Abstract
The enveloped alphavirus Semliki Forest virus (SFV) infects cells via a low-pH-triggered membrane fusion reaction that requires cholesterol and sphingolipid in the target membrane. Cholesterol-depleted insect cells are highly resistant to alphavirus infection and were used to select srf-3, an SFV mutant that is ∼100-fold less cholesterol dependent for infection due to a single amino acid change in the E1 spike subunit, proline 226 to serine. Sensitive lipid-mixing assays here demonstrated that the in vitro fusion of srf-3 and wild-type (wt) virus with cholesterol-containing liposomes had comparable kinetics, activation energies, and sphingolipid dependence. In contrast, srf-3 fusion with sterol-free liposomes was significantly more efficient than that of wt virus. Thus, the srf-3 mutation does not affect its general fusion properties with purified lipid bilayers but causes a marked and specific reduction in cholesterol dependence. Upon exposure to low pH, the E1 spike subunit undergoes distinct conformational changes, resulting in the exposure of an acid conformation-specific epitope and formation of an E1 homotrimer. These conformational changes were strongly cholesterol and sphingolipid dependent for wt SFV and strikingly less cholesterol dependent for srf-3. Our results thus demonstrate the functional importance of fusogenic E1 conformational changes in the control of SFV cholesterol dependence.
In spite of long-standing interest in the role of cholesterol, its functions in eukaryotic cells are not well understood. Cholesterol is essential for mammalian cells and is believed to be required as both a bulk component of cell membranes and a specific modulator of membrane protein function (reviewed in references 40 and 57). Increased evidence for the importance of cholesterol has come from recent studies demonstrating that cholesterol is a covalent adduct critical for the function of embryonic signalling molecules (46) and that cholesterol is involved in the formation and function of caveolae and clathrin-coated vesicles, key membrane specializations in mammalian cells (5, 47, 52). A well-defined example of a membrane protein with a specific functional requirement for cholesterol is the Semliki Forest virus (SFV) spike glycoprotein, which requires cholesterol to mediate the fusion of the virus membrane with a target membrane. Membrane fusion is the mechanism used by enveloped viruses to infect cells and also plays a key role in such important cellular processes as endocytosis, exocytosis, and cell-cell fusion (22). The SFV spike protein thus illustrates the potential importance of specific membrane lipids in the function of a fusion protein and represents a model for cholesterol-membrane protein interactions.
SFV is a member of the alphaviruses, enveloped RNA viruses that infect cells by using receptor-mediated endocytosis and low-pH-mediated fusion of the virus membrane with that of the endosome (reviewed in references 19, 25, 26, and 51). SFV fusion with liposomes has a striking requirement for two specific lipids in the liposome membrane, cholesterol and sphingolipid. Optimal fusion requires about one molecule of cholesterol per two molecules of phospholipid (33 mol%) (55) and is specific for sterols containing the 3β-hydroxyl group (29). In contrast, optimal fusion requires only ∼2 to 5 mol% sphingolipid, of which ceramide is the minimal sphingolipid structure that supports fusion (38, 42).
Low pH triggers a series of conformational changes in the SFV spike protein that lead to the fusion of the virus membrane with the target membrane (reviewed in references 1, 25, 26, and 28). The virus spike proteins are trimers (E1/E2/E3)3 of two type I transmembrane glycoproteins, E1 and E2 (each about 50 kDa), and a peripheral glycoprotein E3 (∼10 kDa). The initial event in fusion appears to be the disruption of the ight interaction between E2 and E1. The released E1 subunit then undergoes distinct acid-dependent conformational changes whose kinetics, biochemical properties, and sequence requirements correlate with the virus fusion reaction both in vitro and in vivo. Previously masked acid conformation-specific monoclonal antibody (MAb) binding sites on E1 are exposed, and E1 forms a homotrimer (E1)3 that is trypsin resistant and highly stable. During fusion, E1 associates with the target membrane, presumably via membrane insertion of a hydrophobic fusion peptide located between amino acids 79 and 97 of E1 (25, 32). A lag period is observed between the completion of these E1 conformational changes and E1 membrane insertion, and the initiation of membrane fusion, and it is believed to reflect additional, as yet uncharacterized steps in the fusion reaction (9).
While the cholesterol dependence of SFV fusion was first observed in fusion experiments with liposomes, it has also been demonstrated in vivo in studies of cholesterol-depleted C6/36 mosquito cells (36, 37, 45, 53). Sterol-depleted cells are about 5,000-fold more resistant to primary infection than are control cells and are also less efficient in the exit of newly synthesized virus. We used such depleted cells to select srf-3 (for “sterol requirement in function”), a mutant virus that grew more efficiently in the absence of cholesterol (37, 53). srf-3 is increased about 100-fold in its ability to infect cells without cholesterol and about 500-fold in its ability to fuse with the depleted cell plasma membrane, and it also shows more efficient exit from cells lacking cholesterol. Sequence analysis showed that the srf-3 mutation is a change of proline 226 to serine (P226S) within the E1 subunit. Studies of Sindbis virus (SIN), another member of the alphaviruses, demonstrated that SIN has comparable cholesterol requirements to those of wild-type (wt) SFV and that mutations in the E1 226 region could similarly increase SIN fusion and exit from sterol-depleted cells (35).
From these studies, it was clear that the P226S mutation significantly reduced the cholesterol dependence of SFV when assayed in the context of sterol-depleted insect cells. However, cholesterol depletion could also produce changes in membrane lipid composition, lipid distribution, or other properties of the cell membrane that could affect its fusion potential. Although the block in wt SFV fusion was reversed by addition of cholesterol to the depleted cells, reversal required overnight culture to redistribute the added cholesterol into the cell plasma membrane (37, 45). Thus, clearly the decrease in srf-3 cholesterol dependence observed in this complex biological system could differ from its fusion properties with a well-defined lipid bilayer. In addition, a key unresolved issue is the mechanism by which the P226S mutation affects virus cholesterol dependence. The mutation could act by making the virus a more efficient overall fusogen, thus increasing its fusion capacity on a number of target membranes including those without cholesterol. Alternatively, the mutation might affect only the spike protein cholesterol requirement, perhaps by affecting the acid-dependent conformational changes in E1. It is also possible that srf-3 has alterations in fusion steps downstream of the E1 conformational changes, such as steps occuring during the lag period.
In the present study, we have characterized the properties of the P226S mutation by using a defined liposome system. This in vitro system enabled us to sensitively measure the fusion properties of srf-3, to determine its overall dependence on both cholesterol and sphingolipid, and to study its E1 conformational changes. Our results demonstrate that while its overall fusion properties were very similar to those of wt SFV, srf-3 showed a significant and specific increase in fusion activity with sterol-deficient liposomes. This increased fusion was due to the increased cholesterol independence of srf-3 E1 conformational changes, including acid-specific epitope exposure and E1 homotrimerization.
MATERIALS AND METHODS
Cells and viruses.
BHK-21 cells were maintained as previously described (53) and used for virus propagation, plaque titer determination, and infectivity experiments. C6/36 mosquito cells were depleted of cholesterol by 4 to 15 passages in medium containing lipoprotein-depleted fetal calf serum, as previously described (53).
wt SFV was previously plaque purified from prototype wt virus obtained from the Department of Virology, University of Helsinki, and has been extensively characterized (20, 53). The srf-3 mutant was derived from this wt stock by successive passages on cholesterol-depleted C6/36 cells, as described in detail previously (53). Previous studies have demonstrated that srf-3 has a decreased requirement for cellular cholesterol for virus infection, fusion, and exit due to a single amino acid change of E1 proline 226 to serine (35–37, 53).
Preparation of pyrene and radiolabeled viruses.
Pyrene-labeled viruses (wt SFV and srf-3) were prepared using a slightly modified version of published procedures (9, 54). BHK-21 cells were cultured for 15 h in 850-cm2 roller bottles containing Dulbecco's modified Eagle's medium supplemented with 100 U of penicillin per ml, 100 μg of streptomycin per ml, 5% fetal calf serum, and 10% tryptose phosphate broth. Cellular phospholipids were labeled by growth for 24 h in the presence of C16-pyrene (Molecular Probes, Eugene, Oreg.) at a final concentration of 10 μg/ml. The cells were then infected with wt SFV or srf-3 at a multiplicity of 5 PFU/cell, and the infection was continued for 24 h in the above BHK growth medium without pyrene. The virus-containing medium was then harvested, the cell debris was removed by centrifugation, and the labeled virus was pelleted and resuspended in TN buffer (50 mM Tris, 100 mM NaCl [pH 7.4]). The virus was then purified by centrifugation on a discontinuous sucrose gradient (10 to 20%/25 to 50% sucrose [wt/wt] in TN buffer) (30). The virus band was harvested, the virus protein concentration was determined (34), and aliquots were snap-frozen in liquid nitrogen and stored at −80°C. The pyrene excimer-to-monomer ratio was determined for each virus preparation by exciting at 343 nm at 37°C and recording the emitted fluorescence at 480 (excimer) and 398 nm (monomer) on an SLM-8000 fluorometer, as described below. The excimer-to-monomer ratios were 0.38 and 0.37 for wt SFV and srf-3, respectively. These ratios are similar to those previously described for pyrene-labeled preparations of wt SFV (9).
[35S]methionine and [35S]cysteine-labeled wt or srf-3 virus was prepared by growth in BHK cells, or in cholesterol-depleted C6/36 cells (srf-3 only) (53). Labeled viruses were purified by banding on a discontinuous sucrose gradient as described above. Radiolabeled srf-3 prepared from cholesterol-depleted cells contained both intact virus and disrupted virus particles (53).
Liposomes.
Large unilamellar vesicles were prepared by freezing-thawing and extrusion using a published procedure (15) with some modifications. Lipid mixtures were washed with chloroform and t-butanol in a rotary evaporator, lyophilized for at least 90 min, hydrated in buffer, and vortexed with glass beads. The suspensions were subjected to 10 cycles of freezing-thawing and extruded 21 times through two stacked polycarbonate filters (pore size, 0.2 μm) using a Liposofast mini-extruder (Avestin, Ottawa, Canada). Lipids were hydrated in MES buffer (20 mM morpholine ethanesulfonic acid [MES] and 130 mM NaCl [pH 7.0] with or without 0.2% bovine serum albumin) for assays of E1 conformational changes or HNE buffer (5 mM HEPES, 150 mM NaCl, 0.1 mM EDTA [pH 7.0]) for fusion studies. Complete liposomes contained a 1:1:1:1.5 molar ratio of phosphatidylcholine (PC; from egg yolk), phosphatidylethanolamine (PE; prepared from egg PC by transphosphatidylation), sphingomyelin (Sph; from bovine brain), and cholesterol. Sterol-free liposomes had a PC/PE/Sph molar ratio of 1:1:1. Sphingolipid-free liposomes had a PC/PE/cholesterol molar ratio of 1:1:1. Liposomes without sphingolipid and sterol contained 1:1 PC/PE. All phospholipids were purchased from Avanti Polar Lipids (Alabaster, Ala.), and cholesterol was purchased from Steraloids Inc. (Wilton, N.H.). Phospholipid quantitation was performed as described previously (39).
Fusion assay.
Lipid mixing during SFV-liposome fusion was assayed by monitoring the decrease in virus pyrene excimer fluorescence (9, 54). Each assay mixture (2 ml) contained purified pyrene-labeled virus (0.6 μM phospholipid as calculated from a phospholipid/protein ratio of 0.45 μmol/μg) and 200 to 800 μM liposomes of the indicated lipid composition. The mixtures were stirred continuously in a thermostatted cuvette at the indicated temperature. Fusion was triggered by the addition of a pretitrated volume of 0.3 M MES (pH 4.8) to give a final pH of 5.5. Pyrene excimer fluorescence was measured in an SLM-8000 fluorometer upgraded to SLM-8100 software (SLM-Aminco, Urbana, Ill.), using excitation and emission wavelengths of 343 and 480 nm, respectively, in the presence of a 470-nm cutoff filter in the emission beam. The 0% fusion level was set at the initial excimer fluorescence, and the 100% fusion level was set at the background intensity of the target liposomes at the concentration used in the reaction.
Sensitivity of virus infection to inhibition by NH4Cl.
The sensitivity of infection by wt SFV and srf-3 to inhibition by NH4Cl was assayed as a marker of the pH dependence of virus fusion, as previously described (20). BHK cells in 24-well trays were preincubated for 15 min with the indicated concentration of NH4Cl and then infected for 90 min with virus at a multiplicity of 1 PFU/cell in the presence of the indicated concentrations of NH4Cl. Virus-specific RNA synthesis was quantitated by labeling for 3.5 h with [3H]uridine in the presence of 2 μg of actinomycin D per ml and 20 mM NH4Cl to prevent secondary infection.
Analysis of low pH-dependent conformational changes in virus spike proteins.
To assess conformational changes in the virus spike proteins, [35S]methionine- and [35S]cysteine-labeled virus was mixed with 800 μM liposomes of the appropriate composition, treated at the indicated pH, and returned to neutral pH. The exposure of acid conformation-specific epitopes was determined by immunoprecipitation with E1a-1, a previously characterized E1 acid conformation-specific MAb (27). Formation of the E1 homotrimer was evaluated by incubating samples in sodium dodecyl sulfate (SDS)-gel sample buffer for 3 min at 30°C and then analyzing them by SDS-polyacrylamide gel electrophoresis (PAGE) (28, 54). Quantitation of SDS-gel assays was performed by PhosphorImager analysis with Image Quant v.1.2 software (Molecular Dynamics, Sunnyvale, Calif.).
RESULTS
Fusion of wt SFV and srf-3 with cholesterol-containing membranes in vitro.
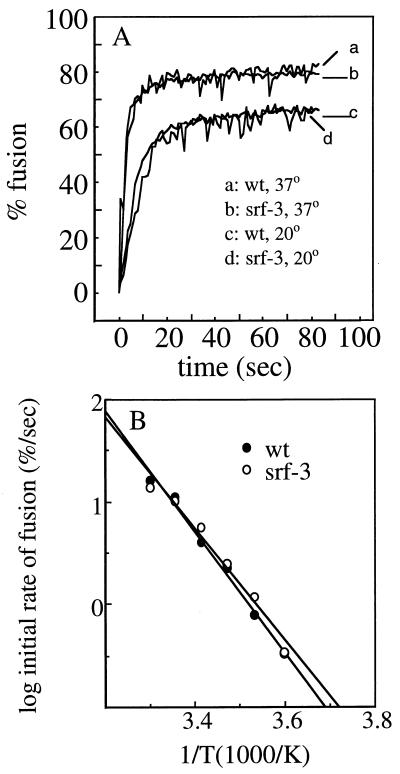
The low-pH-induced fusion of wt SFV with cell plasma membranes is highly cholesterol dependent, with fusion ∼4 to 5 logs lower on depleted cells than on control cells (35, 53). While srf-3 is increased about 1,000-fold in its ability to fuse with depleted cells, it still shows maximal fusion on cells containing cholesterol, with approximately a 100-fold difference between the two cell types (35, 53). To characterize srf-3 fusion in vitro with cholesterol-containing and -depleted membranes, we reasoned that we would need to use a highly sensitive fusion assay that might be capable of detecting srf-3 fusion with sterol-free membranes. We therefore used a previously described lipid-mixing assay based on the fusion of pyrene-labeled virus with unlabeled liposomes (9, 54). This assay is based on the biosynthetic labeling of cells with pyrene fatty acids, which are incorporated into phospholipids in the cell plasma membrane and acquired by the virus during budding. At high concentrations, pyrene displays a characteristic excimer peak at 480 nm, which is lost when the pyrene label is diluted during virus fusion. The loss of excimer fluorescence can be monitored in real time and is a sensitive fusion assay that shows little nonspecific exchange of the fluorescent label (9, 54). We prepared pyrene-labeled wt SFV and srf-3 and determined that both virus preparations had comparable ratios of pyrene excimer to monomer fluorescence, indicating comparable densities of pyrene fluorophore in the virus membranes. The fusion of these virus preparations was then characterized using “complete” liposomes (PC/PE/Sph/cholesterol, 1:1:1:1.5) that contain both cholesterol and sphingolipid and support efficient wt SFV fusion (9, 55). Fusion was initiated by adjusting the virus-liposome mixture to pH 5.5 and plotted as the percentage of maximal pyrene dilution. Real-time fluorescence recordings of fusion reactions with wt SFV and srf-3 at incubation temperatures of 37 and 20°C are shown (Fig. 1A). Both viruses fused rapidly and efficiently and showed comparable rates and extents of fusion at both 37 and 20°C. A series of fusion experiments were performed at temperatures ranging from 5 to 30°C, and the initial rates of fusion were plotted against the reciprocal of the absolute temperature in an Arrhenius diagram (Fig. 1B). As previously described, a slow rate of SFV fusion occurred even at temperatures as low as 5°C (9, 55). The Arrhenius diagrams of wt SFV and srf-3 were indistinguishable, with similar straight lines and slopes for both viruses. Thus, wt SFV and srf-3 have similar, constant activation energies for fusion throughout the temperature range. These results imply that the rate-limiting steps of the overall fusion process are comparable for wt SFV and srf-3, suggestive of similar fusion mechanisms with complete liposomes. We also quantitated the low-pH-dependent association of radiolabeled wt SFV and srf-3 with complete liposomes, using a gradient flotation assay that measures both virus binding and fusion (29). Virus-membrane association was comparable for wt SFV and srf-3 when assayed after a 5-min treatment at pH 5.5 and 37°C (data not shown). Thus, the overall properties of fusion with cholesterol-containing membranes are conserved between wt SFV and the srf-3 mutant.
FIG. 1.
Fusion of wt SFV and srf-3 with complete liposomes. (A) Real-time fluorescence recordings of the fusion of pyrene-labeled wt or srf-3 virus (0.6 μM) with unlabeled complete liposomes (200 μM). Samples were preequilibrated for 3 to 5 min at the indicated temperature in buffer at pH 7.0. At time zero, the mixture was adjusted to pH 5.5 (B) Arrhenius plot of the initial rate of fusion (the slope of the steepest part of the curve) for wt SFV and srf-3 at various temperatures. Each point is the average of two independent determinations.
Lipid dependence of wt SFV and srf-3 fusion.
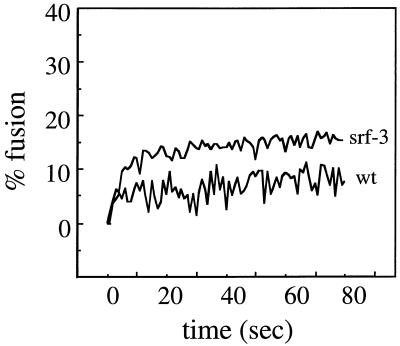
To compare the lipid dependence of wt SFV and srf-3 fusion, liposomes were prepared in the absence of either cholesterol (PC/PE/Sph, 1:1:1) or sphingolipid (PC/PE/cholesterol, 1:1:1). Since wt virus is rapidly acid inactivated at 37°C (9), fusion assays with deficient liposomes were performed at either 20 or 25°C to increase the potential time available for fusion with these suboptimal liposomes. As demonstrated previously (9) and above, fusion at these temperatures occurs by a similar mechanism to that at 37°C and mediates efficient virus infection. To maximize the possibility of virus fusion with the liposome membrane, the liposome concentration was also increased from 200 to 800 μM. While the higher liposome concentration increases the likelihood of a productive interaction between virus and target membrane (9), it also increases the light scattering in the reaction, precluding the use of concentrations above ∼800 μM. Pilot experiments demonstrated that the initial rate of fusion of wt SFV or srf-3 with complete liposomes was increased about threefold using 800 μM liposomes compared to 200 μM liposomes (data not shown). Using these modifications, the fusion characteristics of wt SFV and srf-3 were compared using sterol-free liposomes at 25°C and pH 5.5 (Fig. 2). wt virus, as predicted, showed little fusion even in this optimized assay, with final extents of about 5 to 7%. In contrast, srf-3 consistently showed an increased level of fusion with the sterol-free liposomes, with fusion extents of about 10 to 15%. Thus, although srf-3 fusion in the absence of cholesterol was much less efficient than with complete liposomes, a significant increase in fusion compared to wt SFV was observed. This is the first evidence for decreased cholesterol dependence in srf-3 using a defined lipid bilayer and indicates that the decreased cholesterol requirement for srf-3 fusion is not dependent on the lipid composition or membrane properties of sterol-depleted mosquito cells but can be replicated in vitro.
FIG. 2.
Fusion of wt SFV and srf-3 with cholesterol-free liposomes. Real-time fluorescence recordings of the fusion of pyrene-labeled wt or srf-3 virus (0.6 μM) with unlabeled sterol-free liposomes (800 μM) are shown. Samples were preequilibriated for 5 min at 25°C, and the pH was adjusted to 5.5 at time zero. This is a representative example of four experiments.
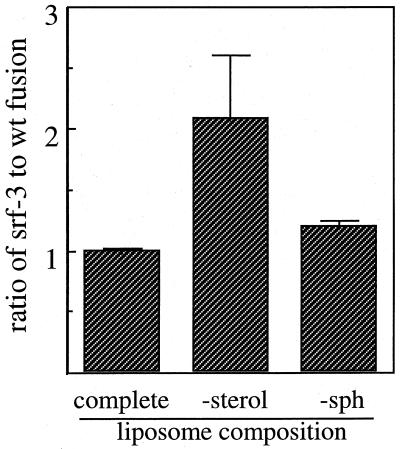
We next examined the sphingolipid dependence for fusion of both wt SFV and srf-3. Fusion experiments were performed in parallel with complete, sterol-free, or sphingolipid-free liposomes at 20°C using liposome concentrations of 800 μM. The ratio of the final extent of fusion of srf-3 versus wt SFV was plotted for each liposome type (Fig. 3). Both viruses showed comparable fusion extents with complete liposomes (ratio ∼1) and sphingolipid-free liposomes (ratio, ∼1.2). In contrast, and in agreement with the results in Fig. 2, on average srf-3 fusion with sterol-free liposomes was ca. twofold higher than that of wt virus. The final fusion extents in the four experiments in this figure varied somewhat between experiments, apparently due to variation between different preparations of liposomes, but the ratios of fusion between srf-3 and wt SFV were quite consistent.
FIG. 3.
Lipid dependence of wt SFV and srf-3 liposome fusion. Pyrene-labeled wt or srf-3 virus (0.6 μM) was mixed with unlabeled liposomes (800 μM) composed of PC-PE-Sph-cholesterol (complete), PC-PE-Sph (−sterol), or PC-PE-cholesterol (−sph). Samples were preequlibriated for 5 min at 20°C, the pH was adjusted to pH 5.5, and the final extents of fusion were measured after 60 s at 20°C. Data were plotted as the ratio of srf-3 to wt SFV fusion for each liposome type. The graph represents the average of four independent determinations for each virus, and the bars show the standard deviation.
Comparison of the initial rates of srf-3 fusion with sterol-free and cholesterol-containing liposomes showed that the rate was about eightfold slower in the absence of cholesterol (data not shown). This result suggests that the final extent of srf-3 fusion is lower with sterol-free than with cholesterol-containing liposomes because the slower fusion rate alters the equilibrium between acid-dependent fusion and acid inactivation (9). It is known that inactivation of virus fusion activity occurs upon exposure of virus to acidic pH in the absence of a fusion-competant membrane and has kinetics slower than those of fusion (9). No reliable comparison could be made to the rate of wt virus fusion with sterol-free liposomes since this rate was below the limit of accurate detection in our assay system.
pH dependence of wt SFV and srf-3 infection.
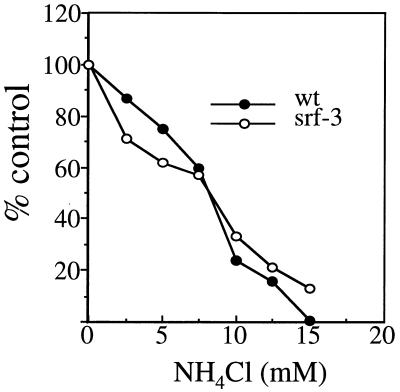
Taken together, the above data indicated that srf-3 was less cholesterol dependent for fusion than wt SFV but had a similar overall fusion mechanism and sphingolipid dependence. In addition to the lipid requirements for fusion, a critical parameter of the SFV fusion reaction is its pH dependence. It was important to determine if the srf-3 phenotype involved a change in the pH dependence of fusion, a parameter that can be sensitively measured in vivo. During virus uptake into host cells, fusion of SFV takes place in the endosome and results in virus infection. The pH dependence of this intracellular fusion event can be assessed by measuring its sensitivity to inhibition by a variety of agents that act to raise the pH of the endosome above the threshold required for fusion (31). For example, this in vivo assay was used to demonstrate differences in the fusion thresholds of wt SFV, a strain of SIN, and a fusion mutant of SFV (20, 21). BHK cells were infected with wt SFV and srf-3 in the presence of various concentrations of the lysosomotropic weak base NH4Cl, and virus infection was quantitated by monitoring the incorporation of [3H]uridine into viral RNA (20, 31). The NH4Cl sensitivities of wt SFV and srf-3 infection were similar, displaying half-maximal inhibition at about ∼8 mM NH4Cl (Fig. 4). This result suggested that the pH dependence of fusion within the endosome was similar for both viruses.
FIG. 4.
Sensitivity of infection by wt SFV and srf-3 to inhibition by NH4Cl. BHK cells were pretreated for 15 min with the indicated concentrations of NH4Cl and infected with wt SFV or srf-3 at 1 PFU/cell for 90 min in the continued presence of NH4Cl. Infection was then quantitated by determining the incorporation of [3H]uridine into viral RNA in the presence of 20 mM NH4Cl to prevent secondary infection. [3H]uridine incorporation was expressed as a percentage of control incorporation in the absence of NH4Cl, with the background incorporation obtained in uninfected cells subtracted from all points. The graph represents the mean of two separate experiments.
Cholesterol dependence of E1 conformational changes in wt SFV and srf-3.
We hypothesized that the increased ability of srf-3 to fuse with cholesterol-depleted membranes is due to an increase in the cholesterol independence of a sterol-requiring step in fusion. To test our hypothesis, we monitored acid conformation-specific epitope exposure and E1 homotrimer formation, two distinct acid-induced conformational changes that are enhanced by cholesterol for the wt E1 protein (14, 26, 32). Since cholesterol affects the rate of these E1 conformational changes, their kinetics were compared in the presence of liposomes with or without cholesterol, using conditions of suboptimal pH and temperature to slow the reaction.
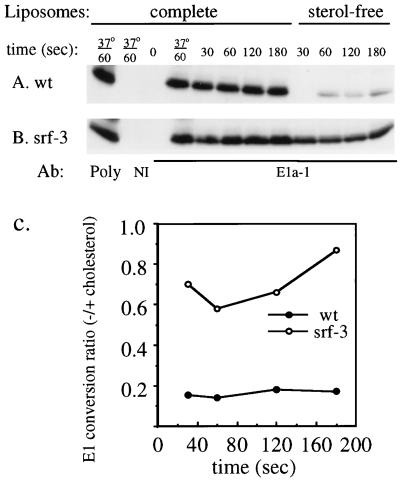
To assay acid epitope exposure, radiolabeled wt SFV and srf-3 were mixed with cholesterol-containing or sterol-free liposomes and treated at pH 5.8 for various times at 4°C. Samples were returned to neutral pH and immunoprecipitated with MAb E1a-1, a MAb that specifically detects the acid conformation of E1 (1, 27), or with a rabbit polyclonal anti-spike antibody that quantitatively precipitates the total E1 present in the reaction mixture. When exposed to low pH in the presence of cholesterol liposomes, wt E1 rapidly converts to a form that reacts with the MAb (Fig. 5A). Conversion after 1 min of low-pH treatment at 4°C was ∼98% of that obtained after similar treatment for 1 min at 37°C. Conversion was efficient, with the maximal MAb E1a-1 precipitation being ∼70% of the total E1. In comparison, the efficiency of srf-3 E1 conversion after 1 min of treatment at 4°C in the presence of cholesterol liposomes was ∼76% of that obtained at 37°C, and the maximal E1a-1 precipitation was ∼70% of the total E1 (Fig. 5B). Thus, the efficiency of the E1 conformational change under cholesterol-containing control conditions was similar for wt SFV and srf-3. However, when the pH treatment was performed in the presence of sterol-free liposomes, almost no wt E1 became immunoreactive after 30 s of pH treatment (Fig. 5A). The conversion of wt E1 slowly increased with time of pH treatment, but even after 3 min of pH treatment, it was considerably below that obtained with complete liposomes. In contrast, srf-3 conversion in the absence of sterol was much more efficient than that of wt virus throughout the entire period (Fig. 5B). Conversion was quantitated by PhosphorImager analysis and plotted for each time point as the ratio of E1 conversion with sterol-free liposomes to E1 conversion with complete liposomes (Fig. 5C). Even after 3 min of low-pH treatment with sterol-free liposomes, wt E1 showed a conversion ratio of only ∼0.18 of that obtained with cholesterol liposomes. The srf-3 E1 conformational change was much less cholesterol dependent, with conversion in sterol-free liposome samples ranging from ∼0.6 to 0.9 of that obtained with cholesterol liposomes. Similar results were obtained when this experiment was performed at 20°C, although the magnitude of the wt SFV difference with and without sterol was smaller than that at 4°C (data not shown).
FIG. 5.
Cholesterol dependence of E1 acid conformation-specific epitope exposure in wt SFV and srf-3. (A and B) [35S]methionine- and [35S]cysteine-labeled wt SFV (A) or srf-3 (B) was mixed with either complete, cholesterol-containing liposomes or liposomes without sterol and treated at pH 5.8 for the indicated times at 4°C unless otherwise indicated. The samples were then adjusted to neutral pH, solubilized in buffer containing 1% Triton X-100, and immunoprecipitated with an acid conformation-specific MAb to the E1 subunit (E1a-1), a rabbit polyclonal antibody to the SFV spike protein (poly), or an unrelated MAb (NI). Immunoprecipitates were analyzed by SDS-PAGE and fluorography. (C) E1 precipitated by MAb E1a-1 was quantitated by PhosphorImager analysis and plotted for each time point as the ratio of the E1 converted in the presence of sterol-free liposomes compared to the E1 converted in the presence of complete liposomes. The graph represents the mean of two independent experiments.
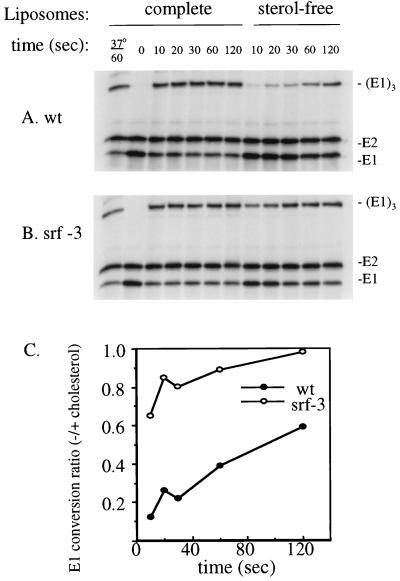
The cholesterol dependence of E1 homotrimer formation was similarly compared between wt SFV and srf-3, using incubation at pH 5.8 and 20°C in the presence of complete or sterol-free liposomes (14, 54). The samples were analyzed by SDS-PAGE under solubilization conditions that preserve the homotrimer (Fig. 6A and B). In the presence of cholesterol liposomes, both viruses efficiently produced E1 homotrimer, with maximal levels in two experiments ranging from 61 to 68% of the total E1 for wt SFV and 53 to 78% for srf-3. The pH dependence of homotrimer formation was assayed by treating virus plus complete liposomes at various pH values and was comparable for wt SFV and srf-3 (data not shown), again indicating that the srf-3 mutation does not affect the virus pH dependence. However, the sterol dependence of wt SFV versus srf-3 homotrimer formation showed a clear difference (Fig. 6A and B). The ratio of the homotrimer obtained with sterol-free liposomes to the homotrimer obtained with complete liposomes was determined for each virus at the various time points (Fig. 6C). wt E1 homotrimer formation in the sterol-free samples was much lower than that in the cholesterol liposome samples, with ratios ranging from an initial level of ∼0.1 to a level of ∼0.6 with longer incubation. In contrast, the efficiency of homotrimer formation with srf-3 was comparable with or without sterol, with ratios ranging from 0.7 to 1.0. Similar results were obtained when this experiment was performed at 4°C (data not shown). Taken together, these data indicate that two distinct acid-dependent conformational changes in E1, epitope exposure and homotrimerization, were less cholesterol dependent in the srf-3 mutant.
FIG. 6.
Cholesterol dependence of E1 homotrimer formation in wt SFV and srf-3. (A and B) [35S]methionine- and [35S]cysteine-labeled wt SFV (A) or srf-3 (B) was mixed with either complete, cholesterol-containing liposomes or liposomes without sterol and treated at pH 5.8 for the indicated times at 20°C unless otherwise indicated. The samples were then adjusted to neutral pH, solubilized in SDS sample buffer for 3 min at 30°C, and analyzed by SDS-PAGE. (C) Homotrimeric E1 was quantitated by PhosphorImager analysis and plotted for each time point as the ratio of homotrimer in the presence of sterol-free liposomes compared to homotrimer in the presence of complete liposomes. The graph represents the mean of four independent experiments.
The alphavirus lipid bilayer is derived from the host cell during virus budding from the plasma membrane. The radiolabeled viruses used in the above experiments were produced in BHK cells and thus contained cholesterol in the virus membranes (25). srf-3, unlike wt virus, can be propagated in sterol-depleted cells. Previous experiments demonstrated that srf-3 virus produced in the absence of cholesterol is viable and morphologically normal although significantly less stable to centrifugal shear force than virus propagated in cholesterol-containing cells (53). We investigated whether virus membrane cholesterol plays a role in E1 conformational changes by assaying radiolabeled srf-3 produced in cholesterol-depleted C6/36 cells. Similar to the results with srf-3 produced in BHK cells, conversion of this sterol-deficient srf-3 to reactivity with MAb E1a-1 was much less cholesterol dependent than for wt virus (data not shown). Thus, it appears that the conformational changes in srf-3 E1 are relatively cholesterol independent regardless of the presence or absence of cholesterol in the virus membrane. This is in keeping with the specific requirement for cholesterol in the target membrane during virus fusion (25, 26).
Sphingolipid dependence of E1 conformational changes in wt SFV and srf-3.
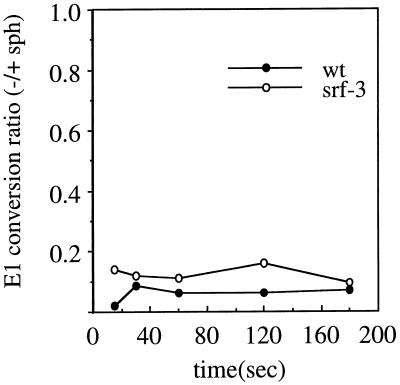
To test the importance of sphingolipid in the conformational changes in the wt and srf-3 E1 proteins, we made use of a system recently described by Corver (14). Radiolabeled wt or srf-3 virus was treated at pH 5.8 and 20°C in the presence of either PC-PE-Sph or PC-PE liposomes. E1 homotrimerization was assayed by SDS-PAGE. Both liposome types are cholesterol free and do not support the maximum efficiency of E1 conformational changes, but, similar to the results obtained by Corver (14), this assay demonstrated a striking enhancement of wt E1 homotrimerization by sphingolipid (Fig. 7). The relative sphingolipid dependence of wt SFV and srf-3 was compared by plotting the ratio of homotrimer obtained without sphingolipid to homotrimer obtained with sphingolipid at each time point (Fig. 7). In the absence of sphingolipid, E1 homotrimer formation by either wt SFV or srf-3 was inefficient, with ratios below 0.2 for both viruses. Acid conformation-specific epitope exposure was assayed using the same liposome system, and a similar, strong sphingolipid dependence for both viruses was observed (data not shown). Thus, the srf-3 mutation does not significantly affect the sphingolipid dependence of the E1 conformational changes, in keeping with the similar sphingolipid dependence of the fusion reactions of wt SFV and srf-3. Taken together, our results suggest that srf-3 fusion and E1 conformational changes show a significant decrease in their cholesterol requirements, whereas the srf-3 sphingolipid requirements and overall fusion properties are unaltered.
FIG. 7.
Sphingolipid dependence of E1 homotrimer formation in wt SFV and srf-3. [35S]methionine- and [35S]cysteine-labeled wt SFV or srf-3 was mixed with sterol-free liposomes with or without sphingolipid, treated at pH 5.8 for various times at 20°C, and analyzed by SDS-PAGE as in Fig. 6. Homotrimeric E1 was plotted as the ratio of homotrimer obtained without sphingolipid compared to that with sphingolipid. The graph represents the average of two experiments.
DISCUSSION
The isolation of the srf-3 mutant and of its previous characterization were dependent on a cholesterol-depleted mosquito cell culture system (45, 53). Published studies of cholesterol requirements in insect cells demonstrate that insects are sterol auxotrophs (41) and that, surprisingly, although insects require cholesterol for proper development, insect cells in tissue culture can be maintained in the absence of sterol for indefinite periods (45, 48, 49). The overall phospholipid composition and fatty acyl chain composition of such sterol-depleted insect cells do not appear significantly altered (49). Thus, these studies indicate that at least some eukaryotic cells do not require bulk sterol to form functional membranes. Although the absence of cholesterol or compensatory sterol in depleted insect cells is clear (36, 45, 49), a number of issues about the lipid composition and membrane functions of such cells remain, and these complicated the previous interpretation of the srf-3 mutant phenotype. First, it is now known that efficient SFV fusion requires not only cholesterol but also sphingolipid (38, 42). Second, under some conditions, sterol-depleted cells can adapt by an unknown mechanism to become more susceptible to SFV infection, suggesting that the membrane lipid composition could play a role in altering the permissiveness of a sterol-depleted membrane (36). Lastly, the original analysis of sterol-depleted cells did not address their membrane sphingolipid composition or distribution (10, 49). Thus, although clearly the srf-3 phenotype is dramatically dependent on cholesterol in depleted versus control insect cells, this phenotype could be due to secondary alterations in the depleted cell membrane, which might involve lipid composition, lipid distribution, or protein alterations. We began these studies to determine the lipid requirement of the srf-3 mutant under controlled in vitro conditions and to examine the function of the E1 protein carrying the critical P226S mutation. Our results demonstrated that the key alteration in srf-3 fusion properties was a specific increase in the fusion of the virus with cholesterol-depleted membranes. The sphingolipid requirement for fusion was maintained in the srf-3 mutant and was similar to that of wt virus. The decrease in the cholesterol requirement for srf-3 fusion correlated with a decrease in the cholesterol requirement for the srf-3 E1 conformational changes at low pH, detected as exposure of an acid-specific epitope and formation of the E1 homotrimer. Thus, even though the isolation of srf-3 was dependent on sterol-depleted insect cells, its phenotype of relative sterol independence could be demonstrated using purified, protein-free liposomes in vitro. Significantly, these results provide an important functional connection between the cholesterol dependence of E1 conformational changes and the cholesterol dependence of SFV fusion.
The data presented here have identified two points in the srf-3 E1 conformational changes that have a decreased dependence on cholesterol: acid-specific epitope exposure and homotrimerization. In support of these being independent steps in fusion, previous studies indicate that acid-specific epitope exposure can occur in a virus fusion peptide mutant that does not form the E1 homotrimer (28). In addition, studies of the binding site for MAb E1a-1, the acid conformation-specific MAb analyzed here, mapped this epitope to position 157 on E1 and indicate that its exposure can be triggered under conditions in which the E1 homotrimer is not formed (1). Thus, the available evidence suggests that epitope exposure and E1 homotrimerization represent two distinct steps in the virus fusion reaction, each of which is less cholesterol dependent in srf-3. The simplest explanation for the relative cholesterol independence of both steps is that both these E1 conformational changes occur subsequent to a point that controls the sterol dependence of fusion, a point that is less cholesterol dependent in srf-3. Previous data suggest that E1 may have a “lipid-sensing” step that occurs prior to epitope exposure, homotrimerization, fusion peptide insertion, and fusion (26). One example of such a potential E1 lipid-sensing function is that the kinetics of E1 epitope exposure and homotrimerization are both strongly enhanced by the presence of sphingolipid when a cholesterol-free target membrane is assayed (14) (Fig. 7). Such cholesterol-free target membranes are negative for both fusion peptide insertion and fusion (9, 26, 32), but the presence or absence of sphingolipid in the membrane nevertheless affects the conformational changes in E1. One model for SFV fusion could be that upon exposure to low pH, the E1-E2 dimer dissociates and the E1 protein senses the target membrane lipid composition and goes through a cholesterol-dependent priming reaction. The primed conformation of E1 then undergoes epitope exposure, homotrimerization, and fusion peptide insertion and finally triggers fusion. This model suggests that once E1 is in the primed configuration, the later conformational changes, fusion peptide insertion, and fusion itself would not require additional cholesterol interactions. This would be in keeping with the evidence presented here suggesting that the overall fusion reaction of srf-3, its activation energy, kinetics, and pH dependence, were all similar to those of wt virus. In this model, only the formation of primed E1, a key cholesterol-dependent step, would be altered in the srf-3 mutant. The decreased sterol dependence of this step in srf-3 would then lead to decreased sterol dependence of epitope exposure, homotrimerization, and fusion. It is possible that one of the E1 sterol-dependent conformational changes detected here actually represents the formation of this primed intermediate and would occur prior to the remaining E1 conformational changes and control their sterol dependence. Our data also do not indicate whether cholesterol could play a further role with E1 during the terminal stages of fusion including the formation of the fusion pore.
Studies of SFV and SIN indicate that both have strongly cholesterol-dependent fusion reactions (26, 29, 35, 45, 53, 55). Since these two alphaviruses are only distantly related, the data suggest that highly cholesterol-dependent fusion and infection may be a general characteristic of alphaviruses. It is not clear why alphaviruses have evolved such a sterol-requiring fusion mechanism. Studies of srf-3 infection by direct intrathoracic injection into the mosquito vector show that the mutant with reduced sterol requirement is not selected against in the insect host and, indeed, has something of a growth advantage compared to wt virus (2). It is possible that highly sterol-dependent viruses are selected in vivo by transmission conditions that are not replicated in the various tissue culture systems that we have characterized. It is also notable that the alphavirus mutants so far characterized, although demonstrating increased fusion with sterol-depleted membranes, all show maximal fusion activity only with a cholesterol-containing membrane. This result suggests that cholesterol may be an intrinsic part of efficient alphavirus fusion, perhaps conferred by a specific priming step during the fusogenic conformational changes in E1.
It is of interest to consider the general importance of specific lipids in the fusion and infection of other enveloped viruses. Virus membrane fusion can be triggered by a variety of mechanisms, including mildly acidic pH, spike protein receptor binding (23), and dual interaction of the virus spike protein with a receptor and coreceptor molecule (8, 18). For many viruses, the potential role of specific lipids in fusion has not been evaluated. This is in part because viruses whose fusion is triggered by mechanisms involving interactions with protein receptors and/or coreceptors are more difficult to study with defined liposome systems (26). It is clear that the low-pH-dependent viruses influenza virus and vesicular stomatitis virus do not require specific lipids for membrane fusion (13, 17, 45, 50, 56). There are several examples of viruses with potential requirements for cholesterol in fusion and/or budding, although clearly the data obtained with these systems are not conclusive at this point (see reference 26 for a review and in-depth discussion). One example is that of human immunodeficiency virus (HIV), which may exit from regions of the plasma membrane that are enriched in cholesterol and sphingolipid (3, 4). Studies with the N-terminal fusion peptide of HIV gp41 also suggest that cholesterol may play a role in HIV fusion (43, 44), although these studies examine the fusion peptide out of the normal context of the intact virus envelope protein and its physiological fusion trigger of receptor and coreceptor (26). Studies of the coronavirus mouse hepatitis virus suggest that susceptibility to a lytic virus infection and generation of virus-induced syncytia may be influenced by cellular cholesterol content (11, 16). Infection and fusion of African swine fever virus are affected by cellular cholesterol content (7), although the restricted host range of this virus of necessity required that these studies be performed in mammalian cells in which cholesterol depletion is limited and may produce toxic effects (26). A variety of in vitro studies suggested that Sendai virus, a paramyxovirus, fuses its membrane in a cholesterol-dependent mechanism (6, 12, 24, 33). For these and other viruses, as well as for cellular fusion proteins, the involvement of cholesterol and other specific lipids remains an important but unresolved issue in our understanding of the fusion reaction (26).
The E1 P226S mutation in srf-3 confers a strong increase in the cholesterol independence of virus fusion and E1 conformational changes. Using several different selection strategies, this mutation has been independently isolated eight times (P. K. Chatterjee and M. Kielian, unpublished data), demonstrating the overall importance of this residue and region in SFV cholesterol interactions. Further understanding of the role of cholesterol in SFV fusion will come from mutagenesis studies of the E1 226 region and from the isolation and characterization of new srf mutants. It will be important to determine if other srf mutations act by similar mechanisms to srf-3 and whether the distinct E1 regions identified as important in the cholesterol requirement actually interact in the three-dimensional structure of the SFV spike protein. Ongoing studies on the structure of the srf-3 virus particle and the properties of the srf-3 fusion pore may also add to our understanding of the means by which the virus takes advantage of the cholesterol present in cell plasma membranes to trigger efficient fusion and infection.
ACKNOWLEDGMENTS
We thank Jan Wilschut for generously sharing unpublished data on the cholesterol and sphingolipid dependence of the SFV E1 conformational changes, and we thank Jan Wilschut and Yolande Smit for their very helpful advice and protocols for the preparation and use of pyrene-labeled SFV in fusion assays. We thank Philip Aisen for the generous use of his fluorometer. We also thank the members of our laboratory for helpful discussions and suggestions, and we thank Duncan Wilson and the members of our laboratory for critical reading of the manuscript.
This work was supported by a grant to M.K. from the National Institutes of Health (R01 GM57454), by the Jack K. and Helen B. Lazar Fellowship in Cell Biology, and by Cancer Center Core Support Grant NIH/NCI P30-CA13330. M.V. was supported by a Martin Foundation Fellowship during a portion of this work.
REFERENCES
- 1.Ahn A, Klimjack M R, Chatterjee P K, Kielian M. An epitope of the Semliki Forest virus fusion protein exposed during virus-membrane fusion. J Virol. 1999;73:10029–10039. doi: 10.1128/jvi.73.12.10029-10039.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn A, Schoepp R J, Sternberg D, Kielian M. Growth and stability of a cholesterol-independent Semliki Forest virus mutant in mosquitoes. Virology. 1999;262:452–456. doi: 10.1006/viro.1999.9932. [DOI] [PubMed] [Google Scholar]
- 3.Aloia R C, Jensen F C, Curtain C C, Mobley P W, Gordon L M. Lipid composition and fluidity of the human immunodeficiency virus. Proc Natl Acad Sci USA. 1988;85:900–904. doi: 10.1073/pnas.85.3.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aloia R C, Tian H, Jensen F C. Lipid composition and fluidity of the human immunodeficiency virus envelope and host cell plasma membranes. Proc Natl Acad Sci USA. 1993;90:5181–5185. doi: 10.1073/pnas.90.11.5181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Anderson R G W. The caveolae membrane system. Annu Rev Biochem. 1998;67:199–225. doi: 10.1146/annurev.biochem.67.1.199. [DOI] [PubMed] [Google Scholar]
- 6.Asano K, Asano A. Binding of cholesterol and inhibitory peptide derivatives with the fusogenic hydrophobic sequence of F-glycoprotein of HVJ (Sendai virus): possible implication in the fusion reaction. Biochemistry. 1988;27:1321–1329. doi: 10.1021/bi00404a035. [DOI] [PubMed] [Google Scholar]
- 7.Bernardes C, António A, De Lima M C P, Valdeira M L. Cholesterol affects African swine fever virus infection. Biochim Biophys Acta. 1998;1393:19–25. doi: 10.1016/s0005-2760(98)00051-4. [DOI] [PubMed] [Google Scholar]
- 8.Binley J, Moore J P. HIV-cell fusion: the viral mousetrap. Nature. 1997;387:346–348. doi: 10.1038/387346a0. [DOI] [PubMed] [Google Scholar]
- 9.Bron R, Wahlberg J M, Garoff H, Wilschut J. Membrane fusion of Semliki Forest virus in a model system: correlation between fusion kinetics and structural changes in the envelope glycoprotein. EMBO J. 1993;12:693–701. doi: 10.1002/j.1460-2075.1993.tb05703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brown K, Havel C M, Watson J A. Isoprene synthesis in isolated embryonic Drosophila cells. II. Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity. J Biol Chem. 1983;13:8512–8518. [PubMed] [Google Scholar]
- 11.Cervin M, Anderson R. Modulation of coronavirus-mediated cell fusion by homeostatic control of cholesterol and fatty acid metabolism. J Med Virol. 1991;35:142–149. doi: 10.1002/jmv.1890350213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Citovsky V, Rottem S, Nussbaum O, Laster Y, Rott R, Loyter A. Animal viruses are able to fuse with prokaryotic cells. J Biol Chem. 1988;263:461–467. [PubMed] [Google Scholar]
- 13.Cleverley D Z, Geller H M, Lenard J. Characterization of cholesterol-free insect cells infectible by baculoviruses: Effects of cholesterol on VSV fusion and infectivity and on cytotoxicity induced by influenza M2 protein. Exp Cell Res. 1997;233:288–296. doi: 10.1006/excr.1997.3573. [DOI] [PubMed] [Google Scholar]
- 14.Corver J. Membrane fusion activity of Semliki Forest virus. Ph.D. thesis. Groningen University; 1998. [Google Scholar]
- 15.Corver J, Bron R, Snippe H, Kraaijeveld C, Wilschut J. Membrane fusion activity of Semliki forest virus in a liposomal model system: specific inhibition by Zn2+ ions. Virology. 1997;238:14–21. doi: 10.1006/viro.1997.8799. [DOI] [PubMed] [Google Scholar]
- 16.Daya M, Cervin M, Anderson R. Cholesterol enhances mouse hepatitis virus-mediated cell fusion. Virology. 1988;163:276–283. doi: 10.1016/0042-6822(88)90267-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eidelman O, Schlegel R, Tralka T S, Blumenthal R. pH-dependent fusion induced by Vesicular Stomatitis virus glycoprotein reconstituted into phospholipid vesicles. J Biol Chem. 1984;259:4622–4628. [PubMed] [Google Scholar]
- 18.Feng Y, Broder C C, Kennedy P E, Berger E A. HIV-1 entry cofactor: functional cDNA cloning of a seven-transmembrane, G protein-coupled receptor. Science. 1996;272:872–877. doi: 10.1126/science.272.5263.872. [DOI] [PubMed] [Google Scholar]
- 19.Garoff H, Wilschut J, Liljeström P, Wahlberg J M, Bron R, Suomalainen M, Smyth J, Salminen A, Barth B U, Zhao H. Assembly and entry mechanisms of Semliki Forest virus. Arch Virol. 1994;9:329–338. doi: 10.1007/978-3-7091-9326-6_33. [DOI] [PubMed] [Google Scholar]
- 20.Glomb-Reinmund S, Kielian M. fus-1, a pH-shift mutant of Semliki Forest virus, acts by altering spike subunit interactions via a mutation in the E2 subunit. J Virol. 1998;72:4281–4287. doi: 10.1128/jvi.72.5.4281-4287.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Glomb-Reinmund S, Kielian M. The role of low pH and disulfide shuffling in the entry and fusion of Semliki Forest virus and Sindbis virus. Virology. 1998;248:372–381. doi: 10.1006/viro.1998.9275. [DOI] [PubMed] [Google Scholar]
- 22.Hernandez L D, Hoffman L R, Wolfsberg T G, White J M. Virus-cell and cell-cell fusion. Annu Rev Cell Dev Biol. 1996;12:627–661. doi: 10.1146/annurev.cellbio.12.1.627. [DOI] [PubMed] [Google Scholar]
- 23.Hernandez L D, Peters R J, Delos S E, Young J A T, Agard D A, White J M. Activation of a retroviral membrane fusion protein: soluble receptor-induced liposome binding of the ALSV envelope glycoprotein. J Cell Biol. 1997;139:1455–1464. doi: 10.1083/jcb.139.6.1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu M C, Scheid A, Choppin P W. Fusion of Sendai virus with liposomes: dependence on the viral fusion protein (F) and the lipid composition of liposomes. Virology. 1983;126:361–369. doi: 10.1016/0042-6822(83)90485-3. [DOI] [PubMed] [Google Scholar]
- 25.Kielian M. Membrane fusion and the alphavirus life cycle. Adv Virus Res. 1995;45:113–151. doi: 10.1016/s0065-3527(08)60059-7. [DOI] [PubMed] [Google Scholar]
- 26.Kielian, M., P. K. Chatterjee, D. L. Gibbons, and Y. E. Lu. Specific roles for lipids in virus fusion and exit: examples from the alphaviruses. Subcell. Biochem., in press. [DOI] [PubMed]
- 27.Kielian M, Jungerwirth S, Sayad K U, DeCandido S. Biosynthesis, maturation, and acid-activation of the Semliki Forest virus fusion protein. J Virol. 1990;64:4614–4624. doi: 10.1128/jvi.64.10.4614-4624.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kielian M, Klimjack M R, Ghosh S, Duffus W A. Mechanisms of mutations inhibiting fusion and infection by Semliki Forest virus. J Cell Biol. 1996;134:863–872. doi: 10.1083/jcb.134.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kielian M C, Helenius A. The role of cholesterol in the fusion of Semliki Forest virus with membranes. J Virol. 1984;52:281–283. doi: 10.1128/jvi.52.1.281-283.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kielian M C, Keränen S, Kääriäinen L, Helenius A. Membrane fusion mutants of Semliki Forest virus. J Cell Biol. 1984;98:139–145. doi: 10.1083/jcb.98.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kielian M C, Marsh M, Helenius A. Kinetics of endosome acidification detected by mutant and wild-type Semliki Forest virus. EMBO J. 1986;5:3103–3109. doi: 10.1002/j.1460-2075.1986.tb04616.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Klimjack M R, Jeffrey S, Kielian M. Membrane and protein interactions of a soluble form of the Semliki Forest virus fusion protein. J Virol. 1994;68:6940–6946. doi: 10.1128/jvi.68.11.6940-6946.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kundrot C E, Spangler E A, Kendall D A, MacDonald R C, MacDonald R I. Sendai virus-mediated lysis of liposomes requires cholesterol. Proc Natl Acad Sci USA. 1983;80:1608–1612. doi: 10.1073/pnas.80.6.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 35.Lu Y E, Cassese T, Kielian M. The cholesterol requirement for Sindbis virus entry and exit and characterization of a spike protein region involved in cholesterol dependence. J Virol. 1999;73:4272–4278. doi: 10.1128/jvi.73.5.4272-4278.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marquardt M T, Kielian M. Cholesterol-depleted cells that are relatively permissive for Semliki Forest virus infection. Virology. 1996;224:198–205. doi: 10.1006/viro.1996.0521. [DOI] [PubMed] [Google Scholar]
- 37.Marquardt M T, Phalen T, Kielian M. Cholesterol is required in the exit pathway of Semliki Forest virus. J Cell Biol. 1993;123:57–65. doi: 10.1083/jcb.123.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Moesby L, Corver J, Erukulla R K, Bittman R, Wilschut J. Sphingolipids activate membrane fusion of Semliki Forest virus in a stereospecific manner. Biochemistry. 1995;34:10319–10324. doi: 10.1021/bi00033a001. [DOI] [PubMed] [Google Scholar]
- 39.Morrison W R. A fast, simple, and reliable method for the microdetermination of phosphorus in biological materials. Anal Biochem. 1964;7:218–224. doi: 10.1016/0003-2697(64)90231-3. [DOI] [PubMed] [Google Scholar]
- 40.Nes W R, McKean M L, editors. Biochemistry of steroids and other isopentenoids. Baltimore, Md: University Park Press; 1977. [Google Scholar]
- 41.Nes W R, McKean M L. Occurence, physiology, and ecology of sterols. In: Nes W R, McKean M L, editors. Biochemistry of steroids and other isopentenoids. Baltimore, Md: University Park Press; 1977. pp. 411–533. [Google Scholar]
- 42.Nieva J L, Bron R, Corver J, Wilschut J. Membrane fusion of Semliki Forest virus requires sphingolipids in the target membrane. EMBO J. 1994;13:2797–2804. doi: 10.1002/j.1460-2075.1994.tb06573.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pereira F B, Goni F M, Muga A, Nieva J L. Permeabilization and fusion of uncharged lipid vesicles induced by the HIV-1 fusion peptide adopting an extended conformation: dose and sequence effects. Biophys J. 1997;73:1977–1986. doi: 10.1016/S0006-3495(97)78228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pereira F B, Goni F M, Nieva J L. Membrane fusion induced by the HIV type 1 fusion peptide: modulation of factors affecting glycoprotein 41 activity and potential anti-HIV compounds. AIDS Res Hum Retroviruses. 1997;13:1203–1211. doi: 10.1089/aid.1997.13.1203. [DOI] [PubMed] [Google Scholar]
- 45.Phalen T, Kielian M. Cholesterol is required for infection by Semliki Forest virus. J Cell Biol. 1991;112:615–623. doi: 10.1083/jcb.112.4.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Porter J A, Young K E, Beachy P A. Cholesterol modification of hedgehog signaling proteins in animal development. Science. 1996;274:255–259. doi: 10.1126/science.274.5285.255. [DOI] [PubMed] [Google Scholar]
- 47.Rodal S D, Skretting G, Garred O, Vilhardt F, van Deurs B, Sandvig K. Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formtaiotn of clathrin-coated vesicles. Mol Biol Cell. 1999;10:961–974. doi: 10.1091/mbc.10.4.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rolls M M, Marquardt M T, Kielian M, Machamer C E. Cholesterol-independent targeting of Golgi membrane proteins in insect cells. Mol Biol Cell. 1997;8:2111–2118. doi: 10.1091/mbc.8.11.2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Silberkang M, Havel C M, Friend D S, McCarthy B J, Watson J A. Isoprene synthesis in isolated embryonic Drosophila cells. I. Sterol-deficient eukaryotic cells. J Biol Chem. 1983;258:8303–8311. [PubMed] [Google Scholar]
- 50.Stegmann T, Helenius A. Influenza virus fusion: from models towards a mechanism. In: Bentz J, editor. Viral fusion mechanisms. Boca Raton, Fla: CRC Press, Inc.; 1993. pp. 89–111. [Google Scholar]
- 51.Strauss J H, Strauss E G. The alphaviruses: gene expression, replication, and evolution. Microbiol Rev. 1994;58:491–562. doi: 10.1128/mr.58.3.491-562.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Subtil A, Gaidarov I, Kobylarz K, Lampson M A, Keen J H, McGraw T E. Acute cholesterol depletion inhibits clathrin-coated pit budding. Proc Natl Acad Sci USA. 1999;96:6775–6780. doi: 10.1073/pnas.96.12.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vashishtha M, Phalen T, Marquardt M T, Ryu J S, Ng A C, Kielian M. A single point mutation controls the cholesterol dependence of Semliki Forest virus entry and exit. J Cell Biol. 1998;140:91–99. doi: 10.1083/jcb.140.1.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wahlberg J M, Bron R, Wilschut J, Garoff H. Membrane fusion of Semliki Forest virus involves homotrimers of the fusion protein. J Virol. 1992;66:7309–7318. doi: 10.1128/jvi.66.12.7309-7318.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.White J, Helenius A. pH-dependent fusion between the Semliki Forest virus membrane and liposomes. Proc Natl Acad Sci USA. 1980;77:3273–3277. doi: 10.1073/pnas.77.6.3273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.White J, Kartenbeck J, Helenius A. Membrane fusion activity of influenza virus. EMBO J. 1982;1:217–222. doi: 10.1002/j.1460-2075.1982.tb01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yeagle P L. Modulation of membrane function by cholesterol. Biochimie. 1991;73:1303–1310. doi: 10.1016/0300-9084(91)90093-g. [DOI] [PubMed] [Google Scholar]