Abstract
Infants of mothers with Graves’ disease (GD) may develop central hypothyroidism (CH) due to exposure of the foetal hypothalamic-pituitary-thyroid axis to higher-than-normal thyroid hormone concentrations, primary hypothyroidism (PH) due to transplacental passage of maternal thyroid stimulating hormone receptor antibody (TRAb), antithyroid drugs (ATD) or thyroid dysgenesis secondary to maternal uncontrolled hyperthyroidism. We describe two infants with PH and four infants with CH born to mothers with poorly controlled Graves’ disease. All infants required levothyroxine and had normal developmental milestones. While national guideline consensus for high thyroid stimulating hormone (TSH) on neonatal screening is well-established, thyroid function tests (TFTs) should be serially monitored in infants with low TSH on screening, as not all mothers with Graves’ disease are diagnosed antenatally.
Keywords: infants, central hypothyroidism, primary hypothyroidism, congenital hypothyroidism, maternal Graves’ disease, maternal hyperthyroidism
INTRODUCTION
Graves’ disease in a pregnant woman can lead to either hyper- or hypothyroidism in the foetus and neonate, depending on the control of hyperthyroidism, the presence of maternal stimulatory and/or inhibitory antibodies and antithyroid drug effects.1-5 While hyperthyroidism in neonates of mothers with Graves’ disease (GD) is well-described, hypothyroidism is less commonly reported.
Infants born to mothers with Graves’ disease may develop central hypothyroidism (CH) or primary hypothyroidism (PH).6-10 The most likely cause of central hypothyroidism is exposure of the foetal hypothalamic-pituitary-thyroid axis to higher-than-normal thyroid hormone concentrations impairing physiologic maturation during intrauterine life.11 PH can be transient due to transplacental passage of maternal thyroid stimulating hormone receptor antibody (TRAb) or antithyroid drugs (ATD). However, if maternal hyperthyroidism is severe, with high transplacental passage of maternal free thyroxine (FT4), permanent thyroid dysgenesis may ensue.7
Hypothyroidism is the most common preventable cause of mental retardation in children.12 Therefore, it is imperative to identify and treat them as early as possible. We describe six infants with hypothyroidism born to mothers with Graves’ disease.
METHODOLOGY
A retrospective review was conducted on all infants referred to Paediatric Department Hospital Putrajaya for infants of mothers with Graves’ disease between 1st January 2021 until 30th June 2022. Data was extracted from the electronic medical record (EMR) system and the referral letters from the referring physicians. Demographic profile and treatment course of mothers and infants were obtained. Additional details such as anthropometrics and blood chemistry were also extracted. Informed consent was obtained for each patient.
All samples were analysed with Beckman Coulter UniCel DxI 800 Access Immunoassay System with reference range for TSH (0.770-5.640) mU/L, FT4 (9.5-16.2) pmol/L and FT3 (4.3-6.8) pmol/L.
CASE
Primary hypothyroidism
Case 1
A live baby girl was born via lower segment caesarean section (LSCS) with birth weight of 2.67 kg. Her mother had Graves’ disease (GD) antenatally and was maintained on carbimazole with highest dose of 10 mg once daily (OD). She was later switched to Propylthiouracil (PTU) in early pregnancy until delivery. Thyroid ultrasonography (USG) revealed multinodular goiter with no malignant changes. Maternal TRAb titre was 2.25 IU/L (normal <1.75 IU/L).
Cord thyroid stimulating hormone (TSH) was 3.5 mU/L. Thyroid function test (TFT) on day 9 of life revealed high TSH 56.2 mU/L (reference range 0.770-5.640 mU/L), low free thyroxine (FT4) 6.6 pmol/L (reference range 9.5-16.2 pmol/L), and low-normal free triiodothyronine (FT3) 4.3 pmol/L (reference range 4.3-6.8 pmol/L). TFT on day 18 of life showed high TSH 105.29 mU/L, low FT4 6.3 pmol/L and normal FT3 5.12 pmol/L. TRAb was <0.8 IU/L. She was started on L-thyroxine 55 mcg/m2/day the following day. Her developmental milestones were appropriate. Weight was on the 10th centile and her length was between 25th to 50th centile on the Centers for Disease Control and Prevention (CDC) growth charts.
Case 2
A baby boy was born via LSCS with birth weight of 2.5 kg. His mother had gestational diabetes mellitus, diet-controlled and GD diagnosed in early pregnancy. She was started on PTU with highest dose of 100 mg BD and post-delivery on carbimazole 5 mg OD. Thyroid USG revealed a small cyst at left superior pole measures 0.9 x 0.2 cm. Mother’s TRAb was 9.14 IU/L.
The infant had high cord TSH of 42.18 mU/L and cord FT4 of 12.3 pmol/L. TFT at day 4 of life revealed high TSH 96.55 mU/L and FT4 of 18.2 pmol/L. He was treated with L-thyroxine 49 mcg/m2/day at day 7 of life. As per consensus guidelines for hypothyroidism in Malaysia,13 if venous TSH >40 mIU/L, treatment should be started regardless of FT4 concentration. No TRab was taken. His developmental milestones were appropriate, and his weight and length were on the 10th centile on CDC growth charts.
Central hypothyroidism
Case 3
A baby girl was born term at 38 weeks via spontaneous vaginal delivery with birth weight of 3.41 kg. Mother had gestational diabetes mellitus and hyperthyroidism for 5 years prior to delivery. She was on carbimazole with highest dose of 30 mg per day throughout pregnancy and her TRAb was <0.3 IU/L.
Baby’s cord TSH was 0.07 mU/L. At day 9 of life, TFT revealed normal TSH 3.385 mU/L and low FT4 <3.2 pmol/L. She was started on L-thyroxine 123 mcg/m2/day. TRAb was <0.3 IU/L. Other blood investigations were within normal (random serum cortisol 170 nmol/l, random blood sugar 5.0 mmol/L, urea 3.4 mmol/L, sodium 136 mmol/L, potassium 4.5 mmol/L, insulin like growth factor-1 (IGF-1) 98.1 ng/ml [NV: 16-148 ng/ml] and prolactin 423 uU/ml [NV: 152-1520 uU/ml]).
Developmental milestones were appropriate. Weight and height were within the 50th centile on the CDC growth charts.
Case 4
A baby girl was born preterm at 35 weeks and 2 days with birth weight of 1.98 kg. Antenatally, mother was diagnosed with GD with ophthalmopathy. She underwent radioactive ablation therapy (RAI) twice with total cumulative dose of 50 millicurie (mCi). Last RAI was 1 year and 2 months prior to delivery. She was initially hypothyroid post-treatment, but she became hyperthyroid again preconception. She was on PTU in early pregnancy and was switched to carbimazole in the second trimester. She had serially high TRAb at 10 IU/L in the first trimester and 5.6 IU/L in the second trimester.
Cord TSH was <0.005 mU/L. TFT at day 5 of life revealed low TSH <0.005 mU/L and high FT4 64 pmol/L. Repeat TFT on day 7 of life revealed persistently suppressed TSH of 0.009 mU/L and high free T4 of 54.4 pmol/L.
She was treated with oral carbimazole 0.4 mg (0.2 mg/kg/dose) twice daily (BD) and oral propranolol 0.2 mg (0.1 mg/kg/dose) BD. She was weaned off carbimazole at day 45 of life when TFT revealed TSH 0.005 mU/L and FT4 16.2 pmol/L. However, she developed central hypothyroidism at day 66 of life when TFT revealed TSH 0.018 mU/L, FT4 9.1 pmol/L and FT3 4.9 pmol/L. She was started on L-thyroxine at 39 mcg/m2/day the next day. TFT was only taken three weeks after cessation of carbimazole due to restrictions during the COVID-19 pandemic. No TRAb was taken. Developmental milestones were appropriate. Weight was below 10th centile and length was on 10th centile on the CDC growth charts.
Case 5
A baby boy was born large for gestational age via LSCS at 38 weeks and 1 day with birth weight of 4.21 kg. Mother was para 2 and diagnosed with hyperthyroidism post-delivery. She was hypertensive maintained on methyldopa and aspirin and has been experiencing palpitations for two months. TFT showed low TSH at <0.005 mU/L and high FT4 at 47.2 pmol/L. She was then started on carbimazole. Anti-thyroglobulin (ATG) was high at 307.2 U/ml (normal <1.0 U/ml), anti-thyroid peroxidase (ATPO) taken twice was elevated at 242.4 to 366 U/ml (normal <10.00 U/ml) and TRAb was 21.36 IU/L. Her thyroid USG had features suggestive of Graves’ disease or thyroiditis.
Cord TSH was 0.01 mU/L. At day 28 of life, TSH was inappropriately low at 0.454 mU/L despite low FT4 at 7.5 pmol/L. ATG was 1.9 U/ml and ATPO was 42 U/ml. TRAb was <0.8 IU/L. He was started on L-thyroxine 96 mcg/m2/day the next day. Other blood investigations were within normal (urea 2.0 mmol/L, sodium 136 mmol/L, potassium 4.8 mmol/L, creatinine 64 umol/L and random serum cortisol 364 nmol/L).
Developmental milestones were appropriate. Weight was at 90th centile and length was at 50th centile on the CDC growth charts.
Case 6
A baby girl was delivered via LSCS at 38 weeks and 3 days with a birth weight of 2.85 kg. Mother was para 1+1 and diagnosed with Graves’ disease 3 months prior to pregnancy, initially presenting with tachycardia. Her TRAb was 23.45 IU/L, ATG 10.12 U/ml and ATPO 4.67 U/ml.
Baby’s cord TSH was 2.28 mU/L. At day 4 of life, TSH was normal with high 2FT4 (48.7 pmol/L). TFT at day 10 revealed FT4 16.4 pmol/l with low TSH of 0.11 mU/L. Repeat TFT at day 36 of life revealed FT4 of 11.7 pmol/L and TSH of 1.52 mU/L. No other pituitary hormones were examined. The child was started on L-thyroxine 60 mcg/m2/day that same day. Baby’s TRAb was 6.13 IU/L, ATG <10 U/mL and Anti TPO 5.52 U/mL. The infant’s weight and length were at 50th centile on the CDC growth charts, with appropriate developmental milestones noted.
DISCUSSION
Four mothers were diagnosed with Graves’ disease prior to pregnancy – one during pregnancy and one post-delivery (Table 1). The mother of Case 4 was hypothyroid for 3 months after the second RAI treatment but became hyperthyroid 10 months before conception. The mother of Case 5 was hyperthyroid after delivery and was on carbimazole.
Table 1.
Endocrine profile of mothers with Graves’ disease
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|
| Age | 36 | 42 | 28 | 36 | 34 | 30 |
| Parity | 1 | 5 | 2 | 2 | 2 | 1 |
| Graves’ Diagnosis | 2 years antepartum | pregnancy | 5 years antepartum | 5 years antepartum | postpartum | 3 months antepartum |
| USG | Multinodular goitre | Cyst left superior pole | NA | Diffuse goitre with features of thyroiditis | Suspicious Graves’ disease or Thyroiditis |
NA |
| TSH Receptor Ab (normal <1.75 IU/L) | 2.25 | 9.14 | <0.3 | 10 | 21.36 | 23.45 |
| ATG (normal <1 U/ml) | <0.9 | Not done | 320.4 | 21.9 | 307.2 | Normal |
| ATPO (normal <10 U/ml) | 307.0 | Not done | 266.3 | 36.4 | 366 | Normal |
TSH-Thyroid stimulating hormone, Ab-Antibody, NA-Not Available, ATG-Antithyroglobulin antibodies, ATPO-Anti-thyroid Peroxidase antibodies
All mothers had elevated TRAb except the mother of Case 3. TRAb level in Case 3 may be undetectable due to prolonged ATD use, however, TRAb has been reported to have a poor correlation with disease activity.14 Although four of the mothers were diagnosed prior to pregnancy, they had poorly controlled hyperthyroidism due to lack of compliance. At the end of the study, all mothers were on carbimazole.
All mothers employed mixed feeding except in Case 6 who was exclusively formula-fed prior to weaning. After weaning was started, all infants were on formula feeding. All mothers were from urban residences with presumably adequate dietary iodine intake.
There were four female and two male infants (Table 2). During follow-up, none of the infants had palpable goitres. Among the infants tested, only one had elevated TRAb while two were not tested (Table 3). Two infants developed primary hypothyroidism while four infants developed central hypothyroidism. Among the infants with central hypothyroidism, other pituitary hormones were normal for Case 3 and Case 5 while Case 4 and Case 6 were not tested. Two infants, (Cases 4 and 6) developed transient hyperthyroidism prior to central hypothyroidism at 2 months and 1 month of age, respectively. Case 4 required carbimazole treatment initially, while Case 6 did not require ATD. At 2 years old, both infants required levothyroxine.
Table 2.
Demographic characteristics of hypothyroid infants born to mothers with Graves’ Disease
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|
| Gestation | 38 weeks | 38 weeks | 38 weeks | 35 weeks 2 days | 38 weeks 1 day | 38 weeks 3 days |
| Birth Weight (kg) | 2.67 | 2.5 | 3.41 | 1.98 | 4.21 | 2.85 |
| Ethnicity | Chinese | Chinese | Malay | Malay | Chinese | Malay |
| Gender | Female | Male | Female | Female | Male | Female |
Table 3.
Laboratory profile and treatment of hypothyroid infants born to mothers with Graves’ disease
| Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | |
|---|---|---|---|---|---|---|
| Cord TSH (mU/L) | 3.5 | 42.18 | 0.07 | <0.005 | 0.01 | 2.28 |
| Treatment initiation (day of life) | Day 19 | Day 7 | Day 9 | Day 67 | Day 29 | Day 36 |
| TSH at treatment (mU/L) | 105.29 | 96.55 | 3.385 | 0.018 | 0.454 | 1.52 |
| FT4 at treatment (pmol/L) | 6.3 | 18.2 | <3.2 | 9.1 | 7.8 | 11.7 |
| TRAb | <0.8 | NA | <0.3 | NA | <0.8 | 6.13 |
| L-thyroxine dosage (mcg/m2/day) | 55 | 49 | 123 | 39 | 96 | 60 |
TSH – Thyroid stimulating hormone, FT4 – Free thyroxine, TRAb – Thyroid stimulating hormone receptor antibody, L-Thyroxine – Levothyroxine
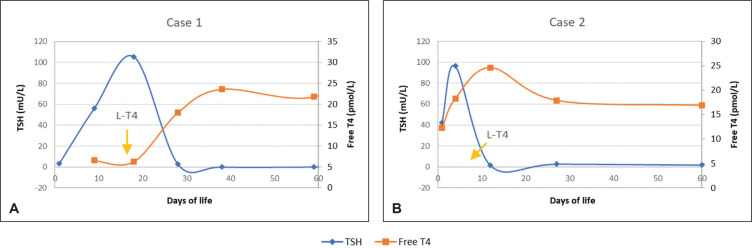
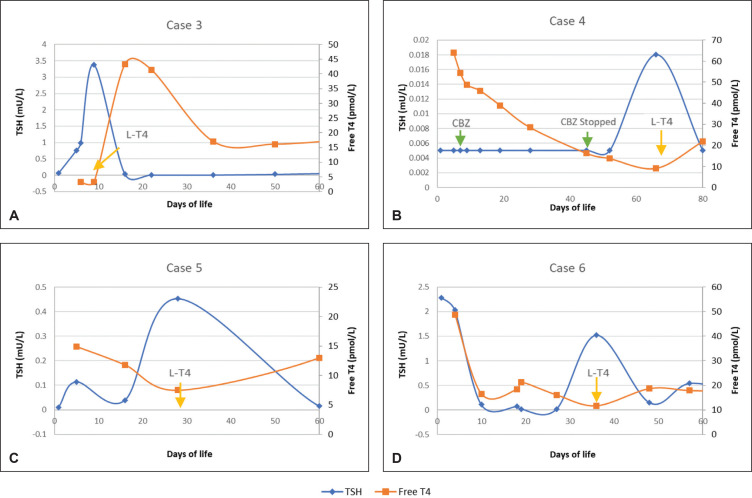
All infants attained normal TSH values (Figure 1) by day 28 of life and FT4 (Figure 2) by day 45 of life. Apart from Case 4, all other infants achieved normal FT4 values by day 27 of life.
Figure 1.
Primary hypothyroidism. (A) Case 1 and (B) Case 2 shows the start of levothyroxine (LT4) and corresponding changes of thyroid stimulating hormone (TSH) and free thyroxine (Free T4).
Figure 2.
Central hypothyroidism. (A) Case 3, (B) Case 4, (C) Case 5 and (D) Case 6 shows start of levothyroxine and changes in TSH and FT4. Case 4 was started on carbimazole (CBZ) at day 7 of life and was weaned off CBZ at day 45 of life when TFT revealed TSH 0.005 mU/L and FT4 16.2 pmol/L. Case 4 was started on levothyroxine at Day 66 of life.
All infants were followed up for at least 2 years except for Case 6 who was 12 months old at the end of the study. All infants had appropriate developmental milestones as per Paediatric Protocols for Malaysian Hospitals, 4th Edition, 2019.15
CONCLUSION
In our case series, all infants required levothyroxine at the end of the study. Developmental assessment was done for at least 1 year and all infants remained under follow-up. Re-evaluation with thyroid ultrasonography to determine permanent or transient hypothyroidism is done at or after 3 years of age when myelination in the central nervous system is complete.13 At the end of the study none of the infants had reached 3 years of age.
Infants of maternal Graves’ disease (GD) should be monitored for both hyperthyroidism and hypothyroidism. Maternal gestational hyperthyroidism causes a hyperthyroid foetal environment due to increased thyroxine transfer which leads to suppression of the foetal hypothalamic-pituitary-thyroid axis and central hypothyroidism in newborns. Certainly, the most effective management would be the preservation of euthyroid status throughout pregnancy.16 Primary hypothyroidism could be a result of transplacental passage of antithyroid drugs (ATD) during pregnancy, transplacental passage of maternal blocking antibodies or thyroid dysgenesis secondary to maternal hyperthyroidism.
While the Malaysia national guideline13 consensus on reevaluation for high TSH in neonatal screening is established, no recommendation exists for low TSH. As illustrated by our case series, the presentation of hypothyroidism in this group of patients is a spectrum and may be delayed. In view of this, it is imperative that TFT be serially monitored in infants with low TSH as not all mothers with Graves’ disease are diagnosed antenatally.
Funding Statement
Funding Source None.
Ethical Consideration
Patients' consent were obtained before submission of the manuscript.
Statement of Authorship
All authors certified fulfillment of ICMJE authorship criteria.
CRediT Author Statement
AAL: Conceptualization, Methodology, Validation, Investigation, Resources, Data Curation, Writing - original draft preparation, Writing - review and editing, Visualization, project administration; JSLW: Conceptualization, Methodology, Resources, Supervision, Project administration; NS: Conceptualization, Methodology, Resources, Supervision, Project administration; JYHH: Conceptualization, Methodology, Resources, Supervision, Project administration.
Authors Disclosure
The authors declared no conflict of interest.
References
- 1.Polak M, Legac I, Vuillard E, Guibourdenche J, Castanet M, Luton D. Congenital hyperthyroidism: The fetus as a patient. Horm Res. 2006;65(5):235-42. PMID: 16582565. 10.1159/000092454. [DOI] [PubMed] [Google Scholar]
- 2.De Groot L, Abalovich M, Alexander EK, et al. Management of thyroid dysfunction during pregnancy and postpartum: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2012;97(8): 2543–65. PMID: 22869843. 10.1210/jc.2011-2803. [DOI] [PubMed] [Google Scholar]
- 3.Ogilvy-Stuart AL. Neonatal thyroid disorders. Arch Dis Child Fetal Neonatal Ed. 2002;87(3):F165–71. PMID: 12390984 PMCID: . 10.1136/fn.87.3.f165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Uenaka M, Tanimura K, Tairaku S, Morioka I, Ebina Y, Yamada H. Risk factors for neonatal thyroid dysfunction in pregnancies complicated by Graves' disease. Eur J Obstet Gynecol Reprod Biol. 2014;177:89-93. PMID: 24726178. 10.1016/j.ejogrb.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 5.Momotani N, Noh JY, Ishikawa N, Ito K. Effects of propylthiouracil and methimazole on fetal thyroid status in mothers with Graves' hyperthyroidism. J Clin Endocrinol Metab. 1997;82(11):3633-6. PMID: 9360518. 10.1210/jcem.82.11.4347. [DOI] [PubMed] [Google Scholar]
- 6.Kempers MJ, van Tijn DA, van Trotsenburg AS, de Vijlder JJ, Wiedijk BM, Vulsma T. Central congenital hypothyroidism due to gestational hyperthyroidism: Detection where prevention failed. J Clin Endocrinol Metab. 2003;88(12):5851-7. PMID: 14671180. 10.1210/jc.2003-030665. [DOI] [PubMed] [Google Scholar]
- 7.Stephanie L. Samuels, Sisi M. Namoc, Andrew J. Bauer. Neonatal thyrotoxicosis. Clin Perinatol. 2018;45(1):31-40. PMID: 29406005. 10.1016/j.clp.2017.10.001. [DOI] [PubMed] [Google Scholar]
- 8.Blizzard RM, Chandler RW, Landing BH, Pettit MD, West CD. Maternal autoimmunization to thyroid as a probable cause of athyrotic cretinism. N Engl J Med. 1960;263:327-36. PMID: 13801488. 10.1056/NEJM196008182630702. [DOI] [PubMed] [Google Scholar]
- 9.Ritzén EM, Mahler H, Alveryd A. Transitory congenital hypothyroidism and maternal thyroiditis. Acta Paediatr Scand. 1981;70(5):765-6. PMID: 7324929. 10.1111/j.1651-2227.1981.tb05785.x [DOI] [PubMed] [Google Scholar]
- 10.Goldsmith RE, McAdams AJ, Larsen PR, McKenzie M, Hess E. Familial autoimmune thyroiditis: Maternal-fetal relationship and the role of generalized autoimmunity. J Clin Endocrinol Metab. 1973;37(2): 265-75. PMID: 4124243. 10.1210/jcem-37-2-265. [DOI] [PubMed] [Google Scholar]
- 11.Kempers MJE, van Trotsenburg ASP, van Tijn DA, et al. Disturbance of the fetal thyroid hormone state has long-term consequences for treatment of thyroidal and central congenital hypothyroidism. J Clin Endocrinol Metabol. 2005;90(7):4094-100. PMID: 15827096. 10.1210/jc.2005-0197. [DOI] [PubMed] [Google Scholar]
- 12.American Academy of Pediatrics; Rose SR; Section on Endocrinology and Committee on Genetics, American Thyroid Association, Brown RS; Public Health, Committee and Lawson Wilkins Pediatric Endocrine Society . Update of newborn screening and therapy for congenital hypothyroidism. Pediatrics. 2006;117(6):2290-303. PMID: 16740880. 10.1542/peds.2006-0915. [DOI] [PubMed] [Google Scholar]
- 13.Guideline Working Committee . Consensus guidelines on screening, diagnosis, and management of congenital hypothyroidism in Malaysia for the Ministry of Health Malaysia; 2021. Accessed April 23, 2023. https://mpedg.mems.my/wp-content/uploads/2021/12/Paeds_consensus_guidelines_CHT_Nov2021.pdf [Google Scholar]
- 14.Mukasa K, Yoshimura Noh J, Kouzaki A, et al. TSH receptor antibody titers measured with a third-generation assay did not reflect the activity of Graves' ophthalmopathy in untreated Japanese Graves' disease patients. Endocr J. 2016;63(2):151-7. PMID: 26581710. 10.1507/endocrj.EJ15-0137. [DOI] [PubMed] [Google Scholar]
- 15.Malaysian Paediatric Association Paediatric Protocols for Malaysian hospitals, 4th ed. 2019. Accessed June 29, 2023. https://mpaeds.my/wp-content/uploads/2019/09/Paediatric_Protocols_4th_Edition_(MPA%20Version)_2nd_Print_Aug_2019.pdf. [Google Scholar]
- 16.Kempers MJ, van Tijn DA, van Trotsenburg AS, de Vijlder JJ, Wiedijk BM, Vulsma T. Central congenital hypothyroidism due to gestational hyperthyroidism: detection where prevention failed. J Clin Endocrinol Metab. 2003;88(12):5851-7. PMID: 14671180. 10.1210/jc.2003-030665. [DOI] [PubMed] [Google Scholar]