Abstract
Background
This review covers two conditions: acute clavicle fractures and non‐union resulting from failed fracture healing. Clavicle (collarbone) fractures account for around 4% of all fractures. While treatment for these fractures is usually non‐surgical, some types of clavicular fractures, as well as non‐union of the middle third of the clavicle, are often treated surgically. This is an update of a Cochrane review first published in 2009.
Objectives
To evaluate the effects (benefits and harms) of different methods of surgical treatment for acute fracture or non‐union of the middle third of the clavicle.
Search methods
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (27 June 2014), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 5), MEDLINE (1966 to June week 3 2014), EMBASE (1988 to 2014 week 25), LILACS (1982 to 27 June 2014), trial registries and reference lists of articles. We applied no language or publication restrictions.
Selection criteria
We considered randomised and quasi‐randomised controlled trials evaluating any surgical intervention for treating people with fractures or non‐union of the middle third of the clavicle. The primary outcomes were shoulder function or disability, pain and treatment failure (measured by the number of participants who had undergone or were being considered for a non‐routine secondary surgical intervention for symptomatic non‐union, malunion or other complications).
Data collection and analysis
Two review authors selected eligible trials, independently assessed risk of bias and cross‐checked data. Where appropriate, we pooled results of comparable trials.
Main results
We included seven trials in this review with 398 participants. Four trials were new in this update.
The four new trials (160 participants) compared intramedullary fixation with open reduction and internal fixation with plate for treating acute middle third clavicle fractures in adults. Low quality evidence from the four trials indicated that intramedullary fixation did not result in a clinically important improvement in upper arm function (despite a statistically significant difference in its favour: standardised mean difference 0.45, 95% confidence interval (CI) 0.08 to 0.81; 120 participants, three trials) at long term follow‐up of six months or more. Very low quality evidence indicated little difference between intramedullary fixation and plate fixation in pain (one trial), treatment failure resulting in non‐routine surgery (2/68 with intramedullary fixation vs. 3/65 with plate fixation; risk ratio 0.69, 95% CI 0.16 to 2.97, four trials) or time to clinical fracture consolidation (three trials). There was very low quality evidence of a lower incidence of participants with adverse events (mainly infection, poor cosmetic result and symptomatic hardware) in the intramedullary fixation group (18/68 with intramedullary fixation vs. 27/65 with plate fixation; RR 0.64, 95% CI 0.39 to 1.03) but the CI of the pooled results also included the small possibility of a lower incidence in the plate fixation group. None of the four trials reported on quality of life or return to previous activities. Evidence is pending from two ongoing trials, with planned recruitment of 245 participants, testing this comparison.
There was low or very low quality evidence from three small trials, each testing a different comparison. The three trials had design features that carried a high risk of bias, potentially limiting the reliability of their findings. Low‐contact dynamic compression plates appeared to be associated with significantly better upper‐limb function throughout the year following surgery, earlier fracture union and return to work, and a reduced incidence of implant‐associated symptoms when compared with a standard dynamic compression plate in 36 adults with symptomatic non‐union of the middle third of the clavicle. One quasi‐randomised trial (69 participants) compared Knowles pin versus a plate for treating middle third clavicle fractures or non‐union. Knowles pins appeared to be associated with lower pain levels and use of postoperative analgesics and a reduced incidence of implant‐associated symptoms. One study (133 participants) found that a three‐dimensional technique for fixation with a reconstruction plate was associated with a significantly lower incidence of symptomatic delayed union than a standard superior position surgical approach. Evidence is pending from two ongoing trials, with planned recruitment of 130 participants, comparing anterior versus superior plates for acute fractures.
Authors' conclusions
There is very limited and low quality evidence available from randomised controlled trials regarding the effectiveness of different methods of surgical fixation of fractures and non‐union of the middle third of the clavicle. The evidence from four ongoing trials is likely to inform practice for the comparisons of intramedullary versus plate fixation and anterior versus superior plates for acute fractures in a future update. Further randomised trials are warranted, but in order to optimise research effort, these should be preceded by research that aims to identify priority questions.
Keywords: Adult; Female; Humans; Male; Middle Aged; Bone Plates; Clavicle; Clavicle/injuries; Clavicle/surgery; Fracture Fixation; Fracture Fixation/methods; Fracture Fixation, Intramedullary; Fracture Fixation, Intramedullary/methods; Fractures, Bone; Fractures, Bone/surgery; Fractures, Ununited; Fractures, Ununited/surgery; Randomized Controlled Trials as Topic; Treatment Failure
Plain language summary
Surgical interventions for treating fractures and non‐union of the collarbone
Background and aims
Collarbone (middle third clavicle) fractures are a common injury and account for up to 4% of all fractures. Although the majority of acute (recent injury) fractures can be treated conservatively, for instance by using a sling, there are some types of fracture that need to be surgically treated. Non‐union of the collarbone, which results from failed fracture healing, is usually treated surgically when a person has pain and difficulties in using their shoulder.
This review set out to evaluate the effects, primarily on pain and long‐term function, of different methods for surgically treating collarbone fractures and non‐union.
Search results
We searched the scientific literature up to 27 June 2014 and found seven relevant studies with 398 participants. The seven small studies had methodological limitations that may affect the reliability of their findings. The types of surgical fixation evaluated were dynamic compression plates, low‐contact dynamic compression plates, and intramedullary nails. Dynamic compression plates are screwed to the collarbone and apply pressure between the fractured ends; low‐contact dynamic compression plates are similar, but are designed to have less contact with the underlying bone. Some compression plates can be customised to the three‐dimensional contours of the bone before application. Unlike a compression plate, which is fixed to the external surface of the collarbone, an intramedullary nail is inserted into the bone's internal 'cavity' to span and stabilise the fracture.
Key results
Four poor quality studies compared intramedullary fixation with plate fixation in 160 people with acute collarbone fractures. Pooled data from three studies did not show a clinically important difference between the two types of surgery in upper arm function at long term follow‐up of six months or more. The studies found little difference between intramedullary fixation and plate fixation in pain, treatment failure resulting in non‐routine surgery or in time to fracture healing (three trials). Pooled data from all four studies indicated that fewer people had adverse events, such as infection or prominent or troublesome hardware, after intramedullary fixation but the converse result where fewer people had adverse events after plate fixation could not be ruled out.
One poor quality trial that involved 36 participants compared two types of plates for treating non‐union of fractures of the middle third of the collarbone. The trial found that participants treated with a low‐contact dynamic compression plate reported better upper arm function during the year after surgery and returned to work earlier than those people treated with a standard dynamic compression plate. The second trial, which was also of poor quality, concluded that there were advantages in using intramedullary nail fixation compared with plate fixation in 69 people with either acute fractures or non‐union. The third trial, involving 133 participants, was well conducted but did not include enough participants to be conclusive. It compared two different techniques for placement of plates to fix displaced collarbone fractures. This trial found that a technique in which the plate was contoured in three dimensions before fixation to the collarbone gave better results than placing the plate along the upper surface of the collarbone.
Conclusions and quality of evidence
We judged the evidence for all four comparisons was low or very low quality because the studies were at risk of bias due to flawed methods and the data too few to be sure that the results were not due to chance. This means that we are unsure that the results gave a true picture of the clinically important differences between the methods of surgery under comparison. Hence, we conclude that the evidence regarding the effectiveness of different methods of surgical interventions for treating fracture and non‐union of the collarbone is very limited and that further studies are justified.
Summary of findings
Summary of findings for the main comparison. Intramedullary fixation versus open reduction and internal fixation with plate for treating acute middle third clavicle fractures.
| Intramedullary fixation versus open reduction and internal fixation with plate for treating acute middle third clavicle fractures | ||||||
|
Patient or population: adults (aged ≥ 16 years) with acute middle third clavicle fractures Settings: hospital Intervention: intramedullary fixation Comparison: plate fixation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| Plate Fixation | Intramedullary fixation | |||||
|
Function or disability (overall) Various tools (Constant score and Oxford Shoulder Score) Follow‐up: 6‐12 months |
Mean (SD) population Constant score 89 (7)1 | Mean function or disability (overall) in the intervention groups was 0.45 standard deviations higher (0.08 lower to 0.81 higher) | SMD 0.45 (0.08 to 0.81) | 120 (3 studies) | ⊕⊕⊝⊝ low2 | SMD 0.45 (95% CI 0.08 to 0.81); translates to an absolute improvement of 3.2 points (0.6 to 5.7 points improvement) in the Constant score (0 to 100 points: higher = better) in the intramedullary fixation group This is not a clinically significant difference3 |
|
Pain ‐ using the section of Constant score Scale from 0 to 15; with 15 being the best positive score Follow‐up: 12 months |
Mean pain in the control group was 13.1 points | Mean pain ‐ using the section of constant score in the intervention groups was 0.6 higher (0.8 lower to 2 higher) |
MD 0.60 points (‐0.80 to 2.00) |
32 (1 study) | ⊕⊝⊝⊝ very low4 | A second trial (13 participants) reported no difference between the 2 groups in VAS at 4 months |
|
Treatment failure (participants who have a non‐routine secondary surgical intervention) ‐ Overall treatment failure Follow‐up: 6‐12 months |
53 per 10005 | 37 per 1000 (9 to 158) | RR 0.69 (0.16 to 2.97) | 133 (4 studies) | ⊕⊝⊝⊝ very low6 | 1 of the 4 trials (50 participants) had no treatment failures in either group |
| Clinical healing ‐ time to clinical/radiographic fracture consolidation (weeks) | Mean clinical healing ranged across control groups from 10.1 to 29.2 weeks | Mean clinical healing: time to clinical/radiographic fracture consolidation (weeks) in the intervention groups was 1.22weeks lower (3.83 lower to 1.39 higher) | MD ‐1.22 weeks (‐3.83 to 1.39) | 98 (3 studies) | ⊕⊝⊝⊝ very low7 | ‐ |
|
Adverse events ‐ total of adverse events (various: mainly infection; cosmetic result ‐ e.g. prominent metalwork ‐ and symptomatic hardware) Follow‐up: 6‐12 months |
431 per 10005 |
276 per 1000 (168 to 444) |
RR 0.64 (0.39 to 1.03) | 133 (4 studies) | ⊕⊝⊝⊝ very low8 | Definition, description, and distribution of adverse events and their sequelae varied considerably in the 4 trials. In 1 trial, all 17 pins were removed for undisclosed reasons but probably routinely whereas 8 plates were removed only for complications or by request |
| Quality of life ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured in any trial |
| Return to previous activities ‐ not measured | See comment | See comment | Not estimable | ‐ | See comment | Not measured in any trial |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: confidence interval; MD: mean difference; RR: risk ratio SD: standard deviation; SMD: standardised mean difference; VAS: visual analogue scale. | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1. This is based on the Constant score in healthy people as reported by the SD of the Constant score in healthy people as reported in Yian 2005.
2. We downgraded the evidence for this outcome 2 levels for high risk of bias reflecting serious study limitations, which included inadequately concealed treatment allocation and lack of assessor blinding.
3. For the purposes of this review, the minimally clinical important difference was considered to be 10 points for the Constant score (Kukkonen 2013).
4. We downgraded the evidence for this outcome 2 levels for high risk of bias reflecting serious study limitations, which included inadequately concealed treatment allocation and lack of blinding. We downgraded the evidence 1 further level for imprecision given the wide confidence interval and that the available data were from only 1 trial.
5. Basis for assumed risk was the median baseline risk from the studies in the meta‐analysis.
6. We downgraded the evidence for this outcome 2 levels for high risk of bias reflecting serious study limitations, which included inadequately concealed treatment allocation and lack of assessor blinding. We downgraded the evidence 1 further level for imprecision given the total number of events were small and the wide confidence interval includes both no clinical effect and 'appreciable benefit'.
7. We downgraded the evidence for this outcome 2 levels for high risk of bias reflecting serious study limitations, which included inadequately concealed treatment allocation and lack of assessor blinding. We downgraded the evidence 1 further level for inconsistency given the significant heterogeneity between the results of the 3 trials.
8. We downgraded the evidence for this outcome 2 levels for high risk of bias reflecting serious study limitations, which included inadequately concealed treatment allocation, lack of assessor blinding, and the possible unit of analysis issues that could have resulted in double counting for a few participants with ≥ 2 adverse events. We downgraded the evidence 1 further level for imprecision given the total number of events were small and the wide confidence interval includes both no clinical effect and 'appreciable benefit'.
Background
The clavicle (collarbone) has several important functions. It acts as a bridge connecting the upper limb to the thoracic cage, which helps to stabilise the shoulder girdle, while allowing the arm to perform a full range of movement. In addition, it functions as an attachment for muscles, provides protection to vital neurovascular structures, supports respiratory function, and has a significant aesthetic role in a person's physical appearance. These functions can be damaged by the occurrence of fractures and non‐union (Kotelnicki 2006; Lazarus 2001).
This review deals with two conditions: acute fractures and non‐union resulting from failed fracture healing.
Description of the condition
The clavicle is a commonly fractured bone, accounting for 2.6% to 4% of all fractures (Nordqvist 1994; Postacchini 2002). The incidence of clavicle fractures in adults is 71 per 100,000 men and 30 per 100,000 women (Neer 1984). Court‐Brown 2006, in an epidemiological study of fractures in people over 12 years of age, observed a bimodal distribution curve for the incidence of clavicle fractures in males with age; with a high incidence in young males and, to a lesser extent, in older males. The curve was unimodal in females with a high incidence in older women.
Clavicle fractures occur after indirect trauma or direct to the bone itself. The most common mechanism occurs after a fall from a standing height onto the outer side of the shoulder and corresponds to around 90% of the cases. The other mechanism of clavicle injury is indirect trauma, which happens after a fall onto an outstretched arm. The force of the fall is transmitted through the upper extremity to the clavicle, producing the fracture. Although this was previously believed to be the most frequent cause of injury, it represents only 2% to 5% of clavicle fractures (Jeray 2007; Kotelnicki 2006). Sporting activities such as bicycling and skiing are common causes of falls resulting in a fracture (Nowak 2000).
Allman 1967 proposed a classification for clavicle fractures, by dividing them into three groups according to their location along the bone. Group I are fractures in the middle third of the bone, group II are fractures in the outer or lateral third of the bone and group III are fractures in the inner or medial third. In one large epidemiological study, Nordqvist 1994 classified 76% of all fractures as group I fractures; the median age was 13 years for people in this group. Due to the absence of a single system that had both prognostic and therapeutic value, Robinson 1998 proposed his own classification. It was based on Allman's categories but included prognostically important variables, such as the degree of displacement and comminution (fragmentation of the bone).
One possible complication of middle third clavicle fractures is non‐union. In 1986, the US Food and Drug Administration (FDA) defined non‐union to be "established when a minimum of nine months has elapsed since injury and the fracture shows no visible progressive signs of healing for three months". However, these criteria cannot be applied to every fracture (LaVelle 2003). Even though non‐union of the clavicle has not been definitively defined in the literature so far, many investigators agree that a diagnosis can be made if consolidation does not happen within six months after the injury (Jeray 2007; Manske 1985; Pyper 1978; Wilkins 1983). The verification of the non‐union is made when there is clinical or radiographic evidence showing that healing has ceased and that union is highly improbable.
Description of the intervention
Indications for operative treatment of middle third clavicle fractures include: open fracture, severe displacement caused by comminution, an imminent lesion of the skin by a sharp edge of the clavicle, and neurovascular injuries. The relative (not absolute) indications for surgery are: multiple trauma, floating shoulder, and symptomatic malunion and non‐union. More recently, other relative indications have been adopted, including high energy fractures such as clavicle shortening greater than 20 mm, complete displacement, and severe comminution. When the surgical approach is chosen to treat these fractures, there are several techniques of fixation that can be implemented (Bradbury 1996; Ebraheim 1997; Jupiter 1987; Mullaji 1994). These include internal fixation with screws, pins, wire loops, or plates; and external fixation with external fixators. The most common implants in current practice have been reported to be dynamic compression or titanium nails or locking plates specially designed for treating clavicle fractures (Donnelly 2013; Khan 2008; Khan 2009; King 2015). The use of bridging plates, a minimally invasive method, could also be an option to treat clavicle fractures (Sökücü 2014). Bone grafting may also be used.
The primary indications for treatment of an established non‐union are pain and functional impairment. Usually there is no indication for treating an asymptomatic non‐union. Surgical treatment of clavicle non‐union includes a bone graft with or without fixation, clavicle excision, and, more rarely, a free‐fibular vascularised graft. The graft involves using bone from the fibula (one of the two bones of the lower leg), which includes blood vessels that can be connected to the blood vessels in the locality of the clavicle. Each treatment has documented advantages and disadvantages (Lazarus 2001).
How the intervention might work
While studies show incidences of non‐union ranging from 0.03% to 5.9% for undisplaced fractures (Nordqvist 1998; Robinson 2004; Zlowodzki 2005), studies of displaced fractures reveal non‐union rates up to 15% (Canadian 2007; Hill 1997; McKee 2006). Aetiological factors that predispose to the development of non‐union include open fracture, associated poly‐traumatic lesions, re‐fracture, initial fracture displacement, comminution, shortening, older age, smoking, and an inadequate period of immobilisation (Jupiter 1987; Marti 2003; Murray 2013). Robinson 2004 observed that intrinsic factors, such as advanced age and female gender, are more likely to be predisposing factors for non‐union. These findings have prompted an increase in preference for operative treatments by surgeons through the usual techniques of open reduction and internal fixation (using a plate and screw) or intramedullary fixation (either approaching the focus of the fracture or not) (Canadian 2007; Meier 2006).
Complications of surgical treatments include wound infection or dehiscence, deep infection, and problems with the hardware used for fixation. The rate of infection ranges from 0% to 18% (Böstman 1997; Poigenfürst 1992; Verborgt 2005; Wu 1998), and the rate of hardware irritation that requires part or total hardware removal ranges from 50% to 100% (Ali Khan 1978; Böstman 1997; Canadian 2007). Other potential drawbacks of surgical interventions include scarring, complex regional pain syndrome, transient brachial plexus symptoms, non‐union and re‐fracture after hardware removal, and hardware migration (Lazarus 2001).
Why it is important to do this review
Middle third fracture of the clavicle is one of the most common fractures of the body. It frequently results in short‐term disability and pain, and can lead to longer‐term deformity and disability. Although the majority of acute fractures can be treated conservatively, there are some types of fracture that need to be treated surgically. Surgical treatment for symptomatic non‐union is also performed. Hence, it is important to review the available evidence in the literature on surgical interventions systematically in order to inform management decisions for these injuries.
This is an update of a Cochrane review first published in 2009. Before this update (2015), two other Cochrane reviews had separately considered different conservative interventions (Lenza 2014), and had compared surgery with conservative treatment for these fractures (Lenza 2013). Lenza 2014 found there was insufficient evidence to inform decisions on conservative treatment including the choice between a figure‐of‐eight bandage and an arm sling tested in two trials. Based on evidence from eight trials with high risk of bias, Lenza 2013 provided some low quality evidence that surgical interventions may not result in significant improvement in upper arm function.
Objectives
To evaluate the effects (benefits and harms) of different methods of surgical interventions for fractures and non‐union of the middle third of the clavicle.
Methods
Criteria for considering studies for this review
Types of studies
Any randomised or quasi‐randomised (method of allocating participants to a treatment that is not strictly random, e.g. by date of birth, hospital record number, or alternation) controlled trials comparing surgical interventions for treating middle third clavicle fractures or non‐union.
Types of participants
Trials with adolescents or adults diagnosed with an acute middle third clavicle fracture or non‐union. We excluded trials involving young children only (aged less than 10 years). However, we included trials that involved young children provided the proportion of young children was clearly under 10%, or separate data were available. We excluded people with polytrauma or other shoulder injuries or disorders.
Types of interventions
All surgical interventions for treating middle third clavicle fractures or non‐union. Examples included internal fixation using a plate, Kirschner wires, titanium nails, Knowles pins, and external fixation with an external fixator. We considered all possible comparisons between these surgical strategies (applied either singly or combined), with or without bone grafting.
Types of outcome measures
Primary outcomes
Function or disability evaluated by upper limb functional outcome measures. Ideally, these were participant‐reported measures of function validated for people with clavicle fractures (however, we are not aware of any outcome measures in this category). Examples of validated participant‐reported measures of upper limb function were the Disability of the Arm, Shoulder and Hand questionnaire (DASH) (Hudak 1996), and the Oxford Shoulder Score (OSS) (Dawson 1996). A commonly used instrument for assessing shoulder function is the Constant score (Constant 1987), which is a composite score for shoulder function that includes subjectively rated pain and activities of daily living, as well as objectively rated range of movement and strength.
Pain. We gave preference to reports of pain measured using validated pain scales (visual analogue scale (VAS) or numerical rating scale (NRS)) and reported in terms of a clinically important change in pain score in the acute/short‐term phase (e.g. proportion of people with at least 30% improvement in pain) or participant‐reported long‐term pain (e.g. proportion of people above 30/100 mm VAS scale, i.e. worse than mild pain). These examples were drawn from recommendations in Eccleston 2010 and Moore 2010.
Treatment failure measured by the number of participants who had undergone or were being considered for a non‐routine secondary surgical intervention for symptomatic non‐union, malunion, or other complications (e.g. mechanical failure defined as a condition in which an implant lost its capacity to carry a load).
Timing of primary outcomes measurement
We extracted outcome data at the following time periods: short term follow‐up (up to six weeks following treatment); intermediate follow‐up (more than six weeks and up to six months after the end of treatment), and long term (longer than six months after the end of treatment). We chose the time period of six weeks because normally people initiate rehabilitation after this time, and six months because a diagnosis of non‐union of the clavicle can be made after this time.
Secondary outcomes
Clinical fracture healing: time to clinical and radiographic union, we treated this as a proxy for recovery of function in this review.
-
Adverse events, measured by:
wound infection or dehiscence;
cosmetic result: poor outcome such as deformity, asymmetrical result, and skin problems;
asymptomatic non‐union (i.e. the fracture has not healed radiographically) or symptomatic non‐union that was not considered for surgery, radiographic malunion;
stiffness/restricted of range of shoulder movement;
symptomatic hardware and hardware irritation requiring removal;
other reported complication.
Health‐related quality of life, such as Short Form‐36 (Ware 1992) and EuroQol (EQ‐5D) (EuroQol Group 1990; Johnson 1998).
Return to previous activities (work, sport, activities of daily living, etc.), including time to return.
Participant satisfaction with method of treatment.
Search methods for identification of studies
Electronic searches
We searched the Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (27 June 2014), the Cochrane Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2014, Issue 5), MEDLINE (1966 to June week 3 2014), MEDLINE In‐Process and Other Non‐Indexed Citations (26 June 2014), EMBASE (1988 to 2014 week 25), and Latin American and Caribbean Health Sciences (LILACS) (1982 to 27 June 2014). We also searched the ISRCTN Registry (14 September 2014), ClinicalTrials.gov (14 September 2014), and the World Health Organization (WHO) International Clinical Trial Registry Platform (14 September 2014) for ongoing and recently completed trials, and the UK National Research Register Archive for records up to September 2007. For this update, we limited the search results from 2008 onwards. Details of the previous search strategies are available in Lenza 2009. We did not apply any restrictions based on language or publication status.
In MEDLINE, the sensitivity‐maximising version of the Cochrane Highly Sensitive Search Strategy for identifying randomised trials (Lefebvre 2011) was combined with the subject‐specific search (seeAppendix 1). Appendix 1 shows the search strategies for CENTRAL, EMBASE, and LILACS.
Searching other resources
We checked the reference lists of articles, reviews, and textbooks for possible relevant studies. We handsearched abstracts for the annual meetings of the British Elbow and Shoulder Society (2001 to September 2014), the American Orthopaedic Trauma Association (1996 to September 2014), American Academy of Orthopaedic Surgeons (September 2014), and the British Trauma Society (September 2014).
Data collection and analysis
Selection of studies
Two review authors (ML and FF) independently selected and assessed, using a piloted form, potentially eligible studies for inclusion in the review. We resolved any disagreements by discussion. The review authors were not blinded to the journal or to the authors.
Data extraction and management
Two review authors (ML and FF) extracted the following data using a pre‐piloted data extraction form: characteristics of the study methods including study design, duration of the study, whether the protocol was published before recruitment of participants, funding sources, and details of trial registration; characteristics of the study participants including place of study, number of participants assigned, number of participants assessed, inclusion criteria, exclusion criteria, age, and classification of injury; characteristics of the study interventions including timing of intervention, type of surgical interventions, rehabilitation, and any co‐interventions; characteristics of the study outcomes including length of follow‐up, loss to follow‐up, and outcome measures; as well as the methodological domains as outlined later in Assessment of risk of bias in included studies.
We resolved any disagreements by discussion. Two review authors (ML and FF) entered data into Review Manager 5 (RevMan 2012). We sent requests seeking additional information or data to trial authors.
Assessment of risk of bias in included studies
Two review authors (FF and ML) independently assessed the risk of bias of included studies. As recommended by The Cochrane Collaboration's 'Risk of bias' tool (Higgins 2011), we assessed the following domains:
random sequence generation;
allocation concealment;
blinding of participants and personnel;
blinding of outcome assessment;
incomplete outcome data;
selective reporting;
other bias (e.g. major baseline imbalance; inappropriate influence of funders; risk of bias associated with inexperience of care providers with the interventions, differences in rehabilitation).
We explicitly judged each of these criteria on the basis of low risk of bias, high risk of bias, and unclear risk of bias (either lack of information or uncertainty over the potential for bias). We resolved disagreements between review authors regarding the risk of bias for domains by consensus.
Measures of treatment effect
We calculated risk ratios (RRs) together with 95% confidence intervals (CIs) for dichotomous outcomes. We expressed continuous outcome data as mean differences (MDs) with 95% CIs.
For illustrative purposes and when appropriate, we reported the number needed to treat for an additional beneficial outcome (NNTB) with 95% CIs and the number needed to treat for an additional harmful outcome (NNTH) with 95% CIs.
Unit of analysis issues
The unit of randomisation for all the included trials was the individual participants. There were no unit of analysis issues in the analysis of studies such as with cluster‐randomised trials or for people with bilateral fractures, where data could have been presented for fractures or limbs instead of individual participants. We avoided, where possible, unit of analysis problems with multiple reporting of outcomes such as at different follow‐up times by presenting these separately. However, lack of clarity in some trial reports on the incidence of complications and incidence of participants with complications may mean that we have inadvertently double counted a very few participants with two or more complications in our estimates of total adverse events.
Dealing with missing data
With the purpose of including all participants randomised to any intervention, we performed an intention‐to‐treat analysis. When there was insufficient information relative to estimate effects, such as number of participants, means, measures of uncertainty (standard deviation or error), or number of events and participants, we contacted the main authors of the included trials.
When it was impossible to acquire adequate data for the forest plot (e.g. means and standard deviations), we presented the data in the text.
We investigated the effects of drop‐outs and exclusions by conducting worst‐case and best‐case scenario analyses. For dichotomous outcomes, we analysed the worst‐case scenario using the number randomly assigned as denominator, with the assumption that any participants missing at the end of treatment did not have a positive outcome (e.g. for the outcome number of participants experiencing treatment failure, we assumed that any missing participants had an adverse event). We analysed the best‐case scenario using the number randomly assigned in the denominator, and ignoring the drop‐outs in our analyses of dichotomous outcomes (overall treatment failure).
Assessment of heterogeneity
We assessed the heterogeneity of estimate effects between the included studies by visual inspection of the forest plot, and using the Chi² test and the I² statistic. We quantified the possible magnitude of inconsistency across studies using the I² statistic as follows: 0% to 40% might not be important, 30% to 60% may represent moderate heterogeneity, 50% to 90% may represent substantial heterogeneity, and 75% to 100% may represent considerable heterogeneity (Deeks 2011). In cases of considerable heterogeneity (defined as I² ≥ 75%), we planned to explore the data further by comparing the characteristics of individual studies and conducting subgroup analyses.
Assessment of reporting biases
In meta‐analyses with more than 10 studies, we planned to draw funnel plots of primary outcomes to assess the potential publication bias (small‐study effects). However, the small number of included studies precluded this analysis.
Data synthesis
When considered appropriate, we pooled the results of comparable groups of trials using the fixed‐effect model and 95% CIs. We also checked the results using the random‐effects model where there was diversity in clinical or methodological characteristics, and presented random‐effects results where there was significant heterogeneity.
Subgroup analysis and investigation of heterogeneity
We investigated surgical management of both acute clavicle fractures and non‐union of clavicle fractures. We planned, where possible, to carry out subgroup analyses by: age (adolescent, adult, and elderly), type of fracture (two fragments and more than two fragments), type of non‐union (hypervascular/hypertrophic or avascular/atrophic), mechanism of injury, and the surgeon's level of experience. We planned to investigate whether the results of subgroups were significantly different by inspecting the overlap of CI values and by performing the test for subgroup differences available in the Review Manager 5 software (RevMan 2012).
Sensitivity analysis
We also planned, where possible, to conduct sensitivity analyses exploring aspects of trial and review methodology, including the effects of missing data and study quality (specifically allocation concealment and outcome assessor blinding).
'Summary of findings' tables and assessment of the quality of the evidence
We used the GRADE approach to assess the quality of evidence related to each of the key outcomes listed in the Types of outcome measures (see Section 12.2, Schunemann 2011).
We presented the main results of intramedullary fixation versus plate fixation for treating acute middle third clavicle fractures in a 'Summary of findings' table. The 'Summary of findings' table provides key information concerning the quality of evidence, the magnitude of effect of the interventions examined, and the sum of available data on the main outcomes.
Outcomes for the 'Summary of findings' table
We included the following outcomes in the 'Summary of findings' table: upper limb functional outcomes, pain, treatment failure (non‐routine secondary surgical intervention for symptomatic non‐union, malunion, or other complications), clinical fracture healing, total of adverse events, health‐related quality of life, and return to previous activities. We converted the standardised mean difference (SMD) for the upper limb function outcome to a clinically meaningful measure of function (Constant score) by multiplying the SMD by the standard deviation of the Constant score in healthy people as reported in Yian 2005.
Results
Description of studies
Results of the search
We updated the search from December 2008 to June 2014. We screened 448 records from the following databases: Cochrane Bone, Joint and Muscle Trauma Group Specialised Register (9), CENTRAL (66), MEDLINE (129), EMBASE (104), LILACS (19), ISRCTN Registry (9), ClinicalTrials.gov (65), and WHO International Clinical Trials Registry Platform (47). We did not identify any potentially eligible studies from other sources.
After screening the results, we identified 19 new studies. Of these, we included four (Assobhi 2011; Ferran 2010; Silva 2011; Tabatabaei 2011), and excluded 11 (Böhme 2011; Cho 2010; Flikweert 2009; Fu 2012; Jiang 2012; Kraus 2013; Liu 2010; Ma 2008; NCT01311219; NCT01405703; Pai 2009). Four studies were ongoing trials (ChiCTR‐TRC‐12001973; NCT00871468; NCT01015924; Wijdicks 2011). Figure 1 shows a flow diagram summarising the study selection process.
1.
Study flow diagram.
Overall, we included seven trials, excluded 16 studies, found four ongoing trials, and found no studies awaiting classification.
Appendix 2 shows the results from the previous searches (up to 2008).
Included studies
We detailed the seven included studies in the Characteristics of included studies table. All trials were reported in English, except Silva 2011 (in Portuguese). One review author (ML) translated it into English.
Design of the studies
All trials were randomised except Lee 2007 and Tabatabaei 2011, which were quasi‐randomised. All seven single‐centre trials randomised individual participants into one of two intervention groups. The seven trials were each conducted in hospitals located in one of seven countries: Egypt (Assobhi 2011), UK (Ferran 2010), Turkey (Kabak 2004), Taiwan (Lee 2007), China (Shen 2008), Brazil (Silva 2011), and Iran (Tabatabaei 2011).
Sample sizes
The seven trials enrolled 398 participants; outcome data allowing analysis by the trial authors were available for a maximum of 345 participants (86.7%).
Participants
Age and gender
With a probably only one exception in Ferran 2010, where the youngest participant was 13 years old, trial participants were adults. Three trials did not specify the age limits in the inclusion criteria of (Ferran 2010; Kabak 2004; Tabatabaei 2011). Assobhi 2011 included participants aged 16 to 60 years old; Shen 2008, participants aged 18 to 60 years old; and Silva 2011, participants aged 16 to 65 years old. Lee 2007, which stipulated a lower age limit of 50 years, included the oldest participant who was aged 81 years.
Overall, six trials reported that 230/332 (69.3%) participants with outcome data were male (Assobhi 2011; Ferran 2010; Kabak 2004; Lee 2007; Shen 2008; Tabatabaei 2011). Silva 2011 did not report on gender.
Types of fractures and non‐union
Participants in five trials had sustained an acute, displaced, middle third clavicle fracture (Assobhi 2011; Ferran 2010; Shen 2008; Silva 2011; Tabatabaei 2011). Kabak 2004 included only participants with non‐union of the middle third of the clavicle (as early as six months after the initial fracture). Lee 2007 included participants with either acute fractures or non‐union.
Kabak 2004 classified non‐union into two groups: avascular/atrophic, when there was little or no visible callus; and hypervascular/hypertrophic, with excessive callus. Lee 2007 divided fracture patterns into: open fractures, transverse fractures, oblique and spiral fractures, comminuted fractures, and symptomatic non‐union. Shen 2008 classified the acute dislocated fractures as comminuted and spiral. The other four trials did not report on the classification of the fractures.
Mechanisms of injury
The most common mechanism of injury was motor vehicle accident in three trials: 45.5% of the study population in Kabak 2004, 42.1% in Assobhi 2011, and 38% in Tabatabaei 2011. Ferran 2010 described the main cause of injury as sporting activities in 53.1% of participants. Shen 2008 reported that the most common mechanism of fracture was a fall from a standing height in 63.9% of participants. Lee 2007 and Silva 2011 did not report information on mechanism of injuries.
Interventions
Types of comparison
The included trials allowed four comparisons:
Comparison 1: intramedullary fixation versus open reduction plus internal fixation with plate for treating acute middle third clavicle fractures (Assobhi 2011; Ferran 2010; Silva 2011; Tabatabaei 2011).
Comparison 2: low‐contact dynamic compression plate (LC‐DCP) versus dynamic compression plate (DCP) for treating non‐union of the middle third of the clavicle (Kabak 2004).
Comparison 3: Knowles pin versus DCP for treating middle third clavicle fractures and non‐union (Lee 2007).
Comparison 4: three‐dimensional (3D) plate versus superior‐positioned plate for treating acute dislocated middle third clavicle fractures (Shen 2008). This trial compared two techniques of plate fixation. In one group, the plate was three‐dimensionally positioned and superiorly fixed on the main distal fragment and anteriorly on the main proximal fragments. In the other group, the plate was shaped in the form of an 'S' and fixed on the superior surface.
The included trials did not evaluate comparisons using new‐generation implants, such as site‐specific pre‐contoured locking plates.
Surgeons' levels of experience
Only two trials gave information on the surgeons' levels of experience; Lee 2007 described that participants were randomly assigned to four senior surgeons and Shen 2008 reported that the surgery was performed in most cases by an experienced orthopaedic surgeon.
Outcome measures
The studies varied in timing of follow‐up. Five studies specified follow‐up time points at 12 months (Assobhi 2011; Ferran 2010; Shen 2008), six months (Tabatabaei 2011), and four months (Silva 2011). Two studies reported mean follow‐up: Kabak 2004 presented a mean follow‐up of 44.2 months and Lee 2007 reported a mean follow‐up of 30 months.
Primary outcomes
-
Six trials evaluated function or disability:
Assobhi 2011 and Lee 2007 used the Constant score;
Ferran 2010 used the Constant score and OSS;
Kabak 2004 used the DASH questionnaire;
Silva 2011 used DASH and Constant scores but did not report the results of these; and
Tabatabaei 2011 used DASH and OSS.
-
Four trials evaluated pain:
Lee 2007 and Silva 2011 used a VAS scale to assess pain;
Kabak 2004 measured pain with dichotomous outcomes (presence or not);
Ferran 2010 reported the pain section of Constant score.
All trials reported treatment failure (e.g. re‐operation for symptomatic non‐union or implant loosening).
Secondary outcomes
All trials measured clinical fracture healing.
All trials collected data on adverse events.
None of the trials evaluated health‐related quality of life.
One trial evaluated return to previous activities (Kabak 2004).
The trials did not evaluate participant satisfaction with method of treatment.
Excluded studies
We excluded 16 studies because they did not meet the inclusion criteria. For the full reasons for excluding these studies, see the Characteristics of excluded studies table.
Ongoing studies
Our search for ongoing trials found nine studies on Current Controlled Trials, 47 on the WHO International Clinical Trials Registry Platform, and 65 on the ClinicalTrials.gov register. We excluded 54 duplicates and 63 were either not relevant or did not meet our inclusion criteria, leaving four studies to be included in an updated version of this review when they are published (ChiCTR‐TRC‐12001973; NCT00871468; NCT01015924; Wijdicks 2011). One published protocol, indexed in PubMed, for one of the ongoing trials is available (Wijdicks 2011).
All ongoing studies are parallel randomised controlled trials with two intervention groups. Wijdicks 2011 is a multicentre trial in the Netherlands. The other ongoing studies are single‐centre trials, taking place in China (ChiCTR‐TRC‐12001973), the US (NCT00871468), and Norway (NCT01015924). The four ongoing studies should enrol 375 participants; two trials are comparing anterior versus superior plates in 130 participants (ChiCTR‐TRC‐12001973; NCT00871468), and two trials are comparing intramedullary fixation versus open reduction plus internal fixation with plate in 245 participants (NCT01015924; Wijdicks 2011).
For further details of the four ongoing studies, see the Characteristics of ongoing studies table.
Risk of bias in included studies
All trials had methodological flaws rendering them at high risk of bias (seeFigure 2 and Figure 3). For details of the method of randomisation, assessor blinding, intention‐to‐treat analysis, loss to follow‐up, and length of follow‐up, see the Characteristics of included studies table. We presented a summary of the results and impressions of the likelihood of bias below.
2.
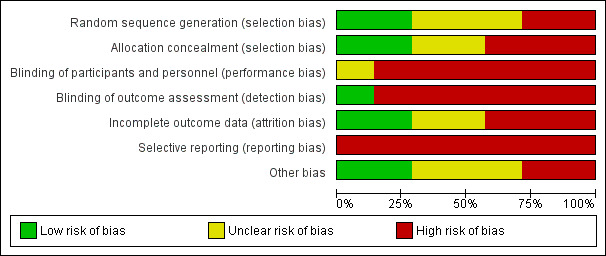
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies.
3.
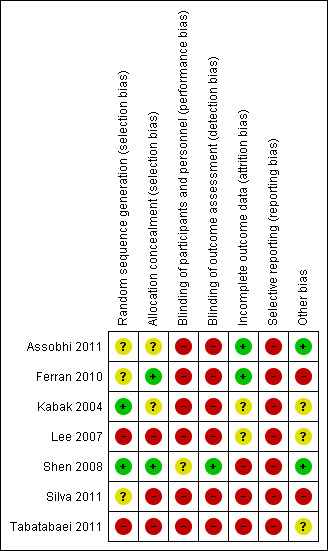
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Kabak 2004 and Shen 2008 reported that random sequence generation was performed using a computer random number generator. Lee 2007 and Tabatabaei 2011 were quasi‐randomised based on alternation and thus at high risk of bias for this item. The three remaining trials did not provide sufficient information about the sequence generation process to permit a judgement about risk of bias (Assobhi 2011; Ferran 2010; Silva 2011).
Concealment of allocation prior to assignment adequate for Ferran 2010 and Shen 2008 (opaque and sealed envelopes); we judged both to be at low risk of bias. Assobhi 2011 and Kabak 2004 did not describe their methods of allocation concealment. There was no concealment of allocation in Lee 2007, Silva 2011, and Tabatabaei 2011.
Blinding
We judged all trials at high risk of performance and detection bias, except Shen 2008, which we considered at unclear risk of performance bias and at low risk of detection bias. As all trials compared surgical interventions, it was not possible to blind treatment providers. While it may have been possible to blind outcome assessors and participants, only one trial mentioned participants and assessor blinding ‐ this information was provided from author contact and was not given in the trial report (Shen 2008).
Incomplete outcome data
We considered trials at low risk of attrition bias if more than 80% of participants completed the follow‐up, missing outcomes data were balanced in number across intervention groups, and an intention‐to‐treat analysis was reported for the primary outcomes. As a result, two trials were at low risk of attrition bias (Assobhi 2011; Ferran 2010); three were at high risk (Shen 2008; Silva 2011; Tabatabaei 2011); and two were at unclear risk (Kabak 2004; Lee 2007).
Losses to follow‐up were reported in all trials, except in Assobhi 2011 and Ferran 2010, where the authors did not report missing data. The losses to follow‐up were 8% in Kabak 2004, 10% in Lee 2007, 12% in Shen 2008, and 26.5% in Tabatabaei 2011. Silva 2011 reported preliminary results at four months of follow‐up only ‐ therefore, the authors described the results of 13 of 22 participants (59.1% only). Kabak 2004 and Lee 2007 did not mention in which groups the losses occurred. In Shen 2008, the loss of participants was dissimilar (four vs. 12) in the two groups because of the exclusion of participants (one vs. eight) who had re‐operations. However, none of the trials presented outcome data for participants who were withdrawn from the trial or were lost to follow‐up.
Selective reporting
We classified all included trials as at high risk of selective reporting bias because the study protocols were not available and some of important outcomes were not evaluated using a validated tool or they were reported incompletely.
Other potential sources of bias
Two trials were at low risk of other bias (Assobhi 2011; Shen 2008), three trials were at unclear risk of bias (Kabak 2004; Lee 2007; Tabatabaei 2011), and two trials were at high risk of other potential threats to validity (Ferran 2010; Silva 2011).
Four trials did not provide baseline characteristics for all randomised participants and pre‐specify time points of outcomes (Kabak 2004;Lee 2007;Silva 2011; Tabatabaei 2011). Ferran 2010 was at high risk of bias because the authors reported major baseline imbalance in age of participants. The interim nature of the reporting for just 13 participants who had completed four months' follow‐up put Silva 2011 at high risk of other bias.
Effects of interventions
See: Table 1
We judged the evidence as low or very low quality for the outcomes reported for each comparison. We based our assessment of the clinical importance of results from the Constant score, DASH questionnaire, and OSS on minimal clinically important differences (MCID) reported in the literature for shoulder‐related conditions. However, we did not find MCIDs for clavicle fractures. For the purposes of this review, we considered the MCID to be 10 points for the Constant score (Kukkonen 2013), 10 points for the DASH questionnaire (Gummesson 2003; Hudak 1996), and 6 points for the OSS (Van Kampen 2013).
Comparison 1: intramedullary fixation versus open reduction plus internal fixation with plate for treating acute middle third clavicle fractures
Four trials with 160 participants assessed intramedullary fixation versus open reduction plus internal fixation with plate for treating acute middle third clavicle fractures (Assobhi 2011; Ferran 2010; Silva 2011; Tabatabaei 2011). Follow‐up data were available for 133 participants (68 with intramedullary fixation and 65 with plate fixation).
Function or disability
For the purpose of pooling data, where trials included more than one measure of function, we preferentially included one measure according to the following hierarchy: Constant score, DASH questionnaire, and OSS. This is because the Constant score is more specific for shoulder function than the DASH questionnaire and it was more commonly used than the OSS.
Pooled data (Constant scores at 12 months (Assobhi 2011; Ferran 2010); and OSS at six months (Tabatabaei 2011)) for overall shoulder function demonstrated a statistically significant difference in favour of the intramedullary group at long‐term follow‐up of six months or more (SMD 0.45, 95% CI 0.08 to 0.81; 120 participants; Analysis 1.1). However, this result was not clinically significant, upon conversion using data from Yian 2005 of the SMD scores to Constant scores. Silva 2011 did not report on this outcome.
1.1. Analysis.
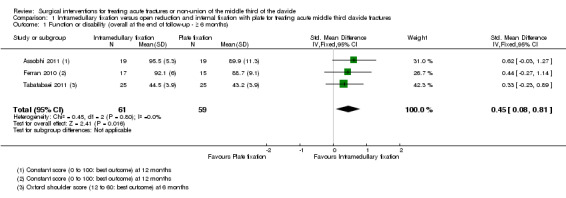
Comparison 1 Intramedullary fixation versus open reduction and internal fixation with plate for treating acute middle third clavicle fractures, Outcome 1 Function or disability (overall at the end of follow‐up ‐ ≥ 6 months).
Specific function endpoints
Constant score (0 to 100 scale: higher scores mean a better outcome): Assobhi 2011 found higher scores favouring intramedullary nailing at short‐term follow‐up (MD 7.00 points, 95% CI 0.41 to 13.59), and at intermediate‐ and long‐term follow‐up of six months; the CIs for all three follow‐up times included a slight possibility of a clinically important effect as they exceeded the MCID of 10 points. Pooled Constant score data from two trials at 12 months' follow‐up also favoured the intramedullary group (MD 4.46 points, 95% CI 0.56 to 8.36, 70 participants); however, this did not include a clinically important difference (Assobhi 2011; Ferran 2010) (Analysis 1.2).
1.2. Analysis.
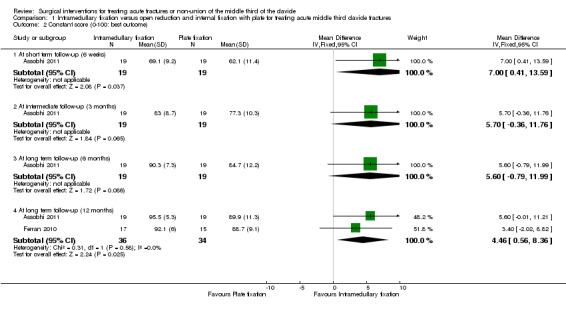
Comparison 1 Intramedullary fixation versus open reduction and internal fixation with plate for treating acute middle third clavicle fractures, Outcome 2 Constant score (0‐100: best outcome).
DASH questionnaire: Tabatabaei 2011 found no clinically important difference between the two intervention groups at six months' follow‐up (MD 1.40 points, 95% CI ‐0.90 to 3.70; 50 participants; 20‐ to 100‐point scale used in Tabatabaei 2011: lower scores mean a better outcome; Analysis 1.3).
1.3. Analysis.
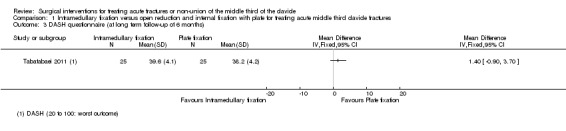
Comparison 1 Intramedullary fixation versus open reduction and internal fixation with plate for treating acute middle third clavicle fractures, Outcome 3 DASH questionnaire (at long term follow‐up of 6 months).
OSS (48 points; high scores mean better outcome): pooled data (12 months' follow‐up for Ferran 2010, and six months' follow‐up for Tabatabaei 2011) demonstrated no clinically important difference between the two groups at long‐term follow‐up (MD 0.86 points, 95% CI ‐0.59 to 2.31; 84 participants; Analysis 1.4).
1.4. Analysis.
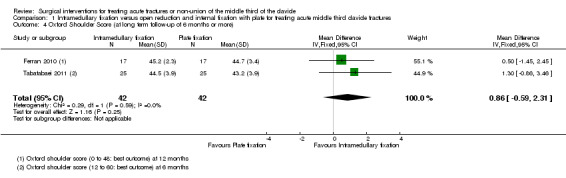
Comparison 1 Intramedullary fixation versus open reduction and internal fixation with plate for treating acute middle third clavicle fractures, Outcome 4 Oxford Shoulder Score (at long term follow‐up of 6 months or more).
Pain
Two trials reported on pain. Data could not be extracted from Silva 2011, which reported similar results in both groups (mean VAS was 2.5 in both groups, 13 participants).
Ferran 2010 (32 participants) found no significant differences between the two groups at 12 months' follow‐up using pain section of the Constant score (MD 0.60, 95% CI ‐0.80 to 2.00; 0 to 15 scale, with higher scores meaning less pain; Analysis 1.5).
1.5. Analysis.

Comparison 1 Intramedullary fixation versus open reduction and internal fixation with plate for treating acute middle third clavicle fractures, Outcome 5 Pain ‐ using the section of Constant score at 12 months.
Treatment failure
The difference in overall treatment failure did not show a difference between the two surgical interventions (2/68 with intramedullary fixation vs. 3/65 with open reduction plus internal fixation with plate; RR 0.69, 95% CI 0.16 to 2.97; Analysis 1.6). The most common cause of treatment failure in the two groups was early mechanical failure or re‐fracture. Tabatabaei 2011 did not report any failure of treatment of all included participants.
1.6. Analysis.
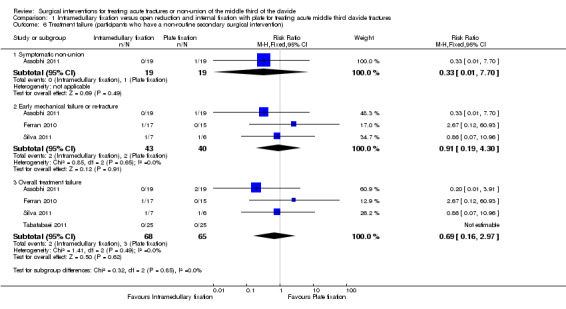
Comparison 1 Intramedullary fixation versus open reduction and internal fixation with plate for treating acute middle third clavicle fractures, Outcome 6 Treatment failure (participants who have a non‐routine secondary surgical intervention).
Clinical fracture healing
Pooled data from three trials demonstrated no significant difference between the two groups in the time to clinical fracture consolidation (MD ‐1.22 weeks, 95% CI ‐3.83 to 1.39; Analysis 1.7) (Assobhi 2011; Silva 2011; Tabatabaei 2011). We presented the random‐effects result are there was substantial heterogeneity (I2 = 68%) and variation in the time to union in the three trials. Ferran 2010 reported that union was achieved in all participants.
1.7. Analysis.
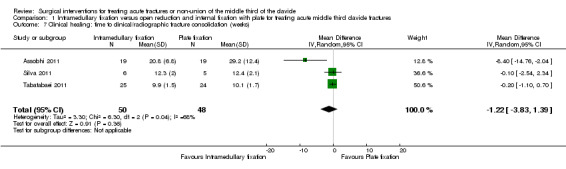
Comparison 1 Intramedullary fixation versus open reduction and internal fixation with plate for treating acute middle third clavicle fractures, Outcome 7 Clinical healing: time to clinical/radiographic fracture consolidation (weeks).
Adverse events
The three most common causes of adverse events were infection, cosmetic result of final treatment (mainly hypertrophic scar or prominent implant under skin and symptomatic hardware. The definitions and distributions of adverse events in the two groups differed between trials; as shown also by visual inspection of Analysis 1.8. Overall, the total adverse events tended to favour the intramedullary fixation group (18/68 with intramedullary fixation vs. 27/65 with open reduction plus internal fixation with plate; RR 0.64, 95% CI 0.39 to 1.03; Analysis 1.8). However, we cannot confirm that the data from pooled studies apply to the total number of participants with complications rather than total complications as a few participants may have had more than one complication. In addition, the reasons for removing pins in all 17 participants of the intramedullary group in Ferran 2010 were not disclosed, although it may have been routine.
1.8. Analysis.
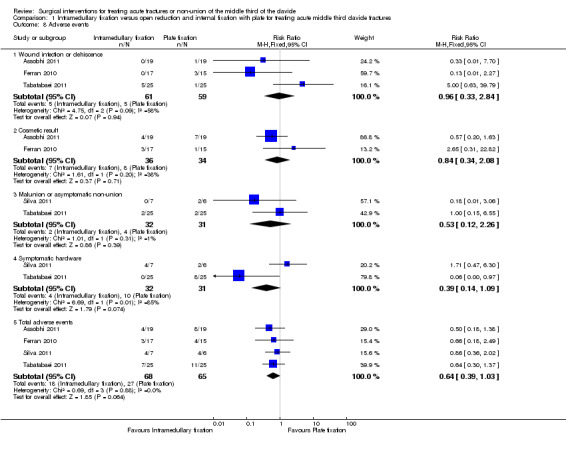
Comparison 1 Intramedullary fixation versus open reduction and internal fixation with plate for treating acute middle third clavicle fractures, Outcome 8 Adverse events.
Health‐related quality of life
The included trials did not evaluate health‐related quality of life.
Return to previous activities
The included trials did not evaluate return to previous activities.
Patient satisfaction with method of treatment
The included trials did not evaluate patient satisfaction with method of treatment.
Other outcomes assessed
There were significant differences in favour of the intramedullary fixation group at all other outcomes assessed (duration of surgery, mean wound size, mean blood loss, and mean hospital stay; Analysis 1.9).
1.9. Analysis.
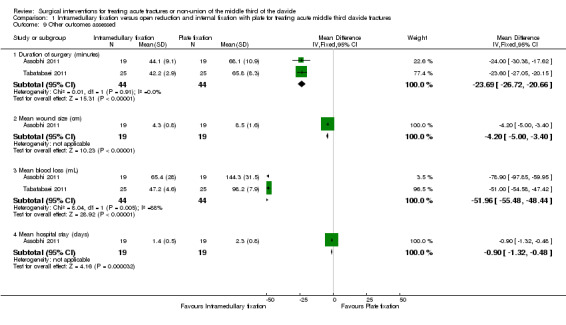
Comparison 1 Intramedullary fixation versus open reduction and internal fixation with plate for treating acute middle third clavicle fractures, Outcome 9 Other outcomes assessed.
Comparison 2: low‐contact dynamic compression plate versus dynamic compression plate for treating non‐union of the middle third of the clavicle
Kabak 2004 assessed open reduction and internal fixation using LC‐DCP versus DCP for treating non‐union of the middle third of the clavicle in 36 patients with mid‐clavicular non‐union. We presented only outcome data that were complete and consistently reported in the analyses. We received no response from the authors following our request for further information or data from this trial. Follow‐up data were available for 33 participants (17 with LC‐DCP fixation and 16 with DCP).
Function or disability
Participant‐assessed upper‐limb function was evaluated using the DASH questionnaire. Participants allocated to LC‐DCP consistently reported statistically significant higher scores at all follow‐up times (Analysis 2.1). At three months (intermediate follow‐up), the MD was ‐13.90 (95% CI ‐17.83 to ‐9.97); at six months (intermediate follow‐up) the MD was ‐13.20 (95% CI ‐16.77 to ‐9.63); at 12 months (long‐term follow‐up) the MD was ‐8.90 (95% CI ‐11.73 to ‐6.07); and at final follow‐up the MD was ‐8.10 (95% CI ‐10.73 to ‐5.47). The best estimates (MDs) of both three and six months are over 10 points, which is considered a clinically relevant difference in favour of the LC‐DCP group (Gummesson 2003). The CIs included the MCID at the later time points and so a slight clinical benefit in favour of the LC‐DCP group was possible in the long term.
2.1. Analysis.
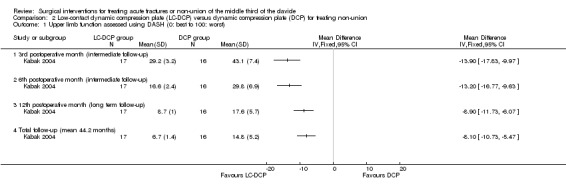
Comparison 2 Low‐contact dynamic compression plate (LC‐DCP) versus dynamic compression plate (DCP) for treating non‐union, Outcome 1 Upper limb function assessed using DASH (0: best to 100: worst).
Pain
Kabak 2004 recorded the presence of pain. No participants in either group reported pain at final follow‐up. However, of the three sportsmen in each group, only those of the DCP group reported mild but not restricting pain after heavy exercise at 12 months. At the same follow‐up time, three other participants of this group complained of occasional pain related to changes in the weather. This resolved after implant removal.
Treatment failure
There were two treatment failures where union was not achieved in the DCP group. Union was achieved subsequently in both participants after a further operation. The difference between the two groups was not statistically significant (RR 0.19, 95% CI 0.01 to 3.36; Analysis 2.2) (Kabak 2004).
2.2. Analysis.

Comparison 2 Low‐contact dynamic compression plate (LC‐DCP) versus dynamic compression plate (DCP) for treating non‐union, Outcome 2 Treatment failure (re‐operation for unresolved non‐union).
Clinical fracture healing
Two participants of the DCP group did not achieve union. The time to clinical and radiographic union was achieved significantly earlier in the LD‐DCP group with a MD of ‐2.70 weeks (95% CI ‐4.09 to ‐1.25; Analysis 2.3) (Kabak 2004).
2.3. Analysis.

Comparison 2 Low‐contact dynamic compression plate (LC‐DCP) versus dynamic compression plate (DCP) for treating non‐union, Outcome 3 Time to clinical and radiological union (weeks).
Adverse events
The treatment groups were not identified for the four participants with superficial infections and the participant who presented with short‐term incomplete brachial palsy (resolved by four months). Significantly fewer participants in the LC‐DCP group required plate removal (two with LC‐DCP fixation vs. eight with DCP fixation), primarily done for cosmesis (two with LC‐DCP fixation vs. five with DCP fixation), with an RR of 0.24 (95% CI 0.06 to 0.95; NNTH 3; Analysis 2.4). Explicit mention in Kabak 2004 of mild or moderate limitation of range of motion was less common for the LC‐DCP group (six with LC‐DCP fixation vs. one with DCP).
2.4. Analysis.

Comparison 2 Low‐contact dynamic compression plate (LC‐DCP) versus dynamic compression plate (DCP) for treating non‐union, Outcome 4 Plate removals (mainly for cosmetic reasons).
Health‐related quality of life
Kabak 2004 did not evaluate health‐related quality of life.
Return to previous activities
All participants of the LC‐DCP group returned to their original occupations, whereas two former truck drivers in the DCP group changed their jobs because of limitations in shoulder mobility. Kabak 2004 reported that the mean time to return to work was statistically significantly shorter in the LC‐DCP group (6.1 weeks with LC‐DCP fixation vs. 9.6 weeks with DCP fixation; reported P value < 0.001).
Participant satisfaction with method of treatment
Kabak 2004 did not assess participant satisfaction with method of treatment.
Comparison 3: Knowles pin versus a dynamic compression plate for treating middle third clavicle fractures and non‐union
Lee 2007 compared open reduction and internal fixation with the Knowles pin (intramedullary fixation) versus the DCP for treating middle third clavicle fractures and non‐union. Follow‐up data were available for 62 participants (32 with Knowles pin and 30 with DCP).
Function or disability
Lee 2007 found no difference between the two groups in the Constant and Murley scores of the affected side at 30 months post operation (mean score (out of 100) for best function: 85 with Knowles pin vs. 84 with DCP).
Pain
Pain, assessed using a VAS, and analgesic consumption were recorded for the first five days after surgery. Without providing data, Lee 2007 reported that there were no significant differences in the pain scores between the two groups on the first three postoperative days; however, results from day four and five showed lower pain scores in favour of the Knowles pin group (reported P value = 0.05 on day four; P value = 0.04 on day five). Lee 2007 reported that all participants were placed on a standard protocol for analgesia, which consisted of participant‐controlled meperidine, paracetamol (acetaminophen), and non‐steroidal anti‐inflammatory drugs (including tiaprofenic acid, celecoxib, and ketoprofen). In the Knowles pin group, a statistically significantly lower total consumption over five days of meperidine (80 mg intramuscular with Knowles pin vs. 221 mg oral with DCP; reported P value = 0.02) and paracetamol (520 mg with Knowles pin vs. 1724 mg with DCP; reported P value = 0.01) was evident; although the clinical significance was less clear.
Treatment failure
No participants in the Knowles pin group required re‐operation, whereas three participants allocated to DCP required re‐operation: for symptomatic non‐union (one case), or implant failure (two cases). The difference between the two groups was not statistically significant (RR 0.13, 95% CI 0.01 to 2.49; Analysis 3.1).
3.1. Analysis.
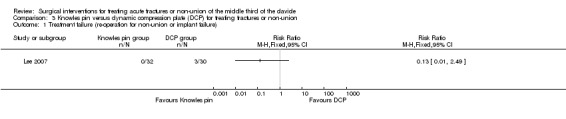
Comparison 3 Knowles pin versus dynamic compression plate (DCP) for treating fractures or non‐union, Outcome 1 Treatment failure (re‐operation for non‐union or implant failure).
Clinical fracture healing
All fractures of participants in the Knowles pin group healed in six months and 29/30 (96.7%) fractures of participants of the DCP group healed in six months (RR 1.03, 95% CI 0.95 to 1.13; Analysis 3.2).
3.2. Analysis.
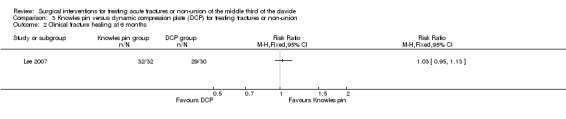
Comparison 3 Knowles pin versus dynamic compression plate (DCP) for treating fractures or non‐union, Outcome 2 Clinical fracture healing at 6 months.
Adverse events
Adverse outcomes other than those resulting in treatment failure were: wound infection (one case that resolved after treatment) and symptomatic hardware problems. Elective removal of hardware was also reported (Analysis 3.3).
3.3. Analysis.
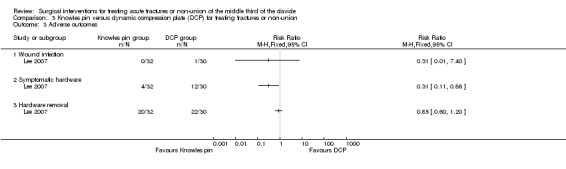
Comparison 3 Knowles pin versus dynamic compression plate (DCP) for treating fractures or non‐union, Outcome 3 Adverse outcomes.
Implant‐associated symptoms were significantly more common after DCP (4/32 with Knowles pin vs. 12/30 with DCP; RR 0.31, 95% CI 0.11 to 0.86; NNTH 4). However, elective removal of hardware did not differ significantly between the two groups (20/32 with Knowles pin vs. 22/30 with DCP; RR 0.85, 95% CI 0.60 to 1.20).
Health‐related quality of life
Lee 2007 did not evaluate health‐related quality of life.
Return to previous activities
Separate data for functional impairment and return to function were not available.
Participant satisfaction with method of treatment
Lee 2007 did not assess patient satisfaction with method of treatment.
Other outcomes assessed
Wound size was significantly smaller in the Knowles pin group (mean incision length: 4.2 cm with Knowles pin vs. 7.8 cm with DCP; reported P value < 0.001). Length of surgery was also significantly shorter in the Knowles pin group (36 minutes with Knowles pin vs. 64 minutes with DCP; reported P value < 0.001).
Hospital stay in the Knowles pin group was on average three days shorter (mean stay 6.2 days (range 5 to 10 days) with Knowles pin vs. 9.1 days (range 5 to 15 days) with DCP; reported P value = 0.03). No other resource or cost data were reported.
Comparison 4: three‐dimensional plate versus superior‐positioned plate for treating acute dislocated middle third clavicle fractures
In Shen 2008, open reduction and internal fixation involved a reconstruction plate which, after shaping, was placed either three dimensionally (3D plate) or superiorly (superior plate) onto the clavicle and fixed. Additional information and data were supplied on this trial by the lead author for length of surgery; length of hospital stay; and definitions of symptomatic people, delayed union, and fracture healing. Follow‐up data were available for 117 participants (63 with 3D plate fixation and 54 with superior plate fixation).
Function or disability
Shen 2008 did not evaluate function or disability.
Pain
Shen 2008 did not report pain outcomes except within the definition of symptomatic participants (see below).
Treatment failure
There were significantly fewer treatment failures, defined as re‐operation within four months after surgery for symptomatic non‐union, in the 3D plate group (1/67 with 3D plate fixation vs. 8/66 with superior plate fixation; RR 0.12, 95% CI 0.02 to 0.96; NNTH 10; Analysis 4.1).
4.1. Analysis.

Comparison 4 Three‐dimensional (3D) plate versus superior plate for treating acute dislocated fractures, Outcome 1 Treatment failure (re‐operation for symptomatic delayed union).
Clinical fracture healing
Significantly fewer participants allocated to 3D plate fixation failed to achieve fracture healing by four months from surgery (4/67 with 3D plate fixation vs. 23/66 with superior plate fixation; RR 0.17, 95% CI 0.06 to 0.47; Analysis 4.2).
4.2. Analysis.
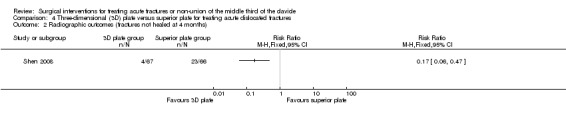
Comparison 4 Three‐dimensional (3D) plate versus superior plate for treating acute dislocated fractures, Outcome 2 Radiographic outcomes (fractures not healed at 4 months).
Adverse events
Functional impairment and clinical outcomes were evaluated in terms of the number of symptomatic participants who had two or more of the following symptoms: pain at rest, pain during activity, strength reduction, and shoulder elevation less than 120°. As shown in Analysis 4.3, there were significantly fewer symptomatic participants in the 3D plate group at both four months (3/67 with 3D plate fixation vs. 15/66 with superior plate fixation; RR 0.20, 95% CI 0.06 to 0.65; NNTH 6) and 12 months after surgery (2/63 with 3D plate fixation vs. 10/54 with superior plate fixation; RR 0.17, 95% CI 0.04 to 0.75; NNTH 7).
4.3. Analysis.
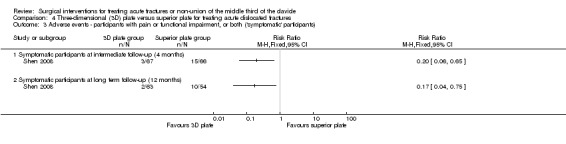
Comparison 4 Three‐dimensional (3D) plate versus superior plate for treating acute dislocated fractures, Outcome 3 Adverse events ‐ participants with pain or functional impairment, or both ('symptomatic' participants).
Health‐related quality of life
Shen 2008 did not evaluate health‐related quality of life.
Return to previous activities
Shen 2008 did not describe return to previous activities.
Patient satisfaction with method of treatment
Shen 2008 did not assess patient satisfaction with method of treatment.
Other outcomes assessed
There was no significant difference between the two groups for length of surgery (Analysis 4.4).
4.4. Analysis.

Comparison 4 Three‐dimensional (3D) plate versus superior plate for treating acute dislocated fractures, Outcome 4 Length of surgery (hours).
There was no significant difference between the two groups in the length of hospital stay (MD 0.20 days, 95% CI ‐0.85 to 1.20; Analysis 4.5).
4.5. Analysis.

Comparison 4 Three‐dimensional (3D) plate versus superior plate for treating acute dislocated fractures, Outcome 5 Length of hospital stay (days).
Discussion
Summary of main results
We found five randomised and two quasi‐randomised controlled trials that involved 398 participants. Four studies compared intramedullary fixation with plate fixation for people with acute clavicle fractures; each of the other three small studies made a different comparison.
Evidence was not available for all important outcomes for any of the comparisons; and all trials were at high risk of bias for at least two domains. The available evidence was generally judged as being of very low quality reflecting both the risk of bias but also the small sample sizes.
Table 1 presents a summary of the evidence for intramedullary fixation with plate fixation for adults (aged 16 years or over) with acute clavicle fractures. Low quality evidence from three of the four included trials making this comparison indicated that intramedullary fixation may not result in a clinically important improvement in upper arm function (despite a statistically significant difference in its favour). Very low quality evidence indicated little difference between intramedullary fixation and plate fixation in pain (one trial), treatment failure resulting in non‐routine surgery (four trials), and time to clinical fracture consolidation (three trials). There was very low quality evidence of a lower incidence of participants with adverse events (mainly infection, cosmetic result, and symptomatic hardware) in the intramedullary fixation group but the CI of the pooled results also included the small possibility of a lower incidence in the plate group. None of the four trials reported on quality of life or return to previous activities (seeTable 1).
In the surgical treatment of non‐union of the middle third of the clavicle, there was very low quality evidence that the use of LC‐DCPs, when compared with a standard DCPs (one study, 36 participants), was associated with statistically significantly better upper limb function scores throughout the year following surgery and at final follow‐up (mean 44 months). However, the clinical importance of this difference was marginal at one year and subsequently. The use of LC‐DCPs was also associated with earlier fracture union and return to work, and a reduced incidence of implant‐associated symptoms. One quasi‐randomised trial (69 participants) that compared Knowles pins versus plates for treating middle third clavicle fractures and non‐union provided very low quality evidence that the use of Knowles pins was associated with less treatment failure and fewer adverse outcomes. One study (133 participants) found low quality evidence that a 3D technique of plate fixation was associated with a significantly lower incidence of symptomatic delayed union than a standard superior surgical approach.
Overall completeness and applicability of evidence
We included only seven trials in this review. These did not allow a comprehensive review of the relative effectiveness of different methods of surgical treatment for fracture and non‐union of the middle third of the clavicle. For the four comparisons for which we found eligible studies, the evidence is not robust due to the risk of bias and the small size of the included studies. Furthermore, outcome data were available for a maximum of 133 participants for the main comparison of intramedullary versus plate fixation. It is notable too that evidence is pending from two ongoing trials, with planned recruitment of 245 participants, testing this comparison.
With probably just one exception, participants of the included trials were adults. Since paediatric fractures have a better prognosis than fractures in adults and are generally treated conservatively (non‐surgically), the results of this review apply only to the surgical treatment of clavicle fractures in adults. Exceptionally, one trial included only participants over 50 years of age (Lee 2007). However, with the data available we could not develop subgroup analyses to check for differences in treatment effect between younger and older adults. Neither were there data available to perform subgroup analysis according to the pattern of the fracture or non‐union sustained by the participants.
All trials failed to measure some outcomes of importance; notably pain was only measured in four trials, and they did not provide data for long‐term pain; plus no trials evaluated health‐related quality of life.
One limitation of our review is that some of the interventions evaluated by the included trials are unlikely to be used in current practice in many parts of the world and, moreover, many implants in current use are being superseded by a new generation of implants, such as site‐specific pre‐contoured locking plates. The most common implants in current practice have been reported to be dynamic compression or locking plates designed for treating acute clavicle fractures and non‐union (Khan 2008; Khan 2009). Reports of high rates of complications, such as migration of the implants, implant breakage, and skin breakdown at the site of nail insertion, have limited the use of intramedullary fixation (Lyons 1990; Strauss 2007). Reconstruction plates are also less accepted nowadays as they are susceptible to deformation at the fracture site, which may lead to healing complications (Khan 2009).
Quality of the evidence
All seven included trials had serious methodological limitations that placed them at high risk of bias (Figure 2; Figure 3). In our assessment of the quality of the available evidence for individual outcomes for each comparison, we invariably downgraded the evidence two levels for serious study limitations to low quality. As detailed in Table 1 for the comparison of intramedullary versus plate fixation, we generally further downgraded the evidence a level for imprecision reflecting a low number of events and small sample size or inconsistency reflecting heterogeneity. Thus, in our judgement, the quality of the evidence is either low quality where "Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate" or, more usually, very low quality, meaning, "We are very uncertain about the estimate".
Therefore, the results of included trials should be interpreted with caution and viewed, at this stage, as requiring confirmation with studies of good methodological quality and adequate power.
Potential biases in the review process
This review was conducted following criteria and methods set out in a published protocol (Lenza 2008). We believe that our search strategy was comprehensive, and it has been maintained properly and regularly updated by the contact author (ML). It has included handsearching of conference proceedings and searches for ongoing and recently completed trials. However, it is possible that we have missed some potentially eligible trials. When necessary, we tried to contact authors of all included trials; however, only the authors of one trial responded. For this trial, we obtained unpublished data (means and standard deviations) and some information about the study design (Shen 2008).
Currently, there is a small number of specific validated scores for assessing shoulder function. When pooling results from trials that included more than one measure of function, for the purpose of pooling data for the summary of findings table, we decided to choose the Constant score as default. This is because it is more specific for shoulder function than the DASH questionnaire; in addition, when compared with the OSS, the Constant score is the most commonly used in the literature. When interpreting the results of these scores, we used MCID obtained from the literature for rotator cuff and other shoulder disorders and not for people recovering after a clavicle fracture. This is unavoidable but of note is that we have found that our selected MCIDs are lower than reported in other publications; for example, those for DASH were estimated as 12.4 rather than 10.0 in Van Kampen 2013.
Agreements and disagreements with other studies or reviews
We found eight published systematic reviews that approached the comparison between surgical interventions to treat acute fractures or non‐union of the middle third of the clavicle in adults (Barlow 2013; Barlow 2014; Chen 2013; Duan 2011; Houwert 2012; Wijdicks 2012; Wijdicks 2013; Zlowodzki 2005). All the systematic reviews included randomised and non‐randomised clinical trials. The results of our review are consistent with the results of the eight non‐Cochrane systematic reviews.
Barlow 2013 analysed four studies (one randomised controlled trial, two quasi‐randomised controlled trials, and two retrospective studies). The authors did not pool the data due to the heterogeneity of the study populations and interventions. They found no difference between intramedullary fixation and plate fixation when shoulder function was evaluated; however, intramedullary fixation presented a lower complication rate when compared with people who underwent to plate fixation.
Barlow 2014 evaluated the outcome of middle third clavicle non‐union when treated with external fixation devices. The review included four studies, among them, three case series and one case‐control study comparing reconstruction plating with external fixation. Despite the very low quality of included studies, the authors concluded, without evidence, that external fixation is an option of treatment for hypertrophic non‐union of the clavicle.
Chen 2013 is a Chinese review that assessed studies comparing intramedullary fixation with plate fixation for treating middle third clavicle fractures. Their results, based on data of one randomised controlled trial and four retrospective controlled trials (388 participants), showed that intramedullary fixation provided significantly higher functional endpoints and less mean bone union time, operation time, incision length, intraoperative blood loss, and the hospital stay when compared with plate fixation. The authors found no significant differences in incidence rate of complications, non‐union, implant failure, wound infection, and malunion between groups.
Duan 2011 is a systematic review that evaluated the results of trials that compared plate fixation versus intramedullary pin or conservative treatment for middle third clavicle fracture. The authors summarised their results based on only two studies that were included in our review (Ferran 2010; Lee 2007). The available evidence showed that stabilisation with intramedullary pinning is associated with better functional results and lower rates of adverse effects when compared with plate fixation.
Houwert 2012 reported their results based on one randomised controlled trial and one case‐series study that evaluated intramedullary fixation versus plate fixation. The available evidence from included studies indicated no difference in functional outcome or complications after intramedullary fixation or plate fixation for middle third displaced clavicle fractures.
Wijdicks 2012 and Wijdicks 2013 reported the complications of surgical interventions for people with clavicle fractures. Wijdicks 2012 included 11 studies (three randomised controlled trials and eight non‐randomised controlled trials) that assessed plate fixation complications; the study found less than 10% of non‐union and malunion rates, the majority of complications were related to implant irritation or failure, accounting for 9% to 64%. Wijdicks 2013 evaluated complications after intramedullary fixation for displaced middle third clavicle fractures; based on the results of six studies, only one of which was a randomised controlled trial, the authors reported major complications such as bone‐healing problems and deep infections requiring implant removal at a rate no higher than 7%; however, the rates for minor complications, such as wound infection and implant irritation that could be resolved without further surgery, were as high as 31%.
Zlowodzki 2005 assessed all types of comparison treatments for clavicle fractures; the authors identified and included 22 studies (randomised controlled trials and non‐randomised controlled trials). The results on types of surgical interventions were based on one non‐randomised controlled trial that compared anterior‐inferior versus superior plating of acute mid‐shaft fractures in 34 people. The authors reported a significantly better visual analogue scale patient symptoms scores in people undergoing anterior‐inferior plating, there were no non‐union in either group, they described two infections and one failed fixation in the superior plating group, and one delayed union in the anterior‐inferior plating group.
Authors' conclusions
Implications for practice.
There was very limited and low quality evidence on the relative effectiveness of only some of the methods of surgical intervention for treating acute fractures or non‐union of the middle third of the clavicle. The relevance of the available evidence depends also on current practices in different parts of the world and the availability of more recently introduced implants.
Based on evidence from four trials with high risk of bias, this review provided some low quality evidence of a lack of a clinically important difference in function between intramedullary fixation and plate fixation. It found very low quality evidence of a lack of difference in pain, treatment failure, and time to union between these two interventions and that overall adverse events may be less after intramedullary fixation but we are very uncertain of the reliability of these findings. Based on very low quality evidence from one small, methodologically weak trial, intramedullary fixation may give better results than plate fixation for acute clavicle fractures and non‐union. Similarly, another small, methodologically weak trial showed that a low‐contact dynamic compression plate may give better results than a dynamic compression plate for treating non‐union of the clavicle. Where plate fixation is considered appropriate, the use of a reconstruction plate fixed superiorly on the main distal fragment and anteriorly on the main proximal fragments may be more effective than fixation with the plate shaped in the form of an 'S' and fixed on the superior surface. Again, the low or very low quality evidence for these comparisons raises questions over the reliability of these findings.
Implications for research.
Further studies on the surgical treatment of acute fracture and non‐union of the middle third of the clavicle appear justified. We suggest that:
acute fracture and non‐union are somewhat different problems, and that individual studies should include participants with one or the other, but not both, unless appropriate randomisation is used and outcomes are reported separately for each condition;
as non‐union is relatively rare, adequately powered multicentre studies with central randomisation should be developed comparing intramedullary versus plate fixation and different techniques for placement of the plate, such as anterior versus superior plate positioning;
a consensus on indications for surgical treatment of mid‐shaft clavicle fractures should be developed to determine the priorities and inclusion criteria for future comparative studies. Multicentre randomised controlled trials of high quality could then be developed to compare different techniques of fixation. Decisions on prioritisation should take into account the four ongoing trials identified in this review;
comparisons of techniques in common use, such as pre‐contoured plates on the superior and the anterior‐inferior aspect of the clavicle, should be done. Furthermore, the efficacy of newer generations of implants (e.g. site‐specific pre‐contoured locking plates and locking screws) should be tested in randomised controlled clinical trials;
validated shoulder function tests (e.g. Disability of the Arm, Shoulder and Hand questionnaire (DASH) and Oxford Shoulder Score), pain scores (using visual analogue scale or numerical rating scale) and health‐related quality of life questionnaires (e.g. Short Form‐36 and EQ‐5D) should be used as outcome measures.
The addition of evidence in future updates of this review from the four ongoing trials, identified from trial registries, should help to inform on the comparisons of intramedullary fixation versus plate fixation and anterior versus superior plate fixation for acute fractures.
What's new
| Date | Event | Description |
|---|---|---|
| 5 May 2015 | New search has been performed | In this update, published in Issue 5, 2015, the following changes were made: 1. The search was updated to June 2014. 2. Nineteen new studies were identified. Of these, four were included, 11 were excluded and four are ongoing studies. 3. The 'Types of outcome measures' section was restructured for consistency with another more recent review on these fractures. 4. The methodology was upgraded, including assessment of risk of bias and use of GRADE for assessment of the quality of the evidence. |
| 4 May 2015 | New citation required and conclusions have changed | 1. All four newly included studies compared intramedullary fixation with open reduction versus internal fixation with plate. The results of this newly included comparison are summarised in a 'Summary of findings' table. 2. Changes were made to the authorship of the review. |
Acknowledgements
We would like to thank Lindsey Elstub, Joanne Elliott, and Amy Kavanagh for their assistance in preparing the protocol, review, and subsequent update. We thank the following people for helpful feedback at editorial review: Bill Gillespie, Nigel Hanchard, Helen Handoll, Peter Herbison, Amar Rangan, and Janet Wale.
Thanks are extended to the authors of one included trial who responded to requests for additional information and data: Dr Jin‐wen Shen (China).
We would like to thank João Baptista Gomes dos Santos, João Carlos Belloti, and Marcelo Hide Matsumoto who were authors on the previous version of this review.
This project was supported by the National Institute for Health Research (NIHR) via Cochrane Infrastructure funding to the Cochrane Bone, Joint and Muscle Trauma Group. The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, or the UK National Health Service or Department of Health.
Appendices
Appendix 1. Search strategies
CENTRAL (CRS Online)
#1 MESH DESCRIPTOR Clavicle EXPLODE ALL TREES (63) #2 (clavic* OR midclavic* OR collarbone):TI,AB,KY (163) #3 #1 OR #2 (163) #4 MESH DESCRIPTOR Fractures, Bone EXPLODE ALL TREES (3050) #5 MESH DESCRIPTOR Fracture Fixation EXPLODE ALL TREES (975) #6 MESH DESCRIPTOR Fracture Healing (327) #7 (fracture* OR pseudoarthros* OR pseudarthros*):TI,AB,KY (8196) #8 #4 OR #5 OR #6 OR #7 (8200) #9 #3 AND #8 (66)
MEDLINE (Ovid Online)
1 Clavicle/ (4357) 2 (clavic* or midclavic* or collarbone).tw. (7309) 3 1 or 2 (8854) 4 exp Fractures, Bone/ or exp Fracture Fixation/ or exp Fracture Healing/ (147172) 5 (fracture* or pseudoarthros* or pseudarthros*).tw. (176792) 6 4 or 5 (218335) 7 3 and 6 (2708) 8 Randomized controlled trial.pt. (376608) 9 Controlled clinical trial.pt. (88576) 10 randomized.ab. (297122) 11 placebo.ab. (155143) 12 Drug therapy.fs. (1708731) 13 randomly.ab. (214813) 14 trial.ab. (308526) 15 groups.ab. (1365989) 16 or/8‐15 (3362041) 17 exp Animals/ not Humans/ (3954113) 18 16 not 17 (2884168) 19 7 and 18 (255) 20 (200812* or 2009* or 2010* or 2011* or 2012* or 2013* or 2014*).ed. (5314287) 21 19 and 20 (129)
EMBASE (Ovid Online)
1 Clavicle/ (4400) 2 (clavic* or midclavic* or collarbone).tw. (8331) 3 or/1‐2 (9687) 4 exp Fracture Healing/ or exp Fracture Treatment/ or exp Fracture/ or exp Pseudarthrosis/ (224994) 5 (fracture* or pseudoarthros* or pseudarthros*).tw. (199015) 6 or/4‐5 (278950) 7 and/3,6 (3024) 8 Clinical Trial/ (831857) 9 Randomized controlled trial/ (343948) 10 Randomization/ (62353) 11 Single blind procedure/ (18403) 12 Double blind procedure/ (113783) 13 Crossover procedure/ (39220) 14 Placebo/ (241145) 15 Randomi?ed controlled trial*.tw. (99401) 16 Rct.tw. (14025) 17 Random allocation.tw. (1311) 18 Randomly allocated.tw. (20228) 19 Allocated randomly.tw. (1925) 20 (allocated adj2 random).tw. (712) 21 Single blind*.tw. (14284) 22 Double blind*.tw. (140702) 23 ((treble or triple) adj blind*).tw. (371) 24 Placebo*.tw. (197733) 25 Prospective study/ (253266) 26 or/8‐25 (1361237) 27 Case study/ (26548) 28 Case report.tw. (258694) 29 Abstract report/ or letter/ (892491) 30 or/27‐29 (1172102) 31 26 not 30 (1323607) 32 limit 31 to human (1213554) 33 and/7,32 (171) 34 (2008* or 2009* or 2010* or 2011* or 2012* or 2013* or 2014*).em. (7622235) 35 33 and 34 (104)
LILACS (Bireme)
Mh clavicle OR Tw clavic$ OR midclavic$ OR Tw collarbone [Words] and Mh fracture healing OR Mh fracture fixation OR Mh fractures OR Tw fracture$ OR Mh Pseudarthrosis OR Tw pseudoarthros$ OR Tw pseudarthros$ [Words] and ((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animals AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animals AND NOT (Ct human and Ct animals)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animals AND NOT (Ct human and Ct animals))) [Words] (19)
Orthopaedic Proceedings (The Bone and Joint Journal)
Title: clavic* or midclavic* or collarbone
Abstract or title: random*
Orthopaedic Proceedings = 9
ISRCTN Registry
Advanced search Search in Title field using: clavic* AND fracture* Total = 9
Clinical Trials
clinicaltrials.gov/
Advanced search Search in Title field using: clavic* AND fracture* Total = 65
WHO International Clinical Trials Registry Platform Search Portal
Advanced search Search in Title field using: clavic* AND fracture* Total = 47
Appendix 2. Search results reported in previous version of the review (Lenza 2009)
The search strategy found 159 references (Figure 1), of which two review authors (JB and ML) excluded 152 through initial screening of reference titles and abstracts. Of those excluded, 115 were duplicates or not relevant, 20 were excluded as they were not randomised controlled trials, and 17 did not meet the inclusion criteria for participants and interventions. Of the seven remaining potentially relevant studies, for which full reports were obtained, three were included and four were excluded. One further study, subsequently excluded, was identified via PubMed related articles.
Data and analyses
Comparison 1. Intramedullary fixation versus open reduction and internal fixation with plate for treating acute middle third clavicle fractures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Function or disability (overall at the end of follow‐up ‐ ≥ 6 months) | 3 | 120 | Std. Mean Difference (IV, Fixed, 95% CI) | 0.45 [0.08, 0.81] |
| 2 Constant score (0‐100: best outcome) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 At short term follow‐up (6 weeks) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 7.00 [0.41, 13.59] |
| 2.2 At intermediate follow‐up (3 months) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 5.70 [‐0.36, 11.76] |
| 2.3 At long term follow‐up (6 months) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | 5.60 [‐0.79, 11.99] |
| 2.4 At long term follow‐up (12 months) | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | 4.46 [0.56, 8.36] |
| 3 DASH questionnaire (at long term follow‐up of 6 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Oxford Shoulder Score (at long term follow‐up of 6 months or more) | 2 | 84 | Mean Difference (IV, Fixed, 95% CI) | 0.86 [‐0.59, 2.31] |
| 5 Pain ‐ using the section of Constant score at 12 months | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 6 Treatment failure (participants who have a non‐routine secondary surgical intervention) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Symptomatic non‐union | 1 | 38 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 7.70] |
| 6.2 Early mechanical failure or re‐fracture | 3 | 83 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.91 [0.19, 4.30] |
| 6.3 Overall treatment failure | 4 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.69 [0.16, 2.97] |
| 7 Clinical healing: time to clinical/radiographic fracture consolidation (weeks) | 3 | 98 | Mean Difference (IV, Random, 95% CI) | ‐1.22 [‐3.83, 1.39] |
| 8 Adverse events | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Wound infection or dehiscence | 3 | 120 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.33, 2.84] |
| 8.2 Cosmetic result | 2 | 70 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.84 [0.34, 2.08] |
| 8.3 Malunion or asymptomatic non‐union | 2 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.12, 2.26] |
| 8.4 Symptomatic hardware | 2 | 63 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.39 [0.14, 1.09] |
| 8.5 Total adverse events | 4 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.39, 1.03] |
| 9 Other outcomes assessed | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 9.1 Duration of surgery (minutes) | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐23.69 [‐26.72, ‐20.66] |
| 9.2 Mean wound size (cm) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐4.2 [‐5.00, ‐3.40] |
| 9.3 Mean blood loss (mL) | 2 | 88 | Mean Difference (IV, Fixed, 95% CI) | ‐51.96 [‐55.48, ‐48.44] |
| 9.4 Mean hospital stay (days) | 1 | 38 | Mean Difference (IV, Fixed, 95% CI) | ‐0.90 [‐1.32, ‐0.48] |
Comparison 2. Low‐contact dynamic compression plate (LC‐DCP) versus dynamic compression plate (DCP) for treating non‐union.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Upper limb function assessed using DASH (0: best to 100: worst) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 1.1 3rd postoperative month (intermediate follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.2 6th postoperative month (intermediate follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.3 12th postoperative month (long term follow‐up) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 1.4 Total follow‐up (mean 44.2 months) | 1 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Treatment failure (re‐operation for unresolved non‐union) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Time to clinical and radiological union (weeks) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 4 Plate removals (mainly for cosmetic reasons) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Knowles pin versus dynamic compression plate (DCP) for treating fractures or non‐union.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure (re‐operation for non‐union or implant failure) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Clinical fracture healing at 6 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse outcomes | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Wound infection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Symptomatic hardware | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.3 Hardware removal | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. Three‐dimensional (3D) plate versus superior plate for treating acute dislocated fractures.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Treatment failure (re‐operation for symptomatic delayed union) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Radiographic outcomes (fractures not healed at 4 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Adverse events ‐ participants with pain or functional impairment, or both ('symptomatic' participants) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3.1 Symptomatic participants at intermediate follow‐up (4 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 3.2 Symptomatic participants at long term follow‐up (12 months) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Length of surgery (hours) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Length of hospital stay (days) | 1 | Mean Difference (IV, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Assobhi 2011.
| Methods |
Study design: randomised controlled trial Duration of the study: December 2003 to September 2008 Protocol was published before recruitment of participants: not reported Details of trial registration: not reported Funding sources: no conflict of interest |
|
| Participants |
Place of study: Cairo, Egypt Number of participants assigned: 38 (19 intramedullary group; 19 plate group) Number of participants assessed: 38 (19 intramedullary group; 19 plate group) Inclusion criteria:
Exclusion criteria:
Age (mean/SD/range):
Gender (male/female):
Side of injury (dominant/non‐dominant):
Classification of injury: not reported |
|
| Interventions |
Timing of intervention (mean/SD/range):
Intervention 1 (Intramedullary group):
Intervention 2 (Plate group):
Rehabilitation: For both groups, participants received arm sling protection for 1‐2 weeks postoperatively, and then light daily activities such as writing or eating were allowed. Participants were encouraged to resume their normal daily activities after the 4th week when the pain was tolerated. Strenuous activities were discouraged before the 6th week from the trauma Any co‐interventions: not reported |
|
| Outcomes |
Length of follow‐up:
Loss of follow‐up: 0 lost to follow‐up Primary outcomes:
Secondary outcomes:
Other outcomes assessed:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of generating the random sequence was not reported |
| Allocation concealment (selection bias) | Unclear risk | It was not reported when the randomisation was done and details were not described to ascertain that allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data |
| Selective reporting (reporting bias) | High risk | Pain (as primary outcome measured by validated score), health‐related quality of life, return to previous activities, and participant satisfaction were not evaluated by the authors |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Ferran 2010.
| Methods |
Study design: randomised controlled trial Duration of the study: 2006‐2008 Protocol was published before recruitment of participants: not reported Details of trial registration: not reported Funding sources: no conflict of interest |
|
| Participants |
Place of study: Wales, UK Number of participants assigned: 32 (17 intramedullary group; 15 plate group) Number of participants assessed: 32 (17 intramedullary group; 15 plate group) Inclusion criteria:
Exclusion criteria:
Age (mean/range):
Gender (male/female):
Side of injury (dominant/non‐dominant): not reported Classification of injury: not reported |
|
| Interventions |
Timing of intervention (mean/SD):
Intervention 1 (Intramedullary group):
Intervention 2 (Plate group):
Rehabilitation: For both groups, participants received arm sling protection for 2 weeks postoperatively and were encouraged to perform regular pendular exercises At 2 weeks, participants initiated gleno‐humeral range of motion exercises. For the first 6 weeks, abduction and forward flexion were limited to 90°. Beyond 6 weeks, full range of gleno‐humeral motion was encouraged Any co‐interventions: not reported |
|
| Outcomes |
Length of follow‐up:
Loss of follow‐up: 0 lost to follow‐up. Primary outcomes:
Secondary outcomes:
|
|
| Notes | After fracture healing, all pins were removed under local anaesthesia | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of generating the random sequence was not reported |
| Allocation concealment (selection bias) | Low risk | Opaque and sealed envelopes were used |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | There were no missing data |
| Selective reporting (reporting bias) | High risk | The authors did not report outcomes at each time point, it was unclear when the outcomes were collected |
| Other bias | High risk | Major baseline imbalance related to age of participants |
Kabak 2004.
| Methods |
Study design: Randomised controlled trial Duration of the study: March 1996 to July 2000 Protocol was published before recruitment of participants: not reported Details of trial registration: not reported Funding sources: not reported |
|
| Participants |
Location: Turkey Number of participants assigned: 36 (number of participants was not reported by groups) Number of participants assessed: 33 (17 LC‐DCP group; 16 DCP group) Inclusion criteria:
Exclusion criteria:
Age (mean/SD/range):
Gender (male/female):
Side of injury (dominant/non‐dominant):not reported. Classification of injury:
|
|
| Interventions |
Timing of intervention (mean/range):
Intervention 1 (LC‐DCP group)
Intervention 2 (DCP group):
Rehabilitation: The postoperative interventions of 2 groups were identical:
Any co‐interventions: not reported |
|
| Outcomes |
Length of follow‐up:
Primary outcomes:
Secondary outcomes:
|
|
| Notes | In participants with atrophic non‐union: sclerotic bone ends were excised and the medullar canals of both fragments were opened up and an intercalary segment of iliac crest graft was fashioned to fit between the 2 clavicular segments In the case of an hypertrophic non‐union: the extra callus built up and excessively hypertrophic bone was shaved down to a normal clavicular size to facilitate fitting of the plate |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The sequence generation was performed by a computer random number generator |
| Allocation concealment (selection bias) | Unclear risk | It was not reported when the randomisation was done and details were not described to ascertain that allocation was concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors did not specify which groups the 3 participants that were lost to follow‐up belonged to |
| Selective reporting (reporting bias) | High risk | Outcomes of interest in the review were reported incompletely |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Lee 2007.
| Methods |
Study design: Quasi‐randomised controlled trial Duration of the study: 1999 to 2002 Protocol was published before recruitment of participants: not reported. Details of trial registration: not reported Funding sources: not reported |
|
| Participants |
Location: Taiwan Number of participants assigned: 69 (number of participants was not reported by groups) Number of participants assessed: 62 (32 intramedullary group; 30 plate group) Inclusion criteria:
Exclusion criteria:
Age (mean/range):
Gender (male/female):
Side of injury (dominant/non‐dominant): not reported Classification of injury: The fractures were classified as:
|
|
| Interventions |
Timing of intervention: not reported ‐ 3 participants with painful non‐union were included Intervention 1 (Intramedullary group):
Intervention 2 (Plate group):
Rehabilitation The postoperative interventions of 2 groups were identical:
Any co‐interventions: not reported |
|
| Outcomes |
Length of follow‐up: total follow‐up was 30 months Loss of follow‐up: 7 participants were lost to follow‐up:
Primary outcomes:
Secondary outcomes:
Other outcomes assessed:
|
|
| Notes | Indications for surgery: 21 participants with persistent separation of the fracture with a gap more than half the diameter of the clavicle; 12 with associated fractures (5 ribs, 4 upper extremities, 2 lower extremities, and 1 scapula); 6 with severe displacements and tenting of the skin; 3 painful non‐unions; 2 open fractures; 2 for cosmetic reasons; and 16 with intolerable pain Iliac crest bone grafts were used around the fracture sites of 7 participants who had severe comminuted fractures or non‐union ‐ not specified to which group they belonged |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence generated by some rule based on date (alternation): "patients were treated with a Knowles pin or a plate in turn" |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | The authors did not specify which groups the 7 participants who were lost to follow‐up belonged to |
| Selective reporting (reporting bias) | High risk | Outcomes of interest in the review were reported incompletely and the author did not report any measure of variance for DASH and Constant scores |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Shen 2008.
| Methods |
Study design: randomised controlled trial Duration of the study: 2003‐2006 Protocol was published before recruitment of participants: not published Details of trial registration: not registered Funding sources: no benefits in any form were received or will be received from a commercial party related directly or indirectly to the subject of this article |
|
| Participants |
Place of study: *Zhejiang Province TCM Hospital, Zhejiang TCM University Hospital and a level ‐ an academic trauma centre located in Hangzhou, Zhejiang Province, China Number of participants assigned: 133 (67 in 3D plate group; 66 in superior plate group) Number of participants assessed: 117 (63 in 3D plate group; 54 in superior plate group); 16 participants were lost to follow‐up at 12 months Inclusion criteria:
Exclusion criteria:
Age (mean/range):
Gender (male/female):
Side of injury (dominant/non‐dominant): not reported Classification of injury: The fractures were classified as:
|
|
| Interventions |
Timing of intervention: The operation was usually performed within 2 days of admission Intervention 1 (3D plate group):
Intervention 2 (Superior plate group):
Rehabilitation: *In hospital, participants were instructed on early motion exercises of the fingers, wrist, and elbow. Shoulder immobilisation was applied for 2‐6 weeks, based on the participants' level of comfort. A sling was applied for 6 weeks. The same instructions were used for all the cases Any co‐interventions: not reported |
|
| Outcomes |
Length of follow‐up: participants underwent clinical and radiological assessment at 4 and 12 months after operation by independent surgeons Loss of follow‐up: 16 participants lost to follow‐up at 12 months: 3D plate group ‐ 4 participants lost to follow‐up:
Superior plate group ‐ 12 participants lost to follow‐up:
Primary outcomes
Secondary outcomes
Other outcomes assessed:
|
|
| Notes | * Data obtained by personal contact with the authors | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | The sequence generation was performed by a computer random number generator |
| Allocation concealment (selection bias) | Low risk | In the operating theatre, the participants were allocated to 1 of 2 treatment groups according to sequentially opened sealed envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | The participants were blinded to the surgical method (information via personal contact with author) It was not possible blinding the care providers due to the type of treatment |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Follow‐up evaluations were done by the same non‐participating surgeons. All participants had sufficient records and, at follow‐up, all participants were interviewed according to protocol and examined by the non‐participating surgeons, who contributed with similar numbers of cases to each group (P value = 0.29), which should have prevented surgeons from being a confounding variable. The cases were randomised after the initial assessment, so the evaluator was blinded to the group allocation (personal contact) |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Missing outcome data were not balanced in numbers across intervention groups; more participants in the superior plate group were lost to follow‐up at 12 months (4/67 (6%) in 3D plate group vs. 12/64 (18.1%) in superior plate group). This may have overestimated the benefits of 3D plate |
| Selective reporting (reporting bias) | High risk | Outcomes of interest in the review are not reported (function, pain, and quality of life) |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Silva 2011.
| Methods |
Study design: randomised controlled trial Duration of the study: May 2010 to February 2011 Protocol was published before recruitment of participants: not reported Details of trial registration: retrospective registration 23 May 2011 in ClinicalTrials.gov (NCT01410032) Funding sources: no conflict of interest |
|
| Participants |
Place of study: Sao Paulo, Brazil Number of participants assigned: 22 (12 intramedullary group; 10 plate group) Number of participants assessed: 13 (7 intramedullary group; 6 plate group) Inclusion criteria:
Exclusion criteria:
Age: not reported Gender: not reported Side of injury (dominant/non‐dominant): not reported Classification of injury: not reported |
|
| Interventions |
Timing of intervention: not reported Intervention 1 (Intramedullary group):
Intervention 2 (Plate group):
Rehabilitation: not reported Any co‐interventions: not reported |
|
| Outcomes |
Length of follow‐up:
Loss of follow‐up: the authors assessed the results of 13 participants only; the results of 9 participants (40.1%) were not reported Primary outcomes:
Secondary outcomes:
|
|
| Notes | All participants of the intramedullary group had hardware removal after 6 months | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | The method of generating the random sequence was not reported |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | The study reported preliminary results at 4 months of follow‐up only; therefore, the authors described the results of 13 of a total of 22 participant (59.1% only) |
| Selective reporting (reporting bias) | High risk | Outcomes of interest in the review are not reported (function, pain, and quality of life) |
| Other bias | High risk | Insufficient information to permit judgement The interim nature of the reporting puts this trial at high risk of other bias |
Tabatabaei 2011.
| Methods |
Study design: quasi‐randomised controlled trial Duration of the study: April 2008 to December 2010 Protocol was published before recruitment of participants: not reported Details of trial registration: prospectively registered in 2007 at WHO International Clinical Trial Registry (IRCT201105165920N3) Funding sources: study was supported by the grant number 2228 from Jundishapur University of Medical Sciences |
|
| Participants |
Place of study: Iran Number of participants assigned: 68 (34 intramedullary group; 34 plate group) Number of participants assessed: 50 (25 intramedullary group; 25 plate group) Inclusion criteria:
Exclusion criteria:
Age (mean/SD/range):
Gender (male/female):
Side of injury (dominant/non‐dominant): not reported Classification of injury: AO classification but the authors did not report |
|
| Interventions |
Timing of intervention: not reported. Intervention 1 (Intramedullary group):
Intervention 2 (Plate group):
Rehabilitation: the postoperative protocol was Velpeau bandage for a few days and then sling and early pendulum exercise. In the 6th week, if there was radiological union, the participant was allowed to perform restricted exercise. The participants were instructed not to perform contact sports until 12th week post operation Any co‐interventions: not reported |
|
| Outcomes |
Length of follow‐up: mean follow‐up was 6 months for all participants with clinical assessment at 2, 4, and 6 weeks and 6 months after operation Loss of follow‐up: 18 participants lost to follow‐up at 6 months: Primary outcomes:
Secondary outcomes:
Other outcomes assessed:
|
|
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Sequence generated by some rule based on date (alternation): "We operated the first patient with pin and the other with plate and continued the operations in the same manner" |
| Allocation concealment (selection bias) | High risk | Allocation was not concealed |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Participants and personnel were not blinded |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Outcomes assessors were not blinded |
| Incomplete outcome data (attrition bias) All outcomes | High risk | > 80% of participants completed the follow‐up (50/68 participants, 73.5%) |
| Selective reporting (reporting bias) | High risk | Outcomes of interest in the review are not reported (function, pain, and quality of life) |
| Other bias | Unclear risk | Insufficient information to permit judgement |
3D: three‐dimensional; AO: Arbeitsgemeinschaft fur Osteosynthesefragen; DASH: Disabilities of the Arm, Shoulder and Hand questionnaire; DCP: dynamic compression plate; LC‐DCP: low‐contact dynamic compression plate; SD: standard deviation; TEN: titanium elastic nail; VAS: visual analogue scale; WHO: World Health Organization.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Böhme 2011 | Design of study not relevant: not a randomised or quasi‐randomised controlled trial |
| Cho 2010 | Design of study not relevant: retrospective comparative study |
| Flikweert 2009 | Design of study not relevant: not a randomised or quasi‐randomised controlled trial ‐ with unpublished data only |
| Fu 2012 | Design of study not relevant: retrospective comparative study |
| Jiang 2012 | Design of study not relevant: not a randomised or quasi‐randomised controlled trial ‐ retrospective comparative study |
| Jubel 2002 | Design of study not relevant: not a randomised or quasi‐randomised controlled trial |
| Jubel 2005 | Design of study not relevant: not a randomised or quasi‐randomised controlled trial |
| Kraus 2013 | Design of study not relevant: retrospective comparative study |
| Lee 2008 | Design of study not relevant: not a randomised or quasi‐randomised controlled trial |
| Liu 2010 | Design of study not relevant: retrospective comparative study |
| Ma 2008 | Design of study not relevant: not a randomised or quasi‐randomised controlled trial |
| NCT01311219 | The registration for a multicentre trial was withdrawn prior to enrolment because it was "a duplicate study". The other trial registration was not identified |
| NCT01405703 | Design of study not relevant: not a randomised or quasi‐randomised controlled trial, it was an observational cohort study |
| Pai 2009 | Design of study not relevant: not a randomised or quasi‐randomised controlled trial |
| Ros Y Codorniu 2000 | Design of study not relevant: narrative review |
| Shen 1999 | Design of study not relevant: not a randomised or quasi‐randomised controlled trial |
Characteristics of ongoing studies [ordered by study ID]
ChiCTR‐TRC‐12001973.
| Trial name or title | The placement of fixation plate in clavicular fracture. A randomized prospective study |
| Methods |
Study design: parallel randomised controlled trial Random sequence generation: SPSS software randomly generated Allocation concealment: not reported Masking: double‐blind (just before surgery) |
| Participants |
Location: Karamay City, China Target sample size: 40 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention 1 (Anterior plate):
Intervention 2 (Superior plate):
|
| Outcomes |
Outcomes: wound healing; fixation fatigue fracture; malunion; non‐union; re‐fracture; vascular and nerve injury; and fracture healing Timing of outcomes measurement: not reported |
| Starting date |
Main ID: ChiCTR‐TRC‐12001973 Date of registration: 1 March 2012 Last refreshed on: 29 June 2014 Date of first enrolment: 1 March 2012 Status: completed |
| Contact information |
Name: Dr Yue Yong Address: People's Hospital Orthopedic of Karamay City of Xinjiang Uygur Autonomous Region 834000 Telephone: +86 18999505353 Email: yueyongenglish@yahoo.com.cn Affiliation: People's Hospital Orthopedic of Karamay City |
| Notes |
NCT00871468.
| Trial name or title | Does anterior‐inferior clavicle plating have a lower rate of soft tissue irritation compared to superior plating? A prospective randomized trial |
| Methods |
Study design: parallel randomised controlled trial Random sequence generation: not reported Allocation concealment: not reported Masking: double‐blind (just before surgery) |
| Participants |
Location: Texas, US Target sample size: 90 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention 1 (Anterior‐inferior plate):
Intervention 2 (Superior plate):
|
| Outcomes |
Outcomes: function or disability measured by: Constant score and DASH score; adverse events measured by: symptomatic hardware and hardware irritation requiring removal Timing of outcomes measurement: 24 months |
| Starting date |
Main ID: NCT00871468 Date of registration: 26 March 2009 Last refreshed on: 31 March 2014 Date of first enrolment: 1 October 2008 Status: active, not recruiting |
| Contact information |
Name: not reported Address: William Beaumont Army Medical Center ‐ El Paso, TX, US, 79920‐5001 Telephone: not reported Email: not reported Affiliation: William Beaumont Army Medical Center |
| Notes |
NCT01015924.
| Trial name or title | Intramedullary nailing compared with plate fixation of displaced mid‐clavicular fractures. A prospective randomized controlled trial |
| Methods |
Study design: parallel randomised controlled trial Random sequence generation: not reported Allocation concealment: not reported Masking: open label |
| Participants |
Location: Akershus, Norway Target sample size: 125 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention 1 (Intramedullary group):
Intervention 2 (Plate group):
|
| Outcomes |
Outcomes: function or disability measured by: Constant score and DASH score is used to evaluate the functional score at 6, 12, 26, and 52 weeks; adverse events measured by: wound infection or dehiscence and thoracic outlet syndrome; and health‐related quality of life measured by Short Form‐36 Timing of outcomes measurement: 12 months |
| Starting date |
Main ID: NCT01015924 Date of registration: 17 November 2009 Last refreshed on: 30 January 2014 Date of first enrolment: 1 July 2009 Status: completed |
| Contact information |
Name: Dr Stein Erik Utvag Address: Hendrik Frolich Fuglesang, University Hospital, Akershus, Norway Telephone: not reported Email: not reported Affiliation: University Hospital, Akershus |
| Notes |
Wijdicks 2011.
| Trial name or title | Rationale and design of the plate or pin (pop) study for dislocated midshaft clavicular fractures: study protocol for a randomised controlled trial |
| Methods |
Study design: parallel randomised multicentre controlled trial in 2 level 1 and 1 level 2 trauma centres Random sequence generation: performed by computerised bock randomisation Allocation concealment: not reported Masking: open label |
| Participants |
Location: 3 hospitals in The Netherlands, including Diakonessenhuis, Utrecht (level 2 trauma centre); Medisch Centrum Haaglanden, The Hague and St. Elisabeth Hospital, Tilburg (both level 1 trauma centres) Target sample size: 120 participants Inclusion criteria:
Exclusion criteria:
|
| Interventions |
Intervention 1 (Intramedullary group):
Intervention 2 (Plate group):
|
| Outcomes |
Primary outcomes: function or disability measured by: Constant score and DASH; pain score; re‐operation after unsatisfying result; complications; time to radiological consolidation; and cosmetic satisfaction Timing of outcomes measurement: participants will undergo clinical assessment at 2 and 6 weeks and 3, 6, and 12 months post operation |
| Starting date |
Main ID: NTR2438 Date of registration: 1 August 2010 Last refreshed on: 21 July 2014 Date of first enrolment: 1 January 2011 Status: completed |
| Contact information |
Name: Dr Verleisdonk Address: University Medical Center Utrecht (UMCU), P.O. Box 85500 , Heidelberglaan 100, 3584 CX, Utrecht, The Netherlands Telephone: +31 (0)30 2506968 Email: e.j.m.m.verleisdonk@umcutrecht.nl Affiliation: UDiakonessenhuis Utrecht, Department of Surgery |
| Notes |
DASH: Disability of the Arm, Shoulder and Hand questionnaire; LCP: locking compression plate.
Differences between protocol and review
There were no data available to carry out the subgroup analyses specified in the protocol, by age (adolescent, adult, or elderly), type of fracture (two fragments and more than two fragments), type of non‐union (hypervascular or avascular), mechanism of injury, or surgical experience.
In this update, we made the following changes from our published protocol.
We adjusted the outcomes to accord with our most current review on these fractures (Lenza 2013); see Types of outcome measures.
To search for ongoing and recently completed trials, we included the WHO International Clinical Trial Registry.
We assessed risk of bias and used the GRADE approach to assess the quality of evidence related to each of the key outcomes listed in the Types of outcome measures.
We listed three potential subgroups, should data become available for subgroup analysis in future.
Contributions of authors
The search strategy for the updated review was developed, in liaison with the Trials Search Co‐ordinator, by Mario Lenza and Flavio Faloppa.
Mario Lenza contacted the authors of eligible trials for additional information and entered data into Review Manager 5.
All authors performed trial selection, quality assessment, and data extraction.
All authors commented on and approved the final version of the review.
Mario Lenza is the guarantor of the review.
Sources of support
Internal sources
Universidade Federal de São Paulo, Brazil.
The University of Manchester, UK.
Hospital Israelita Albert Einstein, Brazil.
External sources
CAPES ‐ Coordenação de Aperfeiçoamento de Pessoal de Nível Superior, Brazil.
Declarations of interest
None known.
New search for studies and content updated (conclusions changed)
References
References to studies included in this review
Assobhi 2011 {published data only}
- Assobhi JE. Reconstruction plate versus minimal invasive retrograde titanium elastic nail fixation for displaced midclavicular fractures. Journal of Orthopaedics and Traumatology 2011;12(4):185‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ferran 2010 {published data only}
- Ferran NA, Hodgson P, Vannet N, Williams R, Evans RO. Locked intramedullary fixation vs plating for displaced and shortened mid‐shaft clavicle fractures: a randomized clinical trial. Journal of Shoulder and Elbow Surgery 2010;19(6):783‐9. [DOI] [PubMed] [Google Scholar]
Kabak 2004 {published data only}
- Kabak S, Halici M, Tuncel M, Avsarogullari L, Karaoglu S. Treatment of midclavicular nonunion: comparison of dynamic compression plating and low‐contact dynamic compression plating techniques. Journal of Shoulder and Elbow Surgery 2004;13(4):396‐403. [DOI] [PubMed] [Google Scholar]
Lee 2007 {published data only}
- Lee YS, Lin CC, Huang CR, Chen CN, Liao WY. Operative treatment of midclavicular fractures in 62 elderly patients: Knowles pin versus plate. Orthopedics 2007;30(11):959‐64. [DOI] [PubMed] [Google Scholar]
Shen 2008 {published and unpublished data}
- Shen JW. Personal communication November 2008.
- Shen JW, Tong PJ, Qu HB. A three‐dimensional reconstruction plate for displaced midshaft fractures of the clavicle. Journal of Bone and Joint Surgery ‐ British Volume 2008;90(11):1495‐8. [DOI] [PubMed] [Google Scholar]
Silva 2011 {published and unpublished data}
- Silva FBA. Reconstruction plate compared with flexible intramedullary nailing for midshaft clavicular fractures: a prospective, randomized clinical trial. clinicaltrials.gov/show/NCT01410032 (accessed 15 September 2014).
- Silva FBA, Kojima KE, Silva JS, Mattar Junior R. Comparison between plate and flexible intramedullary fixation in the treatment of midshaft clavicle fractures ‐ preliminary results [Comparação entre o uso de placas e o de hastes flexíveis para a osteossíntese de fraturas do terço médio da clavícula: resultados preliminares]. Revista Brasileira de Ortopedia 2011;46(Suppl 1):34‐9. [Google Scholar]
Tabatabaei 2011 {published and unpublished data}
- Tabatabaei S, Shalamzari S. To compare the results of internal fixation of midshaft clavicular fractures by pin and plate. apps.who.int/trialsearch/Trial2.aspx?TrialID=IRCT201105165920N3 (accessed 15 September 2014).
- Tabatabaei S, Shalamzari S. Treatment of displaced midshaft clavicular fractures: a comparison between smooth pin and LCDCP and reconstruction plate fixation. Pakistan Journal of Medical Sciences 2011;27(5):1129‐34. [Google Scholar]
References to studies excluded from this review
Böhme 2011 {published data only}
- Böhme J, Bonk A, Bacher GO, Wilharm A, Hoffmann R, Josten C. Current treatment concepts for mid‐shaft fractures of the clavicle ‐ results of a prospective multicentre study [Aktuelle behandlungskonzepte der klavikulaschaftfraktur ‐ ergebnisse einer prospektiven multicenterstudie]. Zeitschrift für Orthopädie und Unfallchirurgie 2011;149(1):68‐76. [DOI] [PubMed] [Google Scholar]
Cho 2010 {published data only}
- Cho CH, Song KS, Min BW, Bae KC, Lee KJ. Operative treatment of clavicle midshaft fractures: comparison between reconstruction plate and reconstruction locking compression plate. Clinics in Orthopedic Surgery 2010;2(3):154‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Flikweert 2009 {unpublished data only}
- Flikweert P, Olden G. Surgical treatment of clavicle fractures: a comparative study of intramedullary nailing and plate fixation. European Journal of Trauma and Emergency Surgery 2009;35(1):80. [Google Scholar]
Fu 2012 {published data only}
- Fu TH, Tan BL, Liu HC, Wang JW. Anatomical reduction for treatment of displaced midshaft clavicular fractures: Knowles pinning vs. reconstruction plating. Orthopedics 2012;35(1):e23‐30. [DOI] [PubMed] [Google Scholar]
Jiang 2012 {published data only}
- Jiang H, Qu W. Operative treatment of clavicle midshaft fractures using a locking compression plate: comparison between mini‐invasive plate osteosynthesis (MIPPO) technique and conventional open reduction. Orthopaedics & Traumatology, Surgery & Research 2012;98(6):666‐71. [DOI] [PubMed] [Google Scholar]
Jubel 2002 {published data only}
- Jubel A, Andermahr J, Schiffer G, Rehm KE. Technique of intramedullary osteosynthesis of the clavicle with elastic titanium nails [Die Technik der intramedullären Osteosynthese der Klavikula mit elastischen Titannägeln]. Der Unfallchirurg 2002;105(6):511‐6. [DOI] [PubMed] [Google Scholar]
Jubel 2005 {published data only}
- Jubel A, Andermahr J, Weisshaar G, Schiffer G, Prokop A, Rehm KE. Intramedullary nailing (ESIN) in clavicular pseudoarthroses. Results of a prospective clinical trial [Die intramedulläre Nagelung (ESIN) von Klavikulapseudar throsen. Ergebnisse einer prospektiven Anwendungsbeobach tung]. Der Unfallchirurg 2005;108(7):544‐50. [DOI] [PubMed] [Google Scholar]
Kraus 2013 {published and unpublished data}
- Kraus TM. Personal communication August 2014.
- Kraus TM, Martetschläger F, Schrödl C, Siebenlist S, Ganslmeier A, Kirchhoff C, et al. Elastic stable intramedullary nailing of clavicular midshaft fractures: comparison of open vs closed fracture reduction [Die elastisch stabile intramedulläre osteosynthese der diaphysären klavikulafraktur. Offene vs. geschlossene reposition]. Der Unfallchirurg 2013;116(2):104‐8. [DOI] [PubMed] [Google Scholar]
Lee 2008 {published data only}
- Lee YS, Huang HL, Lo TY, Hsieh YF, Huang CR. Surgical treatment of midclavicular fractures: a prospective comparison of Knowles pinning and plate fixation. International Orthopaedics 2008;32(4):541‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2010 {published data only}
- Liu HH, Chang CH, Chia WT, Chen CH, Tarng YW, Wong CY. Comparison of plates versus intramedullary nails for fixation of displaced midshaft clavicular fractures. Journal of Trauma 2010;69(6):E82‐7. [DOI] [PubMed] [Google Scholar]
Ma 2008 {published data only}
- Ma Y, Chen HX, Lin L, Chen WF, Cheng FP, Liang JB, et al. Clinical controlled trial on the treatment of comminuted clavicular fracture with acromioclavicular external fixator and clavicle anatomic DCP internal fixation. Zhongguo gu shang = China Journal of Orthopaedics and Traumatology 2008;21(7):494‐6. [PubMed] [Google Scholar]
NCT01311219 {unpublished data only}
- White RA. Study comparing intramedullary nailing, plate fixation, and non‐operative treatment of clavicle fractures. apps.who.int/trialsearch/Trial2.aspx?TrialID=NCT01311219 (accessed 19 December 2014).
NCT01405703 {unpublished data only}
- Kubiak E, Christensen T. Percutaneous versus open plate fixation of diaphyseal clavicle fractures. clinicaltrials.gov/show/NCT01405703 (accessed 15 September 2014).
Pai 2009 {published data only}
- Pai HT, Lee YS, Cheng CY. Surgical treatment of midclavicular fractures in the elderly: a comparison of locking and nonlocking plates. Orthopedics 2009;32(4):1‐7. [PubMed] [Google Scholar]
Ros Y Codorniu 2000 {published data only}
- Ros Y Codorniu AH, Fernandez Domingo A. Intramedullary nailing in clavicular fractures [El enclavijamiento intramedular en las fracturas de clavícula]. Revista de Ortopedia y Traumatologia 2000;44(5):429‐33. [Google Scholar]
Shen 1999 {published data only}
- Shen WJ, Liu TJ, Shen YS. Plate fixation of fresh displaced midshaft clavicle fractures. Injury 1999;30(7):497‐500. [DOI] [PubMed] [Google Scholar]
References to ongoing studies
ChiCTR‐TRC‐12001973 {unpublished data only}
- Yong Y. The placement of fixation plate in clavicular fracture. A randomized prospective study. apps.who.int/trialsearch/Trial2.aspx?TrialID=ChiCTR‐TRC‐12001973 (accessed 15 September 2014).
NCT00871468 {unpublished data only}
- United States Army Institute of Surgical Research. Does anterior‐inferior clavicle plating have a lower rate of soft tissue irritation compared to superior plating? A prospective randomized trial. clinicaltrials.gov/show/NCT00871468 (accessed 15 September 2014).
NCT01015924 {unpublished data only}
- Utvag SE. Intramedullary nailing compared with plate fixation of displaced mid‐clavicular fractures. A prospective randomized controlled trial. clinicaltrials.gov/show/NCT01015924 (accessed 15 September 2014).
Wijdicks 2011 {published data only}
- Verleisdonk EJMM. Surgical treatment of midshaft clavicular fractures with dislocation: Plate fixation versus intramedullary fixation. The Plate Or Pin study: "POP" study. apps.who.int/trialsearch/Trial2.aspx?TrialID=NTR2438 (accessed 15 September 2014).
- Wijdicks FJ, Houwert RM, Dijkgraaf MG, Lange DH, Meylaerts SA, Verhofstad MH, et al. Rationale and design of the plate or pin (POP) study for dislocated midshaft clavicular fractures: study protocol for a randomised controlled trial. Trials 2011;12:177. [DOI] [PMC free article] [PubMed] [Google Scholar]
Additional references
Ali Khan 1978
- Ali Khan MA, Lucas HK. Plating of fractures of the middle third of the clavicle. Injury 1978;9(4):263‐7. [DOI] [PubMed] [Google Scholar]
Allman 1967
- Allman FL Jr. Fractures and ligamentous injuries of the clavicle and its articulation. Journal of Bone and Joint Surgery ‐ American Volume 1967;49(4):774‐84. [PubMed] [Google Scholar]
Barlow 2013
- Barlow T, Beazley J, Barlow D. A systematic review of plate versus intramedullary fixation in the treatment of midshaft clavicle fractures. Scottish Medical Journal 2013;58(3):163‐7. [DOI] [PubMed] [Google Scholar]
Barlow 2014
- Barlow T, Upadhyay P, Barlow D. External fixators in the treatment of midshaft clavicle non‐unions: a systematic review. European Journal of Orthopaedic Surgery and Traumatology 2014;24(2):143‐8. [DOI] [PubMed] [Google Scholar]
Bradbury 1996
- Bradbury N, Hutchinson J, Hahn D, Colton CL. Clavicular nonunion. 31/32 healed after plate fixation and bone grafting. Acta Orthopaedica Scandinavica 1996;67(4):367‐70. [DOI] [PubMed] [Google Scholar]
Böstman 1997
- Böstman O, Manninen M, Pihlajamäki H. Complications of plate fixation in fresh displaced midclavicular fractures. Journal of Trauma ‐ Injury, Infection and Critical Care 1997;43(5):778‐83. [DOI] [PubMed] [Google Scholar]
Canadian 2007
- Canadian Orthopaedic Trauma Society. Nonoperative treatment compared with plate fixation of displaced midshaft clavicular fractures. A multicenter, randomized clinical trial. Journal of Bone and Joint Surgery ‐ American Volume 2007;89(1):1‐10. [DOI] [PubMed] [Google Scholar]
Chen 2013
- Chen H, Zhang JH, He ZL. Elastic stable intramedullary nailing versus plate fixation for midshaft clavicular fractures: a meta‐analysis [弹性髓内钉固定与钢板固定治疗锁骨中段骨折的Meta分析]. Zhongguo Zuzhi Gongcheng Yanjiu 2013;17(13):2407‐14. [Google Scholar]
Constant 1987
- Constant CR, Murley AH. A clinical method of functional assessment of the shoulder. Clinical Orthopaedics and Related Research 1987;(214):160‐4. [PubMed] [Google Scholar]
Court‐Brown 2006
- Court‐Brown CM, Caesar B. Epidemiology of adult fractures: a review. Injury 2006;37(8):691‐7. [DOI] [PubMed] [Google Scholar]
Dawson 1996
- Dawson J, Fitzpatrick R, Carr A. Questionnaire on the perceptions of patients about shoulder surgery. Journal of Bone and Joint Surgery ‐ British Volume 1996;78(4):593‐600. [PubMed] [Google Scholar]
Deeks 2011
- Deeks JJ, Higgins JPT, Altman DG. Chapter 9: Analysing data and undertaking meta‐analyses. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Donnelly 2013
- Donnelly TD, Macfarlane RJ, Nagy MT, Ralte P, Waseem M. Fractures of the clavicle: an overview. Open Orthopaedics Journal 2013;7:329‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
Duan 2011
- Duan X, Zhong G, Cen S, Huang F, Xiang Z. Plating versus intramedullary pin or conservative treatment for midshaft fracture of clavicle: a meta‐analysis of randomized controlled trials. Journal of Shoulder Elbow Surgery 2011;20(6):1008‐15. [DOI] [PubMed] [Google Scholar]
Ebraheim 1997
- Ebraheim NA, Mekhail AO, Darwich M. Open reduction and internal fixation with bone grafting of clavicular nonunion. Journal of Trauma ‐ Injury, Infection and Critical Care 1997;42(4):701‐4. [DOI] [PubMed] [Google Scholar]
Eccleston 2010
- Eccleston C, Moore RA, Derry S, Bell RF, McQuay H. Improving the quality and reporting of systematic reviews. European Journal of Pain 2010;14(7):667‐9. [DOI] [PubMed] [Google Scholar]
EuroQol Group 1990
- EuroQol Group. EuroQol ‐ a new facility for the measurement of health‐related quality of life. Health Policy 1990;16(3):199‐208. [DOI] [PubMed] [Google Scholar]
Gummesson 2003
- Gummesson C, Atroshi I, Ekdahl C. The Disabilities of the Arm, Shoulder and Hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self‐rated health change after surgery. BMC Musculoskeletal Disorders 2003;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Altman DG, Sterne JAC. Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hill 1997
- Hill JM, McGuire MH, Crosby LA. Closed treatment of displaced middle‐third fractures of the clavicle gives poor results. Journal of Bone and Joint Surgery ‐ British Volume 1997;79(4):537‐9. [DOI] [PubMed] [Google Scholar]
Houwert 2012
- Houwert RM, Wijdicks FJ, Steins Bisschop C, Verleisdonk EJ, Kruyt M. Plate fixation versus intramedullary fixation for displaced mid‐shaft clavicle fractures: a systematic review. International Orthopaedics 2012;36(3):579‐85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hudak 1996
- Hudak PL, Amadio PC, Bombardier C. Development of an upper extremity outcome measure: the DASH (Disabilities of the Arm, Shoulder and Dand) [corrected]. The Upper Extremity Collaborative Group (UECG). American Journal of Industrial Medicine 1996;29(6):602‐8. [DOI] [PubMed] [Google Scholar]
Jeray 2007
- Jeray KJ. Acute midshaft clavicular fracture. Journal of the American Academy of Orthopaedic Surgeons 2007;15(4):239‐48. [DOI] [PubMed] [Google Scholar]
Johnson 1998
- Johnson JA, Coons SJ, Ergo A, Szava‐Kovats G. Valuation of EuroQOL (EQ‐5D) health states in an adult US sample. PharmacoEconomics 1998;13(4):421‐33. [DOI] [PubMed] [Google Scholar]
Jupiter 1987
- Jupiter JB, Leffert RD. Non‐union of the clavicle. Associated complications and surgical management. Journal of Bone and Joint Surgery ‐ American Volume 1987;69(5):753‐60. [PubMed] [Google Scholar]
Khan 2008
- Khan SA, Shamshery P, Gupta V, Trikha V, Varshney MK, Kumar A. Locking compression plate in long standing clavicular nonunions with poor bone stock. Journal of Trauma ‐ Injury, Infection and Critical Care 2008;64(2):439‐41. [DOI] [PubMed] [Google Scholar]
Khan 2009
- Khan LA, Bradnock TJ, Scott C, Robinson CM. Fractures of the clavicle. Journal of Bone and Joint Surgery ‐ American Volume 2009;91(2):447‐60. [DOI] [PubMed] [Google Scholar]
King 2015
- King PR, Ikram A, Lamberts RP. The treatment of clavicular shaft fractures with an innovative locked intramedullary device. Journal of Shoulder and Elbow Surgery 2015;24(1):e1‐6. [DOI] [PubMed] [Google Scholar]
Kotelnicki 2006
- Kotelnicki JJ, Bote HO, Mitts KG. The management of clavicle fractures. Journal of the American Academy of Physician Assistants 2006;19(9):50, 53‐4, 56. [DOI] [PubMed] [Google Scholar]
Kukkonen 2013
- Kukkonen J, Kauko T, Vahlberg T, Joukainen A, Aärimaa V. Investigating minimal clinically important difference for Constant score in patients undergoing rotator cuff surgery. Journal of Shoulder and Elbow Surgery 2013;22(12):1650‐5. [DOI] [PubMed] [Google Scholar]
LaVelle 2003
- LaVelle DG. Delayed union and nonunion of fractures. In: Canale ST editor(s). Campbell's Operative Orthopaedics. 10th Edition. Philadelphia: Mosby, 2003:3125‐65. [Google Scholar]
Lazarus 2001
- Lazarus MD. Fractures of the clavicle. In: Bucholz RW, Heckman JD editor(s). Rockwood & Greens Fractures in Adults. 5th Edition. Philadelphia: Lippincott‐Wilkins, 2001:1041‐78. [Google Scholar]
Lefebvre 2011
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Lenza 2013
- Lenza M, Buchbinder R, Johnston RV, Belloti JC, Faloppa F. Surgical versus conservative interventions for treating fractures of the middle third of the clavicle. Cochrane Database of Systematic Reviews 2013, Issue 6. [DOI: 10.1002/14651858.CD009363.pub2] [DOI] [PubMed] [Google Scholar]
Lenza 2014
- Lenza M, Belloti JC, Andriolo RB, Faloppa F. Conservative interventions for treating middle third clavicle fractures in adolescents and adults. Cochrane Database of Systematic Reviews 2014, Issue 5. [DOI: 10.1002/14651858.CD007121.pub3] [DOI] [PubMed] [Google Scholar]
Lyons 1990
- Lyons FA, Rockwood CA Jr. Migration of pins used in operations on the shoulder. Journal of Bone and Joint Surgery ‐ American Volume 1990;72(8):1262‐7. [PubMed] [Google Scholar]
Manske 1985
- Manske DJ, Szabo RM. The operative treatment of mid‐shaft clavicular non‐unions. Journal of Bone and Joint Surgery ‐ American Volume 1985;67(9):1367‐71. [PubMed] [Google Scholar]
Marti 2003
- Marti RK, Nolte PA, Kerkhoffs GM, Besselaar PP, Schaap GR. Operative treatment of mid‐shaft clavicular non‐union. International Orthopaedics 2003;27(3):131‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
McKee 2006
- McKee MD, Pedersen EM, Jones C, Stephen DJ, Kreder HJ, Schemitsch EH, et al. Deficits following nonoperative treatment of displaced midshaft clavicular fractures. Journal of Bone and Joint Surgery ‐ American Volume 2006;88(1):35‐40. [DOI] [PubMed] [Google Scholar]
Meier 2006
- Meier C, Grueninger P, Platz A. Elastic stable intramedullary nailing for midclavicular fractures in athletes: indications, technical pitfalls and early results. Acta Orthopaedica Belgica 2006;72(3):269‐75. [PubMed] [Google Scholar]
Moore 2010
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. "Evidence" in chronic pain ‐ establishing best practice in the reporting of systematic reviews. Pain 2010;150(3):386‐9. [DOI] [PubMed] [Google Scholar]
Mullaji 1994
- Mullaji AB, Jupiter JB. Low‐contact dynamic compression plating of the clavicle. Injury 1994;25(1):41‐5. [DOI] [PubMed] [Google Scholar]
Murray 2013
- Murray IR, Foster CJ, Eros A, Robinson CM. Risk factors for nonunion after nonoperative treatment of displaced midshaft fractures of the clavicle. Journal of Bone and Joint Surgery ‐ American Volume 2013;95(13):1153‐8. [DOI] [PubMed] [Google Scholar]
Neer 1984
- Neer C. Fractures of the clavicle. In: Rockwood CA Jr, Green DP editor(s). Fractures in Adults. 2nd Edition. Philadelphia: Lippincott Williams & Wilkins, 1984:707‐13. [Google Scholar]
Nordqvist 1994
- Nordqvist A, Petersson C. The incidence of fractures of the clavicle. Clinical Orthopaedics and Related Research 1994;(300):127‐32. [PubMed] [Google Scholar]
Nordqvist 1998
- Nordqvist A, Petersson CJ, Redlund‐Johnell I. Mid‐clavicle fractures in adults: end result study after conservative treatment. Journal of Orthopaedic Trauma 1998;12(8):572‐6. [DOI] [PubMed] [Google Scholar]
Nowak 2000
- Nowak J, Mallmin H, Larsson S. The aetiology and epidemiology of clavicular fractures. A prospective study during a two‐year period in Uppsala, Sweden. Injury 2000;31(5):353‐8. [DOI] [PubMed] [Google Scholar]
Poigenfürst 1992
- Poigenfürst J, Rappold G, Fischer W. Plating of fresh clavicular fractures: results of 122 operations. Injury 1992;23(4):237‐41. [DOI] [PubMed] [Google Scholar]
Postacchini 2002
- Postacchini F, Gumina S, Santis P, Albo F. Epidemiology of clavicle fractures. Journal of Shoulder and Elbow Surgery 2002;11(5):452‐6. [DOI] [PubMed] [Google Scholar]
Pyper 1978
- Pyper JB. Non‐union of fractures of the clavicle. Injury 1978;9(4):268‐70. [DOI] [PubMed] [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.2. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2012.
Robinson 1998
- Robinson CM. Fractures of the clavicle in the adult. Epidemiology and classification. Journal of Bone and Joint Surgery ‐ British Volume 1998;80(3):476‐84. [DOI] [PubMed] [Google Scholar]
Robinson 2004
- Robinson CM, Court‐Brown CM, McQueen MM, Wakefield AE. Estimating the risk of nonunion following nonoperative treatment of a clavicular fracture. Journal of Bone and Joint Surgery ‐ American Volume 2004;86(7):1359‐65. [DOI] [PubMed] [Google Scholar]
Schunemann 2011
- Schunemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glaziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Strauss 2007
- Strauss EJ, Egol KA, France MA, Koval KJ, Zuckerman JD. Complications of intramedullary Hagie pin fixation for acute midshaft clavicle fractures. Journal of Shoulder and Elbow Surgery 2007;16(3):280‐4. [DOI] [PubMed] [Google Scholar]
Sökücü 2014
- Sökücü S, Menges Ö, Cetinkaya E, Parmaksızoğlu A, Kabukçuoğlu Y. Treatment of comminuted mid‐diaphyseal clavicle fractures by plate fixation using a bridging technique. Acta Orthopaedica et Traumatologica Turcica 2014;48(4):401‐5. [DOI] [PubMed] [Google Scholar]
Van Kampen 2013
- Kampen DA, Willems WJ, Beers LW, Castelein RM, Scholtes VA, Terwee CB. Determination and comparison of the smallest detectable change (SDC) and the minimal important change (MIC) of four‐shoulder patient‐reported outcome measures (PROMs). Journal of Orthopaedic Surgery and Research 2013;8(40):1‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Verborgt 2005
- Verborgt O, Pittoors K, Glabbeek F, Declercq G, Nuyts R, Somville J. Plate fixation of middle‐third fractures of the clavicle in the semi‐professional athlete. Acta Orthopaedica Belgica 2005;71(1):17‐21. [PubMed] [Google Scholar]
Ware 1992
- Ware JE Jr, Sherbourne CD. The MOS 36‐item short‐form health survey (SF‐36). I. Conceptual framework and item selection. Medical Care 1992;30(6):473‐83. [PubMed] [Google Scholar]
Wijdicks 2012
- Wijdicks FJ, Meijden OA, Millett PJ, Verleisdonk EJ, Houwert RM. Systematic review of the complications of plate fixation of clavicle fractures. Archives of Orthopaedic and Trauma Surgery 2012;132(5):617‐25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wijdicks 2013
- Wijdicks FJ, Houwert RM, Millett PJ, Verleisdonk EJ, Meijden OA. Systematic review of complications after intramedullary fixation for displaced midshaft clavicle fractures. Canadian Journal of Surgery 2013;56(1):58‐64. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wilkins 1983
- Wilkins RM, Johnston RM. Ununited fractures of the clavicle. Journal of Bone and Joint Surgery ‐ American Volume 1983;65(6):773‐8. [PubMed] [Google Scholar]
Wu 1998
- Wu CC, Shih CH, Chen WJ, Tai CL. Treatment of clavicular aseptic nonunion: comparison of plating and intramedullary nailing techniques. Journal of Trauma ‐ Injury, Infection and Critical Care 1998;45(3):512‐6. [DOI] [PubMed] [Google Scholar]
Yian 2005
- Yian EH, Ramappa AJ, Arneberg O, Gerber C. The Constant score in normal shoulders. Journal of Shoulder and Elbow Surgery 2005;14(2):128‐33. [DOI] [PubMed] [Google Scholar]
Zlowodzki 2005
- Zlowodzki M, Zelle BA, Cole PA, Jeray K, McKee MD. Treatment of acute midshaft clavicle fractures: systematic review of 2144 fractures: on behalf of the Evidence‐Based Orthopaedic Trauma Working Group. Journal of Orthopaedic Trauma 2005;19(7):504‐7. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Lenza 2008
- Lenza M, Belloti JC, Gomes dos Santos JB, Matsumoto MH, Faloppa F. Surgical interventions for treating acute fractures or non‐union of the middle third of the clavicle [Protocol]. Cochrane Database of Systematic Reviews 2008, Issue 4. [DOI: 10.1002/14651858.CD007428] [DOI] [PubMed] [Google Scholar]
Lenza 2009
- Lenza M, Belloti JC, Gomes dos Santos JB, Matsumoto MH, Faloppa F. Surgical interventions for treating acute fractures or non‐union of the middle third of the clavicle. Cochrane Database of Systematic Reviews 2009, Issue 4. [DOI: 10.1002/14651858.CD007428.pub2] [DOI] [PubMed] [Google Scholar]


