Abstract
We compare methods of detection of intercellular transport of the herpes simplex virus protein VP22 and of a green fluorescent protein (GFP)-VP22 fusion protein. Spread of both proteins was observed by immunofluorescence (IF) using organic fixatives. Spread of both proteins was also detected by IF after paraformaldehyde (PFA) fixation and detergent permeabilization, albeit at reduced levels. However, while spread of GFP-VP22 was observed by examining intrinsic GFP fluorescence after methanol fixation, little spread was observed after PFA fixation, suggesting that the levels of the fusion protein in recipient cells were below the detection limits of intrinsic-fluorescence or that PFA fixation quenches the fluorescence of GFP-VP22. We further considered whether elution of VP22 from methanol-fixed cells and postfixation binding to surrounding cells contributed to the increased detection of spread observed after methanol fixation. The results show that while this could occur, it appeared to be a minor effect not accounting for the observed VP22 cell-to-cell spread in culture.
VP22, the product of the UL49 gene of herpes simplex virus (8), is a major structural component of the virion. The protein is 301 residues in length, basic, and subject to a number of posttranslational modifications including phosphorylation (7) and nucleotidylylation (2). We previously reported that VP22 exhibits the unusual property of transport between cells (5). Transport was observed after introduction of the VP22 gene by several routes, including transfection or microinjection of the isolated gene in plasmid constructs or by infection with a nonreplicating herpesvirus encoding the native VP22 gene. One of the features of transport was that in cells actively synthesizing the protein, VP22 was located predominantly in the cytoplasm, where it could be observed in filamentous arrays colocalizing with bundled microtubules (4), while in the surrounding cells, VP22 was observed mainly in the nucleus, where it could also be observed colocalizing with chromatin in mitotic cells. A short C-terminal deletion mutant of VP22 lacking 34 residues was expressed normally and exhibited unaltered cytoplasmic localization in the primary cells expressing VP22 but failed to spread to the surrounding cells. Spread of VP22 was also sensitive to treatment of cells with cytochalasin D (5). In addition, we found that this transport activity was retained in a fusion protein consisting of VP22 linked to green fluorescent protein (GFP) which behaved essentially like the native protein with respect to expression, localization, and spread (5).
We subsequently reported that trafficking of the GFP-VP22 fusion protein could not be readily observed in living cells (6), in agreement with the results of Fang and colleagues (9), but was detected in methanol-fixed cells either by examining intrinsic GFP fluorescence or by immunofluorescence (IF) analysis with anti-GFP antibodies (6). More recently, other laboratories have observed spread of a VP22-GFP fusion protein in fixed but not living cells (1), while the spread of VP22-GFP in living cells was reported by fluorescence-activated cell sorting analysis (14). Here we compare methods of fixation and detection in the attempt to reconcile the observations on spread of VP22 and of GFP-VP22 in live cells. We specifically wished to examine whether detection of the GFP fusion protein by intrinsic GFP fluorescence was as sensitive as detection by IF using antibodies, whether the fixation methods influenced sensitivity, and whether fixation itself contributed to spread. The results indicate that while VP22 spread was observed by IF following several different fixation methods, the method of fixation influenced detection. Fixation with organic solvents allowed the most sensitive detection of spread. We further examined whether any postfixation extraction of VP22 could account for enhanced detection of spread in methanol-fixed cells and found evidence for some weak leaching of the protein from VP22-expressing cells to mock-transfected cells. However, this effect did not appear to account for the extent of spread observed in transfected-cell monolayers, which was also observed in paraformaldehyde (PFA)-fixed cells. As with native VP22, spread of the GFP-VP22 fusion protein was also detected in PFA-fixed cells but required a period of rehydration for detection by intrinsic GFP fluorescence analysis.
To examine spread of VP22 and VP22-GFP fusion proteins, COS-1 cells (2 × 105 cells) (on glass coverslips in six-well chambers) were plated in standard culture medium (Dulbecco's modified minimal essential medium containing 10% newborn calf serum) and transfected with expression plasmids for VP22 or GFP-VP22 as previously described (5). For native VP22, plasmids pUL49ep (10) and pAP85H were used as indicated. These vectors contain the VP22 gene driven by the cytomegalovirus (CMV) immediate-early enhancer/promoter and flanked at the C terminus by different epitope tags; pUL49ep (kindly provided by J. McLauchlan) contains the CMV UL83 tag, detected by the monoclonal antibody CMV-018-48151 (Capricorn Products Ltd., Scarborough, Maine), while pAP85H contains VP22 in the background of the commercial vector pcDNA1/Amp (Invitrogen), flanked by the tag from the influenza virus hemagglutinin (HA), enabling detection by the anti-HA monoclonal antibody (Cambridge Biosciences). pEGFPC1 (Clontech) was the parent plasmid for the VP22 fusion pGE155 (GFP-VP22) constructed as described previously (5) except in this case GFP was fused to the N terminus of VP22.
For analysis by indirect IF, transfected cells (approximately 40 h after transfection) were rinsed with phosphate-buffered saline (PBS), fixed in 100% methanol or 100% acetone for 10 min, and then washed in PBS. Alternatively, cells were fixed in 4% PFA in PBS for 10 min, washed in PBS, and then permeabilized in PBS containing 0.5% Triton X-100 (TX-100) for 10 min before a final wash in PBS. The cells were examined by intrinsic GFP fluorescence where appropriate or processed for IF with either AGV30, a rabbit anti-VP22 polyclonal antibody (5), the anti-HA monoclonal antibody, or an anti-GFP polyclonal antibody (Clontech Laboratories). Secondary antibodies were fluorescein isothiocyanate (FITC) (Vector Laboratories) conjugated or tetramethyl rhodamine isocyanate (TRITC) (Sigma) conjugated and used at the recommended dilutions. Confocal microscopy was performed with a Zeiss LSM410 system attached to an inverted Axiovert 135 microscope. Fields were examined by illumination with the 488-nm-wavelength laser at 1:30 or 1:100 attenuation or with the 543-nm-wavelength laser at 1:3 or 1:10 attenuation, using 63× and 40× objectives.
Fixation methods affect detection of VP22-GFP.
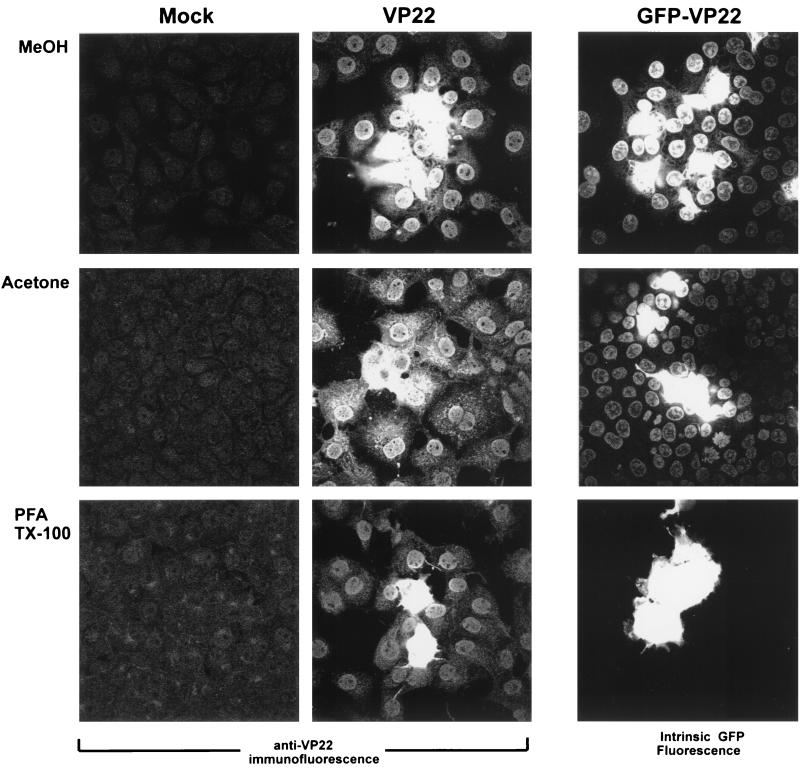
In the first series of experiments, we examined detection of VP22 spread by IF analysis using different fixation methods. Plasmids expressing VP22 were transfected into COS-1 cells, and 40 h after transfection, the cells were fixed with either 100% methanol, 100% acetone, or 4% PFA followed by permeabilization with 0.5% TX-100. Transfected and control monolayers were subsequently stained with anti-VP22 polyclonal antibody. For methanol fixation, the results were as expected from previous analysis, with cells displaying intense cytoplasmic fluorescence surrounded by numerous cells with the VP22 protein mainly in the nucleus (Fig. 1, VP22, MeOH). Very similar results were obtained with acetone fixation, although we noted somewhat greater cytoplasmic staining of VP22 together with the nuclear accumulation in the cells surrounding the intensely staining central cells (Fig. 1, VP22, acetone). Spread of VP22 was also observed using PFA fixation and detergent permeabilization (Fig. 1, VP22, PFA), but generally staining was less intense and fewer positive cells were observed surrounding the more-intense central cells.
FIG. 1.
COS-1 cells were transfected with expression vectors for VP22 (pUL49ep [500 ng]) and GFP-VP22 (pGE155 [200 ng]) or mock transfected as indicated. Forty hours after transfection, the cells were fixed as indicated and stained with AGV30 rabbit anti-VP22 antiserum (Mock and VP22) or examined for intrinsic GFP fluorescence (GFP-VP22) with 63× objective. MeOH, methanol.
Analysis of the GFP-VP22 fusion protein was assessed by intrinsic GFP fluorescence without antibody staining. As for native VP22, we observed spread of the GFP-VP22 fusion protein (Fig. 1) with either methanol or acetone fixation, although the increased cytoplasmic staining after acetone fixation, seen for native VP22 by anti-VP22 IF analysis, was not readily apparent for GFP-VP22. Surprisingly, for PFA fixation, while spread was clearly detected for native VP22 by IF, it was not observed for the GFP-VP22 by intrinsic GFP fluorescence, with the fusion protein being detected in isolated single or double cells (Fig. 1, GFP-VP22).
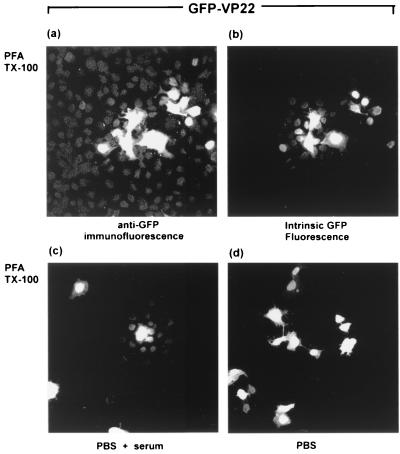
To explain these results, we considered the possibility that the GFP-VP22 fusion protein was present in the surrounding cells but that the levels were below those required for detection by intrinsic GFP fluorescence. Methanol fixation may then result in concentration or dequenching of the fluorescence, for example, by facilitating refolding of denatured protein, while this would not have occurred with PFA fixation and cross-linking. Moreover, PFA fixation may have somehow actively quenched the intrinsic GFP fluorescence of the VP22 fusion protein. We therefore next examined localization of the GFP-VP22 fusion protein in cells fixed with PFA and permeabilized with detergent, using anti-GFP antibody. The results (Fig. 2) show that the fusion protein could now be detected not only in the more intensely staining central cells but also in surrounding cells where it appeared mainly nuclear (Fig. 2a). However, we further noted that after staining with anti-GFP antibody, the GFP-VP22 fusion protein could now be detected in surrounding cells by intrinsic GFP fluorescence (Fig. 2b), although detection by IF remained the more sensitive (compare Fig. 2a and b). This result indicated that a period of rehydration may facilitate recovery of GFP fluorescence of the fusion protein in surrounding cells. To examine this, transfected cells were fixed with PFA and TX-100 as before, incubated in PBS with or without blocking serum, and then examined by intrinsic GFP fluorescence. In the latter case, foci of cells could now be observed by direct GFP fluorescence, with the fusion protein detected in the nuclei of cells surrounding the brightly expressing cells (Fig. 2c). Interestingly, this was not observed after rehydration in PBS alone (Fig. 2d). The results are consistent with the proposal that intrinsic fluorescence of the fusion protein may have been abrogated in recipient cells, and regained by rehydration and/or refolding.
FIG. 2.
COS-1 cells were transfected with the GFP-VP22 expression vector (200 ng), fixed with 4% PFA, permeabilized with 0.5% TX-100, and subsequently stained with anti-GFP antibody followed by TRITC-coupled secondary antibody. The same field is shown for anti-GFP IF (a) and intrinsic GFP fluorescence (b). In parallel, transfected cells were fixed as described above for panels a and b, and subsequently incubated in PBS plus blocking serum (c) or PBS alone (d). These panels were examined for intrinsic GFP fluorescence with 40× objective.
PFA quenches fluorescence of the GFP fusion protein.
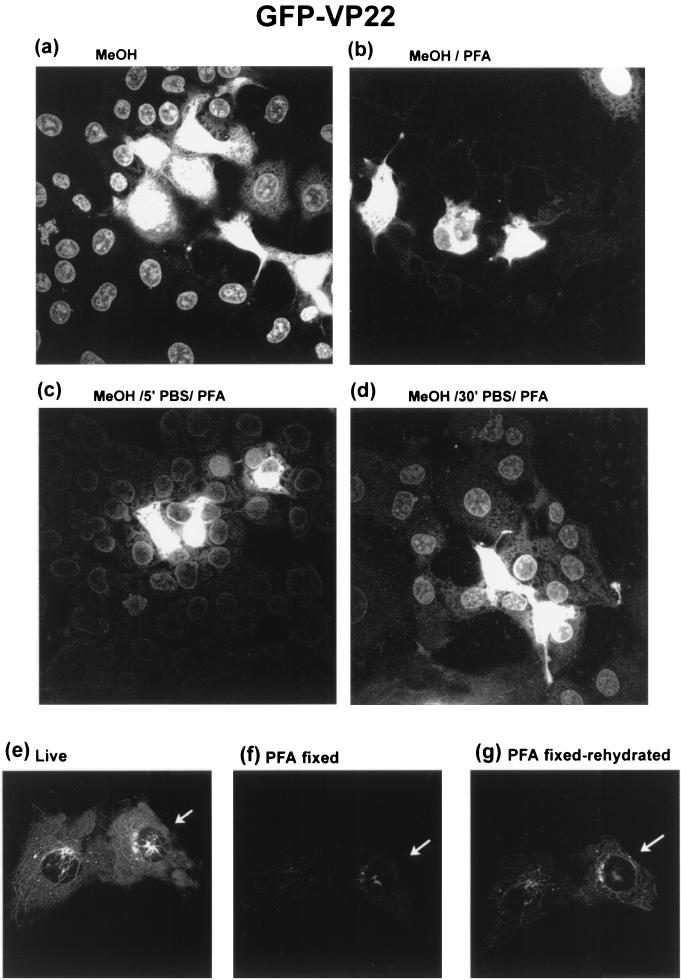
We further reasoned that if PFA fixation was affecting GFP fluorescence of the fusion protein, this might be directly demonstrated by testing the ability of PFA to quench the intrinsic GFP fluorescence that is otherwise be observed in methanol-fixed cells. Cells transfected with the GFP-VP22 construct were therefore fixed with methanol as normal, while parallel coverslips were fixed with PFA immediately after removal of the methanol. Both coverslips were then examined by direct GFP fluorescence. Compared to methanol fixation alone (Fig. 3a), methanol fixation followed directly by PFA fixation (Fig. 3b) resulted in substantial quenching of the fluorescence in the recipient cells. A period of rehydration before PFA application resulted in the partial restoration of fluorescence in recipient cells (Fig. 3c and d). While it is not clearly visible from Fig. 3a and b, a separate experiment demonstrated that this reduction in fluorescence was also observed in the producer cell population. Thus, cells were transfected on coverslips with grids to enable analysis of identical individual cells at all stages. Images were collected of the live producer cells before fixation. The cells were then fixed in PFA and imaged immediately or after a period of rehydration. The results show that the direct fluorescence from the live producer cell (Fig. 3e) was substantially reduced immediately after PFA fixation (Fig. 3f), and although recovery of fluorescence was observed after a period of rehydration in PBS, it was incomplete (Fig. 3g).
FIG. 3.
COS-1 cells were transfected with the expression vector for GFP-VP22 (200 ng). Forty hours after transfection, the cells were fixed with methanol (MeOH) and washed in PBS as normal (a), fixed in methanol followed immediately by fixation in 4% PFA (b), or fixed in methanol and rehydrated in PBS for 5 min (5′) (c) and 30 min (30′) (d) before fixation in PFA. Cells were then examined for spread of GFP-VP22 by intrinsic GFP fluorescence. In a separate experiment transfected cells on coverslips with grids were first examined live by intrinsic fluorescence (e) and then fixed in PFA, and identical cells were examined immediately (f) or after 15 min of rehydration in PBS (g). The panel shows typical results. All images were analyzed at the same attenuation settings.
An explanation consistent with these results taken together is that spread of the GFP-VP22 fusion protein is not detected by intrinsic GFP fluorescence in live recipient cells because it is below the detection limit, that PFA fixation and cross-linking may quench intrinsic fluorescence of the fusion protein which can be partially reversed during rehydration, and that methanol fixation allows detection more readily, possibly by dequenching or renaturation following the rehydration step.
Limited leaching of VP22 from fixed cells.
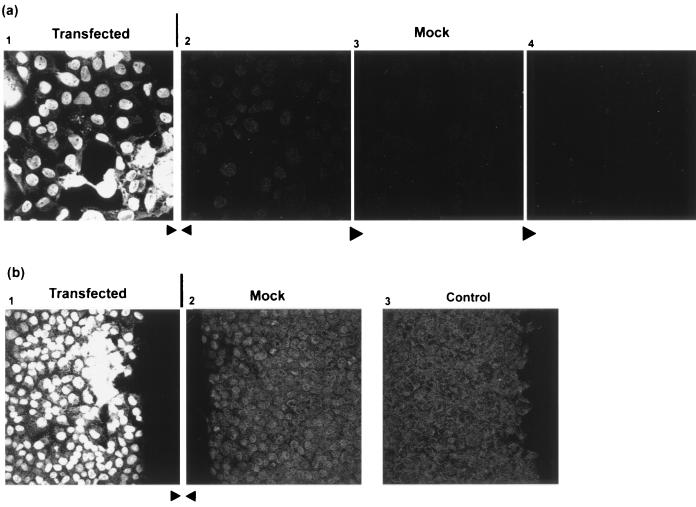
We also considered a possible alternative explanation to account for both the lower detection of the spread of native VP22 by IF after PFA versus methanol fixation and the lack of detection of the spread of GFP-VP22 by intrinsic GFP fluorescence. Although unlikely, it was possible that spread appeared greater in methanol-fixed cells due to some postfixation extraction of VP22 from cells expressing VP2 and subsequent binding to the fixed surrounding cells. Such a possibility has also been considered in a recent study examining VP22 localization by IF in virus-infected cells (12). To examine this possibility, we designed the following experiment. Immediately prior to fixation and processing, a square coverslip of transfected cells was placed directly adjacent to and abutting the edge of a confluent coverslip of mock-transfected cells. With their edges closely abutted, any significant leaching of VP22 during processing from the transfected cells should result in its appearance in the nuclei of naïve cells on the untransfected control coverslip. The results are shown in Fig. 4a, where panel 1 shows the transfected cells, panel 2 shows a typical field in the abutted control coverslip immediately adjacent to the transfected cells, and panels 3 and 4 show fields from the control coverslip moving progressively inward, away from the transfected cells. All the fields were examined in parallel under the same microscopy conditions and laser attenuation. In panel 1 of Fig. 4a, the typical pattern of VP22 spread was observed with intensely staining cells surrounded by cells with the protein present largely in the nucleus. Surprisingly, in panel 2, the mock-transfected cells immediately adjacent to the transfected cells, a weak nuclear pattern was observed. Since this pattern gradually diminished the further the field was from the transfected cells (panels 3 and 4) and was above the background level of mock-transfected cells processed completely separately, we believe this represents a certain amount of postfixation extraction of VP22 from the transfected cells to the adjacent cells. A similar experiment is shown in Fig. 4b, where the edges of the coverslips, shown by the straight edge of cells, in the abutted transfected and mock-transfected cells can be clearly seen. (Note that the cell edges were immediately adjacent during fixation and processing and were slightly separated only to achieve well-resolved images during microscopy). Again the results show a level of nuclear staining and some chromatin association in the abutted mock-transfected cell coverslip (Fig. 4b, panel 2) which was above the background level of a control coverslip processed separately (panel 3). However, compared to the nuclear staining of the cells in the transfected-cell monolayer, the intensity of staining in the adjacent mock-transfected cells appeared to be a very minor effect, not accounting for spread in the transfected-cell monolayer.
FIG. 4.
COS-1 cells, plated on square coverslips, were transfected with the expression vector for native VP22 (600 ng). Forty hours after transfection, the coverslip was transferred to a fresh culture dish and a coverslip of control, untransfected cells was placed directly adjacent to it. The abutted coverslips were then fixed with methanol, washed in PBS, and processed for detection of VP22 by staining with AGV30. The coverslips remained directly abutted during all steps of processing. Panel 1 shows a typical field of view of the edge of the transfected coverslip nearest the abutted coverslip, while panel 2 shows a typical field of view of the edge of the untransfected coverslip directly abutting the transfected cells. Panels 3 and 4 are fields taken progressively towards the center of the untransfected coverslip, away from the transfected cells. (b) Panels 1 and 2 are as described above showing the abutted edges of the transfected and untransfected coverslips, respectively. Panel 3 shows the background from an untransfected coverslip processed separately.
In summary, VP22 spread could be observed by IF using any of a series of standard processing protocols, including PFA fixation. The efficiency of spread detected with PFA fixation was lower than that assessed with methanol fixation, and we sought to determine whether this was due to PFA fixation affecting antibody detection, for which there is much precedent, or whether methanol fixation resulted in some artificial leaching of VP22 protein from the cells expressing VP22 to surrounding cells. While we found evidence for this, it appeared to be a minor effect, insufficient to account for the extent of VP22 spread seen in transfected-cell monolayers. Moreover, if spread was accounted for by such an effect, then it would also have had to occur in PFA-fixed, cross-linked cells, which seems unlikely. Spread of VP22 is also consistent with the enhanced biological activity of VP22-p53 and VP22-TK fusion proteins (3, 10). However, particularly in the case of thymidine kinase (TK), activity of the fusion protein in promoting ganciclovir-induced cell death was much more restricted than would have been predicted from the spread observed by IF with methanol fixation. It may be that the more limited spread observed by PFA fixation gives a more accurate estimate of spread of functional protein.
With regard to the GFP fusion proteins, the failure to observe the spread of a GFP-VP22 in living cells may be explained by the limits of sensitivity of intrinsic GFP fluorescence, and indeed there is precedent for the detection of GFP fusion proteins by IF when none could be detected by intrinsic-fluorescence analysis (11). It has been estimated (13) that even for enhanced GFP, between 105 and 106 molecules of GFP may be required to detect GFP in living cells with any sensitivity above background autofluorescence. Protein turnover together with unfolding or some form of quenching during transport of the GFP-VP22 fusion protein, e.g., related to pH of cell compartments, may also contribute to the lack of detection, and it is possible that in some way fixation with methanol concentrates and/or allows refolding or dequenching of the GFP in the fusion protein in a way that PFA does not. Therefore, as for VP22, detection and localization of certain proteins may not be accurately reflected by intrinsic GFP fluorescence of the corresponding fusion proteins.
REFERENCES
- 1.Aints A, Dilber M S, Smith C I E. Intercellular spread of GFP-VP22. J Gen Med. 1999;1:275–279. doi: 10.1002/(SICI)1521-2254(199907/08)1:4<275::AID-JGM44>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 2.Blaho J A, Mitchell C, Roizman B. An amino acid sequence shared by the herpes simplex virus 1 alpha regulatory proteins 0, 4, 22, and 27 predicts the nucleotidylylation of the UL21, UL31, UL47, and UL49 gene products. J Biol Chem. 1994;269:17401–17410. [PubMed] [Google Scholar]
- 3.Dilber M S, Phelan A, Aints A, Mohamed A J, Elliott G, Smith C I E, O'Hare P. Intercellular delivery of thymidine kinase prodrug activating enzyme by the herpes simplex virus protein, VP22. Gene Ther. 1999;6:12–21. doi: 10.1038/sj.gt.3300838. [DOI] [PubMed] [Google Scholar]
- 4.Elliott G, O'Hare P. Herpes simplex virus type 1 tegument protein VP22 induces the stabilization and hyperacetylation of microtubules. J Virol. 1998;72:6448–6455. doi: 10.1128/jvi.72.8.6448-6455.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Elliott G, O'Hare P. Intercellular trafficking and protein delivery by a herpesvirus structural protein. Cell. 1997;88:223–233. doi: 10.1016/s0092-8674(00)81843-7. [DOI] [PubMed] [Google Scholar]
- 6.Elliott G, O'Hare P. Intercellular trafficking of VP22-GFP fusion proteins. Gene Ther. 1999;6:149–151. doi: 10.1038/sj.gt.3300850. [DOI] [PubMed] [Google Scholar]
- 7.Elliott G, O'Reilly D, O'Hare P. Phosphorylation of the herpes simplex virus type 1 tegument protein VP22. Virology. 1996;226:140–145. doi: 10.1006/viro.1996.0638. [DOI] [PubMed] [Google Scholar]
- 8.Elliott G D, Meredith D M. The herpes simplex virus type 1 tegument protein VP22 is encoded by gene UL49. J Gen Virol. 1992;73:723–726. doi: 10.1099/0022-1317-73-3-723. [DOI] [PubMed] [Google Scholar]
- 9.Fang B, Xu B, Koch P, Roth J A. Intercellular trafficking of VP22-GFP fusion proteins is not observed in cultured mammalian cells. Gene Ther. 1998;5:1420–1424. doi: 10.1038/sj.gt.3300741. [DOI] [PubMed] [Google Scholar]
- 10.Phelan A, Elliott G, O'Hare P. Intercellular delivery of functional p53 by the herpesvirus protein VP22. Nat Biotechnol. 1998;16:440–443. doi: 10.1038/nbt0598-440. [DOI] [PubMed] [Google Scholar]
- 11.Pines J. GFP in mammalian cells. Trends Genet. 1995;11:326–327. doi: 10.1016/s0168-9525(00)89092-7. [DOI] [PubMed] [Google Scholar]
- 12.Pomeranz L E, Blaho J A. Modified VP22 localizes to the cell nucleus during synchronized herpes simplex virus type 1 infection. J Virol. 1999;73:6769–6781. doi: 10.1128/jvi.73.8.6769-6781.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tsein R Y. The green fluorescent protein. Annu Rev Biochem. 1998;67:509–544. doi: 10.1146/annurev.biochem.67.1.509. [DOI] [PubMed] [Google Scholar]
- 14.Wybranietz W A, Prinz F, Speigel M, Schenk A, Bitzer M, Gregor M, Lauer U. Quantification of VP22-GFP spread by direct fluorescence in 15 commonly used cell lines. J Gene Med. 1999;1:265–274. doi: 10.1002/(SICI)1521-2254(199907/08)1:4<265::AID-JGM48>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]