Abstract
Purpose
Globally, the number of children/adolescents exposed to HIV but uninfected (HIV-exposed uninfected, HEU) is growing. The HEU outcomes: population-evaluation and screening strategies study was designed to provide population-level evidence of the impact of HIV and recent antiretroviral therapy regimen exposure on neurodevelopmental, hearing and mental health outcomes from infancy to adolescence.
Participants
The study includes a prospective mother–infant cohort and cross-sectional child/youth–caregiver cohorts conducted in Kenya.
Between 2021 and 2022, the study enrolled 2000 mother–infant pairs (1000 HEU and 1000 HIV-unexposed uninfected (HUU)) for longitudinal follow-up. Infants were eligible if they were aged 4–10 weeks and healthy. Mothers were eligible if their HIV status was known and were ≥18 years. Study visits are 6 monthly until the child reaches age 3 years.
Cross-sectional cohorts spanning ages 3–18 years started enrolment in 2022. Target enrolment is 4400 children/youth (4000 HEU and 400 HUU). Children and youth are eligible if they are HIV negative, maternal HIV status and timing of diagnosis is known, and caregivers are ≥18 years.
Data on infant/child/youth growth, neurodevelopment, mental health, morbidity and hearing are collected at enrolment using standardised tools. Dry blood spots samples are collected for telomere length assessment at baseline and yearly for the longitudinal cohort. Growth z-scores, neurodevelopmental scores, telomere length and prevalence of developmental and hearing problems will be compared between HEU/HUU populations.
Findings to date
Full cohort enrolment for the longitudinal cohort is complete and participants are in follow-up. At 1 year of age, comparing HEU to HUU neurodevelopment using the Malawi developmental assessment tool, we found that HEU infants had higher language scores and comparable scores in fine motor, gross motor and social scores. The cross-sectional cohort has enrolled over 2000 participants and recruitment is ongoing.
Future plans
Longitudinal cohort follow-up and enrolment to the cross-sectional study will be completed in June 2024.
Keywords: INFECTIOUS DISEASES, HIV & AIDS, Epidemiology, Paediatric infectious disease & immunisation, Adolescent, Cross-Sectional Studies
STRENGTHS AND LIMITATIONS OF THIS STUDY.
This is a large prospective study of contemporaneously enrolled mother–infant pairs, including 1000 mothers living with HIV enrolled in the era of optimised antiretroviral therapy including dolutegravir-based (DTG) regimens.
The timing of enrolment enables a comparison of outcomes among infants exposed to newly rolled out DTG regimens versus those exposed to efavirenz enabling us to assess DTG exposure on infant outcomes.
Parallel longitudinal and cross-sectional cohorts allow innovative and efficient approaches to evaluate HIV-exposed uninfected from infancy to adolescence.
The population enrolled may have some selection bias. Participants were not enrolled in pregnancy; therefore, the study will likely underascertain perinatal morbidity and preterm birth outcomes.
Follow-up visits are conducted every 6 months instead of more frequently as infant growth and development are rapid in infancy and early childhood, therefore, longitudinal analysis models will be limited by few time points.
Introduction
Every year, an estimated over 1.2 million children exposed to HIV (HIV-exposed uninfected, HEU) are born; a majority in sub-Saharan Africa (SSA).1 With the expansion of prevention of vertical transmission through HIV programmes—81% of pregnant mothers were receiving antiretroviral therapy (ART) in 20212—most HEU infants are exposed to ART in utero and during breastfeeding. There are an estimated 14.8 million HEU in SSA,1 with continued growth in the population of HEU expected given the high prevalence of HIV among women of reproductive age in SSA.
Evidence from systematic reviews and meta-analysis demonstrate approximately 50% higher mortality among HEU in the era of maternal ART3 and an over 50% higher risk of morbidity from pneumonia and diarrhoeal diseases compared with HIV-unexposed uninfected children (HUU).4 Excess morbidity and mortality could be attributed to lack of or poor breastfeeding practices and poor maternal health,5 challenges that could potentially partly be addressed by newer and more effective ART and breastfeeding guidelines. Additionally, there is evidence of linear growth disparities between HEU and HUU, with consistent evidence of stunting among HEU.6 7 Even in the ART era, there is evidence of a higher stunting prevalence in HEU than HUU, both in areas with high and low general prevalence of stunting.7–9 HEU also have a higher prevalence of underweight and some studies have reported lower mean head circumference scores among HEU associated with specific ART regimens.7 10 Neurodevelopment among HEU may also be impaired, with HEU showing evidence of cognitive impairment, motor and language delays compared with HUU.11–14 However, this evidence is mixed, with some studies showing no differences except for language delays.13 15 16 Some studies have identified hearing differences comparing HEU to HUU; with HEU more likely to have abnormalities or be referred for additional assessments.17 18 Many of these studies have been conducted in infancy or early childhood, and few have studied older HEU. Similar to studies on younger children, the evidence for HEU/HUU differences among older HEU is mixed with some studies showing no differences in cognitive, motor, attention or executive functioning19 and others showing lower math grade scores.20 A systematic review comparing cognitive, neurodevelopmental or behavioural differences among older HEU and HUU identified that HEU had significantly lower performance scores in at least one measure in 7 of 11 studies,21 suggesting concern for future functioning as HEU ages to adulthood.
Prenatal development of mental illness has been studied for illnesses such as schizophrenia, autism spectrum disorders (ASD), attention deficit hyperactivity disorder (ADHD), depression and suicide.22–26 The pathogenesis is thought to be related to maternal infections, maternal stress, placental pathology, fetal brain pathology, genetic predisposition and epigenetic changes due to placental inflammation.22 27 In addition to the risk of opportunistic infections, pregnant women living with HIV in SSA have a high prevalence of mental health challenges and stress and lack support for care.28 Since 2019, guidelines for ART use have switched from first-line efavirenz (EFZ)-based ART to dolutegravir (DTG)-based regimes.29 DTG regimens are associated with fewer adverse drug reactions, better virological profile and are associated with improved maternal health.30 31 However, both DTG and efavirenz are associated with mental health symptoms among mothers living with HIV, but at low prevalence.32–34 Among HEU, there is suggestive evidence that fetal EFZ and DTG exposure is associated with increased neurologic conditions.35 Animal models suggest direct exposure to DTG on the embryo brain during a critical period of development, and therefore, potential for neurotoxicity.36
Many of the existing studies comparing HEU/HUU have examined short-term outcomes among HEU, yet the impact of exposure to drugs or infections in utero could manifest as neurodevelopmental or psychiatric disorders later in childhood, adolescence or in adulthood. Longitudinal studies that track maternal and infant mental and neurodevelopment are lacking; and this gap is compounded by weak health system tracking and reporting for adverse drug reactions for pregnancy exposures.
As HEU populations grow, it is critical to develop robust health services and monitoring systems that can track the health and well-being of this growing population. It is, therefore, imperative to develop screening procedures to support longer-term assessments for neurodevelopment and mental health and enhance referrals for advanced brain and mental healthcare. While differences may be subtle in the era of optimised ART, they could potentially impact future academic and career success. Many countries in SSA lack health systems to track HEU beyond the first 2 years or life or after breast feeding once HIV status is confirmed. Building these systems will require incorporation of simple screening tools useful for long-term surveillance, screening and referral.
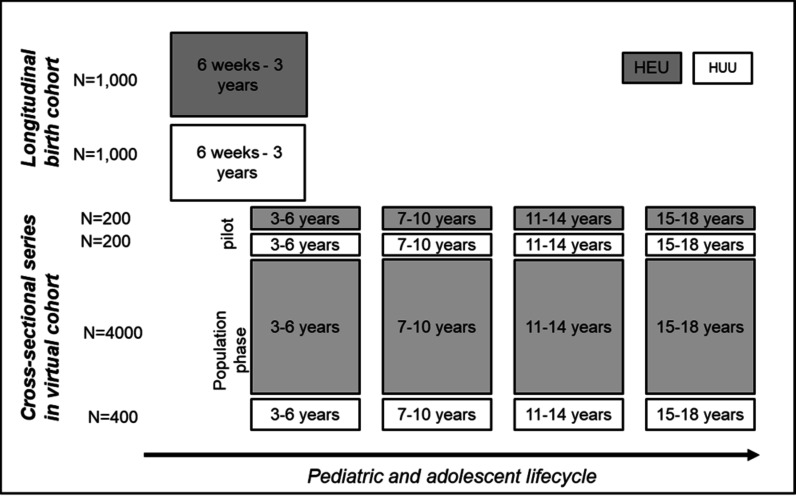
The HEU outcomes: population-evaluation and screening strategies (HOPE) study aims to (1) longitudinally compare growth, hearing, neurodevelopment and telomere length among HEU and HUU enrolled at 4–10 weeks of life and followed 6 monthly to 3 years of age, (2) in a cross-sectional study design, pilot and test a mobile screening strategy to detect neurodevelopmental and mental health outcomes in older HEU (ages 3–18 years) and (3) to support future programmatic scale up and integration of HEU screening, estimate the costs of a population screening strategy for older HEU and convene a stakeholder workshop on programmatic integration of HEU screening. Parallel study designs were selected to provide evidence on early outcomes among HEU with universal maternal ART (longitudinal cohort) and provide an estimate of the prevalence of neurodevelopmental and mental health outcomes among older HEU (cross-sectional cohort). The cross-sectional study was preceded by a pilot phase to test recruitment strategies and identify tools for the larger population-based study. Figure 1 summarises the study design and cohorts.
Figure 1.
HOPE study design and cohorts. HEU, HIV-exposed uninfected; HOPE, HEU outcomes: population-evaluation and screening strategies; HUU, HIV-unexposed uninfected.
Cohort description
Study sites and setting
Study sites are located in three counties in Kenya with varying HIV prevalence (Nairobi 6.1%, Kisumu 16.3% and Homa Bay 20.7%).37 The HOPE study is being conducted in seven public sector maternal and child health (MCH) clinics, three in urban Nairobi (Mathare North Health Centre, Riruta Health Centre, Kayole II Sub-County Hospital) and four in Western Kenya (Kisumu County Referral Hospital, Lumumba Sub-County Hospital, Migosi Sub-County Hospital and Rachuonyo Sub-County Hospital). Additional recruitment facilities near the catchment areas refer participants to primary study sites.
From 2014, the Kenyan National guidelines for HIV treatment recommended universal and lifelong ART for all people living with HIV—including pregnant women as soon as possible after diagnosis. Current first-line regimens for adults/pregnant women include tenofovir/lamivudine/DTG starting 201838 but were largely tenofovir/lamivudine/EFZ since 2014. At the time of the longitudinal cohort recruitment, Kenya was transitioning from EFZ to DTG-based regimens for pregnant women living with HIV.
Longitudinal cohort
Eligibility criteria
Postpartum women living with or not living with HIV presenting at participating MCH clinics were eligible to participate if their infants were aged 4–10 weeks and had no major congenital defects, and for women if they were aged ≥18 years and were planning to reside in the study catchment area for the next 3 years.
Determination of HIV status
A combination of medical records and HIV testing is used to determine HIV status of mothers and children. In Kenyan MCH clinics, maternal HIV testing is part of standard antenatal care and results are recorded in a mother–baby MCH booklet carried by mothers to every MCH clinic visit. HIV status at enrolment is determined from the MCH booklet or clinic records at enrolment and at subsequent visits. Post partum, national guidelines recommend HIV testing for mothers at 6 weeks post partum then every 6 months for 1 year and then yearly or more frequently based on their risk of HIV acquisition. For infants, virological HIV testing is conducted at 6 weeks, 6 and 12 months, and antibody testing at 18 months of age or 6 weeks after the cessation of breast feeding. Infant HIV status is abstracted from MCH/HIV care records.
Recruitment and retention
Enrolment was conducted at the seven participating MCH clinics. A total of 2000 mother–infant pairs (1000 HEU and 1000 HUU) were enrolled between March 2021 and June 2022. To ensure retention, the study collects locator information including individual and next of kin phone numbers and home location, conducts reminder phone calls before scheduled visits and follows up with participants who miss visits either via phone call or home visits. Additionally, staff conduct monthly phone or physical non-study check-ins as needed to maintain rapport. The baseline characteristics of participants are summarised in table 1.
Table 1.
Baseline cohort characteristics for the longitudinal cohort
| Overall (N=2000) N (%) or median (IQR) |
HEU (N=1000) n (%) or median (IQR) |
HUU (N=1000) n (%) or median (IQR) |
|
| n=2000 | n=1000 | n=1000 | |
| Region | |||
| Western Kenya | 1230 (61.5%) | 626 (62.6%) | 604 (60.4%) |
| Nairobi | 770 (38.5%) | 374 (37.4%) | 396 (39.6%) |
| Caregiver characteristics | |||
| Age in years | 29 (24, 33) | 31 (27, 35) | 26 (23, 30) |
| Primary caregiver mother | 1997 (99.9%) | 1000 (100%) | 997 (99.7%) |
| Education | |||
| None | 16 (0.8%) | 12 (1.2%) | 4 (0.4%) |
| Primary | 760 (38.0%) | 495 (49.5%) | 265 (26.5%) |
| Secondary | 900 (45.0%) | 383 (38.3%) | 517 (51.8%) |
| College | 284 (14.2%) | 98 (9.8%) | 186 (18.6%) |
| University | 39 (2.0%) | 12 (1.2%) | 27 (2.7%) |
| Marital status | |||
| Married monogamous | 1534 (76.7%) | 720 (72.0%) | 814 (81.5%) |
| Married polygamous | 158 (7.9%) | 114 (11.4%) | 44 (4.4%) |
| Single, separated, widowed | 216 (10.8%) | 138 (13.8%) | 78 (7.8%) |
| Steady partner | 91 (4.6%) | 28 (2.8%) | 63 (6.3%) |
| Employment status | |||
| Professional | 135 (6.8%) | 56 (5.6%) | 79 (7.9%) |
| Casual | 237 (11.9%) | 136 (13.6%) | 101 (10.1%) |
| Unemployed | 1421 (71.1%) | 714 (71.4%) | 707 (70.7%) |
| Other | 207 (10.4%) | 94 (9.4%) | 113 (11.3%) |
| Food insecurity | |||
| Little to no hunger | 1680 (84.0%) | 770 (77.0%) | 910 (91.0%) |
| Moderate hunger | 309 (15.5%) | 220 (22.0%) | 89 (8.9%) |
| Severe hunger | 11 (0.6%) | 10 (1.0%) | 1 .01%) |
| Infant characteristics | |||
| Sex, female | 1011 (50.6%) | 498 (49.8%) | 513 (51.3%) |
| Age in days | 44 (42, 48) | 43 (42, 49) | 44 (42, 47) |
| Breastfeeding at enrolment | 1995 (99.8%) | 995 (99.5%) | 1000 (100%) |
HEU, HIV-exposed uninfected; HUU, HIV-unexposed uninfected.
Study procedures: longitudinal cohort
Study visits: In-person study visits are conducted every 6 months by trained study staff. Where participants are unable to attend the study visits, phone interviews are conducted. At every visit, standard questionnaires are used to collect data on sociodemographic, family characteristics, medical history and maternal mental health status (table 2).
Table 2.
HOPE longitudinal cohort schedule for procedures and data collection
| Enrolment (6 weeks) |
6 months | 12 months | 18 months | 24 months | 30 months | 36 months | |
| Mother procedures | |||||||
| Informed consent | X | ||||||
| Locator information | X | X | X | X | X | X | X |
| Questionnaire | X | X | X | X | X | X | X |
| Medical record review | X | X | X | X | |||
| Mental health (K-10, HITS, PHQ-9) | X | X | X | X | X | X | X |
| Anthropometry | X | X | X | X | X | X | X |
| Dry blood spot sample* | X | ||||||
| Qualitative interviews* | X | ||||||
| Child procedures | |||||||
| Anthropometry | X | X | X | X | X | X | X |
| MDAT screen | X | X | X | X | X | X | X |
| Hearing screen | X | X | X | X | |||
| ASD assessment | X | X | X | ||||
| Parent SDQ assessment | X | ||||||
| Dry blood spot sample | X | X | X | X |
Maternal dry blood spots between months 12 and 36, and qualitative interviews at one point.
*Procedures could be done at variable times.
ASD, autism spectrum disorder; HITS, Hurt, Insult, Threaten, Scream; HOPE, HEU outcomes: population-evaluation and screening strategies; K-10, Kessler Psychological distress test; MDAT, Malawi developmental assessment tool; PHQ-9, Patient Health Questionnaire version 9; SDQ, Strengths and Difficulties Questionnaire.
Growth assessments: Standard anthropometric measures including weight, height/length, head circumference, and mid-upper arm circumference (MUAC) in infants/children and maternal weight, height (at enrolment), and MUAC are obtained in duplicate by trained study staff.
Neurodevelopmental assessments: Infant/child neurodevelopmental assessments are conducted using the Malawi Developmental Assessment Tool (MDAT), a culturally validated tool developed in Malawi.39 The MDAT assesses gross motor, fine motor, social and language domains. Tests are administered in the preferred language of the child and mother. Scripts for each assessment are available in English, Kiswahili or Dholuo by trained study staff. At 18 and 24 months, children are assessed for ASD using the Modified Checklist for Autism in Toddlers, Revised and with follow-up,40 and at 3 years, the INCLEN diagnostic tool for ASD.41 At 3 years, children are screened for mental health disorders using the parent Strengths and Difficulties Questionnaire (SDQ)42 that includes difficulty scales for emotional symptoms, conduct problems, hyperactivity/inattention and peer relationship problems and a scale for prosocial behaviour.
Hearing: Gross clinical assessment and otoscopy are initially conducted for ear deformities and wax impaction. Distortion Product Otoacoustic Emission is used to screen for hearing disorders at enrolment and yearly in select facilities. Testing is done in each ear at four frequencies of 2, 3, 4 and 5 KHz. A definitive pass requires a 6 dB signal to noise ratio and minimum of −5 dB SPL in three out of four of the frequencies presented. Children who refer on OAE are referred for diagnostic Brain Evoked Response Auditory testing.
Dry blood spots (DBS): DBSs are collected from infants and children at enrolment and yearly and at one time point for mothers. Stored DBSs are batched for telomere analysis as well as infant COVID-19 antibody testing at enrolment (as a proxy for maternal COVID-19 exposure in pregnancy).
Qualitative interviews: We will conduct semistructured focus group discussions with up to 60 mothers and fathers of HOPE children to explore parental caregiving including paternal perspectives on child neurodevelopmental outcomes in HEU and HUU. Interviews will incorporate ‘participatory visual methods’ (eg, photovoice, family mapping) to engage group members in discussions and storytelling. Interviews will be based on the nurturing care framework developed by the WHO and UNICEF43 that describe the necessary components for optimal child development.
Exposure variables
The primary exposure variable is infant HIV exposure status. For women living with HIV, infants are assumed to be HEU unless they test HIV positive at scheduled testing intervals (6 weeks, 6, 12 and 18 months or 6 weeks after cessation of breast feeding). For women not living with HIV, infants are assumed HUU unless their mothers’ test HIV positive at scheduled testing intervals (6 weeks, 6, 12, 24, 36 months post partum) or less than 6 weeks before cessation of breast feeding.
Outcome variables
Primary outcomes include growth z scores (weight-for-age, weight-for-length/height, and length/height-for-age z scores (respectively) calculated using the WHO growth standards), neurodevelopmental scores (MDAT scores), hearing differences (deficit or no deficit) and infant telomere length. Secondary outcomes include the prevalence of ASD, total difficulties scores in children assessed using the parent SDQ and family caregiving experiences assessed using qualitative interviews.
Data analysis
For continuous outcomes (growth z-scores, MDAT domains and telomere length), we will compare mean differences among HEU and HUU using linear mixed-effects models with site included as a random intercept to account for site differences. We will determine if there is an association between telomere length and neurodevelopment. The analysis will adjust for potential confounders including maternal age, preterm birth, infant sex and birth weight. We will determine correlates overall and among HEU/HUU, such as sociodemographic factors and maternal viral suppression, ART regimen and ART duration among HEU. The proportion of HEU/HUU children who are stunted, wasted, or underweight or with hearing loss, ASD, and significant difficulties on the SDQ at 3 years will be compared using generalised linear mixed models with log link and binomial family, and random effect for site.
Study power
Estimated detectable differences and assumptions are summarised in table 3. With 1000 HEU and 1000 HUU in the longitudinal cohort, the study has 80% power to detect differences in MDAT scores, growth Z scores, telomere length and differences in the prevalence of hearing loss, ASD prevalence and prevalence of difficulties in the SDQ under the assumptions provided in table 3. Clinically significant differences in growth and MDAT z-scores are not established; however, we will in addition compare the prevalence of moderate to severe malnutrition and referrals for neurodevelopmental problems.
Table 3.
Expected detectable differences at 80% power and alpha of 0.05 and 20% attrition in the longitudinal cohort
| Minimum detectable difference or estimated prevalence among HEU | Projected sample sizes | Assumptions | |
| Longitudinal cohort | |||
| MDAT and growth z-scores | 0.10 | 1000 HEU, 1000 HUU | SD 1.0 |
| Hearing loss | 2.7% | 500 HEU, 500 HUU | HUU: 1% prevalence |
| ASD prevalence | 4.2% | 500 HEU, 500 HUU | HUU: 2% prevalence |
| SDQ difficulties | 25% | 500 HEU, 500 HUU | HUU: 20% prevalence |
| Telomere length | 0.13 | 500 HEU, 500 HUU | SD 1.0 |
| Cross-sectional cohort | |||
| ASQ delays (Roux AIDS 2019) | 0.14 | 1000 HEU, 100 HUU | HUU: 5% prevalence |
| SDQ difficulties | 29% | 2000 HEU, 200 HUU | HUU: 20% prevalence |
| ASD | 6.1% | 2000 HEU, 200 HUU | HUU: 2% prevalence |
| ADHD | 7.2% | 2000 HEU, 200 HUU | HUU: 2.7% prevalence |
| Depression | 26% | 500 HEU, 50 HUU | HUU: 10% prevalence |
ADHD, attention deficit hyperactivity disorder; ASD, autism spectrum disorder; ASQ, Ages and Stages Questionnaire; HEU, HIV-exposed uninfected; HUU, HIV-unexposed uninfected; MDAT, Malawi developmental assessment tool; SDQ, Strengths and Difficulties Questionnaire.
Cross-sectional cohort
Eligibility criteria
For the cross-sectional cohort, caregivers are eligible to participate if they have a child/adolescent aged 3–18 years who is HIV negative and for whom maternal HIV status during pregnancy or breast feeding is known. Caregivers are required to be aged ≥18 years.
Determination of HIV status
Among mothers living with HIV, maternal HIV-positive status during pregnancy with each child is determined using the mother–baby MCH booklet or HIV care clinic records. In the event no records are available, a maternal report of being diagnosed with HIV either during or prior to the pregnancy with each child is used. The HIV status of children is determined from medical records. If the child was not tested for HIV after the cessation of breastfeeding, HIV testing is offered to the child. Children ages 7–14 years provide assent for HIV testing without mention of HIV while older youth ages 15–17 are asked to provide assent (or consent for 18 years) with explicit mention of HIV testing. For HUU, children are assumed to be HIV negative if their mother is HIV negative and they are <15 years of age. Children ages 15–18 years are asked to provide assent/consent for HIV testing if their HIV test was >1 year ago.
Recruitment and retention
The pilot phase was conducted in 2021–2022 at one facility in western Kenya (Kisumu County Hospital) with an enrolment target of 400 (200 HEU and 200 HUU). This was followed by a population phase enrolment targeting 4400 children/youth (4000 HEU (1000 per age group of 3–5, 6–10, 11–14, 15–18 years) and 400 HUU (100 per age group)) in the three participating counties that began in November 2022. We anticipate a roving model for recruitment where recruitment is conducted at one site for several months before proceeding to a new site. Once enrolled, no study follow-up visits are conducted.
Study procedures: cross-sectional cohort
Data sources
Study visits: In-person study visits are conducted at enrolment by trained study staff during which they collect information on sociodemographics, family characteristics, medical history and maternal mental health status through surveys (table 4).
Table 4.
HOPE cross-sectional cohort schedule for procedures and data collection
| Ages 3–5 | Ages 6–10 | Ages 11–14 | Ages 15–18 | |
| Mother procedures | ||||
| Informed consent | X | X | X | X |
| Questionnaire | X | X | X | X |
| Medical record review | X | X | X | X |
| Anthropometry | X | X | X | X |
| Child/youth procedures | ||||
| Informed consent if age 18 | X | |||
| Child assent (starting age 7) | (X) | X | X | |
| Ages and Stages Questionnaire44 | X | |||
| Child disability screen*45 | X | X | ||
| ASD screen (INCLEN tools)41 | X | X | ||
| ADHD screen (INCLEN tools)46 | X | X | ||
| Parent SDQ42 | X | X | X | |
| Hearing and vision screening | X | X | X | X |
| NIH toolbox52 | X | X | X | |
| Youth SDQ42 | X | |||
| Cohen’s perceived stress score†47 | X | |||
| PHQ-9 depression screen‡48 | X | |||
| GAD-7 anxiety screen‡49 | X | |||
| Psychosis screen‡50 | X | |||
| AUDIT screen‡51 | X |
*Ends at age 9.
†Begins at age 14.
‡Ages 17–18 only.
ADHD, attention deficit hyperactivity disorder; ASD, autism spectrum disorder; AUDIT, Alcohol Use Disorders Identification Test; GAD-7, General Anxiety Disorder 7; HOPE, HEU outcomes: population-evaluation and screening strategies; INCLEN, Trust international tools for ASD/ADHD diagnosis; NIH, National Institutes of Health; PHQ-9, Patient Health Questionnaire version 9; SDQ, Strengths and Difficulties Questionnaire.
Growth assessments: Standard anthropometric measures for mothers and children, including weight, height and MUAC are obtained by trained study staff.
Mental health and neurodevelopmental assessments: A battery of neurodevelopment and mental health assessments are conducted by trained staff. Selected tools are amenable to use by nurses or community health workers without specialised training and are validated as diagnostic or screening tools. Table 4 summarises the tools used in the pilot and population phase and the age group in which they are used. In summary, for children aged 3–5 years, we will assess neurodevelopment using the Ages and Stages Questionnaire (ASQ).44 All children aged 6–10 will be assessed for disability using the children disability screen,45 and for ASD and ADHD using the INCLEN diagnostic tools.41 46 For youth aged 11–14, we will conduct mental health screening using the parent and youth SDQ questionnaire.42 For youth aged 15–18, we will screen for mental health using the Patient Health Questionnaire-9 (PHQ-9) for depression screening, the Generalised Anxiety Disorder-7 (GAD-7) for anxiety screening, Alcohol Use Disorders Identification Test for alcohol use, Cohen’s perceived stress scale for stress and the prodromal questionnaire for psychosis.47–51 All ages will be screened for hearing and vision abnormalities. A sample of 6–18 years will be assessed for neurocognitive scores using the NIH toolbox.52 Where available, diagnostic tests will be conducted either at the same visit or soon after for children who fail screening. For all tools, we will collect data on time taken to administer the tool.
Hearing: Hearing screening is done using HearTest,53 a pure tone tablet-based audiometer with cloud data management for children above 5 years of age. It is a behavioural response-dependent test used to identify conductive hearing loss even at extended high frequencies (8–16 kHZ). Children aged 3–5 years are screened using OAE due to their potential inability to respond to the behavioural hearing test.
Vision: Screening is done using the 3 m LEA or tumbling E eyechart for children aged less or more than 5 years, respectively.
Exposure variables
The primary exposure variable is child/youth HIV exposure status.
Outcome variables
Primary outcomes include the prevalence of any child disability (assessed using UNICEF-10), ASD, ADHD, total and domain-specific scores of the SDQ and moderate to severe depression (PHQ-9 score ≥10) and anxiety (GAD-7 score ≥10) among older children. Secondary outcomes include differences in growth z-scores comparing HEU and HUU.
Data analysis
We will compare prevalence of developmental delays using the ASQ cut-off scores, any disability, SDQ difficulties, ASD, ADHD, hearing and vision problems and mental health symptoms (depression, anxiety, substance use, psychosis) among HEU and HUU using log binomial regression. We will estimate the time spent conducting each of the screening assessments.
Study power
Estimated detectable differences and assumptions are summarised in table 3. With 4000 HEU (1000 HEU per age strata of 3–6, 7–10, 11–14 and 15–18 years) and 400 HUU (100 per age strata), we are powered to detect differences in ASQ for ages 3–6, prevalence of difficulties on the SDQ for ages 3–10 and 11–14, and prevalence of moderate to severe depression and anxiety for ages 15–18 under the assumptions provided in table 3.
Referral criteria for growth, neurodevelopment, mental health and hearing for both the longitudinal and cross-sectional studies have been identified and children meeting criteria are referred for further assessment and evaluation. Referral criteria for aims 1 and 2 are summarised in online supplemental table 1.
bmjopen-2023-081975supp001.pdf (49.5KB, pdf)
Patient and public involvement
Participating clinics and leaders of communities of pregnant and postpartum women living with HIV were informed about the study and were involved in recruitment of study participants. Results of the study will be disseminated to local community advisory boards, participating clinics and to relevant policy-makers in the Kenya Ministry of Health through seminars and policy memos.
Findings to date
Analysis of 1-year neurodevelopment data (using MDAT) for children enrolled in the longitudinal cohort found that HEU infants had higher scores in the language domain but comparable scores in the fine motor, gross motor and social domains. Among HEU, EFZ exposure compared with DTG exposure was associated with lower gross motor scores.54 In the pilot phase of the cross-sectional cohort, we identified significant challenges recruiting older HEU largely because of unknown maternal HIV status during pregnancy/breast feeding and challenges with maternal HIV disclosure to older HEU.55
Future plans
Longitudinal cohort follow-up and enrolment in the cross-sectional study will be completed in June 2024. Study procedures and data collection are ongoing.
Supplementary Material
Acknowledgments
We acknowledge the participating clinics and their leadership for their support. We also thank the University of Washington Global Center for the Integrated Health of Women, Adolescents and Children, (Global WACh) for input provided during study design and manuscript development. We thank the HOPE study staff for their dedication and hard work to achieve the study outcomes. We thank various groups that have supported HOPE study training including the INCLEN TRUST, KEMRI anthropometry monitors, University of Nairobi department of psychiatry, George Mathenge for providing vision training and AMPATH for supporting NIH toolbox training. Most of all, we thank the clinics, health care workers, caregivers and children/youth who will participate in the study.
Footnotes
@GlobalWACh
Contributors: GJ-S is the principal investigator, who designed the study and applied for grant funding. INN, MKi, HM, DW, MKu, SB-N, ADW, CJM, SD, SN, DW and GJ-S participated in designing the trial and data collection tools. MKi, INN and HM coordinate study implementation. MKu and SD provide expertise in mental health, SN in hearing, SB-N in neurodevelopment assessments and ADW in costing methods. AO is responsible for data management. INN wrote the first draft of the manuscript. GJ-S is the guarantor. All authors reviewed and approved the final manuscript.
Funding: This publication was made possible by primary funding from the National Institutes of Health (NIH), 9000 Rockville Pike, Bethesda, Maryland 20892 under award 1R61HD103079-01. This publication was supported in part by Fogarty International Center (FIC) K43TW011422-01A1 to INN.
Disclaimer: The funders have no role in design, collection, analysis or interpretation of data.
Competing interests: None declared.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Cohort description section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
Data availability statement
Data are available on reasonable request. Study data are available on reasonable request. A deidentified participant data set is available on request from the corresponding (irenen@uw.edu) or senior author (gjohn@uw.edu). This publication provides a description of the study protocol and analysis plan.
Ethics statements
Patient consent for publication
Not applicable.
Ethics approval
This study involves human participants and was approved by Kenyatta National Hospital Ethics and Research Committee P400/07/2020, University of Washington Institutional Review Board STUDY00010495. Participants gave informed consent to participate in the study before taking part.
References
- 1. Slogrove AL, Powis KM, Johnson LF, et al. Estimates of the global population of children who are HIV-exposed and uninfected, 2000-18: a Modelling study. Lancet Glob Health 2020;8:e67–75. 10.1016/S2214-109X(19)30448-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Global HIV & AIDS Statistics — fact sheet. 2022. Available: https://www.unaids.org/en/resources/fact-sheet
- 3. Brennan AT, Bonawitz R, Gill CJ, et al. A meta-analysis assessing all-cause mortality in HIV-exposed uninfected compared with HIV-unexposed uninfected infants and children. AIDS Lond Engl 2016;30:2351–60. 10.1097/QAD.0000000000001211 [DOI] [PubMed] [Google Scholar]
- 4. Brennan AT, Bonawitz R, Gill CJ, et al. A meta-analysis assessing diarrhea and pneumonia in HIV-exposed uninfected compared with HIV-unexposed uninfected infants and children. J Acquir Immune Defic Syndr 2019;82:1–8. 10.1097/QAI.0000000000002097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Prendergast AJ, Evans C. Children who are HIV-exposed and uninfected: evidence for action. AIDS Lond Engl 2023;37:205–15. 10.1097/QAD.0000000000003409 [DOI] [PubMed] [Google Scholar]
- 6. Aizire J, Sikorskii A, Ogwang LW, et al. Decreased growth among antiretroviral drug and HIV-exposed uninfected versus unexposed children in Malawi and Uganda. AIDS Lond Engl 2020;34:215–25. 10.1097/QAD.0000000000002405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Evans C, Chasekwa B, Ntozini R, et al. Mortality, human immunodeficiency virus (HIV) transmission, and growth in children exposed to HIV in rural Zimbabwe. Clin Infect Dis 2021;72:586–94. 10.1093/cid/ciaa076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. le Roux SM, Abrams EJ, Donald KA, et al. Growth Trajectories of Breastfed HIV-exposed uninfected and HIV-Unexposed children under conditions of universal maternal antiretroviral therapy: a prospective study. Lancet Child Adolesc Health 2019;3:234–44. 10.1016/S2352-4642(19)30007-0 [DOI] [PubMed] [Google Scholar]
- 9. Wedderburn CJ, Evans C, Yeung S, et al. Growth and neurodevelopment of HIV-exposed uninfected children: a conceptual framework. Curr HIV/AIDS Rep 2019;16:501–13. 10.1007/s11904-019-00459-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Van Dyke RB, Chadwick EG, Hazra R, et al. The PHACS SMARTT study: assessment of the safety of in utero exposure to antiretroviral drugs. Front Immunol 2016;7:199. 10.3389/fimmu.2016.00199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. McHenry MS, McAteer CI, Oyungu E, et al. Neurodevelopment in young children born to HIV-infected mothers: a meta-analysis. Pediatrics 2018;141:e20172888. 10.1542/peds.2017-2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. le Roux SM, Donald KA, Brittain K, et al. Neurodevelopment of Breastfed HIV-exposed uninfected and HIV-Unexposed children in South Africa. AIDS Lond Engl 2018;32:1781–91. 10.1097/QAD.0000000000001872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wedderburn CJ, Yeung S, Rehman AM, et al. Neurodevelopment of HIV-exposed uninfected children in South Africa: outcomes from an observational birth cohort study. Lancet Child Adolesc Health 2019;3:803–13. 10.1016/S2352-4642(19)30250-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Alimenti A, Forbes JC, Oberlander TF, et al. A prospective controlled study of neurodevelopment in HIV-uninfected children exposed to combination antiretroviral drugs in pregnancy. Pediatrics 2006;118:e1139–45. 10.1542/peds.2006-0525 [DOI] [PubMed] [Google Scholar]
- 15. Chaudhury S, Williams PL, Mayondi GK, et al. Neurodevelopment of HIV-exposed and HIV-unexposed uninfected children at 24 months. Pediatrics 2017;140:e20170988. 10.1542/peds.2017-0988 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Boivin MJ, Maliwichi-Senganimalunje L, Ogwang LW, et al. Neurodevelopmental effects of ante-partum and post-Partum antiretroviral exposure in HIV-exposed and uninfected children versus HIV-unexposed and uninfected children in Uganda and Malawi: a prospective cohort study. Lancet HIV 2019;6:e518–30. 10.1016/S2352-3018(19)30083-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Torre P, Zeldow B, Hoffman HJ, et al. Hearing loss in perinatally human immunodeficiency virus-infected and human immunodeficiency virus -Exposed but uninfected children and adolescents. Pediatr Infect Dis J 2012;31:835–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Torre P, Zeldow B, Yao TJ, et al. Newborn hearing screenings in human immunodeficiency virus-exposed uninfected infants. J AIDS Immune Res 2016;1:102. [PMC free article] [PubMed] [Google Scholar]
- 19. Boivin MJ, Barlow-Mosha L, Chernoff MC, et al. Neuropsychological performance in African children with HIV enrolled in a multisite antiretroviral clinical trial. AIDS Lond Engl 2018;32:189–204. 10.1097/QAD.0000000000001683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nicholson L, Chisenga M, Siame J, et al. Growth and health outcomes at school age in HIV-exposed, uninfected Zambian children: follow-up of two cohorts studied in infancy. BMC Pediatr 2015;15:66. 10.1186/s12887-015-0386-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sherr L, Croome N, Parra Castaneda K, et al. A systematic review of psychological functioning of children exposed to HIV: using evidence to plan for tomorrow’s HIV needs. AIDS Behav 2014;18:2059–74. 10.1007/s10461-014-0747-6 [DOI] [PubMed] [Google Scholar]
- 22. Al-Haddad BJS, Oler E, Armistead B, et al. The fetal origins of mental illness. Am J Obstet Gynecol 2019;221:549–62. 10.1016/j.ajog.2019.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Al-Haddad BJS, Jacobsson B, Chabra S, et al. Long-term risk of neuropsychiatric disease after exposure to infection in utero. JAMA Psychiatry 2019;76:594–602. 10.1001/jamapsychiatry.2019.0029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ji Y, Riley AW, Lee L-C, et al. Maternal biomarkers of acetaminophen use and offspring attention deficit hyperactivity disorder. Brain Sci 2018;8:127. 10.3390/brainsci8070127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ji Y, Azuine RE, Zhang Y, et al. Association of cord plasma biomarkers of in utero acetaminophen exposure with risk of attention-deficit/hyperactivity disorder and autism spectrum disorder in childhood. JAMA Psychiatry 2020;77:180–9. 10.1001/jamapsychiatry.2019.3259 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Masarwa R, Levine H, Gorelik E, et al. Prenatal exposure to acetaminophen and risk for attention deficit hyperactivity disorder and autistic spectrum disorder: a systematic review, meta-analysis, and meta-regression analysis of cohort studies. Am J Epidemiol 2018;187:1817–27. 10.1093/aje/kwy086 [DOI] [PubMed] [Google Scholar]
- 27. Murphy SK, Fineberg AM, Maxwell SD, et al. Maternal infection and stress during pregnancy and depressive symptoms in adolescent offspring. Psychiatry Res 2017;257:102–10. 10.1016/j.psychres.2017.07.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Stringer EM, Meltzer-Brody S, Kasaro M, et al. Depression, pregnancy, and HIV: the case to strengthen mental health services for pregnant and post-Partum women in sub-Saharan Africa. Lancet Psychiatry 2014;1:159–62. 10.1016/S2215-0366(14)70273-1 [DOI] [PubMed] [Google Scholar]
- 29. WHO recommends Dolutegravir as preferred HIV treatment option in all populations. 2023. Available: https://www.who.int/news/item/22-07-2019-who-recommends-dolutegravir-as-preferred-hiv-treatment-option-in-all-populations
- 30. Nickel K, Halfpenny NJA, Snedecor SJ, et al. Comparative efficacy, safety and durability of dolutegravir relative to common core agents in treatment-Naïve patients infected with HIV-1: an update on a systematic review and network meta-analysis. BMC Infect Dis 2021;21:222. 10.1186/s12879-021-05850-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Cabello-Ubeda A, de Quirós JCLB, Martín Carbonero L, et al. 48-week effectiveness and tolerability of dolutegravir (DTG) + lamivudine (3Tc) in antiretroviral-Naïve adults living with HIV: a multicenter real-life cohort. PLOS ONE 2022;17:e0277606. 10.1371/journal.pone.0277606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Fettiplace A, Stainsby C, Winston A, et al. Psychiatric symptoms in patients receiving dolutegravir. J Acquir Immune Defic Syndr 2017;74:423–31. 10.1097/QAI.0000000000001269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Menard A, Montagnac C, Solas C, et al. Neuropsychiatric adverse effects on dolutegravir: an emerging concern in Europe. AIDS 2017;31:1201–3. 10.1097/QAD.0000000000001459 [DOI] [PubMed] [Google Scholar]
- 34. Kenedi CA, Goforth HW. A systematic review of the psychiatric side-effects of Efavirenz. AIDS Behav 2011;15:1803–18. 10.1007/s10461-011-9939-5 [DOI] [PubMed] [Google Scholar]
- 35. Crowell CS, Williams PL, Yildirim C, et al. Safety of in-utero antiretroviral exposure: neurologic outcomes in children who are HIV-exposed but uninfected. AIDS 2020;34:1377–87. 10.1097/QAD.0000000000002550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Foster EG, Gendelman HE, Bade AN. HIV-1 Integrase strand transfer inhibitors and Neurodevelopment. Pharmaceuticals (Basel) 2022;15:1533. 10.3390/ph15121533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Kenya County profiles.Pdf. 2022. Available: https://nacc.or.ke/wp-content/uploads/2015/10/KenyaCountyProfiles.pdf
- 38. Scribd . 2022 ART revised Kenyan guidelines. Management Of Hiv/Aids, Diagnosis Of Hiv/Aids. n.d. Available: https://www.scribd.com/document/583401595/2022-ART-Revised-Kenyan-Guidelines [Google Scholar]
- 39. The Malawi Developmental Assessment Tool (MDAT) . The creation, validation, and reliability of a tool to assess child development in rural African settings - PMC. 2023. Available: Https://Www-Ncbi-Nlm-NIH-Gov.Offcampus.Lib.Washington.Edu/Pmc/articles/Pmc2876049/ [DOI] [PMC free article] [PubMed]
- 40. Robins DL, Casagrande K, Barton M, et al. Validation of the modified checklist for autism in toddlers, revised with follow-up (M-CHAT-R/F). Pediatrics 2014;133:37–45. 10.1542/peds.2013-1813 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Juneja M, Mishra D, Russell PSS, et al. INCLEN diagnostic tool for autism spectrum disorder (INDT-ASD): development and validation. Indian Pediatr 2014;51:359–65. 10.1007/s13312-014-0417-9 [DOI] [PubMed] [Google Scholar]
- 42. Hoosen N, Davids EL, de Vries PJ, et al. The strengths and difficulties questionnaire (SDQ) in Africa: a Scoping review of its application and validation. Child Adolesc Psychiatry Ment Health 2018;12:6. 10.1186/s13034-017-0212-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Nurturinnurturing care framework for early childhood development - Homeg care framework for early childhood development - HOME. 2018. Available: https://nurturing-care.org/
- 44. Singh A, Yeh CJ, Boone Blanchard S. Ages and stages questionnaire: a global screening scale. Bol Med Hosp Infant Mex 2017;74:5–12. 10.1016/j.bmhimx.2016.07.008 [DOI] [PubMed] [Google Scholar]
- 45. Belmont L. The International pilot study of severe childhood disability. final report: screening for severe mental retardation in developing countries. 1984.
- 46. Gulati S, Saini L, Kaushik JS, et al. The development and validation of DSM 5-based AIIMS-modified INDT ADHD tool for diagnosis of ADHD: a diagnostic test evaluation study. Neurol India 2020;68:352–7. 10.4103/0028-3886.280638 [DOI] [PubMed] [Google Scholar]
- 47. Cohen S, Kamarck T, Mermelstein R. A global measure of perceived stress. J Health Soc Behav 1983;24:385–96. [PubMed] [Google Scholar]
- 48. The PHQ-9: validity of a brief depression severity measure. 2023. Available: https://pubmed-ncbi-nlm-nih-gov.offcampus.lib.washington.edu/11556941/ [DOI] [PMC free article] [PubMed]
- 49. Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–7. 10.1001/archinte.166.10.1092 [DOI] [PubMed] [Google Scholar]
- 50. Loewy RL, Pearson R, Vinogradov S, et al. Psychosis risk screening with the Prodromal questionnaire--brief version (PQ-B). Schizophr Res 2011;129:42–6. 10.1016/j.schres.2011.03.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Alcohol use disorders identification test. 2023. Available: https://auditscreen.org/
- 52. National Institute on aging. NIH Toolbox, 2023. Available: https://www.nia.nih.gov/research/resource/nih-toolbox
- 53. Patel K, Thibodeau L, McCullough D, et al. Development and pilot testing of smartphone-based hearing test application. Int J Environ Res Public Health 2021;18:5529. 10.3390/ijerph18115529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Bulterys MA, Njuguna I, King’e M, et al. Neurodevelopment of children who are HIV-exposed and uninfected in Kenya. J Int AIDS Soc 2023;26 Suppl 4:e26149. 10.1002/jia2.26149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Moraa H, Kinge M, Onyango A, et al. Identifying HIV-exposed uninfected children and adolescents in resource-limited settings: the HOPE study experience. Afr J AIDS Res 2023;22:244–6. 10.2989/16085906.2023.2276376 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2023-081975supp001.pdf (49.5KB, pdf)
Data Availability Statement
Data are available on reasonable request. Study data are available on reasonable request. A deidentified participant data set is available on request from the corresponding (irenen@uw.edu) or senior author (gjohn@uw.edu). This publication provides a description of the study protocol and analysis plan.