Abstract
Background/Aims:
Phosphate homeostasis is controlled by the renal reabsorption of Pi by the type IIa sodium phosphate cotransporter, Npt2a, which is localized in the proximal tubule brush border membrane. Regulation of Npt2a expression is a key control point to maintain phosphate homeostasis with most studies focused on regulating protein levels in the brush border membrane. Molecular mechanisms that control Npt2a mRNA, however, remain to be defined. We have reported that Npt2a mRNA and protein levels correlate directly with the expression of the Na+/H+ exchanger regulatory factor 1 (NHERF-1) using opossum kidney (OK) cells and the NHERF-1-deficient OK-H cells. The goal of this study was to determine whether NHERF-1 contributes to transcriptional and/or post-transcriptional mechanisms controlling Npt2a mRNA levels.
Methods:
Npt2a mRNA half-life was compared between OK and NHERF-1 deficient OK-H cell lines. oNpt2a promoter-reporter gene assays and electrophoretic mobility shift assays (EMSA) were used identify a NHERF-1 responsive region within the oNpt2a proximal promoter.
Results:
Npt2a mRNA half-life is the same in OK and OK-H cells. The NHERF-1 responsive region lies within the proximal promoter in a region that contains a highly conserved CAATT box and G-rich element. Specific protein-DNA complex formation with the CAATT element is altered by the absence of NHERF-1 (OK v OK-H EMSA) although NHERF-1 does not directly contribute to complex formation.
Conclusion:
NHERF-1 helps maintain steady-state Npt2a mRNA levels in OK cells through indirect mechanisms that help promote protein-DNA interactions at the Npt2a proximal promoter.
Keywords: Npt2a transcription, NHERF-1, Promoter
Introduction
The major mechanism controlling phosphate homeostasis is the renal reabsorption of Pi by the type IIa sodium phosphate cotransporter Npt2a (encoded by the SLC34A1 gene). Npt2a is localized in the proximal tubule brush border membrane (BBM) and Npt2a protein level in the BBM is regulated by dietary phosphate and parathyroid hormone (PTH). Our group and others have established a critical role for Na+/H+ exchanger regulatory factor 1 (NHERF-1) in the expression and trafficking of Npt2a protein to the brush border membrane [1–4]. We have also shown the loss of NHERF-1, either by siRNA silencing in OK cells or using the NHERF-1 deficient OK-H clonal line, resulted in loss (95% reduction) of Npt2a mRNA expression and Npt2a protein expression with significant decrease in functional phosphate uptake. Importantly, Npt2a protein expression and phosphate uptake was regained and Npt2a mRNA levels were restored to 40% of OK levels by re-expression of NHERF-1 in the OK-H cells [5]. These findings support that NHERF-1 protein expression and Npt2a steady-state mRNA expression are directly correlated in the OK and OK-H cell lines. We also showed a unique finding that NHERF-1 has a nuclear location in OK cells [5]. Based on these data, the current study addressed the loss of NHERF-1 on Npt2a promoter protein-DNA interactions and mRNA stability as possible mechanisms controlling Npt2a constitutive mRNA expression.
The limited studies on the molecular mechanisms that regulate Npt2a promoter activity indicate that the Npt2a gene (SLC34A) is regulated in a tissue-specific manner. The 5’-flanking regions of the rat (~4kb rNpt2a), human (~2.4kb hNpt2a), and opossum (~3.7kb oNpt2a) genes were active in reporter gene assays only in the OK cells but not HeLa, NIH-3T3, or HEK293 cells [6–9]. 5’-deletion analyses identified a region within 250bp upstream of the transcription start site as sufficient to drive cell-specific expression of the human and opossum promoters [6, 10]. A transgenic mouse harboring a rat Npt2a promoter-driven LacZ gene showed localized renal cortical-specific expression in the kidney and no other tissue, confirming that 4.7kb of the rat 5’-flanking region is sufficient to control tissue-specific expression in vivo [11]. Comparison of the promoter sequences for human, mouse, and opossum Npt2a revealed a conserved non-consensus CCAAT/enhancer binding protein (C/EBP) element located −128 bp upstream of the transcription start site [6, 10, 12]. AP-1, Sp1, Vitamin D receptor, and cAMP responsive elements have been identified in the human gene promoter but the role of these elements in Npt2a transcription is not known [10, 12, 13]. Electrophoretic mobility shift assays showed specific protein-DNA interactions with nuclear protein from opossum kidney cells and a DNA probe containing the CCAATT conserved region of the oNpt2a promoter [13]. However, lack of cold-competition with a consensus C/EBP element indicates that c/EBPα does not bind to this element [10]. Southwestern analysis detected a 31kDa protein that bound to the CAATT element of oNpt2a promoter but the identity of that protein is not known [10]. Thus the key factors that control the tissue-specific and constitutive expression of Npt2a mRNA levels remain to be determined. To this end, the current study uses the OK and OK-H cells to address the loss of NHERF-1 on Npt2a promoter protein-DNA interactions and mRNA stability. We report herein the novel finding that the presence of NHERF-1 is required to maintain protein-DNA interactions within the highly conserved region of Npt2a the promoter.
Materials and Methods
Reagents
NHERF-1 antibody was a gift from Dr. E. J. Weinman (University of Maryland) [1]. Anti-Sp3 (sc-644x), anti-Sp1 (sc-14027x), and anti- C/EBPβ (sc-150) antibodies were purchased from Santa Cruz Biotechnology, Inc. (Santa Cruz, CA). DNA oligonucleotides were ordered from Integrated DNA Technologies, Inc. (Redwood City, CA).
Cell Culture and treatments
The OK and OK-H cells were maintained in culture as previously described [14, 15]. The OK proximal tubule cell line is derived from Virginia opossum [16], while OK-H cells are a stable clonal subline that lacks NHERF-1 expression [17–19]. For experiments using low phosphate media, cells were switched from normal phosphate (1.0 mM) to low phosphate (0.1 mM) 72 hours prior to initiating treatments. For inhibitor studies, OK or OK-H cells were treated with either actinomycin D (10 μg/ml) or cycloheximide (10 μg/ml) or both for indicated time.
Crude Membrane (CM) and Brush Border Membrane (BBM) Isolation
The cells were washed twice with HBSS and homogenized in 300 mM mannitol, 5 mM HEPES-Tris buffer (pH 7.6) using a 27.5-G needle. The cell lysate was centrifuged at 2000 rpm for 10 min to remove the cell debris, and crude membranes were isolated by centrifugation of the supernatant at 17,000 rpm for 30 min. Brush border membranes were prepared at 4°C using the MgCl2 precipitation method as previously described [14, 20]. In brief, OK or OK-H cells grown on semipermeable membrane inserts were homogenized in 50 mM mannitol and 5 mM Tris-HEPES (pH 7.0) by high-speed homogenizer (20,500 rpm). MgCl2 was added to a final 10 mM concentration and stirred for 20 min. The homogenate was centrifuged at 2000 g and the resultant supernatant was centrifuged at 35,000g for 30 min to recover the BBM pellet. The BBM pellet is enriched 6-fold over the crude membrane preparations for the brush border membrane enzyme γ-glutamyl transpeptidase.
RNA Isolation and RT-qPCR
Total RNA was isolated using MirVana miRNA isolation kit according to the manufacturer’s instructions (Life Technologies). One microgram of RNA was reverse-transcribed using the High Capacity RNA-to-cDNA Master Mix system (Applied Biosystems, Foster City, CA). 200 ng of cDNA was PCR-amplified using TaqMan Gene expression system for Npt2a and 18S rRNA. Real-time PCR was conducted using thermocycling setting of 50°C for 2 min, 95°C for 10 min, and 40 reps of 95°C for 15 s followed by 60°C for 1 min. Target Ct values were normalized to 18S and data expressed relative to control using the 2−ΔΔCt method as described in Applied Biosystems User Bulletin 2: Rev B. For each experiment, RNA was isolated from three wells per treatment group or time point and qPCR reactions were performed in triplicate. For the half-life studies, control was time 0 (t = 0) that was set to 100 for each experiment. The low phosphate treatment studies, control was normal phosphate minus inhibitors (actinomycin D or cycloheximide) within each cell line that was set to 100 (OK NP = 100; OK-H NP = 100). For steady-state mRNA expression, the control was OK cell expression that was set to 100.
Luciferase reporter gene assays
Npt2a promoter-luciferase reporter gene constructs (NpT2a-luc) containing −208 and −4,400 bp were a generous gift of Heine Murer [6]. Npt2a-luc or pGL3-basic and were transfected into OK or OK-H cells using Lipofectamine 2000 (Invitrogen). At 24 h posttransfection, firefly luciferase activities were determined (Promega) according to the manufacturer’s protocol and normalized to protein. The cells were transfected in triplicate and luciferase:protein ratios were determined per well and the average values determined per experiment. Three independent experiments were performed with each independent experiment using different cell culture stocks. In a fourth independent experiment, cells were co-transfected in triplicate with the Npt2a-luc reporter constructs and pSV-β-galactosidase vector (Promega) and the average luciferase:β-gal activity ratios determined (Promega).
Nuclear extract (NE) preparation
NE were prepared as previously described [21, 22] from OK, OK-H, and OK-H cells with re-expressed NHERF-1 (NF) [5]. 0.1% IGEPAL CA-630 rather than homogenization was used to disrupt cell membranes [21]. All buffers contained 1 mM phenylmethyl-sulfonyl fluoride, 0.04 U/ml aprotinin, 0.05 mM dithiothreitol, 10 μl/ml phosphatase inhibitor cocktail set II, 0.1 mM okadaic acid, and 0.1 μg/ml leupeptin (Sigma-Aldrich). Protein concentrations were determined using the bicinchoninic acid assay (Pierce Biotechnology, Rockford, IL).
Electrophoretic mobility shift assay (EMSA)
Double stranded oligonucleotides were generated by mixing equal molar concentrations of top and bottom strand primers in a high salt annealing buffer [10 mM Tris (pH 7.5), 50 mM NaCl, and 1 mM EDTA] and heating for 2 min at 95 C followed by slow equilibration to room temperature. The oligonucleotides were end labeled with T4 DNA kinase and [γ −32P] ATP (NEN Life Science Products). 50 fmol radiolabeled oligonucleotide was incubated with either 10 μg nuclear extract, 0.25 μg GST, or 0.25 μg GST-NHERF-1 for 30 min on ice. The binding buffer contained the following: 50 mM Tris-Cl (pH 8.0), 100 mM KCl, 12.5 mM MgCl2, 1 mM EDTA, 1 mM dithiothreitol, 20% glycerol, and 2.5 μg poly(dI:dC). Cold competition assays were performed with a 250-fold molar excess of unlabeled oligonucleotides. Antibody supershift assays included a 30 min. preincubation of indicated antibodies (4 μg) with nuclear extract prior to addition of the radiolabeled oligonucleotide for an additional 30 min. Binding reactions were resolved on a 4% nondenaturing polyacrylamide gel. The gels were dried and exposed to x-ray film, and the radioactive bands were visualized by autoradiography.
Statistics
Data are shown as means ± SE. The n values represent the number of independent experiments. Each independent experiment begins with different cell culture stocks. Each experiment was performed in triplicate unless otherwise indicated. P values were calculated by one-way ANOVA, followed by indicated post-hoc test using GraphPad Prism software. A P value < 0.05 was a priori considered statistically significant.
Results
NHERF1 does not contribute to Npt2a mRNA stability
Npt2a mRNA expression (Fig. 1A) and Npt2a protein in the membrane fractions (Fig. 1B) is significantly lower the NHERF-1 deficient OK-H cell compared to OK cells. To determine whether the decrease in Npt2a steady-state mRNA levels that we observed in the OK-H cells is due to a decrease in mRNA stability, we compared Npt2a mRNA half-life between OK and OK-H cells. As shown in Fig. 2A, the Npt2a mRNA degradation rate is the same in OK and OK-H cells, indicating that NHERF-1 doesn’t contribute to Npt2a mRNA stability. Of note, Npt2a mRNA half-life in OK cells was the same in the presence of either the protein synthesis inhibitor cycloheximide or transcription inhibitor actinomycin D, or combined cycloheximide plus actinomycin D, indicating there is a requirement for synthesis of a protein factor that enhances transcription.
Fig. 1.
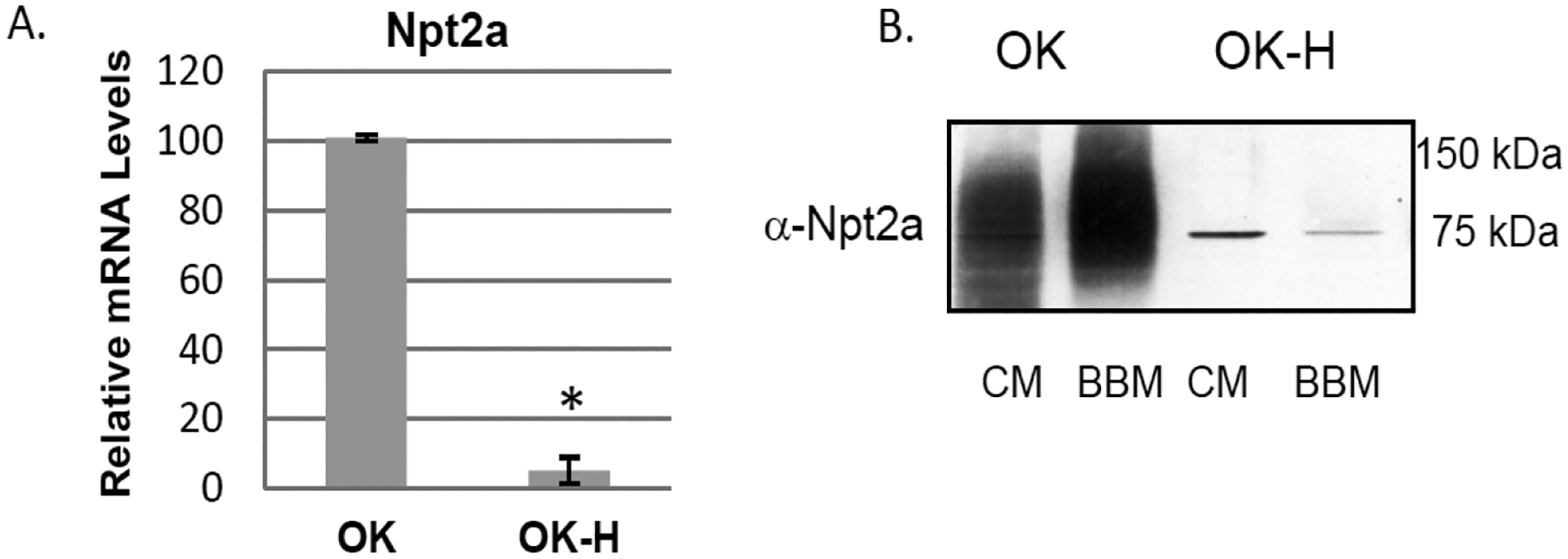
Npt2a expression in OK-H cells. (A) Total RNA was isolated from OK and OK-H cells that were grown to 80% confluency under standard conditions. cDNA was generated for each cell type and qPCR was performed in triplicate and mRNA levels in OK-H cells were expressed relative to levels in OK cells for each experiment. A single experiment represents cells grown from independent cell stocks and shown are the average ± SE values from 3 independent experiments. (B) Western blot analysis of Npt2a protein expression in OK and OK-H cells. Crude membrane (CM) pellets and brush border membranes (BBM) were isolated from the cells as described in the Methods and Npt2a protein detected by standard immunoblot protocol following established procedures [20]. Shown is a blot representative of previously published findings [5].
Fig. 2.
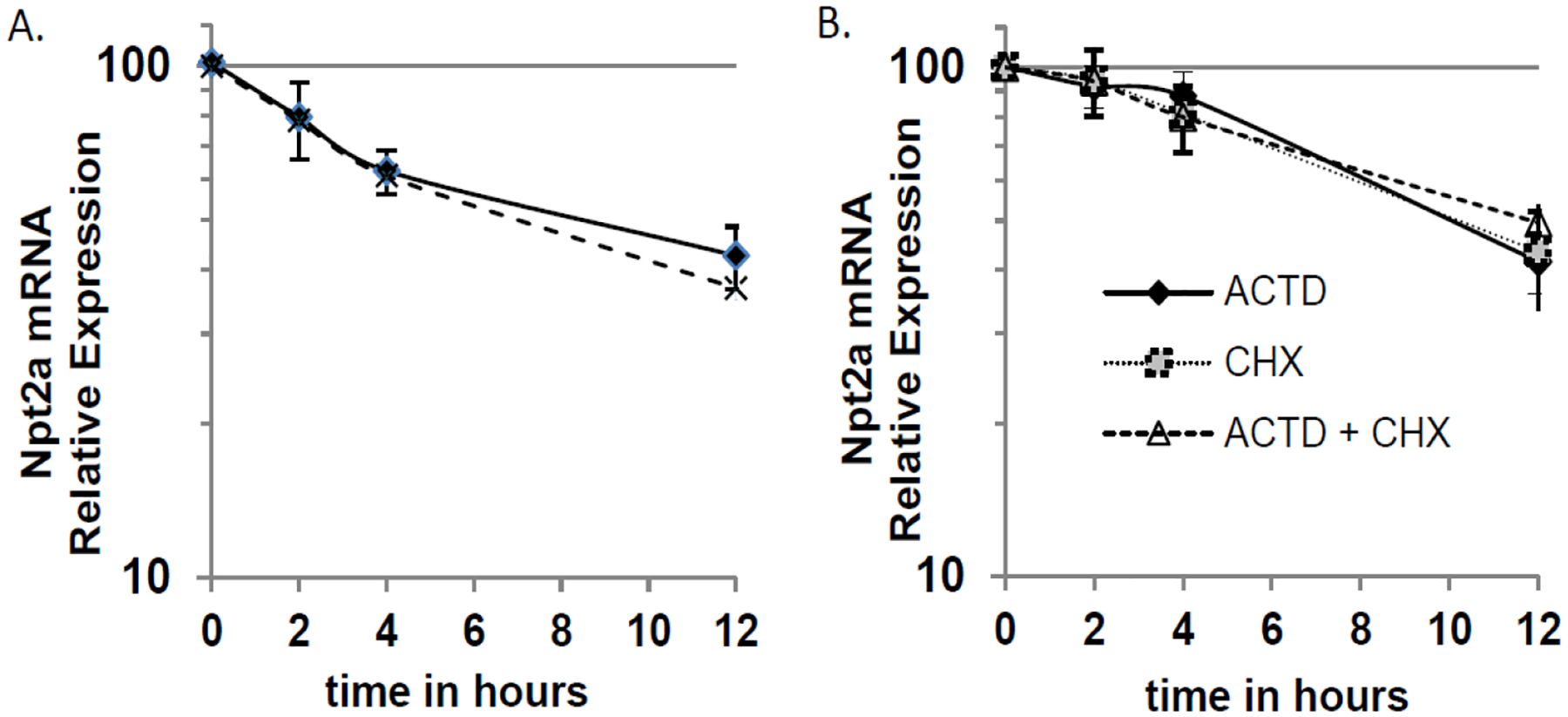
Npt2a mRNA half-life in OK and OK-H Cells. (A) Cells were treated with actinomycin D (ACTD) to inhibit transcription. At the indicated times (0, 2 h, 4 h, and 12 h) total RNA was isolated from the cells (three wells per time point) and relative Npt2a mRNA levels were determined by RT-qPCR as described in the Methods. Relative expression was calculated for each experiment and cell line with time 0 set to 100. The average ± SE values for OK (solid line) and OK-H cells (dashed line) from 3 independent experiments are shown on a semi logarithmic scale. Each independent experiment begins with different cell culture stocks. (B) OK cells were treated with either ACTD (filled diamond, solid line), cycloheximide (CHX) (grey square, dotted line), or ACTD+CHX (open triangle, dashed line) and total RNA was isolated at 0, 2 h, 4 h, and 12 h and relative Npt2a mRNA levels were determined as described for panel A.
Low phosphate treatment does not affect Npt2a mRNA levels in OK or OK-H cells
Npt2a protein expression is not responsive to low phosphate treatment in the NHERF-1 deficient OK-H cells [19] but the effect of loss of NHERF-1 on low phosphate-mediated changes in Npt2 mRNA levels is not known. Therefore, OK or OK-H NHERF-1-deficient cells were treated with low phosphate medium for 24 hours and total RNA was isolated and Npt2a mRNA levels measured by RT-qPCR. As shown in Fig. 3, there was no significant difference in steady-state Npt2a mRNA levels in either OK (Fig. 3A) or OK-H cells (Fig. 3B) after low phosphate treatment. Npt2a steady-state mRNA levels were reduced in the presence of actinomycin D or cycloheximide as expected and low phosphate conditions had no further effect (Fig. 3). These data support previous reports that showed low phosphate doesn’t change Npt2a steady-state mRNA levels in OK cells [6, 23]. Furthermore, these data show for the first time that Npt2a mRNA expression in NHERF-1 deficient cells is also not under low phosphate regulation, suggesting that any potential transcriptional effects for NHERF-1 on Npt2a gene expression is likely not part of the phosphate control of Npt2a protein expression.
Fig. 3.
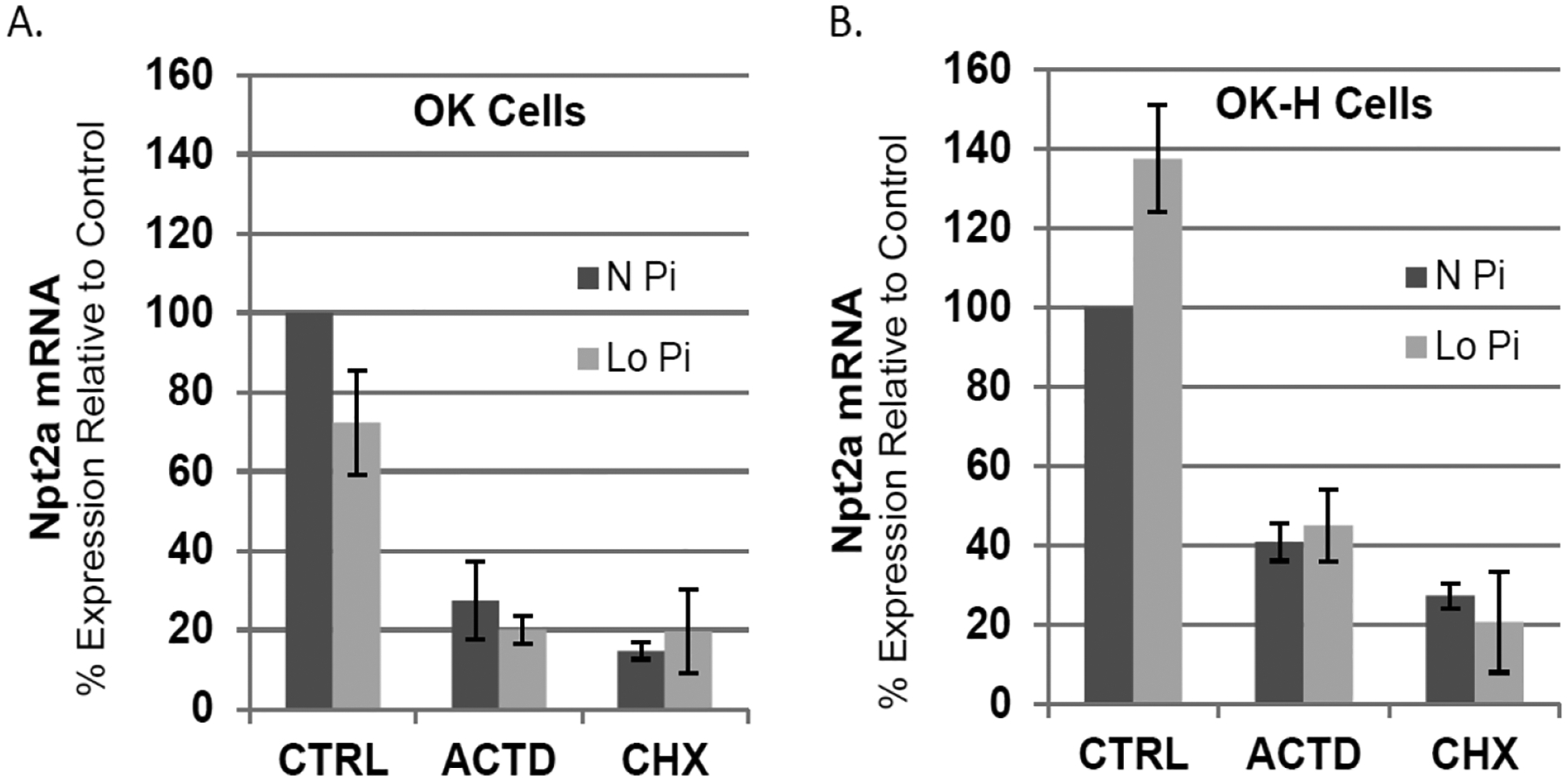
Effect of NHERF-1 on Npt2a steady-state mRNA levels in response to low phosphate. Cells were treated in triplicate with either vehicle control (Ctrl), actinomycin D (ACTD) or cycloheximide (CHX) in Normal phosphate (N Pi, 1.0 mM) or low phosphate (Lo Pi, 0.1 mM) DMEM for 24 hours and total RNA was isolated and Npt2a mRNA levels measured by RT-qPCR as described in the Methods. Relative mRNA expression was calculated for each experiment with control normal phosphate treatment group set to 100. Shown are the average ± SE values from 3 independent experiments that used different cell culture stocks.
Npt2a promoter activity in NHERF-1 deficient cells
Using promoter deletion analysis, the NHERF-1 responsive region of opossum Npt2a (oNpt2a) promoter was examined. OK-WT and OK-H cells were transiently transfected with pGL3 luciferase reporter gene constructs containing either 4.7 kb (p4700), 208 bp (p208), or 121 bp (p121) of the 5’-flanking region of opossum Npt2a gene (oNpt2a). As expected, oNpt2 p4700 and p208 reporter activity was significantly lower in OK-H than OK cells (Fig. 4). The oNpt2a promoter activity in OK cells is significantly decreased (78%) by deletion of the region between −4.7 kb and −208 bp upstream of the transcription start site. The p121 construct has no activity above pGL3 basic indicating no active transcription (Fig. 4). The loss of promoter responsiveness (~ 30%) in the OK-H cells for p4700 and p208 are similar (inset, Fig. 4), indicating that the NHERF-1-dependent region within oNpt2a promoter is between 208 bp – 121 bp of the transcription start site.
Fig. 4.
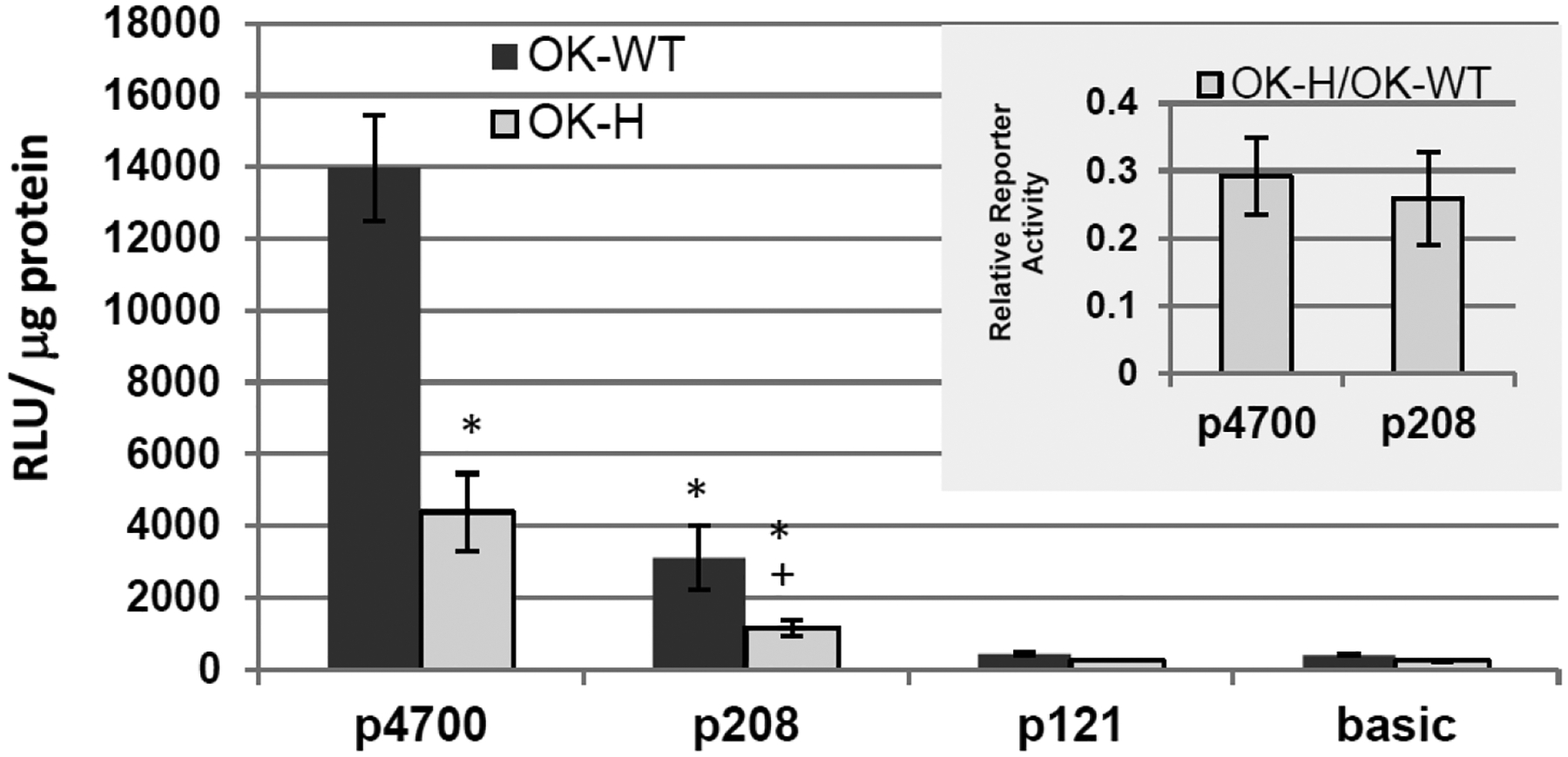
oNpt2a promoter activity in NHERF-1 deficient OK-H cells. Luciferase -reporter gene assays were performed in OK opossum kidney cells (OK-WT) and the NHERF-1-deficient OK-H cells. The cells were transiently transfected with pGL3 luciferase reporter gene constructs containing either 4.7 kb (p4700), 208 bp (p208), or 121 bp (p121) of the 5’-flanking region of opossum Npt2a gene [6]. The pGL3 basic reporter construct was used as negative controls. 24 hours post-transfection cell lysates were collected and luciferase activity determined and normalized to protein concentration. Triplicate transfections were performed for each experiment and the average RLU/μg protein values determined. A single experiment represents cells grown from independent cell stocks and shown are the mean RLU/μg protein values ± SE from 3 independent experiments. A confirmatory experiment using β-galactosidase as a transfection control was performed for normalization and the results were consistent with the data shown. The ratio of OK-H/ OK-WT RLU/μg protein values was determined for each construct to assess the activity in OK-H cells relative to the OK-WT cells. Shown in the inset are the mean relative values ± SEM from the 3 independent experiments. One-way ANOVA followed by Tukey’s post-hoc test was used to determine significant difference in activity. *, p < 0.001 compared to p4700 WT; +, p <0.01 compared to p208 WT.
Protein-DNA interactions at the Npt2a proximal promoter
As stated earlier, the Npt2a promoter has a highly conserved region within −200 bp of the transcription start site that include a non-consensus CAATT enhancer binding protein element and G-rich region for Sp1 binding (Fig. 5A). We used electrophoretic mobility shift assays (EMSA) to characterize protein-DNA interactions at these two conserved sites. Three specific protein-DNA complexes (I-III) formed with the Sp1 element as defined by cold competition with the Sp1 probe (Fig. 5B & 5C). Six protein-DNA complexes were resolved for the CAATT element and NE from OK-WT cells (Fig. 5B). Cold competition with the CAATT probe abolished complexes I-IV, indicating complexes I-IV represent specific protein-DNA interactions (Fig. 5D). Importantly, CAATT complex II was not observed using NE from NHERF-1-deficient OK-H cells (Fig. 5B & 5D). This protein-DNA interaction, however, was reestablished by re-expression of NHERF-1 in the OK-H cells (NF lanes, Fig. 5B & 5D).
Fig. 5.
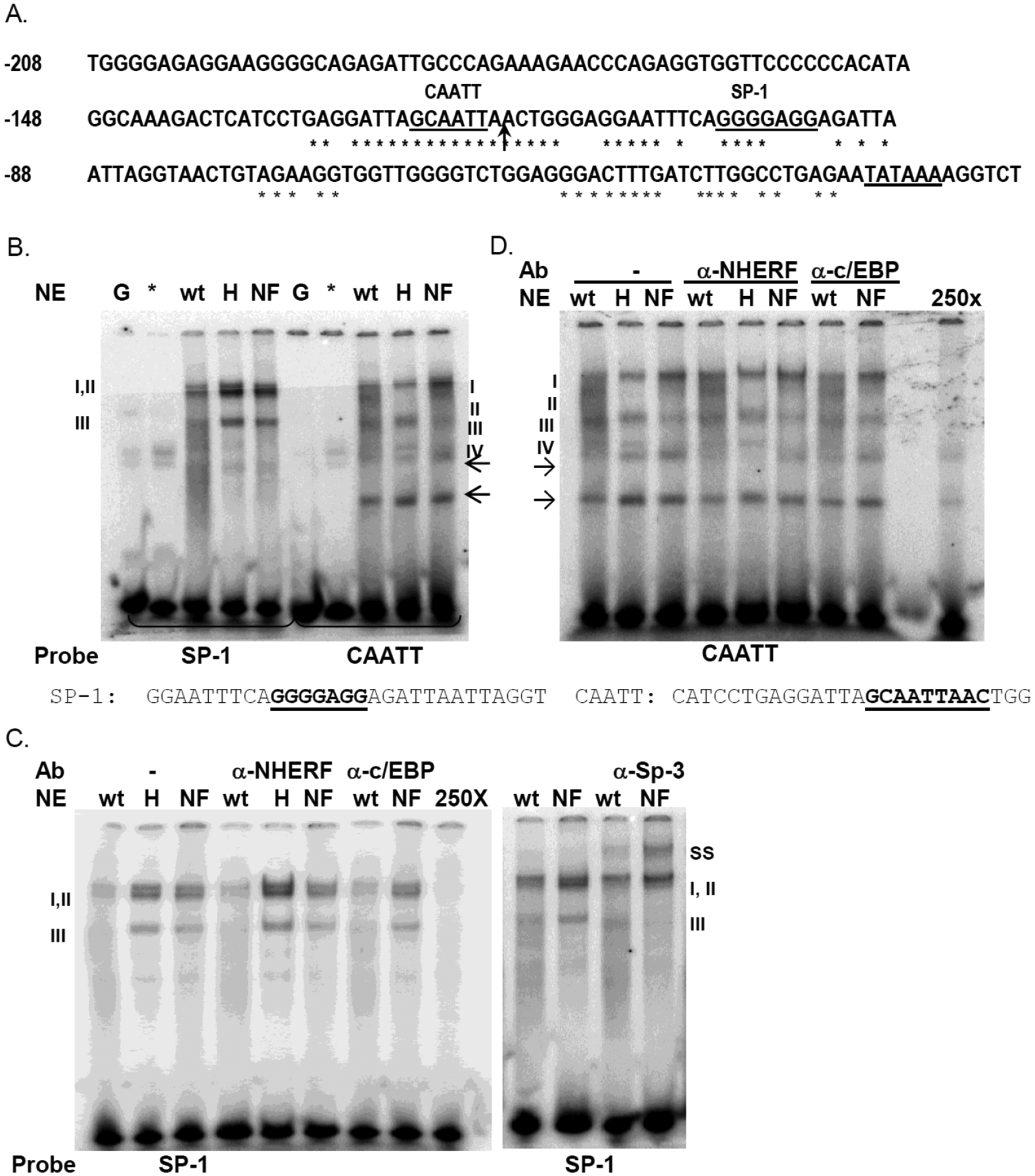
Protein-DNA interactions within the oNpt2a proximal promoter. (A) Shown is the −208 bp region of the 5’-flanking sequence of the opossum Npt2a gene [6]. The asterisks indicate the nucleotides that are 100% conserved between the opossum, mouse, and human Npt2a promoters. The TATA box transcription start site and potential binding elements for Sp1 and C/EBPβ are indicated (bold lettering and underlined). The arrow shows the position of −121 bp. (B-D) EMSA detection of protein-DNA interactions with the Sp1 and CAATT region of oNpt2a proximal promoter. B) Purified recombinant GST-NHERF-1 (*) and GST alone (G) were also tested for DNA binding. Nuclear extracts (NE) isolated from OK-WT (wt), OK-H (H), and OK-H - NHERF-1 stable transformant (NF) were incubated with radiolabeled DNA probes Sp1 or CAATT as indicated. Protein- DNA complexes were resolved by native electrophoresis and detected by autoradiography. Shown are representative images from 3 independent binding experiments using 2 separate NE isolations. (B) Specific complexes were identified by cold competition (see panel D) or antibody supershift (ss) (see panel C) and are indicated as I – II for Sp1 and I-IV for CAATT. Arrows indicate potential non-specific complexes. The sequences of the DNA probes are shown below the gel in panel B. (C) Protein interactions with the Sp1 probe. Binding reactions contained antibodies for either NHERF-1, C/EBPβ, or Sp3 as indicated. 250X, cold competition with 250-fold molar excess of Sp1 probe. ss, supershift. D) Protein interactions with the C/EBP probe. Binding reactions contained antibodies for either NHERF-1 or C/EBPβ as indicated. 250X, cold competition with 250-fold molar excess of C/EBP probe.
To determine if NHERF-1 directly binds to the CAATT or G-rich regions, the Sp1 and CAATT DNA probes were incubated with recombinant, purified GST-NHERF-1. As shown in Fig. 5B, GST-NHERF-1 did not bind either probe. To determine whether NHERF-1 contributed to the protein-DNA complexes detected with the Sp1 and CAATT probes, NHERF-1 antibodies were added to the binding reactions. As shown in Fig. 5C and 5D, NHERF-1 antibody did not alter the protein-DNA complex formation, suggesting that NHERF-1 does not participate directly in establishing oNpt2a promoter-protein interactions within these regions tested.
To further characterize the protein-DNA complexes formed with the Sp1 and CAATT regions of oNpt2a promoter we tested for Sp1 and C/EBPβ binding. Addition of Sp1 or C/EBPβ antibodies had no effect on complex formation with the Sp1 probe (data not shown) or C/EBPβ probe (Fig. 5D), indicating these factors do not contribute to protein-DNA interactions at these sites. However, addition of Sp3 antibody to the binding reactions resulted in a supershift of Sp1 complexes II and III (ss), demonstrating binding of Sp3 to this site (Fig. 5C). Together these data suggest that NHERF-1 indirectly regulates Npt2a transcription, possibly through regulating expression or modification of a transcription factor required for complex II formation at the highly conserved CAATT element of the oNpt2a promoter.
Discussion
This study addressed the potential for a novel role for NHERF-1 in directly contributing to Npt2a transcriptional regulation. We previously reported that NHERF-1 was detected by immunoblot analysis in the nuclear pellet of OK cells and loss of NHERF-1 expression in the OK-H cell line resulted in decreased Npt2a mRNA expression and promoter activity [5], herein). Importantly, Npt2a mRNA levels and promoter activity were partially restored by transfection of the OK-H cells with full-length NHERF-1 [5]. The goal of this study was to determine whether the loss of Npt2a mRNA in NHERF1 deficient OK-H cells may be the result of decreased transcription, increased mRNA degradation, or both. We now show for the first time that the Npt2a mRNA half-life is the same in either the presence (OK) or absence (OK-H) of NHERF-1, indicating mRNA degradation rates are not affected by NHERF-1. Therefore, the loss of Npt2a mRNA in the NHERF-1 deficient OK-H cells is likely due to loss of basal transcription. Using 5’-promoter deletion analysis we localized the NHERF-1 responsive region to within −208bp of the transcription start site. This region is sufficient to drive renal-specific expression of Npt2a and contains the CAATT and G-rich elements that are highly conserved between human, rat, mouse, and opossum Npt2a genes [6–9]. Therefore, constitutive expression of Npt2a is likely controlled via factors binding within 208 bp of the transcription start site. Our EMSA data demonstrate that NHERF-1 does not bind directly to either the CAATT or G-rich (Sp1) element in the proximal promoter of Npt2a. NHERF-1 is also not part of a protein complex binding to these elements as determined by lack of change in complex formation in the presence of NHERF-1 antibody. However, a protein-DNA complex at the highly conserved CAATT element (complex II) in the proximal promoter region of oNpt2a is dependent upon the expression of NHERF-1. This is the first report to demonstrate that NHERF-1 doesn’t bind the proximal promoter of Npt2a yet NHERF-1 expression is required for formation of protein-DNA interactions at the CAATT box that are critical for basal Npt2a transcription. Inhibition of protein synthesis with cycloheximide had the same effect on Npt2a steady-state mRNA levels as inhibition of transcription with actinomycin D, independent of NHERF-1. These data suggest ongoing synthesis of a factor in addition to NHERF-1 is required for Npt2a basal transcription.
We have also identified Sp3 as the factor that binds to the conserved G-rich element adjacent to the CAATT box. Sp3 belongs to the specificity protein/ Krüppel-like (Sp/KLF) transcription factor family that binds to G-rich elements. Sp1 and Sp3 bind to the consensus sequence 5’-G/T-GGGCGG-G/A-G/A-C/T-3’, with similar affinity. These factors are ubiquitously expressed and which Sp factor binds the DNA element must be empirically determined, although the number of DNA elements within a promoter and level of Sp protein expression contributes to target gene selection. Sp1 is the best characterized factor and was originally described to be important in maintaining constitutive transcription of housekeeping genes that lack a TATA-box, although the role for Sp1 has been expanded and can activate target gene expression in a regulated manner. Sp1 recruits coactivator proteins that promote chromatin remodeling to help establish an open chromatin state associated with active transcription. Sp3 functions as both a transcriptional activator and repressor depending on the gene, cellular signals, and splice variant expressed (reviewed in [24, 25]. Sp3 has been reported to be a transcriptional activator of rat NHE3 [26] and human NHE8 genes [27, 28]. For Npt2a, Sp3 binding is not sufficient to drive basal promoter activity based on our data that show the p121 Npt2a promoter construct retains the Sp3 binding element yet is not transcriptionally active in OK cells. We have shown that Sp3 binds to the Npt2a promoter independent of NHERF-1 expression, and it remains to be determined whether Sp3 functions with the NHERF-1-dependent factor to drive basal promoter activity.
Dietary phosphate is a key physiological regulator that controls Npt2a protein expression yet the contribution of transcription for regulated Npt2a mRNA expression is not clear. Indeed, the literature has conflicting data on the impact of low phosphate on Npt2a steady-state mRNA and transcriptional responses [6, 7, 23, 29–31]. Our data are consistent with previous reports that showed low phosphate diet didn’t change Npt2a steady-state mRNA levels in two rodent models or in low phosphate adapted OK cells [6, 23, 29, 30]. However, early work showed low phosphate diets increased steady-state Npt2a mRNA levels in rodent kidney [7, 29, 31]. In rat models the low phosphate diet-induced increase in steady-state mRNA levels was shown to be due to increased Npt2a mRNA stability with no change in Npt2a transcription rates [29]. ICR mice had increased Npt2a steady-state mRNA levels and increased transcription rates after 4 days on a low phosphate diet [7]. In contrast, C57Bl/6 mice on a low phosphate diet for 8 days had no change in in renal Npt2a steady-state mRNA levels [30]. Studies on opossum Npt2a reporter gene expression in OK cells showed that low phosphate media did not affect mRNA levels or promoter activity, supporting the idea that Npt2a transcription is not regulated by changes in phosphate levels [6, 23]. It is likely that species differences (mouse vs. rat) and cell culture conditions contribute to the reported discrepancies in the phosphate-dependent transcriptional regulation of Npt2a. Therefore, using the OK cell model we addressed whether loss of NHERF-1 would alter Npt2a response to low phosphate at the mRNA level. Our data show low phosphate treatment of OK cells had no significant effect on Npt2a steady-state mRNA levels, consistent with early reports for this cell line [6, 23]. These data also support a post-transcriptional mechanism(s) for the increase in Npt2a protein expression and BBM localization in response to low phosphate treatment [5, 23]. The OK-H cells that lack NHERF-1 also did not have a significant change in Npt2a mRNA levels in response to low phosphate treatment, indicating the NHERF-1 requirement for Npt2a mRNA expression is independent of phosphate regulation. Furthermore, inhibition of either general transcription with actinomycin D or general protein synthesis with cycloheximide has similar effects on reducing steady-state Npt2a mRNA levels independent of the NHERF-1 or phosphate levels. We propose that the ongoing synthesis of a factor, in addition to NHERF-1, is required for Npt2a basal transcription and that changes in phosphate levels do not affect this pathway.
A role for NHERF-1 in maintaining basal transcription of Npt2a may represent a regulatory mechanism, one independent of the other well-known physiological regulators of Npt2a protein expression such as dietary phosphate and PTH. In this scenario, the presence of nuclear NHERF-1 signals for organization of a transcriptional complex on Npt2a promoter that maintains steady-state or basal transcription rates to maintain steady-state mRNA levels. The physiological significance of maintaining a steady-state level of Npt2a mRNA is to maintain ongoing steady-state level of Npt2a protein synthesis and membrane insertion. Post-transcriptional mechanisms control both PTH- and low phosphate-mediated changes in Npt2a protein levels. We have shown that PTH treatment decreases the stability of Npt2a mRNA, perhaps as mechanism to help control the level of protein. PTH stimulation also results in Npt2a protein removal from the membrane and degradation. Thus recovery of Npt2a protein in the apical membrane requires new synthesis. Maintaining a basal rate of transcription that is not under PTH control would be important for this recovery. Increased Npt2a protein in the apical membrane under low phosphate conditions in the absence of increased transcription also highlights the importance for maintaining basal transcription rates to assure a sufficient mRNA pool for this recovery. In OK-H cells the loss of NHERF-1 results in intracellular Npt2a protein accumulation and decreased Npt2a mRNA levels. Thus, the loss of Npt2a transcription under conditions where the protein is not properly localized in the membrane may represent a cellular adaptation to the trafficking defect. In this sense, NHERF-1 could serve to coordinate cytoplasmic and nuclear control of Npt2a expression.
In summary, our data provide the first evidence for NHERF-1 requirement to help maintain steady-state Npt2a mRNA levels. We propose the presence of NHERF-1 promotes formation of a protein complex at the CAATT element on the Npt2a proximal promoter but does not directly interact with the complex. Thus the mechanism by which NHERF-1 affects Npt2a transcription is indirect and requires ongoing transcription and translation of a factor that contributes to protein-DNA interactions at the Npt2a promoter. Future studies are necessary to identify the proteins that are part of the NHERF-1-dependent complex to assess the molecular mechanisms that underpin NHERF-1 role in Npt2a constitutive transcription.
Acknowledgments
We thank Caryl Conklin and Lauren Grant for expert technical assistance and Dr. Carolyn M. Klinge, Dept. of Biochemistry and Molecular Genetics, Univ. of Louisville, for critical review of the manuscript. Work is supported by a grant from the VA Merit Review Board to EDL. “The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs or the United States government.”
Footnotes
Disclosure Statement
The Authors have nothing to disclose.
References
- 1.Cunningham R, Steplock D, Wang F, Huang H, X E, Shenolikar S, Weinman EJ: Defective Parathyroid Hormone Regulation of NHE3 Activity and Phosphate Adaptation in Cultured NHERF-1−/− Renal Proximal Tubule Cells. J Biol Chem 2004;279:37815–37821. [DOI] [PubMed] [Google Scholar]
- 2.Ketchem CJ, Khundmiri SJ, Gaweda AE, Murray R, Clark BJ, Weinman EJ, Lederer ED: Role of the sodium hydrogen exchanger regulatory factor 1 (NHERF1) in forward trafficking of the type IIa sodium phosphate cotransporter (NpT2a). Am J Physiol Renal Physiol 2015;309:F109–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mahon MJ, Cole JA, Lederer ED, Segre GV: Na+/H+ exchanger-regulatory factor 1 mediates inhibition of phosphate transport by parathyroid hormone and second messengers by acting at multiple sites in opossum kidney cells. Mol Endocrinol 2003;17:2355–2364. [DOI] [PubMed] [Google Scholar]
- 4.Cunningham R, Steplock D, X E, Biswas RS, Wang F, Shenolikar S, Weinman EJ: Adenoviral expression of NHERF-1 in NHERF-1 null mouse renal proximal tubule cells restores Npt2a regulation by low phosphate media and parathyroid hormone. Am J Physiol Renal Physiol 2006;291:F896–F901. [DOI] [PubMed] [Google Scholar]
- 5.Khundmiri SJ, Ahmad A, Bennett RE, Weinman EJ, Steplock D, Cole J, Baumann PD, Lewis J, Singh S, Clark BJ, Lederer ED: Novel regulatory function for NHERF-1 in Npt2a transcription. Am J Physiol Renal Physiol 2008;294:F840–849. [DOI] [PubMed] [Google Scholar]
- 6.Hilfiker H, Hartmann CM, Stange G, Murer H: Characterization of the 5’-flanking region of OK cell type II Na-Pi cotransporter gene. Am J Physiol 1998;274:F197–204. [DOI] [PubMed] [Google Scholar]
- 7.Kido S, Miyamoto K, Mizobuchi H, Taketani Y, Ohkido I, Ogawa N, Kaneko Y, Harashima S, Takeda E: Identification of regulatory sequences and binding proteins in the type II sodium/phosphate cotransporter NPT2 gene responsive to dietary phosphate. J Biol Chem 1999;274:28256–28263. [DOI] [PubMed] [Google Scholar]
- 8.Shachaf C, Skorecki KL, Tzukerman M: Role of AP2 consensus sites in regulation of rat Npt2 (sodium-phosphate cotransporter) promoter. Am J Physiol Renal Physiol 2000;278:F406–416. [DOI] [PubMed] [Google Scholar]
- 9.Taketani Y, Miyamoto K, Tanaka K, Katai K, Chikamori M, Tatsumi S, Segawa H, Yamamoto H, Morita K, Takeda E: Gene structure and functional analysis of the human Na+/phosphate co-transporter. Biochem J 1997;324:927–934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hilfiker H, Kvietikova I, Hartmann CM, Stange G, Murer H: Characterization of the human type II Na/Pi-cotransporter promoter. Pflugers Arch 1998;436:591–598. [DOI] [PubMed] [Google Scholar]
- 11.Rosenberg T, Shachaf C, Tzukerman M, Skorecki K: A murine transgenic model for transcriptional regulation of the Na/Pi-IIa major renal phosphate cotransporter. Am J Physiol Renal Physiol 2007;292:F1617–1625. [DOI] [PubMed] [Google Scholar]
- 12.Hartmann CM, Hewson AS, Kos CH, Hilfiker H, Soumounou Y, Murer H, Tenenhouse HS: Structure of murine and human renal type II Na+-phosphate cotransporter genes (Npt2 and NPT2). Proc Natl Acad Sci USA 1996;93:7409–7414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Taketani Y, Segawa H, Chikamori M, Morita K, Tanaka K, Kido S, Yamamoto H, Iemori Y, Tatsumi S, Tsugawa N, Okano T, Kobayashi T, Miyamoto K, Takeda E: Regulation of type II renal Na+-dependent inorganic phosphate transporters by 1,25-dihydroxyvitamin D3. Identification of a vitamin D-responsive element in the human NAPi-3 gene. J Biol Chem 1998;273:14575–14581. [DOI] [PubMed] [Google Scholar]
- 14.Lederer ED, Khundmiri SJ, Weinman EJ: Role of NHERF-1 in regulation of the activity of Na-K ATPase and sodium-phosphate co-transport in epithelial cells. J Am Soc Nephrol 2003;14:1711–1719. [DOI] [PubMed] [Google Scholar]
- 15.Murray RD, Holthouser K, Clark BJ, Salyer SA, Barati MT, Khundmiri SJ, Lederer ED: Parathyroid hormone (PTH) decreases sodium-phosphate cotransporter type IIa (NpT2a) mRNA stability. Am J Physiol Renal Physiol 2013;304:F1076–1085. [DOI] [PubMed] [Google Scholar]
- 16.Koyama H, Goodpasture C, Miller MM, Teplitz RL, Riggs AD: Establishment and characterization of a cell line from the american opossum (Didelphys virginiana). In Vitro 1978;14:239–246. [DOI] [PubMed] [Google Scholar]
- 17.Miyauchi A, Dobre V, Rickmeye r M, Cole J, Forte L, Hruska KA: Stimulation of transient elevations in cytosolic Ca2+ is related to inhibition of Pi transport in OK cells. Am J Physiol 1990;259:F485–493. [DOI] [PubMed] [Google Scholar]
- 18.Mahon MJ, Segre GV: Stimulation by Parathyroid Hormone of a NHERF-1-assembled Complex Consisting of the Parathyroid Hormone I Receptor, Phospholipase Cβ, and Actin Increases Intracellular Calcium in Opossum Kidney Cells. J Biol Chem 2004;279:23550–23558. [DOI] [PubMed] [Google Scholar]
- 19.Khundmiri SJ, Ameen M, Delamere NA, Lederer ED: PTH-mediated regulation of Na+-K+-ATPase requires Src kinase-dependent ERK phosphorylation. Am J Physiol Renal Physiol 2008;295:F426–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khundmiri SJ, Rane MJ, Lederer ED: Parathyroid hormone regulation of type II sodium-phosphate cotransporters is dependent on an A kinase anchoring protein. J Biol Chem 2003;278:10134–10141. [DOI] [PubMed] [Google Scholar]
- 21.Meier RK, Clark BJ: Angiotensin II-dependent transcriptional activation of human steroidogenic acute regulatory protein gene by a 25-kDa cAMP-responsive element modulator protein isoform and Yin Yang 1. Endocrinology 2012;153:1256–1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wooton-Kee CR, Clark BJ: Steroidogenic factor-1 influences protein-deoxyribonucleic acid interactions within the cyclic adenosine 3,5-monophosphate-responsive regions of the murine steroidogenic acute regulatory protein gene. Endocrinology 2000;141:1345–1355. [DOI] [PubMed] [Google Scholar]
- 23.Pfister MF, Hilfiker H, Forgo J, Lederer E, Biber J, Murer H: Cellular mechanisms involved in the acute adaptation of OK cell Na/Pi-cotransport to high- or low-Pi medium. Pflugers Arch 1998;435:713–719. [DOI] [PubMed] [Google Scholar]
- 24.Li L, Davie JR: The role of Sp1 and Sp3 in normal and cancer cell biology. Ann Anat 2010;192:275–283. [DOI] [PubMed] [Google Scholar]
- 25.Wierstra I: Sp1: Emerging roles—Beyond constitutive activation of TATA-less housekeeping genes. Biochem Biophys Res Commun 2008;372:1–13. [DOI] [PubMed] [Google Scholar]
- 26.Amin MR, Malakooti J, Sandoval R, Dudeja PK, Ramaswamy K: IFN-γ and TNF-α regulate human NHE3 gene expression by modulating the Sp family transcription factors in human intestinal epithelial cell line C2BBe1. Am J Physiol Cell Physiol 2006;291:C887–C896. [DOI] [PubMed] [Google Scholar]
- 27.Xu H, Chen H, Dong J, Li J, Chen R, Uno JK, Ghishan FK: Tumor necrosis factor-{alpha} downregulates intestinal NHE8 expression by reducing basal promoter activity. Am J Physiol Cell Physiol 2009;296:C489–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Xu H, McCoy A, Li J, Zhao Y, Ghishan FK: Sodium butyrate stimulates NHE8 expression via its role on activating NHE8 basal promoter activity. Am J Physiol Gastrointest Liver Physiol 2015;309:G500–505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Moz Y, Silver J, Naveh-Many T: Protein-RNA interactions determine the stability of the renal NaPi-2 cotransporter mRNA and its translation in hypophosphatemic rats. J Biol Chem 1999;274:25266–25272. [DOI] [PubMed] [Google Scholar]
- 30.Tenenhouse HS, Roy S, Martel J, Gauthier C: Differential expression, abundance, and regulation of Na+-phosphate cotransporter genes in murine kidney. Am J Physiol 1998;275:F527–534. [DOI] [PubMed] [Google Scholar]
- 31.Werner A, Kempson SA, Biber J, Murer H: Increase of Na/Pi-cotransport encoding mRNA in response to low Pi diet in rat kidney cortex. J Biol Chem 1994;269:6637–6639. [PubMed] [Google Scholar]


