Abstract
Introduction
Given the increasing prevalence of both obesity and pre-diabetes in pregnant adults, there is growing interest in identifying hyperglycaemia in early pregnancy to optimise maternal and perinatal outcomes. Multiple organisations recommend first-trimester diabetes screening for individuals with risk factors; however, the benefits and drawbacks of detecting glucose abnormalities more mild than overt diabetes in early gestation and the best screening method to detect such abnormalities remain unclear.
Methods and analysis
The goal of the Glycemic Observation and Metabolic Outcomes in Mothers and Offspring study (GO MOMs) is to evaluate how early pregnancy glycaemia, measured using continuous glucose monitoring and oral glucose tolerance testing, relates to the diagnosis of gestational diabetes (GDM) at 24–28 weeks’ gestation (maternal primary outcome) and large-for-gestational-age birth weight (newborn primary outcome). Secondary objectives include relating early pregnancy glycaemia to other adverse pregnancy outcomes and comprehensively detailing longitudinal changes in glucose over the course of pregnancy. GO MOMs enrolment began in April 2021 and will continue for 3.5 years with a target sample size of 2150 participants.
Ethics and dissemination
GO MOMs is centrally overseen by Vanderbilt University’s Institutional Review Board and an Observational Study Monitoring Board appointed by National Institute of Diabetes and Digestive and Kidney Diseases. GO MOMs has potential to yield data that will improve understanding of hyperglycaemia in pregnancy, elucidate better approaches for early pregnancy GDM screening, and inform future clinical trials of early GDM treatment.
Trial registration number
Keywords: Diabetes in pregnancy, Pregnant Women, Observational Study
STRENGTHS AND LIMITATIONS OF THIS STUDY.
Glycemic Observation and Metabolic Outcomes in Mothers and Offspring (GO MOMs) is a multicentre study that is designed to reflect the demography of the pregnant population in the USA.
GO MOMs participants wear blinded continuous glucose monitoring devices at four timepoints over the course of pregnancy and complete oral glucose tolerance tests at both 10–14 weeks’ and 24–28 weeks’ gestation, generating a uniquely valuable data resource for characterising the longitudinal glycaemic profile of pregnancy.
The study design and sample size for GO MOMs will support development and validation of predictive criteria for gestational diabetes in pregnant individuals at 24–28 weeks’ gestation and large-for-gestational-age birth weight in newborns using early pregnancy data.
Multiple laboratory measures and chart abstraction data will complement glycaemic measurements.
As in any observational, longitudinal study, confounders and missing data may be limitations of the GO MOMs study.
Introduction
Gestational diabetes mellitus (GDM) affects 7.8% of pregnant individuals in the USA1–4 and is associated with an increased risk of adverse pregnancy outcomes.5–8 Further, it is a harbinger of long-term metabolic disease in affected parents and children exposed in utero.5–8 Randomised trials have demonstrated that treatment of GDM in the third trimester results in a reduction in the frequency of large-for-gestational-age birth weight (LGA) and other adverse perinatal outcomes.9–14 Therefore, in the USA, all pregnant individuals accessing prenatal care are typically screened for GDM between 24 and 28 weeks’ gestation.15 16
Due to the increasing prevalence of both obesity and pre-diabetes, along with the recognition that individuals who meet traditional GDM criteria early in pregnancy have a greater risk of adverse outcomes than those diagnosed later, there is growing interest in first trimester identification of hyperglycaemia.17 Although treatment for GDM in the third trimester does not seem to result in improvements in long-term sequelae for exposed neonates, whether treatment of hyperglycaemia in early pregnancy would be associated with decreased metabolic risk in children exposed in utero is not well understood.18–20 Available evidence suggests that the mechanism by which in utero exposure to hyperglycaemia might lead to obesity and metabolic disease later in life is through fetal hyperinsulinaemia and the resultant accrual of excess adipose tissue.21 Studies of amniotic fluid insulin levels demonstrate that fetal hyperinsulinaemia occurs as early as 15 weeks’ gestation, preceding the gestational age at which GDM is usually diagnosed22–24 and suggesting that intervention at earlier gestational ages than the current standard might prevent long-term sequelae. However, available trials of screening and treatment at less than 20 weeks’ gestation based on conventional GDM diagnostic criteria have not consistently demonstrated a beneficial effect on birth weight or other adverse perinatal outcomes.25–28
Although the International Association of Diabetes and Pregnancy Study Groups (IADPSG),29 the American Diabetes Association (ADA)16 and the American College of Obstetricians and Gynecologists (ACOG)15 recommend early pregnancy diabetes screening for individuals at increased risk, a 2021 United States Preventive Services Task Force guideline concluded that evidence was insufficient to assess the balance of benefits and drawbacks of screening for hyperglycaemia before 24 weeks’ gestation.30 In addition, the optimal method and criteria for diagnosing hyperglycaemia in early pregnancy have not been established.31–39 As a result, various testing modalities including haemoglobin A1c (A1c), fasting glucose and oral glucose tolerance tests (OGTTs) are used with variable criteria applied.
In 2017, the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) convened a workshop to identify research gaps in GDM and highlighted three areas related to early diagnosis of GDM as priorities for further investigation: (1) diagnostic criteria and definitions, (2) alternative markers for diagnosis and (3) effects of early diagnosis and treatment on outcomes.20 The NIDDK-supported Glycemic Observation and Metabolic Outcomes in Mothers and Offspring study (GO MOMs) was designed to address these gaps. The goal of GO MOMs is to use early pregnancy glycaemia to predict late pregnancy GDM (diagnosed with traditional criteria) and LGA. A secondary objective is to provide a comprehensive, longitudinal description of changes in glucose throughout pregnancy leveraging continuous glucose monitoring (CGM). A substudy, the GO MOMs Nutrition Study, is collecting dietary information on a subset of participants and will link dietary components to maternal glycaemia and offspring outcomes. It is hoped that these data will elucidate improved approaches for GDM screening in early pregnancy and inform future clinical trials of early GDM treatment.
Methods and analysis
GO MOMs is being conducted at seven clinical centres (table 1), the Biostatistics Research Center (BRC) at Northwestern University Data Analysis and Coordinating Center (Chicago, IL) and the Central Laboratory at the Advanced Research and Diagnostic Laboratory at University of Minnesota (Minneapolis, MN). The protocol and study design were developed by the GO MOMs steering committee, which consists of investigators from each clinical site, the BRC, the Central Laboratory and the NIDDK.
Table 1.
Glycemic Observation and Metabolic Outcomes in Mothers and Offspring study centres
| Clinical centres | |
| Columbia University Irving Medical Center | New York, NY |
| Kaiser Permanente Northwest and Kaiser Permanente Hawaii | Portland, OR/Honolulu, HI |
| Massachusetts General Hospital and Tufts University Medical Center | Boston, MA |
| Northwestern Memorial Hospital | Chicago, IL |
| University of Pittsburgh Medical Center Magee-Women’s Hospital | Pittsburgh, PA |
| Women & Infants Hospital of Rhode Island | Providence, RI |
| Yale University | New Haven, CT |
| Biostatistics research centre | |
| Northwestern University Data Analysis and Coordinating Center | Chicago, IL |
| Central laboratory | |
| Advanced Research and Diagnostic Laboratory at University of Minnesota | Minneapolis, MN |
Eligibility, recruitment process and consent
Inclusion and exclusion criteria are described in table 2. Prior to initial study procedures at 10–14 weeks’ gestation, participants must have an ultrasound confirming dating and a viable, singleton gestation. During the first study visit, participants undergo a 75-gram, 2-hour OGTT and haemoglobin A1c measurement. Both the OGTT and the haemoglobin A1c are performed because the OGTT is the most sensitive method for detection of diabetes and the haemoglobin A1c is currently the most commonly used method to diagnose diabetes. If haemoglobin A1c >6.5%, fasting glucose >126 mg/dL or 2-hour glucose >200 mg/dL, the participant is excluded, having met criteria for overt diabetes.16 Participants who do not meet these criteria are eligible to continue in the study, and results below these thresholds remain masked to participants, their providers and research staff.
Table 2.
Glycemic Observation and Metabolic Outcomes in Mothers and Offspring inclusion and exclusion criteria
| Inclusion | Exclusion |
| Age >18 at consent | Pre-existing diabetes at enrolment |
| Single gestation | Current self-monitoring of blood glucose |
| Gestational age between 10 weeks 0 days and 14 weeks 0 days confirmed by ultrasound and study dating criteria* | Current use of a medication with glycaemic effects |
| Conceived using own oocyte | Fetal malformation evident at or before enrolment that is likely lethal |
| Willing and able to wear continuous glucose monitor as directed and adhere to instructions | Known fetal aneuploidy or high-risk cell-free fetal DNA result for aneuploidy |
| Planning to deliver at a study-affiliated hospital | Participation in another research study that may modify glycaemic profile or study outcomes |
| History of bariatric surgery | |
| Significant allergy to adhesive or extensive skin changes or diseases making continuous glucose monitoring sensor use problematic | |
| Previous participation in the study | |
| Current bulimia or anorexia nervosa | |
| Overnight shift work that alters the sleep/wake periods | |
| Current psychiatric illness/social situation that would limit compliance with study requirements | |
| Haemoglobin A1c >6.5%, or fasting glucose >126 mg/dL, or 2-hour glucose >200 mg/dL during the visit 1 oral glucose tolerance test |
*Participants are required to have a first trimester ultrasound to confirm or establish pregnancy dating and confirm a viable, singleton gestation. The estimated due date established by ultrasound measure of the crown-rump length is used for participants without a sure last menstrual period (LMP) and/or for whom ultrasound dating is discordant with LMP dating according to American College of Obstetricians and Gynecologists criteria. For participants with pregnancies resulting from assisted reproductive technologies (ART), ART-dating is used.73
Recruitment strategies leverage each site’s unique clinical and electronic medical record (EMR) infrastructure. Once identified, potential participants are approached to gauge interest and provide details on study participation. Written informed consent is obtained prior to study procedures.
Study cohort
The study opened to enrolment in April 2021. Recruitment is projected to continue until January 2025. We expect the GO MOMs cohort to represent the demography of the population of US pregnant individuals, including race and ethnicity, body weight distribution and the adult reproductive age spectrum.40
Outcomes
Table 3 summarises the primary, secondary and exploratory outcomes.
Table 3.
GO MOMs primary, secondary and exploratory outcomes
| Primary outcomes | Definition |
| Gestational diabetes mellitus | 100-gram 3-hour OGTT meeting Carpenter-Coustan criteria at 24w0d-28w0d gestation |
| Large for gestational age | Birth weight >90th percentile for gestational age according to Aris et al 42 |
| Secondary outcomes | |
| Hypertensive disorders of pregnancy | Includes pre-eclampsia with and without severe features, gestational hypertension, eclampsia, and haemolysis, elevated liver enzymes and low platelet count (HELLP) syndrome |
| Caesarean delivery | |
| Flank skinfold | Evaluated as a continuous measure and dichotomised as >90th percentile |
| Small for gestational age | Birth weight <10th percentile for gestational age according to Aris et al 42 |
| Preterm birth | Delivery prior to estimated gestational age 37w0d |
| Shoulder dystocia | Defined clinically, requiring documentation that providers applied manoeuvres to reduce the shoulder at delivery |
| Neonatal birth injury | Brachial plexus palsy or clavicular, humeral or skull fracture |
| Neonatal hypoglycaemia | Neonatal hypoglycaemia requiring treatment |
| Neonatal respiratory morbidity | Need for respiratory support within 72 hours after birth and consisting of one or more of the following: the use of continuous positive airway pressure (CPAP) or high-flow nasal cannula for at least two consecutive hours, supplemental oxygen with a fraction of inspired oxygen of at least 0.30 for at least four continuous hours, extracorporeal membrane oxygenation (ECMO) or mechanical ventilation. A high flow of air or blended air and oxygen is defined as more than 1 L/min |
| Neonatal hyperbilirubinaemia | Treatment of hyperbilirubinaemia in the first week of life with phototherapy or exchange transfusion or a diagnosis of kernicterus |
| Exploratory outcomes | |
| NICU admission | Admission to the NICU prior to hospital discharge |
| Neonatal length of admission/length of stay | Includes NICU or entire delivery hospitalisation |
| Spontaneous abortion | Pregnancy loss at <20w0d of gestation |
| Stillbirth | Intrauterine fetal demise ≥20w0d of gestation |
| Neonatal death | Death within the first 28 days of life |
| Major congenital malformation | Birth defects that have significant medical, social or cosmetic consequences for the affected individual, and typically require medical intervention |
| Antepartum admissions or maternal readmissions | Admissions that occur after GO MOMs enrolment and up to 30 days following delivery |
| Low Apgar score | Apgar score <7 at 5 min |
d, days; GO MOMs, Glycemic Observation and Metabolic Outcomes in Mothers and Offspring; NICU, neonatal intensive care unit; OGTT, oral glucose tolerance test; w, weeks.
Primary outcome in pregnant individuals: GDM
GDM is ascertained between 24 and 28 weeks’ gestation according to Carpenter-Coustan criteria applied to a 3-hour, 100-gram OGTT.15 16 We chose the Carpenter-Coustan criteria because they are the most commonly used criteria for GDM diagnosis in the USA. Given that the 1-hour non-fasting glucose challenge test (GCT) has imperfect sensitivity,41 GO MOMs participants forego the 1-hour GCT and all complete the 3-hour diagnostic OGTT for GDM ascertainment.
Primary outcome in newborns LGA
LGA, defined as birth weight greater than the 90th percentile for gestational age and sex according to a 2017 USA based reference,42 is the primary newborn outcome. Fetal overgrowth is the most common clinically relevant consequence of maternal hyperglycaemia, increases morbidity in the perinatal period, and confers long-term metabolic risk in childhood.43 The LGA coprimary outcome will facilitate development of new early pregnancy hyperglycaemia screening criteria for prediction of fetal overgrowth.
Predictors
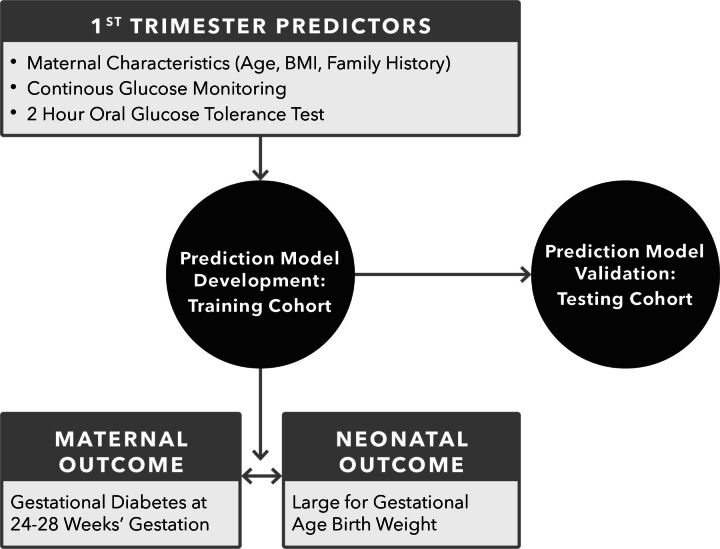
GO MOMs will develop a prediction model, incorporating glycaemic and clinical measures, which can be used in early pregnancy to identify individuals who will have GDM and/or deliver LGA infants (figure 1).
Figure 1.
GO MOMs Primary Analyses Statistical analyses for the primary objectives will evaluate predictive models for the gestational diabetes and large for gestational age birth weight co-primary outcomes. Training and validation data sets will be identified a priori with all observations from a subset of sites used for training, and observations from the remaining sites use for validation.
Glycaemic predictors
Models will incorporate summary measures of CGM or OGTT data obtained at visit 1 (V1). ‘Excess time above range’ will be the primary summary measure of CGM data to best reflect hyperglycaemia. The primary predictor will be nocturnal percentage of time above range, calculated from midnight to 06:00.44 Overnight hyperglycaemia has previously been linked to LGA in GDM.45 A range of cut-off values for both the glucose threshold defining ‘above range’ and the percentage of time spent above a given threshold to define ‘excess’ will be evaluated. Other CGM metrics including 24-hour time above range and mean glucose will also be evaluated. Additionally, timed OGTT glucose measurements obtained at V1 will be evaluated as predictors of outcomes, starting with the IADPSG criteria for GDM46 (fasting ≥92 mg/dL, 1 hour ≥180 mg/dL, 2 hours ≥153 mg/dL) and exploring various cut-offs for each timepoint to develop a dichotomous predictor using the V1 OGTT values. Secondary and exploratory analyses will incorporate continuous versions of CGM and OGTT data, CGM predictors from other gestational time points, and additional glycaemic and metabolic laboratory measures including maternal triglycerides, free fatty acids, haemoglobin A1c, C-peptide, insulin and calculated insulin physiology indices, complete blood count, and alternative glycated markers (glycated CD59, glycated albumin, 1,5-anhydroglucitol).
Clinical predictors
Predictive models that incorporate clinical factors and CGM or OGTT data will be compared with models including only clinical factors, specifically maternal age, body mass index (BMI) and family history of diabetes. These were selected based on clinical factors that have been commonly used in previous reports of clinical predictive models for GDM.47–53 While previously published predictors for GDM and LGA have sometimes included race and ethnicity, GO MOMs will develop a model that does not incorporate race and ethnicity variables. Race and ethnicity are social constructs which, when included in prediction models, could perpetuate healthcare disparities.54 55 Additional clinical factors may also be evaluated for their contributions to predictive accuracy.
Study procedures
Overview
Table 4 summarises the study procedures following enrolment. To capture glycaemic changes across pregnancy, we conduct visits and place CGM devices at four timepoints: 10–14 (V1), 16–20 (visit 2: V2), 24–28 (visit 3: V3) and 32–36 (visit 4: V4) weeks’ gestation. We administer a 2-hour 75-gram OGTT at V1 and a 3-hour 100-gram OGTT at V3. Within 72 hours of delivery, neonatal anthropometric measures are obtained. A postpartum follow-up survey occurs remotely between 4 and 13 weeks after delivery. Medical record abstraction occurs 30–90 days after delivery and captures relevant diagnoses and hospital readmissions. The timing of the gestational windows for the V1 and V3 in-person study visits was chosen to facilitate data collection for the primary analyses. The gestational windows for V2 and V4 were chosen to capture an additional early pregnancy time point (V2) and to facilitate assessment of the impact of GDM treatment (V4) prior to term.
Table 4.
GO MOMs visits and procedures
| Screening visit | V1: 10w0d–14w0d gestation | V2: 16w0d–20w0d gestation | V3: 24w0d–28w0d gestation | V4: 32w0d–36w0d gestation | Delivery | Chart abstraction: 30d–90d after delivery | Remote follow-up: 4w0d–12w6d after delivery | |
| Ultrasound, estimated delivery date (can occur at screening or V1) | ||||||||
| 10-day CGM | ||||||||
| 2-hour 75-gram OGTT, with fasting and hourly timed samples | ||||||||
| 3-hour 100-gram OGTT, with fasting and hourly timed samples | ||||||||
| Non-fasting blood sample | ||||||||
| Maternal urine | ||||||||
| Maternal height | ||||||||
| Maternal weight | ||||||||
| Maternal interview | ||||||||
| Newborn birth weight, length, flank skinfold, abdominal circumference | ||||||||
| Medical chart abstraction |
CGM, continuous glucose monitor; d, days; GO MOMs, Glycemic Observation and Metabolic Outcomes in Mothers and Offspring; OGTT, oral glucose tolerance test; V1, visit 1; V2, visit 2; V3, visit 3; V4, visit 4; w, weeks.
Continuous glucose monitoring
CGM provides a nuanced, detailed determination of dynamic glycaemic patterns by measuring interstitial glucose every 5 min, providing up to 288 glucose readings per day. We chose the Dexcom G6 Pro CGM (Dexcom, San Diego, CA) because of its demonstrated accuracy in pregnancy,56 ability to blind glucose values and lack of need for fingerstick calibration. Based on a participant’s preference, the CGM is placed on the abdomen, upper arm, buttock or lower back/hip. CGM devices are used in ‘blinded’ mode so that participants, providers and the GO MOMs research team do not observe participants’ CGM data. For 10 days after each visit, the participant wears the device and subsequently returns it to the clinical centre. CGM data are downloaded for quality assessment and summary by the BRC. If there are less than 5 days of data, participants are asked to repeat the CGM for a full 10-day wear if this can be accomplished during the study visit window.
Oral glucose tolerance tests
Both the 2-hour 75 g (V1) and 3-hour 100-gram (V3) OGTTs are conducted after an overnight fast (≥8 hours duration). After fasting samples are drawn (table 4), participants consume the oral glucose load within 10 min. Timing of hourly sample collection is based on when OGTT beverage consumption begins. To minimise in-vitro glycolysis,57 blood samples from the OGTT are immediately placed on ice, centrifuged within 15 min of collection and promptly frozen at −80°C. Samples are shipped to the study’s central laboratory where they are assayed within 80 hours of collection. A backup plasma sample from each draw time is stored at each local site at −80°C to allow for re-evaluation of glucose levels in case of primary sample loss or error. Glucose is measured in EDTA plasma by a hexokinase method on the Roche Cobas c502 chemistry analyzer (Roche Diagnostics, Indianapolis, IN); the inter-assay CV is 2.4% at a mean concentration of 98.5 mg/dL and 3.1% at a mean concentration of 229.8 mg/dL. Additional periodic monitoring of the central laboratory glucose assay is assessed via measurement of value-assigned standard reference material from the National Institute of Standards and Technology and accuracy-based proficiency testing programmes that compare results to those obtained by a reference method procedure. Average glucose values for the study population are reviewed periodically by the study’s laboratory committee to evaluate for sample drift over time. Masked OGTT results are reviewed by the same committee to evaluate for potential within-OGTT sample swaps. Both participants and study personnel are masked to the results from the V1 OGTT and A1c, unless the results are consistent with overt type 2 diabetes by ADA criteria.16 Results of the 3-hour OGTT similarly remain masked unless the glucose levels are consistent with GDM by Carpenter-Coustan criteria.16 If overt diabetes (V1) or GDM criteria are met (V3), the BRC shares results with the clinical centre, who notifies the participant’s obstetric provider. Additional safety criteria for unblinding the 3-hour OGTT results include a fasting glucose of ≥126 mg/dL or a 2-hour or 3-hour glucose value of ≥250 mg/dL. If GDM is diagnosed at V3, it is treated according to standard practice by local obstetric providers.
Additional biospecimen collection
Maternal blood and urine are obtained at each visit for additional laboratory measurements and future use (table 5).
Table 5.
Glycemic Observation and Metabolic Outcomes in Mothers and Offspring (GO MOMs) laboratory measures and biospecimens
| Laboratory measure or biospecimen | Visit 1 | Visit 2 | Visit 3 | Visit 4 | ||
| Fasting | Post-load | Non-fasting | Fasting | Post-load | Non-fasting | |
| Plasma glucose | ||||||
| C-peptide | ||||||
| Insulin | ||||||
| Complete blood count | ||||||
| Haemoglobin A1c | ||||||
| Triglycerides | ||||||
| Free fatty acids | ||||||
| Glycated CD59 | ||||||
| 1,5-anhydroglucitol | ||||||
| Glycated albumin | ||||||
| Packed cells (stored) | ||||||
| Plasma and serum (stored) | ||||||
| Urine (stored) | ||||||
Post load indicates hourly specimens after 75-gram (visit 1) or 100-gram (visit 3) glucose load. Biospecimens are shipped to the GO MOMs central laboratory. Glucose, insulin, C-peptide, haemoglobin A1c and complete blood counts are assayed within 80 hours of collection.
Anthropometrics
Participant height is measured at V1 using a Seca Stadiometer 217 (portable) or Detecto Adult Stadiometer (non-portable). Weight is measured using a calibrated Seca Scale 869 at V1 through V4. These measurements are obtained two times; if the first two measurements differ by ≥0.5 cm for height or ≥0.5 kg for weight, a third measurement is taken. Measurements of newborns are obtained within 72 hours of delivery and include length using an Ellard length board, weight using a calibrated Seca 334 scale, flank skinfold using a calibrated Harpenden calliper, and abdominal circumference using the Gulick II tape measure. Newborn measurements are obtained two times; if the first two measurements differ by ≥0.5 cm for length, ≥10 g for weight, ≥0.5 mm for flank skinfold or ≥0.5 cm for abdominal circumference, a third measurement is taken.
Biospecimens are shipped to the GO MOMs Central Laboratory. Glucose, insulin, C-peptide, haemoglobin A1c and complete blood counts are assayed within 80 hours of collection.
Questionnaires
At V1, staff interview participants about obstetrical history, family history of diabetes, alcohol and tobacco use, medical conditions, medication use, food insecurity and other social and demographic information. Information about medical conditions, medication use, alcohol and tobacco use are updated at V2, V3 and V4. To gather data on sleep, which may be associated with glycaemia during pregnancy,58 participants complete the Pittsburgh Sleep Quality Index survey at V1 and V3. At V4, participants complete questionnaires on their perceptions of CGM and OGTT; those diagnosed with GDM also answer questions about nutritional management. The remote postpartum survey includes questions about breast feeding and maternal and newborn hospital admissions. Questionnaires are available in both English and Spanish.
Chart abstraction and adjudication
Data abstracted from each site’s EMR are used to identify the newborn primary outcome and the predefined secondary and exploratory outcomes. Adjudication committees review outcomes requiring decision making beyond what is noted in the EMR and GDM cases diagnosed outside the study.
Nutrition substudy
A subset of participants who enrolled in GO MOMs between February 2023 and February 2024 are participating in the GO MOMs Nutrition Study. Substudy participants complete six 24-hour dietary recalls using the Automated Self-Administered 24-Hour Dietary Assessment Tool.59 Two recalls occur during the V1 and V3 study visits and four unannounced recalls occur after each of the four study visits on a random day during the CGM wear period.
Risk to participants
The study is considered minimal risk to participants; risks are described to participants during the informed consent process. These include risks associated with blood drawing (eg, local pain, irritation, bruising, anxiety, syncope), oral glucose tolerance testing (eg, headache, vomiting, symptoms of hypoglycaemia), collection of health information (eg, loss of confidentiality) and CGM placement/wear (eg, local pain, skin irritation, bleeding). At each visit, participants are asked about their experience with CGM wear and whether any problems occurred, such as skin irritation. If skin irritation from CGM occurs, the study team works with the participant to determine if future CGM placements should occur, with the option of using a barrier film spray to protect skin. Adverse events are reviewed by site investigators, recorded in the study database and reported to the IRB and Observational Study Monitoring Board (OSMB) when appropriate.
Statistical considerations
Statistical analyses for the primary objectives will evaluate predictive models based on logistic regression for GDM and LGA primary outcomes (figure 1). Training and validation data sets will be identified a priori with all observations from a subset of sites used for training, and observations from the remaining sites used for validation. First, predictive models using clinical variables at V1 will be developed for GDM and LGA. Model parameters will be estimated using 10-fold cross-validation in the training data set, maintaining equal GDM and LGA outcome frequencies across rounds of cross-validation.
After finalising predictive models using clinical variables, models that incorporate summary measures of V1 CGM or OGTT data will be developed. Predictive model parameters using CGM or OGTT data summaries will be estimated using 10-fold cross-validation in the training data set, maintaining equal GDM and LGA outcome frequencies across rounds of cross-validation. Improvements in predictive accuracy for models that add CGM or OGTT data to clinical factors will be examined. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) using assumed values of 5.6% GDM (the most recent published prevalence estimate at the time of study design3) and 10% LGA population prevalence will be used for primary reporting. PPV and NPV will also be estimated using the outcome prevalence in GO MOMs. Values of PPV and NPV will be evaluated across the full range of potential cut-offs in logistic regression models, and cut-offs meeting desired NPV and maximising PPV under cross-validation will be selected to define optimal predictive models based on clinical factors alone, CGM and clinical factors, and OGTT and clinical factors. Formal statistical hypothesis testing to compare the optimal predictive model with CGM and clinical factors vs clinical factors alone, and the optimal predictive model with OGTT and clinical factors versus clinical factors alone, will be conducted using methods that account for the paired nature of PPV values under different tests in the same population.60 Predictive accuracy metrics for models optimised in the training data will be independently estimated along with 95% CIs in the validation data set.
Predictive modelling for GDM and LGA will be expanded in secondary analyses to discern whether models including clinical variables and CGM-based or OGTT-based criteria in the V1 time frame demonstrate comparable predictive performance across subgroups according to self-identified race and ethnicity and socioeconomic variables. This will be examined by evaluating statistical interaction terms between the subgroup and the CGM-based and OGTT-based and other clinical variables in logistic regression models, and by examining PPV, NPV, sensitivity and specificity point estimates and 95% CIs within subgroups. In addition to V1 CGM and OGTT metrics and clinical risk factors, the added predictive contribution of continuous summaries of CGM and OGTT data and additional laboratory measures for primary outcomes will be explored.
While outcome prediction is a priority for GO MOMs, statistical analyses will also be performed to explore associations of clinical, CGM and laboratory data obtained at V1–V4 with secondary maternal and newborn outcomes. Statistical methods will include regression modelling and generalised longitudinal linear mixed models. Exploratory dynamic risk prediction models using CGM data over the course of pregnancy will also be examined. Mediation analyses using structural equation models will be conducted to estimate the effects of GDM management, if diagnosed, on the association between maternal glycaemia and newborn outcomes.
Sample size and power
Target enrolment for GO MOMs is 2150 participants. Sample size calculations were based on the primary aims to assess the predictive capacity of CGM and OGTT summary measures at V1 for the primary outcomes. Evaluation of GDM and LGA outcomes will be viewed as separate analyses and 5% two-sided type I error will be used for evaluation of each outcome. Since two formal hypothesis tests will be conducted for each outcome (ie, the evaluation of each outcome with predictive models comparing clinical factors alone to models also including V1 CGM or OGTT data), results will be considered statistically significant at two-sided p<0.025 according to Bonferroni correction to maintain overall 5% type I error.
Sample size was determined based on evaluation of PPV for GDM and LGA at a set NPV for each. Published literature suggests that PPV for GDM using clinical factors that are identifiable early in pregnancy is approximately 0.20.47–53 Less information is available for LGA, but some reports indicate that a PPV of 0.15 is possible using maternal clinical risk factors that are identifiable early in pregnancy.61 Clinical factors in these predictive models uniformly included age and BMI and several also included family history of diabetes. GO MOMs was designed to detect a clinically meaningful increase in PPV from 0.20 to 0.40 for GDM and from 0.15 to 0.30 for LGA. Calculations were performed assuming 5.6% population prevalence for GDM based on the most recent published estimate at the time of study design3 and 10% population prevalence for LGA. NPV was held constant for GDM at 0.97 and for LGA at 0.95. Sample size calculations were based on simulation studies using hypothesis testing methodology that accounts for the paired nature of PPV values under different tests in the same population.60 Dependence was induced between observations of PPV under the null and alternative hypotheses using normally distributed random effects with mean 0 and between-subject variance ranging 0.1–1 (intraclass correlation coefficient 0–0.5). To detect the proposed improvements in PPV for GDM at 90% power, 860 GO MOMs participants with observed data for the primary GDM outcome are required. A sample size of 860 with complete data provides approximately 99% power to detect an increase in PPV from 0.15 to 0.30 at a constant NPV of 0.95 for the LGA primary outcome.
Existing literature does not support hypotheses about the exact CGM-based or OGTT-based criteria at V1 to be evaluated as predictors of GDM and LGA. Thus, a range of statistical summaries for CGM data and cut-off values for OGTT data will be explored. Given this, independent training and validation GO MOMs data sets are paramount. As noted above, 860 GO MOMs dyads will be required for hypothesis testing for improvements in PPV. Two times this number of GO MOMs participants will be enrolled, and training and validation data sets of equal sizes will be identified prior to predictive model development. Training and validation data sets will be designated after completion of data collection, but prior to formal statistical analyses. The full data from each site will be placed either into the training or validation data sets, maintaining comparable demographic and clinical characteristics and outcome frequencies across the two data sets. A sample size of 860 in the validation data set will provide 95% CIs with half-width of 0.10 surrounding the PPV estimate. Precision at this level is thought to be necessary to motivate potential changes in clinical practice for early screening.
In summary, 1720 participants (860 each in training and validation data sets) are required to accomplish the GO MOMs primary objectives. Assuming 80% of participants complete the study with observed outcomes, a total enrolment of 2150 participants is required.
Analyses of associations for secondary outcomes will collectively use observations from all 2150 participants. For analyses investigating associations of predictors with dichotomous secondary outcomes, assuming R2 of the primary predictor with other model covariates of up to 0.4, this sample size affords 90% power at nominal two-sided p<0.05 to detect ORs in the range of 1.25–1.60 for a continuous predictor higher by one SD for outcome frequencies ranging 0.05–0.30. ORs in the range of 1.59–4.31 are detectable for dichotomous predictors with frequencies ranging 0.05–0.30 for both the outcome and predictor. For continuous secondary outcomes, again assuming R2 of the primary predictor with other model covariates of up to 0.4, adjusted mean differences ranging 0.22–0.46 SD are detectable at 90% power at nominal two-sided p<0.05 for dichotomous predictors with frequencies ranging 0.05–0.30. Partial correlation of 0.10 is detectable for a continuous predictor with a continuous outcome. All calculations assume 80% of participants complete the study and have observed outcomes. Analyses of exploratory outcomes are not formally powered.
Patient and public involvement
During study development, participants who had previously enrolled in a similar pregnancy study at the Massachusetts General Hospital site62 were invited to provide feedback and input into study design, recruitment plan and research protocol via focus groups. At the Yale site, investigators convened a stakeholder meeting with community members and leaders from organisations in the local black and Latino communities for feedback on the study plans. The participants in the focus groups and stakeholder meeting strongly supported the scientific rationale and the importance of the study to relevant communities. Input from the focus groups and stakeholder meetings informed the timing of study visits to minimise participant burden, the participant remuneration plan, the strategy for participant engagement and retention, the sharing of individual-level data and the methods for dissemination of study results, among other study details. GO MOMs participants who have consented to future contact will be sent a letter about the results of the study after study completion and publication of results will be made available to the relevant wider patient communities.
Ethics and dissemination
The GO MOMs OSMB, an independent review group appointed by NIDDK, reviewed the study protocol and granted approval in October 2020. Vanderbilt University’s Institutional Review Board (Nashville, TN) granted protocol approval in January 2021 under IRB #202214. The BRC oversees certification of study personnel training to ensure standardisation of study conduct and data collection across sites.
After the study is completed and manuscripts addressing the primary and secondary hypotheses have been developed, a limited dataset will be transmitted to the NIDDK Central Repository, under the supervision of the NIDDK, for use by other researchers. De-identified biological samples will also be stored at the NIDDK Biorepository.
Discussion
Despite clinical guidelines suggesting that at-risk pregnant patients should be screened for hyperglycaemia in the first trimester, there are knowledge gaps that hinder this approach. By gathering extensive data on glycaemia and other related biomarkers starting in the first trimester, GO MOMs may be able to identify better early pregnancy criteria for predicting GDM and hyperglycaemia-associated adverse outcomes. By identifying individuals at risk for GDM and LGA in the first trimester, novel screening strategies, and ultimately, treatments can be developed to improve pregnancy health.
GO MOMs will leverage CGM technology in order to identify glycaemic patterns that may predict GDM, LGA, and other pregnancy complications better than currently employed diagnostic tools. Indeed, CGM is now being leveraged in prediction models for development of type 1 diabetes.63–66 CGM has also been used to describe glycaemic patterns in cohorts of people without diabetes, leading to improved understanding of physiological glycaemic variation.67 Similarly, GO MOMs will describe physiological glycaemic patterns across pregnancy in a large cohort.
GO MOMs will extensively characterise the metabolic profile of pregnant participants. We will use insulin and C-peptide to assess insulin resistance and determine its relationship to the glycaemic patterns identified by CGM and GDM as diagnosed by traditional OGTT. We will also examine alternative GDM biomarkers, including plasma glycated CD59, glycated albumin and 1,5-anhydroglucitol.20 Lipids, which are associated with fetal growth,68 will also be examined. Such novel biomarkers, either alone or combined with CGM, could be a more reliable and efficient way to conduct GDM screening compared with OGTT and improve problems with reproducibility, adherence, patient burden and healthcare resources associated with currently recommended screening protocols.69–72
The GO MOMs cohort will be comprised diverse individuals representing the US birthing population, allowing for generalisability and facilitating translation of findings to US clinical practice. The results will inform future clinical trials designed to prevent GDM and its sequelae. The GO MOMs cohort can also serve as a vehicle for ancillary studies. Such studies could focus on understanding the relationship between in-utero exposure to hyperglycaemia and long-term outcomes in offspring, which would have the potential to help break the intergenerational cycle of obesity and diabetes. Follow-up of the cohort could examine long-term cardiometabolic outcomes in GO MOMs parents which could lead to more effective preventive strategies for type 2 diabetes and cardiovascular disease. The stored samples from the NIDDK Biorepository will be a new resource for research on diabetes, metabolic disease and pregnancy.
Strengths of the GO MOMs study include the large sample size of pregnant participants which will facilitate a training and validation framework for any new criteria developed, the anticipated representativeness of the cohort, the detailed longitudinal glycaemic profiling using multiple assessment methods, and the use of a central laboratory and standardised processing protocol for laboratory analyses. Limitations of the study include its observational nature, which will preclude the ability to make conclusions about causality for the observed associations, limited duration of CGM monitoring in each participant, lack of physical activity data collection, and nutrition data which is limited to a subset of the study population. The decisions about CGM monitoring duration and physical activity and nutrition data collection were made in an effort to minimise participant burden and increase adherence to the study protocol.
GO MOMs has the potential to advance our understanding of the effects of hyperglycaemia throughout gestation on outcomes for birthing persons and their children. Given the association between GDM and both short-term and long-term health consequences5–8 and the large number of people affected by GDM, data generated in GO MOMs could ultimately have a large impact on population health.
Supplementary Material
Acknowledgments
Writing Group: Camille E Powe (Diabetes Unit, Endocrine Division, Department of Medicine, Department of Obstetrics, Gynecology, and Reproductive Biology, Massachusetts General Hospital, Harvard Medical School, Boston, Massachusetts, USA); Kimberly K Vesco, Erin S LeBlanc (Science Programs Department, Kaiser Permanente Center for Health Research, Portland, Oregon, USA); Jennifer Sherr (Department of Pediatrics, Division of Pediatric Endocrinology, Yale University School of Medicine New Haven, Connecticut, USA); Noelia Zork, Uma M Reddy (Department of Obstetrics and Gynecology, Division of Maternal Fetal Medicine, Columbia University Irving Medical Center, New York, New York, USA); Maisa Feghali (Department of Obstetrics, Gynecology, and Reproductive Sciences, Division of Maternal-Fetal Medicine, University of Pittsburgh, Pittsburgh, Pennsylvania, USA); Erika Werner (Department of Obstetrics and Gynecology, Tufts University School of Medicine, Boston, Massachusetts, USA); Denise M Scholtens (Department of Preventive Medicine, Division of Biostatistics, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA).
Footnotes
Collaborators: The GO MOMs Study Group: Erin S LeBlanc, MD, MPH; Kimberly K Vesco, MD, MPH; David Amy, MA; Kristi Bays; Stefan Massimino, MS; Sperry Robinson; Katrina Schell (Center for Health Care Research, Kaiser Permanente Northwest, Portland, Oregon, USA); Teresa A Hillier, MD, MS; Caryn Oshiro, PhD, RD; Sandra Cordero; Connor Howick, MS; Lisa Kim, NP, RN; Yannica Theda Martinez, MS; Olena Pishchalenko; Vladka Wastlova, RN; Gillian A Walters, RN (Center for Integrated Health Care Research, Kaiser Permanente Hawaii, Honolulu, Hawaii, USA); Noelia Zork, MD; Mirella Mourad, MD; Jacqueline Lonier, MD; Uma Reddy, MD, MPH; Jayleen Acevedo; Jose Castillo; Michael Gomez; Belgica Peguero(Columbia University Irving Medical Center, New York, New York, USA); Francesca Facco, MD; Maisa Feghali, MD; Heather Bocan; Savannah Stramowski (Magee-Womens Research Institute/UPMC Magee-Womens Hospital, Pittsburgh, Pennsylvania, USA);Camille E Powe, MD; Andrea Edlow, MD, MSc; William Barth, MD; David Nathan, MD; Robin Azevedo, RN; Arantxa Medina Baez; Chinenye Iroajanma; Mary Larkin, RN, MS; Jacqueline Maya, MD; Chloe Michalopoulos; Nefeli Neamonitaki; Nopporn Thangthaeng, PhD, RN (Massachusetts General Hospital, Boston, Massachusetts, USA); Lynn Yee, MD, MPH; William Lowe, MD; William Grobman, MD, MBA; Emily Szmuilowicz, MD, MS; Alyssa Aguirre; Evelyn Guevara; Gail Mallett; Samantha Matos; Shubhi Tyagi (Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA); Alexandra Spadola, MD; Patrick Catalano, MD; Afshin Azimirad, MD (Tufts University Medical School, Boston, Massachusetts, USA); Erika Werner, MD, MS; Dwight Rouse, MD; Madeline Malloy, MS, RN; Floralba Parra (Women & Infants Hospital of Rhode Island, Providence, Rhode Island, USA); Jennifer Sherr, MD, PhD; Audrey Merriam, MD, MS; Sherrie Bitterman, RN, MBA; Elizabeth Considine; Jessica Leventhal, DNP, APRN; Lauren Perley, MA; Linda Rink, RN; Amy Steffen, RN; Beatrix Thompson; Melinda Zgorski, RN (Yale University School of Medicine, New Haven, Connecticut, USA); Michael Steffes, MD, PhD; Valerie Arends, MS; Anthony Killeen, MD, PhD (Advanced Research and Diagnostic Laboratory, University of Minnesota, Minneapolis, Minnesota, USA); Denise Scholtens, PhD; Juned Siddique, PhD; Patricia Bustamante, MPH; Ying (Jennifer) Cheung, MS; Carmen Edith Freeze, MSEPA; Tania Grott; Alan Kuang, MS; Mary Beth Tull, MS, CCRP (Northwestern University Data Analysis and Coordinating Center, Northwestern University Feinberg School of Medicine, Chicago, Illinois, USA); Barbara Linder, MD, PhD (National Institute of Diabetes and Digestive and Kidney Diseases/National Institutes of Health, Bethesda, Maryland, USA).
Contributors: Individual contributions from members of the Writing Group are reported. Members of the Writing do meet ICMJE criteria for authorship. CEP, KKV and DS were responsible for primary drafting of all sections the manuscript and critical review of all content and suggested edits from the writing group. EL, JS, NZ, MF, EW and UR were all critical contributors to manuscript content review, writing and editing. All authors contributed to study design through cooperative activities of the GO MOMs Study Group. All members of the Writing Group and all members of the GO MOMs Steering Committee approve of this manuscript submission.
Funding: This work is supported by grants from the National Institute of Diabetes and Digestive and Kidney Diseases: U01DK123795 to Massachusetts General Hospital; U01DK123791 to Kaiser Permanente; U01DK123759 and U01DK123745 to Northwestern University; U01DK123799 to Yale University; U01DK123783 to Women & Infants Hospital of Rhode Island. Dexcom provided the CGM systems used in the study free of charge. The GO MOMs Nutrition Study is supported by the National Institutes of Health Office of Nutrition Research.
Competing interests: CEP reports relationships with Mediflix, Endocrine Society, Wolter-Kluwer and American Diabetes Association. JS reports relationships with Medtronic Diabetes, Bigfoot Biomedical, Cecelia Health, Insulet, Vertex, Zealand, Provention Bio, Abbott Diabetes and Start Up Health T1D Moonshot. All other members of the writing group declare they have no known competing interests.
Patient and public involvement: Patients and/or the public were involved in the design, or conduct, or reporting, or dissemination plans of this research. Refer to the Methods section for further details.
Provenance and peer review: Not commissioned; externally peer reviewed.
Contributor Information
Collaborators: Denise Scholtens, Erin S LeBlanc, Kimberly K Vesco, David Amy, Kristi Bays, Stefan Massimino, Sperry Robinson, Katrina Schell, Gillian A Walters, Teresa A Hillier, Caryn Oshiro, Sandra Cordero, Connor Howick, Lisa Kim, Yannica Theda Martinez, Olena Pishchalenko, Vladka Wastlova, Noelia Zork, Mirella Mourad, Jacqueline Lonier, Uma Reddy, Jayleen Acevedo, Jose Castillo, Michael Gomez, Belgica Peguero, Francesca Facco, Maisa Feghali, Heather Bocan, Savannah Stramowski, Camille E Powe, Andrea Edlow, William Barth, David Nathan, Robin Azevedo, Arantxa Medina Baez, Chinenye Iroajanma, Mary Larkin, Jacqueline Maya, Chloe Michalopoulos, Nefeli Neamonitaki, Nopporn Thangthaeng, Lynn Yee, William Lowe, William Grobman, Emily Szmuilowicz, Alyssa Aguirre, Evelyn Guevara, Gail Mallett, Samantha Matos, Shubhi Tyagi, Alexandra Spadola, Patrick Catalano, Afshin Azimirad, Erika Werner, Dwight Rouse, Madeline Malloy, Floralba Parra, Jennifer Sherr, Audrey Merriam, Sherrie Bitterman, Elizabeth Considine, Jessica Leventhal, Lauren Perley, Linda Rink, Amy Steffen, Beatrix Thompson, Melinda Zgorski, Michael Steffes, Valerie Arends, Anthony Killeen, Juned Siddique, Patricia Bustamante, Ying (Jennifer) Cheung, Carmen Edith Freeze, Tania Grott, Alan Kuang, Mary Beth Tull, and Barbara Linder
Ethics statements
Patient consent for publication
Not applicable.
References
- 1. Lawrence JM, Contreras R, Chen W, et al. Trends in the prevalence of preexisting diabetes and gestational diabetes mellitus among a racially/ethnically diverse population of pregnant women, 1999-2005. Diabetes Care 2008;31:899–904. 10.2337/dc07-2345 [DOI] [PubMed] [Google Scholar]
- 2. Correa A, Bardenheier B, Elixhauser A, et al. Trends in prevalence of diabetes among delivery hospitalizations, United States, 1993-2009. Matern Child Health J 2015;19:635–42. 10.1007/s10995-014-1553-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Deputy NP, Kim SY, Conrey EJ, et al. Prevalence and changes in preexisting diabetes and gestational diabetes among women who had a live birth - United States, 2012-2016. MMWR Morb Mortal Wkly Rep 2018;67:1201–7. 10.15585/mmwr.mm6743a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gregory EC, Ely DM. Trends and characteristics in gestational diabetes: United States, 2016-2020. Natl Vital Stat Rep 2022;71:1–15. [PubMed] [Google Scholar]
- 5. Buchanan TA, Xiang AH, Page KA. Gestational diabetes mellitus: risks and management during and after pregnancy. Nat Rev Endocrinol 2012;8:639–49. 10.1038/nrendo.2012.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ehrenberg HM, Durnwald CP, Catalano P, et al. The influence of obesity and diabetes on the risk of cesarean delivery. Am J Obstet Gynecol 2004;191:969–74. 10.1016/j.ajog.2004.06.057 [DOI] [PubMed] [Google Scholar]
- 7. Ehrenberg HM, Mercer BM, Catalano PM. The influence of obesity and diabetes on the prevalence of Macrosomia. American Journal of Obstetrics and Gynecology 2004;191:964–8. 10.1016/j.ajog.2004.05.052 [DOI] [PubMed] [Google Scholar]
- 8. Lowe WL, Scholtens DM, Lowe LP, et al. Association of gestational diabetes with maternal disorders of glucose metabolism and childhood Adiposity. JAMA 2018;320:1005–16. 10.1001/jama.2018.11628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Crowther CA, Hiller JE, Moss JR, et al. Effect of treatment of gestational diabetes mellitus on pregnancy outcomes. N Engl J Med 2005;352:2477–86. 10.1056/NEJMoa042973 [DOI] [PubMed] [Google Scholar]
- 10. Hartling L, Dryden DM, Guthrie A, et al. Benefits and harms of treating gestational diabetes mellitus: a systematic review and meta-analysis for the U.S preventive services task force and the National Institutes of health office of medical applications of research. Ann Intern Med 2013;159:123–9. 10.7326/0003-4819-159-2-201307160-00661 [DOI] [PubMed] [Google Scholar]
- 11. Landon MB. Is there a benefit to the treatment of mild gestational diabetes mellitus Am J Obstet Gynecol 2010;202:649–53. 10.1016/j.ajog.2010.02.006 [DOI] [PubMed] [Google Scholar]
- 12. Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med 2009;361:1339–48. 10.1056/NEJMoa0902430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vääräsmäki M. Is it worth treating gestational diabetes: if so, when and how Diabetologia 2016;59:1391–5. 10.1007/s00125-016-3976-6 [DOI] [PubMed] [Google Scholar]
- 14. Yang X, Tian H, Zhang F, et al. A randomised Translational trial of lifestyle intervention using a 3-tier shared care approach on pregnancy outcomes in Chinese women with gestational diabetes mellitus but without diabetes. J Transl Med 2014;12:290. 10.1186/s12967-014-0290-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. ACOG practice bulletin No.190: gestational diabetes mellitus. Obstet Gynecol 2018;131:e49–64. 10.1097/AOG.0000000000002501 [DOI] [PubMed] [Google Scholar]
- 16. ElSayed NA, Aleppo G, Aroda VR, et al. 2. classification and diagnosis of diabetes: standards of care in Diabetes-2023 . Diabetes Care 2023;46:S19–40. 10.2337/dc23-S002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Simmons D. Paradigm shifts in the management of diabetes in pregnancy: the importance of type 2 diabetes and early hyperglycemia in pregnancy: the 2020 Norbert Freinkel award lecture. Diabetes Care 2021;44:1075–81. 10.2337/dci20-0055 [DOI] [PubMed] [Google Scholar]
- 18. Gillman MW, Oakey H, Baghurst PA, et al. Effect of treatment of gestational diabetes mellitus on obesity in the next generation. Diabetes Care 2010;33:964–8. 10.2337/dc09-1810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Landon MB, Rice MM, Varner MW, et al. Mild gestational diabetes mellitus and long-term child health. Diabetes Care 2015;38:445–52. 10.2337/dc14-2159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wexler DJ, Powe CE, Barbour LA, et al. Research gaps in gestational diabetes mellitus: executive summary of a national Institute of diabetes and digestive and kidney diseases workshop. Obstet Gynecol 2018;132:496–505. 10.1097/AOG.0000000000002726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Josefson JL, Scholtens DM, Kuang A, et al. Newborn Adiposity and cord blood C-peptide as mediators of the maternal metabolic environment and childhood Adiposity. Diabetes Care 2021;44:1194–202. 10.2337/dc20-2398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Carpenter M, Canick J, Star J, et al. Fetal Hyperinsulinism at 14-20 weeks and subsequent gestational diabetes. Obstetrics & Gynecology 1996;87:89–93. 10.1016/0029-7844(95)00361-4 [DOI] [PubMed] [Google Scholar]
- 23. Carpenter MW, Canick JA, Hogan JW, et al. Amniotic fluid insulin at 14-20 weeks' gestation: association with later maternal glucose intolerance and birth Macrosomia. Diabetes Care 2001;24:1259–63. 10.2337/diacare.24.7.1259 [DOI] [PubMed] [Google Scholar]
- 24. Tisi DK, Burns DH, Luskey GW, et al. Fetal exposure to altered amniotic fluid glucose, insulin, and insulin-like growth factor-binding protein 1 occurs before screening for gestational diabetes mellitus. Diabetes Care 2011;34:139–44. 10.2337/dc10-0607 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Rodriguez A, Delgado A, Pressman K, et al. Early gestational diabetes screening in women at risk for gestational diabetes: a randomized controlled trial. American Journal of Obstetrics and Gynecology 2022;226:S41. 10.1016/j.ajog.2021.11.050 [DOI] [Google Scholar]
- 26. Enakpene CA, Della Torre M, DiGiovanni L, et al. Randomization of early diabetes screening among obese pregnant women (REDSOAP study). American Journal of Obstetrics and Gynecology 2022;226:S42. 10.1016/j.ajog.2021.11.100 [DOI] [Google Scholar]
- 27. Harper LM, Jauk V, Longo S, et al. Early gestational diabetes screening in obese women: a randomized controlled trial. Am J Obstet Gynecol 2020;222:495. 10.1016/j.ajog.2019.12.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Simmons D, Immanuel J, Hague WM, et al. Treatment of gestational diabetes mellitus diagnosed early in pregnancy. N Engl J Med 2023;388:2132–44. 10.1056/NEJMoa2214956 [DOI] [PubMed] [Google Scholar]
- 29. International Association of Diabetes and Pregnancy Study Groups Consensus Panel, Metzger BE, Gabbe SG, et al. International Association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–82. 10.2337/dc09-1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Davidson KW, Barry MJ, et al. , Force UPST . Screening for gestational diabetes: US preventive services task force recommendation statement. JAMA 2021;326:531–8. 10.1001/jama.2021.11922 [DOI] [PubMed] [Google Scholar]
- 31. Hadden DR, McLaughlin C. Normal and abnormal maternal metabolism during pregnancy. Seminars in Fetal and Neonatal Medicine 2009;14:66–71. 10.1016/j.siny.2008.09.004 [DOI] [PubMed] [Google Scholar]
- 32. Lowe WL, Karban J. Genetics, Genomics and metabolomics: new insights into maternal metabolism during pregnancy. Diabet Med 2014;31:254–62. 10.1111/dme.12352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Freinkel N. Banting lecture of pregnancy and progeny. Diabetes 1980;29:1023–35. 10.2337/diab.29.12.1023 [DOI] [PubMed] [Google Scholar]
- 34. Freinkel N, Metzger BE, Nitzan M, et al. Facilitated Anabolism in late pregnancy: some novel maternal compensations for accelerated starvation. In: Malaisse WJ, Pirart J, eds. Proceedings of the VIII Congress of the International Diabetes Federation; 1974:474–88. [Google Scholar]
- 35. Haggarty P. Fatty acid supply to the human fetus. Annu Rev Nutr 2010;30:237–55. 10.1146/annurev.nutr.012809.104742 [DOI] [PubMed] [Google Scholar]
- 36. Herrera E, Amusquivar E, López-Soldado I, et al. Maternal lipid metabolism and Placental lipid transfer. Horm Res 2006;65 Suppl 3:59–64. 10.1159/000091507 [DOI] [PubMed] [Google Scholar]
- 37. Kalhan SC. Protein metabolism in pregnancy. Am J Clin Nutr 2000;71:1249S–55S. 10.1093/ajcn/71.5.1249s [DOI] [PubMed] [Google Scholar]
- 38. Powe CE, Huston Presley LP, Locascio JJ, et al. Augmented insulin Secretory response in early pregnancy. Diabetologia 2019;62:1445–52. 10.1007/s00125-019-4881-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bochkur Dratver MA, Arenas J, Thaweethai T, et al. Longitudinal changes in glucose during pregnancy in women with gestational diabetes risk factors. Diabetologia 2022;65:541–51. 10.1007/s00125-021-05622-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Martin JA, Hamilton BE, Osterman MJK, et al. Births: final data for 2018. Natl Vital Stat Rep 2019;68:1–47. [PubMed] [Google Scholar]
- 41. Donovan L, Hartling L, Muise M, et al. Screening tests for gestational diabetes: A systematic review for the U.S. Ann Intern Med 2013;159:115–22. 10.7326/0003-4819-159-2-201307160-00657 [DOI] [PubMed] [Google Scholar]
- 42. Aris IM, Kleinman KP, Belfort MB, et al. US reference for Singleton birth weight Percentiles using obstetric estimates of gestation. Pediatrics 2019;144. 10.1542/peds.2019-0076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Derraik JGB, Maessen SE, Gibbins JD, et al. Large-for-gestational-age phenotypes and obesity risk in adulthood: a study of 195,936 women. Sci Rep 2020;10:2157. 10.1038/s41598-020-58827-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Danne T, Nimri R, Battelino T, et al. International consensus on use of continuous glucose monitoring. Diabetes Care 2017;40:1631–40. 10.2337/dc17-1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Law GR, Alnaji A, Alrefaii L, et al. Suboptimal nocturnal glucose control is associated with large for gestational age in treated gestational diabetes mellitus. Diabetes Care 2019;42:810–5. 10.2337/dc18-2212 [DOI] [PubMed] [Google Scholar]
- 46. Metzger BE, Gabbe SG, et al. , International Association of D, Pregnancy Study Groups Consensus P . International Association of diabetes and pregnancy study groups recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 2010;33:676–82. 10.2337/dc09-1848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Artzi NS, Shilo S, Hadar E, et al. Prediction of gestational diabetes based on nationwide electronic health records. Nat Med 2020;26:71–6. 10.1038/s41591-019-0724-8 [DOI] [PubMed] [Google Scholar]
- 48. Teede HJ, Harrison CL, Teh WT, et al. Gestational diabetes: development of an early risk prediction tool to facilitate opportunities for prevention. Aust N Z J Obstet Gynaecol 2011;51:499–504. 10.1111/j.1479-828X.2011.01356.x [DOI] [PubMed] [Google Scholar]
- 49. Benhalima K, Van Crombrugge P, Moyson C, et al. Estimating the risk of gestational diabetes mellitus based on the 2013 WHO criteria: a prediction model based on clinical and biochemical variables in early pregnancy. Acta Diabetol 2020;57:661–71. 10.1007/s00592-019-01469-5 [DOI] [PubMed] [Google Scholar]
- 50. Donovan BM, Breheny PJ, Robinson JG, et al. Development and validation of a clinical model for Preconception and early pregnancy risk prediction of gestational diabetes mellitus in nulliparous women. PLoS One 2019;14:e0215173. 10.1371/journal.pone.0215173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. van Leeuwen M, Opmeer BC, Zweers EJK, et al. Estimating the risk of gestational diabetes mellitus: a clinical prediction model based on patient characteristics and medical history. BJOG 2010;117:69–75. 10.1111/j.1471-0528.2009.02425.x [DOI] [PubMed] [Google Scholar]
- 52. Griffin ME, Coffey M, Johnson H, et al. Universal vs. risk factor-based screening for gestational diabetes mellitus: detection rates, gestation at diagnosis and outcome. Diabet Med 2000;17:26–32. 10.1046/j.1464-5491.2000.00214.x [DOI] [PubMed] [Google Scholar]
- 53. Tita ATN, Lai Y, Landon MB, et al. Predictive characteristics of elevated 1-hour glucose challenge test results for gestational diabetes. Am J Perinatol 2017;34:1464–9. 10.1055/s-0037-1604243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Vyas DA, Eisenstein LG, Jones DS. Reconsidering the use of race correction in clinical Algorithms. N Engl J Med 2020;383:874–82. 10.1056/NEJMms2004740 [DOI] [PubMed] [Google Scholar]
- 55. Yudell M, Roberts D, DeSalle R, et al. Taking race out of human Genetics. Science 2016;351:564–5. 10.1126/science.aac4951 [DOI] [PubMed] [Google Scholar]
- 56. Castorino K, Polsky S, O’Malley G, et al. Performance of the Dexcom G6 continuous glucose monitoring system in pregnant women with diabetes. Diabetes Technol Ther 2020;22:943–7. 10.1089/dia.2020.0085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Potter JM, Hickman PE, Oakman C, et al. Strict Preanalytical oral glucose tolerance test blood sample handling is essential for diagnosing gestational diabetes mellitus. Diabetes Care 2020;43:1438–41. 10.2337/dc20-0304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Facco F. Sleep duration, sleep timing, and sleep disordered breathing-associations with obesity and gestational diabetes in pregnancy. Clin Obstet Gynecol 2021;64:196–203. 10.1097/GRF.0000000000000587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Automated Self-Administered 24-Hour (ASA24®) Dietary Assessment Tool, 2023. Available: https://epi.grants.cancer.gov/asa24/
- 60. Leisenring W, Alonzo T, Pepe MS. Comparisons of predictive values of binary medical diagnostic tests for paired designs. Biometrics 2000;56:345–51. 10.1111/j.0006-341x.2000.00345.x [DOI] [PubMed] [Google Scholar]
- 61. Meertens L, Smits L, van Kuijk S, et al. External validation and clinical usefulness of first-trimester prediction models for Small- and large-for-gestational-age infants: a prospective cohort study. BJOG 2019;126:472–84. 10.1111/1471-0528.15516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Thaweethai T, Soetan Z, James K, et al. Distinct insulin physiology Trajectories in Euglycemic pregnancy and gestational diabetes mellitus. Diabetes Care 2023;46:2137–46. 10.2337/dc22-2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Steck AK, Dong F, Taki I, et al. Continuous glucose monitoring predicts progression to diabetes in autoantibody positive children. J Clin Endocrinol Metab 2019;104:3337–44. 10.1210/jc.2018-02196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Steck AK, Dong F, Taki I, et al. Early hyperglycemia detected by continuous glucose monitoring in children at risk for type 1 diabetes. Diabetes Care 2014;37:2031–3. 10.2337/dc13-2965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Steck AK, Dong F, Rasmussen CG, et al. CGM Metrics predict imminent progression to type 1 diabetes: Autoimmunity screening for kids (ASK) study. Diabetes Care 2021. 10.2337/figshare.17049941 [DOI] [PubMed] [Google Scholar]
- 66. Insel RA, Dunne JL, Atkinson MA, et al. Staging Presymptomatic type 1 diabetes: a scientific statement of JDRF, the endocrine society, and the American diabetes Association. Diabetes Care 2015;38:1964–74. 10.2337/dc15-1419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Shah VN, DuBose SN, Li Z, et al. Continuous glucose monitoring profiles in healthy nondiabetic participants: A multicenter prospective study. J Clin Endocrinol Metab 2019;104:4356–64. 10.1210/jc.2018-02763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Barbour LA, Hernandez TL. Maternal non-Glycemic contributors to fetal growth in obesity and gestational diabetes: spotlight on lipids. Curr Diab Rep 2018;18:37. 10.1007/s11892-018-1008-2 [DOI] [PubMed] [Google Scholar]
- 69. Sievenpiper JL, McDonald SD, Grey V, et al. Missed follow-up opportunities using a two-step screening approach for gestational diabetes. Diabetes Res Clin Pract 2012;96:e43–6. 10.1016/j.diabres.2012.01.030 [DOI] [PubMed] [Google Scholar]
- 70. Donovan LE, Savu A, Edwards AL, et al. Prevalence and timing of screening and diagnostic testing for gestational diabetes mellitus: A population-based study in Alberta, Canada. Diabetes Care 2016;39:55–60. 10.2337/dc15-1421 [DOI] [PubMed] [Google Scholar]
- 71. Hillier TA, Pedula KL, Ogasawara KK, et al. A pragmatic, randomized clinical trial of gestational diabetes screening. N Engl J Med 2021;384:895–904. 10.1056/NEJMoa2026028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Davis EM, Abebe KZ, Simhan HN, et al. Perinatal outcomes of two screening strategies for gestational diabetes mellitus: A randomized controlled trial. Obstet Gynecol 2021;138:6–15. 10.1097/AOG.0000000000004431 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Committee opinion no 700: methods for estimating the due date. Obstet Gynecol 2017;129:e150–4. 10.1097/AOG.0000000000002046 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.