Abstract
Small RNAs (sRNAs) and riboswitches represent distinct classes of RNA regulators that control gene expression upon sensing metabolic or environmental variations. While sRNAs and riboswitches regulate gene expression by affecting mRNA and protein levels, existing studies have been limited to the characterization of each regulatory system in isolation, suggesting that sRNAs and riboswitches target distinct mRNA populations. We report that the expression of btuB in Escherichia coli, which is regulated by an adenosylcobalamin (AdoCbl) riboswitch, is also controlled by the small RNAs OmrA and, to a lesser extent, OmrB. Strikingly, we find that the riboswitch and sRNAs reduce mRNA levels through distinct pathways. Our data show that while the riboswitch triggers Rho-dependent transcription termination, sRNAs rely on the degradosome to modulate mRNA levels. Importantly, OmrA pairs with the btuB mRNA through its central region, which is not conserved in OmrB, indicating that these two sRNAs may have specific targets in addition to their common regulon. In contrast to canonical sRNA regulation, we find that OmrA repression of btuB is lost using an mRNA binding-deficient Hfq variant. Together, our study demonstrates that riboswitch and sRNAs modulate btuB expression, providing an example of cis- and trans-acting RNA-based regulatory systems maintaining cellular homeostasis.
Graphical Abstract
Graphical Abstract.
Introduction
Bacteria adapt to environmental changes by modulating gene expression at the mRNA and protein levels (1,2). Non-coding RNAs are involved in multiple bacterial regulatory processes and have been shown to be crucial actors in adaptive responses (2). Among these, riboswitches are RNA regulators often located in the 5′ untranslated region (5′ UTR) that modulate gene expression by undergoing structural changes (3,4). These metabolite-binding RNA regulators control gene expression at the levels of transcription termination, translation initiation or mRNA decay (3). In several cases, metabolite binding to riboswitches has been shown to affect multiple regulatory processes, suggesting that these RNA elements orchestrate complex regulatory pathways. For example, lysine binding to the lysC riboswitch downregulates gene expression both by directing RNase E cleavage of lysC mRNA and by inhibiting translation initiation (5). Furthermore, the lysC riboswitch was recently shown to modulate Rho-dependent transcription termination (6–8), indicating that the lysine riboswitch may control at least three regulatory processes to ensure tight genetic regulation. Another example of multiple mechanisms involved in genetic regulation was also characterized in Corynebacterium glutamicum where the flavin mononucleotide (FMN) riboswitch controls both RNase E/G cleavage activity and Rho-dependent transcription termination (9). Although such complex systems involving multiple regulatory factors are expected to be largely used by bacteria, relatively few studies address how they are coordinated during the regulation process, i.e. whether regulatory activities are performed independently or if they require any hierarchical order to achieve a timely and appropriate regulation.
Small RNAs (sRNAs) are regulatory elements that are often involved in cellular adaptative responses (2). In contrast to riboswitches, sRNAs typically control gene expression through intermolecular base pairing with targeted mRNAs and may involve an RNA chaperone, such as ProQ or the Sm-like protein Hfq, to facilitate mRNA recognition (10–13). sRNAs control transcription termination, translation initiation and mRNA degradation by recognizing sequences located in untranslated or coding regions (2). Because sRNAs often bind mRNA sequences using limited base pairing complementarity, this allows for a given sRNA to regulate multiple mRNA targets, thereby enabling a global regulatory response. Surprisingly, although both riboswitches and sRNAs mostly modulate gene expression by targeting mRNA untranslated regions, or early coding sequence, evidence for both effectors controlling the expression of the same mRNAs is lacking, entailing that sRNAs and riboswitch regulatory mechanisms may be mutually exclusive and that they might target globally different mRNA populations.
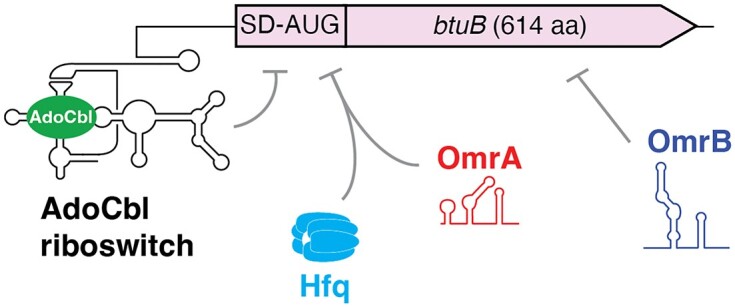
The Escherichia coli btuB riboswitch regulates the synthesis of the BtuB membrane transporter that mediates the influx/efflux of corrinoids such as vitamin B12 (14,15). Upon binding to adenosylcobalamin (AdoCbl)—one of the active forms of vitamin B12—the AdoCbl riboswitch prevents btuB translation initiation by sequestering the Shine-Dalgarno (SD) sequence in a stem-loop structure (Figure 1A) (6,16–18). A putative attenuator at the beginning of the coding sequence was found to be required for transcriptional repression (19,20), suggesting that mRNA levels are repressed as a consequence of translation inhibition. Importantly, previous microarray data (21) suggested that btuB expression is also repressed by two highly similar sRNAs, OmrA and OmrB, whose genes are located adjacently to each other within the aas and galR intergenic region in E. coli (Figure 1A) (21). These sRNAs have nearly identical 5′ and 3′ ends (Supplementary Figure S1A) and are predicted to exhibit specific secondary structures (Supplementary Figure S1B). They are negative regulators of genes encoding outer membrane proteins and proteins involved in biofilm formation and cell motility (21–27). All of the previously validated targets are regulated through binding of the conserved 5′ end region of OmrA/B to their mRNAs. Overall, OmrA and OmrB appear to be important for restructuring the bacterial surface in acidic or high osmolarity environments, i.e. in conditions where they are expressed through transcriptional control by the EnvZ-OmpR two-component system (21,28,29). Like several known OmrA/B-targets such as cirA, fecA and fepA, btuB encodes a TonB-dependent receptor. However, there are currently no available data showing whether OmrA and OmrB directly or indirectly regulate btuB expression and how their cellular function might be coordinated with the regulatory activity of the AdoCbl riboswitch.
Figure 1.
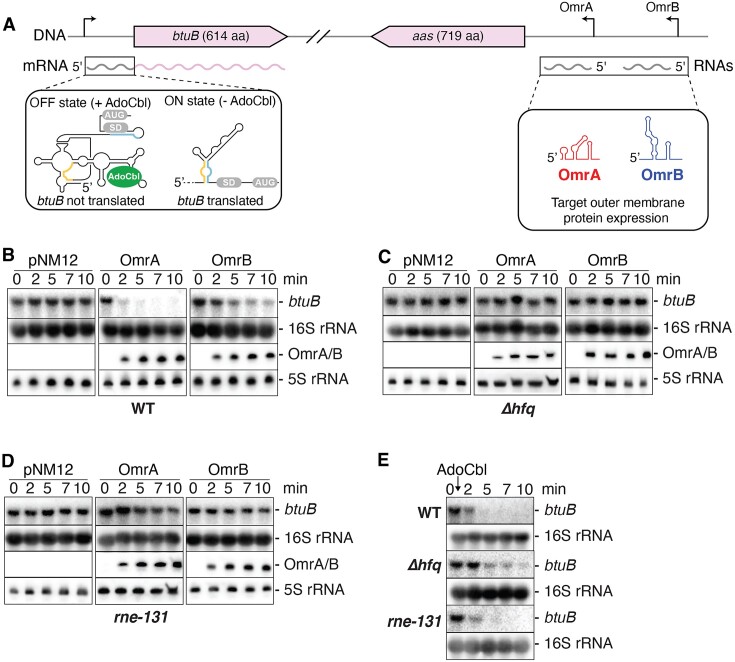
The OmrA and OmrB sRNAs regulate the expression of btuB. (A) Genomic location of the btuB riboswitch and OmrA/B. The left inset shows the ON and OFF riboswitch states when free or bound to adenosylcobalamin (AdoCbl), respectively. While the OFF state prevents translation initiation by sequestering the Shine-Dalgarno (SD) region, the ON state allows efficient translation by releasing the SD sequence through the formation of a helix formed by anti-SD (blue) and anti-anti-SD (yellow) sequences. OmrA and OmrB are shown in red and blue, respectively. (B–D) Northern blot analysis of btuB mRNA levels when expressing OmrA or OmrB. The experiments were performed in E. coli wild-type (WT) (B), Δhfq (C) and rne131 (D) strains. Total RNA was extracted at the indicated times immediately before (0) or (2, 5, 7 and 10 min) after induction of OmrA or OmrB with 0.1% arabinose. pNM12 is the empty vector control used in these experiments. Specific probes were used to detect btuB, OmrA or OmrB. The 16S and 5S rRNA were used as loading controls when monitoring the expression of btuB or OmrA/B, respectively. (E) Northern blot analysis of btuB mRNA levels in the WT, Δhfq and rne131 E. coli strains, after the addition of 5 µM AdoCbl. The 16S rRNA was used as a loading control.
Here, we characterize the genetic regulation exerted by the AdoCbl riboswitch and OmrA/OmrB on btuB expression. Our data indicate that btuB is repressed by OmrA—and to a lesser extent by OmrB—at the level of translation by promoting binding of the Hfq chaperone to the btuB translation initiation region. This sRNA control of btuB is not only independent of the riboswitch regulation but also occurs via a distinct mechanism, even though both regulators primarily target btuB translation initiation. To our knowledge, our study provides the first example of a mechanism where the regulation of gene expression is achieved through both riboswitch and sRNA control.
Materials and methods
Bacterial strains and plasmids
Bacterial strains and plasmids used in this study are described in Supplementary Table S1. Cells were grown in M63 minimal medium containing 0.2% glucose for experiments where AdoCbl concentration was adjusted (Figures 1 and 2), in CAG medium (minimal A salts (30), 0.5% (w/v) glycerol, 0.25% (w/v) casamino acids, 1 mM MgSO4) for fluorescence measurements (Figure 5), or in LB for other experiments. These media were supplemented with antibiotics, AdoCbl, arabinose or IPTG as needed (see Supplementary Information for details).
Figure 2.
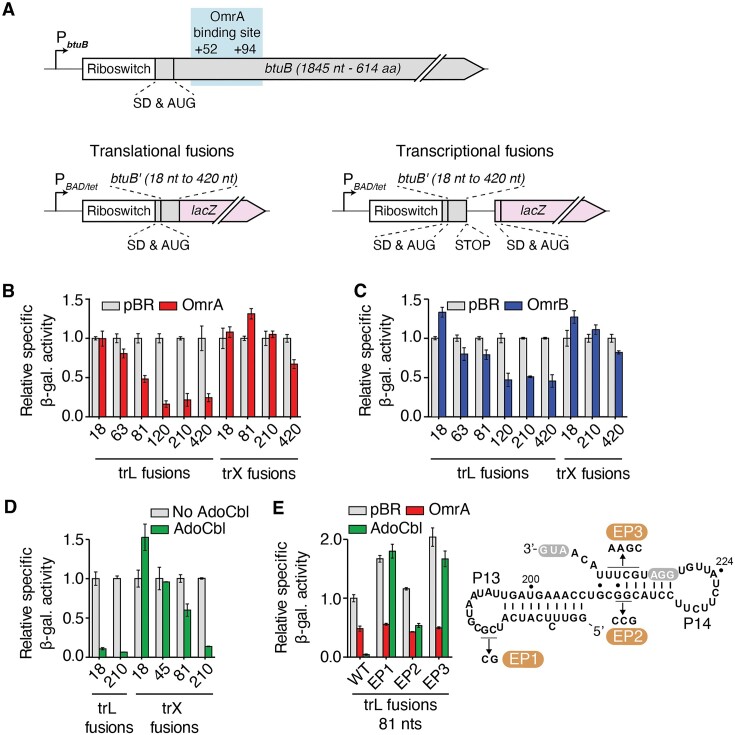
sRNAs and riboswitch regulation rely on different regions of the btuB mRNA. (A) Schematics showing the btuB gene with the location of the OmrA binding site (top) and translational (bottom left) and transcriptional (bottom right) fusions containing various lengths of btuB coding region (btuB'). An arabinose inducible (PBAD) or a constitutive (PLtetO-1) promoter was used to express the constructs. The Shine-Dalgarno (SD) and AUG are indicated. (B–D) β-galactosidase assays of translational (trL) BtuB-LacZ and transcriptional (trX) btuB-lacZ fusions in the presence of OmrA (B), OmrB (C) or AdoCbl (D). The number of nucleotides of the btuB CDS is indicated for each construct. Values were normalized to the activity obtained in the absence of sRNA (pBR, empty vector) or without AdoCbl. The average values and the standard deviations were obtained from three independent experiments. (E) Beta-galactosidase assays of selected btuB mutants destabilizing the stems P13 and P14. Experiments were performed using the BtuB81-LacZ translational fusion in the presence of OmrA or AdoCbl. The predicted secondary structure of the region encompassing stems P13 and P14 is shown to the right, and the EP1, EP2 and EP3 mutations are indicated. The SD sequence (GGA) and the AUG are white in gray.
Figure 5.
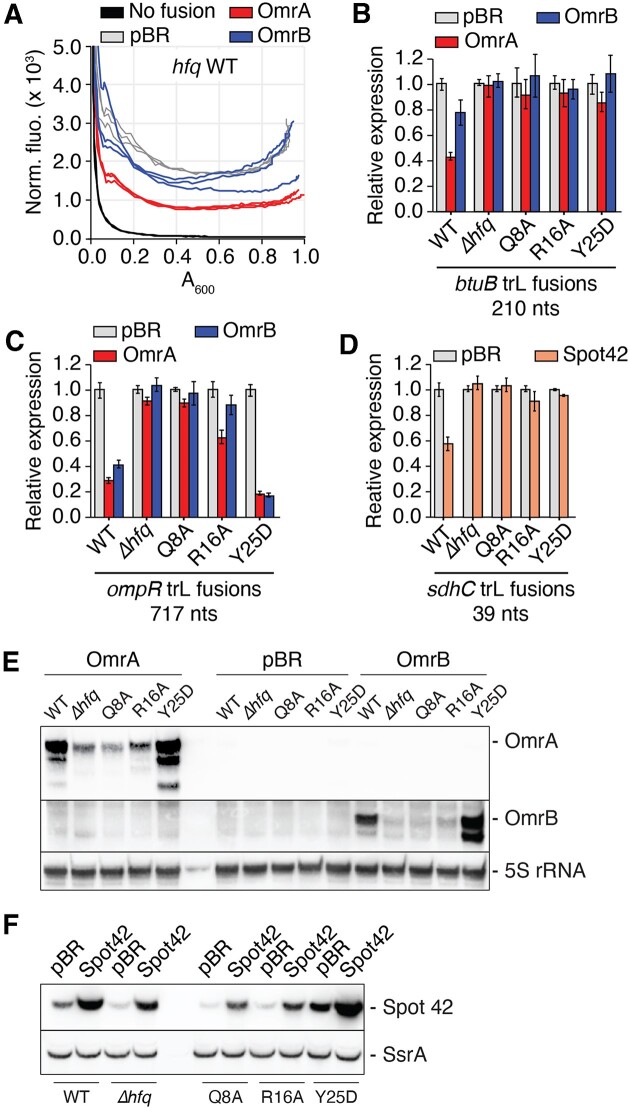
The Hfq distal face is required for the control of btuB by OmrA. (A) Representative fluorescence measurement of a BtuB210-mScarlet translational fusion upon overproduction of OmrA or OmrB over a 16 h time window. Shown is the normalized fluorescence that corresponds to the fluorescence divided by the absorbance at 600 nm, as a function of the absorbance at 600 nm. The corresponding graph showing the normalized fluorescence as a function of time is shown in Supplementary Figure S7A. A background fluorescence control was performed using an isogenic strain lacking the fluorescent construct and carrying the pBRplac (pBR) empty plasmid (‘no fusion’). (B–D) Fluorescence assays of BtuB210- (B), OmpR717- (C) and SdhC39-mScarlet (D) translational fusions upon overproduction of OmrA or OmrB. Experiments were performed in isogenic hfq+ (WT), Δhfq, hfqQ8A, hfqR16A and hfqY25D strains. Measurements were done in triplicate and shown is the fluorescence normalized to the absorbance at 600 nm, at an absorbance at 600 nm close to 0.3 and set arbitrarily at 1.0 for the WT strain transformed with the vector control (pBR). (E, F) Northern blot analysis of levels of OmrA and OmrB (E) and Spot42 (F) in isogenic hfq+ (WT), Δhfq, hfqQ8A, hfqR16A and hfqY25D strains. The 5S rRNA and the SsrA RNA were used as loading controls. Total RNA was extracted from the same set of strains that were used in panels B and D, respectively. A longer acquisition of the OmrA and OmrB northern-blots of panel E is provided in Supplementary Figure S14.
Gene fusions to lacZ or mScarlet reporters were constructed by recombineering using recipient strains carrying a mini-λ-Tet and a cat-sacB allele upstream of the lacZ or mScarlet genes. These recipient strains are PM1205 (for construction of PBAD-driven lacZ fusions), MG1508 (PLtetO-1-driven lacZ fusions), OK868 (PLtetO-1-driven lacZ fusions in the ΔlacY::FRT context) and OK510 (mScarlet fusions). Deletions of omrA, omrB or both were made by recombineering of a kanamycin-resistance cassette, nptI, into the omr loci; these mutations, as well as Δhfq and rne131 alleles, were moved by P1 transduction as necessary. The different hfq point mutants were obtained from D. Schu and N. Majdalani (NIH, Bethesda) and moved by P1 transduction as described in Supplementary Information.
OmrA and OmrB sRNAs were overproduced from either pNM12- or pBRplac-derivative plasmids, where their expression is under the control of an arabinose- or IPTG-inducible promoter, respectively. Mutations were introduced into the pBRplacOmrA plasmid by amplification with mutagenic primers, DpnI digestion, transformation into NEB-5 lacIq strains, and sequencing of the resulting plasmids.
The sequences of DNA oligonucleotides used in this study are in Supplementary Table S2.
ß-Galactosidase assays
The β-galactosidase activity of transcriptional or translational btuB-lacZ fusions expressed from a PBAD promoter (Figure 2) was measured in kinetic assays as previously described (5). Briefly, a bacterial culture was grown overnight in M63 0.2% glycerol minimal medium and was diluted 50-fold into fresh medium, which was then incubated at 37°C until an OD600 of 0.1 was obtained. Arabinose (0.1%) was then added to induce the expression of lacZ constructs. AdoCbl (5 μM) and/or IPTG (1 mM) to allow OmrA/B induction from a pBR plasmid was added when indicated. When using bicyclomycin (BCM; 25 μg/ml), assays were performed in 3 ml of culture media. The β-galactosidase activity of other lacZ fusions (Figure 4 and Supplementary Figure S6) was measured using a standard Miller assay. Briefly, an overnight culture was diluted 500-fold in fresh LB-Tet-IPTG 100 μM medium and cells were grown to mid-exponential phase. Next, 200 μl aliquots were then mixed with 800 μl of Z buffer, and the activity was measured as previously described (31) after cells were lysed with chloroform and SDS.
Figure 4.
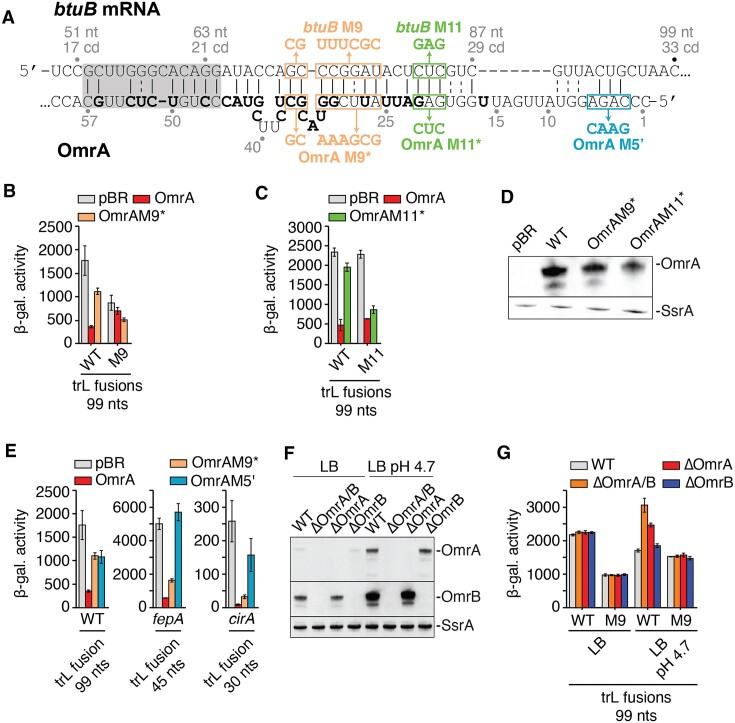
OmrA regulates btuB expression through direct interaction via its central region. (A) Predicted base-pairing between btuB and OmrA. The numbering scheme is relative to the btuB start codon and the OmrA transcription start site. The non-conserved nucleotides between OmrA and OmrB are shown in bold. The regions containing the mutations to investigate the OmrA-btuB interaction are shown in boxes. The grey shadow indicates the interaction that has been predicted by IntaRNA (45), while the other base pairs were predicted using secondary structure predictions of an OmrA-btuB sequence with Unafold and RNAfold and minor adjustment by hand. (B, C and E) β-galactosidase assays of BtuB99-LacZ translational fusions carrying the M9 mutations (B) or the M11 mutations (C), and of FepA45-LacZ and CirA30-LacZ translational fusions (E) upon overproduction of different OmrA variants. All fusions are expressed from a constitutive PLtetO-1 promoter. Strains used in these assays are deleted for omrA and omrB chromosomal copies. The β-galactosidase average values and the standard deviations were obtained from three independent experiments. (D) Northern blot analysis of levels of OmrA M9* and M11* variants using RNA extracted from cell cultures used for corresponding ß-galactosidase assays. SsrA RNA was used as a loading control. (F) Northern blot analysis of OmrA and OmrB levels in LB and in LB pH 4.7. Experiments were done in cells carrying or not the chromosomal omrA and/or omrB genes as indicated and RNA was extracted from the same cultures that were used for the β-galactosidase assays of WT BtuB99-LacZ in (G). (G) β-galactosidase assays of BtuB99-LacZ and BtuB99M9-LacZ translational fusions in cells carrying or not the chromosomal omrA and/or omrB genes, in LB and in acid LB (pH 4.7). Shown are the average β-galactosidase activities and standard deviations of three independent experiments.
Northern blots analysis
Total RNA was extracted from cells grown to midlog phase as described in the previous paragraph using the hot phenol method as in (32). When cells were grown in LB, 650 μl of cell culture were directly mixed with phenol, while cells grown in M63 or CAG were first resuspended in the same volume of sterile water and then mixed with phenol. After one phenol/water and two phenol/chloroform extractions, RNA was precipitated and resuspended in water. A constant amount of total RNA was fractionated on 1% agarose gel and transferred by capillarity onto an Hybond N+ (Amersham) membrane to detect btuB mRNA. For detecting OmrA, OmrB and Spot42 sRNAs, total RNA was separated on a 5% polyacrylamide gel and electro-blotted to an Hybond N + membrane. A radiolabeled probe was used to detect btuB mRNA and OmrA/B sRNAs in Figure 1 as in (5) and biotinylated probes were used to detect OmrA, OmrB or Spot42 in Figures 4 and 5 as previously described (25).
mScarlet fluorescence assays
The protocol for fluorescence measurement is detailed in the Supplementary data and the mScarlet locus is shown in Supplementary Figure S15. Briefly, a saturated culture was diluted 500-fold in 200 μL of CAG medium supplemented with tetracycline and IPTG 250 μM in a dark 96-well plate with clear bottom. Wells were then covered with 50 μl of mineral oil and bacterial growth and fluorescence were followed every 12 min during a 16 h kinetic where the plate was incubated at 37°C with shaking at 500 rpm in a CLARIOstar Plus plate reader. Absorbance was followed at 600 nm, and fluorescence was measured using an excitation wavelength of 560 nm and emission at 600 nm (with a 15 nm bandwidth). Measurements were systematically done in triplicate and normalized to the absorbance at 600 nm. For Northern blot analysis of the sRNA levels in the different hfq mutants (Figure 5E and F), total RNA was extracted from the same strains and same growth media as those used for fluorescence, but grown to mid-exponential phase in Erlenmeyer flasks rather than in 96-well plates.
In vitro transcription
In vitro transcription reactions were performed as previously described (5). Briefly, PCR products were used as DNA templates and contained a T7 RNA polymerase promoter. After transcription reactions were incubated for 3 h at 37°C, RNA products were precipitated with ethanol and purified on denaturing 8% acrylamide gels containing 8M urea. Acrylamide slices containing the RNA were eluted in water at 4°C overnight and recovered by precipitation.
Lead acetate probing assays
5′-radiolabeled btuB RNA (from −80 to +161 relative to the AUG) (10 nM) was incubated with increasing concentrations of Hfq (0, 1, 2.5, 5, 10 and 20 nM). Experiments were performed as previously described (33).
Electrophoresis mobility shift assays (EMSA)
For assessing the btuB-Hfq complex, radiolabeled btuB RNA (10 nM) was incubated 20 min in the absence or presence of Hfq hexamer (2.5, 5, 10, 25, 50 and 100 nM) in the protein buffer (50 mM Tris–HCl pH 7.5, 1 mM EDTA, 250 mM NH4Cl and 10% glycerol). For assessing the btuB-OmrA-Hfq complex, radiolabeled OmrA (10 nM) was incubated 10 min at 37°C in absence or presence of Hfq (10 nM) in the protein buffer. The reaction was then incubated 20 min at 37°C with btuB RNA in Afonyuskin buffer (10 mM Tris–HCl pH 7.5, 5 mM magnesium acetate, 100 mM NH4Cl and 0.5 mM DTT). EMSA reactions were resolved on 5% native acrylamide gels at 4°C TBE 1×.
Toeprint assays
The btuB RNA (0.5 pmol) was mixed with a radiolabeled DNA (3995JG; 10 nM) and incubated 5 min at 37°C. The annealing buffer was added to the reaction and incubated 5 min. When indicated, OmrA (250 nM) and/or Hfq (250 nM) were added to the mixture. Next, 30S ribosomal subunits (100 nM) and tRNA-fMet (250 nM) were added and incubated for 10 min at 37°C. The reverse transcription step was initiated by adding dNTPs (500 μM each) and M-MulV-RT (10 U) and the reaction was incubated 15 min at 37°C. Reaction products were resolved on 8% denaturing polyacrylamide-urea gels.
Results
OmrA/B sRNAs and an AdoCbl-dependent riboswitch negatively control btuB mRNA levels
To study the molecular basis of OmrA/B effects on btuB expression, we first analyzed by northern blot assays the levels of endogenous btuB mRNA in a strain expressing OmrA or OmrB from a plasmid using an arabinose-inducible promoter (PBAD). We performed these experiments in WT, Δhfq and rne131 mutant strains, the last of which contains an RNase E variant lacking the C-terminal domain. This mutation prevents the assembly of the RNA degradosome complex (34) and was found to strongly reduce the regulation of several mRNAs targeted by OmrA/B (22). In the WT strain, induction of OmrA resulted in a large decrease in btuB mRNA levels (Figure 1B), consistent with previous microarray data (21). Induction of OmrB also led to a smaller, but clearly visible reduction in btuB mRNA (Figure 1B). In contrast, btuB mRNA levels remained largely unaffected when OmrA/B were induced in Δhfq (Figure 1C) and rne131 mutant strains (Figure 1D), showing that both Hfq and the RNA degradosome are important for OmrA/B regulation of btuB mRNA levels. Of note, the levels of the OmrA/B sRNAs are strongly decreased in an hfq null strain ((22,24), and see later), which may explain the loss of regulation in the Δhfq background. Together, these results show that OmrA and, to a lesser extent OmrB, reduce btuB mRNA levels and rely on Hfq and the RNA degradosome to achieve genetic regulation at the mRNA level.
Previous studies have shown that AdoCbl sensing by the btuB riboswitch modulates both translation initiation (6,14,17,35) and mRNA levels (14,19). In agreement with this, the addition of AdoCbl promoted a decrease in btuB mRNA levels in the WT strain (Figure 1E). In contrast to OmrA/B regulation, we found that this AdoCbl-dependent decrease in mRNA levels was not affected in either Δhfq or rne131 mutant strains (Figure 1E), showing that Hfq and the degradosome are not involved in the riboswitch regulation. These data indicate that the AdoCbl riboswitch relies on a different molecular mechanism than OmrA/B to regulate the levels of the btuB mRNA. Using a BtuB-mScarlet fluorescent reporter fusion as a readout for btuB expression (described later with Figure 5), we also found that deleting the gene for the 3′-5′ exoribonuclease PNPase, an integral part of the degradosome, caused a decrease in the OmrA-mediated control of BtuB-mScarlet as repression dropped from 2.5-fold in the WT strain to 1.4-fold in the pnp mutant even though the OmrA levels are visibly increased in the mutant (Supplementary Figure S5, panels A–C). This is consistent with the known requirement for PNPase for several other sRNA-dependent regulations (36,37). In contrast, regulation by 1 μM or 5 μM AdoCbl was about 5-fold in the WT and more than 8-fold in the pnp mutant (Supplementary Figure S5, panels D–F), thus confirming that sRNA and riboswitch control of btuB are due to different mechanisms.
sRNAs and riboswitch regulation requires different regions of the btuB mRNA
To further characterize the regulation of btuB by OmrA/B, we next employed translational BtuB-LacZ fusions (Figure 2A and see Supplementary Figure S2 for a detailed view of constructs) containing the intact btuB 5′ UTR and increasing portions of btuB coding sequence (CDS) fused in frame to the 10th codon of lacZ. These fusions were expressed under the control of the PBAD arabinose-inducible promoter to ensure that the observed effects did not originate from an endogenous promoter control. In these experiments, OmrA/B were overexpressed from a plasmid with an IPTG-inducible promoter. Our data revealed that OmrA represses by at least 50% the expression of translational fusions containing 81 nucleotides (nt) or more of the CDS (Figure 2B and Supplementary Figure S3A). Stronger OmrA effects were observed when longer regions of the btuB CDS were used: the expression of BtuB120-, BtuB210- and BtuB420-LacZ fusions were reduced by ∼6-, ∼5- and ∼5-fold, respectively (Figure 2B), indicating that full regulation requires more than the first 81 nt of btuB CDS. Overproduction of OmrB showed a similar trend, but with a systematically lower efficiency, as the maximal repression reached only 50% for the BtuB120-, BtuB210- and BtuB420-LacZ translational fusions (Figure 2C and Supplementary Figure S3B). With a similar strategy, we next investigated the region of btuB necessary to regulate mRNA levels by using transcriptional btuB-lacZ fusions (Figure 2A). These constructs were designed with the same regions of btuB mRNA, followed by four codons ending with a UAA stop and fused to lacZ containing its own translation initiation signals. We observed that only the longest fusion, carrying 420 nt of the CDS, was marginally affected by OmrA and OmrB (reduction of lacZ expression by only ∼33% and ∼20%, respectively), while none of the shorter fusions were repressed by the sRNAs (Figure 2B and C). Together, these data confirm that OmrA represses btuB expression more efficiently than OmrB, consistent with their effect on btuB mRNA levels (Figure 1C). Furthermore, a smaller region of btuB CDS was required to control the expression of the translational fusions when compared to the transcriptional fusions. These results strongly suggest that these sRNAs primarily act at the translational level and that the decrease in btuB mRNA likely results from RNase E cleavage of the messenger devoid of ribosomes, downstream of the 210th nt of the btuB CDS.
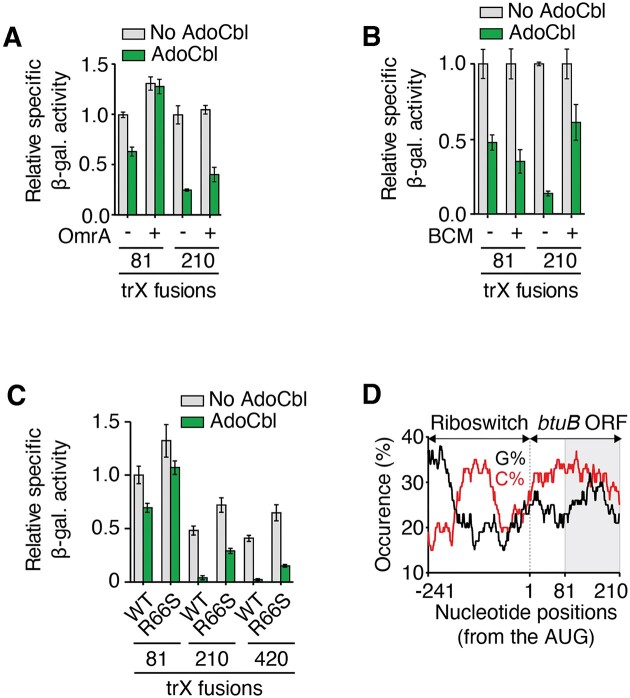
We also determined the minimal btuB region required for riboswitch regulation. Our reporter gene assays revealed that only 18 and 210 nt of btuB CDS are sufficient to trigger strong AdoCbl-dependent regulation of translational or transcriptional constructs, respectively (Figure 2D and Supplementary Figure S3C), in complete agreement with a previous study (14). These results indicate that riboswitch control requires a much smaller region of btuB CDS than sRNA control and confirm that regulation by the riboswitch does not depend on the sRNAs. We further investigated the importance of several 5′-UTR secondary structure elements for riboswitch and OmrA-mediated regulation. Since both regulators modulate the expression of the translational BtuB81-LacZ fusion (Figure 2B and D), we used this construct to characterize the role of two structural elements (helices P13 and P14) that are involved in riboswitch-dependent translation regulation (14,16,17,38). While helix P13 is part of a pseudoknot structure located in the riboswitch aptamer domain, helix P14 directly modulates the access of ribosomes to the SD (Figure 2E) (39). As expected, AdoCbl strongly repressed the WT fusion (by 97%, Figure 2E), and this regulation was lost when the pseudoknot was destabilized via the EP1 mutant. In contrast, repression by OmrA was not affected in the EP1 mutant since it still decreased btuB expression by ∼50%, as observed for the WT (Figure 2E). Similarly, the destabilization of the P14 stem (EP2 and EP3 mutants) resulted in loss or decrease of AdoCbl-dependent btuB gene repression while retaining OmrA regulation (Figure 2E and Supplementary Figure S4A). The partial loss of AdoCbl-dependent repression in the EP2 mutant is most probably caused by the lower degree of structural perturbation of the P14 stem. No AdoCbl-dependent regulation was observed in the context of EP1 and EP3 mutant transcriptional constructs (Supplementary Figure S4B), indicating that mRNA levels remain unaffected as well, which is consistent with the transcriptional effect of the riboswitch being a consequence of the translational effect. Together, these results clearly show that OmrA regulatory activity does not rely on a functional riboswitch to inhibit btuB expression.
OmrA and AdoCbl effectors rely on different regulatory mechanisms
We next assessed whether OmrA binding to btuB mRNA could interfere with the AdoCbl riboswitch regulation. To do so, we employed the 81 nt btuB-lacZ transcriptional fusion whose expression is not regulated by OmrA, but is efficiently repressed by AdoCbl (Figure 2B and D). Remarkably, when OmrA was overexpressed, the effect of AdoCbl was completely abolished (Figure 3A), suggesting that OmrA binding prevents the riboswitch from modulating the mRNA levels of this 81 nt btuB-lacZ transcriptional fusion. However, when repeating these experiments using a longer btuB transcriptional fusion of 210 nt, the addition of AdoCbl decreased the expression of the fusion even in presence of OmrA (Figure 3A). These results indicate that btuB sequence elements located between positions 81 and 210 of the CDS enable a more robust mRNA regulation by the AdoCbl riboswitch that is no longer affected by OmrA. Therefore, we investigated which regulatory mechanisms are at play in this region. We reasoned that, in addition to the known translational repression, the regulation could involve the transcription termination factor Rho, as previously determined for several riboswitches (6), and as suggested for btuB by in vitro assays (40). Accordingly, we monitored the regulation of btuB expression using transcriptional btuB-lacZ fusions in the presence of the Rho inhibitor bicyclomycin (BCM) (41). We found that, while BCM does not perturb AdoCbl regulation of the 81 nt construct, it relieved AdoCbl-dependent repression by ∼4-fold in the 210 nt fusion (Figure 3B). To characterize the regulation of btuB by Rho, we also used the R66S Rho mutant strain. This version of Rho is defective in transcription termination (6,8,42). Similarly to BCM assays (Figure 3B), we found that the AdoCbl-dependent regulation was less efficient in the R66S strain compared to the WT, especially for constructs carrying 210 nts or more of the btuB coding sequence, while the effect of the rho mutant was much less pronounced on the 81 nt fusion (Figure 3C). This supports the idea of Rho regulating the 210 nt (and longer) constructs. Furthermore, to determine if the RNA degradosome was involved in the riboswitch regulation mechanism, we performed β-galactosidase assays in the rne131 strain that is defective in the degradosome assembly (5,34). In this case, similar AdoCbl-dependent repression was obtained in the WT and rne131 strains (Supplementary Figure S4C), indicating that the RNA degradosome is not involved in the regulation of these constructs. Hence, our results suggest that following the AdoCbl-dependent translation inhibition, Rho could target the 81–210 nt region of the btuB ORF to terminate transcription. These results are in complete agreement with recent in vitro experiments showing that Rho transcription termination occurs early within btuB ORF (40). Interestingly, this + 81 to + 210 region of btuB is characterized by a C-rich and G-poor sequence (Figure 3D) that is often associated with the presence of Rho utilization (rut) sites (43,44). Together, our results suggest that the AdoCbl riboswitch and OmrA modulate btuB mRNA levels using different mechanisms that exploit specific btuB sequences to allow Rho-dependent transcription termination (for riboswitch control), and RNA decay mediated by RNase E and the degradosome (for sRNA control).
Figure 3.
Riboswitch regulation relies on Rho to regulate btuB. (A, B) β-Galactosidase assays performed in the absence and presence of AdoCbl when using the btuB81- and btuB210-lacZ transcriptional fusions. Experiments were performed either when expressing OmrA (A) or when adding bicyclomycin (BCM) (B). (C) β-Galactosidase assays performed without and with AdoCbl when using the btuB81-, btuB210- and btuB420-lacZ transcriptional fusions in the WT and rhoR66S strains. (D) Sequence analysis of cytidine (%C) and guanine (%G) distribution in the btuB sequence. A scanning window of 25 nt was used to determine the C and G occurrences as a function of positions from the AUG start site. The gray region highlights btuB region between residues 81 and 210.
OmrA recognizes btuB mRNA via its specific central region
To decipher how OmrA could control btuB expression, we used the IntaRNA in silico prediction tool (45) to search for a potential base-pairing interaction between OmrA and the first 100 nt of the btuB CDS, and extended the prediction using secondary structures prediction with Unafold and RNAfold (46,47) and by hand (Figure 4A). The btuB–OmrA interaction is predicted to rely mostly on the central region of OmrA, which diverges from the OmrB sequence, in complete agreement with the different regulation efficiencies observed for the two sRNAs (Figures 1B, 2B and C). To validate this prediction, different mutations were introduced in OmrA and in a translational fusion of btuB mRNA region (−240 to +99) followed by lacZ coding sequence only a few nts downstream of the pairing prediction (BtuB99-LacZ). This fusion is transcribed from a constitutively expressed PLtetO-1 promoter. We first found that mutating positions 26–36 of OmrA (OmrAM9* mutant) negatively affected the regulation, since the WT fusion was more efficiently repressed by OmrA (5-fold) than by OmrAM9* (1.6-fold) (Figure 4B). Similarly, mutating btuB positions +71 to +78 (btuBM9 mutant) strongly impaired control by the WT OmrA sequence (1.2-fold repression), in agreement with this sequence being important for OmrA recognition. However, combining both the OmrAM9* and btuBM9 mutants to restore the predicted OmrA-btuB interaction only slightly improved repression (1.7-fold) compared to the mutant fusion/WT OmrA pair, and did not lead to significant gain in regulation compared to the WT fusion/OmrAM9* pair (Figure 4B). This could be explained by the presence of the unpaired residues 32–34 and 38–41 in OmrA (Figure 4A), adjacent to the mutated positions in the M9* variant and possibly unfavourable for compensation. Consistent with this, removing these unpaired nucleotides in the OmrAM9* context restored control of the btuBM9 variant (see OmrAopt and OmrAoptM9* mutants in Supplementary Figure S6).
We thus used an additional pair of mutants to assess the OmrA–btuB interaction (mutants btuBM11 and OmrAM11*, Figure 4A). The mutation btuBM11 is not sufficient to prevent OmrA regulation, which is most likely explained by the existence of alternative pairing in this case. Nonetheless, the OmrAM11* variant no longer repressed the WT BtuB-LacZ fusion, but was efficient in regulating the BtuBM11-LacZ fusion (∼2.6-fold repression, Figure 4C), despite the lower levels of OmrAM11* compared to OmrA WT (Figure 4D). Thus, taken together, the M9 and M11 sets of mutants strongly support the hypothesis that the central region of OmrA interacts with residues +66 to +79 of the btuB CDS in vivo.
This base-pairing is highly unusual as all previously reported targets of the OmrA/B sRNAs, such as cirA, fepA, ompR, ompT, csgD, flhDC, dgcM and flgM mRNAs, interact with the conserved 5′-end of OmrA/B (22–27). This specificity of the OmrA-btuB interaction was further examined by comparing the effects of mutations in OmrA 5′ end or central region (Figure 4A, see M5' and M9* mutants, respectively) on btuB or cirA and fepA, two known targets of OmrA 5′-end. The OmrA M5' mutation alleviated the repression of BtuB99-LacZ fusion (Figure 4E) similar to what we observed with OmrA M9*. Hence, neither 5′-end or central region mutation alone abolished regulation completely, which could be explained by the length of the predicted interaction between OmrA and btuB, that may also involve pairing of OmrA 5′-end with btuB residues +83 to +94 (Figure 4A). Consistent with the importance of the conserved OmrA/B 5′-end for regulation, introducing the M5' mutation in OmrB abolished btuB regulation (Supplementary Figure S6C). This further supports both a role of the conserved 5′-end or OmrA/B in btuB control and the fact that the regulation by the central region does not occur with OmrB.
In contrast to btuB, translational fusions FepA45-LacZ and CirA30-LacZ were efficiently repressed by OmrAM9* but were not regulated by OmrAM5' (Figure 4E), as expected with classical OmrA 5′-end targets. These data confirm the crucial and unique role played by the OmrA-specific central region in the control of btuB.
Of note, we also tested the riboswitch regulation of the btuB M9 mutant and found that it was not as strongly (if at all) affected as regulation by OmrA (Supplementary Figure S4D: AdoCbl represses the WT or the M9 fusion by ∼7- and ∼5-fold, respectively). This is consistent with this region of btuB being involved in regulation by sRNAs, but not by the riboswitch. Conversely, mutants that impair riboswitch control do not affect OmrA/B-mediated regulation (Figure 2E and Supplementary Figure S4A). These data further show that sRNA and AdoCbl controls of btuB are independent processes.
Physiological levels of OmrA and OmrB regulate btuB expression
We next analyzed the role of OmrA/B in btuB regulation under more physiological conditions, i.e. when the sRNAs are expressed from their native chromosomal loci. For these assays, we followed the activity of the BtuB99-LacZ translational fusion in an omrAB +strain, or in strains deleted for omrA, omrB or both. These experiments were performed in standard LB medium (measured pH of 6.8), or in acidified LB (pH 4.7) where OmrA/B expression is expected to be strongly induced through OmpR activation (29,48), which was confirmed by Northern blot (Figure 4F). No difference in expression of the WT BtuB99-LacZ fusion was observed in the presence or absence of OmrA and/or OmrB in standard LB (Figure 4G), consistent with the relatively low levels of OmrA/B under this condition (Figure 4F) (29,48). In contrast, when these experiments were performed in acidified LB, the activity of the WT fusion was increased by ∼1.9-fold and ∼1.5-fold in the ΔomrAB and ΔomrA deletion strains, respectively (Figure 4G), confirming the repression of btuB expression by physiological levels of OmrA. No such effect was observed with the sole deletion of OmrB (Figure 4G), further supporting that btuB is preferentially targeted by OmrA rather than by OmrB. Finally, the expression of the M9 mutant version of the BtuB99-LacZ fusion, which is not repressed by OmrA/B, was not significantly affected in any of the omrA/B deletion strains (Figure 4G), in agreement with the direct base-pairing interaction shown in Figure 4A.
The distal face of Hfq is required for btuB regulation by OmrA
Given that the OmrA pairing site is within the btuB CDS, downstream of the translation initiation region (TIR), we next investigated the molecular mechanism by which OmrA represses btuB expression. We first analyzed the role of Hfq in this sRNA regulation by taking advantage of previously characterized point mutants in three different RNA-binding surfaces of Hfq: the proximal face (mutant Q8A), the rim (R16A) and the distal face (Y25D) (49,50). The proximal face and the rim are important for binding to many Hfq-dependent sRNAs, referred to as Class I sRNAs, while the distal face has been implicated in binding successive A–R–N (R = purine, N = any nucleotide) motifs present in the mRNA targets of such sRNAs (49). Of note, OmrA and OmrB display the features of Class I sRNAs as their levels are strongly decreased in the proximal and rim mutants, but not in the distal face mutant (26).
To assess the role of Hfq in the regulation of btuB expression, we employed a BtuB210-mScarlet-I (mSc) translational fusion and monitored mSc fluorescence upon overexpression of OmrA in strains carrying one of the following five hfq alleles: WT hfq, Δhfq, Q8A, R16A, or Y25D. As a control, we also monitored the repression of ompR and sdhC, which are controlled by OmrA/B (22) and Spot42 (51), respectively, using translational mSc fusions in the same hfq backgrounds. Importantly, ompR is a canonical OmrA/B target, because the sRNA pairing site overlaps with the TIR, most likely allowing direct competition with the binding of the ribosomal 30S subunit (22). In contrast, sdhC is repressed by Spot42 sRNA in a non-canonical manner: the sRNA pairs upstream of the TIR and recruits Hfq to the TIR, thus allowing translation repression (51).
In the hfq WT background, the fluorescence of the BtuB-mSc fusion was decreased more than 2-fold upon OmrA overproduction, whereas OmrB had a marginal effect (∼1.3-fold) (Figure 5A and B and Supplementary Figure S7). Under the same conditions, the expression of the OmpR-mSc fusion was decreased more than 3- and 2-fold, respectively (Figure 5C and Supplementary Figure S8). The sRNA-dependent regulation of both btuB and ompR was abolished, or strongly reduced, in the absence of Hfq (Δhfq) or when it was mutated in the proximal (Q8A) or rim (R16A) faces (Figure 5B and C). This is explained, at least in part, by the much lower accumulation of OmrA/B in these mutants (Figure 5E). In contrast, OmrA and OmrB accumulated to higher levels in the context of the Hfq distal face mutant (Y25D) (Figure 5E), as observed previously (26). In this context, the expression of the BtuB-mSc fusion was no longer regulated by OmrA or OmrB (Figure 5B), while ompR-mSc was still repressed more than 5-fold by both sRNAs (Figure 5C). The regulation of ompR in the Y25D mutant strain indicates that OmrA/B do not rely on the Hfq distal face to regulate ompR, consistent with the model that these sRNAs bind directly to the ompR TIR and compete with the 30S ribosomal subunit (28). In contrast, the lack of regulation of btuB in the hfq Y25D mutant suggests that OmrA/B may instead use a different mechanism in this case, in which Hfq binding to the mRNA is required. Such a non-canonical control mechanism was observed for instance in the modulation of sdhC expression by Spot42 (51) or of manX by SgrS and DicF sRNAs (52), with the sRNAs recruiting Hfq to the TIR for translation inhibition. Interestingly, the repression of sdhC and btuB by their respective sRNAs was similarly strongly reduced in all four hfq mutants (Figure 5B and D and Supplementary Figure S9), hinting that the regulatory mechanism involved in both cases could be similar. In support of an important role of Hfq in btuB control, we also found that btuB expression was increased in the Δhfq or in the hfqY25D strain (Supplementary Figure S10), while hfq overexpression from a plasmid (53) led to btuB repression (Supplementary Figure S11). Again, the same pattern was observed with the expression of SdhC-mSc, while no appreciable effect of the Hfq overexpression was visible on OmpR-mSc in these experiments.
Hfq interacts with the btuB mRNA and represses its expression
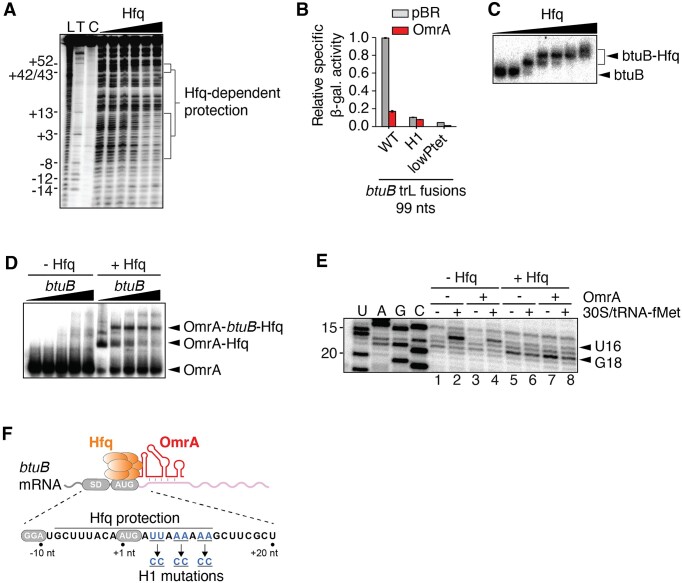
These results prompted us to investigate the interaction between Hfq and the btuB mRNA, and its function in btuB regulation, in greater detail using in vitro approaches. We first performed lead acetate footprinting experiments to identify the Hfq binding site on the btuB mRNA, using a btuB mRNA fragment ranging from nts –80 to +161. An Hfq-dependent protection was observed between positions –8 to +12 of the btuB mRNA, thus overlapping with the btuB start codon (Figure 6A). An additional protected region was observed between nts ∼ +45 to +50, suggesting that Hfq may bind to non-adjacent RNA regions. These two protected regions could be close to each other due to the formation of a stem-loop structure between nucleotides +18 to +44 (as predicted in silico). Importantly, the −8 to +12 protected region contains repetitive A–R–N motifs that are consensus binding sites for the Hfq distal face. We thus tested in vivo the effect of a mutation that eliminates these A–R–N motifs on the control by OmrA. The introduction of three CC pairs within the Hfq binding site of the BtuB99-LacZ fusion (Figure 6F, H1 mutant) strongly reduced the regulation by OmrA, ∼5.8-fold and ∼1.3-fold for the WT and H1 mutant (Figure 6B), respectively. However, this H1 change also strongly decreased the expression of the BtuB99-LacZ fusion. To rule out the possibility that the reduced repression of the H1 mutant by OmrA is due to its lower expression, we verified that a low expression of btuB, for instance with a mutation in the PLtetO-1 promoter (lowPtet), still allowed OmrA regulation (Figure 6B, ∼4.2-fold). Using translational fusions carrying increasing portions of btuB coding sequence, we next investigated the regions of btuB mRNA that are important for the repression upon Hfq overexpression. The expression of btuB was repressed in various constructs upon inducing Hfq overexpression, with the effect being the strongest when the two Hfq binding sites identified in Figure 6A are present on the fusion: a ∼2- and ∼2.9-fold reduction were obtained in the 45 and the 99 nt constructs, respectively (Supplementary Figure S12A). In addition, Hfq overproduction also led to btuB down-regulation in the absence of OmrA and OmrB chromosomal genes, raising the possibility of additional roles for Hfq in btuB control (Supplementary Figure S12A). Furthermore, the H1 mutant abolished regulation (Supplementary Figure S12B), consistent with the ARN sites being important for the repression. Combined, these data strongly suggest that OmrA regulation of btuB requires Hfq and its binding in the vicinity of the TIR to repress btuB.
Figure 6.
btuB mRNA interacts with OmrA and Hfq in vitro. (A) Lead acetate probing of Hfq binding to the btuB mRNA. Radioactively labeled btuB RNA corresponding to nts –80 to +161 relative to start codon was incubated with increasing concentrations of Hfq (0 to 20 nM). Lanes L, T and C correspond to an alkaline hydroxide ladder, a partial RNase T1 digestion and a non-reacted control, respectively. The positions relative to the AUG are indicated on the left. (B) β-galactosidase assays of BtuB99-LacZ translational fusions either WT or carrying the H1 mutation, or weakened version of the PLtetO-1 promoter (lowPtet) in the presence of OmrA. Shown are the average β-galactosidase activities and standard deviations of two independent experiments. (C) EMSA assays performed using radiolabeled btuB RNA with increasing concentrations of Hfq. Arrows on the right of the gel indicate the free btuB RNA and the btuB-Hfq complex. The presence of multiple species when increasing Hfq concentration suggests that Hfq may have multiple btuB RNA binding sites. (D) EMSA assays performed with radiolabeled OmrA incubated with increasing concentrations of btuB RNA in the absence or presence of Hfq. The arrows on the right show the free OmrA and the OmrA-Hfq-btuB complexes. (E) Toeprint assays monitoring the effect of Hfq and OmrA on the formation of the translation initiation complex. Experiments were performed in the absence or presence of Hfq, OmrA or 30S/tRNA-fMet. Lanes U, A, G and C represent sequencing ladders. The full gel is shown in Supplementary Figure S13A. (F) Scheme representing the btuB mRNA with Hfq and OmrA. The Hfq-protected region, the Shine-Dalgarno (SD) and AUG are shown. Nucleotides changed in the H1 mutant are shown in blue.
We next used electrophoresis mobility shift assays (EMSA) to characterize the interaction between btuB, OmrA and Hfq. Our results showed that a bi-partite btuB-Hfq complex is formed upon increasing the concentration of Hfq (Figure 6C). Furthermore, the btuB-OmrA interaction was only detected in the presence of Hfq (Figure 6D), indicating that Hfq is required for the formation of a stable btuB-OmrA complex. These results suggest a regulatory mechanism in which OmrA and Hfq interact directly with btuB to modulate its genetic expression.
To directly assess the role of Hfq in inhibiting translation initiation, we used in vitro toeprinting assays in the presence or absence of Hfq (Figure 6E, see full gel in Supplementary Figure S13A). The assay was performed using the 30S ribosomal subunit, btuB mRNA and the initiator tRNA (tRNA-fMet) (54). In the presence of 30S and tRNA-fMet, a reverse transcription stop (toeprint) was detected at position U16 (Figure 6E, lane 2), indicating that the 30S subunit is bound to the start codon of the btuB mRNA, in agreement with previous data (39). In the presence of Hfq, a clear loss of the U16 toeprint was observed (Figure 6E, lane 6), showing that Hfq prevents the 30S subunit from binding the btuB mRNA. Instead, a new reverse transcription stop was observed at position G18, likely due to Hfq blocking the reverse transcriptase at this position, in good agreement with our lead probing data (Figure 6A). When the experiment was performed with OmrA, the addition of Hfq still resulted in the loss of U16 toeprint and the generation of the G18 signal (Figure 6E, lanes 4 and 8), corresponding to Hfq binding. Toeprint assays performed using the H1 btuB variant did not show the U16 toeprint but rather a different profile of reverse transcriptase stops (Supplementary Figure S13B), suggesting that translation initiation on the H1 btuB variant is compromised. This goes along with the fact that activity of the H1 BtuB-LacZ fusion is 10-fold reduced compared to the WT control (Figure 6B).
Together, these results demonstrate that Hfq binds directly to btuB mRNA upstream of the OmrA pairing site and prevents the 30S subunit from binding to the btuB AUG start codon in vitro (Figure 6E). This, coupled with the requirement for Hfq for OmrA-mediated regulation in vivo (Figures 1C, 5B and 6B), suggests that binding of OmrA helps recruit Hfq to the btuB TIR, similar to the way Spot42 or SgrS/DicF recruit Hfq to regulate sdhC or manX expression, respectively (51,52).
Discussion
The findings reported here identify btuB as a new member of the Omr regulon. Even though the physiological role of these two sRNAs is still not fully understood, btuB is reminiscent of other previously validated targets of OmrA/B. First, BtuB is an outer membrane protein (OMP), like several other known targets (OmpT, CirA, FecA and FepA). Second, like CirA, FecA and FepA, BtuB depends on the TonB system for the uptake of its substrate, namely AdoCbl.
Importantly, there is one notable difference between btuB and the other OmrA/B targets. While the pairing of OmrA/B to all previously recognized targets relies on the sRNAs 5′ end ((22–27) and our unpublished data for fecA), control of btuB involves the central region of OmrA, that distinguishes it from OmrB. Consistent with this, deletion of OmrA is sufficient to increase btuB expression in acidified medium, while under the same conditions, the deletion of OmrB alone had no effect (Figure 4G). However, in the absence of OmrA, deletion of OmrB allowed a further increase in btuB expression, but not that of btuB carrying the M9 mutation (Figure 4G), suggesting that OmrB can still pair to btuB mRNA, although with a much lower efficiency than OmrA. In this regard, a 9 bp complementarity exists between residues 29 to 37 of OmrB and +69 to +77 of btuB (shown in Supplementary Figure S6E), but whether this participates in regulation is still unclear. Furthermore, it is possible that, similarly to OmrA (Figure 4A), OmrB targets the +85 to +94 btuB region through its 5′ domain. While a role for OmrB 5′ end is supported by the mutation M5' abolishing the regulation of btuB (Supplementary Figure S6C), more work would be required to ascertain the OmrB-btuB regulatory interaction. In sum, btuB appears as a preferential OmrA target, while modulation by OmrB is only marginal and possibly requires more specific conditions.
To our knowledge, this is the first report of a preferential OmrA target via pairing to the OmrA specific region, indicating that, in addition to a common regulon, some genes are likely to be regulated by OmrA alone. The reverse could also be true, i.e. that some genes could be controlled by OmrB alone, even though no such examples have been described so far. Differential control of targets such as reported here for btuB is likely to have contributed to the conservation of both OmrA and OmrB sRNAs in enterobacteria.
The stimulation of OmpR activity in acidic conditions (29,48) provides a rationale to explain the induction of OmrA/B following acid stress, which ultimately leads to btuB repression (Figure 4G). In such low pH conditions, btuB transcription is also repressed by the transcriptional regulator GadX (55), thus ensuring that btuB mRNA levels are efficiently regulated. Importantly, low pH conditions also increase the proton motive force (56,57), which is crucial for cobalamin import by BtuB (53) and the function of other TonB-dependent transporters. Therefore, it is expected that the AdoCbl riboswitch regulation mechanism provides an additional level of regulation in conditions that are not acidic enough to repress btuB through OmrA/B and GadX. Globally, with the above results, our data show that btuB expression is highly regulated at multiple levels by the AdoCbl riboswitch and by the sRNAs OmrA, OmrB and GadX.
From a mechanistic perspective, Hfq is strictly required for the control of btuB by OmrA/B. This was expected and has been observed for many of the other Omr targets since OmrA/B levels significantly drop in the absence of this chaperone (Figure 5E) (22,24,26). More unusual, however, is the fact that this control was also abolished in an Hfq distal face mutant (Y25D) as well. This is not generally observed for canonical regulation where sRNAs pair to the RBS (such as ompR control by OmrA/B, Figure 5C). Because the Hfq distal face has been shown to be involved in mRNA binding, this strongly indicates that OmrA control of btuB is dependent on the ability of Hfq to interact with this target mRNA. Interestingly, the same is true for the control of sdhC by Spot42 (Figure 5D) and of dgcM by OmrA/B (26). In the latter case, Hfq was found to mediate a change in the structure of dgcM mRNA facilitating the interaction with OmrA/B (26), whereas in the former case, as mentioned previously, Hfq is directly responsible for the inhibition of 30S binding to sdhC (51). Our in vitro experiments showed that Hfq directly binds to the TIR of btuB mRNA, in a region containing a canonical binding site formed by multiple (A–R–N) motifs, thereby inhibiting 30S binding (Figure 6E). In contrast, OmrA by itself had a much weaker effect on toeprint formation (Figure 6E). Together, these results suggest a mechanism reminiscent of that described for the Spot42-sdhC, SgrS-manX or DicF-manX pairs, where the central region of OmrA binds with btuB mRNA, thereby promoting Hfq recruitment and inhibiting 30S binding. Furthermore, Hfq significantly improves the formation of an OmrA–btuB complex in vitro (Figure 6D). The precise mechanism underlying this observation is still unclear at this stage, but Hfq could be involved in the remodeling of OmrA or btuB mRNA structure to facilitate formation of the duplex, similarly to what has been reported for dgcM (26). It is also worth noting that Hfq overexpression represses btuB, even in the absence of OmrA and OmrB sRNAs (Supplementary Figure S12A). This raises the possibility that Hfq could bind to btuB mRNA, without the need of ‘helper’ sRNAs, and promote gene regulation. Alternatively, sRNAs other than OmrA/B could underly this observation, via binding to btuB mRNA and recruiting Hfq.
While most studies performed in the last decades have led to the idea that sRNAs regulate gene expression at the post-transcriptional level (58–60), it has recently been shown that sRNAs can also operate cotranscriptionally by controlling Rho transcription termination (61–63). For ChiX, Rho transcription termination is indirectly controlled through the modulation of translation initiation in Salmonella enterica (64). However, for several sRNAs such as DsrA, ArcZ and RprA, Rho activity is directly modulated by sRNA association to the mRNA, presumably by inhibiting Rho binding or translocation (7). In contrast, current data indicate that riboswitches mostly regulate gene expression at the co-transcriptional level both in Bacillus subtilis (65,66) by modulating intrinsic transcription terminators, and in E. coli by relying on Rho to downregulate mRNA levels following translation inhibition (6). It was also shown that post-transcriptional regulation may be used by some riboswitches (66), suggesting that like sRNAs, riboswitches may exert control both during and after the completion of the transcriptional process.
It is remarkable that translation initiation of btuB is selectively modulated by both the AdoCbl riboswitch and OmrA/B sRNAs, which affect mRNA levels through different mechanisms. In agreement with previous studies (14,19,20,67), our data suggest that the riboswitch primarily controls translation initiation, which then consequently modulates RNA levels through the action of regulatory regions embedded within btuB ORF. In particular, it was previously suggested that a transcription attenuator (positions +18 to +48) could be involved in decreasing mRNA levels (19), whose formation would presumably be favored upon translation inhibition. Interestingly, the loss of riboswitch regulation in the presence of OmrA in the 81 nt transcriptional construct (Figure 3A) could be attributed to OmrA binding to btuB and disrupting the formation of the attenuator structure. However, for mRNAs carrying longer regions of btuB coding sequence, the Rho-dependent transcription termination plays a major role in the decrease of mRNA levels following riboswitch control (Figure 3).
As the riboswitch-mediated decrease in btuB mRNA is independent of both Hfq and the degradosome (Figure 1), this strongly suggests that blocking translation initiation of btuB is not sufficient to trigger RNase E-mediated decay of this mRNA. Instead, the degradation that is observed upon OmrA/B overexpression most likely relies on the recruitment of RNase E and the degradosome to the btuB mRNA through the Hfq-RNA complex (68,69). Conversely, the fact that much longer regions of btuB mRNA are required for OmrA/B control than for riboswitch control indicates that, while the riboswitch action leads to premature Rho-dependent transcription termination, this may not be the case for the sRNAs. Such a mechanistic difference is compatible with the AdoCbl riboswitch and sRNAs OmrA/B regulating btuB gene expression respectively at the co-transcriptional and post-transcriptional levels. Additional experiments will now be required to fully understand to which extent these regulatory activities are confined to co-transcriptional and post-transcriptional mechanisms.
It has been previously reported that riboswitches and sRNAs may act in concert to control gene expression (70–72). Indeed, in Listeria monocytogenes, transcription termination caused by the S-adenosylmethionine riboswitch generates an sRNA that controls the expression of PrfA, which is involved in virulence gene expression (70). Furthermore, it was shown in Enterococcus faecalis and L. monocytogenes that the AdoCbl riboswitch controls the formation of an sRNA containing ANTAR RNA elements, the latter being important for the regulation of the eut genes involved in ethanolamine utilization (71,72). While these studies revealed that riboswitches might act as ‘pre-sRNA’ regulatory elements, the results presented in our study rather indicate that riboswitches and sRNAs both modulate the expression of the btuB gene.
Riboswitches and sRNAs may participate in the regulation of same mRNA populations, thereby increasing the complexity of gene regulation mechanisms. While riboswitches are in most cases restricted to 5′-UTRs, the combination with sRNA regulation that can target multiple mRNA regions could significantly enhance gene regulation efficiency at both the co-transcriptional and post-transcriptional levels. The great variety of mechanisms through which riboswitches and sRNAs regulate gene expression could therefore be used and combined in bacteria to ensure cellular homeostasis under multiple conditions. In this regard, it is interesting that other E. coli mRNAs known to be regulated by riboswitches were found enriched after immunoprecipitation with the RNA chaperones ProQ or Hfq, and that sRNA-mRNA pairs such as MicA-moaA or CyaR-lysC were identified in the RNA-RNA interactome studied in the Hfq co-IP fraction (73). This suggests that the dual riboswitch-sRNA control of a single gene reported here is not restricted to btuB mRNA, and the study of other systems is likely to be instructive in the future.
Supplementary Material
Acknowledgements
We thank D. Schu and N. Majdalani (NIH, Bethesda) for the hfq variants strains, and E. Massé (Université de Sherbrooke) for strains. We are grateful to N. Fraikin and L. Van Melderen (ULB, Belgium) for the pNF02 plasmid carrying the mScarlet gene, as well as E. Hajnsdorf (IBPC, Paris) for the pCLHfq plasmid. We thank A. Lavigueur, M. Springer and C. Condon for critical reading of the manuscript.
Notes
Present address: Laurène Bastet, Venvirotech Biotechnology SL, 08130 Santa Perpètua de Modoga, Barcelona, Spain.
Present address: Alexey P. Korepanov, Institute of Protein Research RAS, 142290 Pushchino, Moscow Region, Russia.
Contributor Information
Laurène Bastet, Department of Biology, Faculty of Science, Université de Sherbrooke, Sherbrooke, Quebec J1K 2R1, Canada.
Alexey P Korepanov, Expression Génétique Microbienne, UMR8261 CNRS, Université Paris Cité, Institut de Biologie Physico-Chimique, 75005, Paris, France.
Jonathan Jagodnik, Expression Génétique Microbienne, UMR8261 CNRS, Université Paris Cité, Institut de Biologie Physico-Chimique, 75005, Paris, France.
Jonathan P Grondin, Department of Biology, Faculty of Science, Université de Sherbrooke, Sherbrooke, Quebec J1K 2R1, Canada.
Anne-Marie Lamontagne, Department of Biology, Faculty of Science, Université de Sherbrooke, Sherbrooke, Quebec J1K 2R1, Canada.
Maude Guillier, Expression Génétique Microbienne, UMR8261 CNRS, Université Paris Cité, Institut de Biologie Physico-Chimique, 75005, Paris, France.
Daniel A Lafontaine, Department of Biology, Faculty of Science, Université de Sherbrooke, Sherbrooke, Quebec J1K 2R1, Canada.
Data availability
The data underlying this article are available in the article and in its online supplementary material. Further data that support the findings of this study are available from the corresponding authors, M.G and D.A.L., upon reasonable request.
Supplementary data
Supplementary Data are available at NAR Online.
Funding
Canadian Institutes of Health Research (PJT153205, PJT183678); Natural Sciences and Engineering Research Council of Canada (RGPIN-2020-06241) (to D.A.L.); European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program [818750 to M.G.]; European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie grant agreement No 101034407; Research in the UMR8261 is also supported by the CNRS and the ‘Initiative d’Excellence’ program from the FrenchState [‘DYNAMO’, ANR-11-LABX-0011]. Funding for open access charge: CIHR, NSERC and ERC.
Conflict of interest statement. None declared.
References
- 1. Waters L.S., Storz G. Regulatory RNAs in bacteria. Cell. 2009; 136:615–628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hör J., Matera G., Vogel J., Gottesman S., Storz G. Trans-acting small RNAs and their effects on gene expression in Escherichia coli and salmonella enterica. EcoSal Plus. 2020; 9: 10.1128/ecosalplus.ESP-0030-2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mccown P.J., Corbino K.A., Stav S., Sherlock M.E., Breaker R.R. Riboswitch diversity and distribution. RNA. 2017; 23:995–1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Serganov A., Nudler E. A decade of riboswitches. Cell. 2013; 152:17–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Caron M.-P., Bastet L., Lussier A., Simoneau-Roy M., Massé E., Lafontaine D.A. Dual-acting riboswitch control of translation initiation and mRNA decay. Proc. Natl. Acad. Sci. U.S.A. 2012; 109:E3444–E3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Bastet L., Chauvier A., Singh N., Lussier A., Lamontagne A.-M.M., Prévost K., Massé E., Wade J.T., Lafontaine D.A. Translational control and Rho-dependent transcription termination are intimately linked in riboswitch regulation. Nucleic Acids Res. 2017; 45:7474–7486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sedlyarova N., Shamovsky I., Bharati B.K., Epshtein V., Chen J., Gottesman S., Schroeder R., Nudler E. sRNA-mediated control of transcription termination in E. coli. Cell. 2016; 167:111–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ghosh T., Jahangirnejad S., Chauvier A., Stringer A.M., Korepanov A.P., Cote J.-P., Wade J.T., Lafontaine D.A. Direct and indirect control of rho-dependent transcription termination by the Escherichia coli lysC riboswitch. RNA. 2024; 30:381–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Takemoto N., Tanaka Y., Inui M. Rho and RNase play a central role in FMN riboswitch regulation in Corynebacterium glutamicum. Nucleic Acids Res. 2015; 43:520–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vogel J., Luisi B.F. Hfq and its constellation of RNA. Nat. Rev. Micro. 2011; 9:578–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Park S., Prévost K., Heideman E.M., Carrier M.-C., Azam M.S., Reyer M.A., Liu W., Massé E., Fei J. Dynamic interactions between the RNA chaperone Hfq, small regulatory RNAs, and mRNAs in live bacterial cells. eLife. 2021; 10:e64207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Małecka E.M., Woodson S.A. Stepwise sRNA targeting of structured bacterial mRNAs leads to abortive annealing. Mol. Cell. 2021; 81:1988–1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Holmqvist E., Berggren S., Rizvanovic A. RNA-binding activity and regulatory functions of the emerging sRNA-binding protein ProQ. Biochim. Biophys. Acta (BBA) - Gene Regul. Mech. 2020; 1863:194596. [DOI] [PubMed] [Google Scholar]
- 14. Nou X., Kadner R.J. Coupled changes in translation and transcription during cobalamin-dependent regulation of btuB expression in Escherichia coli. J. Bacteriol. 1998; 180:6719–6728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bradbeer C., Kenley J.S., Di Masi D.R., Leighton M. Transport of vitamin B12 in Escherichia coli. Corrinoid specificities of the periplasmic B12-binding protein and of energy-dependent B12 transport. J. Biol. Chem. 1978; 253:1347–1352. [PubMed] [Google Scholar]
- 16. Perdrizet 2nd G.A., Artsimovitch I., Furman R., Sosnick T.R., Pan T. Transcriptional pausing coordinates folding of the aptamer domain and the expression platform of a riboswitch. Proc. Natl. Acad. Sci. U.S.A. 2012; 109:3323–3328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lussier A., Bastet L., Chauvier A., Lafontaine D.A. A kissing loop is important for btuB riboswitch ligand sensing and regulatory control. J. Biol. Chem. 2015; 290:26739–26751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Johnson J.E. Jr, Reyes F.E., Polaski J.T., Batey R.T. B12 cofactors directly stabilize an mRNA regulatory switch. Nature. 2012; 492:133–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Franklund C.V., Kadner R.J. Multiple transcribed elements control expression of the Escherichia coli btuB gene. J. Bacteriol. 1997; 179:4039–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ravnum S., Andersson D.I. Vitamin B12 repression of the btuB gene in Salmonellatyphimurium is mediated via a translational control which requires leader and coding sequences. Mol. Microbiol. 1997; 23:35–42. [DOI] [PubMed] [Google Scholar]
- 21. Guillier M., Gottesman S. Remodelling of the Escherichia coliouter membrane by two small regulatory RNAs. Mol. Microbiol. 2006; 59:231–247. [DOI] [PubMed] [Google Scholar]
- 22. Guillier M., Gottesman S. The 5’ end of two redundant sRNAs is involved in the regulation of multiple targets, including their own regulator. Nucleic Acids Res. 2008; 36:6781–6794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. De Lay N., Gottesman S. A complex network of small non-coding RNAs regulate motility in Escherichia coli. Mol. Microbiol. 2012; 86:524–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Holmqvist E., Reimegård J., Sterk M., Grantcharova N., Römling U., Wagner E.G.H. Two antisense RNAs target the transcriptional regulator CsgD to inhibit curli synthesis. EMBO J. 2010; 29:1840–1850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Jagodnik J., Chiaruttini C., Guillier M. Stem-loop structures within mRNA coding sequences activate translation initiation and mediate control by small regulatory RNAs. Mol. Cell. 2017; 68:158–170. [DOI] [PubMed] [Google Scholar]
- 26. Hoekzema M., Romilly C., Holmqvist E., Wagner E.G.H. Hfq-dependent mRNA unfolding promotes sRNA-based inhibition of translation. EMBO J. 2019; 38:e101199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Romilly C., Hoekzema M., Holmqvist E., Wagner E.G.H. Small RNAs OmrA and OmrB promote class III flagellar gene expression by inhibiting the synthesis of anti-Sigma factor FlgM. RNA Biology. 2020; 17:872–880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Brosse A., Korobeinikova A., Gottesman S., Guillier M. Unexpected properties of sRNA promoters allow feedback control via regulation of a two-component system. Nucleic Acids Res. 2016; 44:9650–9666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Stincone A., Daudi N., Rahman A.S., Antczak P., Henderson I., Cole J., Johnson M.D., Lund P., Falciani F. A systems biology approach sheds new light on Escherichia coliacid resistance. Nucleic Acids Res. 2011; 39:7512–7528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Miller J.H. Experiments in Molecular Genetics Laboratory. 1972; NY: Cold Spring Harbor Laboratory Press. [Google Scholar]
- 31. Lemay J.F., Penedo J.C., Tremblay R., Lilley D.M.J., Lafontaine D.A.A. Folding of the adenine riboswitch. Chem. Biol. 2006; 13:857–868. [DOI] [PubMed] [Google Scholar]
- 32. Aiba H., Adhya S., de Crombrugghe B. Evidence for two functional gal promoters in intact Escherichia colicells. J. Biol. Chem. 1981; 256:11905–11910. [PubMed] [Google Scholar]
- 33. Desnoyers G., Morissette A., Prevost K., Masse E. Small RNA-induced differential degradation of the polycistronic mRNA iscRSUA. EMBO J. 2009; 28:1551–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Kido M., Yamanaka K., Mitani T., Niki H., Ogura T., Hiraga S. RNase E polypeptides lacking a carboxyl-terminal half suppress a mukB mutation in Escherichia coli. J. Bacteriol. 1996; 178:3917–3925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Nahvi A., Sudarsan N., Ebert M.S., Zou X., Brown K.L., Breaker R.R. Genetic control by a metabolite binding mRNA. Chem. Biol. 2002; 9:1043. [DOI] [PubMed] [Google Scholar]
- 36. Bandyra K.J., Sinha D., Syrjanen J., Luisi B.F., De Lay N.R. The ribonuclease polynucleotide phosphorylase can interact with small regulatory RNAs in both protective and degradative modes. RNA. 2016; 22:360–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Dendooven T., Sinha D., Roeselová A., Cameron T.A., De Lay N.R., Luisi B.F., Bandyra K.J. A cooperative PNPase-Hfq-RNA carrier complex facilitates bacterial riboregulation. Mol. Cell. 2021; 81:2901–2913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Ravnum S., Andersson D.I. An adenosyl-cobalamin (coenzyme-B12)-repressed translational enhancer in the cob mRNA of Salmonella typhimurium. Mol. Microbiol. 2001; 39:1585–1594. [DOI] [PubMed] [Google Scholar]
- 39. Nou X., Kadner R.J. Adenosylcobalamin inhibits ribosome binding to btuB RNA. Proc. Natl. Acad. Sci. U.S.A. 2000; 97:7190–7195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Nadiras C., Eveno E., Schwartz A., Figueroa-Bossi N., Boudvillain M. A multivariate prediction model for Rho-dependent termination of transcription. Nucleic Acids Res. 2018; 46:8245–8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kohn H., Widger W. The molecular basis for the mode of action of bicyclomycin. Curr. Drug Targets Infect. Disord. 2005; 5:273–295. [DOI] [PubMed] [Google Scholar]
- 42. Martinez A., Burns C.M., Richardson J.P. Residues in the RNP1-like sequence motif of Rho protein are involved in RNA-binding affinity and discrimination. J. Mol. Biol. 1996; 257:909–918. [DOI] [PubMed] [Google Scholar]
- 43. Hollands K., Proshkin S., Sklyarova S., Epshtein V., Mironov A., Nudler E., Groisman E.A. Riboswitch control of Rho-dependent transcription termination. Proc. Nat. Acad. Sci. U.S.A. 2012; 109:5376–5381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Figueroa-Bossi N., Schwartz A., Guillemardet B., D’Heygere F., Bossi L., Boudvillain M. RNA remodeling by bacterial global regulator CsrA promotes Rho-dependent transcription termination. Genes Dev. 2014; 28:1239–1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mann M., Wright P.R., Backofen R. IntaRNA 2.0: enhanced and customizable prediction of RNA-RNA interactions. Nucleic Acids Res. 2017; 45:W435–W439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zuker M. Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 2003; 31:3406–3415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Markham N.R., Zuker M. UNAFold: software for nucleic acid folding and hybridization. Methods Mol. Biol. 2008; 453:3–31. [DOI] [PubMed] [Google Scholar]
- 48. Quinn H.J., Cameron A.D.S., Dorman C.J. Bacterial regulon evolution: distinct responses and roles for the identical OmpR proteins of Salmonella Typhimuriumand Escherichia coliin the acid stress response. PLoS Genet. 2014; 10:e1004215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Schu D.J., Zhang A., Gottesman S., Storz G. Alternative Hfq-sRNA interaction modes dictate alternative mRNA recognition. EMBO J. 2015; 34:2557–2573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Zhang A., Schu D.J., Tjaden B.C., Storz G., Gottesman S. Mutations in interaction surfaces differentially impact E. coli Hfq association with small RNAs and their mRNA targets. J. Mol. Biol. 2013; 425:3678–3697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Desnoyers G., Masse E. Noncanonical repression of translation initiation through small RNA recruitment of the RNA chaperone Hfq. Genes Dev. 2012; 26:726–739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Azam M.S., Vanderpool C.K. Translational regulation by bacterial small RNAs via an unusual Hfq-dependent mechanism. Nucleic Acids Res. 2018; 46:2585–2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Ziolkowska K., Derreumaux P., Folichon M., Pellegrini O., Régnier P., Boni I.V., Hajnsdorf E. Hfq variant with altered RNA binding functions. Nucleic Acids Res. 2006; 34:709–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hartz D., McPheeters D.S., Traut R., Gold L. Extension inhibition analysis of translation initiation complexes. Methods Enzymol. 1988; 164:419–425. [DOI] [PubMed] [Google Scholar]
- 55. Lei G.-S., Syu W.-J., Liang P.-H., Chak K.-F., Hu W.S., Hu S.-T. Repression of btuB gene transcription in Escherichia coliby the GadX protein. BMC Microbiol. 2011; 11:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kashket E.R. Proton motive force in growing Streptococcus lactisand Staphylococcus aureus cells under aerobic and anaerobic conditions. J. Bacteriol. 1981; 146:369–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Minamino T., Imae Y., Oosawa F., Kobayashi Y., Oosawa K. Effect of intracellular pH on rotational speed of bacterial flagellar motors. J. Bacteriol. 2003; 185:1190–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Storz G., Vogel J., Wassarman K.M. Regulation by small RNAs in bacteria: expanding frontiers. Mol. Cell. 2011; 43:880–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Wagner E.G.H., Romby P. Small RNAs in bacteria and archaea: who they are, what they do, and how they do it. Adv. Genet. 2015; 90:133–208. [DOI] [PubMed] [Google Scholar]
- 60. Nitzan M., Rehani R., Margalit H. Integration of bacterial small RNAs in regulatory networks. Annu. Rev. Biophys. 2017; 46:131–148. [DOI] [PubMed] [Google Scholar]
- 61. Reyer M.A., Chennakesavalu S., Heideman E.M., Ma X., Bujnowska M., Hong L., Dinner A.R., Vanderpool C.K., Fei J. Kinetic modeling reveals additional regulation at co-transcriptional level by post-transcriptional sRNA regulators. Cell Rep. 2021; 36:109764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Chen J., Morita T., Gottesman S. Regulation of transcription termination of small RNAs and by small RNAs: molecular mechanisms and biological functions. Front. Cell. Infect. Microbiol. 2019; 9:201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Bossi L., Figueroa-Bossi N., Bouloc P., Boudvillain M. Regulatory interplay between small RNAs and transcription termination factor Rho. Biochim. Biophys. Acta Gene Regul. Mech. 2020; 1863:194546. [DOI] [PubMed] [Google Scholar]
- 64. Bossi L., Schwartz A., Guillemardet B., Boudvillain M., Figueroa-Bossi N. A role for Rho-dependent polarity in gene regulation by a noncoding small RNA. Genes Dev. 2012; 26:1864–1873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Wickiser J.K., Winkler W.C., Breaker R.R., Crothers D.M. The speed of RNA transcription and metabolite binding kinetics operate an FMN riboswitch. Mol. Cell. 2005; 18:49–60. [DOI] [PubMed] [Google Scholar]
- 66. Lemay J.-F., Desnoyers G., Blouin S., Heppell B., Bastet L., St-Pierre P., Massé E., Lafontaine D.A. Comparative study between transcriptionally- and translationally-acting adenine riboswitches reveals key differences in riboswitch regulatory mechanisms. PLos Genet. 2011; 7:e1001278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Lundrigan M.D., Koster W., Kadner R.J. Transcribed sequences of the Escherichia coli btuBgene control its expression and regulation by vitamin B12. Proc. Natl. Acad. Sci. U.S.A. 1991; 88:1479–1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Morita T., Maki K., Aiba H. RNase E-based ribonucleoprotein complexes: mechanical basis of mRNA destabilization mediated by bacterial noncoding RNAs. Genes Dev. 2005; 19:2176–2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Caillet J., Baron B., Boni I.V., Caillet-Saguy C., Hajnsdorf E. Identification of protein-protein and ribonucleoprotein complexes containing Hfq. Sci. Rep. 2019; 9:14054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Loh E., Dussurget O., Gripenland J., Vaitkevicius K., Tiensuu T., Mandin P., Repoila F., Buchrieser C., Cossart P., Johansson J. A trans-acting riboswitch controls expression of the virulence regulator PrfA in Listeria monocytogenes. Cell. 2009; 139:770–779. [DOI] [PubMed] [Google Scholar]
- 71. DebRoy S., Gebbie M., Ramesh A., Goodson J.R., Cruz M.R., van Hoof A., Winkler W.C., Garsin D.A. Riboswitches. A riboswitch-containing sRNA controls gene expression by sequestration of a response regulator. Science. 2014; 345:937–940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Mellin J.R., Koutero M., Dar D., Nahori M.-a.A., Sorek R., Cossart P. Riboswitches. Sequestration of a two-component response regulator by a riboswitch-regulated noncoding RNA. Science. 2014; 345:940–943. [DOI] [PubMed] [Google Scholar]
- 73. Melamed S., Adams P.P., Zhang A., Zhang H., Storz G. RNA-RNA interactomes of ProQ and Hfq reveal overlapping and competing roles. Mol. Cell. 2020; 77:411–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material. Further data that support the findings of this study are available from the corresponding authors, M.G and D.A.L., upon reasonable request.