Abstract
Control of ganglionic herpes simplex virus (HSV) infection depends on CD8+ cells but not on the death of infected neurons. Primarily, perforin and granzyme B mediate CD8+ cell cytotoxicity, whereas the in vivo functions of granzyme A, a third granule protein, are unknown. Here, it is shown that granzyme A restricts the interneuronal spread of HSV and significantly influences ganglionic virus load.
CD8+ T cells and natural killer (NK) cells are major components of the host response to intracellular pathogens (1, 8, 26), including herpes simplex virus (HSV) (19). After experimental inoculation of mouse skin, HSV replicates in epidermal cells and concurrently invades the peripheral nervous system (PNS), where primary sensory neurons are the virus's main target (16, 27). Productive infection of sensory neurons generates the potential for lethal spread of virus throughout the nervous system, but, in immunocompetent hosts, viral replication is usually rapidly terminated by timely development of an adaptive immune response (19). After recovery from primary infection, virus is not eradicated from the PNS; rather, viral DNA persists in a latent form in a proportion of neuronal nuclei, creating a reservoir of virus from which productive infection periodically reactivates (3). It is becoming increasingly apparent that the virus load in the PNS during the primary productive infection directly influences the number of neurons that become latently infected and the number of viral genomes that persist in each latently infected cell (14). Factors that limit the spread of HSV in the PNS are therefore likely to have a profound influence on the ability of the virus to persist in the host and to reactivate. We showed previously that termination of HSV infection in the PNSs of experimentally infected mice depends on CD8+ cells but, paradoxically, not on the death of infected neurons (20). CD8+ cells are generally assumed to kill their target cells by membrane disruption and DNA fragmentation, caused, respectively, by perforin and granzyme B (Gzm B) proteins that are contained within the cytoplasmic granules of cytotoxic lymphocytes (CTLs) (5, 7, 9, 10, 25). A third protein, granzyme A (Gzm A), also found exclusively in the granules of CTLs, is not directly cytolytic (4), and its biological functions in vivo are not well defined. We reasoned that Gzm A might contribute to noncytolytic termination of HSV infection in spinal nerve ganglia, and this hypothesis was addressed by comparing spinal ganglia of C57BL/6 Gzm A knockout mice (4) and congenic immunocompetent animals with respect to virus clearance and virus spread.
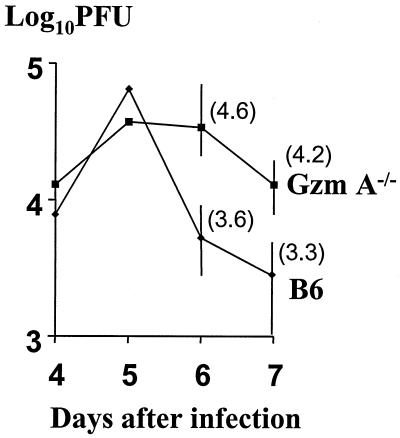
To determine the effect of Gzm A deficiency on virus load in the PNS, several groups of 10 Gzm A knockout mice and congenic C57BL/6 controls were inoculated with HSV-1 strain SC16; various days after inoculation, virus in homogenized dorsal root ganglia was quantified by a standard plaque assay (13). Congenic mice were bred at the Institute of Medical and Veterinary Science from a breeding pair supplied by A. Müllbacher (Australian National University), and their genetic authenticity was determined by PCR, as described previously (4), with tail DNA. The zosteriform model used in these experiments has been described in detail elsewhere (16, 18). Briefly, a small patch of depilated skin on the left flank, innervated by the 8th through the 10th dorsal root ganglia (T8–T10), was scarified with a 27-gauge needle through a 10-μl drop of virus suspension containing 5 × 105 PFU. At 4 and 5 days after inoculation, virus levels were comparable in Gzm A−/− and congenic control mice. However, on days 6 and 7, approximately 10 times more virus was recovered from Gzm A-deficient mice than from congenic controls (Fig. 1). Both groups of mice survived and eventually cleared the infection. Similar outcomes were obtained in three replicate experiments. The major implication of this finding is that Gzm A either facilitates virus clearance or plays a crucial role in restricting virus spread but is not critical for eventual clearance of virus or survival. It is known that development of zosteriform lesions reflects the spread of virus in the PNS (14); significantly, in concomitantly infected groups of 10 mice, zosteriform spread was accelerated in the absence of Gzm A (80% versus 40% of animals on day 6). These data suggest that the major effect is on the spread of virus.
FIG. 1.
Increased virus load in spinal ganglia of Gzm A knockout mice compared with congenic control animals (B6) at 6 and 7 days after flank inoculation with HSV-1 (P < 0.01). Vertical bars indicate ranges of geometric mean titers.
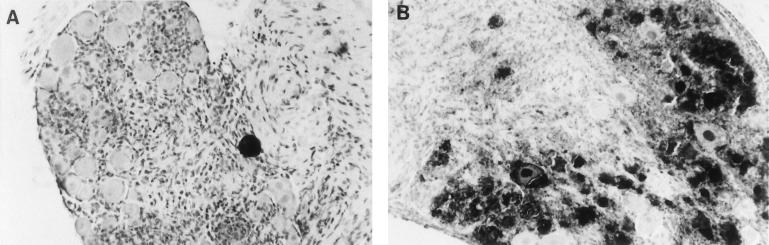
To determine whether the increased virus load in Gzm A−/− mice at 7 days after inoculation was associated with an elevated number of infected neurons, virus antigen-positive neuronal profiles were immunohistochemically quantified (22) in ganglionic sections from Gzm A−/− and congenic control mice. Ganglia (T6–T13) ipsilateral to the inoculation site were fixed in periodate-lysine-paraformaldehyde and processed as described previously (22, 24). In concordance with previously reported observations (24), virus antigen-positive neurons were sparse by day 7 in immunocompetent mice. However, disappearance of antigen-positive neurons was severely impaired by Gzm A deficiency (14 of 18,560 neuronal profiles were antigen positive in control mice versus 959 of 25,856 in Gzm A−/− mice [P < 0.0001] [Fig. 2], implying that Gzm A plays a significant role in reducing interneuronal spread of HSV. Alternative possibilities are that Gzm A contributes to the death of infected neurons or to clearance of virus from the skin. However, the former possibility is not supported by prior data showing that only a minority of viral antigen-positive neurons are killed during termination of ganglionic infection (20), and to date we have found no evidence supporting the latter (data not shown).
FIG. 2.
Sections of spinal ganglia stained for HSV antigens (black areas) at 7 days after flank inoculation, demonstrating approximately 40-fold-fewer antigen-positive neurons in immunocompetent C57BL/6 animals (A) than in congenic Gzm A knockout mice (B).
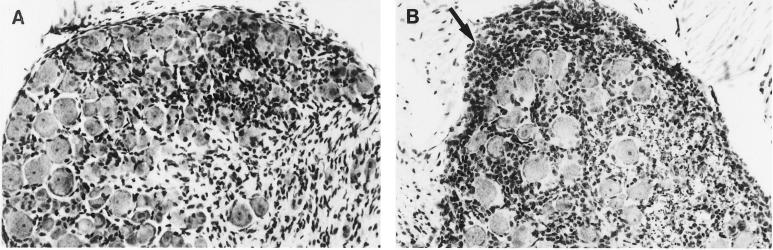
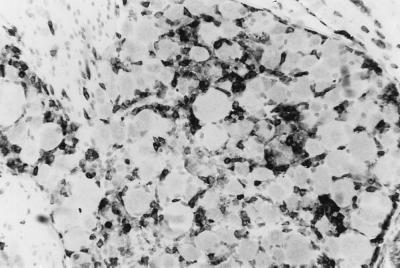
Histological examination showed that the magnitude and distribution of the inflammatory response to HSV infection were profoundly altered by Gzm A deficiency. Seven days after infection, ganglia (T8–T13) were pooled from C57BL/6 and Gzm A−/− mice (five per group) and paraffin embedded, and sections (5 μm thick) were stained with hematoxylin and eosin. Tissue from control mice showed moderate inflammatory infiltration and little edema; i.e., neurons remained closely apposed to each other, as they would appear in uninfected tissue (Fig. 3A). In contrast, Gzm A deficiency was associated with profound edema (demonstrated by increased distance between neuronal somas) and striking mononuclear cell infiltration, particularly under the ganglionic capsule (Fig. 3B, arrow), probably indicative of increased viral load and/or tissue damage. To our knowledge, mononuclear cell infiltration of this magnitude in HSV-infected spinal ganglia has not been previously reported. Despite the increased infiltration and edema in Gzm A−/− mice noted at the microscopic level, there was no overt discernable macroscopic difference in the ganglia of either group, owing to the inherent ganglionic swelling always associated with HSV infection. To determine the phenotypes of the infiltrating inflammatory cells, both in immunocompetent and Gzm A−/− mice, ganglia were stained with an antibody to the cytoplasmic component of the CD3 epsilon chain (Dakopatts, Glostrup, Denmark), as described previously (12). This experiment showed that, in both groups of mice, many of the infiltrating cells were CD3+ and were therefore either T lymphocytes or NK T cells (e.g., Gzm A−/− mice [Fig. 4]).
FIG. 3.
Gzm A deficiency increases the severity of inflammation in spinal ganglia at 7 days after infection. (A) Tissue from control mice showed moderate inflammatory infiltration and little edema, with neurons closely apposed to each other, as they would be in an uninfected ganglion. (B) In contrast, Gzm A deficiency was associated with massive edema (neuronal somas widely separated) and striking inflammation, concentrated under the ganglionic capsule (arrow). Infiltration of mononuclear inflammatory cells was also particularly prominent in nerve fibers of Gzm A−/− mice, illustrated as fine punctate staining surrounding the ganglion.
FIG. 4.
Dorsal root ganglia stained with antibody directed against the epsilon chain of CD3 (black) at 7 days after inoculation of HSV into flank skin of Gzm A−/− mice. The majority of infiltrating cells were CD3+; i.e., they were either T or NK T cells. Sections (5 μm thick) were lightly counterstained with hematoxylin.
The mechanism by which Gzm A might restrict the spread of HSV in the PNS is unknown. In this respect, we note that Gzm A is known to activate prourokinase (2), initiating a proteolytic cascade leading to the production of plasmin, a protease known to directly inactivate enveloped ectromelia virus (11). Preliminary data (not illustrated) suggest that plasmin might also inactivate extracellular HSV. Generation of plasmin in response to release of Gzm A from CD8+ cells might therefore represent a general noncytolytic mechanism for restricting extracellular virus spread, and further experiments with a wider range of concentrations of plasmin will be required to test this hypothesis.
Several other potential mechanisms of action of Gzm A merit discussion because, in vitro, purified Gzm A has a diverse range of properties. The possibility that Gzm A might directly inactivate HSV has not been formally excluded, though it is noteworthy that Gzm A has no direct antiviral activity against enveloped ectromelia virus (11). Importantly, T cells from Gzm A knockout mice are not impaired in the ability to lyse ectromelia virus-infected targets; nevertheless, control of ectromelia by these mice is impaired (11). Recent data suggest that Gzm A can play an auxiliary role in the induction of apoptosis (15), raising the possibility that the death of infected neurons also contributes to clearance of infectious virus from the PNS. However, prior data do not support this conclusion (20).
Gzm A has also been shown to process interleukin 1β precursor molecules into active cytokines (6), which might in turn qualitatively enhance other antiviral host defenses. Other established properties of Gzm A include cleavage of extracellular matrix proteins (22) and sensitization of B cells to proliferate (21), which could be envisioned to enhance local antibody production. Of potential relevance to this point, we showed previously that B-cell suppression increases virus load and virus spread in ganglia, not skin, of HSV-infected mice (17). Finally, in vivo Gzm A is believed to potentiate the activity of Gzm B (23).
In conclusion, it is shown here, for the first time, that Gzm A deficiency is associated with impaired ability to restrict the spread of HSV between neurons, resulting in accelerated development of zosteriform lesions, increased inflammation and edema in sensory nerve ganglia, and increased virus load in the PNS. These data indicate that Gzm A, a noncytolytic serine protease of T-cell granules, plays a crucial role in limiting the spread of HSV in the nervous system, perhaps by limiting the number of infected neurons and hence virus load, at the peak of infection.
Acknowledgments
This work was supported by grants 96-0535 and 98-1300 from the National Health and Medical Research Council of Australia.
We thank Arno Müllbacher (JCSMR, Canberra, Australia) for helpful discussion and for providing breeding pairs of Gzm A knockout mice, and we thank Lidia Mathews for technical assistance.
REFERENCES
- 1.Blanden R V. T cell response to viral and bacterial infection. Transplant Rev. 1974;19:56–88. doi: 10.1111/j.1600-065x.1974.tb00128.x. [DOI] [PubMed] [Google Scholar]
- 2.Brunner G, Simon M, Kramer M D. Activation of pro-urokinase by the human T cell-associated serine proteinase HuTSP-1. FEBS Lett. 1990;260:141–144. doi: 10.1016/0014-5793(90)80087-y. [DOI] [PubMed] [Google Scholar]
- 3.Cook M L, Bastone V B, Stevens J G. Evidence that neurons harbor latent herpes simplex virus. Infect Immun. 1974;9:946–951. doi: 10.1128/iai.9.5.946-951.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ebnet K, Hausmann M, Lehmann-Grube F, Müllbacher A, Kopf M, Lamers M, Simon M. Granzyme A-deficient mice retain potent cell-mediated cytotoxicity. EMBO J. 1995;14:4230–4239. doi: 10.1002/j.1460-2075.1995.tb00097.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heusel J W, Wesselschmidt R L, Shresta S, Russell J H, Ley T J. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell. 1994;76:977–987. doi: 10.1016/0092-8674(94)90376-x. [DOI] [PubMed] [Google Scholar]
- 6.Irmler M, Hertig S, MacDonald H R, Sadoul R, Becherer J D, Proudfoot A, Solari R, Tschopp J. Granzyme A is an Interleukin 1-converting enzyme. J Exp Med. 1995;181:1917–1922. doi: 10.1084/jem.181.5.1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kagi D, Ledermann B, Bürki K, Seiler P, Odermatt B, Olsen K J, Podack E R, Zinkernagel R M, Hengartner H. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin deficient mice. Nature (London) 1994;369:31–37. doi: 10.1038/369031a0. [DOI] [PubMed] [Google Scholar]
- 8.Karupiah G, Coupar B E H, Andrew M E, Boyle D B, Phillips S M, Mullbacher A, Blanden R V, Ramshaw I A. Elevated natural killer cell responses in mice infected with recombinant vaccinia virus encoding murine IL-2. J Immunol. 1990;144:290–298. [PubMed] [Google Scholar]
- 9.Kojima H, Shimohara N, Hanaoka S, Someya-Shirota Y, Takagaki Y, Ohnoa H, Saito T, Katayama T, Yagita H, Okomura K, Shiukai Y, Alt F W, Matsuzawa A, Yonehara S, Takayama H. Two distinct pathways of specific killing revealed by perforin mutant cytotoxic T lymphocytes. Immunity. 1994;1:357–364. doi: 10.1016/1074-7613(94)90066-3. [DOI] [PubMed] [Google Scholar]
- 10.Lowin B, Hahne M, Mattman C, Tschopp J. T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature (London) 1994;370:650–652. doi: 10.1038/370650a0. [DOI] [PubMed] [Google Scholar]
- 11.Müllbacher A, Ebnet K, Blanden R V, Tha Hla R, Stehle T, Museteanu C, Simon M. Granzyme A is critical for recovery of mice from infection with the natural cytopathic viral pathogen, ectromelia. Proc Natl Acad Sci USA. 1996;93:5783–5787. doi: 10.1073/pnas.93.12.5783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pereira R A, Simmons A. Cell surface expression of H2 antigens on primary sensory neurons in response to acute but not latent herpes simplex virus infection in vivo. J Virol. 1999;73:6484–6489. doi: 10.1128/jvi.73.8.6484-6489.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell W C. A sensitive and precise assay for herpesvirus. Nature (London) 1962;195:1028–1029. doi: 10.1038/1951028a0. [DOI] [PubMed] [Google Scholar]
- 14.Sawtell N M. Comprehensive quantification of herpes simplex virus latency at the single-cell level. J Virol. 1997;71:5423–5431. doi: 10.1128/jvi.71.7.5423-5431.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shresta S, Graubert T A, Thomas D A, Raptis S Z, Ley T J. Granzyme A initiates an alternative pathway for granule-mediated apoptosis. Immunity. 1999;10:595–605. doi: 10.1016/s1074-7613(00)80059-x. [DOI] [PubMed] [Google Scholar]
- 16.Simmons A, Nash A A. Zosteriform spread of herpes simplex virus as a model of recrudescence and its use to investigate the role of immune cells in prevention of recurrent disease. J Virol. 1984;52:816–821. doi: 10.1128/jvi.52.3.816-821.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simmons A, Nash A A. Effect of B cell suppression on primary infection and reinfection of mice with herpes simplex virus. J Infect Dis. 1987;155:649–654. doi: 10.1093/infdis/155.4.649. [DOI] [PubMed] [Google Scholar]
- 18.Simmons A, La Vista A B. Neural infection in mice after cutaneous inoculation with HSV-1 is under complex host genetic control. Virus Res. 1989;13:263–270. doi: 10.1016/0168-1702(89)90020-8. [DOI] [PubMed] [Google Scholar]
- 19.Simmons A, Tscharke D C, Speck P G. Role of immune mechanisms in control of HSV infection in the peripheral nervous system. Curr Top Microbiol Immunol. 1991;179:31–56. doi: 10.1007/978-3-642-77247-4_3. [DOI] [PubMed] [Google Scholar]
- 20.Simmons A, Tscharke D C. Anti-CD8 impairs clearance of herpes simplex virus from the peripheral nervous system: implications for the fate of virally infected neurons. J Exp Med. 1992;175:1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Simon M M, Hoschützky H, Fruth U, Simon H G, Kramer M D. Purification and characterisation of a T cell specific serine proteinase (TSP-1) from cloned cytolytic T lymphocytes. EMBO J. 1986;5:3267–3274. doi: 10.1002/j.1460-2075.1986.tb04638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simon M M, Simon H G, Fruth U, Epplen J, Müller-Hermelink H K, Kramer M D. Cloned cytolytic effector cells and their malignant variants produce an extracellular matrix degrading trypsin-like serine proteinase. Immunology. 1987;60:219–230. [PMC free article] [PubMed] [Google Scholar]
- 23.Simon M M, Hausmann M, Tran T, Ebnet K, Tschopp J, Tha Hla R, Müllbacher A. In vitro- and ex vivo-derived cytolytic leukocytes from granzyme A × B double knockout mice are defective in granule-mediated apoptosis but not lysis of target cells. J Exp Med. 1997;186:1781–1786. doi: 10.1084/jem.186.10.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Speck P G, Simmons A. Synchronous appearance of antigen positive and latently infected neurons in spinal ganglia of mice infected with a virulent strain of herpes simplex virus. J Gen Virol. 1992;73:1281–1285. doi: 10.1099/0022-1317-73-5-1281. [DOI] [PubMed] [Google Scholar]
- 25.Walsh C M, Matloubian M, Liu C-C, Ueda R, Kurahara C G, Christensen J L, Huang M T F, Young J D-E, Ahmed R, Clark W R. Immune function in mice lacking the perforin gene. Proc Natl Acad Sci USA. 1994;91:10854–10858. doi: 10.1073/pnas.91.23.10854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Welsh R M. Natural cell-mediated immunity during viral infections. Curr Top Microbiol Immunol. 1981;92:83–97. doi: 10.1007/978-3-642-68069-4_6. [DOI] [PubMed] [Google Scholar]
- 27.Wildy P, Field H J, Nash A A. Classical herpes latency revisited. Symposium 33. Cambridge, England: SGM, Cambridge University Press; 1992. pp. 133–167. [Google Scholar]