Abstract
Objectives
Migrants from high HIV, hepatitis B virus (HBV) or hepatitis C virus (HCV) endemicity regions have a great burden of these infections and related diseases in the host countries. This study aimed to assess the predictive capacity of the Test Rapide d'Orientation Diagnostique (TROD) Screen questionnaire for HIV, HBV and HCV infections among migrants arriving in France.
Design
An observational and multicentre study was conducted among migrants. A self-questionnaire on demographic characteristics, personal medical history and sexual behaviours was completed.
Setting
The study was conducted in the centres of the French Office for Immigration and Integration (OFII).
Participants
Convenience sampling was used to select and recruit adult migrants between January 2017 and March 2020.
Outcome measures
Participants were tested for HIV, HBV and HCV with rapid tests. For each infection, the test performance was assessed using receiver operating characteristics curves, using area under the curve (AUC) as a measure of accuracy.
Results
Among 21 133 regular migrants seen in OFII centres, 15 343 were included in the study. The participants’ mean age was 35.6 years (SD±11.1). The prevalence (95% CI) of HBV, HCV and HIV was 2.0% (1.8% to 2.2%), 0.3% (0.2% to 0.4%) and 0.3% (0.2% to 0.4%), respectively. Based on the sensitivity–specificity curve analysis, the cut-off points (95% CI) chosen for the risk score were: 2.5 (2.5 to 7.5) for HBV infection in men; 6.5 (0.5 to 6.5) for HBV infection in women; 9.5 (9.5 to 12.5) for HCV infection; and 10.5 (10.0 to 18.5) for HIV infection. Test performance was highest for HIV (AUC=82.15% (95% CI 74.54% to 87.99%)), followed by that for HBV in men (AUC=79.22%, (95% CI 76.18% to 82.26%)), for HBV in women (AUC=78.83 (95% CI 74.54% to 82.10%)) and that for HCV (AUC=75.95% (95% CI 68.58% to 83.32%)).
Conclusion
The TROD screen questionnaire showed good overall performance for predicting HIV, HBV and HCV infections among migrants in OFII centres. It could be used to optimise screening for these infections and to propose rapid screening tests to those who are at high risk.
Trial registration number
Keywords: HIV & AIDS, hepatology, infectious diseases, sexually transmitted disease
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This study included a large number of migrants from several different countries, which may capture estimates from different profiles of migrants in several centres.
The migrants participating in the study are regular migrants receiving permits to stay; they might have a higher socioeconomic status than other categories, such as asylum seekers, and these risk scores should be tested in this population.
Not all migrants having their medical consultation at French Office for Immigration and Integration (OFII) centres were proposed to participate in the study due to a shortage of time, staff or language barriers.
There may be a bias related to under-reporting information, as some participants, being at the OFII centre, may feel uncomfortable disclosing some sexual behaviours.
Using these tools, a considerable number of infections could be missed, which could cause an ethical issue.
Background
Viral hepatitis (including hepatitis B and hepatitis C virus (HBV and HCV)) as well as HIV infection is among the most common chronic infectious diseases worldwide and a major public health problem.1 2 In 2015, HIV infection was involved in 1.06 million deaths, while viral hepatitis led to 1.34 million deaths due to chronic or long-term complications such as cirrhosis, liver failure and hepatocellular carcinoma.2 These infections disproportionately affect regions of the world, with a high prevalence in low-income countries.2 3 In low-endemicity regions, the mobile population or migrants have a great burden of these infections and related diseases.4 5 A meta-analysis on HBV prevalence among immigrants found an overall pooled seroprevalence of infection of 7.2% (95% CI 6.3% to 8.2%). In addition, a subgroup analysis showed that HBV prevalence reflected that in the region of origin, particularly for those from intermediate or high-prevalence regions including the Middle East, East Asia and sub-Saharan Africa.6 Another meta-analysis found an overall anti-HCV antibody prevalence among migrants of 1.9% (95% CI 1.4 to 2.7%), with a higher prevalence among migrants from Sub-Saharan Africa, Asia and Eastern Europe.7
France is a country with low endemicity for these infections; the estimated prevalence of HIV infection was 0.41%, of chronic HBV infection was 0.93%, and of chronic HCV infection was 0.60% in 2020 in the general adult population.8 People born abroad and mobile populations such as immigrants and travellers from countries with a relatively high HIV, HBV or HCV endemicity are vulnerable groups for these infections.9 10 In a cross-sectional survey (AfroBaromètre 2016) conducted among Afro-Caribbeans living in the Paris area in 2016, the prevalence of HIV and hepatitis B surface antigen (HBsAg) was 1.4% and 1.7%, respectively, among participants born in France, both 2.6% among those born in Haiti, and 1.7% and 7.0%, respectively, for those born in sub-Saharan Africa.10 Almost 20% of them have never been screened for HIV or HBV. In addition, 40% of HIV-positive participants and 77% of those living with HBV were unaware of their seropositive status.
In recent years, with the development of rapid screening tests, HIV, HBV and HCV screening rates have highly increased in France.11 However, many people remain undiagnosed and unaware of their infection status, including people from sub-Saharan Africa or Asia who were less likely to receive HIV, HBV or HCV screening, fearing mostly discrimination but also because most of them did not have a regular residence permit. It is also known that migrant populations have a lack of knowledge about these infections and are less likely to be screened for them.10 12 Given the high prevalence of these infections in migrant populations, they might be at increased risk of transmission or acquisition.
In many European countries, it is common for migrants from countries with high HBV endemicity to be systematically offered screening for HIV, HBV and HCV. Morbidity and mortality from these infections can be reduced by early diagnosis through screening at-risk people for these infections and offering appropriate medical management,6 13 which should also contribute to the secondary prevention of HIV, HBV and HCV infections. In addition, for at-risk patients, a vaccination against HBV should be proposed.
The STRADA study, which was designed to evaluate a new strategy for screening for infectious diseases among the migrants admitted in the French Office for Immigration and Integration (OFII) departments and to validate a self-screening questionnaire for tuberculosis Screen as well as HIV, HBV and HCV (‘Test Rapide d'Orientation Diagnostique (TROD) screen’), showed a high acceptability of the participants for HIV and viral hepatitis screening.14
The objective of this study is to assess the predictive capacity of the TROD screen risk factor self-reported questionnaire for HIV, HBV and HCV infection among migrants during their medical visit in the OFII.
Methods
Study design and participants
This was a prospective multicentre and observational study carried out between January 2017 and March 2020 among migrants in the OFII centres. There are 32 OFII centres, including 28 in mainland France and four overseas. During our study, all OFII centres were invited to participate, but only 21 OFII centres have accepted, including three centres in Ile-de-France (Cergy, Melun, Montrouge), 16 centres outside Ile-de-France (Bordeaux, Dijon, Grenoble, Lille, Limoges, Lyon, Marseille, Montpellier, Nantes, Nice, Orléans, Rennes, Reims, Rouen, Strasbourg, Toulouse) and 2 overseas centres (Cayenne, Pointe-à-Pitre). Individuals were included during the compulsory medical visit at the time of the delivery of their first residence permit. Eligible participants were migrants aged 18 years or more who consented to participate in this study.
Study intervention and data collection
Participants completed the anonymous TROD screen questionnaire online. This self-administrated questionnaire was translated into 10 languages (English, Arabic, Chinese, Bengali, Russian, Lingala, Portuguese, Spanish, Turkish and Haitian Creole) and included data related to sociodemographic, personal medical history and sexual behaviours. A few pieces of data were retrieved from medical records (year of birth, gender, height, weight and nationality). Then, a rapid screening test for HBV, HCV or HIV infections was proposed to the participants, who could refuse or choose between tests. The participants who reported prior testing but forgot the result of the test were encouraged to be tested again. However, those who were aware of their status (eg, HIV or HBV) or those who documented a vaccination against HBV were not tested.
A nurse performed the rapid screening test using the TOYO HCV test (for HCV),15 the TOYO HBsAg test (for HBV)16 and the INSTI HIV1/HIV2 test (for HIV).17 Then the doctor or nurse announced the results. In the event of a positive result, the participant was referred to a specialised hospital consultation to confirm the diagnosis and initiate adapted treatments.
Variables definition
The outcome was a positive rapid screening test for HBV, HCV or HIV infections. It was used as the gold standard for calculating the sensitivity and specificity of various combinations of participants’ characteristics for predicting HBV, HCV or HIV infection.
A number of independent predictor factors were used for the prediction of HBV, HCV or HIV infection. (1) Sociodemographic characteristics: age (years), gender (male or female), weight status (underweight (body mass index (BMI) <18 kg/m2); Normal weight (BMI 18 and 25 kg/m2) or overweight/obesity (BMI>25 kg/m2)), endemic area for each infection, knowledge of HIV, HBV and HCV. (2) Personal history: HIV, HBV or HCV screening, vaccination against HBV, dental treatment, surgery, abortion, caesarean section or difficult childbirth, history of liver disease, tattoos or piercings, prison, blood transfusion, living with a person infected with viral hepatitis, psychoactive substance use (injection or snorting). (3) Sexual behaviours: geographical origin of sexual partner, number of sexual partners during the last 12 months, and sexual practices and orientation.
Statistical analysis
Statistical analyses were performed by using R software version R V.3.6.3. The participants’ characteristics were described using absolute frequencies, proportions for categorical variables or means and SD for continuous variables.
A cross-analysis between explanatory factors and each infection (HBV, HCV and HIV) was performed using a Student’s t-test or Wilcoxon for the means and a χ2 or Fisher’s exact test for the proportions.
Binary logistic regression models were fitted to identify factors associated with HBV, HCV or HIV infection. In the univariate analysis, independent variables with a p<0.25 were included in the multivariable logistic regression analysis in order to control potential confounders. The final multivariable model was performed using a stepwise selection procedure, which was based on the likelihood ratio test (p<0.05). The Akaike information criterion was to select the final model. Results were reported as unadjusted OR, and adjusted ORs (aOR) with 95% CI. There was no missing data in all predictor variables and outcomes, and we carried out a complete-case analysis for the outcomes.
Model performance was evaluated in terms of discrimination. The discriminating capacity of the TROD screen questionnaire for HBV, HCV and HIV was evaluated using the predictive value of the questionnaire, its sensitivity and specificity, as well as the 95% CIs. An ROC curve was used to quantify discrimination and determine the cut-off score of the questionnaire for each infection. That also assesses whether those with higher predicted risks are more likely to have an HBV, HCV or HIV infection.
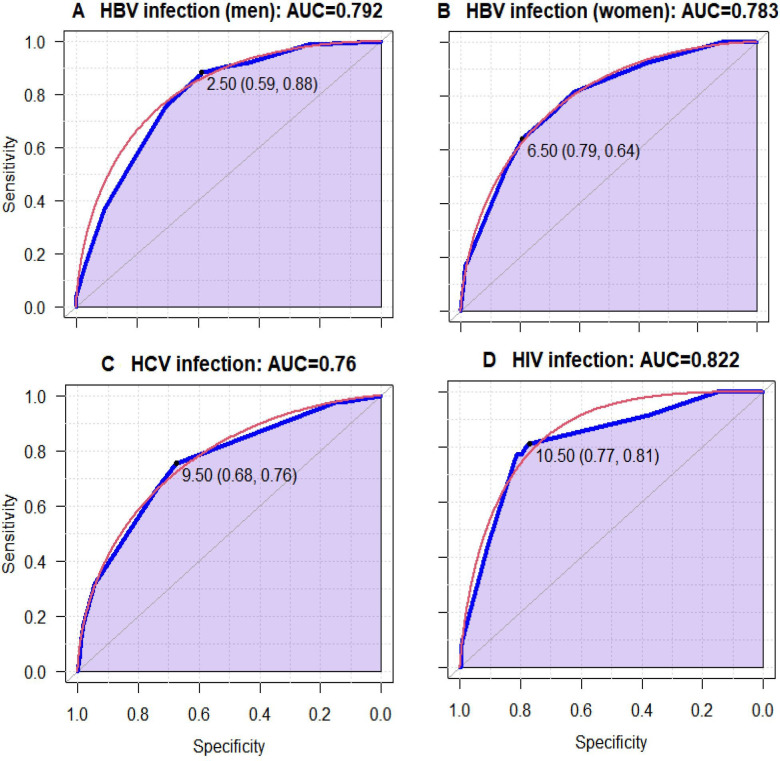
To make the models easier to use in clinical practice, we created a risk score for evaluating the likelihood of HBV, HCV or HIV infection based on multivariable regression coefficients, which were rescaled and rounded to the nearest whole number. To determine the score for each level of the variables, weighted points were assigned to each of the final associated factors. The β-coefficients of each variable were multiplied by a constant (we have chosen 5), and rounded to the nearest integer.18 19 Based on the sensitivity–specificity curve analysis (figure 1), the cut-offs point was chosen for the risk score for HBV (both in men and women), HCV and for HIV infections, with maximum sensitivity and specificity.
Figure 1.
Mean receiver operating characteristics (ROC) curve for multivariable logistic regression models. Each blue line indicates the ROC curve. (A) The area under the curve (AUC) for the HBV infection in men, the AUC for this model is 0.792, indicating a good fit. The threshold was 2.50, specificity=0.88 and sensitivity=0.59. (B) The AUC for HBV in women, which was 0.783. The threshold was 6.50, specificity=0.79 and sensitivity=0.64. (C) The curve for HCV has an AUC of 0.760, indicating a fair fit for the score. The threshold was 9.50, specificity=0.76 and sensitivity=0.68. (D) The AUC for the HIV infection. The AUC for this model is 0.822, indicating a good fit. The threshold was 10.50, specificity=0.81 and sensitivity=0.77. HBV, hepatitis B virus; HCV, hepatitis C virus.
By assuming that the HBV prevalence is higher in men than in women, we carried out the analysis on HBV infection by stratifying by sex in order to obtain risk scores specific to the men and women included in this study.
Patient and public involvement
None.
Results
Characteristics of study participants
A total of 21 133 participants realised a rapid test during their medical visit. Among them, 15 343 participants who had the rapid screening test and completed the TROD screen questionnaire were included in this analysis. Their sociodemographic characteristics are described in table 1. The mean age of the participants was 35 years (SD±11), and 62.8% were female. More than one-third (36.5%) of them were overweight or obese. Among the participants, 23.9%, 16.3% and 2.7% came from a high endemicity area of HCV, HBV and HIV, respectively. History of dental care (72.0%), surgery (32.7%), piercing and tattooing (31.0%), blood transfusion (3.7%), psychoactive substance consumption (3.1%) and liver problems (2.2%) were reported. A little more than one-fifth of the female participants reported a history of abortion, caesarean section or difficult childbirth (21.4%), and 3.9% of them declared being pregnant at the moment of the survey.
Table 1.
Participants’ characteristics
| Participants’ characteristics | Total=15 343 | HBV-positive n=293 |
HCV-positive n=42 |
HIV-positive n=47 |
|
| N | n (%) or mean (±SD) | n (prevalence) | n (prevalence) | n (prevalence) | |
| Sociodemographic characteristics | |||||
| Age, years | 15 343 | 35 (±11) | 37.3 (±9.5)* | 44.5 (±15.0)*** | 37.6 (±9.4) |
| Gender | 15 343 | *** | ° | ° | |
| Male | 5707 (37.2) | 164 (3.0) | 17 (0.3) | 20 (0.4) | |
| Female | 9636 (62.8) | 129 (1.4) | 25 (0.3) | 27 (0.3) | |
| BMI (kg/m2) | 15 343 | ° | ° | ° | |
| Underweight | 907 (5.9) | 12 (1.8) | 2 (0.2) | 2 (0.2) | |
| Normal weight | 8830 (57.6) | 167 (2.0) | 23 (0.3) | 24 (0.3) | |
| Overweight/obesity | 5606 (36.5) | 114 (2.1) | 12 (0.3) | 21 (0.4) | |
| Endemic area of HCV | 15 327 | *** | *** | *** | |
| Low | 2393 (15.6) | 8 (0.3) | 1 (4.10-4) | 1 (4.10-4) | |
| Medium | 9278 (60.5) | 114 (1.3) | 16 (0.2) | 14 (0.2) | |
| High | 3656 (23.9) | 171 (4.9) | 25 (0.7) | 31 (0.9) | |
| Endemic area HBV | 13 884 | *** | *** | *** | |
| Low | 7686 (55.3) | 39 (0.5) | 10 (0.1) | 4 (5.10-4) | |
| Medium | 3938 (28.4) | 117 (3.1) | 22 (0.6) | 24 (0.6) | |
| High | 2260 (16.3) | 122 (5.6) | 7 (0.3) | 17 (0.8) | |
| Endemic area HIV | 11 390 | *** | ** | *** | |
| Low | 8317 (73.0) | 102 (1.3) | 17 (0.2) | 11 (0.1) | |
| Medium | 2763 (24.3) | 142 (5.4) | 18 (0.7) | 29 (1.1) | |
| High | 310 (2.7) | 25 (8.1) | 0 (0.0) | 1 (0.3) | |
| Knowledge of hepatitis B infection | 15 343 | 10 852 (70.7) | 200 (1.9)° | 32 (0.3)° | 30 (0.3)° |
| Knowledge of hepatitis C infection | 15 343 | 10 335 (67.5) | 175 (1.8)** | 29 (0.3)° | 26 (0.3)° |
| Knowledge of HIV infection | 15 343 | 13 168 (85.8) | 246 (1.9)° | 36 (0.3)° | 43 (0.3)° |
| Personal history | |||||
| Screened for hepatitis B infection | 12 631 | 4044 (32.0) | 101 (2.6)*** | 11 (0.3)° | 13 (0.3)° |
| Screened for hepatitis C infection | 12 286 | 3239 (26.4) | 50 (1.6)° | 13 (0.4)° | 8 (0.2)° |
| Screened for HIV infection | 14 176 | 6733 (47.5) | 169 (2.6)*** | 27 (0.4)* | 28 (0.4)* |
| Vaccination against HBV | 9446 | 4291 (45.4) | 45 (1.1)*** | 8 (0.2)° | 9 (0.2)* |
| History of dental care | 14 990 | 10 797 (72.0) | 161 (1.5)*** | 32 (0.3)° | 26 (0.2)* |
| History of surgery | 15 061 | 4930 (32.7) | 78 (1.6)° | 17 (0.3)° | 17 (0.4)° |
| History of piercing or tattoo | 15 343 | 4751 (31.0) | 65 (1.4)*** | 15 (0.3)° | 22 (0.5)* |
| History of blood transfusion | 15 343 | 572 (3.7) | 15 (2.7)° | 8 (1.4)*** | 7 (1.2)*** |
| History liver problems | 15 343 | 333 (2.2) | 22 (7.0)*** | 3 (0.9)° | 0 (0.0)° |
| Abortion or caesarean section or difficult childbirth† | 9635 | 2057 (21.4) | 39 (2.0)* | 14 (0.7)*** | 16 (0.8)*** |
| Pregnant† | 9443 | 2057 (3.9) | 5 (1.4)° | 1 (0.3)° | 0 (0.0)° |
| Living with someone who has had viral hepatitis‡ | 15 343 | 369 (2.4) | 26 (7.3)*** | 2 (0.6)° | 0 (0.0)° |
| Healthcare worker or barber | 15 343 | 1066 (6.9) | 15 (1.5)° | 4 (0.4)° | 4 (0.4)° |
| Being in jail | 15 048 | 80 (0.5) | 4 (5.0)° | 0 (0.0)° | 0 (0.0)° |
| Behaviours | |||||
| Inject or snort psychoactive substance | 14 987 | 462 (3.1) | 9 (2.0)° | 2 (0.4)° | 0 (0.0)° |
| Number of sexual partners in last 12 previous months | 13 037 | ° | ° | ° | |
| 0 | 990 (7.6) | 19 (2.0) | 2 (0.2) | 2 (0.2) | |
| 1 | 10 700 (82.1) | 194 (1.9) | 24 (0.2) | 36 (0.3) | |
| 2 and more | 1347 (10.3) | 23 (1.8) | 5 (0.4) | 5 (0.4) | |
| Sexual partner born in Asia Middle East or Africa | 13 889 | 5360 (38.6) | 137 (2.6)*** | 19 (0.4)° | 25 (0.5)* |
| Sexual identity | 14 768 | *** | ° | *** | |
| No sex | 2011 (13.6) | 26 (1.3) | 7 (0.4) | 1 (0.1) | |
| Heterosexual women | 7777 (52.7) | 109 (1.5) | 18 (0.2) | 27 (0.4) | |
| Heterosexual men | 4632 (31.4) | 139 (3.1) | 11 (0.2) | 13 (0.3) | |
| Homosexual and bisexual | 348 (2.4) | 4 (1.2) | 2 (0.6) | 6 (1.7) | |
| Having anal sex, yes | 12 469 | 1211 (9.7) | 16 (1.4)° | 4 (0.3)° | 8 (0.7)* |
| Outcomes | |||||
| HBV-positive | 14 849 | 293 (2.0) | 2 (0.7)° | 3 (1.1)* | |
| HCV-positive | 15 214 | 42 (0.3) | 2 (5.3)° | 3 (7.1)*** | |
| HIV-positive | 15 099 | 47 (0.3) | 3 (7.1)* | 3 (6.8)*** | |
*P(Χ2 or Fisher’s exact tests)<0.05, **p<0.01, ***p<0.001, °NS.
†Only in female participants.
‡Mother, sexual partner, household member.
HBV, hepatitis B virus; HCV, hepatitis C virus.
Regarding sexual behaviours, 10.3% of the migrants seen reported two sexual partners or more during the last 12 months, while 38.6% reported a sexual partner born in Asia, the Middle East or Africa. About 10% (1211) of participants reported anal intercourses.
Almost one-third of the participants (32.0%) reported a history of screening for HBV, 26.4% for HCV and 47.5% for HIV. In addition, 4291 migrants reported a vaccination against HBV. The overall seroprevalence (95% CI) of HBV, HCV and HIV was 2.0% (1.8% to 2.2%), 0.3% (0.2% to 0.4%) and 0.3% (0.2% to 0.4%), respectively.
Development of the risk score for HBV, HCV and HIV infection
Univariable logistic regression analyses were used to select explanatory variables for adjusted models. Multivariable logistic regression models were fitted to determine factors associated with each infection. The coefficient values and aOR of each variable were obtained (table 2). Only significant variables in the parsimonious models were selected to be used in the development of the risk score in each infection, which were as follows (table 3):
Table 2.
Associated factors with HBV, HCV and HIV among migrants in France
| Participants’ characteristics | HBV infection Men (n=5707) |
HBV infection Women (n=9636) |
HCV infection (n=15 343) |
HIV infection (n=15 343) |
| aOR (95%CI) | aOR (95%CI) | aOR (95%CI) | aOR (95%CI) | |
| Endemic area of HBV (low: aOR=1) | ||||
| Medium | 7.62 (4.51 to 13.67*** | 4.65 (2.86 to 7.79)*** | ||
| High | 15.83 (9.40 to 28.35)*** | 6.68 (4.06 to 11.29)*** | ||
| Endemic area of HCV (low: aOR=1) | ||||
| Medium | 4.09 (0.83 to 73.85)° | |||
| High | 13.97 (2.94 to 250.21)** | |||
| Endemic area of HIV (low: aOR=1) | ||||
| Medium | 8.31 (4.25 to 17.50)*** | |||
| High | 2.23 (0.12 to 11.57)° | |||
| History of dental care (no: aOR=1) | 0.65 (0.47 to 0.89)** | |||
| History of blood transfusion (no: aOR=1) | 4.67 (1.96 to 9.90)*** | 3.65 (1.47 to 7.79)*** | ||
| History liver problems (no: aOR=1) | 3.13 (1.46 to 6.07)*** | 3.40 (1.72 to 6.19)*** | ||
| Abortion or caesarean section or difficult childbirth† (no: aOR=1) | 2.40 (1.20 to 4.60)* | |||
| Living with someone who has had viral hepatitis‡ (no: aOR=1) | 6.03 (3.43 to 10.08)*** | |||
| Sexual identity (no sex: aOR=1) | ||||
| Heterosexual women | 5.88 (1.24 to 105.03)° | |||
| Heterosexual men | 4.75 (0.95 to 86.29)° | |||
| Homosexual and bisexual | 44.60 (7.45 to 849.71)*** | |||
| Vaccination against HBV (no: aOR=1) | 0.63 (0.41 to 0.96)* | 0.21 (0.11 to 0.36)*** |
*P<0.05, **p<0.01, ***p<0.001, °NS.
†Only in female participants.
‡Mother, sexual partner, household member.
aOR, adjusted OR; HBV, hepatitis B virus; HCV, hepatitis C virus.
Table 3.
Score assignment
| β-coefficient | β-coefficient multiplied by 5 | Score mark | |
| HBV infection (men) | |||
| Endemic area of HBV (low=0) | |||
| Medium | 2.03 | 10.15 | 10 |
| High | 2.76 | 13.80 | 14 |
| History of dental care (no=0) | −0.44 | −2.20 | −2 |
| History liver problems (no=0) | 1.14 | 5.70 | 6 |
| Vaccination for hepatitis B (no=0) | −0.46 | −2.30 | −2 |
| Risk score (endemic area of HBV: low=0; medium=10; high=14)–2×(history of dental care)+6×(history liver problems)–2×(vaccination for hepatitis B) | |||
| HBV female (women) | |||
| Endemic area of HBV (low=0) | |||
| Medium | 1.54 | 7.70 | 8 |
| High | 1.90 | 9.50 | 10 |
| History liver problems (no=0) | 1.22 | 6.10 | 6 |
| Living with someone who has had viral hepatitis (no=0) | 1.80 | 9.00 | 9 |
| Vaccination for hepatitis B (no=0) | −1.58 | −7.90 | −8 |
| Risk score (female): (endemic area of HBV: low=0; medium=8; high=10)+6×(history liver problems)+9×(living with someone who has had viral hepatitis)–8×(vaccination for hepatitis B) | |||
| HCV infection | |||
| Endemic area of HCV (low=0) | |||
| Medium | 1.41 | 7.05 | 7 |
| High | 2.64 | 13.20 | 13 |
| History of blood transfusion (no=0) | 1.54 | 7.70 | 8 |
| Abortion or caesarean section or difficult childbirth* (no=0) | 0.87 | 4.35 | 4 |
| Risk score: (endemic area of HCV: low=0; medium=7; high=13)+8×(history of blood transfusion)+4×(abortion or cesarean section or difficult childbirth) | |||
| HIV infection | |||
| Endemic area of HIV (low=0) | |||
| Medium | 2.12 | 10.60 | 11 |
| High | 0.80 | 4.0 | 4 |
| History of blood transfusion (no=0) | 1.29 | 6.45 | 6 |
| Sexual identity (no sex=0) | |||
| Heterosexual women | 1.77 | 8.85 | 9 |
| Heterosexual men | 1.56 | 7.80 | 8 |
| Homosexual and bisexual | 3.80 | 19.0 | 19 |
| Risk score: (endemic area of HIV: low=0; medium=11; high=4)+6×(history of blood transfusion)+(sexual identity: no sex=0; heterosexual women=9; heterosexual men=8; homosexual and bisexual=19) | |||
*Only in female participants.
HBV, hepatitis B virus; HCV, hepatitis C virus.
For HBV infection in men: endemic area of HBV, history of dental care, history of liver problems and vaccination against HBV.
For HBV infection in women: endemic area of HBV, history of liver problems, living with someone who has had viral hepatitis and vaccination against HBV.
For HCV infection: endemic area of HCV, history of blood transfusion and abortion, or caesarean section, or difficult childbirth.
For HIV infection: endemic area of HIV, history of blood transfusion and sexual identity.
Determination of cut-off points
The sensitivity, specificity and area under the curve (AUC) values and their 95% CI corresponding to each cut-off are detailed in table 4. These cut-off points were used to differentiate participants with a high risk of each infection (ie, HBV, HCV and HIV infection) from those with a low risk. Indeed, participants whose score was less than the cut-off were supposed to have low risk.
Table 4.
Performance parameters
| Optimal threshold (95% CI) |
Sensitivity (95% CI) |
Specificity (95% CI) |
AUC (95% CI) |
Score ≥optimal threshold | Negative rapid test | Positive rapid test | Total | |
| HBV infection (men) | 2.5 (2.5 to 7.5) | 88.34 (73.62 to 92.64) | 58.81 (57.76 to 71.49) | 79.22 (76.18 to 82.26) | No | 3144 (TN) | 19 (FN) | 3163 |
| Yes | 2202 (FP) | 144 (TP) | 2346 | |||||
| Total | 5346 | 163 | 5509 | |||||
| HBV infection (women) | 6.5 (0.5 to 6.5) | 63.85 (60.00 to 86.15) | 79.38 (60.95 to 80.10) | 78.83 (74.54 to 82.10) | No | 7312 (TN) | 47 (FN) | 7359 |
| Yes | 1899 (FP) | 83 (TP) | 1982 | |||||
| Total | 9211 | 130 | 9341 | |||||
| HCV infection | 9.5 (9.5 to 12.5) | 75.61 (58.54 to 87.80) | 67.58 (66.90 to 74.83) | 75.95 (68.58 to 83.32) | No | 10 256 (TN) | 10 (FN) | 10 266 |
| Yes | 4919 (FP) | 31 (TP) | 4950 | |||||
| Total | 15 175 | 41 | 15 216 | |||||
| HIV infection | 10.5 (10.0 to 18.5) | 81.25 (68.75 to 91.67) | 77.12 (76.59 to 82.85) | 82.15 (74.54 to 87.99 | No | 11 608 (TN) | 9 (FN) | 11 617 |
| Yes | 3444 (FP) | 39 (TP) | 3483 | |||||
| Total | 15 052 | 48 | 15 100 |
AUC, area under the curve; FN, false negative; FP, false positive; HBV, hepatitis B virus; HCV, hepatitis C virus; TN, true negative; TP, true positive.
Furthermore, 5509 men realised a rapid test for HBV and completed the TROD screen questionnaire. The optimal threshold for HBV was 2.5 (95% CI 2.5% to 7.5%) (table 4). At this cut-off, we would have performed 2346 tests, detected 144 HBV infections, avoided 3163 tests, and missed 19 HBV infections (table 4).
Regarding women, 9341 individuals realised a rapid test for HBV and completed the TROD screen questionnaire during their visits. The optimal threshold for HBV was 6.5 (95% CI 0.5 to 6.5) (table 4). At this cut-off, we would have performed 1982 tests, detected 83 HBV infections and avoided 7359 tests but missed 47 HBV infections (table 4).
For HCV, 15 216 migrants realised a rapid test during their visits and completed the TROD screen questionnaire. The optimal threshold for HCV was 9.5 (95% CI 9.5 to 12.5) (table 4). At this cut-off, we would have performed 4950 tests, detected 31 HCV infections and avoided 10 266 tests but missed 10 HCV infections (table 4).
For HIV infection, 15 100 migrants realised a rapid test during their visits and completed the TROD screen questionnaire. The optimal threshold for HBV was 10.5 (95% CI 10.0 to 18.5) (table 4). At this cut-off, we would have performed 3483 tests, detected 39 HIV infections, avoided 11 617 tests and missed 9 HIV infections (table 4).
Discussion
This large French nationwide study was carried out among migrants in order to evaluate the capacity of the TROD Screen questionnaire to predict HBV, HCV or HIV infection, which may assist health practitioners and policy-makers in optimal screening of these infections in migrants. Therefore, we developed a risk score for these infections using a combination of participants’ characteristics, including sociodemographic, personal health-related history and behaviours, mainly sexual behaviours. With the determination of a cut-off point, this score allowed us to classify participants into subgroups at low and high risk for these infections.
In view of the AUC, the specificity and the sensitivity, our scoring models showed good discrimination and calibration, particularly for HBV infection in men and HIV infection. For a predictive questionnaire, it is expected to have a sensitivity greater than 80% with lower specificity compared with the rapid test. For HBV infection in women and HCV infection, even though the specificity and the sensitivity were not good enough, their discriminatory capacity remained acceptable (AUC>70%). Globally, as demonstrated by our results, the use of these tools could avoid a considerable number of rapid tests, resulting in a reduction in the workload of health professionals.
The endemic area of origin of the participants remains an important characteristic in the construction of these scores. Indeed, the endemic area is predictive of the risk of HBV, HCV and HIV infection among migrants in France. Participants from medium and/or high endemicity areas were the most likely to have a positive rapid test. In medium-endemicity or high-endemicity areas, which are generally countries with limited resources, strategies for the prevention and diagnosis of these infections remain poorly available or accessible.20 Therefore, there is a low rate of screening tests for viral hepatitis as well as the availability of verified blood products. Migrants from these areas have a high probability of having been in contact with or being chronic carriers of one of these viruses before their migration process.21
For HBV infection, either in men or women, vaccination against HBV and a history of liver problems were predictive of the risk of HBV. Vaccination against HBV was associated with a low probability of having an HBV-positive test in both men and women. In low-income countries, availability and access to HBV vaccines remain a challenge.22 In these regions, contact with this virus occurred for the most part in the perinatal period and during early childhood, most often leading to an evolution to chronicity.22–24 Even though vaccination against HBV (with a complete or incomplete schedule) predicted a low risk for HBV infection, participants who reported a history of liver pathology were more likely to have a positive test for HBV. Even if only 2%–10% of HBV infections are symptomatic or evolve to chronic form,25 it is important to make further investigations in migrants who have reported a history of liver problems, as suggested in our study.
In addition, the history of blood transfusions is also predictive of HCV and HIV infection. Till the last decade, in low-income countries, the risk of transfusion-transmitted infections remained high due to unsafe transfusion practices. HIV or HCV are the main transfusion-transmitted infections reported and must be at the centre of prevention strategies.26 27 These unsafe transfusion practices in those regions are more often correlated to other unsafe practices, especially in invasive procedures like abortion or caesarean section. Thus, in our predictive analysis, in migrant women, history of abortion, caesarean section or difficult childbirth and living with someone who had viral hepatitis have predicted, respectively, HCV and HBV infection.
Reporting being homosexual or bisexual has been found to be highly predictive of HIV infection among migrants. This is in line with several studies, which highlighted that men who have sex with men are at higher risk of sexually transmitted infections, including HIV infection, especially when having multiple sexual partners and unprotected anal intercourse.28 29
Despite our study providing useful risk score for predicting HIV, HBV and HCV infections, several limitations need to be addressed. It is possible that answers to questions may be subject to subdeclarations. Even though it is a nationwide study, participants may not be representative of all migrants in France, and this tool needs external validation before using it in another context. Furthermore, variables such as sexually transmitted infections and alcohol have not been included in the questionnaire, since they may be the predictors of HIV, HBV or HCV infection.
Conclusion
The TROD screen questionnaire showed acceptable overall performance for predicting HIV, HBV and HCV infections in migrants seen in OFII centres. That should provide the OFII’s medical staff and other healthcare workers receiving migrants, optimal diagnosis tools based on the risk assessment, leading to the proposal of a rapid screening test for these infections. It could also be helpful for orienting those at high risk for biological confirmation.
Supplementary Material
Acknowledgments
We sincerely thank the study participants and Nephrotek.
Footnotes
Contributors: MD: conceptualisation, methodology, validation, writing-review and editing, project administration and is responsible for the overall content as guarantor. IY: formal analysis, data curation, writing-original draft preparation. LY-K: data collection, formal analysis, data curation, writing-review and editing. PB: data collection, formal analysis, data curation, writing-review and editing. FT: conceptualisation, methodology, validation, writing-review and editing, project administration. OR-T: writing-review and editing, project administration. FR-T: writing-review and editing. FL: writing-review and editing. DZ: writing-review and editing. OC conceptualisation, methodology, validation, writing-review and editing. All authors approved the final version of the manuscript for publication.
Funding: The STRADA study was funded by the Asylum, Migration and Integration Funds (AMIF), the French Office for Immigration and Integration (OFII), ViiV Healthcare, Gilead Sciences and Abbvie.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were not involved in the design, or conduct, or reporting, or dissemination plans of this research.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement
Data are available upon reasonable request. The datasets used during the current study are not publicly available but could be available from the corresponding author on reasonable request.
Ethics statements
Patient consent for publication
Consent obtained directly from patient(s).
Ethics approval
This study involves human participants and all procedures of this study were in accordance with the ethical approval granted by an Independent Ethics Committees (CPP Ile de France IV, No IRB 3835, Ref. 2016/43NI) and by the French data protection authority (CNIL) (no 2008669). All methods were carried out in accordance with relevant guidelines and regulations. Participants gave informed consent to participate in the study before taking part.
References
- 1. World Health Organization (WHO) . Hepatitis B, Available: https://www.who.int/news-room/fact-sheets/detail/hepatitis-b
- 2. World Health Organization (WHO) . Global hepatitis report 2017. Geneva: WHO. 2017.
- 3. Ott JJ, Stevens GA, Groeger J, et al. Global epidemiology of hepatitis B virus infection: new estimates of age-specific HBsAg Seroprevalence and Endemicity. Vaccine 2012;30:2212–9. 10.1016/j.vaccine.2011.12.116 Available: 10.1016/j.vaccine.2011.12.116 [DOI] [PubMed] [Google Scholar]
- 4. Sharma S, Carballo M, Feld JJ, et al. Immigration and viral hepatitis. J Hepatol 2015;63:515–22. 10.1016/j.jhep.2015.04.026 Available: 10.1016/j.jhep.2015.04.026 [DOI] [PubMed] [Google Scholar]
- 5. Nørredam M. Migration and health: exploring the role of migrant status through register-based studies. Dan Med J 2015;62:B5068. [PubMed] [Google Scholar]
- 6. Rossi C, Shrier I, Marshall L, et al. Seroprevalence of chronic hepatitis B virus infection and prior immunity in immigrants and refugees: a systematic review and meta-analysis. PLoS One 2012;7:e44611. 10.1371/journal.pone.0044611 Available: 10.1371/journal.pone.0044611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Greenaway C, Thu Ma A, Kloda LA, et al. The Seroprevalence of hepatitis C antibodies in immigrants and refugees from intermediate and high Endemic countries: A systematic review and meta-analysis. PLoS ONE 2015;10:e0141715. 10.1371/journal.pone.0141715 Available: 10.1371/journal.pone.0141715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Delmas G, Ndeikoundam Ngangro N, Brouard C, et al. n.d. Surveillance Surcegidd: Dépistage et diagnostic Du VIH, des Hépatites B et C et des IST Bactériennes en Cegidd en 2020. Bull Epidémiol Hebd;2021:401–12. [Google Scholar]
- 9. Saboni L, Brouard C, Gautier A, et al. Prévalence des Hépatites Chroniques C et B, et Antécédents de Dépistage en population Générale en 2016: contribution À une Nouvelle Stratégie de Dépistage, Baromètre de Santé Publique France-Barotest. Bull Epidémiol Hebd 2019:469–77. [Google Scholar]
- 10. Larsen C, Limousi F, Rahib D, et al. Infections VIH et VHB Parmi LES Afro-Caribéens D’Île-de-France: des Prévalences Élevées et des Dépistages Insuffisants. Bull Epidémiol Hebd 2017:609–16. [Google Scholar]
- 11. Pioche C, Léon L, Vaux S, et al. Dépistage des Hépatites B et C en France en 2016, Nouvelle Édition de L’Enquête Labohep. Bull Épidémiologique Hebd 2018:188–95. [Google Scholar]
- 12. van der Veen YJ, Voeten HA, de Zwart O, et al. Awareness, knowledge and self-reported test rates regarding hepatitis B in Turkish-Dutch: a survey. BMC Public Health 2010;10:512. 10.1186/1471-2458-10-512 Available: 10.1186/1471-2458-10-512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Weinbaum CM, Williams I, Mast EE, et al. Recommendations for identification and public health management of persons with chronic hepatitis B virus infection. MMWR Recomm Rep Morb Mortal Wkly Rep Recomm Rep 2008;57:1–20. [PubMed] [Google Scholar]
- 14. Duracinsky M, Thonon F, Bun S, et al. Good acceptability of HIV, HBV, and HCV screening during immigration medical check-up amongst migrants in France in the STRADA study. PLoS One 2020;15:e0235260. 10.1371/journal.pone.0235260 Available: 10.1371/journal.pone.0235260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Chevaliez S, Poiteau L, Rosa I, et al. Prospective assessment of rapid diagnostic tests for the detection of antibodies to hepatitis C virus, a tool for improving access to care. Clin Microbiol Infect 2016;22:459. 10.1016/j.cmi.2016.01.009 Available: 10.1016/j.cmi.2016.01.009 [DOI] [PubMed] [Google Scholar]
- 16. Poiteau L, Soulier A, Roudot-Thoraval F, et al. Performance of rapid diagnostic tests for the detection of anti-HBs in various patient populations. J Clin Virol 2017;96:64–6. 10.1016/j.jcv.2017.09.012 Available: 10.1016/j.jcv.2017.09.012 [DOI] [PubMed] [Google Scholar]
- 17. Stafylis C, Bristow CC, Natoli LJ, et al. Field evaluation of a dual rapid human immunodeficiency virus and Treponemal Syphilis rapid test in community-based clinics in Los Angeles and New York. Diagn Microbiol Infect Dis 2019;93:325–8. 10.1016/j.diagmicrobio.2018.10.002 Available: 10.1016/j.diagmicrobio.2018.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Madan P, Elayda MA, Lee V-V, et al. Predicting major adverse cardiac events after percutaneous coronary intervention: the Texas heart Institute risk score. Am Heart J 2008;155:1068–74. 10.1016/j.ahj.2008.01.034 Available: 10.1016/j.ahj.2008.01.034 [DOI] [PubMed] [Google Scholar]
- 19. Austin PC, Lee DS, D’Agostino RB, et al. Developing Points‐Based Risk‐Scoring systems in the presence of competing risks. Stat Med 2016;35:4056–72. 10.1002/sim.6994 Available: 10.1002/sim.6994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Fopa D, Candotti D, Tagny CT, et al. Occult hepatitis B infection among blood donors from Yaoundé, Cameroon. Blood Transfus 2019;17:403–8. 10.2450/2019.0182-19 Available: 10.2450/2019.0182-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klok S, van Dulm E, Boyd A, et al. Hepatitis B virus (HBV), hepatitis C virus (HCV) and human immunodeficiency virus (HIV) infections among Undocumented migrants and uninsured legal residents in the Netherlands: A cross-sectional study, 2018-2019. PLoS One 2021;16:e0258932. 10.1371/journal.pone.0258932 Available: 10.1371/journal.pone.0258932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zampino R, Boemio A, Sagnelli C, et al. Hepatitis B virus burden in developing countries. World J Gastroenterol 2015;21:11941–53. 10.3748/wjg.v21.i42.11941 Available: 10.3748/wjg.v21.i42.11941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peto TJ, Mendy ME, Lowe Y, et al. Efficacy and effectiveness of infant vaccination against chronic hepatitis B in the Gambia hepatitis intervention study (1986–90) and in the nationwide Immunisation program. BMC Infect Dis 2014;14:7. 10.1186/1471-2334-14-7 Available: 10.1186/1471-2334-14-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Franco E. Epidemiology and prevention in developing countries. WJH 2012;4:74. 10.4254/wjh.v4.i3.74 Available: 10.4254/wjh.v4.i3.74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Trépo C, Chan HLY, Lok A. Hepatitis B virus infection. Lancet 2014;384:2053–63. 10.1016/S0140-6736(14)60220-8 Available: 10.1016/S0140-6736(14)60220-8 [DOI] [PubMed] [Google Scholar]
- 26. Birhaneselassie M. Prevalence of transfusion-transmissible infections in donors to an Ethiopian blood bank between 2009 and 2013 and donation factors that would improve the safety of the blood supply in underdeveloped countries. Lab Med 2016;47:134–9. 10.1093/labmed/lmw003 Available: 10.1093/labmed/lmw003 [DOI] [PubMed] [Google Scholar]
- 27. Abdella S, Moshago Berheto T, Tolera G, et al. Sero-prevalence of transfusion transmittable infections: HIV, hepatitis B, C and Treponema Pallidum and associated factors among blood donors in Ethiopia: A retrospective study. PLoS One 2020;15:e0241086. 10.1371/journal.pone.0241086 Available: 10.1371/journal.pone.0241086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Keshinro B, Crowell TA, Nowak RG, et al. High prevalence of HIV, Chlamydia and Gonorrhoea among men who have sex with men and Transgender women attending trusted community centres in Abuja and Lagos, Nigeria. J Int AIDS Soc 2016;19:21270. doi:21270 Available: 10.7448/IAS.19.1.21270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yaya I, Diallo F, Kouamé MJ-B, et al. Decrease in incidence of sexually transmitted infections symptoms in men who have sex with men enrolled in a quarterly HIV prevention and care programme in West Africa (Cohmsm ANRS 12324-expertise France). Sex Transm Infect 2022;98:85–94. 10.1136/sextrans-2020-054755 Available: 10.1136/sextrans-2020-054755 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available upon reasonable request. The datasets used during the current study are not publicly available but could be available from the corresponding author on reasonable request.