Abstract
VP1 is the major viral coat protein of murine polyomavirus and can be used for the generation of virus-like particles in vitro. Here, we demonstrate that capsid assembly is an equilibrium reaction followed by oxidation of intracapsomere disulfide bonds, which are not essential for the formation of virus-like particles but enable complete particle assembly and prevent capsid dissembly.
Murine polyomavirus is a nonenveloped, double-stranded DNA virus with a circular genome 5.3 kb in size. The icosahedral polyomavirus shell has a diameter of approximately 45 nm and consists of 72 pentameric VP1 proteins and the minor core proteins VP2 and VP3 (9). Virion disruption experiments indicated the importance of disulfide bonds and bound calcium in capsid stability for polyomavirus shells (1, 2, 8). In vitro assembly of polyomavirus VP1 into virus-like particles is induced by adding CaCl2 and inhibited by EDTA (15, 16). Capsomere contacts within the virus shell are accomplished by VP1's flexible C-terminal arm, which protrudes into a neighboring pentamer (10, 20). Although intrapentamer disulfide bonds are observed in the crystal structure between residues C19 and C114′ of neighboring subunits (18, 20), it is not clear whether they are sufficient or essential for capsid stabilization, or if there is also involvement of intercapsomere disulfide bonds which are not resolved in the crystal structure.
MATERIALS AND METHODS
Cloning and vector construction.
The mouse polyomavirus VP1 gene was amplified by PCR, changing the two C-terminal residues of VP1 from Gly383-Asn384 to Pro383-Gly384, since Asn at the position preceding the intein sequence inhibits in vitro splicing (6). Sequences of the oligonucleotides used were 5′-TAT ACA TAT GGC CCC CAA AAG AAA AAG C-3′ and 5′-ATA TCC CGG GAG GAA ATA CAG TCT TTG TTT TTC C-3′. The PCR product was cloned into plasmid pCYB2 (New England Biolabs), which contains the genes for the intein and the chitin-binding domain (CBD). The fusion protein VP1-intein-CBD was amplified in a second PCR and cloned into plasmid pET21a (Novagen). Sequences of the oligonucleotides used were 5′-TAT ACA TAT GGC CCC CAA AAG AAA AAG C-3′ and 5′-ATA TGA ATT CCA GTC ATT GAA GCT GCC ACA AGG-3′.
Site-directed mutagenesis.
Mutants VP1-CallS (C11S, C15S, C19S, C114S, C273S, C282S), and VP1-2C (C11S, C15S, C273S, C282S) were generated by using a Quickchange site-directed mutagenesis kit (Stratagene) according to the standard protocol. Oligonucleotide sequences were as follows: C11S, C15S, and C19S, 5′-GTC TCT AAA AGC GAG ACA AAA AGC ACA AAG GCT AGC CCA AGA CCC-3′; C114S, 5′-GAG GAC CTC ACG TCT GAC ACC CTA C-3′; C273S and C282S, 5′-GGG CCC CTC AGC AAA GGA GAA GGT CTA TAC CTC TCG AGC GTA GAT ATA ATG-3′; S19C, 5′-GCA CAA AGG CTT GTC CAA GAC CCG C-3′.
Protein expression and purification.
VP1 production was carried out in 5-liter flasks containing 2 liters of Luria-Bertani medium. At an optical density at 600 nm of >2.0, expression of the fusion protein was induced by addition of 1 mM isopropyl-β-d-thiogalactopyranoside. The cultures were further incubated at 15°C for 20 h. Purification of the recombinant protein was performed by chitin affinity chromatography as specified by the manufacturer (New England Biolabs). Cleavage of the fusion protein was induced with a mixture of 30 mM hydroxylamine and 30 mM dithiothreitol (DTT) and was allowed to proceed for 14 h at 6°C.
Assembly of VP1 capsids.
Empty polyomavirus-like particles were generated from VP1 capsomeres by several rounds of dialysis, with some modifications to the originally described procedures (19, 20). After purification, the protein was precipitated with 40% (wt/vol) (NH4)2SO4 and resolubilized in a buffer containing 20 mM HEPES, 1 mM EDTA, 200 mM NaCl, 100 mM DTT, and 5% (wt/vol) glycerol (pH 7.2). Protein concentrations were adjusted to 0.4 to 0.5 mg/ml. For in vitro assembly, protein solutions were dialyzed for 3 days at room temperature against 10 mM HEPES–0.5 mM CaCl2–50 mM NaCl–5% (wt/vol) glycerol (pH 7.2), with renewal of the buffer every day.
Analytical size exclusion chromatography.
Twenty- to 50-μl samples with protein concentrations of between 0.4 and 0.5 mg/ml were applied to an analytical high-pressure liquid chromatography (HPLC) column (14-ml volume; TSKgel G5000PWXL; TosoHaas) with an appropriate buffer (10 mM HEPES, 0.5 mM CaCl2, 200 mM NaCl, 5% [wt/vol] glycerol [pH 7.2]) at a flow rate of 0.7 ml/min.
Electron microscopy.
For electron microscopy studies, an EM 912 instrument (Zeiss) was used with a magnification factor of 63,000. Staining of the specimen was performed with uranyl acetate on bacitracin-incubated (0.1 mg/ml, 1 min) copper-carbon grids according to standard protocols.
Fluorescence labeling.
VP1-2C pentamers were labeled with fluorescein, using a thiol-specific maleimide conjugate (Molecular Probes), as described earlier (17).
RESULTS
Recombinant expression of VP1 in Escherichia coli.
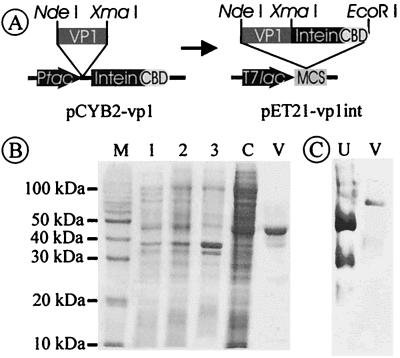
VP1 protein was produced in recombinant E. coli as an N-terminal fusion protein with a modified Saccharomyces cerevisiae VMA1 intein, which can be used for in vitro splicing (4, 7), and a Bacillus circulans CBD for affinity purification. This construct allows self-cleavage of the fusion protein without the need of a protease and therefore a single-step purification of the authentic protein (5). The VP1 gene was cloned into the vector pCYB2, for expression of the fusion protein from a tac promoter. To obtain sufficiently high expression levels, the VP1-intein fusion construct was cloned into the T7 expression vector pET21a (pET21-vp1int [Fig. 1A]). Protein yields depended significantly on the postinduction growth temperature of the cultures, increasing from 100 μg of purified VP1 per liter of culture medium at 30°C to 6 mg at 15°C. Probably, in vivo cleavage which is affected by the residues adjacent to the intein cleavage site of the fusion protein (6) is significantly increased at higher temperatures, thus resulting in lower yields. A single affinity purification step via the fused CBD was sufficient to produce VP1 in nearly homogeneous form, as determined by sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) (Fig. 1B) and native PAGE (Fig. 1C). The native molecular mass of VP1 was approximately 210 kDa, as determined by native PAGE, indicating correct folding and assembly of the pentameric capsomere.
FIG. 1.
Recombinant expression of VP1. (A) Construct pCYB-2-vp1 allows expression of a fusion protein consisting of (i) VP1, (ii) modified S. cerevisiae VMA1 intein, and (iii) B. circulans CBD. Since the recombinant protein had only low expression rates, the whole fusion protein was cloned into the multiple cloning site (MCS) of pET21a, an E. coli expression vector with a T7 lac promoter (T7lac). (B) SDS-PAGE of the purification procedure. It is difficult to detect the fusion protein (97 kDa) in whole cell and crude extract fractions due to a strong E. coli protein background in this region. After chitin affinity chromatography, VP1 elutes in one major and some minor bands which are due to in vivo proteolytic cleavage. Lanes: M, molecular mass marker; 1, cell lysate without induction; 2, cell lysate 20 h postinduction; 3, insoluble cell fraction; 4, crude cell extract; V, purified VP1 protein. (C) Native PAGE (6% tris-glycine-buffered separating gel) of purified VP1. After a single-step purification, VP1 shows a single band in native PAGE corresponding to its native molecular mass of approximately 210 kDa. Molecular masses of VP1-wt and VP1 mutants were determined by standard proteins. Here, only urease is represented (lane U; trimer = 272 kDa, hexamer = 545 kDa); analysis of VP1 (lane V) demonstrates homogeneity of the capsomere.
Variants VP1-CallS and VP1-2C.
Two VP1 variants were constructed to examine the dependence of the virion assembly process on disulfide bonds. All six cysteine residues occurring in the wild-type protein (VP1-wt) monomer were replaced by serines in the variant VP1-CallS. A second mutant (VP1-2C) contained only C19 and C114, the two residues supposed to form an intrapentamer disulfide bond (20). Both mutants were expressed and purified as VP1-intein-CBD fusions in a native pentameric form.
In vitro assembly and size exclusion chromatography.
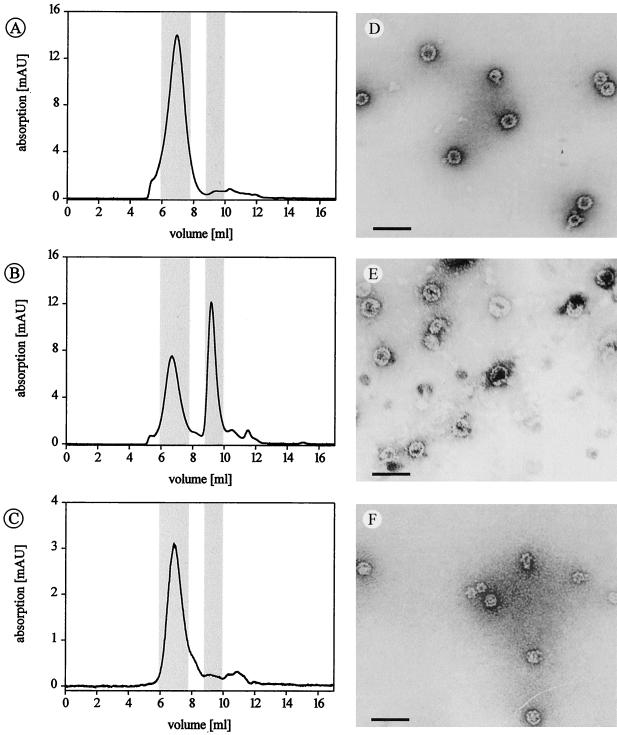
To monitor and quantify the assembly under different conditions, HPLC-based size exclusion chromatography was established to separate capsids from capsomeres, using a column with a molecular mass resolution capacity up to 20 MDa. Standard proteins were used for calibration for masses less than 1 MDa; no standard proteins were available for higher masses. Unassembled, pentameric VP1 eluted at a volume according to a mass of 220 kDa, in good agreement with the calculated mass of 212.5 kDa (Table 1). VP1 virus-like particles eluted at significantly lower volumes. The putative capsid peaks were collected and analyzed by electron microscopy for verification, revealing a population of virus-like particles (Fig. 2).
TABLE 1.
Elution volumes of VP1 and calibration proteins on a TSKgel G5000PWXL size exclusion chromatography column
| Protein | Molecular mass (kDa) | Elution vol (ml) |
|---|---|---|
| VP1 | ||
| Pentamer | 212.5 | 9.4 |
| Capsid of 72 pentamers | 15,300 | 6.7 |
| Urease | ||
| Hexamer | 545.0 | 8.7 |
| Trimer | 272.0 | 9.4 |
| Bovine serum albumin (dimer) | 66.0 | 10.1 |
| Chicken egg albumin | 45.0 | 10.2 |
| α-Lactalbumin | 14.2 | 10.3 |
FIG. 2.
Size exclusion chromatography with polyomavirus-like particles. (A to C) Elution profiles of VP1-wt, VP1-CallS, and VP1-2C, respectively. Elution volumes of capsids and capsomeres are highlighted and also listed in Table 1, together with calibration data. Peaks with elution volumes of >11 ml are caused by buffer agents. (D to F) Electron micrographs of isolated capsid peaks presenting VP1-wt (D), VP1-CallS (E), and VP1-2C (F). VP1-CallS capsids dissociated partially after isolation. Scale bar = 100 nm.
VP1-wt, VP1-CallS, and VP1-2C all formed virus-like particles in vitro (Fig. 2). However, under identical conditions, VP1-CallS assembled only to an extent of about 50%, whereas VP1-wt and VP1-2C assembled quantitively into virus-like particles. Varying assembly parameters such as incubation time, ionic strength, and sample concentration did not result in more than 55% capsids with VP1-CallS (data not shown).
Influence of disulfide bonds on in vitro assembly.
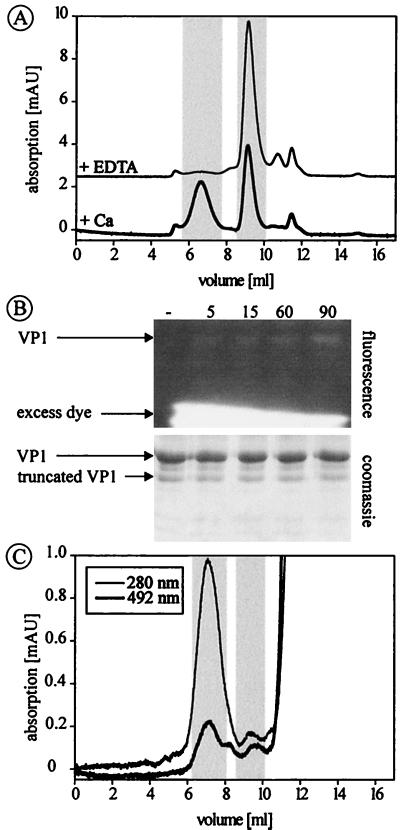
The assembly of VP1-CallS is completely reversible when Ca2+ ions are removed from the solution by adding an excess of EDTA (Fig. 3A). However, neither the addition of 10 mM EDTA to a capsid solution of VP1-wt as well as VP1-2C nor the addition of 50 mM DTT affected the integrity of the capsids. When both EDTA and DTT were added simultaneously to wild-type or 2C capsids, dissembly into free pentamers was achieved (data not shown).
FIG. 3.
Dissembly of VP1-CallS and oxidation of disulfide bond C19-C114′. (A) Elution of optimally assembled VP1-CallS, indicating a near 1:1 ratio of capsids and capsomeres. Addition of 10 mM EDTA resulted in complete dissembly of the virus-like particles. Elution volumes of capsids and capsomeres are highlighted (see also Table 1). (B) Thiol-specific fluorescence labeling of fully assembled VP1-2C results in weak labeling of the VP1 protein, demonstrating that C19 and C114 are only partially oxidized. Fluorescence- and Coomassie blue-stained SDS-polyacrylamide gels of unlabeled VP1-2C (lane 1) and of protein after a labeling reaction for 5, 15, 30, and 90 min are shown. (C) The elution profile of fluorescein-labeled VP1-2C demonstrates that the dye is attached to capsids and not to remaining capsomeres. Excess dye elutes beyond 11 ml.
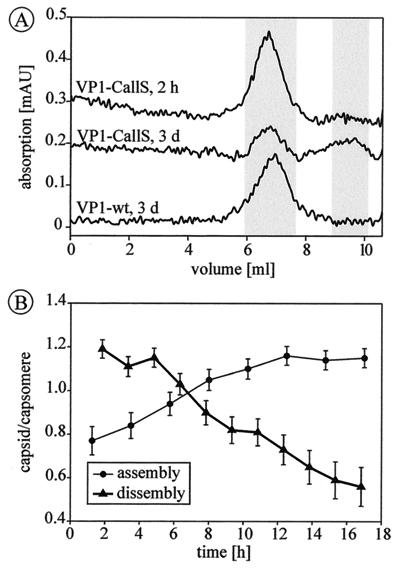
To test whether all possible disulfide bonds of C19-C114′ are formed, fully assembled VP1-2C capsids were labeled with fluorescein-C2-maleimide, an agent which attaches to free SH groups in a highly specific reaction (17). Under conditions allowing complete labeling of all available SH groups (17), a significant amount of fluorescein was coupled to VP1-2C, as shown by SDS-PAGE (Fig. 3B) and size exclusion chromatography (Fig. 3C). Therefore, not all possible disulfide bonds in the capsid are actually formed or are necessary for complete assembly. This conclusion is further supported by experiments of mixed assemblies of VP1-2C with VP1-CallS. Capsids containing 50% cysteine-free VP1-CallS assembled completely, and the resulting capsids were resistant to EDTA treatment (data not shown). Immediately after isolation, VP1-CallS capsids eluted in a single peak at the same volume as before (Fig. 4A). After further incubation for 3 days at room temperature, the VP1-CallS capsids had spontaneously dissembled to an extent of about 50%, indicating equilibration between these two forms. Under the same conditions, capsids of VP1-wt and VP1-2C remained intact. Therefore, it may be assumed that pentamers are fixed in the assembled state by a disulfide bond between C19 and C114′ once capsids have formed (Fig. 4A). The assembly and dissembly reaction of VP1-CallS was further analyzed by sequentially determining capsid-to-capsomere ratios by integration of the respective peak areas. The assembly of incompletely equilibrated samples proceeded over several hours until a maximum of 55% capsids was reached, a level which did not increase further (Fig. 4B). The reverse reaction of completely equilibrated samples started at the maximum of 55% capsids, which decreased gradually at a comparable speed upon addition of EDTA (Fig. 4B).
FIG. 4.
Assembly and dissembly of VP1-CallS. (A) Elution profiles of capsids isolated by size exclusion chromatography after 2 h (VP1-CallS) and 3 days (VP1-CallS and VP1-wt). Elution volumes of capsids and capsomeres are highlighted (see also Table 1). After 3 days, VP1-CallS is partially dissembled, reaching a capsid-to-capsomere ratio of 1:1, indicating adjustment of an equilibrium, whereas capsids of VP1-wt (and of VP1-2C [data not shown]) remain intact. (B) Kinetic studies of the equilibrium between capsids and capsomeres of VP1-CallS. The maximum amount of capsid which could be assembled is 55%. After addition of 10 mM EDTA, capsids gradually dissembled; after 16 h, half of the capsids were disrupted.
DISCUSSION
The novel purification strategy for recombinant polyomavirus protein VP1 using a self-splicing fusion protein provides a convenient and fast system which allows expression and purification of different VP1 mutants in a single step with yields of up to 6 mg per liter of culture medium. Purification strategies for recombinant VP1 described previously by Leavitt et al. (11) and Braun et al. (3) either are laborious or result only in significantly lower protein yields.
Analysis of the in vitro assembly process was done by HPLC-based size exclusion chromatography, which allows separation of capsids and capsomeres and an exact quantification of the different particle populations (Table 1). This method is comparatively fast and therefore well suited for the study of slow reactions, e.g., virus assembly.
All variants of murine polyomavirus VP1 described here were found to be capable of in vitro assembly. Although disulfide bonds play a major role in capsid assembly, the cysteine-free mutant formed virus-like particles similar to those formed by the wild-type protein. This is a remarkable feature because it was recently demonstrated for the related bovine papillomavirus that mutation of an essential cysteine residue resulted in completely assembly-deficient capsomeres (12).
The mutant VP1-2C shows assembly characteristics the same as those of VP1-wt and distinct from those of the cysteine-free mutant VP1-CallS (Fig. 2). Therefore, we conclude that the intrapentamer disulfide bond C19-C114′ is responsible for complete and (under nonreducing conditions) irreversible virion assembly by fixing the otherwise flexible CD loop, thus contributing to a rigid conformation of the pentamer which locks the invading C-terminal arm from the neighboring pentamer in its correct position (18, 20). There are no interpentamer disulfide bonds (e.g., involving C11 or C15 on the flexible N-terminal part of the protein whose structure is unresolved) necessary for capsid stabilization. This feature distinguishes murine polyomavirus from the related viruses simian virus 40 and bovine papillomavirus, where interpentamer disulfide bonds contribute to the stability of the virion (12, 13, 19). Our results demonstrate that cysteine-free capsids of VP1-CallS are in equilibrium with free capsomeres. This conclusion is based on three major observations: (i) capsids of VP1-CallS can assemble only to an extent of 55%, while VP1-wt and VP1-2C can assemble completely under the same conditions (Fig. 2); (ii) the cysteine-free capsid of VP1-CallS can be completely dissembled by removing Ca2+ ions with the chelating agent EDTA, whereas VP1-wt and VP1-2C capsids are resistant to EDTA as long as the disulfide bridges are oxidized (Fig. 3); and (iii) isolated capsids of VP1-CallS spontaneously dissemble until a capsid-to-capsomere ratio of 1:1 is reached, while VP1-wt and VP1-2C capsids were stable for several days.
In accordance with these results, we propose a two-step mechanism for in vitro virus-like particle assembly. In a first step, independent of redox conditions and disulfide bonds, an equilibrium between capsids and capsomeres is reached. The equilibrium is on the side of capsomeres in the absence of Ca2+ and shifts to 55% capsid formation when Ca2+ is present. This reaction is comparatively slow, taking about 2 days at room temperature (Fig. 4). Any intermediates formed on the way from free capsomeres to whole capsids must have short half-lives, because partially assembled capsids could not be detected in any experiment. In a second step, capsids are fixed by the formation of disulfide bond C19-C114′. This oxidation inhibits capsid dissembly by EDTA and constantly removes capsids from the equilibrium reaction, which in turn results in complete particle assembly.
This two-step mechanism may have a biological function during virion dissembly in vivo. Successful replication requires uncoating of the virus and transport of its DNA into the cellular nucleus. It has been demonstrated for polyomavirus that after endocytosis the whole virion is transported into the nucleus, where uncoating and subsequent gene transcription occur (14). It is still unclear how this is accomplished, because virions are too large to pass through the nuclear pore complex. Therefore, a conformational change in the virus shell is required prior to entry into the nucleus. When a virus enters the cytosol of a host cell, it encounters reducing conditions, and at least partial reduction of disulfide bond C19-C114′ should occur, increasing the overall flexibility of the virus shell. This increased flexibility may be required for a capsid deformation for successful transport into the nucleus. When a critical number of disulfide bonds is not maintained, virions spontaneously begin to uncoat without further aid of proteases and the DNA genome is released. However, testing of this hypothesis requires additional experiments.
ACKNOWLEDGMENTS
This work was supported by a grant from Land Sachsen-Anhalt.
We thank Robert Garcea, University of Colorado, for providing a plasmid containing the original VP1 gene and Thilo Stehle, Harvard University, for sending the coordinates of the VP1 structure prior to publication. We also thank Dieter Neumann, Institut für Pflanzenbiochemie, Halle, Germany, for help with electron microscopy.
REFERENCES
- 1.Brady J N, Winston V D, Consigli R A. Dissociation of polyomavirus by the chelation of calcium ions found associated with purified virions. J Virol. 1977;23:717–724. doi: 10.1128/jvi.23.3.717-724.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brady J N, Winston V D, Consigli R A. Characterization of a DNA-protein complex and capsomere subunits derived from polyomavirus by treatment with ethyleneglycol-bis-N,N′-tetraacetic acid and dithiothreitol. J Virol. 1978;27:193–204. doi: 10.1128/jvi.27.1.193-204.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braun H, Boller K, Lower J, Bertling W M, Zimmer A. Oligonucleotide and plasmid DNA packaging into polyoma VP1 virus-like particles expressed in Escherichia coli. Biotechnol Appl Biochem. 1999;29:31–43. [PubMed] [Google Scholar]
- 4.Chong S, Xu M-Q. Protein splicing of the Saccharomyces cerevisiae VMA intein without the endonuclease motifs. J Biol Chem. 1997;272:15587–15590. doi: 10.1074/jbc.272.25.15587. [DOI] [PubMed] [Google Scholar]
- 5.Chong S, Mersha F B, Comb D G, Scott M E, Landry D, Vence L M, Perler F B, Benner J, Kucera R B, Hirvonen C A, Pelletier J J, Paulus H, Xu M-Q. Single-column purification of free recombinant proteins using a self-cleavable affinity tag derived from a protein splicing element. Gene. 1997;192:271–281. doi: 10.1016/s0378-1119(97)00105-4. [DOI] [PubMed] [Google Scholar]
- 6.Chong S, Williams K S, Wotkowicz C, Xu M-Q. Modulation of protein splicing of the Saccharomyces cerevisiae vacuolar membrane ATPase intein. J Biol Chem. 1998;273:10567–10577. doi: 10.1074/jbc.273.17.10567. [DOI] [PubMed] [Google Scholar]
- 7.Chong S, Yang S, Paulus H, Benner J, Perler F B, Xu M-Q. Protein splicing involving the Saccharomyces cerevisiae VMA intein. The steps in the splicing pathway, side reactions leading to protein cleavage, and establishment of an in vitro splicing system. J Biol Chem. 1996;271:22159–22168. doi: 10.1074/jbc.271.36.22159. [DOI] [PubMed] [Google Scholar]
- 8.Christiansen G, Landers T, Griffith J, Berg P. Characterization of components released by alkali disruption of simian virus 40. J Virol. 1977;21:1079–1084. doi: 10.1128/jvi.21.3.1079-1084.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Eckhart W. Polyomaviridae and their replication. In: Fields B N, Knipe D M, editors. Fundamental virology. 2nd ed. New York, N.Y: Raven Press; 1991. pp. 727–741. [Google Scholar]
- 10.Garcea R L, Salunke D M, Caspar D L D. Site-directed mutation affecting polyomavirus capsid self-assembly in vitro. Nature. 1987;329:86–87. doi: 10.1038/329086a0. [DOI] [PubMed] [Google Scholar]
- 11.Leavitt A D, Roberts T M, Garcea R L. Polyomavirus major capsid protein, VP1. Purification after high level expression in Escherichia coli. J Biol Chem. 1985;260:12803–12809. [PubMed] [Google Scholar]
- 12.Li M, Beard P, Estes P A, Lyon M K, Garcea R L. Intercapsomeric disulfide bonds in papillomavirus assembly and disassembly. J Virol. 1998;72:2160–2167. doi: 10.1128/jvi.72.3.2160-2167.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liddington R C, Yan Y, Moulai J, Sahli R, Benjamin T L, Harrison S C. Structure of simian virus 40 at 3.8 Å resolution. Nature. 1991;354:278–284. doi: 10.1038/354278a0. [DOI] [PubMed] [Google Scholar]
- 14.Mackay R L, Consigli R A. Early events in polyomavirus infection: attachment, penetration, and nuclear entry. J Virol. 1976;19:620–636. doi: 10.1128/jvi.19.2.620-636.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salunke D M, Caspar D L, Garcea R L. Polymorphism in the assembly of polyomavirus capsid protein VP1. Biophys J. 1989;56:887–900. doi: 10.1016/S0006-3495(89)82735-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salunke D M, Caspar D L D, Garcea R L. Self-assembly of purified polyomavirus capsid protein VP1. Cell. 1986;46:895–904. doi: 10.1016/0092-8674(86)90071-1. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt U, Kenklies J, Rudolph R, Böhm G. Site-specific fluorescence labeling of recombinant polyomavirus-like particles. Biol Chem. 1999;380:397–401. doi: 10.1515/BC.1999.053. [DOI] [PubMed] [Google Scholar]
- 18.Stehle T, Harrison S C. Crystal structures of murine polyomavirus in complex with straight-chain and branched-chain sialyloligosaccharide receptor fragments. Structure. 1996;4:183–194. doi: 10.1016/s0969-2126(96)00021-4. [DOI] [PubMed] [Google Scholar]
- 19.Stehle T, Gamblin S J, Yan Y, Harrison S C. The structure of simian virus 40 refined at 3.1 Å resolution. Structure. 1996;4:165–182. doi: 10.1016/s0969-2126(96)00020-2. [DOI] [PubMed] [Google Scholar]
- 20.Stehle T, Yan Y, Benjamin T L, Harrison S C. Structure of murine polyomavirus complexed with an oligosaccharide receptor fragment. Nature. 1994;369:160–163. doi: 10.1038/369160a0. [DOI] [PubMed] [Google Scholar]