Abstract
The COVID-19 pandemic has caused hundreds million cases and millions death as well as continues to infect human life in the world since late of 2019. The breakthrough infection caused from mutation of SARS-CoV-2 is rising even the vaccinated population has been increasing. Currently, the severe threat posed by SARS-CoV-2 has been alleviated worldwide, and the situation has transitioned to coexisting with the virus. The dietary food with antiviral activities may improve to prevent virus infection for living with COVID-19 pandemic. Teas containing enriched phenolic ingredients such as tannins have been reported to be antitumor agents as well as be good inhibitors for coronavirus. This study developed a highly sensitive and selective ultra-high performance liquid chromatography-high resolution mass spectrometric method for quantification of tannic acids, a hydrolysable tannin, and proanthocyanidins, a condense tannin, in teas with different levels of fermentation. The in vitro pseudoviral particles (Vpp) infection assay was used to evaluate the inhibition activities of various teas. The results of current research demonstrate that the tannins in teas are effective inhibitors against infection of SARS-CoV-2 and its variants.
Keywords: SARS-CoV-2, tea, proanthocyanidin, tannic acid, UHPLC-HRMS
Introduction
In December 2019, Coronavirus Disease 2019 (COVID-19) caused by Severe Acute Respiratory Syndrome Coronavirus Type 2 (SARS-CoV-2) was first reported in Wuhan city of China [1,2]. Since that, the COVID-19 pandemic has become a significant issue because the pandemic impacts human health all the world. Coronavirus is one group of viruses that causes respiratory infections in humans and animals [3]. SARS-CoV-2, an enveloped single-stranded RNA virus with four structural proteins including spike (S), membrane (M), nucleocapsid (N), and envelope (E) protein, was reported to bind to the angiotensin-converting enzyme 2 (ACE2) of the host cell and further trigger by the transmembrane protease serine 2 (TMPRSS2) [4,5]. Several vaccines designed based on the wile-type SARS-CoV-2 have been successfully used to inhibit the COVID-19 pandemic. However, the antiviral immunity ability of developed vaccines decreases rapidly due to the mutation of SARS-CoV-2, which consequently leads to the breakthrough cases happen in the fully vaccinated population [6]. Until 2024, COVID-19 pandemic has caused more than 770 million cases and 7 million deaths worldwide. Due to high transmission and infection rate, SARS-CoV-2 variants alpha strain (B.1.1.7), beta strain (B.1.351), gamma strain (B.1.1.28.1), delta strain (B.1.617.2), and omicron strain (B.1.1.529) have been declared as the major global variants of concern (VOC) by the World Health Organization (WHO) [7-9]. Omicron variant is the predominant variant in the world currently, the most common sublineages XBB.1.5, XBB.1.16, EG.5, BA.2.86 and JN.1 with high immune evasion properties are tracked by the WHO as variants of interest (VOI) [10,11]. Apart from the development of next-generation vaccines, food intake with antiviral activity may improve protection against SARS-CoV-2 infection.
Tea (Camellia sinensis L.), one of the most popular non-alcoholic beverage in the world for more than thousand years, is mainly classified into non-fermented (green tea), semi-fermented (Oolong tea), and fully-fermented (black tea) based on the degree of fermentation. Taiwan is a prime tea-growing region due to the favourable climate, soil, and geography, producing a wide variety of teas through processions involving different levels of fermentation such as Sun-Link-Sea High-Mountain tea (15-25% fermentation degree), Tongding Oolong tea (25-30% fermentation degree), Oriental Beauty tea (50-60% fermentation degree), and Ruby black tea (> 95% fermentation degree). The different levels of fermentation significantly affect the active polyphenols in tea, impacting both its flavour profile and health benefits [12]. The polyphenolic compounds are well characterized with the strong antioxidant and antitumor activities [13,14]. For instance, epigallocatechin-3-gallate (EGCG), a primary active ingredient in green tea, potentially prevents colorectal cancer development through AKT, VEGFR, and STAT3 inhibition [15]. Tannic acids (TAs) attenuate NF-κB-mediated stemness property in breast cancer [16]. Proanthocyanidins (PACs) block β-catenin activation in melanoma to inhibit tumor migration and metastasis [17]. Therefore, tea consumption has been associated with cancer prevention and cancer survival [18,19], although inconsistent results were also reported [20,21].
Emerging evidence indicates that the polyphenolic compounds in tea including EGCG, TAs, and PACs are associated with anti-SARS-CoV-2 activities. EGCG can inhibit the activity of the main protease (MPro) in SARS-CoV-2 and suppress virus replication in vitro [22,23]. TAs function as a potent dual inhibition for TMPRSS2 and MPro of SARS-CoV-2 [24,25]. PACs suppress SARS-CoV-2 spike protein binding to ACE2 as well as inhibiting ACE2 and MPro activity [26,27]. Additionally, both TAs and PACs show efficacy against SARS-CoV-2 infection in the clinical evaluation [28]. Several studies described the correlation between tea consumption and low COVID-19 morbidity and mortality [29-31], which led to our hypothesis that different ratios of TAs and PACs in tea could impact the effect of anti-SARS-CoV-2 infection. In this study, we characterized TAs and PACs in teas with different levels of fermentation by an ultra-high performance liquid chromatography-high resolution mass spectrometry (UHPLC-HRMS) method. Additionally, a pseudoviral particle (Vpp) functional assay was conducted to verify the effects of TAs and PAC on the infectious inhibition of SARS-CoV-2 and its variants.
Materials and methods
Chemicals and reagents
Tannic acid (USP puriss), purified grape seeds oligomeric proanthocyanidins (USP reference standard), and formic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). HPLC grade acetonitrile and ethyl acetate were obtained from J.T. Baker (Radnor, PA, USA) and Honeywell (Charlotte, NC, USA). Double distilled water (R > 18.2 MΩ) was made from Milli-Q water purified system (Millipore ADVANTAGE, Millipore, Burlington, MA, USA). Tea samples with different fermentation process were purchased from local tea dealer in Taiwan, including Sun-Link-Sea High-Mountain tea, Lishan High-Mountain tea, and Alishan High-Mountain tea (15-20% fermentation degree), Paochong tea (15-30% fermentation degree), Tongding Oolong tea (25-30% fermentation degree), Jhinhsuan tea (25-30% fermentation degree), Tieh-Kuan-Yin tea (40% fermentation degree), Oriental Beauty tea (50-60% fermentation degree), Red Oolong tea (70-80% fermentation degree), and Ruby black tea (> 95% fermentation degree).
Tea preparation and ethyl acetate processing
Tea infusion was prepared by adding 1 g of tea sample to 18 mL of boiling water [32]. After shaking for 6 min, the tea brew was filtered through syringe filter with 0.22 µm polyvinylidene difluoride (PVDF) membrane (Paul, Port Washington, NY, USA) and then analysed by UHPLC-HRMS. In this study, ethyl acetate was used as extraction solvent for removing the tannins in tea brew [33]. An aliquot of 0.5 mL ethyl acetate was added into 1 mL of filtered tea infusion and then the mixture was vigorously shaken for 1 min. After centrifuging for 2 min, the ethyl acetate layer was discarded by micropipette. This procession was carried out in duplicate and the water layer was analysed by UHPLC-HRMS.
UHPLC-HRMS analysis
The UHPLC separation was performed on an Ultimate 3000 UHPLC system equipped with pump, degasser, autosampler, and column oven (Thermo Scientific, San Jose, CA, USA). The components in teas were separated by Waters ACQUITY UPLC C18 column (2.1 × 100 mm, 1.7 µm, Waters, Milford, MA, USA). The deionized water containing 0.1% formic acid and acetonitrile served as mobile phase A and B, respectively. For quantification of TAs and PACs, the chromatographic gradient was 0-1 min, hold at 5% B; 1-15 min, from 5% B to 40% B; 15-16 min, from 40% B to 80% B; 16-21 min, hold at 80% B. For identification of phytochemicals in tea, the separating gradient was 0-1 min, hold at 2% B; 1-25 min, from 2% B to 30% B; 25-30 min, from 30% B to 95% B; 30-40 min, hold at 95% B. The flow rate was set at 0.4 mL/min and the injection volume was 5 µL. The column and sample temperature were set as 25°C and 5°C, respectively. The HRMS detection was performed on a Thermo Scientific Q-Exactive Plus high-resolution mass spectrometer with heated-electrospray ionization (HESI) ion source. The compounds of LC eluent were ionized by both positive and negative ionization mode with the spray voltage of 4.0 kV. The full scan mode of HRMS with range of m/z 150-2000 under the mass resolution of 70 000 was used detection.
HPLC fractionation
The Agilent 1260 Infinity II HPLC system (Agilent, Santa Clara, CA, USA) was used for fractionation of tea in this study. The separation was performed by the Agilent Poroshell C18 column (4.6 × 150 mm, 4 μm, Agilent, Santa Clara, CA, USA) and the mobile phase A and B were 0.1% formic acid aqueous solution and acetonitrile, respectively. The chromatographic gradient was the same as the gradient used for identification of phytochemicals described in UHPLC-HRMS section. The flow rate was 0.7 mL/min and the injection volume was 100 μL. The wavelength of 254 nm was utilized for detection of phytochemical in tea. The collected fraction was freeze-dried, and subsequently reconstituted in equal volumes of water for further Vpp assay.
Pseudoviral particles (Vpp) infection assay
The wile-type and VOC variants of SARS-CoV-2 pseudoviral particles were purchased from the RNAi core of the Academia Sinica in Taiwan (http://rnai.genmed.sinica.edu.tw/). The HEK 293T cell line that was expressed recombinant human ACE2 (HEK293T/hACE2) and maintained in Dulbecco’s Modified Eagle Medium (DMEM) containing 100 U/mL penicillin, 100 μg streptomycin, and 10% fetal bovine serum. The HEK293T/hACE2 cells were seeded into 96-well plates then pretreated with different doses of test tea samples for 1 h. After dosing tea samples, the cells were inoculated with 50 μL of normal media containing the pseudovirions (MOI=0.1, multiplicity of infection). After incubation for 24 h, cell viability assay was carried out by the Cell Counting Kit-8 (CCK-8) assay kit (ab228554, Cambridge, UK). In assay procedure, each sample was mixed with 100 μL luciferase substrate (Bright-Glo Luciferase Assay System, Promega, Madison, WI, USA), and luminescence was immediately detected by the GloMax Navigator System (Promega). Viability-normalized relative light unit (RLU) was set as 100% for the control group assay, and the relative infection rate of the treated group assays was calculated. All experiments were performed in triplicate and repeated three times.
Correlation analysis between annual per capita consumption of tea and COVID-19-related morbidity/mortality
The information of annual per capita tea consumption of different countries was obtained from the report of “Annual per capita tea consumption worldwide as of 2016, by leading countries (in pounds)” by the Statista website (https://www.statista.com/aboutus/our-research-commitment). Information about COVID-19 morbidity (expressed as cases per population) and mortality (expressed as deaths per cases) were obtained from the Worldometer website (worldometers.info/Coronavirus) in the end of October 2011 and in the end of April 2022, respectively. The number of cases and deaths in every nation is based on official daily reports and regarded as reliable.
Results
Annual per capita consumption of tea is reversely correlated with COVID-19-related mortality
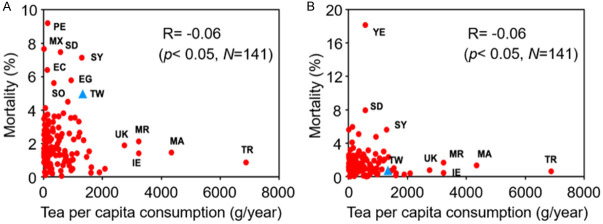
The history of tea spreads across multiple cultures for thousands of years and the habit of drinking tea have become a part of life in people all over the world. Teas contain polyphenolic compounds that have been identified with multiple functions including an antiviral activity [22-28]. Accordingly, we wondered whether tea as a dietary supplement could enhance protection against SARS-CoV-2 infection. To verify the potential of tea, we determined the correlation between annual per capita consumption of tea and the COVID-19-related morbidity/mortality by integrating the epidemiological statistical data reported in the Statista and Worldometer websites [34-37]. Tea consumption was not significantly associated with risk of SARS-CoV-2 morbidity in this analysis (data not shown). Surprisingly, the epidemiological statistics revealed that annual per capita consumption of tea was reversely correlated with COVID-19-related mortality no matter the original SARS-CoV-2 (Figure 1A) or its omicron variant (Figure 1B) infection. This result suggested that the components of tea could protect cells against SARS-CoV-2 infection and proliferation.
Figure 1.
Annual per capita consumption of tea is reversely correlated with COVID-19-related mortality. The association between annual per capita consumption of tea and COVID-19-related mortality in individual country was analyzed before omicron infection (A) and after omicron infection (B). EC: Ecuador; EG: Egypt; IE: Republic of Ireland; MA: Morocco; MR: Mauritania; MX: Mexico; PE: Peru; SC: Seychelles; SD: Sudan; SO: Somalia; SY: Syria; TR: Turkey; TW: Taiwan; UK: United Kingdom; YE: Yemen.
Characterization of TAs and PACs in teas by UHPLC-HRMS
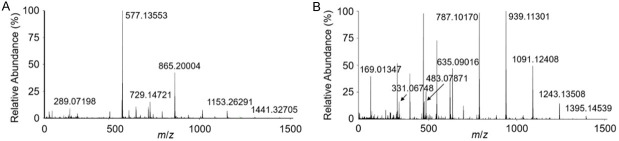
Our previous studies showed the inhibitory effects of TAs and PACs on TMPRSS2 and MPro of SARS-CoV-2 including human trial data [23,28,38]. To evaluate whether TAs and PACs in teas impact SARS-CoV-2 infection, we determined their levels using a MS-based quantitative strategy. Firstly, we characterized the MS profiles of TA and PAC standard compounds using HESI-HRMS. The solutions of TA and PAC standard compounds in the concentration of 50 µg/mL were directly injected into HESI-HRMS by a syringe pump. PACs are oligomeric flavonoids, which are usually composed of catechins and epicatechins and their gallic acid esters. As suspected, the full mass spectrum of PAC standard compounds showed multiple oligomer compositions, including the deprotonated molecules ([M-H]-) of monomer (catechin and epicatechin, MW=290.07), dimer (procyanidin B, MW=578.13 and procyanidin-O-gallate, MW=730.14), trimer (procyanidin C, MW=866.19), tetramer (MW=1154.26), and pentamer (MW=1442.32) (Figure 2A). TAs are usually composed of hydrolyzed gallic acid and polygalloyl glucose derivatives. The full scan profile of MS analysis revealed that TA standard compounds contained gallic acid (GA, MW=170.01), monogalloylglucose (1GG, MW=332.06), digalloylglucose (2GG, MW=484.07), trigalloylglucose (3GG, MW=636.09), tetragalloylglucose (4GG, MW=788.10), pentagalloylglucose (5GG, MW=940.11), hexagalloylglucose (6GG, MW=1092.12), heptagalloylglucose (7GG, MW=1244.13), and octagalloylglucose (8GG, MW=1396.14) (Figure 2B). The information of chemical formula, experimental mass-to-charge (m/z) values, theoretical m/z value, and mass tolerance for TAs and OPCs detection were summarized in Table 1. The HRMS analysis provided accurate MS determination and the mass tolerance less than 3 ppm for individual compound (Table 1), suggesting that it could serve as a suitable analytic method to precisely quantify the TA and OPC levels in the complexes of tea extracts.
Figure 2.
Full mass spectra of TAs and PACs in the standard sample. The compositions of the oligomeric PACs (A) and TAs (B) standard samples (50 μg/mL) were analyzed by HRMS using negative ionization mode.
Table 1.
The component analysis of tannic acids and proanthocyanidins by HRMS
| Compound | Chemical formula | Theoretical value (m/z) | Experimental value (m/z) | Mass tolerance (ppm) |
|---|---|---|---|---|
| Tannic acids | ||||
| Gallic acid (GA) | C7H6O5 | 169.01370 | 169.01347 | -1.36 |
| Galloylglucose (1GG) | C13H16O10 | 331.06652 | 331.06748 | 2.90 |
| Digalloylglucose (2GG) | C20H20O14 | 483.07748 | 483.07871 | 2.55 |
| Trigalloylglucose (3GG) | C27H24O18 | 635.08844 | 635.09016 | 2.71 |
| Tetragalloylglucose (4GG) | C34H28O22 | 787.09940 | 787.10170 | 2.92 |
| Pentagalloylglucose (5GG) | C41H32O26 | 939.11036 | 939.11301 | 2.82 |
| Hexagalloylglucose (6GG) | C48H36O30 | 1091.12132 | 1091.12408 | 2.52 |
| Heptagalloylglucose (7GG) | C55H40O34 | 1243.13228 | 1243.13508 | 2.25 |
| Octagalloylglucose (8GG) | C62H44O38 | 1395.14324 | 1395.14539 | 1.54 |
| Proanthocyanidins | ||||
| Catechin or Epicatechin | C15H14O6 | 289.07121 | 289.07198 | 2.66 |
| Procyanidin B | C30H26O12 | 577.13460 | 577.13553 | 1.61 |
| Procyanidin B-O-gallate | C37H30O16 | 729.14556 | 729.14721 | 2.26 |
| Procyanidin C | C45H38O18 | 865.19799 | 865.20004 | 2.37 |
| Tetramer | C60H50O24 | 1153.26138 | 1153.26291 | 1.33 |
| Pentamer | C75H62O30 | 1441.32477 | 1441.32705 | 1.58 |
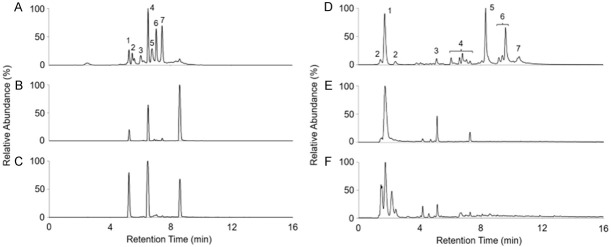
Due to precise MS detection, the total ion chromatogram (TIC) profiles of the PAC and TA standard samples can be well resolved by UHPLC-HRMS (Figure 3A and 3D). Based on retention time and molecular weight of individual compound, the PAC and TA contents in tea brew samples can be determined by comparing with the standard compounds under the same analytic condition. The mass characteristic signals representing the component of PACs in Oriental Beauty tea and Sun-Link-Sea High-Mountain tea were showed in Figure 3B and 3C, respectively. Additionally, the mass characteristic signals representing the component of TAs in Oriental Beauty tea and Sun-Link-Sea High-Mountain tea were showed in Figure 3E and 3F, respectively. The mass intensity of individual compound in the PAC and TA standard samples was used to represent its amounts, the sum of total intensity of MS signals in various doses of standard samples was used to establish the calibration curve in this study. According to the corresponding MS intensities, we obtained perfectly linear calibration curves in the range of 0.5-100 μg/mL with R2 value 0.9989 and 0.9987 for PACs and TAs, respectively. We then calculated the levels of PACs and TAs in every tea extracts based on both calibration curves. The concentrations of PACs and TAs in the tea samples ranged 1.1-14.1 mg/g and 0-4.8 mg/g, respectively (Figure 4). Teas with a lower degree of fermentation contained higher levels of PACs, while those with a higher degree of fermentation contained more TAs (Figure 4), which was consistent with the previous findings [39,40]. Sun-Link-Sea High-Mountain tea exhibited the highest levels of PACs, while Oriental Beauty tea had the highest levels of TAs in the current study. Accordingly, we chose both of them for the subsequent functional validation.
Figure 3.
Total ion chromatograms of PACs and TAs in the standard samples and teas. (A) The TIC profile of PACs standard sample (25 μg/mL) in UHPLC-HRMS analysis, the indicated peaks representing: catechin (1), procyanidin C (2 and 6), procyanidin (3), epicatechin (4), and procyanidin B-gallate (5 and 7). The corresponding PAC signals in Oriental Beauty tea (B) and Sun-Link-Sea High-Mountain tea (C). (D) The TIC profile of TAs standard sample (25 μg/mL) in UHPLC-HRMS analysis, the indicated peaks representing: GA (1), 1GG (2), 2GG (3), 3GG (4), 4GG (5), 5GG (6), and 6GG (7). The corresponding TA signals in Oriental Beauty tea (E) and Sun-Link-Sea High-Mountain tea (F).
Figure 4.
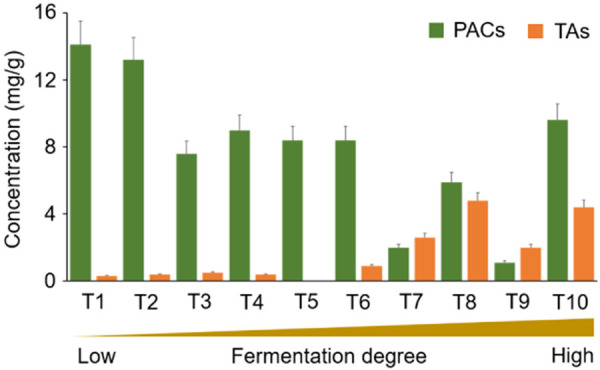
Concentrations of PACs and TAs in differential degrees of fermented teas. T1: Sun-Link-Sea High-Mountain tea; T2: Lishan High-Mountain tea; T3: Alishan High-Mountain tea; T4: Paochong tea; T5: Tongding oolong tea; T6: Jhinhsuan tea; T7: Tieh-Kuan-Yin tea; T8: Oriental Beauty tea; T9: Red Oolong tea; T10: Ruby black tea.
Tea extracts reduce infection of SARS-CoV-2 and its variants
To explore the functional consequences of tea extract treatment, we determined their inhibitory activities against viral infection by a Vpp assay, which was developed to measure the infection rate of SARS-CoV-2 pseudoviral particle into mammalian cell line 293T-ACE2, an ACE2-overexpressing human embryonic kidney cell [41-44]. Due to the relative higher mortality in comparison with other SARS-CoV-2 variants [45,46], we determined the effect of tea extracts on suppressing the delta variant infection. Both tea extracts of Sun-Link-Sea High-Mountain tea (T1) and Oriental Beauty tea (T8) effectively reduced pseudoviral infection of wild-type as well as the delta variant SARS-CoV-2. Additionally, the inhibitory activities exhibited a dose-dependent manner, despite an inconsistency at low-dose of T1 in the wild-type (Figure 5A and 5B). This result suggested the effective compounds of tea extracts involved in protecting mammalian cells from viral infection.
Figure 5.
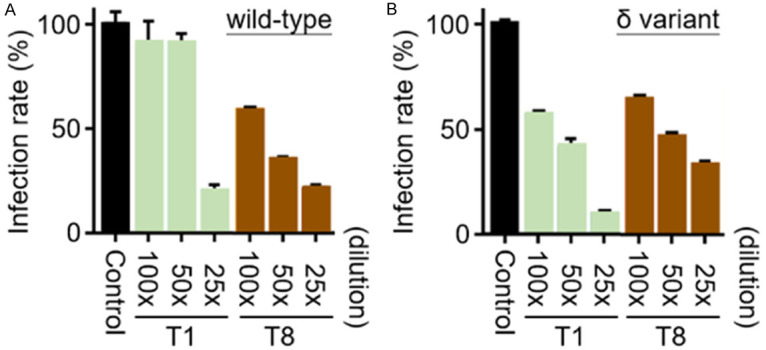
The Vpp infection assay of wild-type and the delta variant SARS-CoV-2. Sun-Link-Sea High-Mountain tea (T1) and Oriental Beauty tea (T8) at indicated fold-dilution doses were used to treat cells, the untreated group served as normalized control and its infection rate was set as 100%.
TAs and PACs in teas contribute to suppress infection of SARS-CoV-2 and its variants
To verify the critical roles of PACs and TAs of tea extracts in reducing viral infection, we used ethyl acetate (EA) to remove tannins such as PACs and TAs from tea extracts. PACs belonging to condensed tannins have the higher hydrophobicity than hydrolysable TAs, thus PACs have higher solubility than TAs by EA extraction. Consistent with this chemical property, UHPLC-HRMS quantitative analysis revealed that EA had better removal efficacy of PACs (69.5% for T1 and 79.6% for T8) than TAs (35.0% for T8; very low abundance of TAs in T1) from tea extracts (Figure 6).
Figure 6.
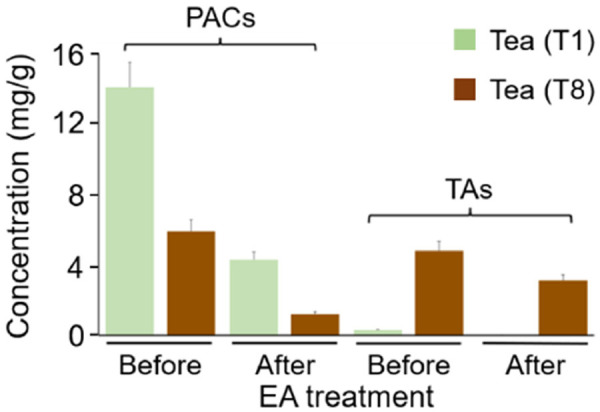
Concentrations of PACs and TAs in Sun-Link-Sea High-Mountain tea (T1) and Oriental Beauty tea (T8) before and after ethyl acetate (EA) extraction.
We next determined the impact of PACs and TAs removal from tea extracts on viral infection. The Vpp assays revealed that both Sun-Link-Sea High-Mountain tea (T1) and Oriental Beauty tea (T8) had markedly inhibitory effect on viral infection not only against wild-type SARS-CoV-2 but also to its variants including alpha, beta, gamma, delta, and omicron (Figure 7A and 7B). PACs and TAs removal significantly impaired the inhibitory efficacy of T1 and T8, but not completely abolish their inhibitory abilities (gray vs. blue, Figure 7A and 7B). This result indicated that the effective components were partially removed by EA, which was consistent with our aforementioned finding (Figure 6). To verify the causal regulation of PACs and TAs in viral infection, we supplemented an equal amount of PACs and TAs into the EA-extracted tea solution. Indeed, PACs and TAs supplement significantly rescued the inhibitory abilities of T1 and T8 against infection of SARS-CoV-2 and all its variants (Figure 7A and 7B). Our result strongly suggested the functions of PACs and TAs of tea extracts in protecting from SARS-CoV-2 viral infection.
Figure 7.
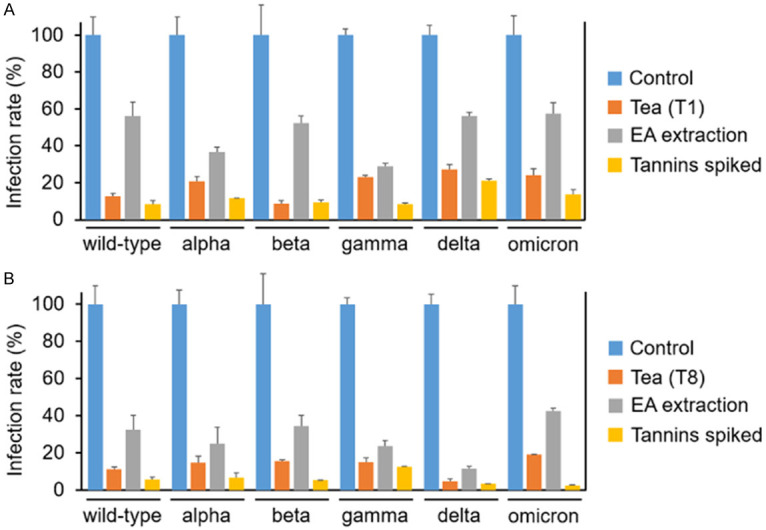
The effects of PACs and TAs in tea extracts on protecting cells from SARS-CoV-2 and its variants infection were determined by the Vpp assay. (A) Sun-Link-Sea High-Mountain tea (T1) and (B) Oriental Beauty tea (T8) (used as the positive control) were extracted by ethyl acetate (EA) to remove their tannins (EA extraction group), and then supplemented with equal amounts of PACs and TAs (Tannins spiked group). The untreated group served as normalized control and its infection rate was set as 100%.
Tea fractions contribute to suppress infection of SARS-CoV-2 XBB variant
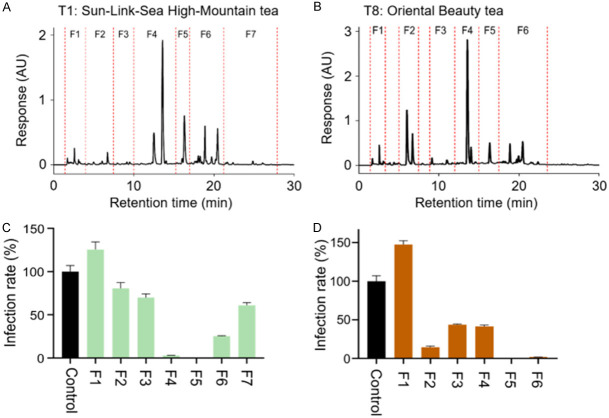
We further examined the ability of phytochemicals in teas to combat the infection of SARS-CoV-2 variant omicron XBB.1.5 virus, which enhanced binding to ACE2 and vaccine evasion remains the dominant variant globally [47]. The component fractionations of Sun-Link-Sea High-Mountain tea (T1) and Oriental Beauty tea (T8) were performed by HPLC system, the fingerprint chromatograms of T1 and T8 were shown in Figure 8A and 8B, respectively. The tea components were further divided into seven fractions (F1-F7) in T1 and six fractions (F1-F6) in T8 based on the fingerprint profiles. The Vpp assay revealed that the F4, F5, and F6 of T1 as well as F2, F5, and F6 of T8 showed significant inhibition to omicron XBB.1.5 infection (Figure 8C and 8D).
Figure 8.
Fractionation of tea component and the Vpp assay. The HPLC fingerprint chromatogram of Sun-Link-Sea High-Mountain tea (A) and Oriental Beauty tea (B). The Vpp assay was used to determine the effect of indicated HPLC fractions of Sun-Link-Sea High-Mountain tea (C) and Oriental Beauty tea (D) on inhibiting omicron XBB.1.5 variant infection. The untreated group served as normalized control and its infection rate was set as 100%.
To understand the phytochemical composition, different fractions of T1 and T8 were assayed by UHPLC-HRMS and the MS spectra were searched by the databases Chemspider, PlantCyc, mzVault, and mzCloud. The analytic results revealed the organic acid (quinic acid), alkaloids (theobromine and caffeine), hydrolysable tannins (TAs), condense tannins (flavonoid), and flavonoid glycosides in T1 and T8 (Table 2). EGCG was the main component in F5 fraction of T1 and T8, which showed the most efficacy of suppressing omicron XBB.1.5 infection. This result is consistent with the previous finding that the neutralizing effect of EGCG on omicron variants [48]. The F4 fraction of T1 contained high levels of PACs epigallocatechin and catechin, while the F6 fraction of T8 included TA 3GG and PACs procyanidin B-2-O-gallate and epicatechin-3-O-gallate, which was in line with our previous findings (Figures 3 and 4) as well as supported our hypothesis that TAs and PACs in tea could impact the effect of anti-SARS-CoV-2 infection. Moreover, aside from anticancer activity, theobromine and gallic acid in the F2 fraction of T8 have been identified to potentially serve as an anti-coronavirus agent and an anti-influenza virus agent, respectively [49,50]. Therefore, both compounds could contribute to a significant inhibition of viral infection in the F2 fraction of T8 (Figure 8D).
Table 2.
The phytochemicals in different fraction of Sun-Link-Sea High-Mountain tea (T1) and Oriental Beauty tea (T8)
| Compound | RT (min) | Theoretical value (m/z) | Experimental value (m/z) | Mass tolerance (ppm) | T1 fraction | T8 fraction | Relative content1 |
|---|---|---|---|---|---|---|---|
| Quinic acid | 1.51 | 191.05556 | 191.05513 | -2.3 | F1 | F1 | 1.17 |
| Galloylglucose (1GG) | 2.63 | 331.06652 | 331.06695 | 1.3 | F2 | F2 | 2.19 |
| Theasinensin C | 2.63 | 609.12443 | 609.12464 | 0.3 | F2 | F2 | 1.24 |
| Gallic acid (GA) | 3.15 | 169.01370 | 169.01311 | -3.5 | F2 | F2 | 28.29 |
| 5-galloylquinic acid | 3.89 | 343.06653 | 343.06667 | 0.4 | F2 | F2 | 2.29 |
| Theobromine | 5.94 | 181.07255 | 181.07197 | -3.2 | F3 | F2 | 6.15 |
| Gallocatechin | 6.09 | 305.06613 | 305.06622 | 0.3 | F3 | F3 | 0.11 |
| Prodelphinidin B-4 | 7.25 | 609.12444 | 609.12456 | 0.2 | F3 | N.D.2 | -3 |
| Theasinensin B | 8.06 | 761.13540 | 761.13554 | 0.2 | N.D. | F3 | - |
| Epigallocatechin | 9.27 | 305.06613 | 305.06625 | 0.4 | F4 | F4 | 0.17 |
| 3-coumaroylquinic acid | 9.27 | 337.09235 | 337.09256 | 0.6 | F4 | F4 | 4.51 |
| Catechin | 9.88 | 289.07122 | 289.07136 | 0.5 | F4 | F4 | 0.23 |
| 5-caffeoylquinic acid | 9.91 | 353.08726 | 353.08741 | 0.4 | N.D. | F4 | - |
| Digalloylglucose (2GG) | 9.91 | 483.07749 | 483.07750 | 0.1 | F4 | F4 | 14.79 |
| Caffeine | 9.96 | 195.08820 | 195.08757 | -3.2 | F4 | F4 | 2.27 |
| Strictinin | 10.72 | 633.07280 | 633.07292 | 0.2 | F4 | F4 | 6.71 |
| (Epi)catechin-(epi)catechin | 10.98 | 577.13461 | 577.13459 | -0.1 | F4 | F4 | 0.3 |
| 4-coumaroylquinic acid | 11.45 | 337.09235 | 337.09278 | 1.3 | F4 | F4 | 0.54 |
| Epicatechin | 12.32 | 289.07122 | 289.07148 | 0.9 | F5 | F5 | 0.2 |
| 5-coumaroylquinic acid | 12.39 | 337.09235 | 337.09266 | 0.9 | F5 | F5 | 1.64 |
| Epigallocatechin-3-O-gallate (EGCG) | 12.65 | 457.07709 | 457.07734 | 0.5 | F5 | F5 | 0.8 |
| Gallocatechin-3-O-gallate | 13.27 | 457.07709 | 457.07745 | 0.8 | F5 | F5 | 0.59 |
| Trigalloylglucose (3GG) | 14.24 | 635.08845 | 635.08886 | 0.6 | F6 | F6 | 48.05 |
| Procyanidin B-2 (or 4) 3’-O-gallate | 14.29 | 729.14557 | 729.14600 | 0.6 | F6 | F6 | 1.38 |
| Myricitin-3-O-glucoside | 14.42 | 479.08257 | 479.08241 | -0.3 | F6 | F6 | 0.48 |
| 6-C-arabinoside-8-C-glucosyl apigenin | 14.42 | 563.14009 | 563.13987 | -0.4 | F6 | F6 | 0.66 |
| Quercetin-3-O-glucosyl-rhamnosyl-glucoside | 15.54 | 771.19839 | 771.19877 | 0.5 | F6 | F6 | 0.83 |
| Epigallocatechin-3-O-(4-O-methyl) gallate | 15.31 | 471.09274 | 471.09257 | -0.4 | F6 | F6 | 0.33 |
| Rutin | 16.25 | 609.14557 | 609.14571 | 0.2 | F6 | F6 | 1.24 |
| Epicatechin-3-O-gallate | 16.50 | 441.08218 | 441.08213 | -0.1 | F6 | F6 | 1.34 |
| Kaempferol-3-O-glucosyl-rhamnosyl-glucoside | 17.09 | 755.20348 | 755.20355 | 0.1 | F6 | F6 | 0.83 |
| Kaempferol-3-O-glucoside | 18.43 | 447.09274 | 447.09275 | 0.1 | F7 | F6 | 2.11 |
| Epicatechin-3-O-(3-O-methyl) gallate | 18.91 | 455.09783 | 455.09796 | 0.3 | F7 | F6 | 1.01 |
relative content: content ratio of T8 over T1;
N.D.: not detected;
-: not available.
Discussion
The COVID-19 pandemic caused by SARS-CoV-2, especially infected by omicron variant, remains the most important issue of would public health. Several vaccines and drugs have been developed for preventing and curing COVID-19, however, the developing of specific and high efficient vaccines for SARS-CoV-2 variants is still in progress [51]. Apart from vaccines, the antioxidant ingredients such as catechins and polyphenols in teas have been shown to exhibit the biological activity to inhibit SRAS-CoV-2 infection by in silico molecular docking and dynamic studies [52,53]. Moreover, tannins in natural products have been reported as potential anti-SARS-CoV-2 components [24,25,28]. Our epidemiological analysis revealed a weak correlation between annual per capita consumption of tea and the COVID-19-related morbidity (data not shown), which may result from different personal non-pharmaceutical interventions (NPIs) in different chronological order in different countries [54]. In consistent with the recent finding [55], our data showed that annual per capita consumption of tea is reversely correlated with COVID-19-related mortality for SARS-CoV-2 and its variant (Figure 1), providing a strong rationale behind tea drinking as a dietary supplement to prevent SARS-CoV-2 viral infection.
In this study, the concentrations of TAs and PACs were successfully determined by the UHPLC-HRMS method. In comparison to previous reported methods, the current developed method is easy and fast to quantify the trace of TAs and PACs in tea extracts within 30 min without derivatization step during analysis. As Figure 4 shown, the PACs concentration in teas decreased along with an increase of the tea fermentation degree, whereas the TAs concentration exhibited the opposite trend. The fermentation in tea preparation steps refers to the natural browning reactions induced by oxidative enzymes in the fresh tea leaves and then the subsequent fired and dried steps [32,39]. The oxidation reaction and heating bring about releasing of gallic acid from galloylated catechin and formation of other phenolic glycosides in teas [56,57], consequently resulting in different compositions in teas.
Accumulating evidence indicates the roles of TAs and PACs in the anticancer functions. TAs can not only negatively regulate stemness by inhibiting NF-κB activation [16] but also synergistically enhance anticancer efficacy of chemotherapeutic drugs such as cisplatin and paclitaxel in diverse cancers, including lung cancer, liver cancer, and breast cancer [58-60]. PACs have been shown to associate with anticancer properties through modulating cellular metabolism and activating ferroptosis pathways [61-63]. These activities may add their value for dietary benefits. According to the infection rates of T1 and T8 on SARS-CoV-2 wild-type and delta variant (Figure 5), we approximately estimated the half maximal effective concentration (EC50) of T1 and T8 as 1.9 g/L and 0.8 g/L for wild-type and 0.8 g/L and 1.0 g/L for delta variant, respectively. The average blood volume in typical adult human body is 5 L, thus, the estimated effective dose is about 10 g (≈ 1.9 g/L × 5 L) in an ideal condition. Consequently, 4 cups of teas (3 g per commercial tea bag) for daily supplement may achieve an effective dose to provide antiviral protection. Interestingly, this result can also be supported by recent studies that daily intake more than 4 cups of tea may reduce the odds of SRAS-CoV-2 infection [30,64].
Despite different fingerprint profiles, similar phytochemicals have been identified in the related HPLC fractions of T1 and T8. Fraction F5 of both T1 and T8 had the best capacity of suppressing SARS-CoV-2 virus infection, whose main component EGCG could was involved in inhibiting the MPro activity and virus replication of SARS-CoV-2 wild-type and omicron variant [22,23,48]. Except fraction F5, other fractions of T1 and T8 showed different abilities against infection of SARS-CoV-2 XBB.1.5 variant (Figure 8C and 8D), which could result from different levels of effective compounds. For instance, T1 had higher levels of PACs epigallocatechin (6-fold) and catechin (4-fold) than T8 in fraction F4, which could be associated with a superior inhibition activity of T1 in comparison with T8 (Table 2). On the other hand, T8 contained more than 48-fold of TAs 3GG than T1 in fraction F6, which could contribute to better efficacy of infection inhibition in T8. These findings support the functions of PACs and TAs as the key effectors in tea samples for preventing SARS-CoV-2 infection. Additionally, there are nine mutations identified in the spike protein of the Delta variant. Among these mutations, L452R, T478K, D614G, and P681R have been identified to critically enhance transmissibility of virus [65]. Omicron variant XBB.1.5 has three mutation sites T478K, D614G, and P681R identical with the Delta variant. Omicron JN.1 variant, the major virus strain spreading worldwide since the end of 2023, has all four mutations in the critical mutation sites of the spike protein of Delta variant. This result may support our current finding that despite the spike protein mutations, TAs and PACs still have the potential to inhibit SARS-CoV-2 infection.
Conclusions
The composition of polyphenols in teas can be significantly affected by fermentation process. PACs are enriched in teas with low fermentation degree such as Sun-Link-Sea High-Mountain tea, while high levels of TAs are identified in high fermentation degree of teas such as Oriental Beauty tea. Our current findings demonstrate the potential of both OPCs and TAs in teas for reducing viral infection of SARS-CoV-2 wild-type and its variants. Currently, the severe threat posed by SARS-CoV-2 has been alleviated worldwide, and the situation has transitioned to coexisting with the virus. Tea drinking may serve as a dietary supplement to enhance protection against SARS-CoV-2 infection.
Acknowledgements
We would like to acknowledge the Instrument Center of National Chung Hsing University for the assistance of LC-HRMS analysis. We also acknowledge the National RNAi Core Facility of Academia Sinica in Taiwan for supplying all SARS-CoV-2 spike-pseudotyped lentiviruses and related services. This work was assisted by grants from the National Science and Technology Council, Taiwan [NSTC 112-2314-B-039-026-MY3, NSTC 112-2639-B-039-001-ASP, T-Star Center NSTC 113-2634-F-039-001], and the Ministry of Health and Welfare [MOHW113-TDU-B-222-134016]. Also, this work was supported from The Featured Areas Research Center Program by the Ministry of Education (MOE) in Taiwan.
Disclosure of conflict of interest
None.
References
- 1.Wu F, Zhao S, Yu B, Chen YM, Wang W, Song ZG, Hu Y, Tao ZW, Tian JH, Pei YY, Yuan ML, Zhang YL, Dai FH, Liu Y, Wang QM, Zheng JJ, Xu L, Holmes EC, Zhang YZ. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature. 2020;579:270–273. doi: 10.1038/s41586-020-2012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cui J, Li F, Shi ZL. Origin and evolution of pathogenic coronaviruses. Nat Rev Microbiol. 2019;17:181–192. doi: 10.1038/s41579-018-0118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li F. Structure, function, and evolution of coronavirus spike proteins. Annu Rev Virol. 2016;3:237–261. doi: 10.1146/annurev-virology-110615-042301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S, Schiergens TS, Herrler G, Wu NH, Nitsche A, Müller MA, Drosten C, Pöhlmann S. SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell. 2020;181:271–280. e8. doi: 10.1016/j.cell.2020.02.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davies NG, Klepac P, Liu Y, Prem K, Jit M CMMID COVID-19 working group. Eggo RM. Age-dependent effects in the transmission and control of COVID-19 epidemics. Nat Med. 2020;26:1205–1211. doi: 10.1038/s41591-020-0962-9. [DOI] [PubMed] [Google Scholar]
- 7.Fernandes Q, Inchakalody VP, Merhi M, Mestiri S, Taib N, Moustafa Abo El-Ella D, Bedhiafi T, Raza A, Al-Zaidan L, Mohsen MO, Yousuf Al-Nesf MA, Hssain AA, Yassine HM, Bachmann MF, Uddin S, Dermime S. Emerging COVID-19 variants and their impact on SARS-CoV-2 diagnosis, therapeutics and vaccines. Ann Med. 2022;54:524–540. doi: 10.1080/07853890.2022.2031274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Callaway E. Heavily mutated omicron variant puts scientists on alert. Nature. 2021;600:21. doi: 10.1038/d41586-021-03552-w. [DOI] [PubMed] [Google Scholar]
- 9.Shuai H, Chan JF, Hu B, Chai Y, Yuen TT, Yin F, Huang X, Yoon C, Hu JC, Liu H, Shi J, Liu Y, Zhu T, Zhang J, Hou Y, Wang Y, Lu L, Cai JP, Zhang AJ, Zhou J, Yuan S, Brindley MA, Zhang BZ, Huang JD, To KK, Yuen KY, Chu H. Attenuated replication and pathogenicity of SARS-CoV-2 B.1.1.529 Omicron. Nature. 2022;603:693–699. doi: 10.1038/s41586-022-04442-5. [DOI] [PubMed] [Google Scholar]
- 10.Wang X, Jiang S, Ma W, Li X, Wei K, Xie F, Zhao C, Zhao X, Wang S, Li C, Qiao R, Cui Y, Chen Y, Li J, Cai G, Liu C, Yu J, Li J, Hu Z, Zhang W, Jiang S, Li M, Zhang Y, Wang P. Enhanced neutralization of SARS-CoV-2 variant BA.2.86 and XBB sub-lineages by a tetravalent COVID-19 vaccine booster. Cell Host Microbe. 2024;32:25–34. e5. doi: 10.1016/j.chom.2023.11.012. [DOI] [PubMed] [Google Scholar]
- 11.Planas D, Staropoli I, Michel V, Lemoine F, Donati F, Prot M, Porrot F, Guivel-Benhassine F, Jeyarajah B, Brisebarre A, Dehan O, Avon L, Bolland WH, Hubert M, Buchrieser J, Vanhoucke T, Rosenbaum P, Veyer D, Péré H, Lina B, Trouillet-Assant S, Hocqueloux L, Prazuck T, Simon-Loriere E, Schwartz O. Distinct evolution of SARS-CoV-2 omicron XBB and BA.2.86/JN.1 lineages combining increased fitness and antibody evasion. Nat Commun. 2024;15:2254. doi: 10.1038/s41467-024-46490-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.LIczbiński P, Bukowska B. Tea and coffee polyphenols and their biological properties based on the latest in vitro investigations. Ind Crops Prod. 2022;175:114265. doi: 10.1016/j.indcrop.2021.114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Higdon JV, Frei B. Tea catechins and polyphenols: health effects, metabolism, and antioxidant functions. Crit Rev Food Sci Nutr. 2003;43:89–143. doi: 10.1080/10408690390826464. [DOI] [PubMed] [Google Scholar]
- 14.Di Domenico F, Foppoli C, Coccia R, Perluigi M. Antioxidants in cervical cancer: chemopreventive and chemotherapeutic effects of polyphenols. Biochim Biophys Acta. 2012;1822:737–747. doi: 10.1016/j.bbadis.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 15.Wang Y, Jin SS, Li DT, Jiang XC, Afrasiyab, Khalid A, Liu X, Wang HL, Wang HY, Wang ZG, Xie ZW, Huang SJ. Improving the anti-tumor effect of EGCG in colorectal cancer cells by blocking EGCG-induced YAP activation. Am J Cancer Res. 2023;13:1407–1424. [PMC free article] [PubMed] [Google Scholar]
- 16.Kim DA, Choi HS, Ryu ES, Ko J, Shin HS, Lee JM, Chung H, Jun E, Oh ES, Kang DH. Tannic acid attenuates the formation of cancer stem cells by inhibiting NF-κB-mediated phenotype transition of breast cancer cells. Am J Cancer Res. 2019;9:1664–1681. [PMC free article] [PubMed] [Google Scholar]
- 17.Vaid M, Singh T, Prasad R, Kappes JC, Katiyar SK. Therapeutic intervention of proanthocyanidins on the migration capacity of melanoma cells is mediated through PGE2 receptors and β-catenin signaling molecules. Am J Cancer Res. 2015;5:3325–3338. [PMC free article] [PubMed] [Google Scholar]
- 18.Mao X, Xiao X, Chen D, Yu B, He J. Tea and its components prevent cancer: a review of the redox-related mechanism. Int J Mol Sci. 2019;20:5249. doi: 10.3390/ijms20215249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang JY, Liao YH, Lin Y, Liu Q, Xie XM, Tang LY, Ren ZF. Effects of tea consumption and the interactions with lipids on breast cancer survival. Breast Cancer Res Treat. 2019;176:679–686. doi: 10.1007/s10549-019-05253-5. [DOI] [PubMed] [Google Scholar]
- 20.Sheerah H, Keyang L, Eshak ES, Cui R, Shirai K, Muraki I, Iso H, Tamakoshi A. Association of tea consumption and the risk of gastric cancer in Japanese adults: the Japan Collaborative Cohort Study. BMJ Open. 2020;10:e038243. doi: 10.1136/bmjopen-2020-038243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Filippini T, Malavolti M, Borrelli F, Izzo AA, Fairweather-Tait SJ, Horneber M, Vinceti M. Green tea (Camellia sinensis) for the prevention of cancer. Cochrane Database Syst Rev. 2020;3:CD005004. doi: 10.1002/14651858.CD005004.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jang M, Park YI, Cha YE, Park R, Namkoong S, Lee JI, Park J. Tea polyphenols EGCG and theaflavin inhibit the activity of SARS-CoV-2 3CL-protease in vitro. Evid Based Complement Alternat Med. 2020;2020:5630838. doi: 10.1155/2020/5630838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jang M, Park R, Park YI, Cha YE, Yamamoto A, Lee JI, Park J. EGCG, a green tea polyphenol, inhibits human coronavirus replication in vitro. Biochem Biophys Res Commun. 2021;547:23–28. doi: 10.1016/j.bbrc.2021.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang SC, Chen Y, Wang YC, Wang WJ, Yang CS, Tsai CL, Hou MH, Chen HF, Shen YC, Hung MC. Tannic acid suppresses SARS-CoV-2 as a dual inhibitor of the viral main protease and the cellular TMPRSS2 protease. Am J Cancer Res. 2020;10:4538–4546. [PMC free article] [PubMed] [Google Scholar]
- 25.Haddad M, Gaudreault R, Sasseville G, Nguyen PT, Wiebe H, Van De Ven T, Bourgault S, Mousseau N, Ramassamy C. Molecular interactions of tannic acid with proteins associated with SARS-CoV-2 infectivity. Int J Mol Sci. 2022;23:2643. doi: 10.3390/ijms23052643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xie Z, Li Y, Liu Z, Zeng M, Moore JC, Gao B, Wu X, Sun J, Wang TTY, Pehrsson P, He X, Yu LL. Bioactive compositions of Cinnamon (Cinnamomum verum J. Presl) extracts and their capacities in suppressing SARS-CoV-2 spike protein binding to ACE2, inhibiting ACE2, and scavenging free radicals. J Agric Food Chem. 2023;71:4890–4900. doi: 10.1021/acs.jafc.3c00285. [DOI] [PubMed] [Google Scholar]
- 27.Sugamoto K, Tanaka YL, Saito A, Goto Y, Nakayama T, Okabayashi T, Kunitake H, Morishita K. Highly polymerized proanthocyanidins (PAC) components from blueberry leaf and stem significantly inhibit SARS-CoV-2 infection via inhibition of ACE2 and viral 3CLpro enzymes. Biochem Biophys Res Commun. 2022;615:56–62. doi: 10.1016/j.bbrc.2022.04.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chen HF, Wang WJ, Chen CY, Chang WC, Hsueh PR, Peng SL, Wu CS, Chen Y, Huang HY, Shen WJ, Wang SC, Hung MC. The natural tannins oligomeric proanthocyanidins and punicalagin are potent inhibitors of infection by SARS-CoV-2. Elife. 2023;12:e84899. doi: 10.7554/eLife.84899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kagawa Y. Influence of nutritional intakes in Japan and the United States on COVID-19 infection. Nutrients. 2022;14:633. doi: 10.3390/nu14030633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nanri A, Yamamoto S, Konishi M, Ohmagari N, Mizoue T. Green tea consumption and SARS-CoV-2 infection among staff of a referral hospital in Japan. Clin Nutr Open Sci. 2022;42:1–5. doi: 10.1016/j.nutos.2022.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Storozhuk M, Lee S, Lee JI, Park J. Green tea consumption and the COVID-19 omicron pandemic era: pharmacology and epidemiology. Life (Basel) 2023;13:852. doi: 10.3390/life13030852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dou J, Lee VS, Tzen JT, Lee MR. Identification and comparison of phenolic compounds in the preparation of oolong tea manufactured by semifermentation and drying processes. J Agric Food Chem. 2007;55:7462–7468. doi: 10.1021/jf0718603. [DOI] [PubMed] [Google Scholar]
- 33.Dong JJ, Lu JU, Lu JL, Zheng X, Liang YR. Isolation of antioxidant catechins from green tea and its decaffeination. Food Bioprod Process. 2011;89:62–66. [Google Scholar]
- 34.McIntyre RS, Lui LM, Rosenblat JD, Ho R, Gill H, Mansur RB, Teopiz K, Liao Y, Lu C, Subramaniapillai M, Nasri F, Lee Y. Suicide reduction in Canada during the COVID-19 pandemic: lessons informing national prevention strategies for suicide reduction. J R Soc Med. 2021;114:473–479. doi: 10.1177/01410768211043186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahmad S, Zahiruddin S, Parveen B, Basist P, Parveen A, Gaurav, Parveen R, Ahmad M. Indian medicinal plants and formulations and their potential against COVID-19-preclinical and clinical research. Front Pharmacol. 2021;11:578970. doi: 10.3389/fphar.2020.578970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sooriyaarachchi P, Jeyakumar DT, King N, Jayawardena R. Impact of vitamin D deficiency on COVID-19. Clin Nutr ESPEN. 2021;44:372–378. doi: 10.1016/j.clnesp.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liu XD, Wang W, Yang Y, Hou BH, Olasehinde TS, Feng N, Dong XP. Nesting the SIRV model with NAR, LSTM and statistical methods to fit and predict COVID-19 epidemic trend in Africa. BMC Public Health. 2023;23:138. doi: 10.1186/s12889-023-14992-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang SC, Chou IW, Hung MC. Natural tannins as anti-SARS-CoV-2 compounds. Int J Biol Sci. 2022;18:4669–4676. doi: 10.7150/ijbs.74676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dou J, Lee VS, Tzen JT, Lee MR. Rapid identification of acylated flavonol tetraglycosides in oolong teas using HPLC-MSn. Phytochem Anal. 2008;19:251–257. doi: 10.1002/pca.1044. [DOI] [PubMed] [Google Scholar]
- 40.Lee RJ, Lee VS, Tzen JT, Lee MR. Study of the release of gallic acid from (-)-epigallocatechin gallate in old oolong tea by mass spectrometry. Rapid Commun Mass Spectrom. 2010;24:851–858. doi: 10.1002/rcm.4442. [DOI] [PubMed] [Google Scholar]
- 41.Huang ST, Chen Y, Chang WC, Chen HF, Lai HC, Lin YC, Wang WJ, Wang YC, Yang CS, Wang SC, Hung MC. Scutellaria barbata D. Don inhibits the main proteases (Mpro and TMPRSS2) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection. Viruses. 2021;13:826. doi: 10.3390/v13050826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wang WJ, Chen Y, Su WC, Liu YY, Shen WJ, Chang WC, Huang ST, Lin CW, Wang YC, Yang CS, Hou MH, Chou YC, Wu YC, Wang SC, Hung MC. Peimine inhibits variants of SARS-CoV-2 cell entry via blocking the interaction between viral spike protein and ACE2. J Food Biochem. 2022;46:e14354. doi: 10.1111/jfbc.14354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu CS, Chiang HM, Chen Y, Chen CY, Chen HF, Su WC, Wang WJ, Chou YC, Chang WC, Wang SC, Hung MC. Prospects of coffee leaf against SARS-CoV-2 infection. Int J Biol Sci. 2022;18:4677–4689. doi: 10.7150/ijbs.76058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu CS, Li YC, Peng SL, Chen CY, Chen HF, Hsueh PR, Wang WJ, Liu YY, Jiang CL, Chang WC, Wang SC, Hung MC. Coffee as a dietary strategy to prevent SARS-CoV-2 infection. Cell Biosci. 2023;13:210. doi: 10.1186/s13578-023-01154-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zali A, Khodadoost M, Gholamzadeh S, Janbazi S, Piri H, Taraghikhah N, Hannani K, Looha MA, Mohammadi G. Mortality among hospitalized COVID-19 patients during surges of SARS-CoV-2 alpha (B.1.1.7) and delta (B.1.617.2) variants. Sci Rep. 2022;12:18918. doi: 10.1038/s41598-022-23312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Muntean M, Briciu V, Lupse M, Colcear D, Macicasan RV, Csiszer A, Manole A, Radulescu A. Effects of COVID-19 on the liver and mortality in patients with SARS-CoV-2 pneumonia caused by delta and non-Delta variants: an analysis in a single centre. Pharmaceuticals (Basel) 2023;17:3. doi: 10.3390/ph17010003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yue C, Song W, Wang L, Jian F, Chen X, Gao F, Shen Z, Wang Y, Wang X, Cao Y. ACE2 binding and antibody evasion in enhanced transmissibility of XBB.1.5. Lancet Infect Dis. 2023;23:278–280. doi: 10.1016/S1473-3099(23)00010-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang Z, Hao M, Zhang X, He Y, Chen X, Taylor EW, Zhang J. Potential of green tea EGCG in neutralizing SARS-CoV-2 Omicron variant with greater tropism toward the upper respiratory tract. Trends Food Sci Technol. 2023;132:40–53. doi: 10.1016/j.tifs.2022.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Li J, Wang Y, Rajpoot S, Lavrijsen M, Pan Q, Li P, Baig MS. Investigating theobromine as a potential anti-human coronaviral agent. Microbiol Immunol. 2023;67:404–412. doi: 10.1111/1348-0421.13086. [DOI] [PubMed] [Google Scholar]
- 50.Lee JH, Oh M, Seok JH, Kim S, Lee DB, Bae G, Bae HI, Bae SY, Hong YM, Kwon SO, Lee DH, Song CS, Mun JY, Chung MS, Kim KH. Antiviral effects of black raspberry (Rubus coreanus) seed and its gallic acid against influenza virus infection. Viruses. 2016;8:157. doi: 10.3390/v8060157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kuhlmann C, Mayer CK, Claassen M, Maponga T, Burgers WA, Keeton R, Riou C, Sutherland AD, Suliman T, Shaw ML, Preiser W. Breakthrough infections with SARS-CoV-2 omicron despite mRNA vaccine booster dose. Lancet. 2022;399:625–626. doi: 10.1016/S0140-6736(22)00090-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ghosh R, Chakraborty A, Biswas A, Chowdhuri S. Evaluation of green tea polyphenols as novel corona virus (SARS CoV-2) main protease (Mpro) inhibitors - an in silico docking and molecular dynamics simulation study. J Biomol Struct Dyn. 2021;39:4362–4374. doi: 10.1080/07391102.2020.1779818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh R, Bhardwaj VK, Sharma J, Purohit R, Kumar S. In-silico evaluation of bioactive compounds from tea as potential SARS-CoV-2 nonstructural protein 16 inhibitors. J Tradit Complement Med. 2022;12:35–43. doi: 10.1016/j.jtcme.2021.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nader IW, Zeilinger EL, Jomar D, Zauchner C. Onset of effects of non-pharmaceutical interventions on COVID-19 infection rates in 176 countries. BMC Public Health. 2021;21:1472. doi: 10.1186/s12889-021-11530-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Storozhuk M. Green tea catechins against COVID-19: lower COVID-19 morbidity and mortality in countries with higher per capita green tea consumption. Coronaviruses. 2022;3:e240122200469. [Google Scholar]
- 56.Lee VS, Dou J, Chen RJ, Lin RS, Lee MR, Tzen JT. Massive accumulation of gallic acid and unique occurrence of myricetin, quercetin, and kaempferol in preparing old oolong tea. J Agric Food Chem. 2008;56:7950–7956. doi: 10.1021/jf801688b. [DOI] [PubMed] [Google Scholar]
- 57.Lo YH, Chen YJ, Chang CI, Lin YW, Chen CY, Lee MR, Lee VS, Tzen JT. Teaghrelins, unique acylated flavonoid tetraglycosides in chin-shin oolong tea, are putative oral agonists of the ghrelin receptor. J Food Agric Chem. 2014;62:5085–5091. doi: 10.1021/jf501425m. [DOI] [PubMed] [Google Scholar]
- 58.Zheng X, Yang L, Zhai W, Geng N, Zhang Z, Li X, Wu M. Synergistic anticancer activity of cisplatin combined with tannic acid enhances apoptosis in lung cancer through the PERK-ATF4 pathway. Eur J Med Res. 2023;28:462. doi: 10.1186/s40001-023-01420-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Geng N, Zheng X, Wu M, Yang L, Li X, Chen J. Tannic acid synergistically enhances the anticancer efficacy of cisplatin on liver cancer cells through mitochondria-mediated apoptosis. Oncol Rep. 2019;42:2108–2116. doi: 10.3892/or.2019.7281. [DOI] [PubMed] [Google Scholar]
- 60.Chowdhury P, Nagesh PKB, Hatami E, Wagh S, Dan N, Tripathi MK, Khan S, Hafeez BB, Meibohm B, Chauhan SC, Jaggi M, Yallapu MM. Tannic acid-inspired paclitaxel nanoparticles for enhanced anticancer effects in breast cancer cells. J Colloid Interface Sci. 2019;535:133–148. doi: 10.1016/j.jcis.2018.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ravindranathan P, Pasham D, Balaji U, Cardenas J, Gu J, Toden S, Goel A. Mechanistic insights into anticancer properties of oligomeric proanthocyanidins from grape seeds in colorectal cancer. Carcinogenesis. 2018;39:767–777. doi: 10.1093/carcin/bgy034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rupasinghe HPV, Parmar I, Neir SV. Biotransformation of cranberry proanthocyanidins to probiotic metabolites by Lactobacillus rhamnosus enhances their anticancer activity in HepG2 cells in vitro . Oxid Med Cell Longev. 2019;2019:4750795. doi: 10.1155/2019/4750795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shimura T, Sharma P, Sharma GG, Banwait JK, Goel A. Enhanced anti-cancer activity of andrographis with oligomeric proanthocyanidins through activation of metabolic and ferroptosis pathways in colorectal cancer. Sci Rep. 2021;11:7548. doi: 10.1038/s41598-021-87283-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vu TT, Rydland KJ, Achenbach CJ, Van Horn L, Cornelis MC. Dietary behaviors and incident COVID-19 in the UK biobank. Nutrients. 2021;13:2114. doi: 10.3390/nu13062114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dhawan M, Sharma A, Priyanka, Thakur N, Rajkhowa TK, Choudhary OP. Delta variant (B.1.617.2) of SARS-CoV-2: mutations, impact, challenges and possible solutions. Hum Vaccin Immunother. 2022;18:2068883. doi: 10.1080/21645515.2022.2068883. [DOI] [PMC free article] [PubMed] [Google Scholar]