INTRODUCTION
Alcohol-associated liver disease (ALD) is a well-recognized consequence of heavy alcohol consumption. ALD develops in a dose-dependent manner and has a higher incidence in individuals exhibiting a more advanced degree of liver fibrosis.1 Although most patients with advanced chronic liver disease and reported excessive alcohol use or with alcohol use disorder (AUD) are quickly recognized as ALD, individuals with other underlying etiologies of liver disease can also be affected by alcohol use.2 Thus, alcohol may contribute to the development of chronification of hepatitis infections, steatohepatitis, the progression of liver fibrosis, decompensation in those with established cirrhosis, and increase the risk of HCC. For example, a nationwide cohort study of patients with the biopsy-proven liver disease showed that alcohol use can significantly increase the risk of liver disease–related mortality by 260-fold.3 Although this effect could be more evident in those with steatotic liver disease and metabolic dysfunction, alcohol can also impact other conditions such as viral hepatitis, autoimmune liver disease, and hemochromatosis. In this article, we will discuss the potential mechanisms of alcohol-associated liver injury as a cofactor for other chronic liver diseases; the most cost-effective screening methods for AUD, and the long-term consequences of alcohol use in individuals with chronic liver disease, as well as recipients of liver transplants.
ASSESSMENT OF AUD IN CLINICAL PRACTICE
In 2016, ~3.1 billion people (57% of the global population) aged 15 and over were current drinkers in the last 12 months.4 However, although alcohol use is common, it is often not reported accurately by patients and is frequently overlooked or inaccurately assessed by clinicians, even in patients with advanced chronic liver disease. In addition, the definition of a standard drink varies significantly among different regions worldwide, making standardized assessment of the level of patterns of drinking difficult. Several efforts have been made to address these limitations, including the use of questionnaires, as well as indirect and direct biomarkers to screen for alcohol use.5
The Alcohol Use Disorders Identification Test (AUDIT) is one of the most validated tools for assessing hazardous or harmful alcohol use, including the risk of AUD (Table 1).6 A shortened version of AUDIT, known as AUDIT-C, includes only the first 3 questions (those related to alcohol consumption). It has been shown to be effective in identifying patients at risk of AUD, and it is simpler and quicker to administer than the full version7 (Table 1). Since AUDIT and AUDIT-C are only screening tools for AUD, a formal diagnosis of AUD should be based on meeting at least 2 out of the 11 Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM-V) criteria, which identify problematic patterns of alcohol use leading to clinically significant impairment or distress.6
TABLE 1.
Screening questionnaires for alcohol use in the overall population and patients with chronic liver disease
| Screening method | Description | Evidence in specific populations | Sensitivity and specificity |
|---|---|---|---|
| AUDITa | 10 questions: How much, and how often do they drink? | A score of 8 or more may indicate AUD | Sensitivity of 95% Specificity of 85% |
| AUDIT-Ca | 3 questions, each 4 choices | 3 or more in females; 4 for males likely AUD | Simplified version for screening |
| CAGE | Cut down, Annoyed, Guilty, Eye-opener | Two or more yes | Sensitivity of 79%–97% Specificity of 77%–94% |
| T-ACE | For pregnant, C replaced with T (tolerance) | Similar to CAGE Screening alcohol use during pregnancy |
Sensitivity of 69%–100% Specificity of 19%–89% |
| SBIb | Questions followed by brief interventions | Screen for AUD, and brief intervention/referral to an addiction clinic | A tool that can be integrated with NCD clinics |
| DSM- 5c | 2 of the 11 criteria | The gold standard for diagnosing AUD | Sensitivity of 69%–100% Specificity of 64%–73% |
Validated in individuals with ALD.
Validated tool for primary care practice.
General population, and in ALD as a gold standard test
Abbreviations: ALD, alcohol-associated liver disease; AUDIT, Alcohol Use Disorders Identification Test; CAGE, Cut down, Annoyed, Guilty; Eye-opener, DSM-5, Diagnostic and Statistical Manual of Mental Disorders. 5th ed; NCD, noncommunicable disease; SBI, Screening and Brief Intervention; T-ACE, Tolerance, Annoyance, Cut Down, Eye-opener.
While indirect biomarkers of alcohol use, such as mean corpuscular volume, serum aspartate aminotransferase, alanine aminotransferase, and gamma-glutamyl transferase, show insufficient sensitivity and specificity for monitoring alcohol use in chronic liver disease,8 direct biomarkers assessing alcohol byproducts have shown promise in detecting alcohol use in patients underreporting, for example, individuals with dual etiology or underlying ALD in chronic liver disease. Ethyl glucuronide and ethyl sulfate can detect alcohol consumption in urine for a period of 2–4 days,9 while phosphatidylethanol in the blood can detect alcohol use for up to 20 days, of alcohol consumption (either chronic or episodic drinking) and up to ~4 weeks after cessation.10
ALCOHOL USE AND ITS CONTRIBUTION TO THE PROGRESSION OF CHRONIC LIVER DISEASE
Alcohol significantly exacerbates the progression of chronic liver disease, especially in individuals with metabolic risk factors, such as overweight, obesity, insulin resistance, and type 2 diabetes mellitus.11 Recent findings highlight the importance of combined risk factors for liver disease progression, representing a preventable cause mainly in developed countries.12 For example, alcohol consumption accelerates the progression of metabolic dysfunction–associated steatohepatitis, increasing the risk of liver decompensation.13 Indeed, even low levels of alcohol intake could negatively affect disease progression in a dose-dependent manner across all stages of liver disease in both men and women, especially in those with genetic susceptibility.14
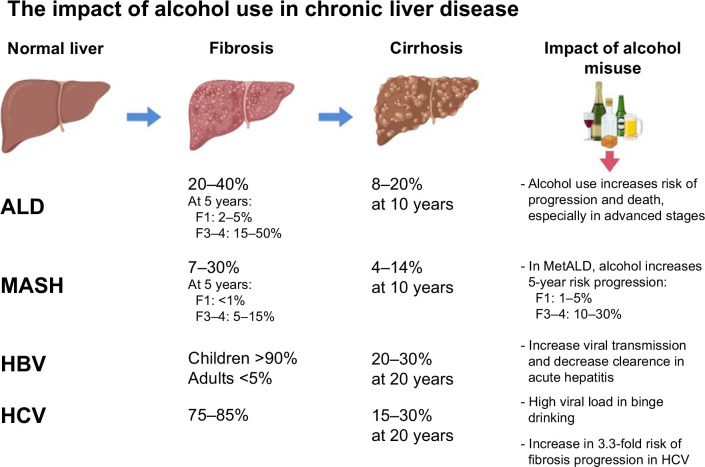
Several studies support the association between alcohol and liver disease progression in patients with metabolic syndrome. Risk estimates for a 5-year period show that decompensation in metabolic dysfunction–associated steatotic liver disease is <1% at the F1 fibrosis stage (according to the METAVIR scoring system). This percentage increases with higher alcohol use and can reach up to 50% in patients with ALD and advanced fibrosis, emphasizing the significant impact of alcohol on disease outcomes.1 Another study, using data from the UK Biobank, found that individuals with obesity, excessive alcohol consumption, and homozygous carriers of the 148M variant of the patatin-like phospholipase domain-containing 3 (PNPLA3) gene had an ~8% risk of developing cirrhosis over a 10-year period.11 This study highlights the synergistic relationship between genetic and environmental factors. In fact, the 2023 definition of steatotic liver disease recognizes this connection and introduces a new term, MetALD, to describe individuals with both metabolic dysfunction and significant alcohol use.15
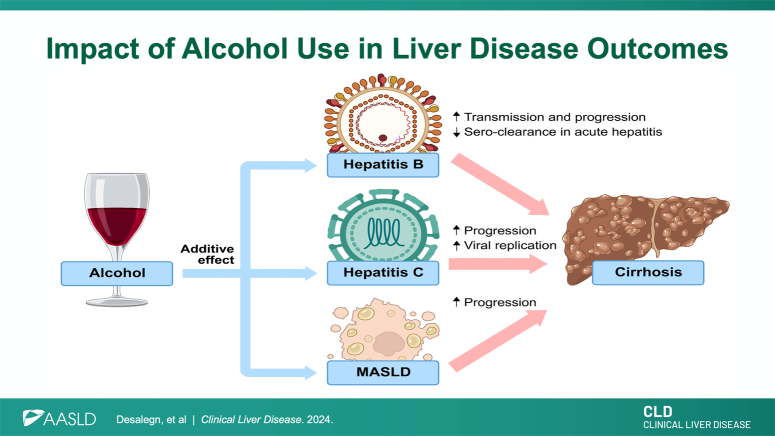
Alcohol not only exerts direct toxic effects on the liver but also alters the clinical course of viral infections, including HBV and HCV, by impairing the immune response and promoting dysregulated inflammatory states, thereby increasing the risk of infection.16 Studies indicate that alcohol consumption also exacerbates the progression of liver disease in individuals with HBV-related cirrhosis.17 In sub-Saharan Africa, increased HBV viral loads have been observed during episodes of binge drinking, suggesting a close relationship between alcohol consumption and viral dynamics. This is a topic that needs further research. Some proposed mechanisms for the higher HBV viral load include the suppression of different components of the immune system, such as reduced cell-mediated immunity and increased oxidative stress. As a result, there is a lack of control over viral replication.18 Indeed, animal models have shown that alcohol increases the HBV viral load.19,20 In addition, high viral replication rates have been observed in over 90% of patients with concurrent ALD and HCV infection.21 In particular, there is evidence of a significant correlation between self-reported alcohol intake and an increase in serum HCV RNA levels.21 Alcohol use also interplays with shared risk factors with viral etiologies: it increases the risk of transmission of the hepatitis viruses, causes decreased clearance after an acute infection, and enhances the progression of liver fibrosis. Alcohol is known to reduce the likelihood of spontaneous clearance following acute hepatitis infection, leading to a greater chance of progression to chronic infection. Alcohol consumption is also associated with an increased risk of contracting HBV and HCV due to high-risk behaviors, including sexual transmission. In addition, alcohol is known to reduce the likelihood of spontaneous clearance following acute hepatitis infection, leading to a greater chance of progression to chronic infection. Furthermore, there is a dose-dependent increase in the severity of liver disease progression and the development of complications such as HCC in individuals who consume alcohol.2,22
THE EFFECT OF ALCOHOL USE ON LIVER-RELATED OUTCOMES IN THE POST-TRANSPLANT PERIOD
ALD is currently the leading cause of liver transplants in the United States, and it is responsible for a significant and increasing portion of the overall burden of liver disease.23 Approximately 12%–33% of recipients of liver transplant for ALD have a relapse to heavy alcohol drinking. Alcohol relapse after liver transplantation (LT) has been associated with graft rejection, graft loss, recurrent ALD, and decreased overall survival. In terms of histopathology, patients with alcohol relapse were more likely to have advanced fibrosis (stage 3 or higher) compared to those who remained abstinent.24 Studies have also found that alcohol use after LT is independent of the LT indications, as people who have not been diagnosed with ALD also drink alcohol after LT.25 Individuals with a previous alcohol use history should be considered by physicians caring for them pretransplant and provided with social support despite the indications of LT. The risk of relapse was comparable to that of individuals with ALD, and the most important factor in relapse was drinking duration and amount before transplantation.26 Therefore, alcohol abstinence should be promoted in all recipients of LT regardless of the underlying etiology, while specific AUD treatment should be sustained and optimized after LT in those with ALD.
FUTURE DIRECTIONS AND KNOWLEDGE GAPS
The attention given to alcohol, both as a standalone cause of liver disease and in combination with other factors, has been limited. Thus, there remain significant gaps in our understanding of the detrimental effects of alcohol on liver disease, including its exacerbation of chronic liver conditions and impact after LT. Future studies should focus on the nuanced interactions between alcohol and various liver disease etiologies, such as viral hepatitis and metabolic dysfunction–associated steatotic liver disease. Research should aim to clarify the mechanisms by which alcohol influences disease progression and treatment outcomes. Furthermore, there is a pressing need to enhance the clinical diagnosis of AUD in patients with liver diseases. The development and implementation of more sensitive and specific diagnostic tools for detecting alcohol consumption, particularly in patients who underreport their intake, would be a crucial step forward. Addressing these knowledge gaps will not only advance our understanding of the pathophysiology of ALD but also improve clinical management and outcomes. Moreover, these insights could inform public health strategies, emphasizing the need for alcohol abstinence as a primary prevention strategy for liver disease and advocating for more robust and accessible treatment options for AUD.
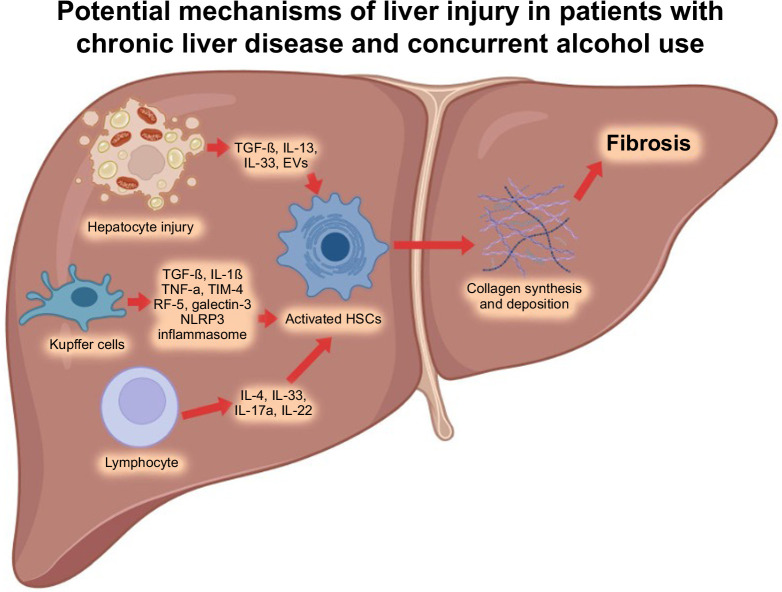
In conclusion, alcohol accelerates liver fibrosis progression and interacts with other risk factors, including obesity, type 2 diabetes mellitus, and viral hepatitis. Abstaining from alcohol should be a primary prevention strategy in patients with significant liver fibrosis caused by HBV, HCV, metabolic dysfunction–associated steatotic liver disease, and other liver diseases. LT programs should evaluate patients with ALD based on clinical criteria rather than the duration of the patient’s alcohol abstinence history. Supportive behavioral, group therapies and pharmacological treatments should be widely available to support patients in alcohol abstinence, including those patients after LT. International guidelines should clearly state that alcohol is a major contributor to cirrhosis and cancer. Furthermore, it is essential for industries and global organizations to actively support clinical trials that focus on developing effective diagnostic and treatment options for ALD (Figure 1 and 2).
FIGURE 1.
Potential pathophysiological mechanisms of liver injury in individuals with chronic liver disease (eg, HBV, HCV, MASLD, among others) and concurrent alcohol use. After a liver injury, inflammatory mediators are released, lymphocytes infiltrate the liver, and Kupffer cells are activated due to apoptosis of hepatocytes. These pathways stimulate fibrogenic cytokines like TGF-β and result in the production of major collagen-producing cells, one of which is HSCs. Subsequently, HSCs undergo significant phenotypic activation and secrete excessive amounts of collagen deposition, leading to liver fibrosis. Created with BioRender.com. Abbreviations: IRF-5, interferon regulatory factor-5; MASLD, metabolic dysfunction–associated steatotic liver disease; NLRP3, NLR family pyrin domain containing 3; TIM-4, T-cell immunoglobulin and mucin-4.
FIGURE 2.
Liver disease progression with different etiologies and impact of alcohol. Created with BioRender.com. Abbreviations: ALD, alcohol-associated liver disease; MASH, metabolic dysfunction–associated steatohepatitis.
Acknowledgments
FUNDING INFORMATION
Juan Pablo Arab receives support from the Chilean government through the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT 1200227 to Juan Pablo Arab).
CONFLICTS OF INTEREST
The authors have no conflicts to report.
Footnotes
Abbreviations: ALD, alcohol-associated liver disease; AUD, alcohol use disorder; AUDIT, alcohol use disorders identification test; DSM-V, Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition; LT, liver transplantation.
Hailemichael Desalegn and Luis Antonio Diaz share first authorship.
Contributor Information
Hailemichael Desalegn, Email: hailed2003@yahoo.com.
Luis Antonio Diaz, Email: luisdiazpiga@gmail.com.
Jürgen Rehm, Email: jtrehm@gmail.com.
Juan Pablo Arab, Email: jparab@gmail.com.
REFERENCES
- 1. Bataller R, Arab JP, Shah VH. Alcohol-associated hepatitis. N Engl J Med. 2022;387:2436–2448. [DOI] [PubMed] [Google Scholar]
- 2. Rehm J, Patra J, Brennan A, Buckley C, Greenfield TK, Kerr WC, et al. The role of alcohol use in the aetiology and progression of liver disease: A narrative review and a quantification. Drug Alcohol Rev. 2021;40:1377–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hagström H, Thiele M, Roelstraete B, Söderling J, Ludvigsson JF. Mortality in biopsy-proven alcohol-related liver disease: A population-based nationwide cohort study of 3453 patients. Gut. 2021;70:170–179. [DOI] [PubMed] [Google Scholar]
- 4. World Health Organization . Global Status Report on Alcohol and Health 2018. World Health Organization; 2019. [Google Scholar]
- 5. Kulkarni AV, Singal AK. Screening for alcohol use disorder and monitoring for alcohol use in the liver clinic. Clin Liver Dis. 2023;22:219–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Arab JP, Izzy M, Leggio L, Bataller R, Shah VH. Management of alcohol use disorder in patients with cirrhosis in the setting of liver transplantation. Nat Rev Gastroenterol Hepatol. 2022;19:45–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Arab JP, Atkinson SR, Bataller R. Alcohol‐related liver disease [Internet]. In: Wang TC, Camilleri M, Lebwohl B, Lok AS, Sandborn WJ, Wang KK, Wu GD, eds. Yamada’s Textbook of Gastroenterology. John Wiley & Sons Ltd. 2022:1966–1978. [Google Scholar]
- 8. Harris JC, Leggio L, Farokhnia M. Blood biomarkers of alcohol use: A scoping review. Curr Addict Rep. 2021;8:500–508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Staufer K, Huber-Schönauer U, Strebinger G, Pimingstorfer P, Suesse S, Scherzer T-M, et al. Ethyl glucuronide in hair detects a high rate of harmful alcohol consumption in presumed non-alcoholic fatty liver disease. J Hepatol. 2022;77:918–930. [DOI] [PubMed] [Google Scholar]
- 10. Kechagias S, Dernroth DN, Blomgren A, Hansson T, Isaksson A, Walther L, et al. Phosphatidylethanol compared with other blood tests as a biomarker of moderate alcohol consumption in healthy volunteers: A prospective randomized study. Alcohol Alcohol. 2015;50:399–406. [DOI] [PubMed] [Google Scholar]
- 11. Díaz LA, Arab JP, Louvet A, Bataller R, Arrese M. The intersection between alcohol-related liver disease and nonalcoholic fatty liver disease. Nat Rev Gastroenterol Hepatol. 2023;20:764–783. [DOI] [PubMed] [Google Scholar]
- 12. Xie J, Huang H, Liu Z, Li Y, Yu C, Xu L, et al. The associations between modifiable risk factors and nonalcoholic fatty liver disease: A comprehensive Mendelian randomization study. Hepatology. 2023;77:949–964. [DOI] [PubMed] [Google Scholar]
- 13. Israelsen M, Torp N, Johansen S, Thiele M, Krag A. MetALD: New opportunities to understand the role of alcohol in steatotic liver disease. Lancet Gastroenterol Hepatol. 2023;8:866–868. [DOI] [PubMed] [Google Scholar]
- 14. Åberg F, Byrne CD, Pirola CJ, Männistö V, Sookoian S. Alcohol consumption and metabolic syndrome: Clinical and epidemiological impact on liver disease. J Hepatol. 2023;78:191–206. [DOI] [PubMed] [Google Scholar]
- 15. Rinella ME, Lazarus JV, Ratziu V, Francque SM, Sanyal AJ, Kanwal F, et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. Hepatology. 2023;78:1966–1986. [DOI] [PubMed] [Google Scholar]
- 16. Chan C, Levitsky J. Infection and alcoholic liver disease. Clin Liver Dis. 2016;20:595–606. [DOI] [PubMed] [Google Scholar]
- 17. Xu H-Q, Wang C-G, Zhou Q, Gao Y-H. Effects of alcohol consumption on viral hepatitis B and C. World J Clin Cases. 2021;9:10052–10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Pasala S, Barr T, Messaoudi I. Impact of alcohol abuse on the adaptive immune system. Alcohol Res. 2015;37:185–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Li Z-M, Kong C-Y, Zhang S-L, Han B, Zhang Z-Y, Wang L-S. Alcohol and HBV synergistically promote hepatic steatosis. Ann Hepatol. 2019;18:913–917. [DOI] [PubMed] [Google Scholar]
- 20. Larkin J, Clayton MM, Liu J, Feitelson MA. Chronic ethanol consumption stimulates hepatitis B virus gene expression and replication in transgenic mice. Hepatology. 2001;34:792–797. [DOI] [PubMed] [Google Scholar]
- 21. Degos F. Hepatitis C and alcohol. J Hepatol. 1999;31(suppl 1):113–118. [DOI] [PubMed] [Google Scholar]
- 22. Aisyah DN, Shallcross L, Hully AJ, O’Brien A, Hayward A. Assessing hepatitis C spontaneous clearance and understanding associated factors—A systematic review and meta-analysis. J Viral Hepat. 2018;25:680–698. [DOI] [PubMed] [Google Scholar]
- 23. Lee BP, Vittinghoff E, Dodge JL, Cullaro G, Terrault NA. National trends and long-term outcomes of liver transplant for alcohol-associated liver disease in the United States. JAMA Intern Med. 2019;179:340–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Rice JP, Eickhoff J, Agni R, Ghufran A, Brahmbhatt R, Lucey MR. Abusive drinking after liver transplantation is associated with allograft loss and advanced allograft fibrosis. Liver Transpl. 2013;19:1377–1386. [DOI] [PubMed] [Google Scholar]
- 25. Lee BP, Im GY, Rice JP, Lazar A, Weinberg E, Han H, et al. Patterns of alcohol use after early liver transplantation for alcoholic hepatitis. Clin Gastroenterol Hepatol. 2022;20:409–418.e5. [DOI] [PubMed] [Google Scholar]
- 26. Russ KB, Chen N-W, Kamath PS, Shah VH, Kuo Y-F, Singal AK. Alcohol use after liver transplantation is independent of liver disease etiology. Alcohol Alcohol. 2016;51:698–701. [DOI] [PMC free article] [PubMed] [Google Scholar]