followed the guidelines of the institutional ethical committee (Tehran University of Medical Sciences, TUMS). The protocols were approved by TUMS ethical committee under registry IR.TUMS.SPH.REC.1399.176.
Conflict of interest statement
The authors declare there is no conflict of interests.
References
- 1.Fernández-Moreno MA, Farr CL, Kaguni LS, Garesse R. (2007) Drosophila melanogaster as a model system to study mitochondrial biology. Methods Mol Biol. 372: 33–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wollard L, Klein B, Carlson DJ, Carlson KA. (2006) Rearing media as a variable in fruit fly fecundity: an activity to introduce scientific methods of inquiry to biology students. Bioscene. 32(3): 24–29. [Google Scholar]
- 3.Ashburner M, Roote J. (2000) Culture of Drosophila: The laboratory setup. adapted from “Laboratory Culture of Drosophila,” chapter 35, in Drosophila Protocols (eds Sullivan et al.) Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, USA. pdb.ip34. [Google Scholar]
- 4.Bridges CB, Darby HH. (1933) Culture media for Drosophila and the pH of media. Am Nat. 67: 437–472. [Google Scholar]
- 5.Ugur B, Chen K, Bellen HJ. (2016) Drosophila tools and assays for the study of human diseases. Dis Model Mech. 9(3): 235–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mirzoyan Z, Sollazzo M, Allocca M, Valenza AM, Grifoni D, Bellosta P. (2019) Drosophila melanogaster: A model organism to study cancer. Front Genet. 10: 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hotaling S, Sproul JS, Heckenhauer J, Powell A, Larracuente AM, Pauls SU, Kelley JL, Frandsen PB. (2021) Long reads are revolutionizing 20 years of insect genome sequencing. Genome Biol Evol. 13(8): evab138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Teixeira L, Ferreira Á, Ashburner M. (2008) The bacterial symbiont Wolbachia induces resistance to RNA viral infections in Drosophila melanogaster. PLoS Biol. 6 (12): e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pimentel AC, Cesar CsS, Martins M, Cogn R. (2021) The antiviral effects of the symbiont bacteria Wolbachia in insects. Front Immunol. 11: 626329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hedges LM, Brownlie JC, O'neill SL, Johnson KN. (2008) Wolbachia and virus protection in insects. Science. 322(5902): 702. [DOI] [PubMed] [Google Scholar]
- 11.Panteleev DI, Goryacheva I, Andrianov BV, Reznik N, Lazebny O, Kulikov A. (2007) The endosymbiotic bacterium Wolbachia enhances the nonspecific resistance to insect pathogens and alters behavior of Drosophila melanogaster. Genetika. 43: 1277–80. [PubMed] [Google Scholar]
- 12.Schnettler E, Sreenu VB, Mottram T, McFarlane M. (2016) Wolbachia restricts insectspecific flavivirus infection in Aedes aegypti cells. J Gen Virol. 97(11): 3024–3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Joshi D, Pan X, McFadden MJ, Bevins D, Liang X, Lu P, Thiem S, Xi Z. (2017) The maternally inheritable Wolbachia wAlbB induces refractoriness to Plasmodium berghei in Anopheles stephensi. Front Microbiol. 8: 366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hughes GL, Koga R, Xue P, Fukatsu T, Rasgon JL. (2011) Wolbachia infections are virulent and inhibit the human malaria parasite Plasmodium falciparum in Anopheles gambiae. PLoS Pathog. 7(5): e1002043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bian G, Joshi D, Dong Y, Lu P, Zhou G, Pan X, Xu Y, Dimopoulos G, Xi Z. (2013) Wolbachia invades Anopheles stephensi populations and induces refractoriness to Plasmodium infection. Science. 340 (6133): 748–751. [DOI] [PubMed] [Google Scholar]
- 16.Kambris Z, Blagborough AM, Pinto SB, Blagrove MS, Godfray HC, Sinden RE, Sinkins SP. (2010) Wolbachia stimulates immune gene expression and inhibits Plasmodium development in Anopheles gambiae. PLoS Pathog. 6(10): e1001143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kambris Z, Cook PE, Phuc HK, Sinkins SP. (2009) Immune activation by life-shortening Wolbachia and reduced filarial competence in mosquitoes. Science. 326 (5949): 134–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Andrews ES, Crain PR, Fu Y, Howe DK, Dobson SL. (2012) Reactive oxygen species production and Brugia pahangi survivorship in Aedes polynesiensis with artificial Wolbachia infection types. PLoS Pathog. 8(12): e1003075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sasaki T, Ishikawa H. (2000) Transinfection of Wolbachia in the mediterranean flour moth, Ephestia kuehniella, by embryonic microinjection. Heredity (Edinb). 85 (Pt 2): 130–135. [DOI] [PubMed] [Google Scholar]
- 20.Flores HA, Taneja de Bruyne J, O'Donnell TB, Tuyet Nhu V, Thi Giang N, Thi Xuan Trang H, Thi Thuy Van H, Thi Long V, Thi Dui L, Le Anh Huy H, Thi Le Duyen H, Thi Van Thuy N, Thanh Phong N, Van Vinh Chau N, Thi Hue Kien D, Thuy Vi T, Wills B, O'Neill SL, Simmons CP, Carrington LB. (2020) Multiple Wolbachia strains provide comparative levels of protection against dengue virus infection in Aedes aegypti. PLoS Pathog. 16(4): e1008433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yen PS, Failloux AB. (2020) A review: Wolbachia-based population replacement for mosquito control shares common points with genetically modified control approaches. Pathogens. 9(5): 404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Walker T, Johnson PH, Moreira LA, Iturbe-Ormaetxe I, Frentiu FD, McMeniman CJ, Leong YS, Dong Y, Axford J, Kriesner P, Lloyd AL, Ritchie SA, O'Neill SL, Hoffmann AA. (2011) The wMel Wolbachia strain blocks dengue and invades caged Aedes aegypti populations. Nature. 476 (7361): 450–453. [DOI] [PubMed] [Google Scholar]
- 23.Xi Z, Dobson SL. (2005) Characterization of Wolbachia transfection efficiency by using microinjection of embryonic cytoplasm and embryo homogenate. Appl Environ Microbiol. 71(6): 3199–3204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hoffmann A, Turelli M, Harshman LG. (1990) Factors affecting the distribution of cytoplasmic incompatibility in Drosophila simulans. Genetics. 126(4): 933–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yuzuki K, Tidon R. (2020) Identification key for drosophilid species (Diptera, Drosophilidae) exotic to the neotropical region and occurring in Brazil. Rev Bras entomol. 64(1): e2019100. [Google Scholar]
- 26.Collins FH, Mendez MA, Rasmussen MO, Mehaffey PC, Besansky NJ, Finnerty V. (1987) A ribosomal RNA gene probe differentiates member species of the Anopheles gambiae complex. Am J Trop Med Hyg. 37: 37–41. [DOI] [PubMed] [Google Scholar]
- 27.Simon C, Frati F, Beckenbach A, Crespi B, Liu H, Flook P. (1994) Evolution, weighting, and phylogenetic utility of mitochondrial gene sequences and a compilation of conserved polymerase chain reaction primers. Ann Entomol Soc Am. 87 (6): 651–701. [Google Scholar]
- 28.Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG. (2011) Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol Syst Biol. 7(1): 539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chaudhary GR, Pandey A, Singh A, Yadav V, Dwivedi V, Arya R, Lakhotiaet SC. (2021) Experiments with Drosophila for biology courses (Eds: Lakhotia S. C., Ranganath H. A.) Indian Academy of Sciences, Bengaluru, India, pp. 21–31. [Google Scholar]
- 30.Dhami MK, Kumarasinghe L. (2014) A HRM real-time PCR assay for rapid and specific identification of the emerging pest spotted-wing Drosophila (Drosophila suzukii). PLoS One. 9(6): e98934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kim S, Tripodi AD, Johnson DT, Szalansk AL. (2014) Molecular diagnostics of Drosophila suzukii (Diptera: Drosophilidae) using PCR-RFLP. J Econ Entomol. 107 (3): 1292–1294. [DOI] [PubMed] [Google Scholar]
- 32.Kim HJ, Shin SE, Ko KS, Park SH. (2020) The application of mitochondrial COI gene-based molecular identification of forensically important scuttle flies (Diptera: Phoridae) in Korea. Biomed Res Int. 2020: 6235848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Talebzadeh F, Oshaghi MA, Akbarzadeh K, Panahi-Moghadam S. (2020) Molecular species identification of six forensically important iranian flesh flies (Diptera). J Arthropod Borne Dis. 14(4): 416–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flagg RO. (2005) Carolina Drosophila Manual. Carolina Biological Supply Company, Burlington. [Google Scholar]
- 35.Ashburner M. (1989) Drosophila, a laboratory handbook. Cold Spring Harbor Laboratory Press, New York. [Google Scholar]
- 36.Markow TA, O'Grad PM. (2016) Drosophila: A guide to species identification and use. Elsevier, Netherlands. [Google Scholar]
- 37.Parchami-Araghi M, Gilasian E, Bächli G. (2016) Drosophilids of the lake urmia national park, Iran (Diptera: Drosophilidae). Dros Inf Serv. 99: 63–69. [Google Scholar]
- 38.Ghavami MB, Djalilvand A. (2015) First record of urogenital myiasis induced by Megaselia scalaris (Diptera: Phoridae) from Iran. J Arthropod-Borne Dis. 9(2): 274–280. [PMC free article] [PubMed] [Google Scholar]
- 39.Oshaghi MA, Yaaghoobi F, Abai MR. (2006) Pattern of mitochondrial DNA variation between and within Anopheles stephensi (Diptera: Culicidae) biological forms suggests extensive gene flow. Acta Trop. 99 (2–3): 226–233. [DOI] [PubMed] [Google Scholar]
- 40.Oshaghi MA, Shemshad K, Yaghoobi-Ershadi MR, Pedram M, Vatandoost H, Abai MR, Akbarzadeh K, Mohtarami F. (2007) Genetic structure of the malaria vector Anopheles superpictus in Iran using mitochondrial cytochrome oxidase (COI and COII) and morphologic markers: a new species complex? Acta Trop. 101(3): 241–248. [DOI] [PubMed] [Google Scholar]
- 41.Karimian F, Oshaghi MA, Sedaghat MM, Waterhouse RM, Vatandoost H, Hanafi-Bojd AA, Maleki-Ravasan N, Chavshin AR. (2014) Phylogenetic analysis of the Oriental-Palearctic-Afrotropical members of Anopheles (Culicidae: Diptera) based on nuclear rDNA and mitochondrial DNA characteristics. Jpn J Infect Dis. 67(5): 361–367. [DOI] [PubMed] [Google Scholar]
- 42.Hashemi-Aghdam SS, Oshaghi MA. (2015) A checklist of Iranian cockroaches (Blattodea) with description of Polyphaga sp as a new species in Iran. J Arthropod Borne Dis. 9(2): 161–75. [PMC free article] [PubMed] [Google Scholar]
- 43.Hashemi-Aghdam SS, Rafie G, Akbari S, Oshaghi MA. (2017) Utility of mtDNA-COI barcode region for phylogenetic relationship and diagnosis of five common pest cockroaches. J Arthropod Borne Dis. 11(2): 182–193. [PMC free article] [PubMed] [Google Scholar]
- 44.Khanzadeh F, Khaghaninia S, Maleki-Ravasan N, Oshaghi MA, Adler PH. (2020) Black flies (Diptera: Simuliidae) of the Aras River Basin: species composition and floral visitation. Acta Trop. 209: 105536. [DOI] [PubMed] [Google Scholar]
- 45.Koosha M, Oshaghi MA, Sedaghat MM, Vatandoost H, Azari-Hamidian S, Abai MR, Hanafi-Bojd AA, Mohtarami F. (2017) Sequence analysis of mtDNA COI barcode region revealed three haplotypes within Culex pipiens assemblage. Exp Parasitol. 181(1848): 102–110. [DOI] [PubMed] [Google Scholar]
- 46.Jafari S, Oshaghi MA, Akbarzadeh K, Abai MR, Koosha M, Mohtarami F. (2019) Identification of forensically important flesh flies using the cytochrome c oxidase subunits I and II genes. J Med Entomol. 56 (5): 1253–1259. [DOI] [PubMed] [Google Scholar]
- 47.Bazrafkan S, Vatandoost H, Heydari A, Bakhshi H, Panahi-Moghadam S, Hashemi-Aghdam S, Mohtarami F, Rahimiforoushan A, Anlaandş S, Shayeghi M, Oshaghi MA, Abtahi SM. (2016) Discrimination of Paederus fuscipes and Paederus littoralis by mtDNA-COI PCR-RFLP. J Arthropod Borne Dis. 10(4): 454–461. [PMC free article] [PubMed] [Google Scholar]
- 48.Ashburner M, Carson HL, Thompson JN. (1981) The genetics and biology of Drosophila. London, Academic Press. 3. [Google Scholar]
- 49.Morais PB, Rosa CA, Hagler AN, Mendonca-Hagler LC. (1995) Yeast commu-nities as descriptors of habitat use by the Drosophila-Fasciola subgroup (Repleta group) in Atlantic rainforests. Oecologia. 104(1): 45–51. [DOI] [PubMed] [Google Scholar]
- 50.Harrington LC, Axtell RC. (1994) Comparisons of sampling methods and seasonal abundance of Drosophila repleta in caged-layer poultry houses. Med Vet Entomol. 8(4): 331–339. [DOI] [PubMed] [Google Scholar]
- 51.Pimentel D, Fay RW. (1995) Dispersion of radioactively tagged Drosophila from pit privies. J Econ Entomol. 48: 19–22. [Google Scholar]
- 52.Ewing WH. (1962) Sources of Escherichia coli cultures that belonged to O antigen groups associated with infantile diarrheal disease. J Infect Dis. 110(2): 114–120. [DOI] [PubMed] [Google Scholar]
- 53.Black E, Ecolab, Hinrichs GJ, Barcay SJ, Gardner DB. (2018) Fruit flies as potential vectors of foodborne illness. J Food Prot. 81(3): 509–514. [DOI] [PubMed] [Google Scholar]
- 54.Spicer GS, Pitnick S. (1996) Molecular systematics of the Drosophila hydei subgroup as inferred from mitochondrial DNA sequences. J Mol Evol. 43(3): 281–286. [DOI] [PubMed] [Google Scholar]
- 55.Pitnick S, Markow TA. (1994) Large-male advantages associated with costs of sperm production in Drosophila hydei, a species with giant sperm. Proc Natl Acad Sci U S A. 91(20): 9277–9281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhao L, Begun DJ. (2017) Genomics of parallel adaptation at two timescales in Drosophila. PLoS Genet. 13(10): e1007016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Suvorov A, Kim BY, Wang J, Armstrong EE, Peede D, D'Agostino ERR, Price DK, Waddell P, Lang M, Courtier-Orgogozo V, David JR, Petrov D, Matute DR, Schrider DR, Comeault AA. (2022) Widespread introgression across a phylogeny of 155 Drosophila genomes. Curr Biol. 32(1): 111–123.e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bock IR, Wheeler M. (1972) The Drosophila melanogaster species group. Unv Texas Publ. 7213: 1–102. [Google Scholar]
- 59.Tobari YN. (1993) Geographic distribution. In “Drosophia ananassae: genetical and biological aspects. Japan Sci Soc Press, Tokyo. 1993: 19–22. [Google Scholar]
- 60.Matsuda M, Ng C-S, Doi M, Kopp A, Tobari YN. (2009) Evolution in the Drosophila ananassae species subgroup. Fly. 3 (2): 157–169. [DOI] [PubMed] [Google Scholar]
- 61.McEvey S, Schiffer M. (2015) New species in the Drosophila ananassae subgroup from northern Australia, New Guinea and the south Pacific (Diptera: Drosophilidae), with historical overview. Rec Austral Mus. 67(5): 129–161. [Google Scholar]
- 62.Sawamura K, Sato H, Lee C-Y, Kamimura Y, Matsuda M. (2016) A natural population derived from species hybridizationin the Drosophila ananassae species complexon Penang Island, Malaysia. Zoolog Sci. 33(5): 467–475. [DOI] [PubMed] [Google Scholar]
- 63.Tavakoli M, Sattari S, Hosseini-Chegen A. (2020) Additional records of the African fig fly Zaprionus indianus Gupta, 1970 (Diptera: Drosophilidae) for western Iran supported by DNA barcoding. J Animal Divers (JAD). 2(3): 16–23. [Google Scholar]
- 64.Kremmer L, David JR, Borowiec N, Thaon M, Ris N, Poirié M, Gatti JL. (2017) The African fig fly Zaprionus indianus: a new invasive pest in France? Bull Insectology. 70(1): 57–62. [Google Scholar]
- 65.Kordshouli RS, Grzywacz A, Akbarzadeh K, Azam K, AliMohammadi A, Pasha MG, Oshaghi MA. (2021) Thermal requirements of immature stages of Chrysomya albiceps (Diptera: Calliphoridae) as a common forensically important fly. Sci Justice. 61(3): 227–234. [DOI] [PubMed] [Google Scholar]
- 66.Shiravi A, Mostafavi R, Akbarzadeh K, Oshaghi MA. (2011) Temperature requirements of some common forensically important blow and flesh flies (Diptera) under laboratory conditions. Iran J Arthropod Borne Dis. 5(1): 54–62. [PMC free article] [PubMed] [Google Scholar]
- 67.Oshaghi MA, Maleki-Ravasan N, Javadian E, Rassi Y, Sadraei J, Enayati AA, Vatandoost H, Zare Z, Emami SN. (2009) Application of predictive degree day model for field development of sandfly vectors of visceral leishmaniasis in northwest of Iran. J Vector Borne Dis. 46(4): 247–255. [PubMed] [Google Scholar]
- 68.Perez-Leanosa A, Loustalot-Laclettea MR, Nazario-Yepiza N, Markow TA. (2017) Ectoparasitic mites and their Drosophila hosts. Fly. 11(1): 10–18. [DOI] [PMC free article] [PubMed] [Google Scholar]