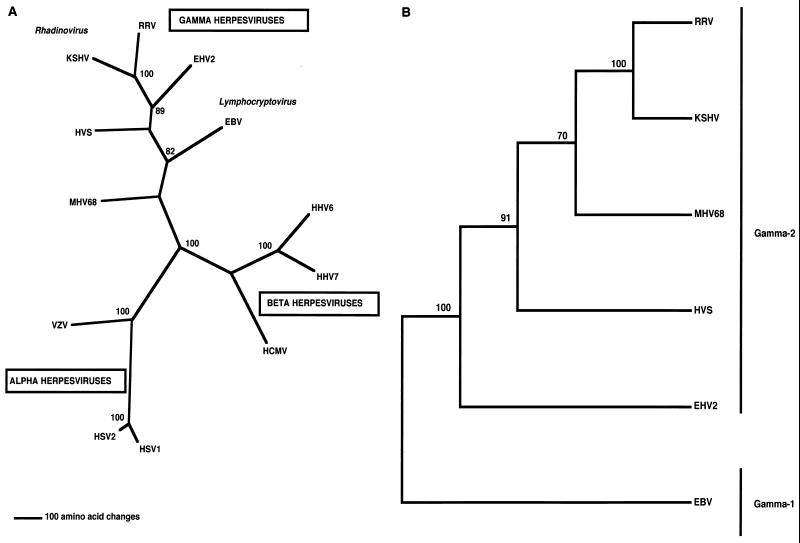
Members of the Herpesviridae family have large double-stranded DNA genomes and replicate in the nucleus of the host cell. Based on genomic organization and biological characteristics, herpesviruses are classified into three subfamilies: alpha, beta, and gamma (Fig. 1A). The gammaherpesviruses replicate and persist in lymphoid cells, but some are capable of undergoing lytic replication in epithelial or fibroblast cells. These viruses can be subdivided into two genera: lymphocryptoviruses (gamma-1) and rhadinoviruses (gamma-2) (Fig. 1A). The lymphocryptoviruses (gamma-1) include Epstein-Barr virus (EBV) or Human herpesvirus 4, Lymphocryptovirus of rhesus monkeys, and Herpesvirus papio of baboons, whereas the rhadinoviruses (gamma-2) include Herpesvirus saimiri (HVS), Kaposi's sarcoma-associated herpesvirus (KSHV) or Human herpesvirus 8, Rhesus monkey rhadinovirus (RRV), Equine herpesvirus 2, and Mouse herpesvirus 68 (Fig. 1B).
FIG. 1.
Classification of herpesviruses. (A) Phylogenetic tree depicting the three subfamilies of herpesviruses: alpha, beta, and gamma. The phylogram was constructed using the viral DNA polymerase gene by parsimony analysis with the neighbor-joining method. The number of amino acid changes can be determined using the scale shown at the bottom of the tree. (B) Cladogram of the gammaherpesvirus subfamilies. A cladogram of gamma-1 (lymphocryptoviruses) and gamma-2 (rhadinoviruses) herpesviruses was constructed by using the conserved glycoprotein B (gB) gene and a distance analysis. HHV6 and -7, human herpesvirus 6 and 7; MHV68, mouse herpesvirus 68.
The gammaherpesviruses, including HVS, EBV, KSHV, and RRV, are capable of establishing latent infection in lymphocytes. Both HVS and EBV have also been shown to transform lymphoid cells and to induce lymphoproliferative diseases in the natural or experimental host. EBV has been shown to be associated with several diseases in humans (16, 40, 78). These include infectious mononucleosis (IM), Burkitt's lymphoma (BL), nasopharyngeal carcinoma (NPC), Hodgkin's disease (HD), and T-cell lymphomas (1, 11, 65, 83, 84, 95, 96, 108, 116, 141). Primary EBV infection is usually asymptomatic, but a proportion of EBV-infected individuals develop IM, a disease characterized by lymphadenopathy and fatigue, later in life. A rare disease called fatal IM or X-linked lymphoproliferative (XLP) syndrome is an EBV-dependent malignancy characterized by uncontrolled immunoblastic lymphomas which are consistently EBV positive (115). The genetic defect in XLP is in the SLAM-associated protein, SAP. The mutated SAP protein in XLP patients affects T/B-cell interactions induced by SLAM, leading to an inability to control the B-cell proliferation caused by EBV infections (123, 133). BL is a malignancy that principally affects children, especially those that live in regions of Africa with a high incidence of malaria (16). The tumor cells of these lymphomas are closely associated with EBV, with 100% of the lymphomas scoring positive for EBV. In other areas of the world, however, the occurrence of BL is more sporadic and a lower percentage of these lymphomas are EBV positive (65). BL is characterized by distinct chromosomal translocations of the c-myc oncogene and immunoglobulin promoter sequences, resulting in the deregulation of c-myc expression (23). Another EBV-associated disease is NPC, a malignancy of the squamous epithelium situated in the nasopharynx (117). EBV is consistently present in cases of epithelial dysplasia, and it is thought to have arisen from clonal expansion of latently infected cells (113, 116). The incidence of NPC is high in Southern China, Northern Africa, and Eskimo populations. HD is the most common malignancy in the Western world, with about 30 to 90% of all HD lymphomas being EBV positive. Like NPC, the EBV genomes in these tumor cells are monoclonal (139). EBV is also present in about 70% of all immunoblastic lymphomas in human immunodeficiency virus (HIV)-infected individuals and in 100% of immunoblastic lymphomas of immune-suppressed posttransplant patients. Recently, EBV, a primarily B-cell-tropic virus, has been detected in different types of human T-cell lymphomas. About 100% of all nasal T-cell lymphomas in Southeast Asia and 100% of T-cell tumors in XLP males contain EBV (65).
Epidemiological studies from many laboratories, using antibody prevalence assays, PCR, and immunohistochemistry, suggest that KSHV is the etiologic agent responsible for Kaposi's sarcoma (KS). KSHV DNA sequences have been widely identified in KS tumors from HIV-positive and HIV-negative patients (20, 93, 132). KSHV has also consistently been found in specific lymphoproliferative diseases such as body cavity-based lymphomas (BCBLs), also called pleural effusion lymphomas, and lymphoblastic variants of multicentric Castleman's disease (MCD) (17, 46, 102, 112, 130). These are principally or exclusively of B-cell origin. There are reasons to believe that the abnormal cell proliferation in KS may differ from the traditional virus-transformed cell paradigms of other transforming viruses. The KS lesion has a mixed cell phenotype. One unusual cell consistently present, and thought to be critical, is a spindle-shaped cell believed to be of endothelial origin. While cells cultured from KS lesions do not contain KSHV, spindle cells in the KS lesion do contain KSHV genetic information. Several studies have suggested a high level of cytokines and chemokines within KS lesions and a dependence on these cytokines and chemokines for maintenance of the lesion (20, 106). BCBLs were first identified in patients with AIDS and were later found to have a high incidence of EBV and KSHV coinfection, although some lymphomas were only positive for KSHV (17, 69). BCBLs are thought to be monoclonal in origin and lack many B-lymphocyte antigens like CD19, CD20, and cell homing and adhesion markers (13, 34). MCD is an atypical lymphoproliferative disorder that includes hyperplasia, lymphadenopathy, and splenomegaly. Both HIV-infected and -uninfected individuals develop MCD, although there is a high rate of KSHV infection in the lymph nodes of HIV patients with MCD (18, 66, 130). It has been demonstrated that viral interleukin-6 (IL-6) acts as an autocrine or paracrine factor in the lymphoproliferative processes common to both BCBLs and MCD (111, 131). In addition, KSHV has been shown to immortalize primary human endothelial cells to long-term proliferation and survival (43). Interestingly, KSHV is found to be present in only a subset of cells, and paracrine mechanisms have been shown to be responsible for the survival of uninfected cells (43).
Recently, a herpesvirus called RRV was isolated that is related to but distinct from KSHV (30). Two homologues of KSHV from two different macaque species have also been identified in retroperitoneal fibromatosis (119). RRV replicates lytically and to high titers in cultured rhesus monkey fibroblasts. Complete DNA sequence analysis of this virus shows that it is much closer to KSHV than to HVS or other rhadinoviruses (4a, 127). A serological study showed that over 95% of rhesus monkeys are strongly positive for the presence of antibodies to this herpesvirus, suggesting that rhesus monkeys are a natural host for RRV infection (31). While specific diseases associated with RRV remain to be determined, some of the naïve seronegative rhesus macaques inoculated with RRV developed lymphadenopathy and vascular hyperplasia (85, 140) similar to that observed in KSHV-infected men (18, 66, 130). Furthermore, inoculated monkeys remained persistently infected with RRV (85).
HVS resides in the T lymphocytes of its natural host, the squirrel monkey, without causing any disease (29). However, HVS infection of other New World primates, e.g., common marmosets, tamarins, and owl monkeys, results in rapidly progressing lymphomas, lymphosarcomas, and leukemias (26, 27, 41). Subgroups A and C are highly oncogenic and are able to immortalize common marmoset T lymphocytes to IL-2-independent growth in vitro (27, 35, 62). In addition, HVS subgroup C is further capable of immortalizing human, rabbit, and rhesus monkey lymphocytes into continuously proliferating T-cell lines (2, 8, 35, 91). Because of the presence of a permissive cell culture system and in vitro and in vivo transformation assays, HVS provides a unique opportunity to investigate the mechanisms of cancer induction by oncogenic herpesviruses.
Gammaherpesviruses share an ability to transform lymphocytes in natural or experimental hosts. The correlation between gammaherpesviruses and disease induction in primates enables a study of the contributions of individual herpesvirus genes to cell growth transformation in a meaningful fashion. A striking feature of these four gammaherpesviruses is that they contain distinct open reading frames (ORFs) at the left and right ends of their respective genomes. Each of these ORFs is involved in lymphocyte signaling events. At the left end of each viral genome are located ORFs encoding distinct transforming proteins. These include the saimiri transformation protein (STP) of HVS, latent membrane protein 1 (LMP1) of EBV, K1 of KSHV, and R1 of RRV. These proteins do not share sequence relatedness, with the exception of limited structural homology between R1 and K1, which is reflective of the fact that RRV is the rhesus homologue of KSHV. In addition, the STP, LMP1, and K1 proteins show high sequence divergence among individual isolates from the same species (29, 31, 63, 71, 103, 105, 107, 143). This may be a consequence of their proximity to the terminal repeats of the viral genome, a region of high mutagenicity arising from the fact that these repetitive sequences undergo homologous recombination at an increased frequency. In contrast, in a limited survey, the R1 protein did not appear to be highly divergent (24, 127).
EBV LMP1
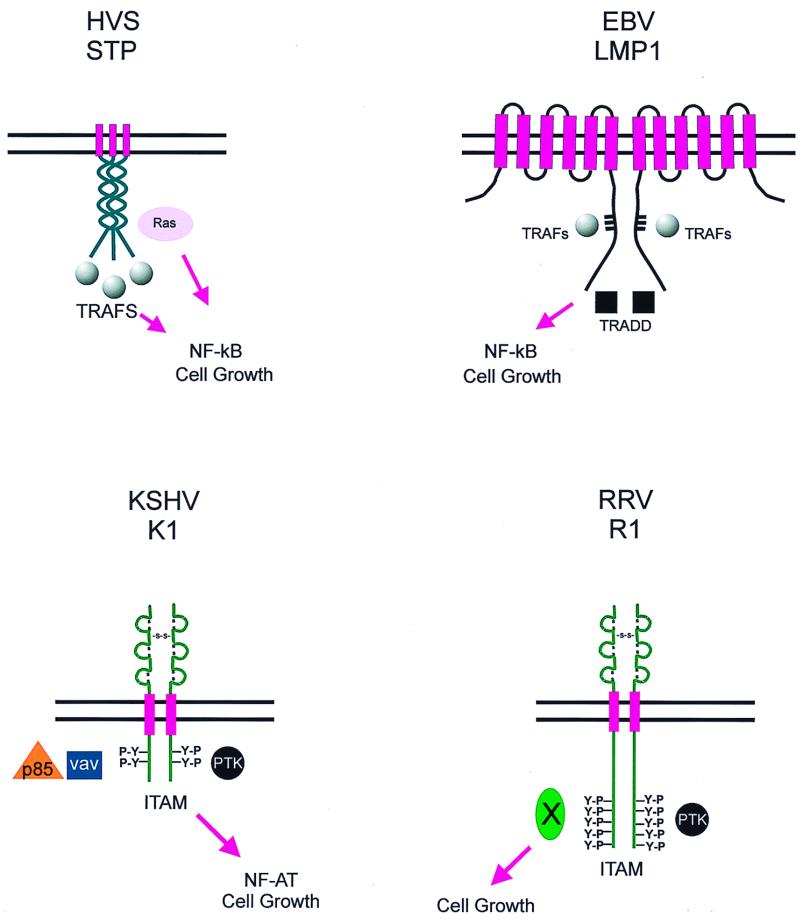
EBV was the first human gammaherpesvirus to be discovered and hence has been studied extensively. The first ORF of EBV encodes a well-characterized transforming protein, LMP1. The LMP1 protein has six transmembrane-spanning domains and a 199-amino-acid cytoplasmic domain. LMP1 has been shown to transform rodent fibroblasts and to be essential for the immortalization of primary B lymphocytes to lymphoblastoid cell lines (5, 137, 138). LMP1 has recently been shown to mimic the B-lymphocyte activation antigen CD40 (47, 53, 67). Like CD40 and other members of the tumor necrosis factor (TNF) receptors, the carboxy-terminal domain of LMP1 is capable of interacting with TNF receptor-associated factors (TRAFs) and with the TNF receptor-associated death domain (TRADD) (32, 33, 37, 38, 56, 122) (Fig. 2). The interaction of LMP1 with TRAFs and TRADD has been shown to be essential for the activation of the NF-κB pathway and for EBV-induced immortalization of B lymphocytes (56, 57, 64). However, unlike CD40, the transduction of signals occurs in the absence of extracellular ligands or cross-linking. This is caused by multimerization of the LMP1 protein through its transmembrane domains, a property that mimics ligand-induced CD40 receptor aggregation (Fig. 2). Multimerization generates a constitutively active signal that results in pleiotropic effects, including the activation of NF-κB and JNK activity, and induction of bcl-2, bclx, mcl1, and A20 gene expression (37, 39, 42, 53–57, 73). Hence, by mimicking the function of the B-lymphocyte CD40 receptor, LMP1 contributes to EBV-induced transformation of B lymphocytes.
FIG. 2.
Schematic representation of the LMP1, STP, K1, and R1 proteins. Interactions with cellular partners and activation of cellular pathways are indicated. Y-P represents the presence of phosphorylated tyrosine residues in K1 and R1.
HVS STP
Before the discovery of KSHV, HVS had been the most extensively studied gamma-2 herpesvirus (58). Based on the extent of sequence divergence at the left end of the HVS genome, the virus has been further classified into subgroups A, B, and C (86). Subgroup A and C strains immortalize common marmoset lymphocytes, but none of the subgroup B strains scored positive in in vitro immortalization assays. The first ORF of the HVS genome codes for highly related STPs in all three subgroups (27, 28, 70, 99). Deletion of the STP genes from subgroup A and C viruses results in viruses that are capable of replication but unable to induce lymphomas in common marmosets and unable to transform primary T lymphocytes in vitro (35, 99). STPs of HVS subgroups A and C have transforming and tumor-inducing activities independent of those of the rest of the herpesvirus genome. Specifically, the STP of HVS subgroup C, STP-C, can transform Rat-1 cells, resulting in apparent loss of contact inhibition, formation of foci, growth at reduced serum concentrations, and formation of invasive tumors in nude mice. The STP of HVS subgroup A, STP-A, is less potent than STP-C in its transforming ability. Furthermore, transgenic mice expressing STP-A developed peripheral pleomorphic T-cell lymphomas, while transgenic mice expressing STP-C developed extensive epithelial cell tumors (62, 99).
Both STP-A and STP-C proteins are predicted to have three distinct domains: an acidic amino terminus, collagen-like repeats in the central region, and a hydrophobic carboxy terminus (Fig. 2). The primary amino acid sequence of STP-A11 has nine copies of a collagen-like motif (Gly-X-Y, where X and/or Y is a proline residue) that are not contiguous. In STP-C488, it is directly repeated 18 times and comprises more than 50% of the protein. These collagen-like repeats are found to be a primary determinant for the transforming activity of the STP gene (J. K. Choi and J. U. Jung, unpublished data). STP-A and STP-C also contain a hydrophobic stretch at their carboxy termini sufficient for a membrane-spanning domain.
As a result of its essential role in HVS transformation, STP-C has been extensively studied. It has been shown to associate with cellular Ras (59), and this interaction is critical for its transforming activity in cell culture (Fig. 2). Furthermore, oncogenic v-ras can complement the HVS STP oncogene to induce lymphocyte transformation and does so more efficiently than normal c-ras (52). STP-C has also been shown to activate NF-κB transcriptional activity by interacting with TRAFs 1, 2, and 3 (75) (Fig. 2). While STP-A can also interact with these TRAFs, it is unable to upregulate NF-κB transcriptional activity (75). To add more complexity, STP-A does not interact with Ras but interacts with Src family kinases through its SH2 binding motif, YAEV/I (74). However, the potential role for the Src-STP-A interaction in transformation remains to be elucidated.
KSHV K1
At a position equivalent to that of the STP of HVS and the LMP1 of EBV, KSHV contains a distinct ORF called K1 (71, 77, 143). K1 is a 46-kDa transmembrane glycoprotein. Sequence analysis has recently demonstrated that the K1 gene is extremely variable, showing as much as 40% divergence at the amino acid level (143). While the amino-terminal extracellular domain of K1 is extremely variable, the carboxy-terminal short cytoplasmic tail is relatively well conserved (63, 77, 103, 143). This carboxy-terminal cytoplasmic tail contains a functional immunoreceptor tyrosine-based activation motif (ITAM) (72, 76) (Fig. 2). The ITAM is capable of transducing signals to induce cellular activation, calcium mobilization, and tyrosine phosphorylation, events that are indicative of lymphocyte activation (72, 76). However, unlike other ITAM-based signal transduction events which require a ligand-receptor interaction, K1 signaling appears to occur constitutively (72). The K1 protein has been shown to interact with several cellular signal transduction proteins that include Vav, p85 and Syk kinase (76) and to induce nuclear factor of activated T cells (NFAT) activity (72) (Fig. 2). In addition to the transformation of rodent fibroblasts, K1 can also functionally replace STP in HVS for the immortalization of common marmoset T lymphocytes to IL-2-independent growth and for the induction of lymphomas in common marmosets (77).
RRV R1
Like that of KSHV, the first ORF of the RRV genome also encodes a transforming gene, R1 (24). R1 shows limited sequence homology to K1 in its extracellular domain. The amino-terminal extracellular domains of both K1 and R1 closely resemble those of members of the immunoglobulin receptor superfamily (24, 77). R1 has also been shown to transform rodent fibroblasts and to functionally replace STP-C of HVS in immortalizing common marmoset peripheral blood mononuclear cells to IL-2-independent growth in vitro (24). Injection of R1-expressing rodent fibroblasts into nude mice resulted in the formation of multifocal and disseminated tumors in these mice (24). While the extracellular domains of R1 and K1 structurally resemble each other, the cytoplasmic tail of R1 is significantly longer than that of K1 and contains several potential SH2 binding motifs which function as ITAMs (Fig. 2) (24a). Further biochemical studies are needed to determine the detailed mechanisms of the alteration of cellular signaling pathways by R1 and K1 and their contribution to virus-induced cell growth transformation.
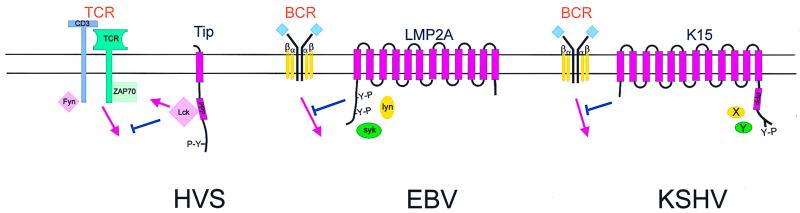
With the exception of the HVS tyrosine kinase-interacting protein (Tip) gene which is expressed as a bicistronic transcript with STP in HVS subgroup C, the genes for EBV LMP2a, KSHV K15, and RRV R15 are located at the right ends of the viral genomes. The LMP2a, K15, and Tip proteins are all capable of associating with the major B- or T-cell receptor-associated kinases and blocking their signaling activity. Cross-linking of the B-cell antigen receptor (BCR) and the T-cell antigen receptor (TCR) triggers a signal transduction cascade that leads to the activation of B and T lymphocytes, respectively. The EBV LMP2a, KSHV K15, and HVS Tip proteins can antagonize these signaling events, thus potentially preventing the reactivation of viral lytic infection from latently infected cells.
EBV LMP2A
LMP2a is expressed in B cells latently infected with EBV. LMP2a contains 12 transmembrane domains linked by loops and a short stretch of amino-terminal and carboxy-terminal domains (Fig. 3). LMP2a is expressed in aggregates in the plasma membranes of latently infected B cells. The amino-terminal cytoplasmic region of LMP2a has been shown to contain three tyrosine-based SH2 domain binding sites, two of which form a functional ITAM (45). This motif is tyrosine phosphorylated and is required for LMP2a association with the SH2 domain of the Lyn, Fyn, Syk, and Csk kinases (Fig. 3) (15, 79, 124). This interaction has been shown to be necessary for intracellular calcium mobilization and cytokine production by LMP2a (7). It has also been suggested that LMP2a is phosphorylated at serine residues by MAP kinases (109). While LMP2a is dispensable for EBV immortalization of B lymphocytes (80–82), its expression blocks normal BCR signaling in EBV-negative B cells (90). In addition, studies using EBV-positive B lymphocytes have shown that this signaling block prevents the reactivation of lytic replication, indicating that EBV LMP2a may play a significant role in the establishment and maintenance of viral latency in vivo (88, 89).
FIG. 3.
Schematic representation of the LMP2a, Tip, and K15 proteins. Interactions with cellular partners and activation of cellular pathways are indicated. Y-P represents the presence of phosphorylated tyrosine residues in these proteins. Blue-colored T-bar, blocking of signal transduction.
HVS TIP
The HVS Tip gene is only present in HVS subgroup C virus, not in HVS subgroups A and B. HVS Tip has been shown to associate with a major T-cell tyrosine kinase, Lck, and this interaction inhibits the TCR-mediated signal transduction pathway (Fig. 3). Two motifs of Tip are responsible for interacting with Lck. These include the carboxy-termini of Src-family kinases (CSKH) motif and the SH3 binding motif (60, 61). Cell lines stably expressing Tip show a reduced level of TCR signal transduction (61). This negative effect on Lck-mediated TCR signal transduction has been shown to be enhanced by a point mutation in Tip which enhances Lck-binding affinity (51). In contrast, a mutation in the Lck-binding motif of Tip that abolishes Lck binding augments the transforming activity of HVS C488 in vitro and in vivo (36). This suggests that an interaction of Tip with Lck modulates the transforming ability of HVS. In addition, Tip has been shown to interact with the nuclear RNA export factor, Tap (Tip-associated factor), independently of Lck binding (49, 142). Expression of Tip and Tap in T cells upregulates surface expression of cellular adhesion molecules, leading to lymphocyte aggregation (142). However, the relevance of the Tip-Tap association for viral transformation remains to be elucidated.
KSHV K15
KSHV encodes a distinct ORF called K15 or latency-associated membrane protein (LAMP) which is located in the same genomic position as the EBV LMP2a (22, 48, 114). While K15 isolates exhibit a complex splicing pattern, they all consist of 4 to 12 transmembrane spanning domains and a short stretch of cytoplasmic domain (Fig. 3) (22, 48, 114). K15 is weakly expressed in latently infected BCBLs, and the level of its expression was significantly increased by tetradecanoyl phorbol acetate stimulation (22, 48). K15 proteins from different KSHV isolates exhibit dramatic sequence variation, showing as much as 60 to 70% divergence at the amino acid level (114). Like EBV LMP2a, the cytoplasmic domain of K15 contains signaling motifs that are highly conserved in most isolates (114). These include potential SH2 and SH3 binding motifs and a YASIL sequence (20, 48, 114). The cytoplasmic domain of K15 is constitutively tyrosine phosphorylated, and the tyrosine residue within the putative SH2 binding motif is a major site of phosphorylation by cellular tyrosine kinases (22). In addition, experiments with CD8-K15 chimeras demonstrate that unlike that of EBV LMP2a, the cytoplasmic domain of K15 is unable to elicit cellular signal transduction upon antibody stimulation. However, like EBV LMP2a, it is capable of inhibiting BCR signal transduction (22). Thus KSHV K15 is likely to be a distant evolutionary relative of EBV LMP2a.
RRV contains a gene named R15 at a genomic location equivalent to that of KSHV K15. R15 also contains multiple transmembrane domains and a cytoplasmic tail containing signal-transducing motifs (unpublished data). The functional role of R15 remains to be deciphered.
FUNCTIONAL COMMONALTIES
Except for the limited homology of the structural motifs seen between KSHV K1 and RRV R1, there is no discernible homology between the proteins encoded by the first ORFs at the left ends of the gammaherpesvirus genomes. The most interesting property they share is their ability to self-oligomerize (Fig. 2). EBV LMP1 has been shown to aggregate through its membrane-spanning domains, mimicking a ligand-induced activated CD40 receptor (47, 67). KSHV K1 and RRV R1 have also been shown to oligomerize through disulfide bonding of their extracellular domains (24, 71, 76, 77). The STP-C protein is capable of oligomerizing through its collagen repeats, and the integrity of this domain has been demonstrated to be essential for the transforming function of the protein (59; Choi et al., unpublished data). However, as an alternative, oligomerization of these proteins may be caused by endogenous ligands. In addition, both LMP-1 and STP-C488 can activate the NF-κB pathway through the binding of TRAFs (32, 33, 37, 38, 57, 75, 122), while the K1 and R1 proteins interact with Syk, a B-cell specific kinase, to induce cellular tyrosine phosphorylation and B-cell activation events (24a, 72, 76). Thus, these proteins share the ability to interact with host factors and to activate cellular signaling pathways. Such similarities exist in the lack of any discernible sequence homology as to imply that these first ORFs are not ancestral herpesvirus genes but have been recently acquired by the individual viral genomes. In either event, through self- or ligand-induced oligomerization and interaction with host cellular factors, these viral transforming proteins appear to have adopted and modified cellular pathways as a means of transforming T and B lymphocytes (Fig. 2).
EBV LMP2A, KSHV K15, and HVS Tip also share a common function in the absence of any sequence homology. All three proteins contain SH2-binding and/or SH3-binding motifs that are capable of interacting with either the Src or Syk family kinases (Fig. 3). These motifs appear to be involved in the down-regulation of lymphocyte receptor signaling. The impairment of cellular signal transduction pathways by these viral proteins may help to reduce or delay the onset of aberrant lytic replication as a result of cellular proliferation triggered by external signals.
OTHER GROWTH-DEREGULATING GENES OF GAMMAHERPESVIRUSES
Herpesviruses have large genomes containing a wide array of genes. Although the first ORFs in these gammaherpesviruses have oncogenic potential, other viral genes may also play a role in viral transformation. These viral genes can be classified into two groups, those that are homologous to cellular genes and those that are unique to the virus. EBV encodes several unique viral genes, e.g., EBNA-1, EBNA-2, and EBNA-3, which all appear to play a role in viral oncogenesis (15, 80, 81, 87, 89, 110, 118, 134, 136). Another such gene is the KSHV K12 (Kaposin) gene, which has been shown to have transforming ability (97), although its contribution to viral pathogenesis is not yet clear. Furthermore, the K12 gene has been shown to undergo a complex translation program (121).
The second set of potential growth-deregulating genes encoded by these viruses are those that resemble cellular genes. EBV, HVS, KSHV, and RRV all harbor a viral Bcl-2 gene that has anti-apoptotic activity in cell culture (21, 25, 98, 127, 135; Alexander et al., submitted). While EBV induces expression of IL-6, cyclin D, complement-control protein (CCP), and IL-8 receptor expression (10, 14, 68, 128, 129, 135), the three rhadinoviruses appear to have come prepared with their own viral homologues of cellular genes: v-cyclin, v-CCP, and v-IL-8R (3, 4, 4a, 19, 100, 101, 120). In addition, the rhadinoviruses contain genes encoding a latency-associated nuclear antigen (LANA) and a FLICE-inhibitor protein (v-FLIP) (3, 4, 120, 127). Furthermore, KSHV and RRV encode genes for viral IL-6, interferon regulatory factors (v-IRFs), and viral chemokines (v-MIP-I, -II, and -III) (12, 50, 92, 102, 104, 125–127). Thus, the proteins encoded by genes near the ends of the gammaherpesvirus genomes may act in concert with a number of other virus-encoded protein products to achieve cell growth transformation.
CONCLUSION
Infected hosts induce numerous antiviral responses that include apoptosis, immune activation, and cell growth arrest. The Gammaherpesvirinae have evolved means of altering these signal transduction pathways by deregulating expression of a subset of cellular signaling genes or encoding their own viral counterparts to these genes. Thus, acting in concert with other viral genes, STP and Tip of HVS, LMP1 and LMP2a of EBV, K1 and K15 of KSHV, and R1 and R15 of RRV are capable of modulating cellular signals such that cell proliferation and viral replication occur at the appropriate times in the viral life cycle.
ACKNOWLEDGMENTS
We thank R. Desrosiers for critical reading of the manuscript, Marcy Auerbach for assistance with the phylogenetic analysis, and K. Toohey for photography support.
This work was supported by NIH grants CA31363, CA82057, CA86841, and RR00168. B. Damania is a fellow of the Cancer Research Institute.
REFERENCES
- 1.Ablashi D V, Levine P H, Papas T, Pearson G R, Kottaridis S D. First international symposium on Epstein-Barr virus and associated malignant diseases. Cancer Res. 1985;45:3981–3984. [PubMed] [Google Scholar]
- 2.Ablashi D V, Schirm S, Fleckenstein B, Faggioni A, Dahlberg J, Rabin H, Loeb W, Armstrong G, Peng J W, Aulahk G, et al. Herpesvirus saimiri-induced lymphoblastoid rabbit cell line: growth characteristics, virus persistence, and oncogenic properties. J Virol. 1985;55:623–633. doi: 10.1128/jvi.55.3.623-633.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Albrecht J C, Fleckenstein B. New member of the multigene family of complement control proteins in herpesvirus saimiri. J Virol. 1992;66:3937–3940. doi: 10.1128/jvi.66.6.3937-3940.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Albrecht J C, Nicholas J, Cameron K R, Newman C, Fleckenstein B, Honess R W. Herpesvirus saimiri has a gene specifying a homologue of the cellular membrane glycoprotein CD59. Virology. 1992;190:527–530. doi: 10.1016/0042-6822(92)91247-r. [DOI] [PubMed] [Google Scholar]
- 4a.Alexander, L., L. Denekamp, A. Knapp, M. Auerbach, S. Czajak, B. Damania, and R. C. Desrosiers. The primary sequence of rhesus rhadinovirus isolate 26-95: sequence similarities to Kaposi's sarcoma-associated herpesvirus and rhesus monkey rhadinovirus isolate 17577. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 5.Baichwal V R, Sugden B. Transformation of Balb 3T3 cells by the BNLF-1 gene of Epstein-Barr virus. Oncogene. 1988;2:461–467. [PubMed] [Google Scholar]
- 6.Bais C, Santomasso B, Coso O, Arvanitakis L, Raaka E G, Gutkind J S, Asch A S, Cesarman E, Gershengorn M C, Mesri E A, Gerhengorn M C. G-protein-coupled receptor of Kaposi's sarcoma-associated herpesvirus is a viral oncogene and angiogenesis activator. Nature. 1998;391:86–89. doi: 10.1038/34193. [DOI] [PubMed] [Google Scholar]
- 7.Beaufils P, Choquet D, Mamoun R Z, Malissen B. The (YXXL/I)2 signalling motif found in the cytoplasmic segments of the bovine leukaemia virus envelope protein and Epstein-Barr virus latent membrane protein 2A can elicit early and late lymphocyte activation events. EMBO J. 1993;12:5105–5112. doi: 10.1002/j.1460-2075.1993.tb06205.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biesinger B, Muller-Fleckenstein I, Simmer B, Lang G, Wittmann S, Platzer E, Desrosiers R C, Fleckenstein B. Stable growth transformation of human T lymphocytes by herpesvirus saimiri. Proc Natl Acad Sci USA. 1992;89:3116–3119. doi: 10.1073/pnas.89.7.3116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biesinger B, Tsygankov A Y, Fickenscher H, Emmrich F, Fleckenstein B, Bolen J B, Broker B M. The product of the herpesvirus saimiri open reading frame 1 (tip) interacts with T cell-specific kinase p56lck in transformed cells. J Biol Chem. 1995;270:4729–4734. doi: 10.1074/jbc.270.9.4729. [DOI] [PubMed] [Google Scholar]
- 10.Birkenbach M, Josefsen K, Yalamanchili R, Lenoir G, Kieff E. Epstein-Barr virus-induced genes: first lymphocyte-specific G protein-coupled peptide receptors. J Virol. 1993;67:2209–2220. doi: 10.1128/jvi.67.4.2209-2220.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Blacklow N R, Watson B K, Miller G, Jacobson B M. Mononucleosis with heterophil antibodies and EB virus infection. Acquisition by an elderly patient in hospital. Am J Med. 1971;51:549–552. doi: 10.1016/0002-9343(71)90260-9. [DOI] [PubMed] [Google Scholar]
- 12.Boshoff C, Endo Y, Collins P D, Takeuchi Y, Reeves J D, Schweickart V L, Siani M A, Sasaki T, Williams T J, Gray P W, Moore P S, Chang Y, Weiss R A. Angiogenic and HIV-inhibitory functions of KSHV-encoded chemokines. Science. 1997;278:290–294. doi: 10.1126/science.278.5336.290. [DOI] [PubMed] [Google Scholar]
- 13.Boshoff C, Weiss R A. Kaposi's sarcoma-associated herpesvirus. Adv Cancer Res. 1998;75:57–86. doi: 10.1016/s0065-230x(08)60739-3. [DOI] [PubMed] [Google Scholar]
- 14.Burgstahler R, Kempkes B, Steube K, Lipp M. Expression of the chemokine receptor BLR2/EBI1 is specifically transactivated by Epstein-Barr virus nuclear antigen 2. Biochem Biophys Res Commun. 1995;215:737–743. doi: 10.1006/bbrc.1995.2525. [DOI] [PubMed] [Google Scholar]
- 15.Burkhardt A L, Bolen J B, Kieff E, Longnecker R. An Epstein-Barr virus transformation-associated membrane protein interacts with Src family tyrosine kinases. J Virol. 1992;66:5161–5167. doi: 10.1128/jvi.66.8.5161-5167.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Burkitt D. Burkitt's lymphoma outside the known endemic areas of Africa and New Guinea. Int J Cancer. 1967;2:562–565. doi: 10.1002/ijc.2910020603. [DOI] [PubMed] [Google Scholar]
- 17.Cesarman E, Chang Y, Moore P S, Said J W, Knowles D M. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in AIDS-related body-cavity-based lymphomas. N Engl J Med. 1995;332:1186–1191. doi: 10.1056/NEJM199505043321802. [DOI] [PubMed] [Google Scholar]
- 18.Cesarman E, Knowles D M. Kaposi's sarcoma-associated herpesvirus: a lymphotropic human herpesvirus associated with Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. Semin Diagn Pathol. 1997;14:54–66. [PubMed] [Google Scholar]
- 19.Cesarman E, Nador R G, Bai F, Bohenzky R A, Russo J J, Moore P S, Chang Y, Knowles D M. Kaposi's sarcoma-associated herpesvirus contains G protein-coupled receptor and cyclin D homologs which are expressed in Kaposi's sarcoma and malignant lymphoma. J Virol. 1996;70:8218–8223. doi: 10.1128/jvi.70.11.8218-8223.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chang Y, Cesarman E, Pessin M S, Lee F, Culpepper J, Knowles D M, Moore P S. Identification of herpesvirus-like DNA sequences in AIDS-associated Kaposi's sarcoma. Science. 1994;266:1865–1869. doi: 10.1126/science.7997879. [DOI] [PubMed] [Google Scholar]
- 21.Cheng E H, Nicholas J, Bellows D S, Hayward G S, Guo H G, Reitz M S, Hardwick J M. A Bcl-2 homolog encoded by Kaposi sarcoma-associated virus, human herpesvirus 8, inhibits apoptosis but does not heterodimerize with Bax or Bak. Proc Natl Acad Sci USA. 1997;94:690–694. doi: 10.1073/pnas.94.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Choi J-K, Lee B-S, Shim S N, Li M, Jung J U. Identification of the novel K15 gene at the rightmost end of the Kaposi's sarcoma-associated herpesvirus genome. J Virol. 1999;74:436–446. [PMC free article] [PubMed] [Google Scholar]
- 23.Dalla-Favera R, Lombardi L, Pelicci P G, Lanfrancone L, Cesarman E, Neri A. Mechanism of activation and biological role of the c-myc oncogene in B-cell lymphomagenesis. Ann N Y Acad Sci. 1987;511:207–218. doi: 10.1111/j.1749-6632.1987.tb36249.x. [DOI] [PubMed] [Google Scholar]
- 24.Damania B, Li M, Choi J K, Alexander L, Jung J U, Desrosiers R C. Identification of the R1 oncogene and its protein product from the rhadinovirus of rhesus monkeys. J Virol. 1999;73:5123–5131. doi: 10.1128/jvi.73.6.5123-5131.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24a.Damania, B., M. DeMaria, J. U. Jung, and R. C. Desrosiers. Activation of lymphocyte signaling by the R1 protein of rhesus monkey rhadinovirus. J. Virol., in press. [DOI] [PMC free article] [PubMed]
- 25.Dawson C W, Eliopoulos A G, Dawson J, Young L S. BHRF1, a viral homologue of the Bcl-2 oncogene, disturbs epithelial cell differentiation. Oncogene. 1995;10:69–77. [PubMed] [Google Scholar]
- 26.Desrosiers R C. Herpesvirus saimiri DNA in tumor cells—deleted sequences and sequence rearrangements. J Virol. 1981;39:497–509. doi: 10.1128/jvi.39.2.497-509.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Desrosiers R C, Bakker A, Kamine J, Falk L A, Hunt R D, King N W. A region of the herpesvirus saimiri genome required for oncogenicity. Science. 1985;228:184–187. doi: 10.1126/science.2983431. [DOI] [PubMed] [Google Scholar]
- 28.Desrosiers R C, Burghoff R L, Bakker A, Kamine J. Construction of replication-competent herpesvirus saimiri deletion mutants. J Virol. 1984;49:343–348. doi: 10.1128/jvi.49.2.343-348.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Desrosiers R C, Falk L A. Herpesvirus saimiri strain variability. J Virol. 1982;43:352–6. doi: 10.1128/jvi.43.1.352-356.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desrosiers R C, Sasseville V G, Czajak S C, Zhang X, Mansfield K G, Kaur A, Johnson R P, Lackner A A, Jung J U. A herpesvirus of rhesus monkeys related to the human Kaposi's sarcoma-associated herpesvirus. J Virol. 1997;71:9764–9769. doi: 10.1128/jvi.71.12.9764-9769.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Desrosiers R C, Silva D P, Waldron L M, Letvin N L. Nononcogenic deletion mutants of herpesvirus saimiri are defective for in vitro immortalization. J Virol. 1986;57:701–705. doi: 10.1128/jvi.57.2.701-705.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Devergne O, Hatzivassiliou E, Izumi K M, Kaye K M, Kleijnen M F, Kieff E, Mosialos G. Association of TRAF1, TRAF2, and TRAF3 with an Epstein-Barr virus LMP1 domain important for B-lymphocyte transformation: role in NF-κB activation. Mol Cell Biol. 1996;16:7098–7108. doi: 10.1128/mcb.16.12.7098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Devergne O, McFarland E C, Mosialos G, Izumi K M, Ware C F, Kieff E. Role of the TRAF binding site and NF-κB activation in Epstein-Barr virus latent membrane protein 1-induced cell gene expression. J Virol. 1998;72:7900–7908. doi: 10.1128/jvi.72.10.7900-7908.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Drexler H G, Uphoff C C, Gaidano G, Carbone A. Lymphoma cell lines: in vitro models for the study of HHV-8+ primary effusion lymphomas (body cavity-based lymphomas) Leukemia. 1998;12:1507–1517. doi: 10.1038/sj.leu.2401160. [DOI] [PubMed] [Google Scholar]
- 35.Duboise S M, Guo J, Czajak S, Desrosiers R C, Jung J U. STP and Tip are essential for herpesvirus saimiri oncogenicity. J Virol. 1998;72:1308–1313. doi: 10.1128/jvi.72.2.1308-1313.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Duboise S M, Lee H, Guo J, Choi J K, Czajak S, Simon M, Desrosiers R C, Jung J U. Mutation of the Lck-binding motif of Tip enhances lymphoid cell activation by herpesvirus saimiri. J Virol. 1998;72:2607–2614. doi: 10.1128/jvi.72.4.2607-2614.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Eliopoulos A G, Blake S M, Floettmann J E, Rowe M, Young L S. Epstein-Barr virus-encoded latent membrane protein 1 activates the JNK pathway through its extreme C terminus via a mechanism involving TRADD and TRAF2. J Virol. 1999;73:1023–1035. doi: 10.1128/jvi.73.2.1023-1035.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eliopoulos A G, Dawson C W, Mosialos G, Floettmann J E, Rowe M, Armitage R J, Dawson J, Zapata J M, Kerr D J, Wakelam M J, Reed J C, Kieff E, Young L S. CD40-induced growth inhibition in epithelial cells is mimicked by Epstein-Barr virus-encoded LMP1: involvement of TRAF3 as a common mediator. Oncogene. 1996;13:2243–2254. [PubMed] [Google Scholar]
- 39.Eliopoulos A G, Young L S. Activation of the cJun N-terminal kinase (JNK) pathway by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) Oncogene. 1998;16:1731–1742. doi: 10.1038/sj.onc.1201694. [DOI] [PubMed] [Google Scholar]
- 40.Epstein M A, Achong B, Barr Y. Virus particles in culture lymphoblasts from Burkitt's lymphoma. Lancet. 1964;i:702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 41.Fleckenstein B. Oncogenic herpesviruses of non-human primates. Biochim Biophys Acta. 1979;560:301–342. doi: 10.1016/0304-419x(79)90007-6. [DOI] [PubMed] [Google Scholar]
- 42.Floettmann J E, Ward K, Rickinson A B, Rowe M. Cytostatic effect of Epstein-Barr virus latent membrane protein-1 analyzed using tetracycline-regulated expression in B cell lines. Virology. 1996;223:29–40. doi: 10.1006/viro.1996.0452. [DOI] [PubMed] [Google Scholar]
- 43.Flore O, Rafii S, Ely S, O'Leary J J, Hyjek E M, Cesarman E. Transformation of primary human endothelial cells by Kaposi's sarcoma-associated herpesvirus. Nature. 1998;394:588–592. doi: 10.1038/29093. [DOI] [PubMed] [Google Scholar]
- 44.Franken M, Devergne O, Rosenzweig M, Annis B, Kieff E, Wang F. Comparative analysis identifies conserved tumor necrosis factor receptor-associated factor 3 binding sites in the human and simian Epstein-Barr virus oncogene LMP1. J Virol. 1996;70:7819–7826. doi: 10.1128/jvi.70.11.7819-7826.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fruehling S, Longnecker R. The immunoreceptor tyrosine-based activation motif of Epstein-Barr virus LMP2A is essential for blocking BCR-mediated signal transduction. Virology. 1997;235:241–251. doi: 10.1006/viro.1997.8690. [DOI] [PubMed] [Google Scholar]
- 46.Gessain A, Sudaka A, Briere J, Fouchard N, Nicola M A, Rio B, Arborio M, Troussard X, Audouin J, Diebold J, et al. Kaposi sarcoma-associated herpes-like virus (human herpesvirus type 8) DNA sequences in multicentric Castleman's disease: is there any relevant association in non-human immunodeficiency virus-infected patients? Blood. 1996;87:414–416. [PubMed] [Google Scholar]
- 47.Gires O, Zimber-Strobl U, Gonnella R, Ueffing M, Marschall G, Zeidler R, Pich D, Hammerschmidt W. Latent membrane protein 1 of Epstein-Barr virus mimics a constitutively active receptor molecule. EMBO J. 1997;16:6131–6140. doi: 10.1093/emboj/16.20.6131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Glenn M, Rainbow L, Aurad F, Davison A, Schulz T F. Identification of a spliced gene from Kaposi's sarcoma-associated herpesvirus encoding a protein with similarities to latent membrane proteins 1 and 2A of Epstein-Barr virus. J Virol. 1999;73:6953–6963. doi: 10.1128/jvi.73.8.6953-6963.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gruter P, Tabernero C, von Kobbe C, Schmitt C, Saavedra C, Bachi A, Wilm M, Felber B K, Izaurralde E. TAP, the human homolog of Mex67p, mediates CTE-dependent RNA export from the nucleus. Mol Cell. 1998;1:649–659. doi: 10.1016/s1097-2765(00)80065-9. [DOI] [PubMed] [Google Scholar]
- 50.Guo H G, Browning P, Nicholas J, Hayward G S, Tschachler E, Jiang Y W, Sadowska M, Raffeld M, Colombini S, Gallo R C, Reitz M S., Jr Characterization of a chemokine receptor-related gene in human herpesvirus 8 and its expression in Kaposi's sarcoma. Virology. 1997;228:371–378. doi: 10.1006/viro.1996.8386. [DOI] [PubMed] [Google Scholar]
- 51.Guo J, Duboise M, Lee H, Li M, Choi J K, Rosenzweig M, Jung J U. Enhanced downregulation of Lck-mediated signal transduction by a Y114 mutation of herpesvirus saimiri tip. J Virol. 1997;71:7092–7096. doi: 10.1128/jvi.71.9.7092-7096.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guo J, Williams K, Duboise S M, Alexander L, Veazey R, Jung J U. Substitution of ras for the herpesvirus saimiri STP oncogene in lymphocyte transformation. J Virol. 1998;72:3698–3704. doi: 10.1128/jvi.72.5.3698-3704.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hatzivassiliou E, Miller W E, Raab-Traub N, Kieff E, Mosialos G. A fusion of the EBV latent membrane protein-1 (LMP1) transmembrane domains to the CD40 cytoplasmic domain is similar to LMP1 in constitutive activation of epidermal growth factor receptor expression, nuclear factor-kappa B, and stress-activated protein kinase. J Immunol. 1998;160:1116–1121. [PubMed] [Google Scholar]
- 54.Hsu H, Xiong J, Goeddel D V. The TNF receptor 1-associated protein TRADD signals cell death and NF-kappa B activation. Cell. 1995;81:495–504. doi: 10.1016/0092-8674(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 55.Huen D S, Henderson S A, Croom-Carter D, Rowe M. The Epstein-Barr virus latent membrane protein-1 (LMP1) mediates activation of NF-kappa B and cell surface phenotype via two effector regions in its carboxy-terminal cytoplasmic domain. Oncogene. 1995;10:549–560. [PubMed] [Google Scholar]
- 56.Izumi K M, Kaye K M, Kieff E D. The Epstein-Barr virus LMP1 amino acid sequence that engages tumor necrosis factor receptor associated factors is critical for primary B lymphocyte growth transformation. Proc Natl Acad Sci USA. 1997;94:1447–1452. doi: 10.1073/pnas.94.4.1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Izumi K M, Kieff E D. The Epstein-Barr virus oncogene product latent membrane protein 1 engages the tumor necrosis factor receptor-associated death domain protein to mediate B lymphocyte growth transformation and activate NF-kappaB. Proc Natl Acad Sci USA. 1997;94:12592–12597. doi: 10.1073/pnas.94.23.12592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jung J U, Choi J K, Ensser A, Biesinger B. Herpesvirus saimiri as a model for gammaherpesvirus oncogenesis. Semin Cancer Biol. 1999;9:231–239. doi: 10.1006/scbi.1998.0115. [DOI] [PubMed] [Google Scholar]
- 59.Jung J U, Desrosiers R C. Association of the viral oncoprotein STP-C488 with cellular ras. Mol Cell Biol. 1995;15:6506–6512. doi: 10.1128/mcb.15.12.6506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jung J U, Lang S M, Friedrich U, Jun T, Roberts T M, Desrosiers R C, Biesinger B. Identification of Lck-binding elements in tip of herpesvirus saimiri. J Biol Chem. 1995;270:20660–20667. doi: 10.1074/jbc.270.35.20660. [DOI] [PubMed] [Google Scholar]
- 61.Jung J U, Lang S M, Jun T, Roberts T M, Veillette A, Desrosiers R C. Downregulation of Lck-mediated signal transduction by tip of herpesvirus saimiri. J Virol. 1995;69:7814–7822. doi: 10.1128/jvi.69.12.7814-7822.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jung J U, Trimble J J, King N W, Biesinger B, Fleckenstein B W, Desrosiers R C. Identification of transforming genes of subgroup A and C strains of herpesvirus saimiri. Proc Natl Acad Sci USA. 1991;88:7051–7055. doi: 10.1073/pnas.88.16.7051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kasolo F C, Monze M, Obel N, Anderson R A, French C, Gompels U A. Sequence analyses of human herpesvirus-8 strains from both African human immunodeficiency virus-negative and -positive childhood endemic Kaposi's sarcoma show a close relationship with strains identified in febrile children and high variation in the K1 glycoprotein. J Gen Virol. 1998;79:3055–3065. doi: 10.1099/0022-1317-79-12-3055. [DOI] [PubMed] [Google Scholar]
- 64.Kaye K M, Devergne O, Harada J N, Izumi K M, Yalamanchili R, Kieff E, Mosialos G. Tumor necrosis factor receptor associated factor 2 is a mediator of NF-kappa B activation by latent infection membrane protein 1, the Epstein-Barr virus transforming protein. Proc Natl Acad Sci USA. 1996;93:11085–11090. doi: 10.1073/pnas.93.20.11085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kieff E. Epstein Barr virus and its replication. In: Fields B N, Knipe D M, Howley P M, et al., editors. Fundamental virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 1109–1163. [Google Scholar]
- 66.Kikuta H, Itakura O, Taneichi K, Kohno M. Tropism of human herpesvirus 8 for peripheral blood lymphocytes in patients with Castleman's disease. Br J Haematol. 1997;99:790–793. doi: 10.1046/j.1365-2141.1997.4653269.x. [DOI] [PubMed] [Google Scholar]
- 67.Kilger E, Kieser A, Baumann M, Hammerschmidt W. Epstein-Barr virus-mediated B-cell proliferation is dependent upon latent membrane protein 1, which simulates an activated CD40 receptor. EMBO J. 1998;17:1700–1709. doi: 10.1093/emboj/17.6.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Klein S C, Kube D, Abts H, Diehl V, Tesch H. Promotion of IL8, IL10, TNF alpha and TNF beta production by EBV infection. Leuk Res. 1996;20:633–636. doi: 10.1016/0145-2126(96)00029-x. [DOI] [PubMed] [Google Scholar]
- 69.Knowles D M, Inghirami G, Ubriaco A, Dalla-Favera R. Molecular genetic analysis of three AIDS-associated neoplasms of uncertain lineage demonstrates their B-cell derivation and the possible pathogenetic role of the Epstein-Barr virus. Blood. 1989;73:792–799. [PubMed] [Google Scholar]
- 70.Koomey J M, Mulder C, Burghoff R L, Fleckenstein B, Desrosiers R C. Deletion of DNA sequence in a nononcogenic variant of herpesvirus saimiri. J Virol. 1984;50:662–665. doi: 10.1128/jvi.50.2.662-665.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lagunoff M, Ganem D. The structure and coding organization of the genomic termini of Kaposi's sarcoma-associated herpesvirus. Virology. 1997;236:147–154. doi: 10.1006/viro.1997.8713. [DOI] [PubMed] [Google Scholar]
- 72.Lagunoff M, Majeti R, Weiss A, Ganem D. Deregulated signal transduction by the K1 gene product of Kaposi's sarcoma-associated herpesvirus. Proc Natl Acad Sci USA. 1999;96:5704–5709. doi: 10.1073/pnas.96.10.5704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Laherty C D, Hu H M, Opipari A W, Wang F, Dixit V M. The Epstein-Barr virus LMP1 gene product induces A20 zinc finger protein expression by activating nuclear factor kappa B. J Biol Chem. 1992;267:24157–24160. [PubMed] [Google Scholar]
- 74.Lee H, Trimble J, Yoon D W, Regier D, Desrosiers R C, Jung J U. Genetic variation of herpesvirus saimiri subgroup A transforming protein and its association with cellular src. J Virol. 1997;71:3817–3825. doi: 10.1128/jvi.71.5.3817-3825.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lee H, Choi J K, Li M, Kaye K, Kieff E, Jung J U. Role of cellular tumor necrosis factor receptor-associated factors in NF-κB activation and lymphocyte transformation by herpesvirus saimiri STP. J Virol. 1999;73:3913–3919. doi: 10.1128/jvi.73.5.3913-3919.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Lee H, Guo J, Li M, Choi J K, DeMaria M, Rosenzweig M, Jung J U. Identification of an immunoreceptor tyrosine-based activation motif of K1 transforming protein of Kaposi's sarcoma-associated herpesvirus. Mol Cell Biol. 1998;18:5219–5228. doi: 10.1128/mcb.18.9.5219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee H, Veazey R, Williams K, Li M, Guo J, Neipel F, Fleckenstein B, Lackner A, Desrosiers R C, Jung J U. Deregulation of cell growth by the K1 gene of Kaposi's sarcoma-associated herpesvirus. Nat Med. 1998;4:435–440. doi: 10.1038/nm0498-435. [DOI] [PubMed] [Google Scholar]
- 78.Lekstrom-Himes J A, Dale J K, Kingma D W, Diaz P S, Jaffe E S, Straus S E. Periodic illness associated with Epstein-Barr virus infection. Clin Infect Dis. 1996;22:22–27. doi: 10.1093/clinids/22.1.22. [DOI] [PubMed] [Google Scholar]
- 79.Longnecker R, Druker B, Roberts T M, Kieff E. An Epstein-Barr virus protein associated with cell growth transformation interacts with a tyrosine kinase. J Virol. 1991;65:3681–3692. doi: 10.1128/jvi.65.7.3681-3692.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Longnecker R, Miller C L, Miao X Q, Marchini A, Kieff E. The only domain which distinguishes Epstein-Barr virus latent membrane protein 2A (LMP2A) from LMP2B is dispensable for lymphocyte infection and growth transformation in vitro; LMP2A is therefore nonessential. J Virol. 1992;66:6461–6469. doi: 10.1128/jvi.66.11.6461-6469.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Longnecker R, Miller C L, Miao X Q, Tomkinson B, Kieff E. The last seven transmembrane and carboxy-terminal cytoplasmic domains of Epstein-Barr virus latent membrane protein 2 (LMP2) are dispensable for lymphocyte infection and growth transformation in vitro. J Virol. 1993;67:2006–2013. doi: 10.1128/jvi.67.4.2006-2013.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Longnecker R, Miller C L, Tomkinson B, Miao X Q, Kieff E. Deletion of DNA encoding the first five transmembrane domains of Epstein-Barr virus latent membrane proteins 2A and 2B. J Virol. 1993;67:5068–5074. doi: 10.1128/jvi.67.8.5068-5074.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Magrath I. Molecular basis of lymphomagenesis. Cancer Res. 1992;52:5529s–5540s. [PubMed] [Google Scholar]
- 84.Magrath I, Jain V, Bhatia K. Epstein-Barr virus and Burkitt's lymphoma. Semin Cancer Biol. 1992;3:285–295. [PubMed] [Google Scholar]
- 85.Mansfield K, Westmoreland S V, DeBakker C D, Czajak S, Lackner A A, Desrosiers R C. Experimental infection of rhesus and pig-tailed macaques with macaque rhadinoviruses. J Virol. 1999;73:10320–10328. doi: 10.1128/jvi.73.12.10320-10328.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Medveczky P, Szomolanyi E, Desrosiers R C, Mulder C. Classification of herpesvirus saimiri into three groups based on extreme variation in a DNA region required for oncogenicity. J Virol. 1984;52:938–944. doi: 10.1128/jvi.52.3.938-944.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Middleton T, Gahn T A, Martin J M, Sugden B. Immortalizing genes of Epstein-Barr virus. Adv Virus Res. 1991;40:19–55. doi: 10.1016/s0065-3527(08)60276-6. [DOI] [PubMed] [Google Scholar]
- 88.Miller C L, Burkhardt A L, Lee J H, Stealey B, Longnecker R, Bolen J B, Kieff E. Integral membrane protein 2 of Epstein-Barr virus regulates reactivation from latency through dominant negative effects on protein-tyrosine kinases. Immunity. 1995;2:155–166. doi: 10.1016/s1074-7613(95)80040-9. [DOI] [PubMed] [Google Scholar]
- 89.Miller C L, Lee J H, Kieff E, Longnecker R. An integral membrane protein (LMP2) blocks reactivation of Epstein-Barr virus from latency following surface immunoglobulin crosslinking. Proc Natl Acad Sci USA. 1994;91:772–776. doi: 10.1073/pnas.91.2.772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Miller C L, Longnecker R, Kieff E. Epstein-Barr virus latent membrane protein 2A blocks calcium mobilization in B lymphocytes. J Virol. 1993;67:3087–3094. doi: 10.1128/jvi.67.6.3087-3094.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Mittrucker H W, Muller-Fleckenstein I, Fleckenstein B, Fleischer B. Herpes virus saimiri-transformed human T lymphocytes: normal functional phenotype and preserved T cell receptor signalling. Int Immunol. 1993;5:985–990. doi: 10.1093/intimm/5.8.985. [DOI] [PubMed] [Google Scholar]
- 92.Moore P S, Boshoff C, Weiss R A, Chang Y. Molecular mimicry of human cytokine and cytokine response pathway genes by KSHV. Science. 1996;274:1739–1744. doi: 10.1126/science.274.5293.1739. [DOI] [PubMed] [Google Scholar]
- 93.Moore P S, Chang Y. Detection of herpesvirus-like DNA sequences in Kaposi's sarcoma in patients with and without HIV infection. N Engl J Med. 1995;332:1181–1185. doi: 10.1056/NEJM199505043321801. [DOI] [PubMed] [Google Scholar]
- 94.Moore P S, Kingsley L A, Holmberg S D, Spira T, Gupta P, Hoover D R, Parry J P, Conley L J, Jaffe H W, Chang Y. Kaposi's sarcoma-associated herpesvirus infection prior to onset of Kaposi's sarcoma. AIDS. 1996;10:175–180. doi: 10.1097/00002030-199602000-00007. [DOI] [PubMed] [Google Scholar]
- 95.Mueller N. An epidemiologist's view of the new molecular biology findings in Hodgkin's disease. Ann Oncol. 1991;2(Suppl.2):23–28. doi: 10.1007/978-1-4899-7305-4_3. [DOI] [PubMed] [Google Scholar]
- 96.Mueller N, Evans A, Harris N L, Comstock G W, Jellum E, Magnus K, Orentreich N, Polk B F, Vogelman J. Hodgkin's disease and Epstein-Barr virus. Altered antibody pattern before diagnosis. N Engl J Med. 1989;320:689–695. doi: 10.1056/NEJM198903163201103. [DOI] [PubMed] [Google Scholar]
- 97.Muralidhar S, Pumfery A M, Hassani M, Sadaie M R, Azumi N, Kishishita M, Brady J N, Doniger J, Medveczky P, Rosenthal L J. Identification of kaposin (open reading frame K12) as a human herpesvirus 8 (Kaposi's sarcoma-associated herpesvirus) transforming gene. J Virol. 1998;72:4980–4988. doi: 10.1128/jvi.72.6.4980-4988.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Murray P G, Swinnen L J, Constandinou C M, Pyle J M, Carr T J, Hardwick J M, Ambinder R F. BCL-2 but not its Epstein-Barr virus-encoded homologue, BHRF1, is commonly expressed in posttransplantation lymphoproliferative disorders. Blood. 1996;87:706–711. [PubMed] [Google Scholar]
- 99.Murthy S C, Trimble J J, Desrosiers R C. Deletion mutants of herpesvirus saimiri define an open reading frame necessary for transformation. J Virol. 1989;63:3307–3314. doi: 10.1128/jvi.63.8.3307-3314.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Neipel F, Albrecht J C, Ensser A, Huang Y Q, Li J J, Friedman-Kien A E, Fleckenstein B. Human herpesvirus 8 encodes a homolog of interleukin-6. J Virol. 1997;71:839–842. doi: 10.1128/jvi.71.1.839-842.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Neipel F, Albrecht J C, Fleckenstein B. Cell-homologous genes in the Kaposi's sarcoma-associated rhadinovirus human herpesvirus 8: determinants of its pathogenicity? J Virol. 1997;71:4187–4192. doi: 10.1128/jvi.71.6.4187-4192.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Neipel F, Albrecht J C, Fleckenstein B. Human herpesvirus 8--the first human rhadinovirus. J Natl Cancer Inst Monogr. 1998;23:73–77. doi: 10.1093/oxfordjournals.jncimonographs.a024178. [DOI] [PubMed] [Google Scholar]
- 103.Nicholas J, Ruvolo V, Zong J, Ciufo D, Guo H G, Reitz M S, Hayward G S. A single 13-kilobase divergent locus in the Kaposi sarcoma-associated herpesvirus (human herpesvirus 8) genome contains nine open reading frames that are homologous to or related to cellular proteins. J Virol. 1997;71:1963–1974. doi: 10.1128/jvi.71.3.1963-1974.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Nicholas J, Ruvolo V R, Burns W H, Sandford G, Wan X, Ciufo D, Hendrickson S B, Guo H G, Hayward G S, Reitz M S. Kaposi's sarcoma-associated human herpesvirus-8 encodes homologues of macrophage inflammatory protein-1 and interleukin-6. Nat Med. 1997;3:287–292. doi: 10.1038/nm0397-287. [DOI] [PubMed] [Google Scholar]
- 105.Nicholas J, Zong J C, Alcendor D J, Ciufo D M, Poole L J, Sarisky R T, Chiou C J, Zhang X, Wan X, Guo H G, Reitz M S, Hayward G S. Novel organizational features, captured cellular genes, and strain variability within the genome of KSHV/HHV8. J Natl Cancer Inst Monogr. 1998;23:79–88. doi: 10.1093/oxfordjournals.jncimonographs.a024179. [DOI] [PubMed] [Google Scholar]
- 106.Noel J C, Hermans P, Andre J, Fayt I, Simonart T, Verhest A, Haot J, Burny A. Herpesvirus-like DNA sequences and Kaposi's sarcoma: relationship with epidemiology, clinical spectrum, and histologic features. Cancer. 1996;77:2132–2136. doi: 10.1002/(SICI)1097-0142(19960515)77:10<2132::AID-CNCR26>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 107.Palefsky J M, Berline J, Penaranda M E, Lennette E T, Greenspan D, Greenspan J S. Sequence variation of latent membrane protein-1 of Epstein-Barr virus strains associated with hairy leukoplakia. J Infect Dis. 1996;173:710–714. doi: 10.1093/infdis/173.3.710. [DOI] [PubMed] [Google Scholar]
- 108.Pallesen G, Hamilton-Dutoit S J, Zhou X. The association of Epstein-Barr virus (EBV) with T cell lymphoproliferations and Hodgkin's disease: two new developments in the EBV field. Adv Cancer Res. 1993;62:179–239. doi: 10.1016/s0065-230x(08)60319-x. [DOI] [PubMed] [Google Scholar]
- 109.Panousis C G, Rowe D T. Epstein-Barr virus latent membrane protein 2 associates with and is a substrate for mitogen-activated protein kinase. J Virol. 1997;71:4752–4760. doi: 10.1128/jvi.71.6.4752-4760.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Parker G A, Crook T, Bain M, Sara E A, Farrell P J, Allday M J. Epstein-Barr virus nuclear antigen (EBNA)3C is an immortalizing oncoprotein with similar properties to adenovirus E1A and papillomavirus E7. Oncogene. 1996;13:2541–2549. [PubMed] [Google Scholar]
- 111.Parravinci C, Corbellino M, Paulli M, Magrini U, Lazzarino M, Moore P S, Chang Y. Expression of a virus-derived cytokine, KSHV vIL-6, in HIV-seronegative Castleman's disease. Am J Pathol. 1997;151:1517–1522. [PMC free article] [PubMed] [Google Scholar]
- 112.Pastore C, Gloghini A, Volpe G, Nomdedeu J, Leonardo E, Mazza U, Saglio G, Carbone A, Gaidano G. Distribution of Kaposi's sarcoma herpesvirus sequences among lymphoid malignancies in Italy and Spain. Br J Haematol. 1995;91:918–920. doi: 10.1111/j.1365-2141.1995.tb05410.x. [DOI] [PubMed] [Google Scholar]
- 113.Pathmanathan R, Prasad U, Sadler R, Flynn K, Raab-Traub N. Clonal proliferations of cells infected with Epstein-Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. N Engl J Med. 1995;333:693–698. doi: 10.1056/NEJM199509143331103. [DOI] [PubMed] [Google Scholar]
- 114.Poole L J, Zong J C, Ciufo D M, Alcendor D J, Cannon J S, Ambinder R, Orenstein J M, Reitz M S, Hayward G S. Comparison of genetic variability at multiple loci across the genomes of the major subtypes of Kaposi's sarcoma-associated herpesvirus reveals evidence for recombination and for two distinct types of open reading frame K15 alleles at the right-hand end. J Virol. 1999;73:6646–6660. doi: 10.1128/jvi.73.8.6646-6660.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Purtilo D T, Cassel C K, Yang J P, Harper R. X-linked recessive progressive combined variable immunodeficiency (Duncan's disease) Lancet. 1975;i:935–940. doi: 10.1016/s0140-6736(75)92004-8. [DOI] [PubMed] [Google Scholar]
- 116.Raab-Traub N. The human DNA tumor viruses: human papilloma virus and Epstein-Barr virus. Cancer Treat Res. 1989;47:285–302. doi: 10.1007/978-1-4613-1599-5_12. [DOI] [PubMed] [Google Scholar]
- 117.Raab-Traub N, Hood R, Yang C-S, Henry II B, Pagano J S. Epstein-Barr virus transcription in nasopharyngeal carcinoma. J Virol. 1983;48:580–590. doi: 10.1128/jvi.48.3.580-590.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Rickinson A B, Young L S, Rowe M. Influence of the Epstein-Barr virus nuclear antigen EBNA 2 on the growth phenotype of virus-transformed B cells. J Virol. 1987;61:1310–1317. doi: 10.1128/jvi.61.5.1310-1317.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rose T M, Strand K B, Schultz E R, Schaefer G, Rankin G W, Jr, Thouless M E, Tsai C C, Bosch M L. Identification of two homologs of the Kaposi's sarcoma-associated herpesvirus (human herpesvirus 8) in retroperitoneal fibromatosis of different macaque species. J Virol. 1997;71:4138–4144. doi: 10.1128/jvi.71.5.4138-4144.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Russo J J, Bohenzky R A, Chien M C, Chen J, Yan M, Maddalena D, Parry J P, Peruzzi D, Edelman I S, Chang Y, Moore P S. Nucleotide sequence of the Kaposi sarcoma-associated herpesvirus (HHV8) Proc Natl Acad Sci USA. 1996;93:14862–14867. doi: 10.1073/pnas.93.25.14862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sadler R, Wu L, Forghani B, Renne R, Zhong W, Herndier B, Ganem D. A complex translational program generates multiple novel proteins from the latently expressed kaposin (K12) locus of Kaposi's sarcoma-associated herpesvirus. J Virol. 1999;73:5722–5730. doi: 10.1128/jvi.73.7.5722-5730.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sandberg M, Hammerschmidt W, Sugden B. Characterization of LMP-1's association with TRAF1, TRAF2, and TRAF3. J Virol. 1997;71:4649–4656. doi: 10.1128/jvi.71.6.4649-4656.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Sayos J, Wu C, Morra M, Wang N, Zhang X, Allen D, van Schaik S, Notarangelo L, Geha R, Roncarolo M G, Oettgen H, De Vries J E, Aversa G, Terhorst C. The X-linked lymphoproliferative-disease gene product SAP regulates signals induced through the co-receptor SLAM. Nature. 1998;395:462–469. doi: 10.1038/26683. [DOI] [PubMed] [Google Scholar]
- 124.Scholle F, Longnecker R, Raab-Traub N. Epithelial cell adhesion to extracellular matrix proteins induces tyrosine phosphorylation of the Epstein-Barr virus latent membrane protein 2: a role for C-terminal Src kinase. J Virol. 1999;73:4767–4775. doi: 10.1128/jvi.73.6.4767-4775.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schulz T F. Future trends in Kaposi's sarcoma and lymphoma. Int J STD AIDS. 1998;9:32–34. [PubMed] [Google Scholar]
- 126.Schulz T F. Kaposi's sarcoma-associated herpesvirus (human herpesvirus-8) J Gen Virol. 1998;79:1573–1591. doi: 10.1099/0022-1317-79-7-1573. [DOI] [PubMed] [Google Scholar]
- 127.Searles R P, Bergquam E P, Axthelm M K, Wong S W. Sequence and genomic analysis of a rhesus macaque rhadinovirus with similarity to Kaposi's sarcoma-associated herpesvirus/human herpesvirus 8. J Virol. 1999;73:3040–3053. doi: 10.1128/jvi.73.4.3040-3053.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sinclair A J, Palmero I, Holder A, Peters G, Farrell P J. Expression of cyclin D2 in Epstein-Barr virus-positive Burkitt's lymphoma cell lines is related to methylation status of the gene. J Virol. 1995;69:1292–1295. doi: 10.1128/jvi.69.2.1292-1295.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sinclair A J, Palmero I, Peters G, Farrell P J. EBNA-2 and EBNA-LP cooperate to cause G0 to G1 transition during immortalization of resting human B lymphocytes by Epstein-Barr virus. EMBO J. 1994;13:3321–3328. doi: 10.1002/j.1460-2075.1994.tb06634.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Soulier J, Grollet L, Oksenhendler E, Cacoub P, Cazals-Hatem D, Babinet P, d'Agay M F, Clauvel J P, Raphael M, Degos L, et al. Kaposi's sarcoma-associated herpesvirus-like DNA sequences in multicentric Castleman's disease. Blood. 1995;86:1276–1280. [PubMed] [Google Scholar]
- 131.Staskus K A, Sun R, Miller G, Racz P, Jaslowski A, Metroka C, Brett-Smith H, Haase A T. Cellular tropism and viral interleukin-6 expression distinguish human herpesvirus 8 involvement in Kaposi's sarcoma, primary effusion lymphoma, and multicentric Castleman's disease. J Virol. 1999;73:4181–4187. doi: 10.1128/jvi.73.5.4181-4187.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Staskus K A, Zhong W, Gebhard K, Herndier B, Wang H, Renne R, Beneke J, Pudney J, Anderson D J, Ganem D, Haase A T. Kaposi's sarcoma-associated herpesvirus gene expression in endothelial (spindle) tumor cells. J Virol. 1997;71:715–719. doi: 10.1128/jvi.71.1.715-719.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tangye S G, Lazetic S, Woollatt E, Sutherland G R, Lanier L L, Phillips J H. Cutting edge: human 2B4, an activating NK cell receptor, recruits the protein tyrosine phosphatase SHP-2 and the adaptor signaling protein SAP. J Immunol. 1999;162:6981–6985. [PubMed] [Google Scholar]
- 134.Tomkinson B, Robertson E, Kieff E. Epstein-Barr virus nuclear proteins EBNA-3A and EBNA-3C are essential for B-lymphocyte growth transformation. J Virol. 1993;67:2014–2025. doi: 10.1128/jvi.67.4.2014-2025.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tosato G, Tanner J, Jones K D, Revel M, Pike S E. Identification of interleukin-6 as an autocrine growth factor for Epstein-Barr virus-immortalized B cells. J Virol. 1990;64:3033–3041. doi: 10.1128/jvi.64.6.3033-3041.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Tsang S F, Wang F, Izumi K M, Kieff E. Delineation of the cis-acting element mediating EBNA-2 transactivation of latent infection membrane protein expression. J Virol. 1991;65:6765–6771. doi: 10.1128/jvi.65.12.6765-6771.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wang D, Liebowitz D, Kieff E. An EBV membrane protein expressed in immortalized lymphocytes transforms established rodent cells. Cell. 1985;43:831–840. doi: 10.1016/0092-8674(85)90256-9. [DOI] [PubMed] [Google Scholar]
- 138.Wang D, Liebowitz D, Wang F, Gregory C, Rickinson A, Larson R, Springer T, Kieff E. Epstein-Barr virus latent infection membrane protein alters the human B-lymphocyte phenotype: deletion of the amino terminus abolishes activity. J Virol. 1988;62:4173–4184. doi: 10.1128/jvi.62.11.4173-4184.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Weiss L M, Chang K L. Molecular biologic studies of Hodgkin's disease. Semin Diagn Pathol. 1992;9:272–278. [PubMed] [Google Scholar]
- 140.Wong S W, Bergquam E P, Swanson R M, Lee F W, Shiigi S M, Avery N A, Fanton J W, Axthelm M K. Induction of B cell hyperplasia in simian immunodeficiency virus-infected rhesus macaques with the simian homologue of Kaposi's sarcoma-associated herpesvirus. J Exp Med. 1999;190:827–840. doi: 10.1084/jem.190.6.827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Yao Q Y, Ogan P, Rowe M, Wood M, Rickinson A B. The Epstein-Barr virus:host balance in acute infectious mononucleosis patients receiving acyclovir anti-viral therapy. Int J Cancer. 1989;43:61–66. doi: 10.1002/ijc.2910430114. [DOI] [PubMed] [Google Scholar]
- 142.Yoon D W, Lee H, Seol W, DeMaria M, Rosenzweig M, Jung J U. Tap: a novel cellular protein that interacts with tip of herpesvirus saimiri and induces lymphocyte aggregation. Immunity. 1997;6:571–582. doi: 10.1016/s1074-7613(00)80345-3. [DOI] [PubMed] [Google Scholar]
- 143.Zong J C, Ciufo D M, Alcendor D J, Wan X, Nicholas J, Browning P J, Rady P L, Tyring S K, Orenstein J M, Rabkin C S, Su I J, Powell K F, Croxson M, Foreman K E, Nickoloff B J, Alkan S, Hayward G S. High-level variability in the ORF-K1 membrane protein gene at the left end of the Kaposi's sarcoma-associated herpesvirus genome defines four major virus subtypes and multiple variants or clades in different human populations. J Virol. 1999;73:4156–4170. doi: 10.1128/jvi.73.5.4156-4170.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]