Abstract
BACKGROUND:
Studies on grade 2 rectal neuroendocrine tumors are limited, and the optimal treatment for these tumors is not well established.
OBJECTIVE:
We aimed to compare the oncologic results of local excision versus radical resection for the treatment of grade 2 rectal neuroendocrine tumors.
DESIGN:
Retrospective multicenter propensity score–matched study to minimize heterogeneity between groups and focus on the differences between surgery strategies.
SETTINGS:
Seventeen large-scale Chinese medical centers participated in this study.
PATIENTS:
A total of 144 patients with pathologically confirmed grade 2 rectal neuroendocrine tumors were retrospectively analyzed.
MAIN OUTCOME MEASURES:
Cancer-specific survival and relapse-free survival were assessed to compare surgery strategies.
RESULTS:
A total of 144 patients with grade 2 rectal neuroendocrine tumors were enrolled in this study. Twenty-seven patients underwent endoscopic resection, 55 underwent transanal excision, 50 underwent radical resection, and 12 underwent palliative surgery or biopsy for distant metastasis. Of the 50 patients who underwent radical resection, 30 (60.0%) had clinically positive lymph nodes on the basis of the histopathology results. The optimal cutoff value for tumor size to predict cancer-specific survival was 1.5 cm. In patients with grade 2 rectal neuroendocrine tumors of ≤1.5-cm size, there were no significant differences in cancer-specific survival and relapse-free survival between local excision and radical resection groups (p > 0.05). In patients with grade 2 rectal neuroendocrine tumors of >1.5-cm size, relapse-free survival was significantly lower in the local excision group than in the radical resection group (p = 0.04).
LIMITATIONS:
The nature of retrospective reviews and a relatively short follow-up period are limitations of this study.
CONCLUSIONS:
Grade 2 rectal neuroendocrine tumors have a nonnegligible rate of lymph node metastasis. Local excision is a feasible choice for tumors of ≤1.5 cm size without metastasis, whereas radical resection is more beneficial in those of >1.5 cm size. See Video Abstract.
ESCISIÓN LOCAL VERSUS RESECCIÓN RADICAL PARA TUMORES NEUROENDOCRINOS RECTALES GRADO 2: ANÁLISIS MULTICÉNTRICO CON PUNTUACIÓN DE PROPENSIÓN COINCIDENTE
ANTECEDENTES:
Los estudios sobre los tumores neuroendocrinos rectales de grado 2 son limitados y el tratamiento óptimo para estos tumores no está bien establecido.
OBJETIVO:
Comparar los resultados oncológicos de la escisión local versus la resección radical para el tratamiento de tumores neuroendocrinos rectales grado 2.
DISEÑO:
Estudio multicéntrico retrospectivo emparejado por puntuación de propensión para minimizar la heterogeneidad entre grupos y centrarse en la diferencia entre estrategias quirúrgicas.
ESCENARIO:
Diecisiete centros médicos chinos de gran tamaño participaron en este estudio.
PACIENTES:
Se analizaron retrospectivamente un total de 144 pacientes con tumores neuroendocrinos rectales grado 2 patológicamente confirmados.
PRINCIPALES MEDIDAS DE RESULTADO:
Se evaluaron la supervivencia específica del cáncer y la supervivencia libre de recaída para comparar las estrategias quirúrgicas.
RESULTADOS:
En este estudio se inscribieron un total de 144 pacientes con tumores neuroendocrinos rectales grado 2. Veintisiete pacientes se sometieron a resección endoscópica, 55 a escisión transanal, 50 a resección radical y 12 a cirugía paliativa o biopsia por metástasis a distancia. De los 50 pacientes que se sometieron a resección radical, 30 (60,0%) tenían ganglios linfáticos clínicamente positivos según los resultados histopatológicos. El valor de corte óptimo para el tamaño del tumor para predecir la supervivencia específica del cáncer fue de 1,5 cm. En pacientes con tumores neuroendocrinos rectales grado 2 ≤ 1,5 cm, no hubo diferencias significativas en la supervivencia específica del cáncer y la supervivencia libre de recaída entre los grupos de escisión local y resección radical (p >0,05). En pacientes con tumores neuroendocrinos rectales grado 2 > 1,5 cm, la supervivencia libre de recaída fue significativamente menor en el grupo de escisión local que en el grupo de resección radical (p = 0,04).
LIMITACIONES:
La naturaleza de la revisión retrospectiva y el período de seguimiento relativamente corto son limitaciones de este estudio.
CONCLUSIONES:
Los tumores neuroendocrinos rectales grado 2 tienen una tasa no despreciable de metástasis en los ganglios linfáticos. La escisión local es una opción factible para tumores ≤ 1,5 cm sin metástasis, mientras que la resección radical es más beneficiosa en aquellos > 1,5 cm. (Traducción—Dr. Felipe Bellolio)
Keywords: Local excision, Lymph node metastasis, Radical resection, Rectal neuroendocrine tumors
Video Abstract
Video Abstract.
Neuroendocrine tumors (NETs) are rare tumors arising from peptidergic neurons and neuroendocrine cells, exhibiting neuroendocrine differentiation, and expressing neuroendocrine markers.1 These tumors can manifest throughout the body but are predominantly localized in the GI tract, pancreas, and lungs.2 The rectum is the third most frequent site of NETs, with an incidence of 1.20 of 100,000 in 2012.2 On the basis of the Ki-67 index and mitotic count, rectal neuroendocrine tumors (RNETs) can be stratified into 3 subgroups that exhibit substantial heterogeneity: well-differentiated G1 RNETs with indolent behavior and favorable prognosis, moderately differentiated G2 RNETs with intermediate risk of metastasis, and poorly differentiated G3 RNETs (also known as neuroendocrine carcinoma [NEC]) with frequent metastasis and dismal outcomes.3,4 Tumor grade is a major predictor of metastasis and prognosis in RNETs.5,6
Currently, both the European Neuroendocrine Tumor Society and the Chinese Society of Clinical Oncology recommend similar therapeutic approaches for G1 RNETs and G2 RNETs.7 Additionally, the scarcity of G2 RNETs has led most studies to analyze G1 RNETs and G2 RNETs together, which might underestimate the risk of metastasis and poor prognosis in G2 RNETs. Therefore, a more precise characterization of this subgroup is imperative for guiding appropriate therapy.
In this study, we used data from a large multicenter database of 17 Chinese large-scale medical centers to elucidate the clinicopathologic features and prognostic factors of G2 RNETs and suggest a reasonable selection mode of resection type for these patients.
MATERIALS AND METHODS
Patients and Data Collection
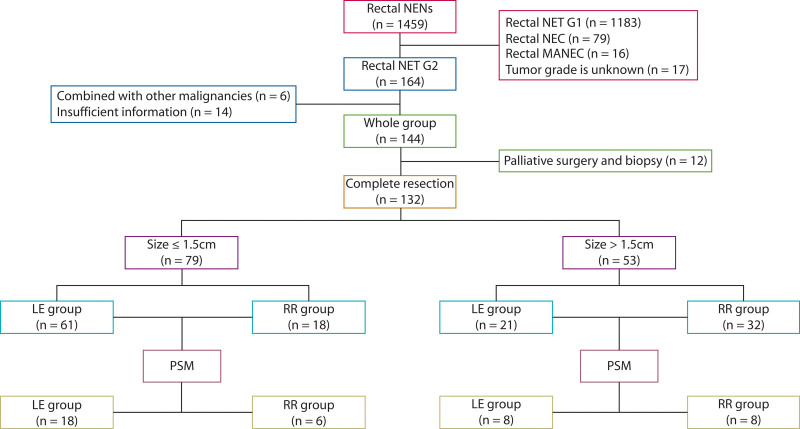
This study retrospectively reviewed the data of patients diagnosed with G2 RNETs at 17 large-scale medical centers in China between January 1, 2010, and April 30, 2022. Demographic, clinicopathologic, treatment, and outcome data were extracted from the electronic medical records of each hospital by surveyors with expertise in NETs, using standardized data collection templates. The inclusion criteria were as follows: (1) confirmation of NETs by pathology, (2) tumor grade of G2, and (3) primary RNETs. The exclusion criteria were as follows: (1) comorbidity with other malignancies and (2) incomplete clinical data or follow-up information. Of 1459 patients, 144 met the inclusion criteria and were enrolled in this study. The procedure for identifying eligible patients is shown in Figure 1. Our work was approved by the Ethics Committees of all 17 participating centers. At the last follow-up, we informed the living patients about the study in detail and obtained their signed informed consent. For patients who were not alive at the time of the study, we contacted their immediate family members, explained the study in detail, and obtained their signed informed consent.
FIGURE 1.
Patient selection flow chart. LE = local excision; MANEC = mixed adeno-neuroendocrine carcinoma; NEC = neuroendocrine carcinoma; NEN = neuroendocrine neoplasm; NET = neuroendocrine tumor; PSM = propensity score matching; RR = radical resection.
Criteria
Tumor size was determined on the basis of the longest diameter of the tumor as recorded in the pathologic reports. In cases where only biopsy was performed, tumor size was determined on the basis of endoscopic findings before treatment. For patients who underwent palliative surgery, tumor size was assessed on the basis of the imaging report.
Tumor stage was classified in accordance with the cancer staging manual from the American Joint Committee on Cancer, whereas tumor grade was classified on the basis of the World Health Organization 2010 classification. The mitosis count was expressed as the number of mitotic cells observed in 10 high-power fields from hematoxylin and eosin stained slides examined under a microscope. The Ki-67 index was calculated as the percentage of cells labeled by immunohistochemistry. Patients with a T stage of T2 or more, lymph node metastasis, neurovascular invasion, or tumor size >2 cm were regarded as having a high risk of recurrence, and a multidisciplinary team was involved in formulating adjuvant therapy plans for these patients. For somatostatin receptor-positive patients, somatostatin analog therapy was recommended.
Follow-up
Cancer-specific survival (CSS) was defined as the interval between diagnosis and death attributed to NETs. Relapse-free survival (RFS) was defined for patients who underwent neoplasm removal and was calculated from the interval between the date of intervention and the date of recurrence. All patients were followed up via telephone and via outpatient and inpatient means. After complete resection, patients were followed up every 6 months and annually after 5 years; patients who underwent palliative resection or biopsy were followed up every 3 months. The last follow-up was conducted in July 2022. Follow-up examinations included routine blood tests, Chromogranin A tests, chest CT, and whole abdominal and pelvic contrast-enhanced CT or MRI; PET-CT was performed if recurrence or metastasis was suspected. Loss to follow-up was defined as failure to contact either the patients or their family.
Statistical Analysis
Continuous variables are presented as medians with interquartile ranges and were evaluated using either the t test or Mann-Whitney U test. Categorical data are expressed as numbers and percentages and were analyzed using either the χ2 test or Fisher exact test. CSS and RFS were analyzed using the Kaplan-Meier method, and variables were compared using the log-rank test in univariable analysis and Cox proportional hazard regression in multivariable analysis. The influence of other confounders on the outcomes of the local excision (LE) and radical resection (RR) groups were minimized by propensity score matching (PSM).8 To complete the matching of patients, we adopted the nearest neighbor matching algorithm according to the logic of propensity score. An SD less than 10% was considered acceptable in the assessment of the matching covariate balance.9
Statistical analysis was performed using R software (version 3.1.0). The optimal cutoff value for continuous variables was determined using X-tile software. Variables with a p value of <0.10 in the univariate analysis were included in the multivariate analysis. P values of <0.05 were considered statistically significant.
RESULTS
Demographic and Clinicopathological Characteristics
A total of 144 patients with G2 RNETs were included in this study, including 88 men (61.1%) and 56 women (38.9%). The median age was 51.0 (40.0–64.0) years, the median tumor size was 1.5 (0.7–2.2) cm, and the median distance from the anus was 5.0 (3.5–8.0) cm. According to TNM staging, 89 (61.8%) patients were staged T1, 24 (16.7%) patients were staged T2, 22 (15.3%) patients were staged T3, and 9 (6.3%) patients were staged T4. Twenty-seven (18.8%) of the patients underwent endoscopic resection, 55 (38.2%) underwent transanal excision (TAE), and 50 (34.7%) underwent RR. Palliative surgery and biopsy due to distant metastasis were performed in 12 (8.4%) patients, of whom 9 (75.0%) had only liver metastasis, 2 (16.7%) had only bone metastasis, and 1 (8.3%) had both liver metastasis and bone metastasis. Of the 50 patients who underwent RR, 30 (60.0%) had clinically positive lymph nodes on the basis of the histopathology results. Demographic and clinicopathological characteristics of patients with G2 RNETs are summarized in Table 1.
TABLE 1.
Clinicopathological data of patients with G2 RNETs
| Clinicopathological factors | Total N = 144 | |
|---|---|---|
| n | IQR or % | |
| Sex, n (%) | ||
| Male | 88 | 61.1% |
| Female | 56 | 38.9% |
| Age, y (IQR) | 51.0 | (40.0–64.0) |
| Tumor size, cm (IQR) | 1.5 | (0.7–2.2) |
| Distance from the anus, cm (IQR) | 5.0 | (3.5–8.0) |
| T stage, n (%) | ||
| T1 | 89 | 61.8% |
| T2 | 24 | 16.7% |
| T3 | 22 | 15.3% |
| T4 | 9 | 6.3% |
| Distant metastasis, n (%) | ||
| Negative | 132 | 91.7% |
| Positive | 12 | 8.3% |
| Resection type, n (%) | ||
| Endoscopic resection | 27 | 18.8% |
| Transanal excision | 55 | 38.2% |
| RR | 50 | 34.7% |
| Only biopsy | 3 | 2.1% |
| Palliative surgery | 9 | 6.3% |
| Microvascular invasion, n (%) | ||
| Negative | 126 | 87.5% |
| Positive | 18 | 12.5% |
| Perineural invasion, n (%) | ||
| Negative | 131 | 91.0% |
| Positive | 13 | 9.0% |
IQR = interquartile range; RNET = rectal neuroendocrine tumor; RR, radical resection.
Prognostic Factors for CSS
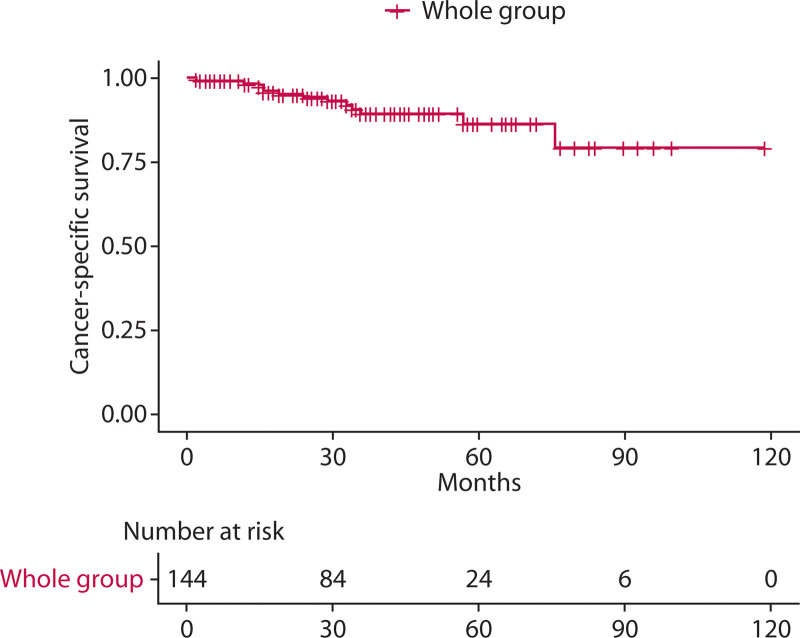
The shortest follow-up time was 2 months, the longest was 119 months, and the median follow-up time was 34.0 months. A total of 13 patients (9.0%) died of tumor-related causes during the follow-up period. The 1-year, 3-year, and 5-year cancer-specific survival rates of the whole group were 98.5%, 89.6%, and 86.5%, respectively (Fig. 2).
FIGURE 2.
Cancer-specific survival for the whole group.
The optimal cutoff value for tumor diameter to predict CSS was 1.5 cm. Tumor size (p = 0.004), T stage (p = 0.008), distant metastasis (p < 0.001), and microvascular invasion (p = 0.046) were all associated with CSS in univariable analysis. Tumor size >1.5 cm (HR: 5.654; 95% CI, 1.228–26.043; p = 0.03) and distant metastasis (HR: 4.191; 95% CI, 1.233–14.244; p = 0.02) were significant prognostic factors in multivariable analysis. Log-rank test and Cox proportional hazard regression analysis of CSS are shown in Table 2.
TABLE 2.
Prognostic factors for cancer-specific survival
| Clinicopathological factors | Univariate analysis | Multivariable analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95% CI | p | HR | 95% CI | p | |
| Sex (male/female) | 2.845 | 0.939 to 8.625 | 0.07 | 3.697 | 0.771 to 17.733 | 0.10 |
| Age (≥65/<65 y) | 2.951 | 0.770 to 11.300 | 0.11 | - | - | - |
| Tumor size (>1.5/≤1.5 cm) | 5.052 | 1.688 to 15.120 | 0.004 | 5.654 | 1.228 to 26.043 | 0.03 |
| Distance from the anus (>7/≤7 cm) | 0.833 | 0.243 to 2.864 | 0.77 | - | - | - |
| T stage (T3+T4/T1+T2) | 5.753 | 1.576 to 21.000 | 0.008 | 1.202 | 0.271 to 5.337 | 0.82 |
| Distant metastasis (positive/negative) | 36.410 | 4.364 to 303.800 | <0.001 | 4.191 | 1.233 to 14.244 | 0.02 |
| Microvascular invasion (positive/negative) | 5.344 | 1.028 to 27.780 | 0.046 | 0.695 | 0.165 to 2.927 | 0.93 |
| Perineural invasion (positive/negative) | 3.250 | 0.403 to 26.200 | 0.268 | - | - | - |
Statistically significant (p <0.05) values are in bold.
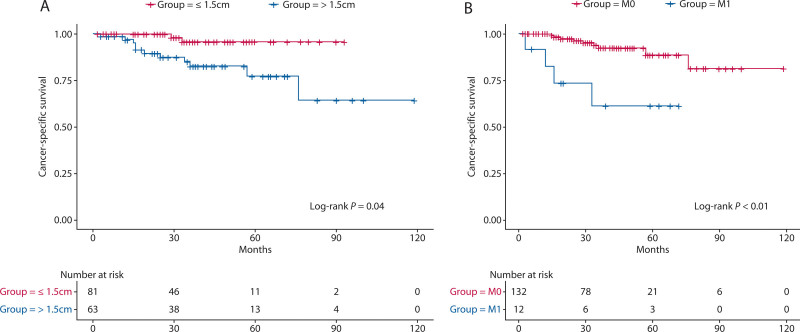
The 3-year CSS rate was 95.7% for patients whose tumor size was ≤1.5 cm and 82.3% for patients whose tumor size was >1.5 cm. The 3-year CSS rate for patients without distant metastasis was 93.8%, and the 3-year CSS rate for patients with distant metastasis was 61.1%. The survival curves according to tumor size and distant metastasis are shown in Figure 3.
FIGURE 3.
Kaplan-Meier CSS curves for patients with G2 RNETs. A, CSS stratified by tumor size. B, CSS stratified by distant metastasis. CSS = cancer-specific survival.
Comparison of Resection Types
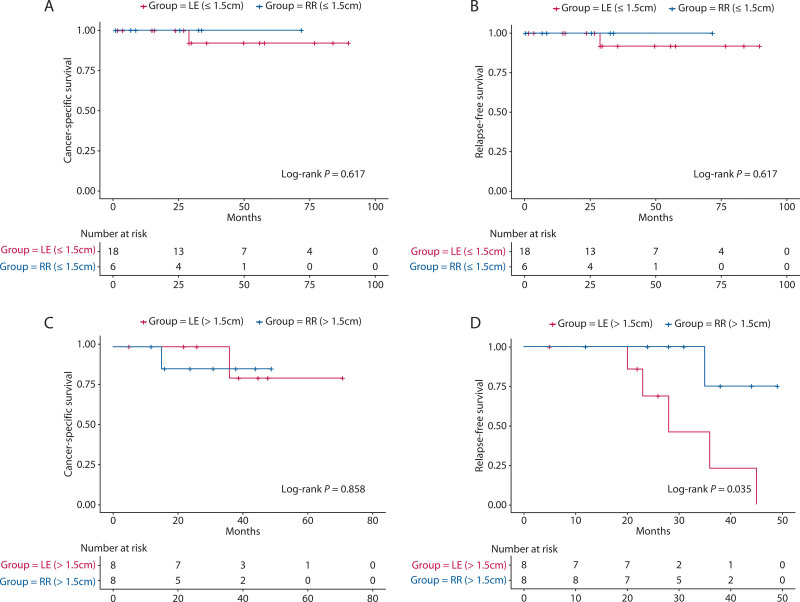
Complete tumor resection was performed in 132 (91.7%) of 144 patients, including endoscopic resection in 27 (20.4%), transanal excision in 55 (41.7%), and RR in 50 (37.9%). Thirty of 132 patients (22.7%) received adjuvant therapy. Of the 132 patients, 79 had tumors with a diameter ≤1.5 cm. Of the 79 patients, 61 underwent LE (including endoscopic resection and transanal excision) and 18 underwent RR. Before PSM, the distance from the anus in the LE group was significantly lower than that in the RR group (p = 0.001); the T stage was also significantly lower than that in the RR group (p = 0.01). There were no significant differences after matching. The clinicopathologic characteristics of the 2 groups before and after matching are shown in Table 3. The 3-year CSS rate was 91.7% in the LE group and 100.0% in the RR group; the difference was not statistically significant (p = 0.62). The 3-year recurrence-free survival rate was 91.7% in the LE group and 100.0% in the RR group; the difference was not statistically significant (p = 0.62). The survival curves according to resection type are shown in Figures 4A and B.
TABLE 3.
Comparison of variables between LE and RR groups for G2 RNETs 1.5 cm or less
| Variable | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
|
LE (n = 61) |
RR (n = 18) |
p |
LE (n = 18) |
RR (n = 6) |
p | |
| Sex, n (%) | 0.60 | 0.07 | ||||
| Female | 25 (80.6%) | 6 (19.4%) | 8 (100.0%) | 0 (0.0%) | ||
| Male | 36 (75.0%) | 12 (25.0%) | 10 (62.5%) | 6 (37.5%) | ||
| Age, y, median (IQR) | 51.0 (40.3–63.8) |
51.0 (41.0–64.0) |
0.58 | 61.0 (36.0–62.0) |
58.0 (37.3–64.8) |
0.38 |
| Distance from the anus, cm, median (IQR) | 5.0 (3.0–8.0) |
6.2 (4.0–8.5) |
0.001 | 5.0 (4.3–7.5) |
5.0 (2.0–8.0) |
0.14 |
| T stage, n (%) | 0.01 | – | ||||
| T1 + T2 | 61 (80.3%) | 15 (19.7%) | 18 (75.0%) | 6 (25.0%) | ||
| T3 + T4 | 0 (0.0%) | 3 (100.0%) | 0 | 0 | ||
| Lymph node metastasis, n (%) | <0.001 | – | ||||
| Negative | 61 (88.4%) | 8 (11.6%) | 18 (75.0%) | 6 (25.0%) | ||
| Positive | 0 (0.0%) | 10 (100.0%) | 0 | 0 | ||
| Microvascular invasion, n (%) | 0.13 | – | ||||
| Negative | 60 (78.9%) | 16 (21.1%) | 18 (75.0%) | 6 (25.0%) | ||
| Positive | 1 (33.3%) | 2 (66.7%) | 0 | 0 | ||
| Perineural invasion, n (%) | 0.23 | – | ||||
| Negative | 61 (78.2%) | 17 (21.8%) | 18 (75.0%) | 6 (25.0%) | ||
| Positive | 0 (0.0%) | 1 (100.0%) | 0 | 0 | ||
IQR = interquartile range; LE = local excision; PSM = propensity score matching; RNET = rectal neuroendocrine tumor; RR = radical resection.
Statistically significant (p <0.05) values are in bold.
FIGURE 4.
Kaplan-Meier survival curves for the matched comparisons. A, CSS stratified by resection type for G2 RNETs ≤1.5 cm. B, RFS stratified by resection type for G2 RNETs ≤1.5 cm. C, CSS stratified by resection type for G2 RNETs > 1.5 cm. D, RFS stratified by resection type for G2 RNETs >1.5 cm. CSS = cancer-specific survival; LE = local excision; RFS = relapse-free survival; RR = radical resection; RNET = rectal neuroendocrine tumor.
Of the 132 patients, 53 had tumors >1.5-cm diameter. In the 53 patients, 21 underwent LE and 32 underwent RR. Before PSM, patients in the LE group had significantly lower T stage (p = 0.0003) than those in the RR group. There were no significant differences after matching. The clinicopathologic characteristics of the 2 groups before and after matching are shown in Table 4. The 3-year CSS rate was 80.0% in the LE group and 85.7% in the RR group, and the difference was not statistically significant (p = 0.86). The 3-year recurrence-free survival rates were 22.9% in the LE group and 75.0% in the RR group, and the difference was statistically significant (p = 0.04). Of the 8 patients after PSM with tumors larger than 1.5 cm and who underwent LE, 5 patients developed recurrence. Among these patients, 3 developed only liver metastases whereas 2 developed abdominal and liver metastases. The survival curves according to resection type are shown in Figures 4C and 4D.
TABLE 4.
Comparison of variables between LE and RR groups for G2 RNETs more than 1.5 cm
| Variable | Before PSM | After PSM | ||||
|---|---|---|---|---|---|---|
| LE (n = 21) |
RR (n = 32) |
p | LE (n = 8) |
RR (n = 8) |
p | |
| Sex, n (%) | 0.26 | 0.13 | ||||
| Female | 11 (50.0%) | 11 (50.0%) | 6 (75.0%) | 2 (25.0%) | ||
| Male | 10 (32.3%) | 21 (67.7%) | 2 (25.0%) | 6 (75.0%) | ||
| Age, y, median (IQR) | 51.0 (40.0–64.0) |
53.5 (44.8–66.0) |
0.27 | 66.0 (46.0–69.5) |
63.5 (47.0–70.0) |
0.78 |
| Distance from the anus, cm (IQR) | 5.0 (3.3,8.0) |
5.0 (3.5–8.0) |
0.21 | 7.0 (4.0–8.0) |
8.0 (4.5–11.5) |
0.06 |
| T stage, n (%) | <0.001 | >0.99 | ||||
| T1+T2 | 20 (57.1%) | 15 (42.9%) | 7 (50.0%) | 7 (50.0%) | ||
| T3+T4 | 1 (5.6%) | 17 (94.4%) | 1 (50.0%) | 1 (50.0%) | ||
| Lymph node metastasis, n (%) | <0.001 | – | ||||
| Negative | 21 (63.6%) | 12 (36.4%) | 8 (50.0%) | 8 (50.0%) | ||
| Positive | 0 (0.0%) | 20 (100.0%) | 0 | 0 | ||
| Microvascular invasion, n (%) | 0.29 | >0.99 | ||||
| Negative | 19 (43.2%) | 25 (56.8%) | 7 (50.0%) | 7 (50.0%) | ||
| Positive | 2 (22.2%) | 7 (77.8%) | 1 (50.0%) | 1 (50.0%) | ||
| Perineural invasion, n (%) | 0.07 | 0.48 | ||||
| Negative | 21 (44.7%) | 26 (55.3%) | 8 (57.1%) | 6 (42.9%) | ||
| Positive | 0 (0.0%) | 6 (100.0%) | 0 (0.0%) | 2 (100.0%) | ||
IQR = interquartile range; LE = local excision; PSM = propensity score matching; RR = radical resection.
Statistically significant (p <0.05) values are in bold.
DISCUSSION
In this study, we aimed to investigate the occurrence of lymph node metastasis in G2 RNETs. As lymph node metastasis may not be detectable by imaging in patients who underwent LE, biopsy, and palliative surgery, only patients who underwent RR + lymphadenectomy were included in the analysis of lymph node metastasis. Pathological examination was used as the criterion for determining lymph node metastasis status. Of the 50 patients who underwent RR + lymphadenectomy, 30 (60.0%) had clinically positive lymph nodes on the basis of the histopathology results. Previous studies have shown that the incidence of lymph node metastasis in G2 RNETs ranges from 20% to 44%,1,7,10 suggesting that G2 RNETs have a significant lymph node metastasis rate. Both CT and MRI were accurate in determining the lymph node metastasis status of RNETs, with metastatic lymph nodes exhibiting an abnormal size or morphology.11 Ushigome et al12 demonstrated that CT had a sensitivity of up to 0.820 in diagnosing lymph node metastasis in RNETs. On the basis of this, the European Neuroendorine Tumor Society recommended routine CT or MRI examination for RNETs >1 cm.13 In addition, endoscopic ultrasonography was found to be helpful in assessing regional lymph nodes in RNETs and identifying possible signs of metastasis.11 PET-CT was also useful in determining the status of systemic lymph node metastasis. Zhou et al14 reported that 68Ga-DOTANOC PET-CT was an effective tool for predicting lymph node metastasis, with a sensitivity of 0.778 and a specificity of 0.917. Therefore, clinicians should be cautious when considering LE. Conventional contrast-enhanced imaging, endoscopic ultrasonography, PET-CT, pathological biopsy, and other examinations may be useful in assessing tumors to select the most appropriate treatment because this study does not evaluate the predictive nature of these modalities.
RNETs are the subtype of GI NETs with the best prognosis, having a 5-year overall survival (OS) rate of 96%.15 However, recent studies have shown that not all RNETs behave indolently, and the prognosis largely depends on the tumor size. Sohn et al6 retrospectively analyzed 64 patients with RNETs and showed that patients with RNETs ≥2 cm had a significantly lower 5-year OS rate of only 31.3% compared with the <2 cm group. Additionally, distant metastasis is an independent risk factor for the prognosis of patients with RNETs. The American Joint Committee on Cancer staging system classifies all RNETs with distant metastases as stage IV.16 Modlin et al17 found that patients with distant metastases had a poor prognosis, with a 5-year OS rate of only 30.0%. On the basis of the results of this study, it was found that patients with G2 RNETs generally had a favorable prognosis, with a 5-year CSS of 86.5%. However, the prognosis was significantly worse for patients with a larger tumor size and distant metastasis. This highlights the importance of early monitoring for G2 RNETs, particularly when the tumor is still in its early stages and is characterized by a small tumor diameter and the absence of distant metastasis. Early detection and appropriate treatment can greatly improve the patient’s prognosis when compared with late-stage diagnosis.
RR, including low anterior resection and abdominoperineal resection, is a commonly used treatment modality for RNETs. However, compared with LE, RR may lead to a higher incidence of postoperative complications, although it can achieve a more complete tumor dissection and improve patient survival.18,19 In addition, RR can lead to poor functional outcomes, with up to 90% of patients experiencing changes in bowel habits after sphincter-saving surgery for rectal cancer,20 and many patients experience fecal urgency, frequency, and incontinence, and abnormal defecation function can seriously affect patients' quality of life.21,22 Therefore, the choice between LE and RR is critical for managing patients with RNETs. The European Neuroendocrine Tumor Society recommends endoscopic resection or transanal excision for G2 RNETs <2 cm if the T stage is early, which can achieve complete resection and long-term satisfying prognosis.13 Similarly, the National Comprehensive Cancer Network also recommends endoscopic resection or transanal excision for patients with tumors <2 cm size.23 However, the Japanese Neuroendocrine Tumor Society believes that RNETs larger than 1 cm have the same risk of lymph node metastasis as adenocarcinoma at the same site and, thus, recommends RR.24,25
In this study, the preoperative imaging results are an important reference for selecting surgical methods for patients with G2 RNETs. Through effective communication with patients and their families, we determined the optimal surgical approach after carefully assessing tumor size, T stage, and lymph node metastasis status. For tumors with a diameter smaller than 1 cm, LE was recommended. For tumors with a diameter larger than 2 cm, RR was suggested. For tumors with diameters between 1 and 2 cm, we recommended RR for patients with a T stage of T2 or more or with lymph node metastasis, whereas LE was suggested for patients with T stage = T1 and negative lymph node. However, this would clearly bias the cohort in favor of a particular outcome for the comparison of LE group and the RR group. Therefore, to minimize potential selection bias, we conducted a matching analysis on the basis of the clinicopathological characteristics of the patients. Our findings indicate that 1.5 cm is the optimal cutoff value for prognostic analysis in G2 RNETs. In addition, we found no statistically significant differences in CSS and RFS between patients who underwent LE and RR for G2 RNETs <1.5 cm. However, for G2 RNETs >1.5 cm, RFS was significantly better in the RR group than in the LE group. On the basis of our analysis, we recommend LE for G2 RNETs <1.5 cm confirmed by biopsy, whereas RR is recommended to reduce the postoperative recurrence rate for G2 RNETs >1.5 cm.
This study has some limitations. First, although we used PSM to reduce potential bias and used subgroup analysis to investigate the impact of the surgical approach on patient outcomes, the nature of the retrospective study still impacts the statistical power of this study and reduces clinical value. In addition, RNETs are very slow-growing tumors26; thus, a longer follow-up period is required for the clinical evaluation of patient status. However, to our knowledge, this is currently the largest study for G2 RNETs and can provide some guidance for the treatment of G2 RNETs.
CONCLUSIONS
In summary, G2 RNETs have a nonnegligible rate of lymph node metastasis, and clinicians should be cautious when considering LE. LE is recommended for G2 RNETs ≤1.5 cm without metastasis to achieve CSS and RFS similar to those of RR; for G2 RNETs >1.5 cm, RR is more suitable because better RFS can be achieved. However, the findings of this study require further validation by retrospective studies with larger sample sizes or by randomized controlled trials.
Footnotes
Funding/Support: This study was funded by National Natural Science Foundation of China (grant no. 81702386 and 81874184) and the Key Project of Hubei Health Commission (grant no. WJ2019Q030).
Financial Disclosure: None reported.
Xinyu Zeng and Rui Zhang contributed equally to this article.
Contributor Information
Xinyu Zeng, Email: zengxy@hust.edu.cn.
Rui Zhang, Email: weizhang2000cn@163.com.
Weizhong Jiang, Email: wb002554@whu.edu.cn.
Chengguo Li, Email: liyong@gdph.org.cn.
Minhao Yu, Email: yubinsswe@163.com.
Weizhen Liu, Email: weizhenliu@hust.edu.cn.
Maojun Di, Email: dimaojun@163.com.
Hongxue Wu, Email: wuhx1978@163.com.
Yueming Sun, Email: sunyueming@njmu.edu.cn.
Zhiguo Xiong, Email: xiongzhiguo120@163.com.
Congqing Jiang, Email: wb002554@whu.edu.cn.
Bin Yu, Email: yubinsswe@163.com.
Shengning Zhou, Email: zhoushn3@mail.sysu.edu.cn.
Yong Li, Email: liyong@gdph.org.cn.
Xiaofeng Liao, Email: liaoxiaofeng66@163.com.
Lijian Xia, Email: xiaalbert2758@163.com.
REFERENCES
- 1.Wei G, Feng X, Wang W, et al. Analysis of risk factors of lymph node metastasis in rectal neuroendocrine neoplasms using multicenter data. Future Oncol. 2018;14:1817–1823. [DOI] [PubMed] [Google Scholar]
- 2.Dasari A, Shen C, Halperin D, et al. Trends in the incidence, prevalence, and survival outcomes in patients with neuroendocrine tumors in the United States. JAMA Oncol. 2017;3:1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ramage JK, Ahmed A, Ardill J, et al. ; UK and Ireland Neuroendocrine Tumour Society. Guidelines for the management of gastroenteropancreatic neuroendocrine (including carcinoid) tumours (NETs). Gut. 2012;61:6–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Anthony LB, Strosberg JR, Klimstra DS, et al. ; North American Neuroendocrine Tumor Society (NANETS). The NANETS consensus guidelines for the diagnosis and management of gastrointestinal neuroendocrine tumors (NETs): well-differentiated nets of the distal colon and rectum. Pancreas. 2010;39:767–774. [DOI] [PubMed] [Google Scholar]
- 5.Wu Z, Wang Z, Zheng Z, Bi J, Wang X, Feng Q. Risk factors for lymph node metastasis and survival outcomes in colorectal neuroendocrine tumors. Cancer Manag Res. 2020;12:7151–7164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sohn B, Kwon Y, Ryoo SB, et al. Predictive factors for lymph node metastasis and prognostic factors for survival in rectal neuroendocrine tumors. J Gastrointest Surg. 2017;21:2066–2074. [DOI] [PubMed] [Google Scholar]
- 7.Li YW, He YP, Liu FQ, et al. Grade G2 rectal neuroendocrine tumor is much more invasive compared with G1 tumor. Front Oncol. 2021;11:646536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Agostino RB, Jr. Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. [DOI] [PubMed] [Google Scholar]
- 9.Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28:3083–3107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li P, Wu F, Zhao H, et al. Analysis of the factors affecting lymph node metastasis and the prognosis of rectal neuroendocrine tumors. Int J Clin Exp Pathol. 2015;8:13331–13338. [PMC free article] [PubMed] [Google Scholar]
- 11.de Mestier L, Lorenzo D, Fine C, et al. Endoscopic, transanal, laparoscopic, and transabdominal management of rectal neuroendocrine tumors. Best Pract Res Clin Endocrinol Metab. 2019;33:101293. [DOI] [PubMed] [Google Scholar]
- 12.Ushigome H, Fukunaga Y, Nagasaki T, et al. Difficulty of predicting lymph node metastasis on CT in patients with rectal neuroendocrine tumors. PLoS One. 2019;14:e0211675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caplin M, Sundin A, Nillson O, et al. ; Barcelona Consensus Conference participants. ENETS Consensus Guidelines for the management of patients with digestive neuroendocrine neoplasms: colorectal neuroendocrine neoplasms. Neuroendocrinology. 2012;95:88–97. [DOI] [PubMed] [Google Scholar]
- 14.Zhou Z, Wang Z, Zhang B, Wu Y, Li G, Wang Z. Comparison of 68Ga-DOTANOC and 18F-FDG PET-CT scans in the evaluation of primary tumors and lymph node metastasis in patients with rectal neuroendocrine tumors. Front Endocrinol (Lausanne). 2021;12:727327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsikitis VL, Wertheim BC, Guerrero MA. Trends of incidence and survival of gastrointestinal neuroendocrine tumors in the United States: a SEER analysis. J Cancer. 2012;3:292–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amin MB, Greene FL, Edge SB, et al. AJCC Cancer Staging Manual. 8th ed. New York, NY: Springer; 2016. [Google Scholar]
- 17.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97:934–959. [DOI] [PubMed] [Google Scholar]
- 18.Caulfield H, Hyman NH. Anastomotic leak after low anterior resection: a spectrum of clinical entities. JAMA Surg. 2013;148:177–182. [DOI] [PubMed] [Google Scholar]
- 19.Phillips BR, Harris LJ, Maxwell PJ, Isenberg GA, Goldstein SD. Anastomotic leak rate after low anterior resection for rectal cancer after chemoradiation therapy. Am Surg. 2010;76:869–871. [PubMed] [Google Scholar]
- 20.Bryant CL, Lunniss PJ, Knowles CH, Thaha MA, Chan CL. Anterior resection syndrome. Lancet Oncol. 2012;13:e403–e408. [DOI] [PubMed] [Google Scholar]
- 21.Pucciani F. A review on functional results of sphincter-saving surgery for rectal cancer: the anterior resection syndrome. Updates Surg. 2013;65:257–263. [DOI] [PubMed] [Google Scholar]
- 22.Emmertsen KJ, Laurberg S. Bowel dysfunction after treatment for rectal cancer. Acta Oncol. 2008;47:994–1003. [DOI] [PubMed] [Google Scholar]
- 23.Soga J. Carcinoids of the rectum: an evaluation of 1271 reported cases. Surg Today. 1997;27:112–119. [DOI] [PubMed] [Google Scholar]
- 24.Tsukamoto S, Fujita S, Yamaguchi T, et al. Clinicopathological characteristics and prognosis of rectal well-differentiated neuroendocrine tumors. Int J Colorectal Dis. 2008;23:1109–1113. [DOI] [PubMed] [Google Scholar]
- 25.Konishi T, Watanabe T, Nagawa H, et al. Treatment of colorectal carcinoids: a new paradigm. World J Gastrointest Surg. 2010;2:153–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nam SJ, Kim BC, Chang HJ, Jeon HH, Kim J, Kim SY. Risk factors for lymph node metastasis and oncologic outcomes in small rectal neuroendocrine tumors with lymphovascular invasion. Gut Liver. 2022;16:228–235. [DOI] [PMC free article] [PubMed] [Google Scholar]