Abstract
Background:
Arm transplantation has been proposed as a valid therapeutic option for arm amputees. A bilateral arm transplantation including reconstruction of the left shoulder was performed on January 13, 2021 in Lyon (France).
Methods:
The recipient was a 48-year-old man with bilateral amputation at proximal arm level on both sides following an electric shock in 1998. He had received a liver transplant in 2002. The donor was a 35-year-old man. On the right side, the donor humerus was fixed on the remaining 9-cm-long proximal stump, and was reinforced with the donor fibula in an intramedullary fashion. On the left side, the whole donor humerus (including the humeral head) was transplanted with reconstruction of the gleno-humeral joint, including a suspension ligamentoplasty. The immunosuppressive protocol was based on antithymocyte globulins as induction therapy, and tacrolimus, steroids and mycophenolate mofetil as maintenance therapy.
Results:
Good bone healing and a well-positioned ligamentoplasty on the left side were achieved. At 2 years, the recipient was able to flex both elbows, and wrist extension, finger flexion, and extension were appreciated on both sides. Intrinsic muscle activity was detectable by electromyography during the eighth posttransplant month, and sensitivity was recovered. The patient is satisfied with his autonomy in some daily activities, but his greatest satisfaction is the recovery of his body image.
Conclusions:
These results confirm that it is possible to propose this transplantation to proximal-level arm amputees. The patients’ information about risks and limits as well as their compliance and determination remain important prerequisites.
Takeaways
Question: Arm transplantation at a high level is a challenge. Outcomes in proximal bilateral arm allotransplantation with reconstruction of the shoulder are reported.
Findings: The functional results were encouraging. Wrist extension and elbow flexion and extension were complete and possible also against resistance on both sides. Partial sensitivity recovery was achieved. Although the patient’s capacity to eat alone and to take care of his personal hygiene dramatically improved, the most important results are his satisfaction and increase of self-esteem.
Meaning: Bilateral arm transplantation may be proposed to proximal-level arm amputees.
INTRODUCTION
Since the first case in 1998,1 hand transplantation has proven able to restore form and function, achieving good success rates with functional recovery, body image restoration, and social acceptance. An arm loss causes severe disability and compromise of body image. For many years, arm allotransplantation was considered to entail many challenges, including quality of nerve regeneration, hand function, and the high immunogenicity of the transplant due to the mass of transplanted tissues.2
The first bilateral arm transplantation was performed in July 2008 with encouraging results; it was followed by another case of arm and forearm allotransplantation in November 2008.3 To our knowledge, until now, 17 patients with bilateral or unilateral arm amputation at different levels have received arm allografts worldwide.4–7
A bilateral arm transplantation, including reconstruction of the left shoulder, was performed on January 13, 2021 in Lyon, France, 21 years after the first bilateral hand transplantation.8 We report here the 2-year outcomes of this patient.
MATERIALS AND METHODS
The patient was a 48-year-old man who had sustained a high-voltage electrical injury (a working accident) on January 12, 1998. The upper extremities were burned, then several infections occurred, necessitating several surgical procedures and amputations, which finally ended in amputation of both arms at a proximal level. (See figure, Supplemental Digital Content 1, which displays patient picture before transplantation. http://links.lww.com/PRSGO/D269.) In addition, the patient sustained fracture of the cervical and thoracic spine (which required osteosynthesis at the thoracic level), fracture of the right clavicle (which evolved into a nonunion) and lesions of the left pectoralis major and latissimus dorsi. Subsequently, the patient developed alcoholic cirrhosis and received liver transplantation in July 2002, followed by portal thrombosis requiring hepatic re-transplantation in August 2002.
Patient Assessment before the Transplantation
The immunosuppressive therapy at the moment of the bilateral arm transplantation included tacrolimus and mycophenolate mofetil (2 mg/d and 2 gr/d, respectively). The patient had a normal renal function (creatinine was 54 µM with a clearance >90 mL/min/1.73 m²) and euglycemia. He underwent a hepatic biopsy on January 7, 2021, which showed minimal signs of rejection, concurrent with a slight increase of hepatic enzymes. He used upper extremity mechanic prostheses with poor satisfaction. His DASH and independence scores were 66 and 93 out of 126, respectively.
The recipient met the medical team for the first time 14 years before the arm transplantation, and during this period he underwent several interviews. He showed neither signs of posttraumatic stress disorder nor symptoms of anxiety or depression during this long period. After adequate informed consent, he entered the waiting list, where he remained for 5 years.
This procedure was financed by a national grant (Programme Hospitalier de Recherche Clinique) and approved by the Comité Consultatif de Protection des Personnes Participant à la Recherche Biomédicale (CCPPRB).
Transplantation
The donor was a 35-year-old man, who died of anoxia. He had the same blood group as the recipient (donor: A-, recipient: A+). There were five HLA mismatches, but the crossmatch was negative.
Both donor and recipient were positive for CMV and EBV and negative for HBV, HCV, HIV, and SARS-CoV-2. The size of the limbs and the skin complexion of the donor were similar to those of the patient.
The recovery of the donor arms was performed simultaneously with that of the left fibula and both peroneal tendons. All structures were identified, isolated, and tagged during the recovery procedure. The grafts were perfused and preserved in IGL-1 solution. The procedure time was 4 hours on the right side and 4 hours 32 minutes on the left side.
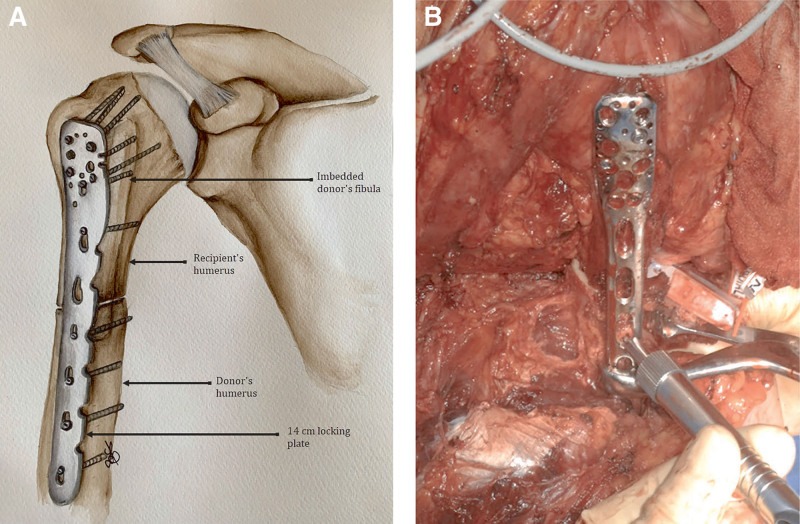
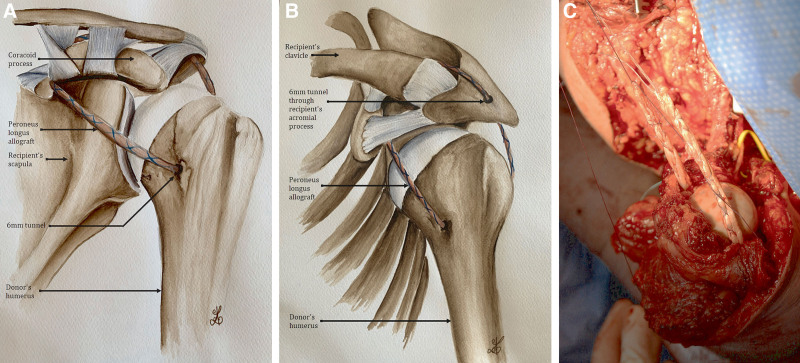
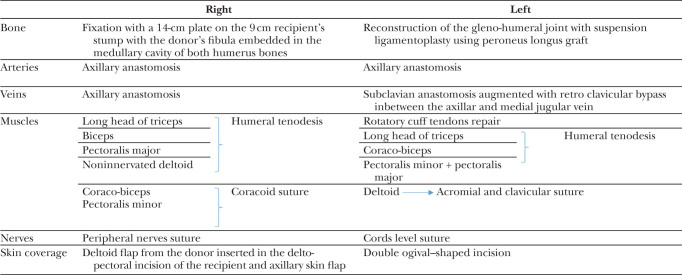
Simultaneously in the recipient, all the structures were identified, isolated, and tagged on both stumps. On the right side, the donor humerus was fixed on the remaining 9-cm-long proximal stump, and was reinforced with the donor fibula in an intramedullary fashion as bone graft to improve consolidation and increase stability (Fig. 1). On the left side, the whole donor humerus (including the humeral head) was transplanted, with reconstruction of the gleno-humeral joint, including a suspension ligamentoplasty using a 5-mm-thick peroneus longus graft slipped into the humeral head in a 6-mm tunnel. The graft runs through the rotator interval, bridges the coracoid process, and reaches the upper surface of the acromion where a second 6-mm tunnel was made. Repair of rotator cuff ligaments was performed at the level of the gleno-humeral joint (Fig. 2). On the right side, vascular anastomoses were performed between the donor and recipient axillary arteries and veins. On the left side, the arterial anastomoses were performed between the donor and recipient subclavian arteries, and the venous anastomoses between the donor axillary vein and the recipient subclavian vein; moreover, a venous bypass was performed using the donor iliac vein, which was anastomosed between the donor axillary vein and the recipient’s jugular vein to improve veinous drainage. Vascular anastomoses were performed by overedge Prolene 6.0 stitches under surgical loupes. Heparin was injected intraoperatively before performing the sutures.
Fig. 1.
Osteosynthesis of the right humerus. A, Anterior view. B, Intraoperative view.
Fig. 2.
Suspensive ligamentoplasty of the left shoulder. A, Anterior view of the reconstruction. B, Supero-lateral view. C, Intraoperative view.
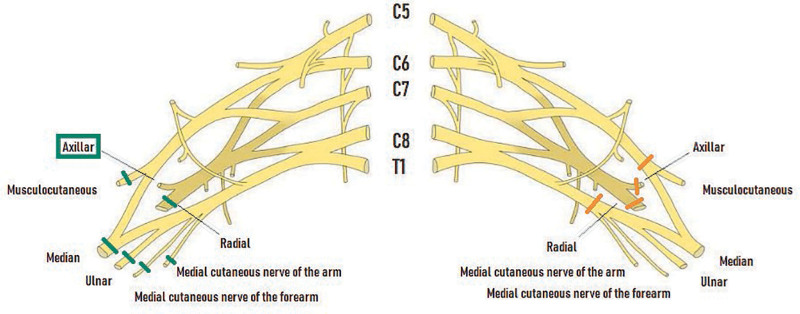
Nervous repair was performed at the origin of the radial, median, ulnar, musculocutaneous and medial cutaneous nerves of the arm and the forearm on the right side. The recipient’s axillary nerve was preserved. On the left side, sutures were performed at the level of the anteromedial and anterolateral cords and axillary and radial nerves. Sutures were performed by separate Ethilon 9.0 stitches under a microscope (Fig. 3).
Fig. 3.
Nerve sutures. On the right side: the recipient’s axillary nerve was preserved.
On the right side, recipient humeral tenodesis of pectoralis major, long head triceps, biceps, and deltoid was performed. The coraco-brachial and pectoralis minor were sutured on the coracoid process. On the left side, humeral tenodesis of the coraco-biceps and triceps was performed. The recipient deltoid was fixed to the acromion and clavicula. Myorraphy of recipient major and minor pectoralis muscles to the donor’s pectoralis major was performed. The donor deltoid was reported posteriorly with its innervation (Table 1).
Table 1.
Summary of the Surgical Procedure
Cold ischemia time was 46 minutes on the right side and 47 minutes on the left side. Anastomosis time was 55 minutes on the right side and 1 hour and 26 minutes on the left side. The transplantation procedure lasted 9 hours and 22 minutes on the right side and 12 hours and 58 minutes on the left side.
To avoid metabolic complications, a continuous veno-venous hemodialysis session was initiated immediately after anesthesia induction. The dialysis catheter was inserted in the right femoral vein, and an arterial catheter and a central venous line were placed at the left femoral site.
The patient remained 6 days in the intensive care unit. No metabolic complications developed during this period. The patient was weaned from mechanical ventilation and vasopressor support after 48 hours.
The immunosuppressive protocol included an induction therapy based on antithymocyte globulins (1 mg/kg/d) for 6 days and a maintenance therapy based on tacrolimus (trough blood levels between 8 and 10 ng/mL), steroids (5 mg/d at 6 posttransplant months) and mycophenolate mofetil (MMF; 1.5 g/d).
Rehabilitation therapy started on postoperative day 1; it progressively included manual lymphatic drainage and passive motion of all joints in a total range (except for the shoulders) during the first 6 weeks. Pressure therapy was applied during the first 6 months to reduce edema of the grafted arms. Electrostimulation on denervated muscles started 7 weeks after the transplantation.
After 6 weeks, the rehabilitation protocol included physiotherapy, electrostimulation, occupational therapy, physical activities, and psychomotricity. The sensorimotor recovery was stimulated by several simulation tools, such as motor imagery, virtual mirror therapy, and virtual reality.
The patient worked 5 hours per day for 5 days per week during the first 12 months after the transplantation, and thereafter, 4 days per week. Electromyography (EMG) of the grafted upper extremities was performed 6, 12, 18, and 24 months after the transplantation.
RESULTS
After the transplantation, the patient was installed in a special bed with customized arm elevation cushions. Both arms were in a splint to support them and to limit graft movements.
He quickly recovered mobilization of the extrinsic musculature of the right shoulder, but the healed tendons permitted passive mobilization only 8 weeks after the transplantation. At 3 months, bony consolidation allowed active mobilization under professional guidance to develop mobility and strengthening. At 6 months, motor recovery started at the proximal level in the deltoid, biceps, and triceps muscles bilaterally. At 2 years, good bone healing on the right side and a well-positioned ligamentoplasty on the left side were seen. A slight ptosis of the right humeral head was evidenced. The patient also reported pain and sensation of instability at the level of the right shoulder, partly due to the previous clavicle nonunion.
Functional Recovery
Two years after the transplantation, passive ranges of motion of upper limbs were considered normal (no functional limitations). Wrist extension and elbow flexion and extension were complete and possible also against resistance on both sides (Table 2). Movements of the extrinsic flexors and extensor muscles of the fingers have been evidenced on both sides, and are improving, particularly on the right side, where finger flexion against gravity is possible (Tables 3 and 4). Recovery of muscular strength at the shoulder, arm and forearm level on both sides (particularly on the right one) was noted (Table 5). [See Video 1 (online), which displays the patient working to improve his functional recovery.]
Table 2.
Functional Motion Range of Shoulder, Elbow, and Wrist (Degrees) at 24 Posttransplant Months
| Joint | Motion | Passive (Degrees) | Active (Degrees) | ||
|---|---|---|---|---|---|
| Right | Left | Right | Left | ||
| Shoulder | Flexion Global Gleno-humeral |
145 95 |
145 100 |
145 95 |
135 NA |
| Extension | 75 | 70 | 40 | 25 | |
| Abduction Frontal plane Scapular plane |
100 130 |
100 100 |
90 130 |
65 95 |
|
| Adduction | 65 | 40 | 50 | 0 | |
| Internal rotation | 110 | 130 | 90 | 110 | |
| External rotation (RE1) | 45 | 55 | 45 | -10 | |
| Elbow | Flexion | 140 | 140 | 140 | 140 |
| Extension | 0 | 0 | -10 | 0 | |
| Pronation | 70 | 45 | 70 | 40 | |
| Supination | 90 | 90 | 70 | 20 | |
| Wrist | Flexion | 85 | 80 | 45 | 40 |
| Extension | 80 | 85 | 65 | 50 | |
| Radial deviation | 10 | 15 | 0 | 0 | |
| Ulnar deviation | 25 | 30 | 0 | 0 | |
NA, not available; RE1, external rotation elbow to body.
Table 3.
Passive Range of Motion of Hands (Degrees) at 24 Posttransplant Months
| Right Hand | I | II | III | IV | V | Trapezo-metacarpal Joint | |
|---|---|---|---|---|---|---|---|
| MCP (degrees) | Flex | 65 | 90 | 95 | 95 | 95 | Abduction: 40 Adduction: 25 |
| Ext | 0 | 35 | 30 | 30 | 40 | ||
| PIP (degrees) | Flex | 90 | 130 | 135 | 140 | 140 | Antepulsion: 20 |
| Ext | 0 | 0 | 0 | 0 | 0 | ||
| DIP (degrees) | Flex | 45 | 45 | 45 | 45 | Retropulsion: 30 | |
| Ext | 5 | 0 | 0 | 0 | |||
| Passive Kapandji index: 0 1 2 3 4 5 6 7 8 9 10 | |||||||
| Left Hand | I | II | III | IV | V | Trapezo-metacarpal joint | |
| MCP (degrees) | Flex | 60 | 95 | 110 | 110 | 100 | Abduction: 45 Adduction: 20 |
| Ext | 15 | 40 | 60 | 60 | 60 | ||
| PIP (degrees) | Flex | 90 | 120 | 120 | 120 | 120 | Antepulsion: 45 |
| Ext | 0 | 0 | 0 | 0 | 0 | ||
| DIP (degrees) | Flex | 80 | 80 | 80 | 90 | Retropulsion: 30 | |
| Ext | 0 | 0 | 0 | 0 | |||
| Passive Kapandji index : 0 1 2 3 4 5 6 7 8 9 10 | |||||||
DIP, distal interphalangeal joint; MCP, metacarpophalangeal joint; PIP, proximal interphalangeal joint.
Table 4.
Active Range of Motion of Hands (Degrees) at 24 Posttransplant Months
| Right Hand | I | II | III | IV | V | Trapezo-metacarpal Joint | |
|---|---|---|---|---|---|---|---|
| MCP (degrees) | Flex | 10 | 70 | 90 | 90 | 90 | Abduction 0 Adduction − 20 |
| Ext | 10 | 20 | 20 | 30 | 30 | ||
| PIP (degrees) | Flex | 45 | 130 | 135 | 140 | 140 | |
| Ext | 0 | −15 | −10 | −20 | −30 | Antepulsion 0 Retropulsion 10 |
|
| DIP (degrees) | Flex | 45 | 45 | 45 | 45 | ||
| Ext | −10 | −20 | 20 | 10 | |||
| PDPCD (cm) | 1.5 | 0 | 0 | 0.5 | |||
| Kapandji Index | 0 1 2 3 4 5 6 7 8 9 10 | ||||||
| Left Hand | I | II | III | IV | V | Trapezo-metacarpal Joint | |
| MCP (degrees) | Flex | 40 | 30 | 30 | 30 | 10 | Abduction: 0 |
| Ext | −15 | 25 | 25 | 35 | 25 | ||
| PIP (degrees) | Flex | 80 | 90 | 90 | 90 | 90 | |
| Ext | 0 | −15 | −15 | −15 | −15 | Antepulsion 10 Retropulsion 10 |
|
| DIP (degrees) | Flex | 45 | 45 | 45 | 45 | ||
| Ext | 0 | 0 | 0 | 0 | |||
| PDPCD (cm) | 3 | 3.5 | 2.5 | 2 | |||
| Kapanji Index | 0 1 2 3 4 5 6 7 8 9 10 | ||||||
The patient has almost a full flexion of long fingers on the right side as shown by the pulp-to-palm distance.
Flex, flexion; Ext, extension; DIP, distal interphalangeal joint; MCP, metacarpophalangeal joint; PIP, proximal interphalangeal joint; PDPCD, pulp to distal palmar crease distance.
Table 5.
Muscular Strength (MRC Grading) at Shoulder, Arm and Forearm Level at 24 Posttransplant Months
| Muscles | Right | Left | |
|---|---|---|---|
| Forearm | Extensor carpi radialis longus and brevis | 4 | 4 |
| Extensor carpi ulnaris | 3 | 3 | |
| Flexor carpi radialis and palmaris longus | 4 | 1 | |
| Flexor carpi ulnaris | 4 | 1 | |
| Pronator teres and pronator quadratus | 4 | 1 | |
| Supinator | 3 | 1 | |
| Abductor pollicis longus | 0 | 0 | |
| Extensor digitorum communis | 3 | 3 | |
| Extensor pollicis brevis | 0 | 0 | |
| Extensor pollicis longus | 3 | 1 | |
| Flexor digitorum profundus | 4 | 2 | |
| Flexor digitorum superficialis | 4 | 1 | |
| Flexor pollicis longus | 3 | 2 | |
| Muscles | Right | Left | |
| Shoulder | Deltoideus anterior | 4- | 2 |
| Deltoideus medius | 3 | 2 | |
| Deltoideus posterior | 3 | 3- | |
| Coracobrachialis | 4- | 2 | |
| Infraspinatus and Teres minor | 4 | 2 | |
| Latissimus dorsi | 3 | * | |
| Teres major | 4 | 2 | |
| Supraspinatus | 4 | 2 | |
| Subscapularis | 4 | 4 | |
| Pectoralis major | 4 | 1 | |
| Arm | Biceps brachii | 4 | 4 |
| Brachialis | 4 | 4 | |
| Brachioradialis | 4 | 4 | |
| Triceps brachii | 2- | 4 | |
Extrinsic muscles of the hands are recovering (particularly on the right side) while contraction of the intrinsic muscles of both hands was not detected.
The patient has no latissimus dorsi on the left side.
Video 1. The patient still works to improve his functional recovery.
Intrinsic muscle activity was detectable by EMG during the 24th posttransplant month.
Sensitivity recovery was detected 8 months after the transplantation. Protective sensibility for pain was recovered on both upper limbs at 24 months, whereas thermal sensibility was recovered only on the anterolateral side of the left upper limb. At 2 posttransplant years, the Semmes-Weinstein monofilament test for sensory threshold was 4.56 and 6.65 on the right and left palm, respectively. Deep pressure sensation reappeared in both hands at 1 year after the transplantation, whereas discriminative sensibility reappeared only on D1 and D2 of the left side (15 mm) at 24 months after the transplantation.
The patient is now able to perform pinch and power grip bilaterally, and on the left upper extremity he can grasp 10 kg. Two years after the transplantation, he can perform the daily activities, which were possible with his prostheses before the transplantation. The DASH score9 is 50.8, and the IRHCTT score10 is 63 (good) on the right side and 56.5 (fair) on the left side. The patient’s capacity to eat alone and to take care of his personal hygiene without help dramatically improved, and he is satisfied of the acquired ability to perform these daily activities as shown in the Canadian Occupational Performance Measure11 (Table 6). [See Video 2 (online), which shows that the patient is able to perform some daily activities which were impossible with the prostheses before the transplantation.] [See Video 3 (online), which shows that the patient’s capacity to take care of his personal hygiene without help improved.)
Table 6.
Canadian Occupational Performance Measure
| Activities | Importance | Performance Before |
Performance After |
Satisfaction Before |
Satisfaction After |
|---|---|---|---|---|---|
| Using a toilet | 10 | 5 | 7 | 2 | 7 |
| Eating alone | 8 | 4 | 5 | 2 | 6 |
| Personal hygiene | 8 | 1 | 7 | 1 | 7 |
| Meal preparation | 6 | 2 | 4 | 1 | 5 |
| Taking care of his granddaughter | 8 | 1 | 10 | 1 | 10 |
Performance and satisfaction evaluated by the patient (before and after the transplantation) considering the most important activities for him. Twenty-four months after transplantation was the considered follow-up point.
Video 2. The patient is able to perform some daily activities which were impossible with the prostheses before the transplantation.
Video 3. The patient’s capacity to take care of his personal hygiene without help improved.
With yet some difficulties, he can use a mobile phone and a credit card, take care of a pet, and drive a car, but he is unable to prepare a meal. His score of independence (Table 7) is 109 out of 126.
Table 7.
Functional Independence Measure
| Activity | Score |
|---|---|
| Eating | 2 |
| Grooming | 4 |
| Bathing | 6 |
| Dressing (upper body) | 4 |
| Dressing (lower body) | 3 |
| WC use | 6 |
| Bladder management | 7 |
| Bowel management | 7 |
| Bed, chair, wheelchair | 7 |
| Toilet | 7 |
| Tub, shower | 7 |
| Walk, wheelchair | 7 |
| Stairs | 7 |
| Comprehension | 7 |
| Expression | 7 |
| Social interaction | 7 |
| Problem solving | 7 |
| Memory | 7 |
| Total score | 109/126 |
7: Complete independence (timely, safely); 6: Modified independence (device); 5: Supervision; 4: Minimal assist; 3: Moderate assist; 2: Maximal assist; 1: Total assist.
Psychological Issues
The patient was psychologically tested during the follow-up using the Montgomery-Åsberg Depression Rating Scale, Hamilton and Rosenberg tests12 (Table 8). During the early posttransplant period the patient was very tired, experienced pain and insomnia, and developed slight depression, which was easily reversed with amitriptyline (30 mg/d). During the first 6 months, the patient experienced all the difficulties associated with the transplantation, the complete dependence on the nursing staff, and the hard rehabilitation program. Thereafter, he started to appreciate his “new” image and to use his grafted upper extremities; particularly, he was very glad to explore the “surrounding world” by touching it. At 1 posttransplant year, he had no signs of depression and was satisfied with his body image with strong self-esteem (Table 8). At 2 posttransplant years, the patient developed very slight depression, due to the heavy rehabilitation program and the pain at the level of the right shoulder; however, he is still satisfied with his body image and has strong self-esteem (Table 8). Body image scores (BIQ20)13 were 21 out of 40 on Rejecting Body Image and 37 out of 60 on Body Vital Dynamics14 at 2 years. The impact of his image on daily life was assessed by the Body Image Quality of Life Inventory.15 The score was -8 before transplantation and +40 two years after the transplantation.
Table 8.
Results of the Psychological Tests at 3, 6, 12, and 24 Months
| M3 | M6 | M12 | M24 | |
|---|---|---|---|---|
| MADRS | 20/60 | 24/60 | 10/60 | 11/60 |
| Hamilton test | 13/50 | 18/50 | 5/50 | 12/50 |
| Rosenberg test | 21/30 | 23/30 | 20/30 | 19/30 |
Montgomery-Åsberg Depression Rating Scale and Hamilton rating scale were used to evaluate depression severity; Rosenberg self-esteem scale is a self-esteem measure.
Clinical Course and Complications
The hepatic function was carefully monitored. A biopsy was performed 10 days after the bilateral arm transplantation because of increased values of hepatic enzymes secondary to his treatment drugs (namely Bactrim), which normalized after its withdrawal. No signs of hepatic rejection were observed. The hepatic function remained stable during the follow-up. The renal function was also monitored. His serum creatinine values were 72 µM (eGFR> 90 mL/min/1.73 m²), 92 µM (eGFR 86 mL/min/1.73 m²), and 98 µM (eGFR 77 mL/min/1.73 m²) at 3, 12, and 24 months, respectively.
On the second postoperative day, partial thrombosis of the venous bypass performed on the left side occurred. Anticoagulant treatment was started, causing a hematoma in the left pectoralis, which was successfully treated with percutaneous drainage.
A specific antibiotic therapy (amoxicilline) was given against Propionibacterium acnes, which was detected in the biopsies performed in the recipient’s bones during the transplantation. In the early postoperative period, the patient experienced neuropathic pain of the grafted upper extremities.
Fungal folliculitis developed during the first postoperative period on both proximal arms and was successfully treated with local applications of ketoconazole gel and oral itraconazole treatment. At 13 months posttransplantation, the patient also developed also two molluscum contagiosum lesions on the cheek, which were treated with excisional biopsy and cryotherapy. During the early posttransplant period, the patient developed two acute rejection episodes diagnosed on days 26 and 87, respectively. They manifested clinically with erythematous skin macules on both arms. Histologically, the Banff rejection grades16 were II and I, respectively. The lesions completely regressed after IV steroids and topical treatment with clobetasol cream and tacrolimus ointment. No donor-specific antibodies have been detected so far.
DISCUSSION
Functional recovery in upper extremity transplantation is considered better and faster in recipients with a distal level of amputation; consequently, a bilateral arm transplantation at a very high level on both sides, requiring also the reconstruction of the left shoulder, was considered a considerable challenge. Initially, the patient was not considered an ideal candidate because of the uncertain functional result, rendering the risk/benefit balance unfavorable. Finally, after careful evaluation and exhaustive information of the candidate, he was accepted, firstly because he was already immunosuppressed (he had undergone liver transplantation in 2002) and compliant to the treatment, and because his expectations of the transplantation were realistic, body image restoration being the main one. He was not deterred by the probable limitations in functional recovery. Interestingly, in upper extremity transplantation, the aesthetic aspect of the grafted extremities and the recovered body image play an important role in patients’ satisfaction.
The initial posttransplant period was difficult because of the neuropathic pain, the complete dependence on the nursing staff, and the hard rehabilitation program. Thereafter, the patient was very satisfied with his “new and complete” body image and adhered to the rehabilitation program. Only two episodes of mild acute rejection occurred, which were easily reversed. The hepatic graft function was not influenced by the new transplantation, although a slight decrease in renal function occurred. Several uni- and bilateral arm transplantations have been performed worldwide, but reconstruction of the gleno-humeral joint has been performed only in one other case.5 In our patient, reconstruction of the left shoulder was realized using a suspension ligamentoplasty and repair of rotator cuff ligaments; the donor deltoid was reported posteriorly and separately reinnervated. During the follow-up, different imaging studies showed a perfectly-positioned gleno-humeral joint. The ischemia times were short, reducing significantly the ischemia/reperfusion injury, particularly on the musculature. In the other case,5 suture of the capsuloligamentous structures and rotator cuff and reinnervated deltoid transplantation on the right side associated with trans-humeral transplantation on the left side were performed. The authors reported, at 18 months, ptosis of the right humeral head with focal damage of the posterior labrum and good consolidation of the left humerus.5 The functional recovery was encouraging also in that case, and the functional results were similar to those achieved in our patient.
Our patient has recovered a functional active range of motion. Since the 18th posttransplantation month, he has been able to flex both elbows. Normal flexion and extension of the elbow were considered the goal of motor recovery in arm transplantation when this procedure was initially performed. Wrist extension was appreciated on both sides (particularly on the right one) as well as finger motion. At 2 posttransplant years, recovery of hand intrinsic muscles was not detected clinically, but was evidenced by EMG. Sensitivity recovery is slowly improving, and at the 24th posttransplant month, partial recovery of the protective sensibility on both arms, and discriminative sensibility in some fingers was evidenced. The functional results are encouraging, although the most important results are the patient’s satisfaction and increase of self-esteem; indeed, although the patient is satisfied with his autonomy in some daily activities, which are important for him, his greatest satisfaction is restoration of his body image.
Although significant technical progress has been achieved with myoelectric prostheses and targeted reinnervation without any risk, compliance to these devices remains poor,17 and human assistance is still required to set them up. The great difference between upper extremity transplantation and prostheses are the recovery of sensibility, and overall, the restitution of a complete body image.18
In conclusion, two years after transplantation, the functional results are unexpectedly encouraging. The patient has no complete autonomy yet, but he can spend several days alone, something that was impossible before the transplantation. He is now able to perform those daily activities which he considers essential, and is satisfied with his new body image and his abilities.
Our immunological results in a liver-grafted patient are similar to those reported in the other arm transplantations, suggesting that it is possible to propose this transplantation to proximal-level arm amputees not yet on immunosuppression for other reasons. The patients’ information about risks and limits, as well as their compliance and determination, remain important prerequisites.
DISCLOSURE
The authors have no financial interest to declare in relation to the content of this article.
ACKNOWLEDGMENTS
We wish to thank Franck Plotard, Thomas Rimmelle, Marion Charvin, all the members of the rehabilitation team, and Eléonore Daux and Christian Seulin for their precious help, and Mrs. Céline Dagot for her coordination work.
Supplementary Material
Footnotes
Published online 10 June 2024.
Presented at the ISVCA meeting, June 4–6, 2022, Cancun, Mexico, and at the TTS meeting, September 10–14, 2022, Buenos Aires, Argentina.
Disclosure statements are at the end of this article, following the correspondence information.
Related Digital Media are available in the full-text version of the article on www.PRSGlobalOpen.com.
REFERENCES
- 1.Dubernard JM, Owen ER, Herzberg G, et al. Human hand allograft: report on first 6 months. Lancet. 1999;353:1315–1320. [DOI] [PubMed] [Google Scholar]
- 2.Jones NF, Schneeberger S. Arm transplantation: prospects and visions. Transplant Proc. 2009;41:476–480. [DOI] [PubMed] [Google Scholar]
- 3.Landin L, Cavadas PC, Nthumba P, et al. Preliminary results of bilateral arm transplantation. Transplantation. 2009;88:749–751. [DOI] [PubMed] [Google Scholar]
- 4.Cavadas PC, Ibáñez J, Thione A, et al. Bilateral trans-humeral arm transplantation: result at 2 years. Am J Transplant. 2011;11:1085–1090. [DOI] [PubMed] [Google Scholar]
- 5.Iglesias M, Ramírez-Berumen M, Butrón P, et al. Functional outcomes 18 months after total and midarm transplantation: a case report. Transplant Proc. 2018;50:950–958. [DOI] [PubMed] [Google Scholar]
- 6.Jablecki J, Kaczmarzyk L, Domanasiewicz A, et al. Result of arm-level upper-limb transplantation in two recipients at 19- and 30-month follow-up. Ann Transplant. 2012;17:126–132. [DOI] [PubMed] [Google Scholar]
- 7.International Registry on Hand and Composite Tissue Transplantation. Homepage. Available at https://handregistry.com/. Accessed June 2, 2023. [Google Scholar]
- 8.Dubernard JM, Petruzzo P, Lanzetta M, et al. Functional results of the first human double-hand transplantation. Ann Surg. 2003;238:128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gummesson C, Atroshi I, Ekdahl C. The disabilities of the arm, shoulder and hand (DASH) outcome questionnaire: longitudinal construct validity and measuring self-rated health change after surgery. BMC Musculoskelet Disord. 2003;4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lanzetta M, Petruzzo P. A comprehensive functional score system in hand transplantation. In: Lanzetta M, Dubernard JM, eds. Hand Transplantation. Milan, Italy: Springer-Verlag; 2007:355–362. [Google Scholar]
- 11.Law M, Baptiste S, McColl M, et al. The Canadian occupational performance measure: an outcome measure for occupational therapy. Can J Occup Ther. 1990;57:82–87. [DOI] [PubMed] [Google Scholar]
- 12.Ottenbacher KJ, Hsu Y, Granger CV, et al. The reliability of the functional independence measure: a quantitative review. Arch Phys Med Rehabil. 1996;77:1226–1232. [DOI] [PubMed] [Google Scholar]
- 13.Davidson J, Turnbull CD, Strickland R, et al. The Montgomery-Åsberg depression scale: reliability and validity. Acta Psychiatr Scand. 1986;73:544–548. [DOI] [PubMed] [Google Scholar]
- 14.Clement U, Löwe B. Validation of the FKB-20 as scale for the detection of body image distortions in psychosomatic patients. Psychother Psychosom Med Psychol. 1996;46:254–259. [PubMed] [Google Scholar]
- 15.Cash TF, Fleming EC. The impact of body image experiences: development of the body image quality of life inventory. Int J Eat Disord. 2002;31:455–460. [DOI] [PubMed] [Google Scholar]
- 16.Cendales LC, Kanitakis J, Schneeberger S, et al. The Banff 2007 working classification of skin-containing composite tissue allograft pathology. Am J Transplant. 2008;8:1396–1400. [DOI] [PubMed] [Google Scholar]
- 17.Salminger S, Sturma A, Roche AD, et al. Functional and psychosocial outcomes of hand transplantation compared with prosthetic fitting in below-elbow amputees: a multicenter cohort study. PLoS One. 2016;11:e0162507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Agnew SP, Ko J, De La Garza M, et al. Limb transplantation and targeted reinnervation: a practical comparison. J Reconstr Microsurg. 2012;28:63–68. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.