Abstract
Flavobacterium is a genus within the phylum Bacteroidota that remains relatively unexplored. Recent analyses of plant microbiota have identified the phylum Bacteroidota as a major bacterial group in the plant rhizosphere. While Flavobacterium species within the phylum Bacteroidota have been recognized as pathogens in the aquatic habitats, microbiome analysis and the characterization of novel Flavobacterium species have indicated the great diversity and potential of their presence in various environments. Many Flavobacterium species have positively contribute to plant health and development, including growth promotion, disease control, and tolerance to abiotic stress. Despite the well-described beneficial interactions of the Flavobacterium species with plants, the molecular mechanisms and bacterial determinants underlying these interactions remain unclear. To broaden our understanding of the genus Flavobacterium’s role in plant health, we review the recent studies focusing on their ecological niche, functional roles, and determinants in plant-beneficial interactions. Additionally, this review discusses putative mechanisms explaining the interactions between plants and Flavobacterium. We have also introduced the importance of future research on Flavobacterium spp. and its potential applications in agriculture.
Keywords: Flavobacterium, plant-beneficial interactions, plant microbiome
The genus Flavobacterium was initially proposed by Bergey (Bergey et al., 1923). It belongs to the domain Bacteria, phylum Bacteroidota, class Flavobacteriia, order Flavobacteriales, and family Flavobacteriaceae (NCBI, https://www.ncbi.nlm.nih.gov/datasets/taxonomy/tree/?taxon=237). Members of the Flavobacterium genus are characterized as Gram-negative, obligately aerobic, rod-shaped bacteria that do not form spores (Bernardet and Bowman, 2015). Colonies formed by Flavobacterium species vary in color from pale to bright yellow due to the production of carotenoid, flexirubin, or a combination of both pigments (Bernardet and Bowman, 2015).
Bacterial species of the genus Flavobacterium are found in diverse habitats, including water, soil, and even plant tissues. Initially, Flavobacterium was primarily focused as pathogenic bacteria of various fish species in aquatic environments (Bernardet and Bowman, 2015). However, they have not received much attention in the context of plant-associated microbes compared to well-known plant growth-promoting rhizobacteria (PGPR) such as Pseudomonas or Bacillus (Marín et al., 2021). Recently, there has been a shift in focus, with Flavobacterium being identified as a key taxonomic group in plant microbiota (Carrión et al., 2019; Kwak et al., 2018). This recognition has sparked growing interest in investigating interaction between plants and Flavobacterium species. In this review, we will provide general information on the beneficial interactions between the plant-associated Flavobacterium species and the host plant. Especially, we will discuss potential mechanisms for plant-beneficial interactions of Flavobacterium in plant microbiota.
Ecological Niche
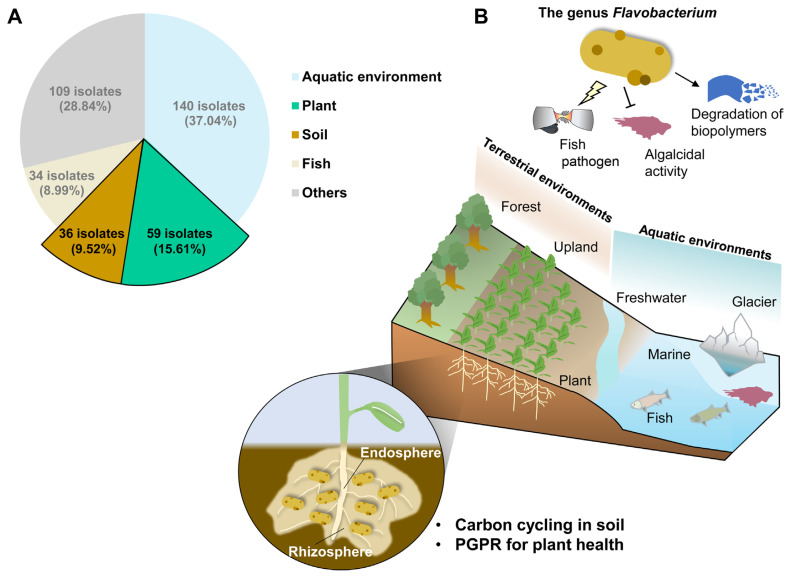
The genome information of 240 species (NCBI, https://www.ncbi.nlm.nih.gov) and 378 isolated strains (BacDive, https://bacdive.dsmz.de/) within the genus Flavobacterium has been reported (Fig. 1A). Members of the Flavobacterium genus have extensively been investigated in aquatic ecosystems, including freshwater and marine environments (Bernardet and Bowman, 2015) (Fig. 1B). Some of the Flavobacterium species, such as F. psychrophilum and F. columnare, have been identified as pathogens that affect many freshwater fish species, including rainbow trout and coho salmon (Chen et al., 2008; Strepparava et al., 2014) (Fig. 1B). Interestingly, the specific Flavobacterium species also showed an algicidal effect in freshwater environments (Fukami et al., 1992) (Fig. 1B). For example, Flavobacterium sp. 5N-3 exhibited the high inhibitory activity on the growth of a red-tide forming Gymnodinium nagasakiense, especially when the alga was in the logarithmic growth phase (Fukami et al., 1992). Furthermore, members of the genus Flavobacterium also play a role as decomposers in aquatic environments (Fig. 1B). It is well known that cultured isolates of Flavobacterium spp. can degrade biopolymers like cellulose and chitin, which are parts of the high molecular mass fraction of dissolved organic matter (Kirchman, 2002).
Fig. 1.
Composition of the genus Flavobacterium in various ecosystems. (A) The number of Flavobacterium isolates from different habitats, based on data from BacDive (https://bacdive.dsmz.de/). A total of 387 Flavobacterium isolates were obtained from aquatic environments, plants, soil, fish, and other sources. Light blue box, the isolates from aquatic environments; green box, the isolates from plants; light brown box, the isolates from soil; beige box, the isolates from fish; gray box, the isolates from others. (B) Geographic distribution and ecological roles of the genus Flavobacterium. Bacteria of the genus Flavobacterium are primarily found in aquatic environments where they serve various ecological roles. Flavobacterium species act as a pathogen of fish, algicidal organism, and a decomposer of biopolymers. Besides, certain Flavobacterium species contribute to carbon cycling and promote plant health in soil environments. PGPR, plant growth-promoting rhizobacteria.
Flavobacterium species are found not only in aquatic ecosystems but also in terrestrial environments, including sludge, frozen soil, polar soil, temperate soil, forest soil, and the rhizosphere soil of plants (Bernardet and Bowman, 2015; Chaudhary et al., 2019; Madhaiyan et al., 2010). Flavobacterium species are widely distributed in terrestrial environments, and the isolated portion of Flavobacterium species range 15.61% and 9.52% in the plants and soils, respectively (Fig. 1A). Large-scale genomic analysis predicts that the genus Flavobacterium in aquatic environments exhibits high activity in peptide and protein utilization, while their counterparts in terrestrial environments are expected to show high activity in carbohydrate metabolism, such as xylose, arabinose, and pectin (Kolton et al., 2013). This suggests their potential contribution to carbon cycling and the promotion of host plant growth in soil (Kolton et al., 2013; Kraut-Cohen et al., 2021) (Fig. 1B).
Functional Roles of Flavobacterium in Plant Microbiome
Bacterial species in the genus Flavobacterium are widely distributed in soil and the plant rhizosphere. Some of Flavobacterium species have recently been considered as PGPR (Kolton et al., 2016); however, the role of most Flavobacterium species in plant functioning is not well-described. Plant-wide microbiota analysis also suggests that Bacteriodota, including members of the Flavobacteriacea family, are predominant in the microbiota of various plant species (Pérez-Jaramillo et al., 2018), implying that Flavobacterium species may have specific plant-beneficial interactions. Extensive microbiome analysis of the plant-rhizosphere suggests that members of the genus Flavobacterium have an increased relative abundance in the rhizosphere and have been recognized as a core taxon in the complex rhizosphere microbiome (Carrión et al., 2019; Kwak et al., 2018). Therefore, we will present recent studies on the beneficial effects of plant-associated Flavobacterium on host plants and their putative modes of action.
Plant disease control
Certain Flavobacterium species protect plants against various diseases caused by bacteria or fungi (Carrión et al., 2019; Choi et al., 2023; Gunasinghe et al., 2004; Hahm et al., 2012; Kwak et al., 2018; Nishioka et al., 2019; Sang and Kim, 2012; Wang et al., 2023) (Fig. 2). A tomato microbiota study reported that the family Flavobacteriaceae is a key taxon in the rhizosphere of a tomato cultivar resistant to bacterial wilt in tomato plants (Kwak et al., 2018). Particularly, Flavobacterium sp. TRM1-10, isolated in the resistant tomato rhizosphere, effectively suppressed bacterial wilt caused by Ralstonia pseudosolanacearum in a bacterial wilt susceptible tomato cultivar (Kwak et al., 2018). Members of the genus Flavobacterium can also protect host plants from various pathogens, including Plasmodiophora brassicae and Fusarium oxysporum (Hahm et al., 2012; Nishioka et al., 2019). Treatment with F. hercynium EPB-C313 reduced the severity of clubroot disease caused by P. brassicae in Kimchi cabbage (Hahm et al., 2012). Individual treatments of Flavobacterium sp. GUAF6005, GUAF6009, and GUAC6072 also reduced the severity of fungal wilt caused by F. oxysporum f. sp. cucumerinum isolate GUS77 in cucumber seedlings (Nishioka et al., 2019). These beneficial Flavobacterium species showed antifungal activity against F. oxysporium, Colletotrichum musae, Botryodiplodia theobromae, and Cladosporium cladosporioides by inhibiting spore formation and the multiplication of fungal pathogens (Gunasinghe et al., 2004; Hahm et al., 2012; Nishioka et al., 2019) (Fig. 2). Furthermore, Flavobacterium species have been considered a keystone in determining disease resistance in host plants, however, their precise mechanisms have yet to be fully understood (Kwak et al., 2018).
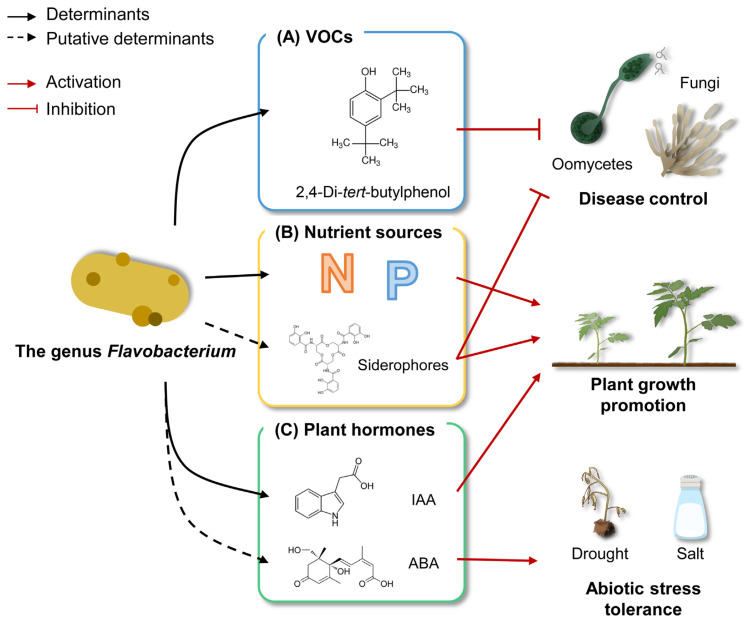
Fig. 2.
An overview of diverse determinants and functional roles within the genus Flavobacterium in plant-beneficial interactions. (A) Volatile organic compounds (VOCs). Bacteria in the genus Flavobacterium release 2,4-Di-tert-butylphenol, which exhibits biological control activity. (B) Nutrient sources. Bacteria in the genus Flavobacterium can provide inorganic nitrogen and solubilized phosphorus for plant growth promotion. They may supply ferric ions to the host plant via the secretion of siderophores. (C) Plant hormones. Plant hormones such as indole acetic acid and abscisic acid produced by Flavobacterium species contribute to plant growth and abiotic stress tolerance. Flavobacterium species secrete determinants (indicated by black arrows) or putative determinants (indicated by black dashed arrows) for plant-beneficial interactions. These various determinants either promote plant health (indicated by red arrows) or inhibit plant pathogens (indicated by red blunt-ended lines). IAA, indole-3-acetic acid; ABA, abscisic acid.
Plant growth promotion
Flavobacterium species have been studied as biostimulants in diverse monocot and dicot plants (Choi et al., 2023; Kwak et al., 2018; Menon et al., 2020; Samad et al., 2017; Yakubovskaya et al., 2019; Youseif, 2018; Zhang et al., 2021) (Fig. 2). For example, all 32 Flavobacterium strains, isolated from tomato rhizosphere soil, including F. anhuiense, F. aquidurense, F. beibuense, F. daejeonense, and F. dauae, showed plant growth promotion (PGP) activity in tomato plants (Jung et al., 2021; Kwak et al., 2018). Similarly, Flavobacterium sp. NGB-31, F. succinicans DSM4002, and Flavobacterium sp. 72, isolated from wheat root and rice rhizosphere, also exhibited the PGP in maize, rice, and ryegrass plants (Yakubovskaya et al., 2019; Youseif, 2018; Zhang et al., 2021). The effects of PGP on plants caused by the Flavobacterium species may be linked to the production of substances that help plants grow, like the phytohormone auxin and nitrogen resources (Yakubovskaya et al., 2019; Youseif, 2018; Zhang et al., 2021) (Fig. 2). However, there have been cases where certain strains showing PGP effects lack genes related to auxin production or nitrogen fixation. Thus, further molecular mechanistic studies are required to understand plant-Flavobacterium interactions better.
Abiotic stress tolerance
Members of the genus Flavobacterium can confer tolerance to abiotic stress, including drought and salt stress, in both monocot and dicot plants (Belimov et al., 2005; Gontia-Mishra et al., 2016; Kim et al., 2020; Walitang et al., 2017; Zhang et al., 2021) (Fig. 2). Flavobacterium sp. IG 15, Flavobacterium sp. IR29-16, Flavobacterium sp. IC27-25, and Flavobacterium sp. IC31-28 enhanced the induction of drought and stress tolerance in monocot plants such as wheat and rice (Gontia-Mishra et al., 2016; Walitang et al., 2017). Besides, F. crocinum HYN0056 activated the stress tolerance to drought and salt stress in Arabidopsis thaliana (Kim et al., 2020). These stress tolerances induced by Flavobacterium species were mainly involved in the activation of the molecular pathway associated with the plant hormone abscisic acid (ABA), which regulates stress tolerance in host plants (Gontia-Mishra et al., 2016; Kim et al., 2020; Yang et al., 2009) (Fig. 2). In addition to ABA signaling, the diverse stress-inducible genes including WRKY transcription factor and the antioxidant enzyme-encoding genes were also regulated by Flavobacterium species (Gontia-Mishra et al., 2016; Kim et al., 2020; Walitang et al., 2017).
Determinants Produced by Flavobacterium for Improving Plant Health
Volatile organic compounds
Bacteria-derived volatile organic compounds (VOCs) play a crucial role in plant-microbe interactions and are known as plant immune elicitors; this is well-documented in 2,3-butanediol-producing Bacillus species (Riu et al., 2022; Ryu et al., 2003, 2004; Sharifi et al., 2022). In addition to Bacillus species, VOC from F. johnsoniae GSE09 can also affect host plants (Sang et al., 2008) (Fig. 2A). F. johnsoniae GSE09, isolated from cucumber root tissues, not only promoted the yield and ripening of pepper fruits but also significantly inhibited the development of phytopathogens, including the oomycete Phytophthora capsici and the fungus C. acutatum (Sang et al., 2008). In particular, 2,4-di-tert-butylphenol derived from the strain GSE09 is a VOC that was identified as the antimicrobial agent against P. capsici and C. acutatum (Sang and Kim, 2012; Sang et al., 2011) (Fig. 2A). Similarly, Flavobacterium sp. R96, isolated from the rhizosphere of potatoes, can produce VOCs inhibiting the mycelial growth of P. infestans, causing potato late blight disease (Hunziker et al., 2015).
Macro- and micronutrient sources
Several plant-associated bacteria, including Flavobacterium species, provide the macro- and micronutrient resources to host plants (Carrión et al., 2019; Choi et al., 2023; Kraut-Cohen et al., 2021; Menon et al., 2020; Tilak et al., 2005; Youseif, 2018; Zhang et al., 2021) (Fig. 2B). For example, Flavobacterium sp. R6S-5-6 can provide a nitrogen source to the host plant by activating nitrogen fixation-related genes, including the nif gene (nifU), fix gene (fixF), and global nitrogen regulator (ntcA) (Choi et al., 2023) (Fig. 2B). Furthermore, solubilized phosphorus produced by Flavobacterium-specific alkaline phosphatase PhoX and PafA can contribute to tolerance against abiotic stress, PGP, and help plants overcome phosphorus depletion (Choi et al., 2023; Lidbury et al., 2021; Youseif, 2018; Zhang et al., 2021) (Fig. 2B). In addition to macronutrients, plant-associated Flavobacterium species are expected to supply the ferric ions to host plant. A study of the functional genomes of Flavobacterium species that are associated with plants found gene clusters that make iron-chelating siderophores, such as the ferric aerobactin, and its receptor lutA gene (Máté et al., 2022; Menon et al., 2020) (Fig. 2B). Therefore, siderophores derived from Flavobacterium species may provide ferric ions to host plants, thereby contributing to photosynthesis, seedling development, and inhibiting the growth of plant pathogens (Arnon, 1965; Connorton et al., 2017; Inoue et al., 2009; Murata et al., 2015; Pahari et al., 2017; Singh et al., 2022).
Plant hormone-mimicking compounds
Plant hormones act as global regulators for cellular processes in plants and can be synthesized not only by plants but also by microorganisms (Costacurta and Vanderleyden, 1995; Tsukanova et al., 2017) (Fig. 2C). A number of plant-associated Flavobacterium species can produce the auxin, indole acetic acid (IAA), affecting plant growth and abiotic stress tolerance in crop plants (Tsukanova et al., 2017; Walitang et al., 2017; Yakubovskaya et al., 2019; Youseif, 2018) (Fig. 2C). The amount of auxin that plant-associated Flavobacterium produces is about 4.88 μg/ml (Lin et al., 2023). Flavobacterium species associated with plants might synthesize auxin through the tryptophan-dependent pathway rather than the tryptophan-independent pathway (Tillmann et al., 2021; Tsavkelova et al., 2007; Xu et al., 2023; Wang et al., 2015). For example, Flavobacterium sp. 11 showed tryptophan-dependent IAA production regulated by the indole-3-glycerol phosphate synthase gene (trpC), which converts 1-(o-ecarboxyphenylamino)-1-deoxyribulose-5-phosphate to indole-3-glycerol-phosphate (Kagan et al., 2008; Xu et al., 2023). In addition to auxin, the treatment of Flavobacterium sp. HYN0056 or Flavobacterium sp. GJW24 activated the expression of ABA-responsive genes in Arabidopsis thaliana, but not ABA-biosynthesis genes, implying the production of ABA by Flavobacterium species (Kim et al., 2020, 2023) (Fig. 2C). Further mechanistic studies on the biosynthesis of various plant hormones, including ABA, in the genus Flavobacterium, should be required to understand the interaction between Flavobacterium species and host plants.
Unveiled Mechanisms for Plant-Flavobacterium Interactions
Colonization in root microbiome
Both culture-dependent and independent studies have shown the potential for root colonization of bacterial strains in the genus Flavobacterium (Acuña et al., 2023; Anzalone et al., 2022; Bodenhausen et al., 2013; Samad et al., 2017; Yuying et al., 2021). Members of the genus Flavobacterium are highly abundant in the rhizosphere, ranging from 5.0% to 20.2% and in root endosphere compartments ranging from 0.53% to 20.9% (Acuña et al., 2023; Anzalone et al., 2022; Bodenhausen et al., 2013; Samad et al., 2017). However, the exact mechanism of the root colonization of the Flavobacterium species is largely unknown. Among speculative mechanisms, we will mainly discuss the potential role of the type IX secretion system (T9SS) in root colonization of the Flavobacterium species. T9SS is a Bacteroidota-specific secretion system, first discovered in F. johnsoniae and Porphyromonas gingivalis (Braun et al., 2005; Sato et al., 2005; Trivedi et al., 2022). Interestingly, the T9SS-related gldJ mutant of Flavobacterium sp. F52 showed reduced colonization activity in the root environment (Kolton et al., 2014). The T9SS of Flavobacterium species is expected to enhance root colonization through gliding motility and the secretion of hydrolytic enzymes. The loss of T9SS showed a reduction in the gliding motility of the Flavobacterium species (Kita et al., 2016; McBride, 2014; McBride and Nakane, 2015). T9SS-mediated gliding motility might correlate with chemotaxis for nutrient searching and surface attachment by biofilm formation of bacterial strains in the genus Flavobacterium in root environments (Eckroat et al., 2021; Nakane et al., 2021). T9SS in the genus Flavobacterium can also facilitate the secretion of hydrolase enzymes, such as pectinase and cellulase, which are plant cell wall-degrading enzymes (Kolton et al., 2014; Kraut-Cohen et al., 2021; Kwak et al., 2018; Mawdsley and Burns, 1994). These hydrolases can confer Flavobacterium species on the utilization of extracellular nutrients and the invasion activity into the plant endosphere. Because of the presence of Flavobacterium species in aboveground tissues and seeds, further investigation of the translocation from roots to flowers and inheritance to offspring of the Flavobacterium species should be elucidated (Hahm et al., 2012; Sang et al., 2008; Wang et al., 2023; Youseif, 2018).
Outer membrane vesicles
Numerous bacteria including the Flavobacterium species can produce small (40–200 nm in diameter) spherical particles derived from the outer membrane called outer membrane vesicles (OMVs) (Jung et al., 2021; McMillan et al., 2021; Møller et al., 2005). Among Flavobacterium species such as F. psychrophilum, F. johnsoniae, and F. columnare, OMVs have been intensively investigated in aquatic conditions (Arias et al., 2012; Møller et al., 2005; Sato et al., 2021). The OMVs have been also detected in plant-associated bacteria. The plant-associated bacterial OMVs contain diverse immune triggers, including lipopolysaccharides and elongation factor Tu (EF-Tu), eliciting plant immunity against pathogenic bacteria and oomycetes (Bahar et al., 2016; Chowdhury and Jagannadham, 2013; McMillan et al., 2021; Rybak and Robatzek, 2019; Sidhu et al., 2008). Interestingly, the OMVs from aquatic Flavobacterium species contain diverse molecules that can modulate host immunity including small RNA, mRNA, various enzymes, antigenic proteins, and oligosaccharides (Chapagain et al., 2021, 2023; Møller et al., 2005). In addition, OMVs have been observed in some Flavobacterium strains with PGP activity (Jung et al., 2021); however, little is known about the role of these OMVs in interactions with plants. Further study is needed to investigate if OMVs of plant-associated Flavobacterium species may play an essential role in beneficial interactions with plants.
Microbiome stimulants
Members of the genus Flavobacterium might not only directly interact with the host plant but also indirectly activate microbe-microbe interactions for plant health and growth (Carrión et al., 2019; Wang et al., 2023; Zhu et al., 2021). Compared to individual treatments, the combination of Flavobacterium species with other bacterial isolates has shown a synergistic effect on disease control activity and immune responses against the bacterial pathogen R. solanacearum and the fungal pathogen Rhizoctonia sp. in crop plants (Carrión et al., 2019; Wang et al., 2023). These synergistic interactions, especially, might enhance the biosynthesis of secondary metabolites. For example, Flavobacterium species can elevate the formation of biofilm, which stimulates the root colonization of PGPR and plant immunity, produced by other bacterial species or a microbial consortium (Wang et al., 2023; Zhu et al., 2021). The T9SS of the Flavobacterium species can contribute to establishing a community either among themselves or with various microorganisms (Li et al., 2021; Shrivastava et al., 2018; Trivedi et al., 2022). This indicates that strains of the genus Flavobacterium might play a role as a network hub in microbe-microbe interactions for plant health and the biosynthesis of specific metabolites.
Perspective and Conclusion
In order to dissect the microbial traits of Flavobacterium species, genetic tools such as mutagenesis, shuttle vectors, and transformation protocols should be established for these specific microbes. While the gene manipulation tools for Proteobacteria did not work in Bacteroidota, techniques for genetic manipulation of Flavobacterium species have been developed (Alvarez et al., 2004; McBride and Kemp, 1996; Shoemaker et al., 1986). Therefore, these techniques would be applicable to plant-associated Flavobacterium species.
To improve our understanding of Flavobacterium-plant interactions, further research should address key questions surrounding the contribution of the Flavobacterium species in the plant microbiome. Here are the key questions. These questions include (1) How can we isolate certain crucial uncultured Flavobacterium species and establish stable, large-scale cultivation methods for agricultural applications?, (2) How do bacteria in the genus Flavobacterium engage in competition or cooperation with indigenous microorganisms in its natural plant environment?, (3) How do plants recognize the Flavobacterium species, and what mechanisms enable the Flavobacterium species to colonize within or around plant tissues? To address these questions, we will need to use both traditional molecular biological methods and multi-omics analyses like metagenomics, metatranscriptomics, proteomics, and metabolomics. Until recently, members of the genus Flavobacterium received little attention as a plant beneficial bacterium in the plant microbiota. However, advances in metagenomic analysis now highlight their significance in improving host plant health. Despite this, compared to well-established PGPR like the genera Pseudomonas or Bacillus, the roles of Flavobacterium species remain enigmatic in the plant microbiome and need to be explored.
Acknowledgments
We thank Dr. Kaari Manigundan and Abah Friday for a critical reading of the paper. This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) grant (No. 2020R1A2C3005453 and 2020R1A6A1A03047729 to S-WL, RS-2023-00249410 to S-ML), Biomaterials Specialized Graduate Program funded by the Korean government (MSIT, MOE, ME), Republic of Korea.
Footnotes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
References
- Acuña J. J., Rilling J. I., Inostroza N. G., Manquian J., Zhang Q., Gupta V. V. S. R., Jorquera M. A. Diversity, community structure, and potential functions of root-associated bacterial communities of different wheat (Triticum aestivum) cultivars under field conditions. Agronomy. 2023;13:1392. [Google Scholar]
- Alvarez B., Secades P., McBride M. J., Guijarro J. A. Development of genetic techniques for the psychrotrophic fish pathogen Flavobacterium psychrophilum. Appl. Environ. Microbiol. 2004;70:581–587. doi: 10.1128/AEM.70.1.581-587.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anzalone A., Mosca A., Dimaria G., Nicotra D., Tessitori M., Privitera G. F., Pulvirenti A., Leonardi C., Catara V. Soil and soilless tomato cultivation promote different microbial communities that provide new models for future crop interventions. Int. J. Mol. Sci. 2022;23:8820. doi: 10.3390/ijms23158820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arias C. R., LaFrentz S., Cai W., Olivares-Fuster O. Adaptive response to starvation in the fish pathogen Flavobacterium columnare: cell viability and ultrastructural changes. BMC Microbiol. 2012;12:266. doi: 10.1186/1471-2180-12-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnon D. I. Ferredoxin and photosynthesis. Science. 1965;149:1460–1470. doi: 10.1126/science.149.3691.1460. [DOI] [PubMed] [Google Scholar]
- Bahar O., Mordukhovich G., Luu D. D., Schwessinger B., Daudi A., Jehle A. K., Felix G., Ronald P. C. Bacterial outer membrane vesicles induce plant immune responses. Mol. Plant-Microbe Interact. 2016;29:374–384. doi: 10.1094/MPMI-12-15-0270-R. [DOI] [PubMed] [Google Scholar]
- Belimov A. A., Hontzeas N., Safronova V. I., Demchinskaya S. V., Piluzza G., Bullitta S., Glick B. R. Cadmium-tolerant plant growth-promoting bacteria associated with the roots of Indian mustard (Brassica juncea L. Czern.) Soil Biol. Biochem. 2005;37:241–250. [Google Scholar]
- Bergey D. H., Harrison F. C., Breed R. S., Hammer B. W., Huntoon F. M. Bergey’s manual of determinative bacteriology. The Williams and Wilkins Co., MD; USA: 1923. pp. 309–321. [Google Scholar]
- Bernardet J. F., Bowman J. P. Flavobacterium. In: Krieg N. R., Staley J. T., Brown D. R., Hedlund B. P., Paster B. J., Ward N. L., Ludwig W., Whitman W. B., editors. Bergey’s manual of systematics of archaea and bacteria. John Wiley & Sons, Inc; Hoboken, NJ, USA: 2015. pp. 1–75. [Google Scholar]
- Bodenhausen N., Horton M. W., Bergelson J. Bacterial communities associated with the leaves and the roots of Arabidopsis thaliana. PLoS ONE. 2013;8:e56329. doi: 10.1371/journal.pone.0056329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T. F., Khubbar M. K., Saffarini D. A., McBride M. J. Flavobacterium johnsoniae gliding motility genes identified by mariner mutagenesis. J. Bacteriol. 2005;187:6943–6952. doi: 10.1128/JB.187.20.6943-6952.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrión V. J., Perez-Jaramillo J., Cordovez V., Tracanna V., De Hollander M., Ruiz-Buck D., Mendes L. W., Van Ijcken W. F. J., Gomez-Exposito R., Elsayed S. S., Mohanraju P., Arifah A., van der Oost J., Paulson J. N., Mendes R., van Wezel G. P., Medema M. H., Raaijmakers J. M. Pathogen-induced activation of disease-suppressive functions in the endophytic root microbiome. Science. 2019;366:606–612. doi: 10.1126/science.aaw9285. [DOI] [PubMed] [Google Scholar]
- Chapagain P., Ali A., Kidane D. T., Farone M., Salem M. sRNAs enriched in outer membrane vesicles of pathogenic Flavobacterium psychrophilum interact with immune genes of rainbow trout. bioRxiv. 2021 doi: 10.1101/2021.12.22.473952. preprint at . [DOI] [Google Scholar]
- Chapagain P., Ali A., Salem M. Dual RNA-Seq of Flavobacterium psychrophilum and its outer membrane vesicles distinguishes genes associated with susceptibility to bacterial cold-water disease in rainbow trout (Oncorhynchus mykiss) Pathogens. 2023;12:436. doi: 10.3390/pathogens12030436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary D. K., Dahal R. H., Kim J. Flavobacterium silvisoli sp. nov., isolated from forest soil. Int. J. Syst. Evol. Microbiol. 2019;69:2762–2766. doi: 10.1099/ijsem.0.003551. [DOI] [PubMed] [Google Scholar]
- Chen Y.-C., Davis M. A., Lapatra S. E., Cain K. D., Snekvik K. R., Call D. R. Genetic diversity of Flavobacterium psychrophilum recovered from commercially raised rainbow trout, Oncorhynchus mykiss (Walbaum), and spawning coho salmon, O. kisutch (Walbaum) J. Fish Dis. 2008;31:765–773. doi: 10.1111/j.1365-2761.2008.00950.x. [DOI] [PubMed] [Google Scholar]
- Choi A., Cha I.-T., Lee K.-E., Son Y. K., Yu J., Seol D. The role of Flavobacterium enshiense R6S-5-6 in the wetland ecosystem revealed by whole-genome analysis. Curr. Microbiol. 2023;80:83. doi: 10.1007/s00284-022-03157-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury C., Jagannadham M. V. Virulence factors are released in association with outer membrane vesicles of Pseudomonas syringae pv. tomato T1 during normal growth. Biochim. Biophys. Acta. 2013;1834:231–239. doi: 10.1016/j.bbapap.2012.09.015. [DOI] [PubMed] [Google Scholar]
- Connorton J. M., Balk J., Rodríguez-Celma J. Iron homeostasis in plants: a brief overview. Metallomics. 2017;9:813–823. doi: 10.1039/c7mt00136c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costacurta A., Vanderleyden J. Synthesis of phytohormones by plant-associated bacteria. Crit. Rev. Microbiol. 1995;21:1–18. doi: 10.3109/10408419509113531. [DOI] [PubMed] [Google Scholar]
- Eckroat T. J., Greguske C., Hunnicutt D. W. The type 9 secretion system is required for Flavobacterium johnsoniae biofilm formation. Front. Microbiol. 2021;12:660887. doi: 10.3389/fmicb.2021.660887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukami K., Yuzawa A., Nishijima T., Hata Y. Isolation and properties of a bacterium inhibiting the growth of Gymnodinium nagasakiense. Nippon Suisan Gakkaishi. 1992;58:1073–1077. [Google Scholar]
- Gontia-Mishra I., Sapre S., Sharma A., Tiwari S. Amelioration of drought tolerance in wheat by the interaction of plant growth-promoting rhizobacteria. Plant Biol. 2016;18:992–1000. doi: 10.1111/plb.12505. [DOI] [PubMed] [Google Scholar]
- Gunasinghe R. N., Ikiriwatte C. J., Karunaratne A. M. The use of Pantoea agglomerans and Flavobacterium sp. to control banana pathogens. J. Hortic. Sci. Biotechnol. 2004;79:1002–1006. [Google Scholar]
- Hahm S., Kim J., Han K., Kim B., Kim H., Nam Y., Yu S. Biocontrol efficacy of endophytic bacteria Flavobacterium hercynim EPB-C313 for control of Chinese cabbage clubroot. Res. Plant Dis. 2012;18:210–216. (in Korean) [Google Scholar]
- Hunziker L., Bönisch D., Groenhagen U., Bailly A., Schulz S., Weisskopf L. Pseudomonas strains naturally associated with potato plants produce volatiles with high potential for inhibition of Phytophthora infestans. Appl. Environ. Microbiol. 2015;81:821–830. doi: 10.1128/AEM.02999-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inoue H., Kobayashi T., Nozoye T., Takahashi M., Kakei Y., Suzuki K., Nakazono M., Nakanishi H., Mori S., Nishizawa N. K. Rice OsYSL15 is an iron-regulated iron (III)-deoxymugineic acid transporter expressed in the roots and is essential for iron uptake in early growth of the seedlings. J. Biol. Chem. 2009;284:3470–3479. doi: 10.1074/jbc.M806042200. [DOI] [PubMed] [Google Scholar]
- Jung E. J., Choi S. Y., Lee S.-M., Song J. Y., Lee H. J., Kim E., Lee P. A., Choi K., Kim J. F., Lee S.-W. Flavobacterium dauae sp. nov., isolated from rhizosphere soil of a tomato plant. Int. J. Syst. Evol. Microbiol. 2021;71:005033. doi: 10.1099/ijsem.0.005033. [DOI] [PubMed] [Google Scholar]
- Kagan J., Sharon I., Beja O., Kuhn J. C. The tryptophan pathway genes of the Sargasso Sea metagenome: new operon structures and the prevalence of non-operon organization. Genome Biol. 2008;9:R20. doi: 10.1186/gb-2008-9-1-r20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Woo O.-G., Kim J. B., Yoon S.-Y., Kim J.-S., Sul W. J., Hwang J.-Y., Lee J.-H. Flavobacterium sp. strain GJW24 ameliorates drought resistance in Arabidopsis and Brassica. Front. Plant Sci. 2023;14:1257137. doi: 10.3389/fpls.2023.1257137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J.-E., Woo O.-G., Bae Y., Keum H. L., Chung S., Sul W. J., Lee J.-H. Enhanced drought and salt stress tolerance in Arabidopsis by Flavobacterium crocinum HYN0056 T. J. Plant Biol. 2020;63:63–71. [Google Scholar]
- Kirchman D. L. The ecology of Cytophaga–Flavobacteria in aquatic environments. FEMS Microbiol. Ecol. 2002;39:91–100. doi: 10.1111/j.1574-6941.2002.tb00910.x. [DOI] [PubMed] [Google Scholar]
- Kita D., Shibata S., Kikuchi Y., Kokubu E., Nakayama K., Saito A., Ishihara K. Involvement of the type IX secretion system in Capnocytophaga ochracea gliding motility and biofilm formation. Appl. Environ. Microbiol. 2016;82:1756–1766. doi: 10.1128/AEM.03452-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolton M., Erlacher A., Berg G., Cytryn E. The Flavobacterium genus in the plant holobiont: ecological, physiological, and applicative insights. In: Castro-Sowinski S., editor. Microbial models: from environmental to industrial sustainability. Springer-Nature; Berlin, Germany: 2016. pp. 189–207. [Google Scholar]
- Kolton M., Frenkel O., Elad Y., Cytryn E. Potential role of Flavobacterial gliding-motility and type IX secretion system complex in root colonization and plant defense. Mol. Plant-Microbe Interact. 2014;27:1005–1013. doi: 10.1094/MPMI-03-14-0067-R. [DOI] [PubMed] [Google Scholar]
- Kolton M., Sela N., Elad Y., Cytryn E. Comparative genomic analysis indicates that niche adaptation of terrestrial Flavobacteria is strongly linked to plant glycan metabolism. PLoS ONE. 2013;8:e76704. doi: 10.1371/journal.pone.0076704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraut-Cohen J., Shapiro O. H., Dror B., Cytryn E. Pectin induced colony expansion of soil-derived Flavobacterium strains. Front. Microbiol. 2021;12:651891. doi: 10.3389/fmicb.2021.651891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak M.-J., Kong H. G., Choi K., Kwon S.-K., Song J. Y., Lee J., Lee P. A., Choi S. Y., Seo M., Lee H. J., Jung E. J., Park H., Roy N., Kim H., Lee M. M., Rubin E. M., Lee S.-W., Kim J. F. Rhizosphere microbiome structure alters to enable wilt resistance in tomato. Nat. Biotechnol. 2018;36:1100–1109. doi: 10.1038/nbt.4232. [DOI] [PubMed] [Google Scholar]
- Li C., Hurley A., Hu W., Warrick J. W., Lozano G. L., Ayuso J. M., Pan W., Handelsman J., Beebe D. J. Social motility of biofilm-like microcolonies in a gliding bacterium. Nat. Commun. 2021;12:5700. doi: 10.1038/s41467-021-25408-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lidbury I. D. E. A., Borsetto C., Murphy A. RJ., Bottrill A., Jones A. M. E., Bending G. D., Hammond J. P., Chen Y., Wellington E. M. H., Scanlan D. J. Niche-adaptation in plant-associated Bacteroidetes favours specialisation in organic phosphorus mineralisation. ISME J. 2021;15:1040–1055. doi: 10.1038/s41396-020-00829-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin S.-Y., Hameed A., Tsai C.-F., Young C.-C. Description of Flavobacterium agricola sp. nov., an auxin producing bacterium isolated from paddy field. Antonie van Leeuwenhoek. 2023;116:1345–1357. doi: 10.1007/s10482-023-01891-4. [DOI] [PubMed] [Google Scholar]
- Marín O., González B., Poupin M. J. From microbial dynamics to functionality in the rhizosphere: a systematic review of the opportunities with synthetic microbial communities. Front. Plant Sci. 2021;12:650609. doi: 10.3389/fpls.2021.650609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhaiyan M., Poonguzhali S., Lee J.-S., Lee K. C., Sundaram S. Flavobacterium glycines sp. nov., a facultative methylotroph isolated from the rhizosphere of soybean. Int. J. Syst. Evol. Microbiol. 2010;60:2187–2192. doi: 10.1099/ijs.0.014019-0. [DOI] [PubMed] [Google Scholar]
- Máté R., Kutasi J., Bata-Vidács I., Kosztik J., Kukolya J., Tóth E., Bóka K., Táncsics A., Kovács G., Nagy I., Tóth Á. Flavobacterium hungaricum sp. nov. a novel soil inhabitant, cellulolytic bacterium isolated from plough field. Arch. Microbiol. 2022;204:301. doi: 10.1007/s00203-022-02905-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mawdsley J. L., Burns R. G. Inoculation of plants with a Flavobacterium species results in altered rhizosphere enzyme activities. Soil Biol. Biochem. 1994;26:871–882. [Google Scholar]
- McBride M. J. The family Flavobacteriaceae. Prokaryotes. 2014;4:643–676. [Google Scholar]
- McBride M. J., Kemp M. J. Development of techniques for the genetic manipulation of the gliding bacterium Cytophaga johnsonae. J. Bacteriol. 1996;178:583–590. doi: 10.1128/jb.178.3.583-590.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride M. J., Nakane D. Flavobacterium gliding motility and the type IX secretion system. Curr. Opin. Microbiol. 2015;28:72–77. doi: 10.1016/j.mib.2015.07.016. [DOI] [PubMed] [Google Scholar]
- McMillan H. M., Zebell S. G., Ristaino J. B., Dong X., Kuehn M. J. Protective plant immune responses are elicited by bacterial outer membrane vesicles. Cell Rep. 2021;34:108645. doi: 10.1016/j.celrep.2020.108645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menon R. R., Kumari S., Viver T., Rameshkumar N. Flavobacterium pokkalii sp. nov., a novel plant growth promoting native rhizobacteria isolated from pokkali rice grown in coastal saline affected agricultural regions of southern India, Kerala. Microbiol. Res. 2020;240:126533. doi: 10.1016/j.micres.2020.126533. [DOI] [PubMed] [Google Scholar]
- Møller J. D., Barnes A. C., Dalsgaard I., Ellis A. E. Characterisation of surface blebbing and membrane vesicles produced by Flavobacterium psychrophilum. Dis. Aquat. Org. 2005;64:201–209. doi: 10.3354/dao064201. [DOI] [PubMed] [Google Scholar]
- Murata Y., Itoh Y., Iwashita T., Namba K. Transgenic petunia with the iron (III)-phytosiderophore transporter gene acquires tolerance to iron deficiency in alkaline environments. PLoS ONE. 2015;10:e0120227. doi: 10.1371/journal.pone.0120227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakane D., Odaka S., Suzuki K., Nishizaka T. Large-scale vortices with dynamic rotation emerged from monolayer collective motion of gliding Flavobacteria. J. Bacteriol. 2021;203:e0007321. doi: 10.1128/JB.00073-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishioka T., Marian M., Kobayashi I., Kobayashi Y., Yamamoto K., Tamaki H., Suga H., Shimizu M. Microbial basis of Fusarium wilt suppression by Allium cultivation. Sci. Rep. 2019;9:1715. doi: 10.1038/s41598-018-37559-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pahari A., Pradhan A., Nayak S. K., Mishra B. B. Bacterial siderophore as a plant growth promoter. In: Patra J. K., Vishnuprasad C. N., Das G., editors. Microbial biotechnology. Vol. 1. Applications in agriculture and environment. Springer-Nature; Berlin, Germany: 2017. pp. 163–180. [Google Scholar]
- Pérez-Jaramillo J. E., Carrión V. J., de Hollander M., Raaijmakers J. M. The wild side of plant microbiomes. Microbiome. 2018;6:143. doi: 10.1186/s40168-018-0519-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riu M., Son J.-S., Oh S.-K., Ryu C.-M. Aromatic agriculture: volatile compound-based plant disease diagnosis and crop protection. Res. Plant Dis. 2022;28:1–18. (in Korean) [Google Scholar]
- Rybak K., Robatzek S. Functions of extracellular vesicles in immunity and virulence. Plant Physiol. 2019;179:1236–1247. doi: 10.1104/pp.18.01557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu C.-M., Farag M. A., Hu C.-H., Reddy M. S., Kloepper J. W., Paré P. W. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 2004;134:1017–1026. doi: 10.1104/pp.103.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu C.-M., Farag M. A., Hu C.-H., Reddy M. S., Wei H.-X., Paré P. W., Kloepper J. W. Bacterial volatiles promote growth in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 2003;100:4927–4932. doi: 10.1073/pnas.0730845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samad A., Trognitz F., Compant S., Antonielli L., Sessitsch A. Shared and host-specific microbiome diversity and functioning of grapevine and accompanying weed plants. Environ. Microbiol. 2017;19:1407–1424. doi: 10.1111/1462-2920.13618. [DOI] [PubMed] [Google Scholar]
- Sang M. K., Chun S.-C., Kim K. D. Biological control of Phytophthora blight of pepper by antagonistic rhizobacteria selected from a sequential screening procedure. Biol. Control. 2008;46:424–433. [Google Scholar]
- Sang M. K., Kim J. D., Kim B. S., Kim K. D. Root treatment with rhizobacteria antagonistic to Phytophthora blight affects anthracnose occurrence, ripening, and yield of pepper fruit in the plastic house and field. Phytopathology. 2011;101:666–678. doi: 10.1094/PHYTO-08-10-0224. [DOI] [PubMed] [Google Scholar]
- Sang M. K., Kim K. D. The volatile-producing Flavobacterium johnsoniae strain GSE09 shows biocontrol activity against Phytophthora capsici in pepper. J. Appl. Microbiol. 2012;113:383–398. doi: 10.1111/j.1365-2672.2012.05330.x. [DOI] [PubMed] [Google Scholar]
- Sato K., Naya M., Hatano Y., Kondo Y., Sato M., Narita Y., Nagano K., Naito M., Nakayama K., Sato C. Colony spreading of the gliding bacterium Flavobacterium johnsoniae in the absence of the motility adhesin SprB. Sci. Rep. 2021;11:967. doi: 10.1038/s41598-020-79762-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato K., Sakai E., Veith P. D., Shoji M., Kikuchi Y., Yukitake H., Ohara N., Naito M., Okamoto K., Reynolds E. C., Nakayama K. Identification of a new membrane-associated protein that influences transport/maturation of gingipains and adhesins of Porphyromonas gingivalis. J. Biol. Chem. 2005;280:8668–8677. doi: 10.1074/jbc.M413544200. [DOI] [PubMed] [Google Scholar]
- Sharifi R., Jeon J.-S., Ryu C.-M. Belowground plant–microbe communications via volatile compounds. J. Exp. Bot. 2022;73:463–486. doi: 10.1093/jxb/erab465. [DOI] [PubMed] [Google Scholar]
- Shoemaker N. B., Getty C., Gardner J. F., Salyers A. A. Tn4351 transposes in Bacteroides spp. and mediates the integration of plasmid R751 into the Bacteroides chromosome. J. Bacteriol. 1986;165:929–936. doi: 10.1128/jb.165.3.929-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shrivastava A., Patel V. K., Tang Y., Yost S. C., Dewhirst F. E., Berg H. C. Cargo transport shapes the spatial organization of a microbial community. Proc. Natl. Acad. Sci. U. S. A. 2018;115:8633–8638. doi: 10.1073/pnas.1808966115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidhu V. K., Vorhölter F.-J., Niehaus K., Watt S. A. Analysis of outer membrane vesicle associated proteins isolated from the plant pathogenic bacterium Xanthomonas campestris pv. campestris. BMC Microbiol. 2008;8:87. doi: 10.1186/1471-2180-8-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh P., Chauhan P. K., Upadhyay S. K., Singh R. K., Dwivedi P., Wang J., Jain D., Jiang M. Mechanistic insights and potential use of siderophores producing microbes in rhizosphere for mitigation of stress in plants grown in degraded land. Front. Microbiol. 2022;13:898979. doi: 10.3389/fmicb.2022.898979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strepparava N., Wahli T., Segner H., Petrini O. Detection and quantification of Flavobacterium psychrophilum in water and fish tissue samples by quantitative real time PCR. BMC Microbiol. 2014;14:105. doi: 10.1186/1471-2180-14-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilak K. VBR., Ranganayaki N., Pal K. K., De R., Saxena A. K., Nautiyal C. S., Mittal S., Tripathi A. K., Johri B. N. Diversity of plant growth and soil health supporting bacteria. Curr. Sci. 2005;89:136–150. [Google Scholar]
- Tillmann M., Tang Q., Cohen J. D. Protocol: analytical methods for visualizing the indolic precursor network leading to auxin biosynthesis. Plant Methods. 2021;17:63. doi: 10.1186/s13007-021-00763-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trivedi A., Gosai J., Nakane D., Shrivastava A. Design principles of the rotary type 9 secretion system. Front. Microbiol. 2022;13:845563. doi: 10.3389/fmicb.2022.845563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsavkelova E. A., Cherdyntseva T. A., Botina S. G., Netrusov A. I. Bacteria associated with orchid roots and microbial production of auxin. Microbiol. Res. 2007;162:69–76. doi: 10.1016/j.micres.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Tsukanova K. A., Chebotar V. K., Meyer J. J. M., Bibikova T. N. Effect of plant growth-promoting rhizobacteria on plant hormone homeostasis. S. Afr. J. Bot. 2017;113:91–102. [Google Scholar]
- Walitang D. I., Kim K., Madhaiyan M., Kim Y. K., Kang Y., Sa T. Characterizing endophytic competence and plant growth promotion of bacterial endophytes inhabiting the seed endosphere of rice. BMC Microbiol. 2017;17:209. doi: 10.1186/s12866-017-1117-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B., Chu J., Yu T., Xu Q., Sun X., Yuan J., Xiong G., Wang G., Wang Y., Li J. Tryptophan-independent auxin biosynthesis contributes to early embryogenesis in Arabidopsis. Proc. Natl. Acad. Sci. U. S. A. 2015;112:4821–4826. doi: 10.1073/pnas.1503998112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang N., Ding J., Chen Y., Zhu Y., Zhang L., Wei Y., Li J., Xu T., Ding G.-C. Bacillus velezensis BER1 enriched Flavobacterium daejeonense-like bacterium in the rhizosphere of tomato against bacterial wilt. FEMS Microbiol. Ecol. 2023;99:fiad054. doi: 10.1093/femsec/fiad054. [DOI] [PubMed] [Google Scholar]
- Xu F., Liao H., Yang J., Zhang Y., Yu P., Cao Y., Fang J., Chen S., Li L., Sun L., Du C., Wang K., Dang X., Feng Z., Cao Y., Li Y., Zhang J., Xu W. Auxin-producing bacteria promote barley rhizosheath formation. Nat. Commun. 2023;14:5800. doi: 10.1038/s41467-023-40916-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yakubovskaya A. I., Melnichuk T. N., Kameneva I. A., Shaposhnikov A. I., Puhalsky Y. V., Sazanova A. L. Flavobacterium sp. strain No 72–associative simbiont of Oryza sativa L. plants. Asian J. Microbiol. Biotechnol. Environ. Sci. 2019;21:566–571. [Google Scholar]
- Yang J., Kloepper J. W., Ryu C.-M. Rhizosphere bacteria help plants tolerate abiotic stress. Trends Plant Sci. 2009;14:1–4. doi: 10.1016/j.tplants.2008.10.004. [DOI] [PubMed] [Google Scholar]
- Youseif S. H. Genetic diversity of plant growth promoting rhizobacteria and their effects on the growth of maize plants under greenhouse conditions. Ann. Agric. Sci. 2018;63:25–35. [Google Scholar]
- Yuying M. A., Weisenhorn P., Guo X., Wang D., Yang T., Shi Y., Zhang H., Chu H. Effect of long-term fertilization on bacterial communities in wheat endosphere. Pedosphere. 2021;31:538–548. [Google Scholar]
- Zhang X.-F., She M.-Z., Li H.-Y., Jing S., Jiang H.-N., Gao H., Zhu Y.-X., Fu J.-J. Growth promotion mechanisms of Flavobacterium succinicans and their physiological regulation on the growth and stress tolerance in Lolium perenne. Acta Bot. Sin. 2021;29:1704–1711. [Google Scholar]
- Zhu L., Wang S., Duan H., Lu X. Foliar pathogen-induced assemblage of beneficial rhizosphere consortia increases plant defense against Setosphaeria turcica. Front. Biosci. 2021;26:543–555. doi: 10.52586/4966. [DOI] [PubMed] [Google Scholar]