ABSTRACT
Escherichia coli and Salmonella isolates produce a range of different polysaccharide structures that play important roles in their biology. E. coli isolates often possess capsular polysaccharides (K antigens), which form a surface structural layer. These possess a wide range of repeat-unit structures. In contrast, only one capsular polymer (Vi antigen) is found in Salmonella, and it is confined to typhoidal serovars. In both genera, capsules are vital virulence determinants and are associated with the avoidance of host immune defenses. Some isolates of these species also produce a largely secreted exopolysaccharide called colanic acid as part of their complex Rcs-regulated phenotypes, but the precise function of this polysaccharide in microbial cell biology is not fully understood. E. coli isolates produce two additional secreted polysaccharides, bacterial cellulose and poly-N-acetylglucosamine, which play important roles in biofilm formation. Cellulose is also produced by Salmonella isolates, but the genes for poly-N-acetylglucosamine synthesis appear to have been lost during its evolution toward enhanced virulence. Here, we discuss the structures, functions, relationships, and sophisticated assembly mechanisms for these important biopolymers.
KEYWORDS: extracellular polysaccharides, capsules, exopolysaccharides, complex carbohydrates, bacterial cell surfaces, host-pathogen interactions, polymer biosynthesis, macromolecular trafficking, antigenic diversity, serotype
The cell surfaces of Escherichia coli and Salmonella consist of a complex array of proteins and polysaccharides. Some of the polysaccharides represent major cellular antigens that have been used to distinguish the serotypes of isolates for more than 60 years (1, 2). Most isolates of both species produce O antigens (3) determined by the variable polysaccharide side chains of lipopolysaccharide (LPS) (4). Many isolates of E. coli (but not Salmonella) also produce K-antigen polysaccharides (derived from the German word for capsule, kapsel), which form highly hydrated cell-enveloping capsule structures surrounding the cell (5) (Fig. 1). The O and K antigens were traditionally identified by classical serological typing in whole-cell agglutination tests using defined antisera, but molecular serotyping offers an increasingly attractive advance with access to whole-genome sequencing and the correlation of specific genetic loci with known polysaccharide structures. This is particularly true with established catalogues of E. coli (6) and Salmonella (7) O antigens. Unfortunately, comparable comprehensive information is not currently available for E. coli K antigens; more than 80 K serotypes are distinguished by serology, but the precise number of unique glycan structures in natural populations is uncertain (5). Horizontal gene transfer and recombination events lead to the generation of new O and K-antigen structures, driven by environmental selective pressures, including host immune responses and evasion of bacteriophages, which may use surface polysaccharides as receptors (8). Similar processes also lead to the distribution of polysaccharide biosynthesis loci between E. coli, Salmonella, and related species (9).
FIG 1.
Group 1 capsule of E. coli serotype K30. This capsule was preserved by a freeze-substitution process (5).
E. coli K-antigen capsules have been classified into four groups (groups 1 to 4) based on characteristics including the genetic organization and regulation of the relevant loci and their mechanisms of biosynthesis and assembly (reviewed in reference 5). Later, we argue that this classification should be reduced to three groups. Homologs of characteristic proteins in these assembly pathways are widespread in Gram-negative species, and some are also involved in conserved steps in Gram-positive bacteria (10), making E. coli systems important prototypes. In addition to serotype-specific polysaccharides, isolates with various combinations of O and K antigens can produce other extracellular polysaccharides that lack robust (or covalent) cell association. These include colanic acid, sometimes called M antigen (mucoid antigen), which is widely distributed in E. coli and Salmonella. Colanic acid is structurally and biosynthetically related to group 1 K antigens, distinguished primarily by its environmental regulation as part of the large Rcs regulon (11). Two other exopolysaccharides produced by E. coli and Salmonella are found in many genera, including some Gram-positive bacteria. These are bacterial cellulose (12) and poly-β-1,6-N-acetyl-d-glucosamine (13) (PNAG); both play vital roles in biofilm formation and are regulated by the second messenger, cyclic-di-GMP. They use assembly processes that are fundamentally different from those of capsular polysaccharides and colanic acid.
GROUP 1 K ANTIGENS AND COLANIC ACID SHARE THE SAME ASSEMBLY STRATEGY BUT ARE DISTINGUISHED BY REGULATION
Structure and organization on the cell surface.
Although more than 80 serological K antigens are recognized, fewer than 20 possess structural features consistent with group 1 capsules (14). These are (typically) branched anionic polymers containing hexuronic acids (Fig. 2A). Their diverse repeat-unit structures always contain glucose (Glc) and/or galactose (Gal) residues in the backbone and are frequently modified with noncarbohydrate residues such as acetyl and pyruvyl groups. Colanic acid shares these features. Genome sequence data indicate that the group 1 capsule (cps) locus occurs relatively infrequently in E. coli, and the available evidence supports the conclusion that these have arisen by horizontal transfer of cps loci from Klebsiella (15). As examples, the E. coli K30 group 1 K-antigen prototype shares the same capsule structure as Klebsiella K20 and the Klebsiella K10 genes have been described in a clade of extended-spectrum β-lactamase (ESBL)-producing multidrug-resistant E. coli isolates. Capsules belonging to group 1 are confined mostly to E. coli isolates causing intestinal diseases (particularly enterotoxigenic E. coli; ETEC) and are typically coexpressed with a limited range of O antigens (5). In contrast, colanic acid is produced by different pathotypes of E. coli, which possess a wide range of O antigens, as well as by Salmonella. Production of a group 1 capsule and colanic acid is mutually exclusive because the corresponding genetic loci occupy the same site on the chromosome (near his and gnd). However, the colanic acid locus is also present in (at least some) E. coli isolates with group 2 and 3 capsules (16).
FIG 2.

Colanic acid, group 1 capsules, and group 4 capsules. The repeat-unit structures of colanic acid examples of group 1 and 4 capsular polysaccharides are shown in panel A. Panel B shows the general organization of the genetic loci encoding enzymes and export-translocation proteins required for production of these glycans. Cartoons showing the cellular locations and known/proposed functions of the synthesis-export-translocation proteins are provided in panel C. All examples share the early steps, including initiation on undecaprenol phosphate acceptor by phosphoglycosyltransferase (1), completion of the repeat unit (2), export via a member of the MOPS transporter family (3), and polymerization by a Wzy homologue (4). This is followed by translocation involving a complex comprising PCP-2a and OPX multimers (5 to 7). The PCP-2 protein plays dual roles in polymerization and translocation driven by its reversible phosphorylation (6), which is proposed to result in disassembly of the PCP-2a/OPX complex. Polymerization likely requires direct interaction between Wzy and PCP-2a proteins, and preliminary evidence suggests that Wzy may reside within the lumen of Wzc offering a contiguous channel from the cytoplasmic membrane to the cell surface (see the text for details). However, this hypothesis still requires structural confirmation, so polymerization and translocation are presented here as separate processes for simplicity. Nevertheless, available evidence does support coupled synthesis and export-translocation processes. While capsular polysaccharides are intimately associated with the cell surface, the underlying processes are not fully understood. In group 1 capsules, the lectin-like activity of Wzi is involved, but neither colanic acid nor group 4 capsule systems possess this protein. A substantial number of these glycans (and most of produced colanic acid) is released from the cell. A variable number of the repeat units from these pathways are channeled into LPS assembly; they are ligated to lipid A-core, translocated to the surface via the Lpt machinery, and integrated into the outer membrane (upper left in each model). The structures of Wza (PDB: 2J58, side view and view from the cell surface) and Wzc (PDB: 7NHR, side view and view from the periplasm) from E. coli K30 are shown. Each monomer in the octameric structures is shown in a different color.
The structural motifs in group 1 capsules and colanic acid can be presented on the cell surface in two forms. The predominant form is a high-molecular-weight (>100 kDa) polymer. In micrographs, group 1 capsules form a coherent hydrated polysaccharide layer that extends 200 to 300 nm from the cell surface (Fig. 1). The association of this material with the cell surface is sufficient for it to be retailed by cells after centrifugation, although a covalent linkage between the polysaccharide and a surface molecule has not been discovered. An outer membrane β-barrel protein (designated Wzi [17, 18]) with lectin-like properties is implicated in organization of the capsular structure, but Wzi alone is not sufficient for retention. It has also been suggested that the capsule is stabilized by interactions between glycan strands (19) and, based on studies with similar capsules in Klebsiella pneumoniae, by interaction with negatively charged residues in LPS (20). Whether these together with Wzi are sufficient for the observed robust cell association is still not clear. Indeed, most of the long-chain colanic acid is released from the cell surface as a secreted polymer, yet colanic acid producers differ only in the absence of Wzi. There is clearly more to learn about the molecular details of capsule attachment and the features that distinguish surface attachment versus release of structurally similar polysaccharides from conserved biosynthetic machinery. Interestingly, some E. coli mutants have increased amounts of cell-bound colanic acid (21), and the underlying mechanism may shed light on important details of encapsulation.
Alongside the long-chain polymers, minor forms of the polysaccharide structures are found linked to LPS. This form is called KLPS (22) or MLPS (23) in group 1 capsule and colanic acid producers, respectively. In both, only short oligosaccharides are attached to lipid A-core, distinguishing these molecules from longer conventional O antigens present in the LPS molecules from the same isolates. Currently, the functional and physiological relevance of the LPS forms is uncertain, and they could simply be an accident of relaxed fidelity in the LPS assembly pathway (see below).
Group 1 capsular polysaccharides and colanic acid use a Wzx/Wzy-dependent polymerization mechanism.
These polysaccharides are polymerized in a classical Wzx/Wzy-dependent strategy, which was first reported for LPS O antigens in Salmonella (3) and is now considered to represent the most widespread approach for bacterial polysaccharide biosynthesis in general. The process is modeled in Fig. 2C. Undecaprenol-linked polysaccharide repeat units are built from activated cytosolic precursors (analogous to peptidoglycan biosynthesis) (24). The repeat unit is initiated by a monotopic phosphoglycosyltransferase (PGT) (25); the prototype for this enzyme family is WbaP, best described in the biosynthesis of a subset of galactose-initiated Salmonella O-antigens (7). In group 1 capsule and colanic acid biosynthesis, these enzymes catalyze the transfer of Glc-1-P/Gal-1-P to undecaprenyl phosphate, using UDP-Glc/UDP-Gal donors. Conventional repeat unit-specific glycosyltransferase enzymes (GTs) then complete the carbohydrate structure and acetyl and ketal transferases decorate it. Individual undecaprenyl diphosphate-linked repeat units are exported to the periplasm (prior to polymerization) by Wzx protein transporters. These transporters belong to the multidrug/oligosaccharidyl-lipid/polysaccharide (MOP) flippase family, whose prototype is the peptidoglycan lipid II flippase (MurJ). Wzx is therefore believed to operate in an alternating access mode (26, 27), and the predicted mechanistic similarities in Wzx and MurJ proteins are highlighted by the ability of relaxed substrate-specificity mutants of Wzx from colanic acid biosynthesis to restore peptidoglycan synthesis in murJ-deficient mutants (28). The Wzy polymerase is the other pathway-defining protein. Wzy is an integral membrane protein whose activity has been confirmed in comparable O-antigen systems. In the influential Salmonella prototype, the growing glycan chain is transferred from its undecaprenyl diphosphate carrier to the nonreducing terminus of the incoming undecaprenyl diphosphate-linked repeat unit (29). This reaction presumably occurs at the interface of the periplasm and cytoplasmic membrane, but the mechanistic details await elucidation of a Wzy structure. The polymerization machinery can continue to produce long-chain polymers or provide shorter oligosaccharides used to glycosylate lipid A-core by the LPS O-antigen ligase (3). The resulting KLPS and MLPS molecules are expected to be routed to the cell surface by the LPS-specific translocation pathway (30).
The crucial role of tyrosine phosphorylation in regulating group 1 capsular polysaccharide and colanic acid synthesis.
Although Wzy polymerases are essential for polymerization, they are not sufficient for producing long-chain polymers. They require the participation of a member of the polysaccharide copolymerase (PCP) protein family, which biases Wzy activity toward further polymerization rather than chain termination. Unlike the early biosynthesis steps that require some serotype-specific enzymes, PCP protein sequences are conserved across E. coli K serotypes, i.e., they are not specific for a particular repeat-unit structure. In conventional O-antigen synthesis, the PCP partner is a membrane protein called Wzz (PCP-1 family) (3), but group 1 capsules and colanic acid pathways use Wzc (PCP-2a) proteins, which have more complex structures and additional functions (10). Wzc is thought to direct biosynthetic intermediates into the CPS assembly pathway and is not needed for KLPS or MLPS (Fig. 2C). All PCP family members possess two transmembrane helices flanking a periplasmic domain and form higher order oligomers. Wzc and Wzz form octamers and share similar transmembrane structures and motifs, suggestive of some conserved functions (31–33). Structures of both octamers reveal a large membrane-spanning lumen, and the transmembrane helices contributed by the monomers are not closely packed, which creates portals connecting the lumen within the interior of the membrane (Fig. 2C). This has led to the suggestion that Wzy-mediated polymerization may occur in the lumen of PCP proteins, perhaps with the undecaprenol diphosphate-linked intermediates accessing the polymerase via the portals. This is supported indirectly by evidence that Wzy and Wzz interact (34) and by altered polymerization in mutants with changes in one of the inner ring helices of Wzz (35). However, this proposal awaits structural confirmation.
Wzc proteins are distinguished by a C-terminal cytoplasmic BY-kinase, which is absent in Wzz (10, 36). The C termini of the Wzc homologs also contain a tyrosine-rich motif that is phosphorylated by the kinase in an intermolecular process. This is enabled by an octameric ring of kinase modules, where the tyrosine-rich region of one monomer sits in the catalytic site of the adjacent monomer. This is evident in the structure of the isolated kinase from Wzc from colanic acid production (37) and the full-length protein from E. coli K30 (31). Tyrosine phosphorylation is essential for polysaccharide production (38, 39). In E. coli K30, kinase-null Wzc mutants cannot support high-molecular-weight glycan production (38) and the amount of capsule is affected by the number of C-terminal tyrosine residues available for phosphorylation (40). A cognate phosphotyrosine phosphatase (Wzb) is another essential element of the system (41, 42). This enzyme dephosphorylates Wzc∼P and is also required for capsule production (38), leading to the proposal that cycling of the phosphorylation state of Wzc is required for its activity. In colanic acid production, a kinase-null mutant is also inactive, and Wzc∼P has been proposed to be an inhibitor of polymer production, but cycling is still proposed (43, 44). The structure of Wzc from E. coli K30 provides insight into the role of phosphorylation (31). The kinase ring is the primary contributor to stability of the Wzc octamer, but stable octamers are formed only by dephosphorylated Wzc. The progressive accumulation of phosphotyrosines leads (after 4 or more tyrosines are modified) to disassembly of the octamer and loss of polysaccharide production. Dephosphorylation by Wzb would facilitate reassembly of the octamer to restore polymerization.
Another facet to this system is the possibility that the Wzc kinase phosphorylates other proteins in the assembly system, as has been reported for comparable Gram-positive capsule systems (10). For example, in colanic acid biosynthesis, the Wzc kinase is reported to phosphorylate and activate uridine diphosphoglucose dehydrogenase (Ugd), which generates the activated donor for GlcA residues (45, 46), although the same principle was not confirmed in E. coli K30 (47). If Wzc-mediated phosphorylation of other biosynthetic enzymes is indeed required to support robust polymer production, this cannot be achieved without dissociation of the octamer to allow the target substrate to access the kinase catalytic site, so this regulatory aspect merits further investigation. In addition to its role in synthesis and polymerization, Wzc is also proposed to be part of the pathway for translocating polymer to the cell surface (Fig. 2C) (48).
Wza, the OPX translocon.
Early microscopy studies exploiting conditional mutants of E. coli K29 showed that new capsular polysaccharide was exported at ∼20 sites distributed around the cell surface (48). Translocation of nascent polymer to the cell surface requires an outer membrane channel, or translocon. This is provided by outer membrane polysaccharide export (OPX) proteins (49), with E. coli K30 Wza as the prototype. Wza forms an octameric complex (like Wzc), possessing a 17-Å channel spanning the outer membrane opening into a large periplasmic lumen (50) (Fig. 2C). Unlike typical outer membrane channels (formed by β-barrel proteins), the transmembrane domain of Wza is composed of amphipathic α-helices, and its stability in the membrane is aided by N-terminal acylation. Like Wzc proteins, Wza proteins have no specificity for a particular repeat-unit structure and the lumen possesses conserved polar residues, which may aid in the translocation process. In vivo site-specific cross-linking trapped polymer translocation intermediates, confirming that Wza does indeed function as a translocon (51). However, the lumen in purified Wza octamers is closed at the periplasmic end by a ring of tyrosine residues (Fig. 2C), so access of polysaccharide to the lumen must require participation of an additional component. This may be provided by the periplasmic domain of Wzc, since Wza and Wzc have been shown to interact in vitro (52) and genetic evidence suggests cognate interactions between Wza/Wzc pairs (53). Furthermore, deletion of wza results in downregulation of polymer production resulting in the same phenotype as a wzc mutation (54), implicating a feedback process influenced by Wzc-Wza interaction. Wzc possesses a substantial periplasmic structure compared to Wzz, and three different structural arrangements have been described for this domain of the protein (31). The available structural data have led to a proposed mechanism where phosphorylation and structural status of the kinase ring are relayed to the periplasmic helices. In this process, changes in oligomer stability modulate both the activity of the Wzy polymerase and the interaction with (and opening of) the Wza translocon.
While polymerization and translocation could be coupled events, the growing glycan chain must be released from its undecaprenyl diphosphate carrier at some point, to accommodate the limited undecaprenol phosphate pool and facilitate export of the polymer to the cell surface or beyond. Recent studies with the analogous peptidoglycan assembly pathway Staphylococcus aureus identified a putative release complex (55), but enzymatic release of extracellular polysaccharides might require a dedicated enzyme for each repeat-unit structure. There are currently no candidates for a similar process for group 1 K antigens or colanic acid. Alternatively, release from the undecaprenyl diphosphate carrier might result from a failed Wzy polymerization step that does not complete the glycosyltransfer reaction for chain extension. Another part of the process that is unclear is the steps involved in transition of polysaccharide from a translocation intermediate to a cell-associated group 1 capsule structure. How is Wzi integrated into this process? Wzi is a monomeric outer membrane β-barrel protein that does not form an open channel, but its structure affords no further functional insight (18). There is no indication that Wza can release polymer laterally into the outer membrane (as occurs in LPS insertion into the outer membrane). This implies that surface association must follow completion of translocation through the Wza channel or the polymer never fully leaves the Wza complex. Understanding the biosynthesis-export basis for mutants in the colanic acid system that produce increased amounts of cell-bound colanic acid (21) may inform the mechanism of release from the lipid carrier.
The functional roles of group 1 capsular polysaccharides and colanic acid may be reflected in their differential genetic regulation.
Gene clusters directing production of colanic acid and group 1 capsules begin with a conserved block of (wzi)-wza-wab-wzc; the colanic acid locus is missing wzi (56). This is followed by a serotype-specific region whose gene products build the specific repeat unit (i.e., PGT, Wzx, Wzy, GTs, acetyl and ketal transferases, and enzymes for any unique donor substrates) (56–59) (Fig. 2B). The gene content of this serotype-specific region is comparable to an O-antigen biosynthesis locus. In the transcript, the conserved and variable regions are separated by a predicted stem-loop terminator, potentially allowing production of larger amounts of the multimeric structural proteins, such as Wza and Wzc, than those needed for biosynthesis (60). The operons are preceded by a JUMPStart (just upstream of many polysaccharide starts) sequence (61), containing the ops (operon polarity suppressor) element that recruits RfaH in a common transcription antitermination mechanism for long operons (62, 63).
Colanic acid is not produced in obvious amounts in bacteria grown at 37°C in rich media because of its complex regulation as part of the large Rcs regulon (the Rcs name originates from regulator of capsule synthesis) (reviewed in reference 11). A subset of cellular systems in the Rcs regulon, including colanic acid production, are regulated by heterodimers of RcsB (the response regulator) and RcsA (an auxiliary protein). These proteins activate transcription by binding at the RcsAB box sequence upstream of the transcription start site (64). Rcs proteins also participate in complex regulation of ugd in Salmonella (65). This gene encodes UDP-glucose dehydrogenase, required for production of the UDP-glucuronic acid precursor for colanic acid production.
The Rcs system is activated by factors that influence cell envelope integrity. These include, for example, defects in LPS synthesis and export, peptidoglycan disruption, loss of osmoregulated periplasmic glucans, and treatment with cationic antimicrobials. The appearance of MLPS is a hallmark of these conditions in E. coli K-12 (23). As might be anticipated from its membership in the Rcs regulon, colanic acid is implicated in protection against a range of environmental stressors, including hyperosmolarity, acidic pH, desiccation, oxidative stress, and extreme temperatures (for references, see reference 66). These conditions suggest that colanic acid may be more important in the environment than in the host. However, these roles must be interpreted with care if based solely on phenotypes of mutants in the colanic acid biosynthesis pathway, because some also have adverse effects on general fitness (due to accumulation of undecaprenyl diphosphate-linked intermediates) (67). Furthermore, transcription of the E. coli O157 colanic acid biosynthesis locus is increased in cold and water activity stress, but colanic acid-deficient mutants showed no adverse fitness defects under those conditions (68).
There are conflicting reports of a role for colanic acid in virulence. Several studies have been unable to identify a benefit from colanic acid in the context of pathogenicity (for references, see reference 66). However, recent investigations implicate colanic acid in resistance to serum killing in extraintestinal pathogenic E. coli (ExPEC) isolates (69, 70) and in Salmonella enterica serovar Typhimurium (71). The proposed Rcs-activating stress signal in this setting is provided either by complement compromising the outer membrane barrier and allowing lysozyme to access the peptidoglycan layer or by the action of antimicrobial peptides on the outer membrane (70). These conditions also have detrimental effects on membrane potential, and Rcs-regulated colanic acid production is proposed to help maintain cellular proton motive force under conditions of stress (66). Colanic acid also contributes to the 3-dimensional (3D) architecture (72) and viscoelastic properties (73) of biofilms and participates in the resistance of Salmonella biofilms to host generated oxidative burst (74). In the context of biofilm formation, it is notable that Rcs-mediated upregulation of colanic acid production in E. coli is accompanied by downregulation of motility genes (11).
From the perspective of biosynthesis and assembly, colanic acid can be viewed as a widespread and highly regulated form of group 1 capsule. E. coli group 1 capsule biosynthesis operons lack Rcs-mediated regulation, and as a result, the capsule production is always “on.” Nevertheless, the amount of group 1 capsule is still influenced to some extent by Rcs activation. This is achieved by an RcsAB-box located upstream of the nearby galF gene, a feature shared with K. pneumoniae (60). GalF enhances the activity of GalU (UTP:glucose-1-phosphate uridylyltransferase) in the production of UDP-glucose (75), providing a key donor substrate for glucose residues in polysaccharides and a central intermediate for the synthesis of others. Elevation of GalF activity may be important for increasing the donor substrate pool available to sustain elevated group 1-capsule production without compromising other cellular systems that require UDP-glucose. The presence of group 1 capsules at physiological temperatures has led to the reasonable assumption that they are important for pathogenicity. In livestock, group 1 capsules are important for the colonization by enteropathogenic E. coli (EPEC) isolates (76) and encapsulated bacteria have been observed closely associated with epithelia (77). However, capsule does impair adherence of ETEC to epithelia (78), so the involvement is complicated. Definitive experiments to establish a precise functional role(s) of E. coli group 1 K antigens are lacking, and most of the assumptions rely on analogy to the closely related capsules in K. pneumoniae. These are vital virulence determinants and promote resistance to host defenses, including phagocytosis and complement-mediated killing (79).
GROUP 4 CAPSULAR POLYSACCHARIDES ARE CLOSELY RELATED TO O ANTIGENS
Structure, cell surface organization, and function.
Group 4 capsules are found in EPEC and enterohemorrhagic E. coli (EHEC) isolates, as well as in isolates of Shigella. These bacteria produce a conventional LPS O antigen, together with a (longer) capsular polysaccharide that possesses the same repeat-unit structure. This situation differs from group 1 capsule producers, where the K antigen is expressed with a structurally distinct O antigen. The LPS-linked form of group 4 K antigen is not confined to short oligosaccharides (unlike group 1 KLPS or MLPS) and shows the characteristic regulated modal chain-length distribution of O antigens (see below) (3), while the LPS-free capsular form possesses substantially longer chain length (80). Due to the structural overlaps, group 4 K antigens were referred to as “O-antigen capsules” in their initial description in E. coli serotype O111 (80), and the structural relationships have led to confusing O/K serotype designations (5). Group 4 K antigens (like all known E. coli O antigens) contain N-acetylglucosamine (GlcNAc) and/or N-acetylgalactosamine (GalNAc) residues in their glycan backbone structures, which represent the initial residue in repeat-unit synthesis (Fig. 2A). This distinguishes them from Glu/Gal as the initial sugar in repeat units of their group 1 counterparts. Fewer than 20 group 4 K antigens are currently recognized (14), but some have presumably been overlooked due to the structural identity with O antigens. Group 4 capsules are synthesized by enzymes encoded by the O antigen-biosynthesis locus but also require additional proteins encoded by an unlinked locus dedicated to group 4 capsule export. Therefore, an active version of this unlinked locus has the potential to convert any E. coli isolate with a conventional O antigen into a group 4 capsule-producer. The group 4-dedicated locus is missing in uropathogenic E. coli (UPEC) isolates, and an IS1-element renders it inactive in E. coli K-12 derivatives (81).
There is a degree of general overlap in protective functions provided by LPS O antigens and capsules in Gram-negative bacteria, and this complicates assignment of roles for group 4 capsule producers. Despite the same repeat-unit structures in both glycans, the presence of a group 4 capsule prevents agglutination of E. coli O111 cells by O-specific antibodies, reflecting an effect on antibody interaction with the cell surface (80). However, there are varied outcomes of group 4 capsules in serum sensitivity. For example, when E. coli O111 cells were incubated with purified complement component C3, the capsular form bound the majority of the C3 (82), and group 4 capsule was correlated with increased susceptibility to serum killing (83). In contrast, group 4 capsules in E. coli O78 (84) and Shigella sonnei (85) are required for survival in serum. E. coli group 4 capsules and O antigens are both implicated in resistance to some antimicrobial peptides (86). Type III protein secretion systems (T3SS) play prominent roles in the pathogenesis of Salmonella, EPEC, and EHEC (87). In E. coli O127, the group 4 capsule can impair the delivery of effector proteins by these systems, presumably by masking the needle structure (88). The potentially conflicting requirement for both group 4 capsules and T3SS for virulence is accommodated by differential regulation of these components by Ler (a positive regulator of T3SS), which downregulates genes responsible for the capsule production pathway while leaving O antigen synthesis unaffected (88).
Biosynthesis and export of group 4 capsules.
These polysaccharides are synthesized by the conventional O-antigen biosynthesis locus in a Wzx/Wzy-dependent mechanism (3). In E. coli, this pathway employs a polytopic PGT (WecA), so each repeat unit is initiated by the transfer of GlcNAc/GalNAc-1-P to undecaprenyl phosphate (rather than the Glc/Gal-1-P used for group 1 capsules) (89). The generation of regulated chain-length distribution in the LPS-linked polysaccharide is conferred by a dedicated PCP-1 protein (Wzz) (3).
The exploitation of this pathway to produce a group 4 capsule requires a separate locus containing 7 genes (Fig. 2B and C). The last two of these genes, etp and etk, encode a phosphatase/tyrosine kinase pair homologous to Wzb and Wzc in group 1 capsule production (90). The first five genes (now designated gfcA to E) are named for their role in the pathway (group four capsules) and encode proteins located in the periplasm and outer membrane (81). The functions of GfcABCD are still unknown, but GfcE is a paralog of the group 1 outer membrane export channel protein, Wza. Available evidence suggests that the paralogous proteins share both structure and function with their group 1 counterparts. This includes a requirement for cycling of the phosphorylation state of Etk (90–92). In addition, GfcE can partially compensate for a loss of Wza in group 1 capsule production (91). GfcC is a periplasmic protein that shares some structural elements with the periplasmic region of Wza (92), but how this and the other proteins interface with the biosynthesis platform and why this system is substantially more complex than the Wza-Wzb-Wzc export machinery in group 1 capsule production are subjects for further investigation.
CAPSULAR GLYCOLIPIDS BELONGING TO GROUPS 2 AND 3
Structure, surface organization, and function.
The remaining capsular K antigens are distinguished from group 1 and 4 CPSs based on their repeat-unit structure, attachment to the cell surface, and mode of synthesis. Although they have been separated into two types (groups 2 and 3) in the past (5), the differences between group 2 and 3 capsules are relatively minor. They cannot be distinguished on the basis of carbohydrate structure or characteristic enzymes for biosynthesis and export, and there are no data to suggest differences in general biological functions. Currently, the only discernible differences lie in organization of the genetic loci and thermoregulated expression (see below). As a result, from most perspectives, group 3 capsules can be considered a subgroup of group 2; here, we term them as “classical” and “variant” examples of group 2 capsules.
Group 2 CPSs are glycolipids. The K-antigen structure is linked to a phosphatidylglycerol lipid terminus via a conserved oligosaccharide composed of 3-deoxy-d-manno-oct-2-ulosonic acid (Kdo) residues, with alternating β2,4 and β2,7 linkages (93–96) (Fig. 3). This has been shown by structural and biochemical data for classical examples and is implied in variants by conserved genes. The same linker structure appears to be shared in other Gram-negative mucosal pathogens, including Neisseria meningitidis, Actinobacillus pleuropneumoniae, and others (97). At a superficial level, these CPSs share structural principles with LPS. In both, a lipid moiety is linked to the remaining glycan backbone via one or more Kdo residues (α-linked in LPS and β-linked in CPS) in a conserved linker region, which is capped by a hypervariable structure (the O or K antigen). The group 2 CPS lipid moiety is thought to be responsible for anchoring the serotype-specific glycan to the outer membrane, but in culture, only 20 to 50% of purified glycans are thought to be lipidated (98) and this may reflect the lability of the Kdo linkage under those growth conditions. Exactly how much group 2 CPS is lipid-free and released from the cell is uncertain, but nonlipid molecules may still be retained at the cell surface by ionic interactions with other surface components, including LPS (99). Group 2 capsule synthesis occurs at distinct sites around the cell, generating clusters of CPS molecules which are proposed to surround the export machinery in “capsular rafts” (100). These rafts expand until they converge and completely coat the cell, but the final capsule is not uniform in terms of thickness; equatorial regions of the cell have a capsule thickness of approximately 200 nm, whereas in the polar regions, capsule extends 250 to 400 nm.
FIG 3.
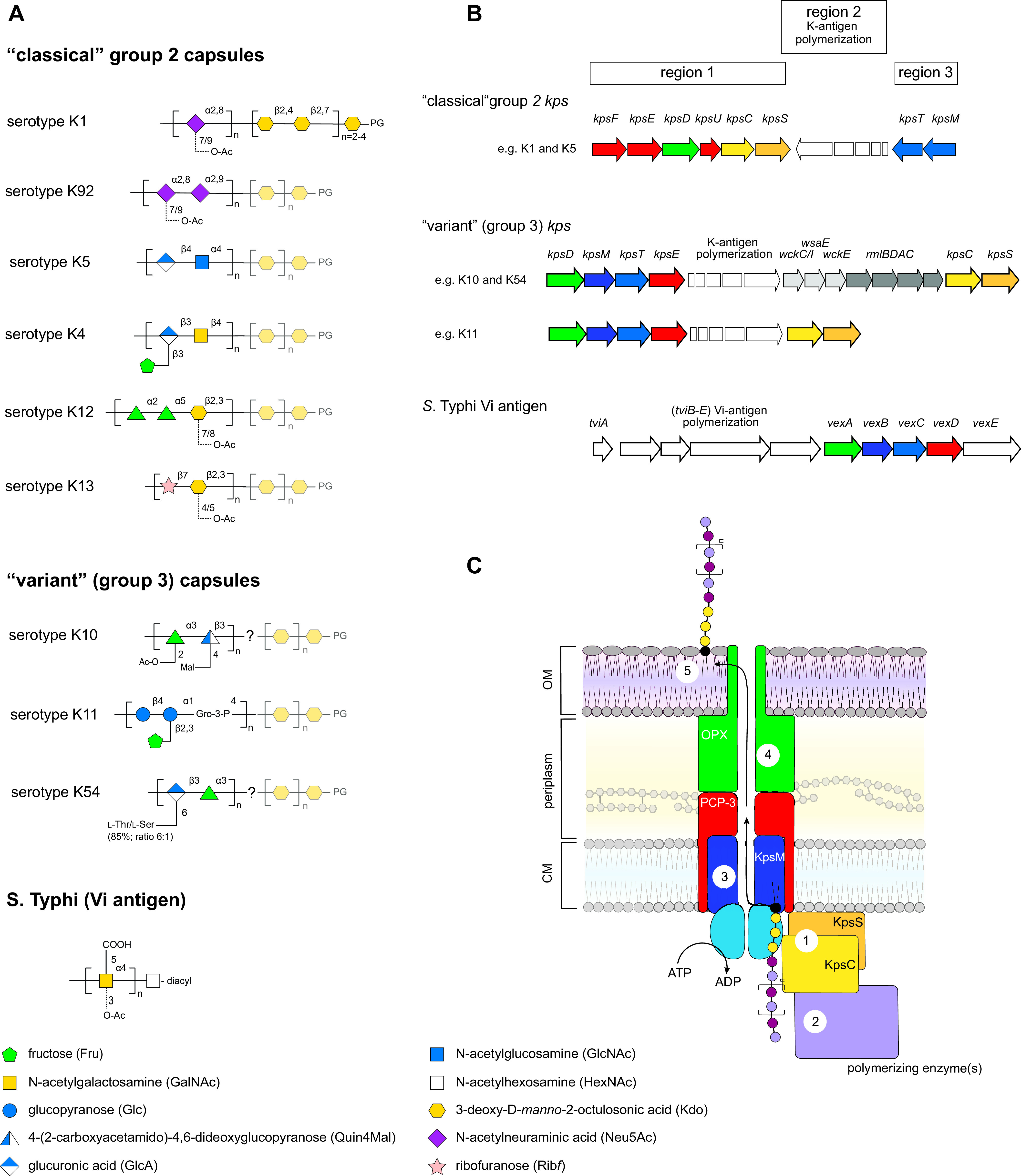
Group 2 capsular glycolipids. Escherichia coli group 2 capsules are separated into classical and variant subgroups (formerly groups 2 and 3). There are no known features in the repeat-unit structures to distinguish the 2 subgroups (panel A), and the presence of kpsSC genes in the genetic loci from both groups (panel B) suggests a shared phosphatidylglycerol-oligo-Kdo terminus. While many serotypes contain the classical locus organization, there are two known subgroups of variant. One subgroup represented by K10 and K54 may introduce some additional sugars between phosphatidylglycerol-oligo-Kdo and the repeat-unit domain (indicated by the question mark in panel A). A cartoon model for synthesis is shown in panel C. The polymer is synthesized at the interface of the cytoplasm and cytoplasmic membrane, initiated by the action of Kdo transferases (1), and extended by repeat unit-specific enzymes (2). Export is performed by an ABC transporter (KpsMT) (3) and is linked to translocation proteins KpsED (4). The terminal glycolipid may help anchor the glycan in the outer membrane (8), but other factors may also participate in generating the morphological capsule structure. While E. coli capsules possess shared assembly structure and assembly features, the sole Salmonella example, Vi antigen, has a different terminal lipid moiety and therefore differs in early steps, but homologs of KpsMTED are required for export-translocation.
Group 2 capsules are commonly associated with extraintestinal pathogenic E. coli (ExPEC) strains, which are responsible for urinary tract infections, meningitis, and other infections outside the digestive tract. Capsules are a key virulence factor in these isolates, allowing them to withstand the harsh environments encountered in the host. In UPEC isolates, bacteria expressing K1 CPS form biofilm-like intracellular bacterial communities promoting persistence in the cytosol of epithelium cells (101) and serotype K2 CPS genes are upregulated in the presence of urea (102). A wealth of data supports the role of group 2 capsules in immune evasion through prevention of complement activation and blocking phagocytosis (103), but the contributions vary according to the combination of O and K antigens (104). Genes responsible for K1 CPS production are upregulated in the presence of human serum (105). Furthermore, mutations reducing K2 CPS decrease survival in serum (106), and chemical inhibition of K1 production attenuates virulence of a UPEC isolate in a mouse sepsis model (107). While the protective role of capsules can be overcome by the presence of anti-CPS antibodies, immune evasion can be achieved though molecular mimicry of host glycan structures by CPS in some serotypes (108). One classical example is the K1 antigen, composed of α-2,8-linked polysialic acid (Fig. 3A), which is identical to the N. meningitidis serotype B CPS. The same structure occurs in the polysaccharide on neural cell adhesion molecules (NCAMs) in mammals (109). Other group 2 CPSs mimic mammalian glycosaminoglycans (GAGs) (108). For example, the K4 CPS backbone (Fig. 3A) is the same as the nonsulfated chondroitin precursor, resembling a GAG that exists primarily as a part of a proteoglycan in the extracellular matrix (110). The glucuronic acid of the K4 repeat unit is decorated with a fructose side chain at C-3, which is easily removed in mild acidic conditions leading to a significant drop in observed serological reactivity in low pH environments. The K5 CPS (Fig. 3A) is composed of heparosan, the precursor to the mammalian heparan sulfate and heparin, which exist as part of proteoglycans found in the extracellular matrix and cytoplasm and on the cell membrane (111). Surface polysaccharides are likely to mediate the initial interactions between pathogens and host cells, so displaying molecules that are recognized as “self” is an effective strategy in avoiding recognition. The potentially damaging consequences of overcoming this recognition barrier make CPS-based vaccines unsuitable for these isolates.
Biosynthesis and export of group 2 CPSs.
Unlike capsules belonging to groups 1 and 4, group 2 glycolipids are synthesized entirely at the cytoplasmic face of the inner membrane before export (Fig. 3C). While synthesis and export are likely coordinated in vivo, they will be discussed separately here for clarity. The conserved poly β-Kdo oligosaccharide is synthesized by two dedicated CMP-Kdo-dependent GTs that transfer β-Kdo residues to phosphatidylglycerol (95, 112, 113). KpsS transfers the first β-Kdo residue, and the remaining β-Kdo residues are transferred by KpsC, which contains two GT99 modules with β2,7 and β2,4 linkage specificities, respectively (96, 97). The linker provides an acceptor for the polymerization of the serotype-specific glycan. Addition of the serotype-specific CPS chain requires two types of GT activity: an enzyme that adds a residue(s) that marks the transition from the β-Kdo linker to the serotype-specific structure and one or more GTs that polymerize the serotype-specific glycan. Capsular glycan chains from a single culture have variable numbers of Kdo residues in their linker regions; in K1, all molecules contain an odd number (5–9, 93), and a recent revised structure for K5 suggests that the same may be true in all serotypes (96). This is likely dictated by the acceptor specificity of the transition enzymes, in a reaction that presumably limits the propensity of KpsC to create unnaturally long linkers. While the identities of transition GTs have not been experimentally established, candidates have been proposed in some systems (97). In a natural situation, the terminal glycolipid is presumed to be an obligate acceptor, but (in E. coli K1 at least) kpsS and kpsC mutants can result in off-pathway polymerization on an unknown nonphysiological acceptor. This acceptor is presumably Kdo or a Kdo-containing molecule. The resulting polymer creates a cytoplasmic aggregate polymer, which cannot be exported (112, 114).
Polymerization of the structurally variable (K antigen) part of glycolipid is achieved by one or more GT enzymes. The polysialyltransferases (PSTs; NeuS) responsible for the polymerization of the K1/K92 antigens are examples of well-characterized single-site polymerizing GTs. K1 NeuS synthesizes a polysialic acid that contains only α-2,8 linkages, while the K92 NeuS generates alternating α-2,8, α-2,9 linkages (115, 116). A single amino acid substitution within the catalytic site of K92-NeuS is sufficient to switch the enzyme to K1-NeuS specificity, indicating a relatively simple mechanism for serotype diversification (117). Although PSTs are single-site enzymes, some studies indicate that they are not processive in the conventional sense, i.e., they do not retain the glycan during polymerization and operate in a distributive mechanism to generate a range of chain lengths. However, enzyme processivity can be influenced by analysis conditions (118), so the true situation in vivo is still uncertain. In contrast to single-enzyme polymerization, K4 CPS biosynthesis requires a dual-domain protein (KfoC) containing two GT modules requiring different sugar-nucleotide precursors (119, 120). The K5 CPS backbone also contains two different sugars, but in this case, synthesis is proposed to be performed by the alternating activity of two separate GTs (KfiA and KfiC), which have different specificities (121). In addition to the GTs required for synthesis of the CPS backbone, some serotypes have enzymes responsible for the addition of decorating residues, such as O-acetylation in K1 (by NeuO/NeuD [122–124]) or β-fructosylation in K4 (by KfoE [125]).
Transenvelope complexes containing ABC transporters translocate capsular glycolipids to the cell surface.
Completed group 2 capsular glycolipids are translocated to the cell surface by an ABC (ATP-binding cassette) exporter (KpsMT) in the inner membrane, together with two accessory proteins bridging the periplasm and outer membrane (KpsED) (Fig. 3C). The export-translocation proteins are conserved across serotypes and therefore operate independently of the structure of the serotype-specific parts of the CPS. Recognition of the conserved terminal glycolipid in the CPSs by the ABC transporter may explain the ability to exchange these transporters between serotypes (126) (and even between species [127, 128]).
ABC transporters are ubiquitous and participate in import and export of a wide range of substrates, including larger molecules such as capsular glycolipids (129, 130). Each transporter contains two transmembrane domains (TMDs; provided by 2 KpsM polypeptides) to form the channel across the membrane and two nucleotide-binding domains (NBDs; KpsT), where ATP is bound and hydrolyzed to drive the pump function. While the number of domains is the same for all ABC transporters, the arrangement of these domains in terms of protomers and structures can vary (131). Typical ABC transporters for small molecules are thought to employ an inward-facing/outward-facing alternating access mechanism, but it seems unlikely that the entire CPS molecule can be contained within the transporter in a single-step export process. Recently determined structures of ABC transporters exporting undecaprenol diphosphate-linked O antigen (132, 133) and teichoic acid (134) may offer insight into how export of long-chain polysaccharides may be achieved. The transporter lumen is lined with aromatic amino acids, allowing π-stacking with the glycan residues, while the lipid component is recognized for substrate engagement but likely remains in the membrane throughout the export process. Binding and hydrolysis of ATP by the NBDs results in conformational changes that may exert a push on the substrate, creating a processive translocase model driven by iterative rounds of ATP hydrolysis until the long glycans chains are exported (132, 133).
Like the Wzx/Wzy-dependent system, the translocation machinery involves proteins belonging to the PCP and OPX families, though there are important structural and (presumably) functional differences. KpsE belongs to the PCP-3 subfamily, which forms oligomers with extensive periplasmic domains (like Wzc). However, KpsE is not required for polymerization, nor does it contain the cytoplasmic kinase domain found in Wzc (135). Instead, KpsE may act as a periplasmic adaptor between the ABC transporter and KpsD, analogous to the proteins bridging membrane pumps to outer membrane channels in tripartite multidrug efflux complexes (136). A simple model of a contiguous translocation pathway from cytoplasm to the cell surface (Fig. 3C) has been proposed, but this is challenged by the recent observation that the translocation substrates in a related capsule system are accessible to periplasmic glycosylhydrolases (137). Whether this applies to all examples of this export-translocation strategy needs further investigation. The OPX protein in this system is encoded by kpsD. Given the interaction of KpsD with a PCP with structure/function properties different from those of Wzc, some differences in KpsD structure might also be anticipated compared to that of Wza. However, KpsD also appears to be a structural outlier compared to other more Wza-like OPX representatives that participate with many PCP-3 proteins in ABC transporter-based translocation systems (49). KpsD is not acylated and possesses an additional C-terminal domain. Structural predictions indicate that this additional domain is related to GfcC, an essential protein in group 4 capsule assembly (138). GfcC shares structural similarity with the periplasmic domains that form the ring structures in Wza. It also contains a C-terminal amphipathic α-helix, although the terminal helix does not form a membrane-spanning pore under the crystallization conditions used (90). A structural prediction of KpsD shows a shorter C-terminal α-helix (compared to that of Wza) that may be too short to create an outer membrane-spanning channel (138), yet immunofluorescence images of nonpermeabilized cells seem to indicate accessibility of KpsD on the cell surface (139). As part of the envelope-spanning export complex, KpsD must be anchored to peptidoglycan (PG) for efficient export of CPS, and Braun’s lipoprotein (Lpp) is important in this tethering (138, 140). The underlying functional implications of the unique properties of KpsD compared to those of other OPX proteins require further investigation.
Group 2 capsule production is subject to complex regulation.
The proteins required for classic group 2 capsule biosynthesis and export are found in the kps locus, which is usually located near serA on the chromosome. This locus is divided into three regions (Fig. 3B). Regions 1 (kpsFEDUCS) and 3 (kpsMT) are conserved across most examples of the classic locus and encode the proteins required for the synthesis of the phosphatidylglycerol-poly-Kdo acceptor and the proteins required for export, which have been described above. Region 1 also encodes additional copies of enzymes required for production of the CMP-Kdo: kpsF and kpsU. KpsF is a d-arabinose 5-phosphate isomerase, which converts d-ribulose 5-phosphate to d-arabinose 5-phophate (141), and KpsU is a CMP-Kdo synthetase (142). In serotype K15, the gene content is preserved but the orientation of kpsC and kpsS is flipped (143). Homologs of both KpsF and KpsU are encoded elsewhere in the E. coli genome (by kdsD and kdsB, respectively), reflecting the requirement for Kdo in the biosynthesis of LPS core, so kpsFU are not essential for CPS biosynthesis. Nevertheless, an increased level of CMP-Kdo synthetase activity is characteristic of bacteria producing classic group 2 capsules (144). Region 2 is serotype specific and encodes proteins required for synthesis and polymerization of the serotype-specific CPS glycan.
In the variant (formerly group 3) kps locus organization, region 1 contains kpsDMTE, and kpsCS are located downstream of the region 2 biosynthesis genes (145) (Fig. 3C). kpsF and kpsU are absent, and there is no group 2-like elevation of CMP-Kdo synthetase activity (144), suggesting that the donor requirements of KpsS and KpsC in CPS production are sustained by KdsB and KdsD alone. In contrast to the high level of conservation in the common genes shared by most classical group 2 isolates, sequence data for gene loci from a small collection of variant serotypes reveal (at least) two divergent forms (145). In these loci, region 2 still carries genes proposed to be involved in the serotype-specific K antigen. However, in most of the variant loci, region 2 also contains a block of seven conserved genes. The last four genes in this block (rmlBDAC) encode enzymes known to produce dTDP-rhamnose precursors, and most of the corresponding K-antigen structures contain Rha. The first three genes encode putative GTs that have been proposed to be involved in synthesizing a variant linker (presumably in addition to the β-Kdo oligosaccharide). This would create a complex structure unique to the variants, and this aspect merits further biochemical and structural investigation.
To date, experimentally based insight into transcription and regulation is confined to classical group 2 kps. In serotypes K1 and K5, kps regions are transcribed as polycistronic mRNAs. kpsFEDUCS constitutes one mRNA, while the promoter upstream of kpsMT allows read-through transcription of regions 3 and 2. This facilitates independent regulation of region 1 and 3 promoters (PR1/PR3), potentially facilitating fine-tuning of this energy-intensive process. Several transcription factors affect kps transcription, affecting either one or both promoters. SlyA and H-NS interact with each other and both PR1 and PR3 for optimal expression (146, 147). Integration host factor (IHF) also has binding sites in both promoter regions (148). The transcription of region 2 depends on an RfaH-dependent antitermination system, as described above for group 1 capsules; a JUMPStart sequence is located downstream from PR3 (149, 150). Other proteins affect regulation by mechanisms that are still unclear, and they may act indirectly. For example, transcription factor MprA apparently does not bind kps promoters but has a strong effect on CPS production (107). Also, BipA, a ribosome-associated GTPase, is proposed to interact with the ribosome to affect Kps protein translation, but the underlying details are not resolved (148). Classical group 2 glycolipid production is characteristically thermoregulated (i.e., produced only above 20°C), and H-NS and BipA have dual roles in this phenotype; they are required for optimal expression at 37°C as well as repression at 20°C (146). The variant (formerly group 3) kps loci are not thermoregulated (144), and it is still unclear how the various group 2 regulatory features will apply to them.
Salmonella Vi antigen, a capsular glycolipid whose synthesis resembles that of E. coli group 2 CPS.
Vi antigen is a linear polymer of α-1,4-2-deoxy-2-N-acetylgalacturonic acid residues nonstoichiometrically O-acetylated at C-3 (Fig. 3A). This polymer is confined to the human host-restricted serovar Typhi and Paratyphi C, but the genetic locus (Fig. 3B) is also present in Citrobacter freundii and some soil bacteria belonging to the Burkholderiales (Bordetella sp. and Achromobacter sp.) (151). Typhoidal serovars infect 10 to 20 million individuals (with ∼2% fatality) each year, and investigation of Vi antigen has been driven by its role as a virulence factor, as well as its application as a protective antigen. Despite being an intracellular pathogen, vaccines are particularly important with the advance of multidrug-resistant isolates of S. Typhi. One of these is a protein:Vi antigen conjugate, and extensive studies have defined the immunological signatures of protection (152), as well as the influence of O-acetyl groups and chain length on physical properties and immunogenicity of the glycan (153, 154).
Like other capsules, Vi antigen is associated with the evasion of components of the innate immune system, including complement-mediated killing, but Vi is not expressed at all phases of infection. The viaB genetic locus (Fig. 3B) directing its production is induced during the transit of the bacteria from the lumen into the ileal mucosa (reviewed in references 155 and 156). Vi-antigen expression is accompanied by repression of T3SS (SPI-1) and flagella and is activated by low osmolarity in the intestinal mucosa by TviA (a transcriptional regulator encoded by viaB), as well as subject to regulation by RcsBC and OmpR-EnvZ.
The viaB locus encodes the machinery for a classical ABC transporter-dependent assembly mechanism with homologs of KpsMT (VexBC) and representatives of the PCP-3 (VexD) and OPX (VexA) families (Fig. 3C) (157). With the exception of the regulator, TviA, the products of the tvi genes encode enzymes for Vi-antigen biosynthesis (157, 158). One fundamental difference distinguishes Vi antigen from E. coli group 2 (and related) systems: Vi-antigen biosynthesis proceeds without the action of KpsSC homologs. This was explained by the finding that instead of the widely conserved phosphatidylglycerol-oligo-β-Kdo terminus of E. coli group 2 capsular glycolipids, Vi antigen possesses a reducing terminal diacyl-HexNAc residue whose precise structure has not yet been fully determined (151). One acyl chain is added by VexE, a lysophospholipid acyltransferase (LPLAT) enzyme, but the source of the second is still unknown and it has been proposed that it could be derived from enzymes involved in LPS lipid A synthesis. There is much still to be learned about this assembly mechanism, including whether it is used to make other repeat-unit structures in nature.
BIOFILM POLYMERS: CELLULOSE AND POLY-N-ACETYLGLUCOSAMINE
Although colanic acid is linked to biofilm architecture, it does not promote surface adhesion (72). Two additional secreted polysaccharides that are major components of the extracellular matrices in E. coli and Salmonella biofilms help confer this property. These are poly-β1,6-d glucose (cellulose) and poly-β1,6-N-acetylglucosamine (PNAG) (Fig. 4A). Both are widespread across bacterial genera.
FIG 4.

Biofilm exopolysaccharides. E. coli and Salmonella both produce bacterial cellulose, while Salmonella spp. lack the genes for PNAG production. The structures of the cellulose repeat-unit structure and a hypothetical structure of the non-uniform PNAG backbone are shown in panel A. Panel B shows the genetic loci. Models for assembly are shown in panel C. Although the details vary, both contain a synthase-translocase that simultaneously polymerizes (1) and translocates (2) the polymer to the periplasm in a cyclic-di-GMP process. The polymers are subject to modification reactions in the periplasm (4) and directed to an outer membrane β-barrel channel protein that also possesses a tetratricopeptide motif domain (5). These polymers are secreted into the intercellular space, where they support biofilm formation and contribute to their architectures.
Cellulose is an abundant and industrially important biopolymer. Although plants provide the main source, bacterial cellulose production by Komagataeibacter xylinus (originally called Acetobacter xylinum) has been known for decades (12). The initial discovery of cellulose production by E. coli and Salmonella resulted from investigation of the multicellular behavioral phenotype of E. coli designated rdar (red, dry, and rough) (159). In this mode of growth, cellulose is coexpressed with thin aggregative (curli) pili (160) and both are important for cell-cell interactions and the capacity of these organisms to adhere to abiotic surfaces. They also contribute to the architecture of the resilient biofilm that can influence fitness and virulence (161). Biofilm formation is a process that integrates many different environmental cues, so as might be anticipated, cellulose production is subject to complex regulation. This includes activation of specific assembly components by cyclic-di-GMP second messenger (see below), direct effects of response regulators such as RpoS, and indirect effects of small RNAs that exert their effects via the master biofilm regulator CsgD (reviewed in reference 162).
PNAG was initially identified in staphylococci, where it contributes to biofilm formation and defense against the innate immune system (163). The same polymer was later found in E. coli (164), and antibodies against PNAG are protective in a mouse model of infection (165). The genes for PNAG production are absent in Salmonella, suggesting that this polymer is unimportant for (or hinders) the capacity of S. enterica serovar Enteritidis to survive in, and colonize, the host (166).
The assembly systems for cellulose and PNAG share important features (167): (i) both exploit a synthase-translocase complex that coordinates synthesis and export across the cytoplasmic membrane, (ii) synthesis is regulated by the secondary messenger, 3'5'-cyclic-di-GMP (c-di-GMP), (iii) secretion across the periplasm and outer membrane is facilitated by a β-barrel channel and tetratricopeptide repeat (TPR) domains, and (iv) the polymer is enzymatically modified in the periplasm during translocation.
Cellulose biosynthesis involves a complex molecular machine (Fig. 4C) encoded by the bcsEFG and bcsRQABZC operons (Fig. 4B). The core biosynthetic machinery includes BcsABC. BcsA is an integral membrane protein possessing a processive polymerizing GT, whose activity is controlled by c-di-GMP binding to a PilZ domain, which modulates access of substrate to the active site (168, 169). BcsB is located predominantly at the membrane-periplasm interface and forms a complex with BcsA to create a synthase-translocase, which extends and extrudes the nascent chain one residue at a time (169). The emerging cellulose product is directed to BcsC (170), a gated β-barrel outer membrane protein channel, to facilitate release of the cellulose product from the cell (171). BcsC possesses a TPR domain, and these are frequently involved in protein-protein interactions. The TPR domain extends into the periplasm and may interact with BcsAB, perhaps assisting in preventing aggregation of the insoluble glycan during passage through the periplasm.
In E. coli and Salmonella, ∼50% of the Glc residues in the cellulose chain are modified in the periplasm by BcsG, which catalyzes the addition of phosphoethanolamine groups derived from phosphatidylethanolamine (172), which strengthens cellulose-curli association in E. coli and enhances bacterial adhesion to host tissues (173). The nascent polysaccharide can also undergo limited hydrolysis by the BcsZ endoglucanase (12). The additional Bcs proteins are responsible for the proper assembly and fine-tuning of the activity of a multimeric megadalton molecular machine for cellulose biosynthesis (170, 174, 175). BcsE is a multifunctional protein; it binds c-di-GMP via a GIL domain to maintain a second messenger pool at the membrane, interacts with BcsQ, targets BcsERG to the membrane, and associates with the transcriptional antitermination component NusB (176). BcsQ, a MinD/ParA/Soj homolog, appears to be involved in localizing the heterocomplex to the cell poles in E. coli (177). Together, these accessory proteins serve to elevate the efficiency of the system. While the mechanism of cellulose synthesis is becoming clear, and impressive progress has been made in understanding the structure of the assembly complex, the molecular features that underpin the observed bundling of glycan chains require further investigation.
The PNAG assembly system (Fig. 4C) is much simpler in composition and is encoded by a locus of 4 dedicated genes, pgaABCD (164, 178) (Fig. 4B). PgaC and PgaD create the synthase-translocase, with the GT domain residing in PgaC, and they interact in a c-di-GMP-regulated manner (179). Like BcsC, the PgaA outer membrane β−barrel channel also possesses a TPR domain (180). Two periplasmic activities affect the final polymer structure, and their coordination is facilitated by being part of a single (dual-domain) PgaB protein. The N-terminal domain is a PNAG N-deacetylase (181) and introduces the heterogeneity observed in the backbone of the secreted product, while the C-terminal domain possesses glycoside hydrolase activity that degrades only de-N-acetylated polymer (182). Like bacterial cellulose, the precise roles of these modifying activities in the context of production and biological function (if any) in biofilm formation have not been definitively established. The glycoside hydrolase (and therefore N-deacetylase) domain is necessary for translocation, but the underlying reason for this requires more investigation. In an initial report, PGA was reported to preferentially appear at cell poles like cellulose (179). However, there is currently no known component that might support localization of the complex, and recent data demonstrated punctate staining around the cell periphery before the PNAG is shed into the intercellular spaces (183).
CONCLUSIONS
Decades of research have generated a solid foundation of knowledge concerning the structure, function, and assembly of the extracellular polysaccharides from E. coli and Salmonella. As the details of the innate immunity are unraveled, knowledge of the contributions of these glycans to host-pathogen and host-commensal interactions advances in parallel. From a microbial cell biology perspective, two main issues require further investigation. The first concerns the interfaces and connections between the dedicated assembly systems and other cellular “housekeeping” processes, such as those involved in outer membrane homeostasis. The other is the architecture of the macromolecular complexes involved in biosynthesis and export. Structural biology initiatives (and the application cryo-electron microscopy) are now revealing the organization and mechanistic principles for protein secretion systems and LPS translocation. The challenge is to match the impressive recent progress on the cellulose assembly system in investigating other extracellular polysaccharide machineries with different functionalities.
ACKNOWLEDGMENTS
C.S. holds a Doctoral scholarship from the Canadian Institutes of Health Research, and C.W. is the recipient of a Canada Research Chair. Research on this topic in the Whitfield lab is generously supported by funding from the Foundation Grant program of the Canadian Institutes of Health Research.
Contributor Information
Chris Whitfield, Email: cwhitfie@uoguelph.ca.
James M. Slauch, University of Illinois at Urbana Champaign
REFERENCES
- 1.Ørskov F, Ørskov I. 1984. Serotyping of Escherichia coli. Methods Microbiol 14:43–112. doi: 10.1016/S0580-9517(08)70447-1. [DOI] [Google Scholar]
- 2.Grimont PAD, Weill F-XA. 2007. Antigenic formulae of the Salmonella serovars (9th ed). World Health Organization, Collaborating Center for Reference and Research on Salmonella, Institut Pasteur, Paris, France. [Google Scholar]
- 3.Whitfield C, Williams DM, Kelly SD. 2020. Lipopolysaccharide O-antigens—bacterial glycans made to measure. J Biol Chem 295:10593–10609. doi: 10.1074/jbc.REV120.009402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertani B, Ruiz N. 2018. Function and biogenesis of lipopolysaccharides. EcoSal Plus 8. doi: 10.1128/ecosalplus.ESP-0001-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitfield C. 2006. Biosynthesis and assembly of capsular polysaccharides in Escherichia coli. Annu Rev Biochem 75:39–68. doi: 10.1146/annurev.biochem.75.103004.142545. [DOI] [PubMed] [Google Scholar]
- 6.Liu B, Furevi A, Perepelov AV, Guo X, Cao H, Wang Q, Reeves PR, Knirel YA, Wang L, Widmalm G. 2020. Structure and genetics of Escherichia coli O antigens. FEMS Microbiol Rev 44:655–683. doi: 10.1093/femsre/fuz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu B, Knirel YA, Feng L, Perepelov AV, Senchenkova SN, Reeves PR, Wang L. 2014. Structural diversity in Salmonella O antigens and its genetic basis. FEMS Microbiol Rev 38:56–89. doi: 10.1111/1574-6976.12034. [DOI] [PubMed] [Google Scholar]
- 8.Mostowy RJ, Holt KE. 2018. Diversity-generating machines: genetics of bacterial sugar-coating. Trends Microbiol 26:1008–1021. doi: 10.1016/j.tim.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Holt KE, Lassalle F, Wyres KL, Wick R, Mostowy RJ. 2020. Diversity and evolution of surface polysaccharide synthesis loci in Enterobacteriales. ISME J 14:1713–1730. doi: 10.1038/s41396-020-0628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Whitfield C, Wear SS, Sande C. 2020. Assembly of bacterial capsular polysaccharides and exopolysaccharides. Annu Rev Microbiol 74:521–543. doi: 10.1146/annurev-micro-011420-075607. [DOI] [PubMed] [Google Scholar]
- 11.Wall E, Majdalani N, Gottesman S. 2018. The complex Rcs regulatory cascade. Annu Rev Microbiol 72:111–139. doi: 10.1146/annurev-micro-090817-062640. [DOI] [PubMed] [Google Scholar]
- 12.Römling U, Galperin MY. 2015. Bacterial cellulose biosynthesis: diversity of operons, subunits, products, and functions. Trends Microbiol 23:545–557. doi: 10.1016/j.tim.2015.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bundalovic-Torma C, Whitfield GB, Marmont LS, Howell PL, Parkinson J. 2020. A systematic pipeline for classifying bacterial operons reveals the evolutionary landscape of biofilm machineries. PLoS Comput Biol 16:e1007721. doi: 10.1371/journal.pcbi.1007721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kunduru BR, Nair SA, Rathinavelan T. 2016. EK3D: an E. coli K antigen 3-dimensional structure database. Nucleic Acids Res 44:D675–D681. doi: 10.1093/nar/gkv1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harris S, Piotrowska MJ, Goldstone RJ, Qi R, Foster G, Dobrindt U, Madec J-Y, Valat C, Rao FV, Smith DGE. 2018. Variant O89 O-antigen of E. coli is associated with group 1 capsule loci and multidrug resistance. Front Microbiol 9:2026. doi: 10.3389/fmicb.2018.02026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keenleyside WJ, Bronner D, Jann K, Jann B, Whitfield C. 1993. Coexpression of colanic acid and serotype-specific capsular polysaccharides in Escherichia coli strains with group II K antigens. J Bacteriol 175:6725–6730. doi: 10.1128/jb.175.20.6725-6730.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rahn A, Beis K, Naismith JH, Whitfield C. 2003. A novel outer membrane protein, Wzi, is involved in surface assembly of the Escherichia coli K30 group 1 capsule. J Bacteriol 185:5882–5890. doi: 10.1128/JB.185.19.5882-5890.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bushell SR, Mainprize IL, Wear MA, Lou H, Whitfield C, Naismith JH. 2013. Wzi is an outer membrane lectin that underpins group 1 capsule assembly in Escherichia coli. Structure 21:844–853. doi: 10.1016/j.str.2013.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hungerer D, Jann K, Jann B, Ørskov F, Orskov I. 1967. Immunochemistry of K antigens of Escherichia coli. 4. The K antigen of E. coli O9:K30:H12. Eur J Biochem 2:115–126. doi: 10.1111/j.1432-1033.1967.tb00115.x. [DOI] [PubMed] [Google Scholar]
- 20.Fresno S, Jiménez N, Izquierdo L, Merino S, Corsaro MM, Castro CD, Parrilli M, Naldi T, Regué M, Tomás JM. 2006. The ionic interaction of Klebsiella pneumoniae K2 capsule and core lipopolysaccharide. Microbiology (Reading) 152:1807–1818. doi: 10.1099/mic.0.28611-0. [DOI] [PubMed] [Google Scholar]
- 21.Kessler NG, Delgado DMC, Shah NK, Dickinson JA, Moore SD. 2021. Exopolysaccharide anchoring creates an extreme resistance to sedimentation. J Bacteriol 203:e00023-21. doi: 10.1128/JB.00023-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.MacLachlan PR, Keenleyside WJ, Dodgson C, Whitfield C. 1993. Formation of the K30 (group I) capsule in Escherichia coli O9:K30 does not require attachment to lipopolysaccharide lipid A-core. J Bacteriol 175:7515–7522. doi: 10.1128/jb.175.23.7515-7522.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Meredith TC, Mamat U, Kaczynski Z, Lindner B, Holst O, Woodard RW. 2007. Modification of lipopolysaccharide with colanic acid (M-antigen) repeats in Escherichia coli. J Biol Chem 282:7790–7798. doi: 10.1074/jbc.M611034200. [DOI] [PubMed] [Google Scholar]
- 24.Garde S, Chodisetti PK, Reddy M. 2021. Peptidoglycan: structure, synthesis, and regulation. Ecosal Plus 9. doi: 10.1128/ecosalplus.ESP-0010-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allen KN, Imperiali B. 2019. Structural and mechanistic themes in glycoconjugate biosynthesis at membrane interfaces. Curr Opin Struct Biol 59:81–90. doi: 10.1016/j.sbi.2019.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuk ACY, Hao A, Guan Z, Lee S-Y. 2019. Visualizing conformation transitions of the lipid II flippase MurJ. Nat Commun 10:1736. doi: 10.1038/s41467-019-09658-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zheng S, Sham L-T, Rubino FA, Brock KP, Robins WP, Mekalanos JJ, Marks DS, Bernhardt TG, Kruse AC. 2018. Structure and mutagenic analysis of the lipid II flippase MurJ from Escherichia coli. Proc Natl Acad Sci U S A 115:6709–6714. doi: 10.1073/pnas.1802192115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sham L-T, Zheng S, Yakhnina AA, Kruse AC, Bernhardt TG. 2018. Loss of specificity variants of WzxC suggest that substrate recognition is coupled with transporter opening in MOP‐family flippases. Mol Microbiol 109:633–641. doi: 10.1111/mmi.14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robbins PW, Bray D, Dankert M, Wright A. 1967. Direction of chain growth in polysaccharide synthesis. Science 158:1536–1542. doi: 10.1126/science.158.3808.1536. [DOI] [PubMed] [Google Scholar]
- 30.Owens TW, Taylor RJ, Pahil KS, Bertani BR, Ruiz N, Kruse AC, Kahne D. 2019. Structural basis of unidirectional export of lipopolysaccharide to the cell surface. Nature 567:550–553. doi: 10.1038/s41586-019-1039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Y, Liu J, Clarke BR, Seidel L, Bolla JR, Ward PN, Zhang P, Robinson CV, Whitfield C, Naismith JH. 2021. The molecular basis of regulation of bacterial capsule assembly by Wzc. Nat Commun 12:4349. doi: 10.1038/s41467-021-24652-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wiseman B, Nitharwal RG, Widmalm G, Högbom M. 2021. Structure of a full-length bacterial polysaccharide co-polymerase. Nat Commun 12:369. doi: 10.1038/s41467-020-20579-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Collins RF, Kargas V, Clarke BR, Siebert CA, Clare DK, Bond PJ, Whitfield C, Ford RC. 2017. Full-length, oligomeric structure of Wzz determined by cryoelectron microscopy reveals insights into membrane-bound states. Structure 25:806–815.e3. doi: 10.1016/j.str.2017.03.017. [DOI] [PubMed] [Google Scholar]
- 34.Nath P, Morona R. 2015. Detection of Wzy/Wzz interaction in Shigella flexneri. Microbiology (Reading) 161:1797–1805. doi: 10.1099/mic.0.000132. [DOI] [PubMed] [Google Scholar]
- 35.Leo V, Tran E, Morona R. 2021. Polysaccharide copolymerase WzzB/WzzE chimeras reveal that the transmembrane 2 region of WzzB Is important for interaction with WzyB. J Bacteriol 203. doi: 10.1128/JB.00598-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Morona R, Purins L, Tocilj A, Matte A, Cygler M. 2009. Sequence-structure relationships in polysaccharide co-polymerase (PCP) proteins. Trends Biochem Sci 34:78–84. doi: 10.1016/j.tibs.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 37.Bechet E, Gruszczyk J, Terreux R, Gueguen-Chaignon V, Vigouroux A, Obadia B, Cozzone AJ, Nessler S, Grangeasse C. 2010. Identification of structural and molecular determinants of the tyrosine-kinase Wzc and implications in capsular polysaccharide export. Mol Microbiol 77:1315–1325. doi: 10.1111/j.1365-2958.2010.07291.x. [DOI] [PubMed] [Google Scholar]
- 38.Wugeditsch T, Paiment A, Hocking J, Drummelsmith J, Forrester C, Whitfield C. 2001. Phosphorylation of Wzc, a tyrosine autokinase, is essential for assembly of group 1 capsular polysaccharides in Escherichia coli. J Biol Chem 276:2361–2371. doi: 10.1074/jbc.M009092200. [DOI] [PubMed] [Google Scholar]
- 39.Soulat D, Jault JM, Geourjon C, Gouet P, Cozzone AJ, Grangeasse C. 2007. Tyrosine-kinase Wzc from Escherichia coli possesses an ATPase activity regulated by autophosphorylation. FEMS Microbiol Lett 274:252–259. doi: 10.1111/j.1574-6968.2007.00841.x. [DOI] [PubMed] [Google Scholar]
- 40.Paiment A, Hocking J, Whitfield C. 2002. Impact of phosphorylation of specific residues in the tyrosine autokinase, Wzc, on its activity in assembly of group 1 capsules in Escherichia coli. J Bacteriol 184:6437–6447. doi: 10.1128/JB.184.23.6437-6447.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hagelueken G, Huang H, Mainprize IL, Whitfield C, Naismith JH. 2009. Crystal structures of Wzb of Escherichia coli and CpsB of Streptococcus pneumoniae, representatives of two families of tyrosine phosphatases that regulate capsule assembly. J Mol Biol 392:678–688. doi: 10.1016/j.jmb.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lescop E, Hu Y, Xu H, Hu W, Chen J, Xia B, Jin C. 2006. The solution structure of Escherichia coli Wzb reveals a novel substrate recognition mechanism of prokaryotic low molecular weight protein-tyrosine phosphatases. J Biol Chem 281:19570–19577. doi: 10.1074/jbc.M601263200. [DOI] [PubMed] [Google Scholar]
- 43.Vincent C, Duclos B, Grangeasse C, Vaganay E, Riberty M, Cozzone AJ, Doublet P. 2000. Relationship between exopolysaccharide production and protein-tyrosine phosphorylation in Gram-negative bacteria. J Mol Biol 304:311–321. doi: 10.1006/jmbi.2000.4217. [DOI] [PubMed] [Google Scholar]
- 44.Obadia B, Lacour S, Doublet P, Baubichon-Cortay H, Cozzone AJ, Grangeasse C. 2007. Influence of tyrosine-kinase Wzc activity on colanic acid production in Escherichia coli K12 cells. J Mol Biol 367:42–53. doi: 10.1016/j.jmb.2006.12.048. [DOI] [PubMed] [Google Scholar]
- 45.Grangeasse C, Obadia B, Mijakovic I, Deutscher J, Cozzone AJ, Doublet P. 2003. Autophosphorylation of the Escherichia coli protein kinase Wzc regulates tyrosine phosphorylation of Ugd, a UDP-glucose dehydrogenase. J Biol Chem 278:39323–39329. doi: 10.1074/jbc.M305134200. [DOI] [PubMed] [Google Scholar]
- 46.Lacour S, Bechet E, Cozzone AJ, Mijakovic I, Grangeasse C. 2008. Tyrosine phosphorylation of the UDP-glucose dehydrogenase of Escherichia coli is at the crossroads of colanic acid synthesis and polymyxin resistance. PLoS One 3:e3053. doi: 10.1371/journal.pone.0003053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mainprize IL, Bean JD, Bouwman C, Kimber MS, Whitfield C. 2013. The UDP-glucose dehydrogenase of Escherichia coli K-12 displays substrate inhibition by NAD that is relieved by nucleotide triphosphates. J Biol Chem 288:23064–23074. doi: 10.1074/jbc.M113.486613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bayer ME, Thurow H. 1977. Polysaccharide capsule of Escherichia coli: microscope study of its size, structure, and sites of synthesis. J Bacteriol 130:911–936. doi: 10.1128/jb.130.2.911-936.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cuthbertson L, Mainprize IL, Naismith JH, Whitfield C. 2009. Pivotal roles of the outer membrane polysaccharide export and polysaccharide copolymerase protein families in export of extracellular polysaccharides in Gram-negative bacteria. Microbiol Mol Biol Rev 73:155–177. doi: 10.1128/MMBR.00024-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dong C, Beis K, Nesper J, Brunkan-Lamontagne AL, Clarke BR, Whitfield C, Naismith JH. 2006. Wza the translocon for E. coli capsular polysaccharides defines a new class of membrane protein. Nature 444:226–229. doi: 10.1038/nature05267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nickerson NN, Mainprize IL, Hampton L, Jones ML, Naismith JH, Whitfield C. 2014. Trapped translocation intermediates establish the route for export of capsular polysaccharides across Escherichia coli outer membranes. Proc Natl Acad Sci U S A 111:8203–8208. doi: 10.1073/pnas.1400341111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Collins RF, Beis K, Dong C, Botting CH, McDonnell C, Ford RC, Clarke BR, Whitfield C, Naismith JH. 2007. The 3D structure of a periplasm-spanning platform required for assembly of group 1 capsular polysaccharides in Escherichia coli. Proc Natl Acad Sci U S A 104:2390–2395. doi: 10.1073/pnas.0607763104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reid AN, Whitfield C. 2005. Functional analysis of conserved gene products involved in assembly of Escherichia coli capsules and exopolysaccharides: evidence for molecular recognition between Wza and Wzc for colanic acid biosynthesis. J Bacteriol 187:5470–5481. doi: 10.1128/JB.187.15.5470-5481.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nesper J, Hill CMD, Paiment A, Harauz G, Beis K, Naismith JH, Whitfield C. 2003. Translocation of group 1 capsular polysaccharide in Escherichia coli serotype K30. Structural and functional analysis of the outer membrane lipoprotein Wza. J Biol Chem 278:49763–49772. doi: 10.1074/jbc.M308775200. [DOI] [PubMed] [Google Scholar]
- 55.Schaefer K, Owens TW, Page JE, Santiago M, Kahne D, Walker S. 2021. Structure and reconstitution of a hydrolase complex that may release peptidoglycan from the membrane after polymerization. Nat Microbiol 6:34–43. doi: 10.1038/s41564-020-00808-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rahn A, Drummelsmith J, Whitfield C. 1999. Conserved organization in the cps gene clusters for expression of Escherichia coli group 1 K antigens: relationship to the colanic acid biosynthesis locus and the cps genes from Klebsiella pneumoniae. J Bacteriol 181:2307–2313. doi: 10.1128/JB.181.7.2307-2313.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Drummelsmith J, Whitfield C. 1999. Gene products required for surface expression of the capsular form of the group 1 K antigen in Escherichia coli (O9a:K30). Mol Microbiol 31:1321–1332. doi: 10.1046/j.1365-2958.1999.01277.x. [DOI] [PubMed] [Google Scholar]
- 58.Scott PM, Erickson KM, Troutman JM. 2019. Identification of the functional roles of six key proteins in the biosynthesis of Enterobacteriaceae colanic acid. Biochemistry 58:1818–1830. doi: 10.1021/acs.biochem.9b00040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Reid AJ, Eade CR, Jones KJ, Jorgenson MA, Troutman JM. 2021. Tracking colanic acid repeat unit formation from stepwise biosynthesis inactivation in Escherichia coli. Biochemistry 60:2221–2230. doi: 10.1021/acs.biochem.1c00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rahn A, Whitfield C. 2003. Transcriptional organization and regulation of the Escherichia coli K30 group 1 capsule biosynthesis (cps) gene cluster. Mol Microbiol 47:1045–1060. doi: 10.1046/j.1365-2958.2003.03354.x. [DOI] [PubMed] [Google Scholar]
- 61.Hobbs M, Reeves PR. 1994. The JUMPstart sequence: a 39 bp element common to several polysaccharide gene clusters. Mol Microbiol 12:855–856. doi: 10.1111/j.1365-2958.1994.tb01071.x. [DOI] [PubMed] [Google Scholar]
- 62.Bailey MJ, Hughes C, Koronakis V. 1997. RfaH and the ops element, components of a novel system controlling bacterial transcription elongation. Mol Microbiol 26:845–851. doi: 10.1046/j.1365-2958.1997.6432014.x. [DOI] [PubMed] [Google Scholar]
- 63.Zuber PK, Artsimovitch I, NandyMazumdar M, Liu Z, Nedialkov Y, Schweimer K, Rösch P, Knauer SH. 2018. The universally-conserved transcription factor RfaH is recruited to a hairpin structure of the non-template DNA strand. Elife 7:e36349. doi: 10.7554/eLife.36349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wehland M, Bernhard F. 2000. The RcsAB box. Characterization of a new operator essential for the regulation of exopolysaccharide biosynthesis in enteric bacteria. J Biol Chem 275:7013–7020. doi: 10.1074/jbc.275.10.7013. [DOI] [PubMed] [Google Scholar]
- 65.Mouslim C, Latifi T, Groisman EA. 2003. Signal-dependent requirement for the co-activator protein RcsA in transcription of the RcsB-regulated ugd gene. J Biol Chem 278:50588–50595. doi: 10.1074/jbc.M309433200. [DOI] [PubMed] [Google Scholar]
- 66.Pando JM, Karlinsey JE, Lara JC, Libby SJ, Fang FC. 2017. The Rcs-regulated colanic acid capsule maintains membrane potential in Salmonella enterica serovar Typhimurium. mBio 8:e00808-17. doi: 10.1128/mBio.00808-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ranjit DK, Young KD. 2016. Colanic acid intermediates prevent de novo shape recovery of Escherichia coli spheroplasts, calling into question biological roles previously attributed to colanic acid. J Bacteriol 198:1230–1240. doi: 10.1128/JB.01034-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kocharunchitt C, King T, Gobius K, Bowman JP, Ross T. 2012. Integrated transcriptomic and proteomic analysis of the physiological response of Escherichia coli O157:H7 Sakai to steady-state conditions of cold and water activity stress. Mol Cell Proteomics 11:M111.009019. doi: 10.1074/mcp.M111.009019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Phan M-D, Peters KM, Sarkar S, Lukowski SW, Allsopp LP, Moriel DG, Achard MES, Totsika M, Marshall VM, Upton M, Beatson SA, Schembri MA. 2013. The serum resistome of a globally disseminated multidrug resistant uropathogenic Escherichia coli clone. PLoS Genet 9:e1003834. doi: 10.1371/journal.pgen.1003834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Miajlovic H, Cooke NM, Moran GP, Rogers TRF, Smith SG. 2014. Response of extraintestinal pathogenic Escherichia coli to human serum reveals a protective role for Rcs-regulated exopolysaccharide colanic acid. Infect Immun 82:298–305. doi: 10.1128/IAI.00800-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ondari EM, Klemm EJ, Msefula CL, Ghany MAE, Heath JN, Pickard DJ, Barquist L, Dougan G, Kingsley RA, MacLennan CA. 2019. Rapid transcriptional responses to serum exposure are associated with sensitivity and resistance to antibody-mediated complement killing in invasive Salmonella Typhimurium ST313. Wellcome Open Res 4:74. doi: 10.12688/wellcomeopenres.15059.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Danese PN, Pratt LA, Kolter R. 2000. Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J Bacteriol 182:3593–3596. doi: 10.1128/JB.182.12.3593-3596.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Horvat M, Pannuri A, Romeo T, Dogsa I, Stopar D. 2019. Viscoelastic response of Escherichia coli biofilms to genetically altered expression of extracellular matrix components. Soft Matter 15:5042–5051. doi: 10.1039/c9sm00297a. [DOI] [PubMed] [Google Scholar]
- 74.Hahn MM, Gunn JS. 2020. Salmonella extracellular polymeric substances modulate innate phagocyte activity and enhance tolerance of biofilm-associated bacteria to oxidative stress. Microorganisms 8:253. doi: 10.3390/microorganisms8020253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Marolda CL, Valvano MA. 1996. The GalF protein of Escherichia coli is not a UDP-glucose pyrophosphorylase but interacts with the GalU protein possibly to regulate cellular levels of UDP-glucose. Mol Microbiol 22:827–840. doi: 10.1046/j.1365-2958.1996.01531.x. [DOI] [PubMed] [Google Scholar]
- 76.Hadad JJ, Gyles CL. 1982. The role of K antigens of enteropathogenic Escherichia coli in colonization of the small intestine of calves. Can J Comp Med 46:21–26. [PMC free article] [PubMed] [Google Scholar]
- 77.Chan R, Acres SD, Costerton JW. 1982. Use of specific antibody to demonstrate glycocalyx, K99 pili, and the spatial relationships of K99+ enterotoxigenic Escherichia coli in the ileum of colostrum-fed calves. Infect Immun 37:1170–1180. doi: 10.1128/iai.37.3.1170-1180.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Runnels PL, Moon PL. 1984. Capsule reduces adherence of enterotoxigenic Escherichia coli to isolated intestinal epithelial cells of pigs. Infect Immun 45:737–740. doi: 10.1128/iai.45.3.737-740.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Paczosa MK, Mecsas J. 2016. Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev 80:629–661. doi: 10.1128/MMBR.00078-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goldman RC, White D, Ørskov F, Orskov I, Rick PD, Lewis MS, Bhattacharjee AK, Leive L. 1982. A surface polysaccharide of Escherichia coli O111 contains O-antigen and inhibits agglutination of cells by O-antiserum. J Bacteriol 151:1210–1221. doi: 10.1128/jb.151.3.1210-1221.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Peleg A, Shifrin Y, Ilan O, Nadler-Yona C, Nov S, Koby S, Baruch K, Altuvia S, Elgrably-Weiss M, Abe CM, Knutton S, Saper MA, Rosenshine I. 2005. Identification of an Escherichia coli operon required for formation of the O-antigen capsule. J Bacteriol 187:5259–5266. doi: 10.1128/JB.187.15.5259-5266.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Joiner KA, Goldman R, Schmetz M, Berger M, Hammer CH, Frank MM, Leive L. 1984. A quantitative analysis of C3 binding to O-antigen capsule, lipopolysaccharide, and outer membrane protein of E. coli O111B4. J Immunol 132:369–375. [PubMed] [Google Scholar]
- 83.Goldman RC, Joiner K, Leive L. 1984. Serum-resistant mutants of Escherichia coli O111 contain increased lipopolysaccharide, lack an O antigen-containing capsule, and cover more of their lipid A-core with O antigen. J Bacteriol 159:877–882. doi: 10.1128/jb.159.3.877-882.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Biran D, Rosenshine I, Ron EZ. 2020. Escherichia coli O-antigen capsule (group 4) is essential for serum resistance. Res Microbiol 171:99–101. doi: 10.1016/j.resmic.2019.12.002. [DOI] [PubMed] [Google Scholar]
- 85.Caboni M, Pédron T, Rossi O, Goulding D, Pickard D, Citiulo F, MacLennan CA, Dougan G, Thomson NR, Saul A, Sansonetti PJ, Gerke C. 2015. An O antigen capsule modulates bacterial pathogenesis in Shigella sonnei. PLoS Pathog 11:e1004749. doi: 10.1371/journal.ppat.1004749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Thomassin J-L, Lee MJ, Brannon JR, Sheppard DC, Gruenheid S, Moual HL. 2013. Both group 4 capsule and lipopolysaccharide O-antigen contribute to enteropathogenic Escherichia coli resistance to human α-defensin 5. PLoS One 8:e82475. doi: 10.1371/journal.pone.0082475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lara-Tejero M, Galán JE. 2019. The injectisome, a complex nanomachine for protein injection into mammalian cells. EcoSal Plus 8. doi: 10.1128/ecosalplus.ESP-0039-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Shifrin Y, Peleg A, Ilan O, Nadler C, Kobi S, Baruch K, Yerushalmi G, Berdichevsky T, Altuvia S, Elgrably-Weiss M, Abe C, Knutton S, Sasakawa C, Ritchie JM, Waldor MK, Rosenshine I. 2008. Transient shielding of intimin and the type III secretion system of enterohemorrhagic and enteropathogenic Escherichia coli by a group 4 capsule. J Bacteriol 190:5063–5074. doi: 10.1128/JB.00440-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Alexander DC, Valvano MA. 1994. Role of the rfe gene in the biosynthesis of the Escherichia coli O7-specific lipopolysaccharide and other O-specific polysaccharides containing N-acetylglucosamine. J Bacteriol 176:7079–7084. doi: 10.1128/jb.176.22.7079-7084.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ilan O, Bloch Y, Frankel G, Ullrich H, Geider K, Rosenshine I. 1999. Protein tyrosine kinases in bacterial pathogens are associated with virulence and production of exopolysaccharide. EMBO J 18:3241–3248. doi: 10.1093/emboj/18.12.3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Drummelsmith J, Whitfield C. 2000. Translocation of group 1 capsular polysaccharide to the surface of Escherichia coli requires a multimeric complex in the outer membrane. EMBO J 19:57–66. doi: 10.1093/emboj/19.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sathiyamoorthy K, Mills E, Franzmann TM, Rosenshine I, Saper MA. 2011. The crystal structure of Escherichia coli group 4 capsule protein GfcC reveals a domain organization resembling that of Wza. Biochemistry 50:5465–5476. doi: 10.1021/bi101869h. [DOI] [PubMed] [Google Scholar]
- 93.Willis LM, Stupak J, Richards MR, Lowary TL, Li J, Whitfield C. 2013. Conserved glycolipid termini in capsular polysaccharides synthesized by ATP-binding cassette transporter-dependent pathways in Gram-negative pathogens. Proc Natl Acad Sci U S A 110:7868–7873. doi: 10.1073/pnas.1222317110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ovchinnikova OG, Doyle L, Huang B-S, Kimber MS, Lowary TL, Whitfield C. 2016. Biochemical characterization of bifunctional 3-deoxy-β-D-manno-oct-2-ulosonic acid (β-Kdo) transferase KpsC from Escherichia coli involved in capsule biosynthesis. J Biol Chem 291:21519–21530. doi: 10.1074/jbc.M116.751115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Doyle L, Ovchinnikova OG, Myler K, Mallette E, Huang B-S, Lowary TL, Kimber MS, Whitfield C. 2019. Biosynthesis of a conserved glycolipid anchor for Gram-negative bacterial capsules. Nat Chem Biol 15:632–640. doi: 10.1038/s41589-019-0276-8. [DOI] [PubMed] [Google Scholar]
- 96.Yan L, Fu L, Xia K, Chen S, Zhang F, Dordick JS, Linhardt RJ. 2020. A revised structure for the glycolipid terminus of Escherichia coli K5 heparosan capsular polysaccharide. Biomolecules 10:1516. doi: 10.3390/biom10111516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Willis LM, Whitfield C. 2013. Structure, biosynthesis, and function of bacterial capsular polysaccharides synthesized by ABC transporter-dependent pathways. Carbohydr Res 378:35–44. doi: 10.1016/j.carres.2013.05.007. [DOI] [PubMed] [Google Scholar]
- 98.Jann B, Jann K. 1990. Structure and biosynthesis of the capsular antigens of Escherichia coli. Curr Top Microbiol Immunol 150:19–42. [DOI] [PubMed] [Google Scholar]
- 99.Jiménez N, Senchenkova SN, Knirel YA, Pieretti G, Corsaro MM, Aquilini E, Regué M, Merino S, Tomás JM. 2012. Effects of lipopolysaccharide biosynthesis mutations on K1 polysaccharide association with the Escherichia coli cell surface. J Bacteriol 194:3356–3367. doi: 10.1128/JB.00329-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Phanphak S, Georgiades P, Li R, King J, Roberts IS, Waigh TA. 2019. Super-resolution fluorescence microscopy study of the production of K1 capsules by Escherichia coli: evidence for the differential distribution of the capsule at the poles and the equator of the cell. Langmuir 35:5635–5646. doi: 10.1021/acs.langmuir.8b04122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Goller CC, Seed PC. 2010. Revisiting the Escherichia coli polysaccharide capsule as a virulence factor during urinary tract infection: contribution to intracellular biofilm development. Virulence 1:333–337. doi: 10.4161/viru.1.4.12388. [DOI] [PubMed] [Google Scholar]
- 102.Withman B, Gunasekera TS, Beesetty P, Agans R, Paliy O. 2013. Transcriptional responses of uropathogenic Escherichia coli to increased environmental osmolality caused by salt or urea. Infect Immun 81:80–89. doi: 10.1128/IAI.01049-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Taylor CM, Roberts IS. 2005. Capsular polysaccharides and their role in virulence. Contrib Microbiol 12:55–66. doi: 10.1159/000081689. [DOI] [PubMed] [Google Scholar]
- 104.Cross AS, Kim KS, Wright DC, Sadoff JC, Gemski P, Ørskov F, Orskov I. 1986. Role of lipopolysaccharide and capsule in the serum resistance of bacteremic strains of Escherichia coli. J Infect Dis 154:497–503. doi: 10.1093/infdis/154.3.497. [DOI] [PubMed] [Google Scholar]
- 105.Ma J, An C, Jiang F, Yao H, Logue C, Nolan LK, Li G. 2018. Extraintestinal pathogenic Escherichia coli increase extracytoplasmic polysaccharide biosynthesis for serum resistance in response to bloodstream signals. Mol Microbiol 110:689–706. doi: 10.1111/mmi.13987. [DOI] [PubMed] [Google Scholar]
- 106.Buckles EL, Wang X, Lane MC, Lockatell CV, Johnson DE, Rasko DA, Mobley HLT, Donnenberg MS. 2009. Role of the K2 capsule in Escherichia coli urinary tract infection and serum resistance. J Infect Dis 199:1689–1697. doi: 10.1086/598524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Arshad M, Goller CC, Pilla D, Schoenen FJ, Seed PC. 2016. Threading the needle: small-molecule targeting of a xenobiotic receptor to ablate Escherichia coli polysaccharide capsule expression without altering antibiotic resistance. J Infect Dis 213:1330–1339. doi: 10.1093/infdis/jiv584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Cress BF, Englaender JA, He W, Kasper D, Linhardt RJ, Koffas MAG. 2014. Masquerading microbial pathogens: capsular polysaccharides mimic host-tissue molecules. FEMS Microbiol Rev 38:660–697. doi: 10.1111/1574-6976.12056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Colley KJ, Kitajima K, Sato C. 2014. Polysialic acid: biosynthesis, novel functions and applications. Crit Rev Biochem Mol Biol 49:498–532. doi: 10.3109/10409238.2014.976606. [DOI] [PubMed] [Google Scholar]
- 110.Rodriguez ML, Jann B, Jann K. 1988. Structure and serological characteristics of the capsular K4 antigen of Escherichia coli O5:K4:H4, a fructose-containing polysaccharide with a chondroitin backbone. Eur J Biochem 177:117–124. doi: 10.1111/j.1432-1033.1988.tb14351.x. [DOI] [PubMed] [Google Scholar]
- 111.Vann WF, Schmidt MA, Jann B, Jann K. 1981. The structure of the capsular polysaccharide (K5 antigen) of urinary tract infective Escherichia coli O10:K5:H4. A polymer similar to desulfo heparin. Eur J Biochem 116:359–364. doi: 10.1111/j.1432-1033.1981.tb05343.x. [DOI] [PubMed] [Google Scholar]
- 112.Willis LM, Whitfield C. 2013. KpsC and KpsS are retaining 3-deoxy-D-manno-oct-2-ulosonic acid (Kdo) transferases involved in synthesis of bacterial capsules. Proc Natl Acad Sci U S A 110:20753–20758. doi: 10.1073/pnas.1312637110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Lanz ND, Ming SA, Thon V, Veeramachineni VM, Azurmendi HF, Vann WF. 2021. Characterization of the β-KDO transferase KpsS, the initiating enzyme in the biosynthesis of the lipid acceptor for Escherichia coli polysialic acid. Biochemistry 60:2044–2054. doi: 10.1021/acs.biochem.1c00088. [DOI] [PubMed] [Google Scholar]
- 114.Cieslewicz M, Vimr E. 1996. Thermoregulation of kpsF, the first region 1 gene in the kps locus for polysialic acid biosynthesis in Escherichia coli K1. J Bacteriol 178:3212–3220. doi: 10.1128/jb.178.11.3212-3220.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Steenbergen SM, Wrona TJ, Vimr ER. 1992. Functional analysis of the sialyltransferase complexes in Escherichia coli K1 and K92. J Bacteriol 174:1099–1108. doi: 10.1128/jb.174.4.1099-1108.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.McGowen MM, Vionnet J, Vann WF. 2001. Elongation of alternating α 2,8/2,9 polysialic acid by the Escherichia coli K92 polysialyltransferase. Glycobiol 11:613–620. doi: 10.1093/glycob/11.8.613. [DOI] [PubMed] [Google Scholar]
- 117.Keys TG, Fuchs HLS, Galuska SP, Gerardy-Schahn R, Freiberger F. 2013. A single amino acid toggles Escherichia coli polysialyltransferases between mono- and bifunctionality. Glycobiol 23:613–618. doi: 10.1093/glycob/cwt003. [DOI] [PubMed] [Google Scholar]
- 118.Yakovlieva L, Walvoort MTC. 2020. Processivity in bacterial glycosyltransferases. ACS Chem Biol 15:3–16. doi: 10.1021/acschembio.9b00619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Ninomiya T, Sugiura N, Tawada A, Sugimoto K, Watanabe H, Kimata K. 2002. Molecular cloning and characterization of chondroitin polymerase from Escherichia coli strain K4. J Biol Chem 277:21567–21575. doi: 10.1074/jbc.M201719200. [DOI] [PubMed] [Google Scholar]
- 120.Osawa T, Sugiura N, Shimada H, Hirooka R, Tsuji A, Shirakawa T, Fukuyama K, Kimura M, Kimata K, Kakuta Y. 2009. Crystal structure of chondroitin polymerase from Escherichia coli K4. Biochem Biophys Res Commun 378:10–14. doi: 10.1016/j.bbrc.2008.08.121. [DOI] [PubMed] [Google Scholar]
- 121.Hodson N, Griffiths G, Cook N, Pourhossein M, Gottfridson E, Lind T, Lidholt K, Roberts IS. 2000. Identification that KfiA, a protein essential for the biosynthesis of the Escherichia coli K5 capsular polysaccharide, is an α-UDP-GlcNAc glycosyltransferase. The formation of a membrane-associated K5 biosynthetic complex requires KfiA, KfiB, and KfiC. J Biol Chem 275:27311–27315. doi: 10.1074/jbc.M004426200. [DOI] [PubMed] [Google Scholar]
- 122.Deszo EL, Steenbergen SM, Freedberg DI, Vimr ER. 2005. Escherichia coli K1 polysialic acid O-acetyltransferase gene, neuO, and the mechanism of capsule form variation involving a mobile contingency locus. Proc Natl Acad Sci U S A 102:5564–5569. doi: 10.1073/pnas.0407428102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Steenbergen SM, Lee YC, Vann WF, Vionnet J, Wright LF, Vimr ER. 2006. Separate pathways for O acetylation of polymeric and monomeric sialic acids and identification of sialyl O-acetyl esterase in Escherichia coli K1. J Bacteriol 188:6195–6206. doi: 10.1128/JB.00466-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bergfeld AK, Claus H, Vogel U, Muhlenhoff M. 2007. Biochemical characterization of the polysialic acid-specific O-acetyltransferase NeuO of Escherichia coli K1. J Biol Chem 282:22217–22227. doi: 10.1074/jbc.M703044200. [DOI] [PubMed] [Google Scholar]
- 125.Liu J, Yang A, Liu J, Ding X, Liu L, Shi Z. 2014. KfoE encodes a fructosyltransferase involved in capsular polysaccharide biosynthesis in Escherichia coli K4. Biotechnol Lett 36:1469–1477. doi: 10.1007/s10529-014-1502-9. [DOI] [PubMed] [Google Scholar]
- 126.Roberts I, Mountford R, High N, Bitter-Suermann D, Jann KKT, Boulnois G. 1986. Molecular cloning and analysis of genes for production of K5, K7, K12, and K92 capsular polysaccharides in Escherichia coli. J Bacteriol 168:1228–1233. doi: 10.1128/jb.168.3.1228-1233.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Silver RP, Prior K, Nsahlai C, Wright LF. 2001. ABC transporters and the export of capsular polysaccharides from Gram-negative bacteria. Res Microbiol 152:357–364. doi: 10.1016/s0923-2508(01)01207-4. [DOI] [PubMed] [Google Scholar]
- 128.Lo RY, McKerral LJ, Hills TL, Kostrzynska M. 2001. Analysis of the capsule biosynthetic locus of Mannheimia (Pasteurella) haemolytica A1 and proposal of a nomenclature system. Infect Immun 69:4458–4464. doi: 10.1128/IAI.69.7.4458-4464.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ford RC, Beis K. 2019. Learning the ABCs one at a time: structure and mechanism of ABC transporters. Biochem Soc Trans 47:23–36. doi: 10.1042/BST20180147. [DOI] [PubMed] [Google Scholar]
- 130.Caffalette CA, Kuklewicz J, Spellmon N, Zimmer J. 2020. Biosynthesis and export of bacterial gycolipids. Annu Rev Biochem 89:741–768. doi: 10.1146/annurev-biochem-011520-104707. [DOI] [PubMed] [Google Scholar]
- 131.Thomas C, Aller SG, Beis K, Carpenter EP, Chang G, Chen L, Dassa E, Dean M, Hoa FDV, Ekiert D, Ford R, Gaudet R, Gong X, Holland IB, Huang Y, Kahne DK, Kato H, Koronakis V, Koth CM, Lee Y, Lewinson O, Lill R, Martinoia E, Murakami S, Pinkett HW, Poolman B, Rosenbaum D, Sarkadi B, Schmitt L, Schneider E, Shi Y, Shyng S, Slotboom DJ, Tajkhorshid E, Tieleman DP, Ueda K, Váradi A, Wen P, Yan N, Zhang P, Zheng H, Zimmer J, Tampé R. 2020. Structural and functional diversity calls for a new classification of ABC transporters. FEBS Lett 594:3767–3775. doi: 10.1002/1873-3468.13935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Bi Y, Mann E, Whitfield C, Zimmer J. 2018. Architecture of a channel-forming O-antigen polysaccharide ABC transporter. Nature 71:635. doi: 10.1038/nature25190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Caffalette CA, Zimmer J. 2021. Cryo-EM structure of the full-length WzmWzt ABC transporter required for lipid-linked O antigen transport. Proc Natl Acad Sci U S A 118:e2016144118. doi: 10.1073/pnas.2016144118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chen L, Hou W-T, Fan T, Liu B, Pan T, Li Y-H, Jiang Y-L, Wen W, Chen Z-P, Sun L, Zhou C-Z, Chen Y. 2020. Cryo-electron microscopy structure and transport mechanism of a wall teichoic acid ABC transporter. mBio 11. doi: 10.1128/mBio.02749-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Larue K, Ford RC, Willis LM, Whitfield C. 2011. Functional and structural characterization of polysaccharide co-polymerase proteins required for polymer export in ATP-binding cassette transporter-dependent capsule biosynthesis pathways. J Biol Chem 286:16658–16668. doi: 10.1074/jbc.M111.228221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Shi X, Chen M, Yu Z, Bell JM, Wang H, Forrester I, Villarreal H, Jakana J, Du D, Luisi BF, Ludtke SJ, Wang Z. 2019. In situ structure and assembly of the multidrug efflux pump AcrAB-TolC. Nat Commun 10:2635. doi: 10.1038/s41467-019-10512-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Liston SD, McMahon SA, Bas AL, Suits MDL, Naismith JH, Whitfield C. 2018. Periplasmic depolymerase provides insight into ABC transporter-dependent secretion of bacterial capsular polysaccharides. Proc Natl Acad Sci U S A 115:E4870–E4879. doi: 10.1073/pnas.1801336115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sande C, Bouwman C, Kell E, Nickerson NN, Kapadia SB, Whitfield C. 2019. Structural and functional variation in outer membrane polysaccharide export (OPX) proteins from the two major capsule assembly pathways present in Escherichia coli. J Bacteriol 201:39. doi: 10.1128/JB.00213-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.McNulty C, Thompson J, Barrett B, Lord L, Andersen C, Roberts IS. 2006. The cell surface expression of group 2 capsular polysaccharides in Escherichia coli: the role of KpsD, RhsA and a multi-protein complex at the pole of the cell. Mol Microbiol 59:907–922. doi: 10.1111/j.1365-2958.2005.05010.x. [DOI] [PubMed] [Google Scholar]
- 140.Diao J, Bouwman C, Yan D, Kang J, Katakam AK, Liu P, Pantua H, Abbas AR, Nickerson NN, Austin C, Reichelt M, Sandoval W, Xu M, Whitfield C, Kapadia SB. 2017. Peptidoglycan association of murein lipoprotein Is required for KpsD-dependent group 2 capsular polysaccharide expression and serum resistance in a uropathogenic Escherichia coli Isolate. mBio 8:e00603-17. doi: 10.1128/mBio.00603-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Meredith TC, Woodard RW. 2006. Characterization of Escherichia coli D-arabinose 5-phosphate isomerase encoded by kpsF: implications for group 2 capsule biosynthesis. Biochem J 395:427–432. doi: 10.1042/BJ20051828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Rosenow C, Roberts IS, Jann K. 1995. Isolation from recombinant Escherichia coli and characterization of CMP-Kdo synthetase, involved in the expression of the capsular K5 polysaccharide (K-CKS). FEMS Microbiol Lett 125:159–164. doi: 10.1111/j.1574-6968.1995.tb07352.x. [DOI] [PubMed] [Google Scholar]
- 143.Azurmendi HF, Veeramachineni V, Freese S, Lichaa F, Freedberg DI, Vann WF. 2020. Chemical structure and genetic organization of the E. coli O6:K15 capsular polysaccharide. Sci Rep 10:12608. doi: 10.1038/s41598-020-69476-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Finke A, Jann B, Jann K. 1990. CMP‐KDO‐synthetase activity in Escherichia coli expressing capsular polysaccharides. FEMS Microbiol Lett 69:129–133. doi: 10.1111/j.1574-6968.1990.tb04188.x. [DOI] [PubMed] [Google Scholar]
- 145.Hong Y, Cunneen MM, Reeves PR. 2019. Two extremely divergent sequence forms of the genes that define Escherichia coli group 3 capsules suggest a very long history since their common ancestor. FEMS Microbiol Lett 366:403. doi: 10.1093/femsle/fnz091. [DOI] [PubMed] [Google Scholar]
- 146.Corbett D, Bennett HJ, Askar H, Green J, Roberts IS. 2007. SlyA and H-NS regulate transcription of the Escherichia coli K5 capsule gene cluster, and expression of slyA in Escherichia coli is temperature-dependent, positively autoregulated, and independent of H-NS. J Biol Chem 282:33326–33335. doi: 10.1074/jbc.M703465200. [DOI] [PubMed] [Google Scholar]
- 147.Xue P, Corbett D, Goldrick M, Naylor C, Roberts IS. 2009. Regulation of expression of the region 3 promoter of the Escherichia coli K5 capsule gene cluster involves H-NS, SlyA, and a large 5′ untranslated region. J Bacteriol 191:1838–1846. doi: 10.1128/JB.01388-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rowe S, Hodson N, Griffiths G, Roberts IS. 2000. Regulation of the Escherichia coli K5 capsule gene cluster: evidence for the roles of H-NS, BipA, and Integration Host Factor in regulation of group 2 capsule gene clusters in pathogenic E. coli. J Bacteriol 182:2741–2745. doi: 10.1128/JB.182.10.2741-2745.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Stevens MP, Hänfling P, Jann B, Jann K, Roberts IS. 1994. Regulation of Escherichia coli K5 capsular polysaccharide expression: evidence for involvement of RfaH in the expression of group II capsules. FEMS Microbiol Lett 124:93–98. doi: 10.1111/j.1574-6968.1994.tb07267.x. [DOI] [PubMed] [Google Scholar]
- 150.Stevens MP, Clarke BR, Roberts IS. 1997. Regulation of the Escherichia coli K5 capsule gene cluster by transcription antitermination. Mol Microbiol 24:1001–1012. doi: 10.1046/j.1365-2958.1997.4241780.x. [DOI] [PubMed] [Google Scholar]
- 151.Liston SD, Ovchinnikova OG, Whitfield C. 2016. Unique lipid anchor attaches Vi antigen capsule to the surface of Salmonella enterica serovar Typhi. Proc Natl Acad Sci U S A 113:6719–6724. doi: 10.1073/pnas.1524665113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Jin C, Hill J, Gunn BM, Yu W-H, Dahora LC, Jones E, Johnson M, Gibani MM, Spreng RL, Alam SM, Nebykova A, Juel HB, Dennison SM, Seaton KE, Fallon JK, Tomaras GD, Alter G, Pollard AJ. 2020. Vi-specific serological correlates of protection for typhoid fever. J Exp Med 218:e20201116. doi: 10.1084/jem.20201116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Hitri K, Kuttel MM, Benedetto GD, Lockyer K, Gao F, Hansal P, Rudd TR, Beamish E, Rijpkema S, Ravenscroft N, Bolgiano B. 2019. O-acetylation of typhoid capsular polysaccharide confers polysaccharide rigidity and immunodominance by masking additional epitopes. Vaccine 37:3866–3875. doi: 10.1016/j.vaccine.2019.05.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Micoli F, Bjarnarson SP, Arcuri M, Pind AAA, Magnusdottir GJ, Necchi F, Benedetto RD, Carducci M, Schiavo F, Giannelli C, Pisoni I, Martin LB, Giudice GD, MacLennan CA, Rappuoli R, Jonsdottir I, Saul A. 2020. Short Vi-polysaccharide abrogates T-independent immune response and hyporesponsiveness elicited by long Vi-CRM197 conjugate vaccine. Proc Natl Acad Sci U S A 117:24443–24449. doi: 10.1073/pnas.2005857117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Keestra-Gounder AM, Tsolis RM, Bäumler AJ. 2015. Now you see me, now you don’t: the interaction of Salmonella with innate immune receptors. Nat Rev Microbiol 13:206–216. doi: 10.1038/nrmicro3428. [DOI] [PubMed] [Google Scholar]
- 156.Johnson R, Mylona E, Frankel G. 2018. Typhoidal Salmonella: distinctive virulence factors and pathogenesis. Cell Microbiol 20:e12939. doi: 10.1111/cmi.12939. [DOI] [PubMed] [Google Scholar]
- 157.Wetter M, Goulding D, Pickard D, Kowarik M, Waechter CJ, Dougan G, Wacker M. 2012. Molecular characterization of the viaB locus encoding the biosynthetic machinery for Vi capsule formation in Salmonella Typhi. PLoS One 7:e45609. doi: 10.1371/journal.pone.0045609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Virlogeux I, Waxin H, Ecobichon C, Popoff MY. 1995. Role of the viaB locus in synthesis, transport and expression of Salmonella typhi Vi antigen. Microbiology 141:3039–3047. doi: 10.1099/13500872-141-12-3039. [DOI] [PubMed] [Google Scholar]
- 159.Zogaj X, Nimtz M, Rohde M, Bokranz W, Römling U. 2001. The multicellular morphotypes of Salmonella typhimurium and Escherichia coli produce cellulose as the second component of the extracellular matrix. Mol Microbiol 39:1452–1463. doi: 10.1046/j.1365-2958.2001.02337.x. [DOI] [PubMed] [Google Scholar]
- 160.Bhoite S, van Gerven N, Chapman MR, Remaut H. 2019. Curli biogenesis: bacterial amyloid assembly by the type VIII secretion pathway. EcoSal Plus 8. doi: 10.1128/ecosalplus.ESP-0037-2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Serra DO, Richter AM, Hengge R. 2013. Cellulose as an architectural element in spatially structured Escherichia coli biofilms. J Bacteriol 195:5540–5554. doi: 10.1128/JB.00946-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Mika F, Hengge R. 2014. Small RNAs in the control of RpoS, CsgD, and biofilm architecture of Escherichia coli. RNA Biol 11:494–507. doi: 10.4161/rna.28867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Vuong C, Voyich JM, Fischer ER, Braughton KR, Whitney AR, DeLeo FR, Otto M. 2004. Polysaccharide intercellular adhesin (PIA) protects Staphylococcus epidermidis against major components of the human innate immune system. Cell Microbiol 6:269–275. doi: 10.1046/j.1462-5822.2004.00367.x. [DOI] [PubMed] [Google Scholar]
- 164.Wang X, Preston JF, 3rd, Romeo T. 2004. The pgaABCD locus of Escherichia coli promotes the synthesis of a polysaccharide adhesin required for biofilm formation. J Bacteriol 186:2724–2734. doi: 10.1128/JB.186.9.2724-2734.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Cerca N, Maira-Litrán T, Jefferson KK, Grout M, Goldmann DA, Pier GB. 2007. Protection against Escherichia coli infection by antibody to the Staphylococcus aureus poly-N-acetylglucosamine surface polysaccharide. Proc Natl Acad Sci U S A 104:7528–7533. doi: 10.1073/pnas.0700630104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Echeverz M, García B, Sabalza A, Valle J, Gabaldón T, Solano C, Lasa I. 2017. Lack of the PGA exopolysaccharide in Salmonella as an adaptive trait for survival in the host. PLoS Genet 13:e1006816. doi: 10.1371/journal.pgen.1006816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Low KE, Howell PL. 2018. Gram-negative synthase-dependent exopolysaccharide biosynthetic machines. Curr Opin Struct Biol 53:32–44. doi: 10.1016/j.sbi.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 168.Morgan JLW, McNamara JT, Zimmer J. 2014. Mechanism of activation of bacterial cellulose synthase by cyclic di-GMP. Nat Struct Mol Biol 21:489–496. doi: 10.1038/nsmb.2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Morgan JLW, McNamara JT, Fischer M, Rich J, Chen H-M, Withers SG, Zimmer J. 2016. Observing cellulose biosynthesis and membrane translocation in crystallo. Nature 531:329–334. doi: 10.1038/nature16966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Krasteva PV, Bernal-Bayard J, Travier L, Martin FA, Kaminski P-A, Karimova G, Fronzes R, Ghigo J-M. 2017. Insights into the structure and assembly of a bacterial cellulose secretion system. Nat Commun 8:2065. doi: 10.1038/s41467-017-01523-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Acheson JF, Derewenda ZS, Zimmer J. 2019. Architecture of the cellulose synthase outer membrane channel and its association with the periplasmic TPR domain. Structure 27:1855–1861. doi: 10.1016/j.str.2019.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Thongsomboon W, Serra DO, Possling A, Hadjineophytou C, Hengge R, Cegelski L. 2018. Phosphoethanolamine cellulose: a naturally produced chemically modified cellulose. Science 359:334–338. doi: 10.1126/science.aao4096. [DOI] [PubMed] [Google Scholar]
- 173.Hollenbeck EC, Antonolis A, Chai C, Thongsomboon W, Fuller GG, Cegelski L. 2018. Phosphoethanolamine cellulose enhances curli-mediated adhesion of uropathogenic Escherichia coli to bladder epithelial cells. Proc Natl Acad Sci U S A 115:10106–10111. doi: 10.1126/science.aao4096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Abidi W, Zouhir S, Caleechurn M, Roche S, Krasteva PV. 2021. Architecture and regulation of an enterobacterial cellulose secretion system. Sci Adv 7:eabd8049. doi: 10.1126/sciadv.abd8049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Acheson JF, Ho R, Goularte NF, Cegelski L, Zimmer J. 2021. Molecular organization of the E. coli cellulose synthase macrocomplex. Nat Struct Mol Biol 28:310–318. doi: 10.1038/s41594-021-00569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Zouhir S, Abidi W, Caleechurn M, Krasteva PV. 2020. Structure and multitasking of the c-di-GMP-sensing cellulose secretion regulator BcsE. mBio 11:e01303-20. doi: 10.1128/mBio.01303-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Quéré BL, Ghigo J-M. 2009. BcsQ is an essential component of the Escherichia coli cellulose biosynthesis apparatus that localizes at the bacterial cell pole. Mol Microbiol 72:724–740. doi: 10.1111/j.1365-2958.2009.06678.x. [DOI] [PubMed] [Google Scholar]
- 178.Itoh Y, Rice JD, Goller C, Pannuri A, Taylor J, Meisner J, Beveridge TJ, Preston JF, Romeo T. 2008. Roles of pgaABCD genes in synthesis, modification, and export of the Escherichia coli biofilm adhesin poly-β-1,6-N-acetyl-D-glucosamine. J Bacteriol 190:3670–3680. doi: 10.1128/JB.01920-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Steiner S, Lori C, Boehm A, Jenal U. 2013. Allosteric activation of exopolysaccharide synthesis through cyclic di‐GMP‐stimulated protein–protein interaction. EMBO J 32:354–368. doi: 10.1038/emboj.2012.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Wang Y, Pannuri AA, Ni D, Zhou H, Cao X, Lu X, Romeo T, Huang Y. 2016. Structural basis for translocation of a biofilm-supporting exopolysaccharide across the bacterial outer membrane. J Biol Chem 291:10046–10057. doi: 10.1074/jbc.M115.711762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Little DJ, Poloczek J, Whitney JC, Robinson H, Nitz M, Howell PL. 2012. The structure- and metal-dependent activity of Escherichia coli PgaB provides insight into the partial de-N-acetylation of poly-β-1,6-N-acetyl-D-glucosamine. J Biol Chem 287:31126–31137. doi: 10.1074/jbc.M112.390005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Little DJ, Pfoh R, Mauff FL, Bamford NC, Notte C, Baker P, Guragain M, Robinson H, Pier GB, Nitz M, Deora R, Sheppard DC, Howell PL. 2018. PgaB orthologues contain a glycoside hydrolase domain that cleaves deacetylated poly-β(1,6)-N-acetylglucosamine and can disrupt bacterial biofilms. PLoS Pathog 14:e1006998. doi: 10.1371/journal.ppat.1006998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Eddenden A, Kitova EN, Klassen JS, Nitz M. 2020. An inactive dispersin B probe for monitoring PNAG production in biofilm formation. ACS Chem Biol 15:1204–1211. doi: 10.1021/acschembio.9b00907. [DOI] [PubMed] [Google Scholar]



