Abstract
Aims
To investigate the roles of neurotrophic factors on cognition in patients with Alzheimer's disease (AD) carrying Apolipoprotein E (APOE) ε4.
Methods
Totals of 173 patients with AD were divided into APOE ε4 carrier and non‐carrier groups, and their demographics, cognition, and neurotrophic factors in cerebrospinal fluid (CSF) were compared. Multiple linear regression analyses were performed to assess correlations among APOE ε4, neurotrophic factors and cognition. Mediation analyses were conducted to assess the sequential associations among APOE ε4, nerve growth factor (NGF), and cognition.
Results
Global cognition and multiple domains were impaired in the APOE ε4 carrier group (all p < 0.05). NGF level in the APOE ε4 carrier group was lower than that in the non‐carrier group (p = 0.016). NGF level showed significant correlations with both global and multiple domains cognitions. Specifically, NGF mediated the association between APOE ε4 and Animal Fluency Test score (β, −0.45; 95% CI [−0.96, −0.07]; p < 0.001) and Trail Making Test‐A (time) (β, 0.15; 95% CI [0.01, 0.33]; p < 0.001).
Conclusion
APOE ε4 is associated with cognitive impairment, and those carrying APOE ε4 have decreased NGF level in CSF. Declined NGF level is correlated with compromised cognition. NGF mediates APOE ε4‐associated cognitive impairment.
Keywords: Alzheimer's disease, apolipoprotein E ε4, cognitive function, nerve growth factor, neurotrophic factors
Apolipoprotein E (APOE) ε4 was associated with cognitive impairment. Patients with Alzheimer's disease carrying APOE ε4 had decreased nerve growth factor (NGF) levels in cerebrospinal fluid. Declined NGF is correlated with compromised cognitive function, including global cognition and multiple cognitive domains. NGF mediated APOE ε4‐associated cognitive impairment.
1. INTRODUCTION
Alzheimer's disease (AD) is the most common cognitive disorder among the elderly. Typical patients with AD are initially characterized by episodic memory decline, followed by overall cognitive impairment, neuropsychiatric symptoms, and impaired activities of daily living. 1
Apolipoprotein E (APOE), encoding the indispensable lipid transporter APOE protein in the brain, includes three alleles of ε2, ε3, and ε4, of which, APOE ε4 is the strongest risk gene for developing sporadic AD. 2 APOE ε4 carriers exhibit an earlier age of onset, a faster rate of cognitive decline, and a poorer cognitive outcome. 2 , 3 Previous studies have linked APOE ε4 to synaptic dysfunction and aggravated cognitive impairment by promoting depositions of neuropathological proteins of AD 4 , 5 and eliciting neuroinflammation. 6 However, the role of APOE ε4 on the neurotrophic state in the brain remains unclear.
Neurotrophic factors constitute a family of proteins, including brain‐derived neurotrophic factor (BDNF), glial cell‐derived neurotrophic factor (GDNF), and nerve growth factor (NGF). These factors played crucial roles in the survival, growth, and differentiation of neurons during development. 7 Decreased levels of BDNF and its precursor were observed in several brain regions and peripheral blood of patients with AD. 8 GDNF level was elevated in the cerebrospinal fluid (CSF) of early‐stage patients with AD but decreased in the middle temporal gyrus and serum of patients with AD. 9 In addition, proNGF levels in the cerebral cortex and CSF of patients with AD were increased, while NGF mRNA was unchanged in the cerebral cortex of patients with AD. 10 , 11 , 12 It came to the limelight that abnormal secretions of these neurotrophic factors were correlated with the progression of neuropathology and cognitive impairment of patients with AD. 13 , 14 However, the relationships between neurotrophic factors in CSF, APOE ε4, and functions of different cognitive domains remain unknown.
In this study, we hypothesized that both APOE ε4 and neurotrophic factors might be related to cognitive impairment, APOE ε4 might be associated with decreased neurotrophic factors in CSF, and the declined neurotrophic factors might play a pivotal role in mediating APOE ε4‐associated cognitive impairment. Based on this hypothesis, patients with AD were divided into APOE ε4 carrier and APOE ε4 non‐carrier groups. Demographic variables were collected, overall cognitive function and individual cognitive domains were assessed by a series of rating scales, and the levels of neurotrophic factors, including BDNF, GDNF, and NGF in CSF were measured by enzyme‐linked immunosorbent assay (ELISA) with aims to figure out the roles of neurotrophic factors on cognitive impairment in patients with AD carrying APOE ε4.
2. METHODS
2.1. Ethics statement
The study was approved by the Ethical Review Board of Beijing Tiantan Hospital. Written informed consent was obtained from patients and their caregivers. All the procedures were conducted in accordance with the guidelines and regulations of ethical principles for medical research involving human subjects of the Declaration of Helsinki.
2.2. Participants
Patients diagnosed with AD according to the National Institute of Aging and Alzheimer's Association (NIA‐AA) criteria 15 , 16 were consecutively enrolled from the Center for Cognitive Neurology, Department of Neurology, Beijing Tiantan Hospital, Capital Medical University. The exclusion criteria included: (1) Patients with a history of diseases that might affect cognitive function besides AD, such as cerebrovascular diseases, Lewy body diseases, frontotemporal neurodegeneration, corticobasal degeneration, multiple sclerosis, epilepsy, substance abuse, etc. (2) Patients with severe systematic diseases, active infections, chronic wasting disease, autoimmune diseases, hematological system diseases, and malignant tumor. (3) Patients suffered from traumatic brain injury and major surgery recently. (4) Patients were undergoing steroid treatment. (5) Patients were unable to cooperate with all the examinations for various reasons.
2.3. Collection of demographic variables
Demographic variables, including gender, age, age of onset, disease duration, education level, drinking, smoking, and body mass index (BMI), were collected. In addition, a history of hypertension, hyperlipidemia, myocardial infarction, atrial fibrillation, diabetes mellitus, et al., was also collected.
2.4. Assessment of cognitive function
The overall cognitive function of patients with AD was assessed by the Mini‐Mental State Examination (MMSE) 17 and the Montreal Cognitive Assessment (MoCA). 18 In the individual cognitive domains, verbal memory was evaluated by the Auditory Verbal Learning Test (AVLT), and visual delayed memory was evaluated by the Rey‐Osterreithm Complex Figure Test (RCFT)‐delayed recall. 19 , 20 The language was evaluated by the Animal Fluency Test (AFT), the Verbal Fluency Test (VFT), and the Boston Naming Test (BNT). 21 , 22 Attention was evaluated by the Symbol Digit Modalities Test (SDMT), 23 the Trail Making Test (TMT)‐A, 24 as well as the Stroop Color‐Word Test (SCWT)‐A and the SCWT‐B. 25 Visuospatial ability was evaluated by the RFT. Executive function was evaluated by the SCWT‐C and the TMT‐B. A detailed description of these rating scales was provided in the Supplementary material.
2.5. Detections of APOE genotypes
The venous blood samples of patients with AD were collected from the median elbow under fasting conditions in the morning following admission and then sent to the clinical laboratory of our hospital.
Genotyping for APOE single nucleotide variants (rs429358 C/T and rs7412 C/T), which define APOE 𝜀2, 𝜀3, and 𝜀4, was performed by real‐time fluorescence quantitative polymerase chain reaction using nucleic acid detection reagents (Youzhiyou company, Wuhan, China). 26
2.6. Collections of CSF samples
Patients were requested to withdraw anti‐cognitive impairment drugs for at least 12–14 hours before lumbar puncture if their conditions allowed. CSF samples were collected under fasting condition through lumbar puncture, followed by being immediately centrifuged at 4°C with 1500 g for 10 min. Each CSF sample was then allocated into separate Nunc cryotubes (Beijing JingkeHongda Biotechnology Co., Ltd, Beijing, China) and frozen for 0.5 mL per tube at −80°C until the assay. 27
2.7. Measurements of neurotrophic factors in CSF
Neurotrophic factors, including BDNF (ProcartaPlex™ Multiplex Immunoassay Kit, Invitrogen, USA), GDNF (ProcartaPlex™ Multiplex Immunoassay Kit, Invitrogen, USA), and NGF (Human NGF DuoSet ELISA Kit, R&D Systems, USA), in CSF were measured by ELISA.
2.8. Statistical analysis
Statistical analyses were performed by SPSS Statistics 25.0 (IBM Corporation, New York, USA). Statistical significance was defined as a two‐sided p < 0.05.
The data were tested for normal distribution using the Kolmogorov–Smirnov test. Demographic variables, cognitive function, and neurotrophic factors in CSF were compared between APOE ε4 carrier and non‐carrier groups. Continuous variables conforming to normal distribution were presented as means ± standard deviations (SD) and compared by two‐tailed t‐test, while non‐normal distributed variables were presented as median (quartile) and compared by a non‐parametric test, and categorical variables were presented as number (percentage) and compared by Chi‐Squared test. Multiple linear regression analyses were performed to assess the correlations among APOE ε4, neurotrophic factors in CSF, and cognitive function of patients with AD. Mediation analysis was conducted to evaluate the sequential associations among APOE ε4, NGF, and cognitive function.
3. RESULTS
3.1. Demographic variables in patients with AD
A total of 173 patients with AD were enrolled in this study, of whom 55 cases (31.79%) carried APOE ε4, 34 cases (61.83%) were female, the mean age was 65.49 ± 9.00 years old, and the median disease duration was 24.00 (12.00, 48.00) months in APOE ε4 carrier group. Demographic variables, including gender, age, age of onset, disease duration, education level, smoking, drinking, BMI, etc., were not significantly different between the two groups (Table 1).
TABLE 1.
Demographic variables of APOE ε4− and APOE ε4+ groups.
| APOE ε4− group (n = 118) | APOE ε4+ group (n = 55) | p | |
|---|---|---|---|
| Female (n [%]) | 68.00 (57.63) | 34.00 (61.82) | 0.602 |
| Age (years, mean ± SD) | 62.71 ± 9.05 | 65.49 ± 9.00 | 0.062 |
| Age of onset (years, median [quartile]) | 59.00 (53.00, 64.75) | 64.00 (53.50, 70.00) | 0.075 |
| Disease duration (months, median [quartile]) | 25.00 (13.50, 49.00) | 24.00 (12.00, 48.00) | 0.460 |
| Education level | 0.311 | ||
| Primary school and below (n [%]) | 25 (21.19) | 11 (20.00) | |
| Middle and high school (n [%]) | 55 (46.61) | 21 (38.18) | |
| Bachelor's degree and above (n [%]) | 38 (32.20) | 13 (23.64) | |
| Smoking (n [%]) | 31 (26.27) | 15 (27.27) | 0.853 |
| Drinking (n [%]) | 28 (23.73) | 14 (25.45) | 0.832 |
| BMI (median [quartile]) | 23.89 (21.91, 25.87) | 23.10 (21.60, 25.16) | 0.252 |
| History | |||
| Hypertension (n [%]) | 39 (33.05) | 13 (23.63) | 0.215 |
| Hyperlipidemia (n [%]) | 15 (12.71) | 13 (23.63) | 0.142 |
| Myocardial infarction (n [%]) | 2 (1.69) | 0 (0.00) | 1.000 |
| Atrial fibrillation (n [%]) | 0 (0.00) | 1 (1.82) | 0.308 |
| Diabetes mellitus (n [%]) | 15 (12.71) | 6 (10.91) | 0.769 |
| Hyperhomocysteinemia (n [%]) | 3 (2.54) | 0 (0.00) | 0.553 |
| Cerebrovascular disease (n [%]) | 17 (14.41) | 5 (9.10) | 0.357 |
| Thyroid disease (n [%]) | 8 (6.78) | 4 (7.27) | 0.968 |
| Chronic obstructive pulmonary disease (n [%]) | 2 (1.69) | 0 (0.00) | 1.000 |
| Asthma (n [%]) | 3 (2.54) | 2 (3.64) | 0.411 |
| Insomnia (n [%]) | 16 (13.56) | 3 (5.45) | 0.074 |
| Sleep apnea syndrome (n [%]) | 4 (4.24) | 1 (1.82) | 0.588 |
| Depression (n [%]) | 8 (6.78) | 3 (5.45) | 0.564 |
| Other mental disorders (n [%]) | 3 (2.54) | 0 (0.00) | 0.223 |
Note: Data were presented as number (percentage), means ± SD, or median (quartile). APOE ε4−, APOE ε4 non‐carriers; APOE ε4+, APOE ε4 carriers.
Abbreviations: APOE, apolipoprotein E; BMI, body mass index; SD, standard deviation.
3.2. Association of APOE ε4 with cognitive function
A comparison of cognitive function between APOE ε4 carrier and APOE ε4 non‐carrier groups was presented (Table 2). Regarding global cognitive function, MMSE (p = 0.042) and MoCA scores (p = 0.015) in APOE ε4 carrier group were lower than those in the APOE ε4 non‐carrier group. In terms of individual cognitive domain function, compared with APOE ε4 non‐carrier group, APOE ε4 carrier group had the lower scores of AVLT N1‐3 (p = 0.012), AVLT N4 (p = 0.027), AVLT N1‐5 (p = 0.003) and AVLT N6 (p = 0.013), lower scores of AFT (p = 0.011), VFT‐H (p = 0.044) and VFT‐alternating fluency (p = 0.046), and longer time for TMT‐A (p = 0.034) and TMT‐B (p = 0.023).
TABLE 2.
Cognitive function of APOE ε4− and APOE ε4+ groups.
| APOE ε4− group (n = 118) | APOE ε4+ group (n = 55) | p | |
|---|---|---|---|
| Global cognitive function | |||
| MMSE (points, median [quartile]) | 20.50 (13.00, 25.00) | 18.00 (10.00, 22.00) | 0.042* |
| MoCA (points, mean ± SD) | 14.44 ± 6.53 | 11.69 ± 6.32 | 0.015* |
| Individual cognitive domain function | |||
| Memory | |||
| AVLT N1‐3 (points, mean ± SD) | 10.55 ± 5.84 | 8.86 ± 5.93 | 0.012* |
| AVLT N4 (points, median [quartile]) | 0.00 (0.00, 3.00) | 0.00 (0.00, 1.00) | 0.027* |
| AVLT N5 (points, median [quartile]) | 0.00 (0.00, 4.00) | 0.00 (0.00, 1.00) | 0.073 |
| AVLT N1‐5 (points, median [quartile]) | 12.00 (8.00, 20.00) | 8.50 (3.00, 14.25) | 0.003** |
| AVLT N6 (points, median [quartile]) | 0.00 (0.00, 3.00) | 0.00 (0.00, 0.75) | 0.013* |
| AVLT N7 (points, median [quartile]) | 9.00 (6.00, 12.00) | 8.00 (0.25, 10.75) | 0.074 |
| RCFT‐delayed (points, median [quartile]) | 0.00 (0.00, 10.00) | 0.00 (0.00, 5.00) | 0.467 |
| Language | |||
| AFT (points, mean ± SD) | 11.79 ± 5.81 | 9.73 ± 6.10 | 0.011* |
| VFT‐H (points, median [quartile]) | 9.00 (6.00, 15.00) | 8.50 (5.00, 12.00) | 0.044* |
| VFT‐alternating fluency (points, mean ± SD) | 7.59 ± 5.34 | 6.38 ± 5.15 | 0.046* |
| BNT (points, median [quartile]) | 21.00 (18.00, 26.00) | 21.00 (15.00, 24.00) | 0.209 |
| Visuospatial ability | |||
| RCFT‐imitation (points, median [quartile]) | 26.00 (1.00, 33.00) | 20.25 (0.00, 32.25) | 0.622 |
| Attention/Executive function | |||
| TMT‐A (points, median [quartile]) | 25.00 (22.00, 25.00) | 25.00 (20.25, 25.00) | 0.683 |
| TMT‐A (time) (minutes, median [quartile]) | 1.88 (1.05, 3.70) | 2.55 (1.45, 4.00) | 0.034* |
| TMT‐B (points, median [quartile]) | 21.00 (5.50, 25.00) | 15.00 (6.00, 24.00) | 0.184 |
| TMT‐B (time) (minutes, median [quartile]) | 2.99 (1.18, 4.00) | 4.00 (2.90, 4.00) | 0.023* |
| SCWT‐A (points, median [quartile]) | 50.00 (49.00, 50.00) | 50.00 (48.00, 50.00) | 0.262 |
| SCWT‐A (time) (minutes, median [quartile]) | 0.67 (0.48, 0.94) | 0.79 (0.50, 1.06) | 0.263 |
| SCWT‐B (points, median [quartile]) | 50.00 (47.25, 50.00) | 50.00 (46.00, 50.00) | 0.523 |
| SCWT‐B (time) (minutes, median [quartile]) | 0.88 (0.62, 1.20) | 0.93 (0.74, 1.51) | 0.461 |
| SCWT‐C (points, median [quartile]) | 47.00 (37.00, 49.00) | 43.00 (26.00, 47.50) | 0.177 |
| SCWT‐C (time) (minutes, median [quartile]) | 1.55 (0.91, 2.11) | 1.74 (0.98, 2.52) | 0.515 |
| SDMT (points, median [quartile]) | 20.00 (0.75, 31.75) | 15.00 (0.00, 26.50) | 0.318 |
Note: Data were presented as means ± SD or median (quartile). APOE ε4−, APOE ε4 non‐carriers; APOE ε4+, APOE ε4 carriers.
Abbreviations: AFT, Auditory Verbal Learning Test; APOE, apolipoprotein E; AVLT, Animal Fluency Test; BNT, Boston Naming Test; MMSE, Mini‐Mental State Examination; MoCA, Montreal Cognitive Assessment; RCFT, Rey‐Osterrieth Complex Figure Test; SCWT, Stroop Color and Word Test; SD, standard deviation; SDMT, Symbol Digit Modalities Test; TMT, Trail Making Test; VFT‐H, Verbal Fluency Test‐Household.
*p < 0.05, **p < 0.01.
Further multiple linear regression analyses revealed the association between APOE ε4 and cognitive function after adjusting for age, gender, age of onset, disease duration, and education level (Table S1). In global cognitive function, APOE ε4 was associated with a lower MoCA score (β, −2.31; 95% CI [−4.58, −0.05]; p = 0.046). In individual cognitive domains, APOE ε4 was associated with the lower scores of AVLT N1‐3 (β, −2.35; 95% CI [−4.51, −0.18]; p = 0.034), AVLT N1‐5 (β, −4.20; 95% CI [−8.14, −0.25]; p = 0.037), AVLT N6 (β, −1.01; 95% CI [−1.94, −0.08]; p = 0.034) and AVLT N7 (β, −2.62; 95% CI [5.02, −0.21]; p = 0.033), the lower score of AFT (β, −2.06; 95% CI [−4.04, −0.09]; p = 0.040), and longer time spend on TMT‐A (β, 0.45; 95% CI [0.21, 0.88]; p = 0.040). APOE ε4 showed no significant association with the scores of rating scales for language and visuospatial ability.
3.3. Association of APOE ε4 with the levels of neurotrophic factors in CSF
The levels of neurotrophic factors in CSF were compared between APOE ε4 carrier and APOE ε4 non‐carrier groups. NGF level in APOE ε4 carrier group was significantly lower than that in APOE ε4 non‐carrier group (p = 0.016) (Figure 1).
FIGURE 1.
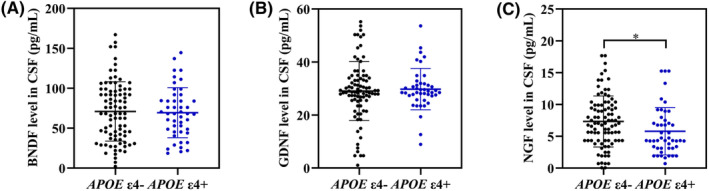
The levels of neurotrophic factors in CSF from APOE ε4− and APOE ε4+ groups. Comparison of the levels of BDNF (A), GDNF (B), and NGF (C) in CSF between APOE ε4− and APOE ε4+ groups. APOE ε4−, APOE ε4 non‐carriers; APOE ε4+, APOE ε4 carriers. APOE, apolipoprotein E; BDNF, brain‐derived neurotrophic factor; CSF, cerebrospinal fluid; GDNF, glial cell‐derived neurotrophic factor; NGF, nerve growth factor. *p < 0.05.
AD patients were further divided into APOE ε4−/−, APOE ε4+/− and APOE ε4+/+ groups. It was found that the NGF level in APOE ε4+/− group was significantly lower than that in APOE ε4−/− group (p = 0.042). Moreover, APOE ε4+/+ group demonstrated the lowest NGF level in CSF compared to the other groups (Figure S1).
Multiple linear regression analyses illustrated that APOE ε4 was associated with a declined NGF level in CSF after adjusting for age, gender, age of onset, disease duration, education level, and BMI (β, −1.98; 95% CI [−3.78, −0.19]; p = 0.031) (Table 3).
TABLE 3.
Association between APOE ε4 and neurotrophic factors in CSF in patients with AD.
| Unadjusted | Adjusted | |||
|---|---|---|---|---|
| β (95% CI) | p | β (95% CI) | p | |
| BDNF (pg/ml) | −0.09 (−14.60, 14.43) | 0.991 | −7.25 (−24.56, 10.05) | 0.408 |
| GDNF (pg/ml) | 2.28 (−2.65, 7.21) | 0.363 | 3.38 (−3.36, 10.12) | 0.322 |
| NGF (pg/ml) | −1.87 (−3.39, −0.35) | 0.016* | −1.98 (−3.78, −0.19) | 0.031* |
Note: Age, gender, age of onset, disease duration, education level, and BMI were adjusted.
Abbreviations: APOE, apolipoprotein E; AD, Alzheimer's disease; BDNF, brain‐derived neurotrophic factor; CI, confidence interval; CSF, cerebrospinal fluid; GDNF, glial cell‐derived neurotrophic factor; NGF, nerve growth factor.
p < 0.05.
3.4. Association between NGF level in CSF and cognitive function
Multiple linear regression analyses were conducted to explore the association between NGF level in CSF and cognitive function in patients with AD after adjusting for age, gender, age of onset, disease duration, education level, and BMI (Figure 2). Regarding global cognitive function, NGF level was positively associated with MMSE score (β, 0.33; 95% CI [0.01, 0.66]; p = 0.046). As far as individual cognitive domain, NGF level was positively associated with the scores of RCFT‐delayed (β, 0.52; 95% CI [0.15, 0.89]; p = 0.007), AFT (β, 0.35; 95% CI [0.107, 0.585]; p = 0.005) and VFT‐alternating fluency (β, 0.28; 95% CI [0.05, 0.52]; p = 0.018), which reflected language function. Furthermore, NGF level was negatively associated with TMT‐A (time) (β, −0.08; 95% CI [−0.14, −0.02]; p = 0.008) and positively associated with SCWT‐B score (β, 0.72; 95% CI [0.06, 1.37]; p = 0.033), which reflected attention/executive function.
FIGURE 2.
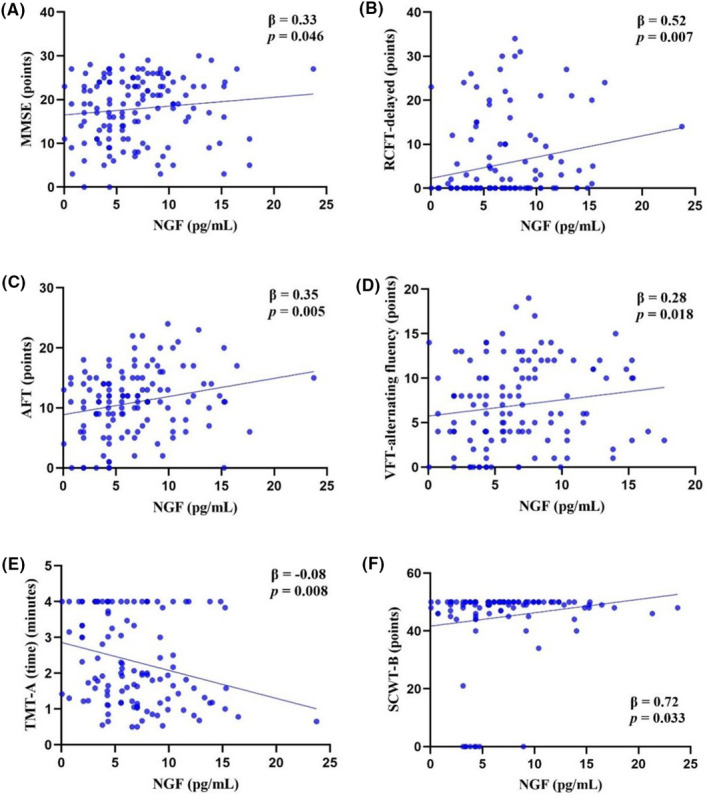
Association between NGF level in CSF and cognitive function in patients with AD. Multiple linear regression analyses were performed between NGF level and MMSE (A), RCFT‐delayed (B), AFT (C), VFT‐alternating fluency (D), TMT‐A (time) (E), and SCWT‐B (F) in patients with AD after adjusting for age, gender, age of onset, disease duration, education level, and BMI. AD, Alzheimer’s disease; AFT, Animal Fluency Test; CSF, cerebrospinal fluid; MMSE, Mini‐Mental State Examination; NGF, nerve growth factor; RCFT, Rey‐Osterrieth Complex Figure Test; SCWT, Stroop Color and Word Test; TMT, Trail Making Test; VFT, Verbal Fluency Test.
Within the subgroup with patients carrying APOE ε4, NGF level was positively associated with the scores of AVLT N1‐3 (β, 0.50; 95% CI [0.11, 0.89]; p = 0.015), AVLT N4 (β, 0.20; 95% CI [0.05, 0.34]; p = 0.010), AVLT N1‐5 (β, 0.63; 95% CI [0.03, 1.24]; p = 0.042), RCFT‐delayed (β, 1.29; 95% CI [0.37, 2.22]; p = 0.010), and VFT‐alternating fluency (β, 0.41; 95% CI [0.02, 0.79]; p = 0.041). NGF level was not associated with visuospatial ability and attention/executive function (Table S2).
3.5. Mediation analyses among APOE ε4, NGF level in CSF, and cognitive function
Mediation analyses were conducted to assess the sequential associations among APOE ε4, NGF level in CSF, and cognitive function in patients with AD (Figure 3). The results showed that NGF level in CSF significantly mediated the associations between APOE ε4 and AFT (β, −0.45; 95% CI [−0.96, −0.07]; p < 0.001) (Figure 3A) as well as TMT‐A (time) (β, 0.15; 95% CI [0.01, 0.33]; p < 0.001) (Figure 3B) after adjusting for age, gender, age of onset, disease duration, education level and BMI. There was no notable mediation effect of NGF level in CSF on the associations between APOE ε4 and functions of other cognitive domains.
FIGURE 3.
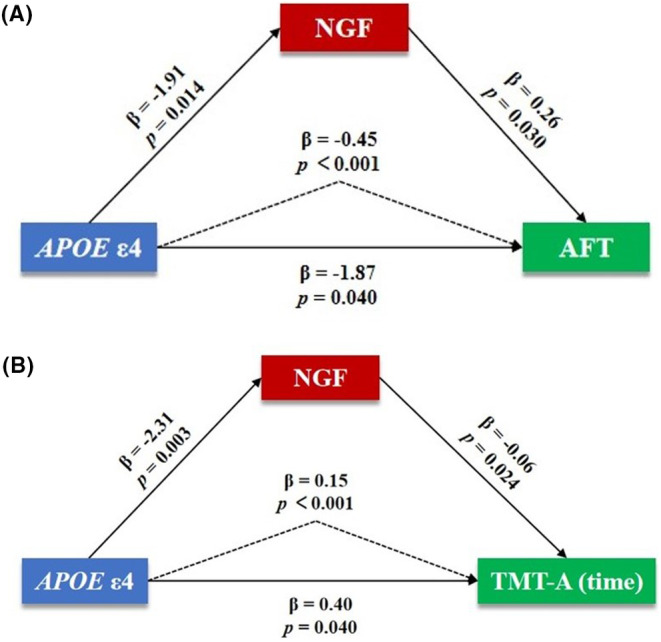
Association among APOE ε4, NGF level in CSF and cognitive function in patients with AD. Mediation analyses among APOE ε4, NGF level in CSF, and AFT score (A) and TMT‐A (time) (B) in patients with AD after adjusting for age, gender, age of onset, disease duration, education level, and BMI. AFT, Animal Fluency Test; AD, Alzheimer's disease; APOE, apolipoprotein E; CSF, cerebrospinal fluid; NGF, nerve growth factor; TMT, Trail Making Test.
4. DISCUSSION
In this study, our exploration delved into the association among APOE ε4, the levels of neurotrophic factors in CSF, and cognitive function in patients with AD. The findings revealed that: (1) APOE ε4 was significantly associated with poorer cognitive function; (2) APOE ε4 was associated with a declined NGF level in CSF; (3) The declined NGF level was significantly associated with cognitive impairment; (4) The decline of NGF level mediated significant association between APOE ε4 and impairments of multiple cognitive domains.
4.1. Relationship between APOE ε4 and cognitive impairment in patients with AD
It is well known that APOE ε4 is the strongest risk gene for sporadic AD. 2 Previous studies demonstrated that patients with AD with APOE ε4 had poorer cognitive function. 28 , 29 , 30 Nonetheless, the precise relationships between APOE ε4 and functions of cognitive domains remained elusive. In this study, we comprehensively evaluated individual cognitive domains by a body of rating scales and found that patients with AD carrying APOE ε4 had particularly more severe memory impairment, which was potentially attributed to the close association of APOE ε4 with temporal lobe (especially hippocampus) atrophy and dysfunction of default mode network. 28 , 29 , 30 In addition to memory, our novel observation indicated a significant association between APOE ε4 and impaired cognitive domains related to language and attention/executive function. These findings might be due to the faster rate of cognitive decline and poorer cognitive function that APOE ε4 carriers had. 2 , 3
4.2. Relationship between APOE ε4 and the levels of neurotrophic factors in CSF in patients with AD
Within the scope of various neurotrophic factors measured in this study, NGF level in CSF from patients with AD with APOE ε4 was significantly declined compared to those without APOE ε4. APOE, a pivotal lipid transport protein in the brain, is secreted primarily by astrocytes and to a lesser extent by microglia. APOE binds with two major metabolic receptors, the low‐density lipoprotein receptor and lipoprotein receptor‐related protein, facilitating lipid transport between cells, redistributing intracellular lipids, and maintaining lipid balance in the brain. 31 Compared to other APOE isoforms, APOE4 encoded by APOE ε4 exerted the weakest effect on lipid transport. 32 Cholesterol homeostasis is essential for the brain, with insufficient accumulation leading to abnormal cellular nutrition and energy metabolism, and excessive accumulation resulting in synaptic plasticity damage and neuronal apoptosis. 33 However, limited studies directly address the relationship between APOE ε4 or APOE4 and NGF. Based on the results of this investigation, we speculate that the impaired cellular nutrient and energy metabolism, stemming from the weakened lipid transport capacity of APOE4, may contribute to the decrease of NGF level in CSF of patients with AD.
4.3. Relationship between NGF level in CSF and cognitive impairment in patients with AD
In this study, we found a close relationship between NGF level in CSF and the cognitive function of patients with AD. It is well known that the main source of acetylcholine in hippocampus and cerebral cortex is the forebrain cholinergic neurons, which dysfunction or degeneration is the prominent cause of decreased acetylcholine level and impaired cognitive function in patients with AD. 1 NGF, a member of the neurotrophin family, supplies the forebrain cholinergic neurons to maintain their cholinergic phenotype. Degeneration of the cholinergic system is associated with dysregulation of the NGF metabolic pathway, which controls the maturation and degradation of NGF. 34 Previous studies have indicated that the disturbed regulation of NGF pathway, including the impaired maturation and increased degradation of NGF, existed in AD, even in the preclinical stage of the disease. 35 , 36 NGF deprivation was associated with poorer cognitive function, and treatment with NGF ameliorated neuropathology and thereafter inhibited memory decline in AD animal models. 11 , 37 However, the relationships between NGF and functions of different cognitive domains remain unclear. In this study, we observed, for the first time, a strong association between NGF level in CSF and impaired global cognition and functions of multiple cognitive domains, including memory, language, attention, and executive function. In addition, the declined NGF level was particularly and fairly associated with compromised memory and language in patients with AD carrying APOE ε4. At the early stage of AD, cognitive domains, such as memory and language, are specifically vulnerable to the decrease of NGF in the individuals carrying APOE ε4, as indicated by the results from this study. The detailed mechanisms underlying their association warrant further investigation in the future.
4.4. Role of NGF on APOE ε4‐associated cognitive impairment in patients with AD
Previous studies highlighted that APOE ε4 exacerbated cognitive impairment by promoting the depositions of neuropathological proteins in AD and facilitating neuroinflammation by activating microglia and astrocytes. 4 , 5 , 6 However, the roles of neurotrophic factors on APOE ε4 and the associated cognitive impairment remain unclear. In the current study, we for the first time found that the declined NGF level in CSF played an important role in mediating APOE ε4‐associated impairments of overall cognition and function of cognitive domains, particularly language and attention/executive function. Given that APOE4 has poorer lipid transport capacity than other APOE isoforms, leading to lipid instability in cells, we speculate that APOE4 may be responsible for the declined NGF level in the brain, and NGF deficiency may further accelerate degeneration of the cholinergic system, eventually propagating cognitive impairment in patients with AD. The potential mechanisms illustrating the mediating role of NGF on APOE ε4‐associated impairments of language and executive function remain to be elucidated.
4.5. Limitations
This study had limitations. First, while measuring neurotrophic factors in CSF is one of the most objective ways to capture their changes in the brain, it is tough to obtain CSF from elderly patients with AD, particularly from people with normal cognition. We will focus on collecting more CSF samples from a larger cohort of patients with AD and cognitively normal controls. Second, unlike a randomized controlled design, this cross‐sectional study may introduce relative selection bias.
5. CONCLUSION
The results from this study indicate a significant association between APOE ε4 and compromised cognitive function in patients with AD. Patients with AD carrying APOE ε4 have prominently decreased NGF level in CSF. Decreased NGF level is markedly associated with drastically impaired overall cognition and individual cognitive domains, including memory, language, and attention/executive function. More importantly, NGF plays a pivotal role in mediating remarkably compromised cognitive function associated with APOE ε4. These novel findings contribute to a better understanding of the mediating role of NGF deficiency on cognitive impairment of patients with AD carrying APOE ε4, thus, replenishing NGF may be an effective therapy for slowing down cognitive impairment in patients with AD carrying APOE ε4.
AUTHOR CONTRIBUTIONS
Mingyue He and Zhan Liu contributed to the conception, design, and data statistics of the study and paper writing; Tenghong Lian, Peng Guo, Wenjing Zhang, Weijiao Zhang, Jinghui Li, Huiying Guan, Weijia Zhang, Dongmei Luo, Jing Qi, and Hao Yue contributed to the acquisition and collation of data; Yue Huang, Yanan Zhang, Gaifen Liu, and Xiaomin Wang contributed to directing paper writing and data statistics; Wei Zhang contributed to the conception, design and implementation of the study and the supervision of paper writing.
FUNDING INFORMATION
This study was supported by the National Key Research and Development Program of China (2016YFC1306300, 2016YFC1306000), the National Natural Science Foundation of China (81970992, 81571229, 81071015, 30770745, 82201639), the Capital's Funds for Health Improvement and Research (CFH) (2022‐2‐2048), the Key Technology R&D Program of Beijing Municipal Education Commission (kz201610025030), the Key Project of Natural Science Foundation of Beijing, China (4161004), the Natural Science Foundation of Beijing, China (7082032), the Project of Scientific and Technological Development of Traditional Chinese Medicine in Beijing (JJ2018‐48), the Capital Clinical Characteristic Application Research (Z121107001012161), the High Level Technical Personnel Training Project of Beijing Health System, China (2009‐3‐26), the Project of Beijing Institute for Brain Disorders (BIBD‐PXM2013_014226_07_000084), the Excellent Personnel Training Project of Beijing, China (20071D0300400076), the Project of Construction of Innovative Teams and Teacher Career Development for Universities and Colleges Under Beijing Municipality (IDHT20140514), the Beijing Healthcare Research Project, China (JING‐15‐2), the Basic‐Clinical Research Cooperation Funding of Capital Medical University, China (2015‐JL‐PT‐X04, 10JL49, 14JL15), the Natural Science Foundation of Capital Medical University, Beijing, China (PYZ2018077), and the Youth Research Funding, Beijing Tiantan Hospital, Capital Medical University, China (2015‐YQN‐14, 2015‐YQN‐15, 2015‐YQN‐17).
CONFLICT OF INTEREST STATEMENT
The authors report no competing interests.
CONSENT FOR PUBLICATION
Not applicable.
Supporting information
Data S1.
ACKNOWLEDGMENTS
We acknowledge all staffs and participants of this study for their contributions.
He M, Liu Z, Lian T, et al. Role of nerve growth factor on cognitive impairment in patients with Alzheimer's disease carrying apolipoprotein E ε4. CNS Neurosci Ther. 2024;30:e14560. doi: 10.1111/cns.14560
The first Mingyue He and Zhan Liu authors contributed equally to this work.
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the first author or the corresponding author upon reasonable request.
REFERENCES
- 1. Davies P, Maloney AJ. Selective loss of central cholinergic neurons in Alzheimer's disease. Lancet. 1976;2:1403. [DOI] [PubMed] [Google Scholar]
- 2. Serrano‐Pozo A, Das S, Hyman BT. APOE and Alzheimer's disease: advances in genetics, pathophysiology, and therapeutic approaches. Lancet Neurol. 2021;20:68‐80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Qian J, Betensky RA, Hyman BT, Serrano‐Pozo A. Association of APOE genotype with heterogeneity of cognitive decline rate in Alzheimer disease. Neurology. 2021;96:e2414‐e2428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Huang YA, Zhou B, Wernig M, Südhof TC. ApoE2, ApoE3, and ApoE4 differentially stimulate APP transcription and Aβ secretion. Cell. 2017;168:427‐441.e421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Shi Y et al. ApoE4 markedly exacerbates tau‐mediated neurodegeneration in a mouse model of tauopathy. Nature. 2017;549:523‐527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu Y, Nwabuisi‐Heath E, Dumanis SB, et al. APOE genotype alters glial activation and loss of synaptic markers in mice. Glia. 2012;60:559‐569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Allen SJ, Dawbarn D. Clinical relevance of the neurotrophins and their receptors. Clin Sci (Lond). 2006;110:175‐191. [DOI] [PubMed] [Google Scholar]
- 8. Peng S, Wuu J, Mufson EJ, Fahnestock M. Precursor form of brain‐derived neurotrophic factor and mature brain‐derived neurotrophic factor are decreased in the pre‐clinical stages of Alzheimer's disease. J Neurochem. 2005;93:1412‐1421. [DOI] [PubMed] [Google Scholar]
- 9. Sharif M, Noroozian M, Hashemian F. Do serum GDNF levels correlate with severity of Alzheimer's disease? Neurol Sci. 2021;42:2865‐2872. [DOI] [PubMed] [Google Scholar]
- 10. Bruno MA, Cuello AC. Activity‐dependent release of precursor nerve growth factor, conversion to mature nerve growth factor, and its degradation by a protease cascade. Proc Natl Acad Sci U S A. 2006;103:6735‐6740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Peng S, Wuu J, Mufson EJ, Fahnestock M. Increased proNGF levels in subjects with mild cognitive impairment and mild Alzheimer disease. J Neuropathol Exp Neurol. 2004;63:641‐649. [DOI] [PubMed] [Google Scholar]
- 12. Pentz R, Iulita MF, Ducatenzeiler A, et al. Nerve growth factor (NGF) pathway biomarkers in Down syndrome prior to and after the onset of clinical Alzheimer's disease: a paired CSF and plasma study. Alzheimers Dement. 2021b;17:605‐617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Choi J, Kwon HJ, Lee JE, Lee Y, Seoh JY, Han PL. Hyperoxygenation revitalizes Alzheimer's disease pathology through the upregulation of neurotrophic factors. Aging Cell. 2019;18:e12888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Nasrolahi A, Javaherforooshzadeh F, Jafarzadeh‐Gharehziaaddin M, Mahmoudi J, Asl KD, Shabani Z. Therapeutic potential of neurotrophic factors in Alzheimer's disease. Mol Biol Rep. 2022;49:2345‐2357. [DOI] [PubMed] [Google Scholar]
- 15. Albert MS, DeKosky ST, Dickson D, et al. The diagnosis of mild cognitive impairment due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:270‐279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. McKhann GM, Knopman DS, Chertkow H, et al. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging‐Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263‐269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cockrell JR, Folstein MF. Mini‐mental state examination (MMSE). Psychopharmacol Bull. 1988;24:689‐692. [PubMed] [Google Scholar]
- 18. Nasreddine ZS, Phillips NA, Bédirian V, et al. The Montreal cognitive assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695‐699. [DOI] [PubMed] [Google Scholar]
- 19. Guo Q, Zhao Q, Chen M, Ding D, Hong Z. A comparison study of mild cognitive impairment with 3 memory tests among Chinese individuals. Alzheimer Dis Assoc Disord. 2009;23:253‐259. [DOI] [PubMed] [Google Scholar]
- 20. Shin MS, Park SY, Park SR, Seol SH, Kwon JS. Clinical and empirical applications of the Rey‐Osterrieth complex figure test. Nat Protoc. 2006;1:892‐899. [DOI] [PubMed] [Google Scholar]
- 21. Mok EH, Lam LC, Chiu HF. Category verbal fluency test performance in chinese elderly with Alzheimer's disease. Dement Geriatr Cogn Disord. 2004;18:120‐124. [DOI] [PubMed] [Google Scholar]
- 22. Katsumata Y, Mathews M, Abner EL, et al. Assessing the discriminant ability, reliability, and comparability of multiple short forms of the Boston naming test in an Alzheimer's disease center cohort. Dement Geriatr Cogn Disord. 2015;39:215‐227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Fellows RP, Schmitter‐Edgecombe M. Symbol digit modalities test: regression‐based normative data and clinical utility. Arch Clin Neuropsychol. 2019;35:105‐115. [DOI] [PubMed] [Google Scholar]
- 24. Wei M, Shi J, Li T, et al. Diagnostic accuracy of the Chinese version of the trail‐making test for screening cognitive impairment. J Am Geriatr Soc. 2018;66:92‐99. [DOI] [PubMed] [Google Scholar]
- 25. Bondi MW, Serody AB, Chan AS, et al. Cognitive and neuropathologic correlates of stroop color‐word test performance in Alzheimer's disease. Neuropsychology. 2002;16:335‐343. [DOI] [PubMed] [Google Scholar]
- 26. Yu SY, Zhu WL, Guo P, et al. Clinical features and brain structural changes in magnetic resonance imaging in Alzheimer's disease patients with apathy. Aging. 2020;12:19083‐19094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lian TH, Zhu WL, Li SW, et al. Clinical, structural, and neuropathological features of olfactory dysfunction in patients with Alzheimer's disease. J Alzheimers Dis. 2019;70:413‐423. [DOI] [PubMed] [Google Scholar]
- 28. Agosta F, Vossel KA, Miller BL, et al. Apolipoprotein E epsilon4 is associated with disease‐specific effects on brain atrophy in Alzheimer's disease and frontotemporal dementia. Proc Natl Acad Sci U S A. 2009;106:2018‐2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Bracco L, Piccini C, Baccini M, et al. Pattern and progression of cognitive decline in Alzheimer's disease: role of premorbid intelligence and ApoE genotype. Dement Geriatr Cogn Disord. 2007;24:483‐491. [DOI] [PubMed] [Google Scholar]
- 30. Wolk DA, Dickerson BC. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional‐executive network function in Alzheimer's disease. Proc Natl Acad Sci U S A. 2010;107:10256‐10261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mahley RW, Weisgraber KH, Huang Y. Apolipoprotein E4: a causative factor and therapeutic target in neuropathology, including Alzheimer's disease. Proc Natl Acad Sci U S A. 2006;103:5644‐5651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Liu L, MacKenzie KR, Putluri N, Maletić‐Savatić M, Bellen HJ. The glia‐neuron lactate shuttle and elevated ROS promote lipid synthesis in neurons and lipid droplet accumulation in glia via APOE/D. Cell Metab. 2017;26:719‐737.e716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li D, Zhang J, Liu Q. Brain cell type‐specific cholesterol metabolism and implications for learning and memory. Trends Neurosci. 2022;45:401‐414. [DOI] [PubMed] [Google Scholar]
- 34. Allard S, Leon WC, Pakavathkumar P, Bruno MA, Ribeiro‐da‐Silva A, Cuello AC. Impact of the NGF maturation and degradation pathway on the cortical cholinergic system phenotype. J Neurosci. 2012;32:2002‐2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mitra S, Behbahani H, Eriksdotter M. Innovative therapy for Alzheimer's disease‐with focus on biodelivery of NGF. Front Neurosci. 2019;13:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Pentz R, Iulita MF, Ducatenzeiler A, Bennett DA, Cuello AC. The human brain NGF metabolic pathway is impaired in the pre‐clinical and clinical continuum of Alzheimers disease. Mol Psychiatry. 2021a;26:6023‐6037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Zhang YW, Chen Y, Liu Y, Zhao Y, Liao FF, Xu H. APP regulates NGF receptor trafficking and NGF‐mediated neuronal differentiation and survival. PLoS One. 2013;8:e80571. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1.
Data Availability Statement
The data that support the findings of this study are available from the first author or the corresponding author upon reasonable request.


