Abstract
Herpes simplex virus type 1 (HSV-1) capsid proteins assemble in vitro into spherical procapsids that differ markedly in structure and stability from mature polyhedral capsids but can be converted to the mature form. Circumstantial evidence suggests that assembly in vivo follows a similar pathway of procapsid assembly and maturation, a pathway that resembles those of double-stranded DNA bacteriophages. We have confirmed the above pathway by isolating procapsids from HSV-1-infected cells and characterizing their morphology, thermal sensitivity, and protein composition. Experiments were carried out with an HSV-1 mutant (m100) deficient in the maturational protease for which it was expected that procapsids—normally, short-lived intermediates—would accumulate in infected cells. Particles isolated from m100-infected cells were found to share the defining properties of procapsids assembled in vitro. For example, by electron microscopy, they were found to be spherical rather than polyhedral in shape, and they disassembled at 0°C, unlike mature capsids, which are stable at this temperature. A three-dimensional reconstruction computed at 18-Å resolution from cryoelectron micrographs showed m100 procapsids to be structurally indistinguishable from procapsids assembled in vitro. In both cases, their predominant components are the four essential capsid proteins: the major capsid protein (VP5), the scaffolding protein (pre-VP22a), and the triplex proteins (VP19C and VP23). VP26, a small, abundant but dispensable capsid protein, was not found associated with m100 procapsids, suggesting that it binds to capsids only after they have matured into the polyhedral form. Procapsids were also isolated from cells infected at the nonpermissive temperature with the HSV-1 mutant tsProt.A (a mutant with a thermoreversible lesion in the protease), and their identity as procapsids was confirmed by cryoelectron microscopy. This analysis revealed density on the inner surface of the procapsid scaffolding core that may correspond to the location of the maturational protease. Upon incubation at the permissive temperature, tsProt.A procapsids transformed into polyhedral, mature capsids, providing further confirmation of their status as precursors.
Herpes simplex virus type 1 (HSV-1) is widely distributed in the human population, where it is the etiological agent of recurrent fever blisters. Infection in neonates can result in more severe, disseminated disease (50). Like all herpesviruses, HSV-1 consists of an icosahedral capsid surrounded by a membrane envelope with the double-stranded DNA (dsDNA) genome contained inside the capsid. During HSV-1 infection, the capsid plays a central role in delivering the virus genome to the host cell nucleus. Following fusion of virus and host cell membranes, the DNA-filled capsid enters the peripheral cytoplasm. From there it is transported to the nucleus, where it docks at a nuclear pore and releases its DNA into the nucleoplasm (35, 38).
Progeny HSV-1 capsids are assembled in the nucleus, where they are also packaged with DNA before further virus maturation takes place (15, 33, 35). Capsids are initially formed with an internal protein scaffold (composed of UL26 and UL26.5 gene products) which is lost from the capsid upon DNA packaging. The scaffold contains the virus protease (UL26 gene), which cleaves both itself (to generate capsid proteins VP24 and VP21) and the UL26.5 gene product (generating VP22a). The protease is essential for DNA packaging, capsid maturation, and virus growth (12).
The events of capsid formation prior to DNA encapsidation have been examined in insect cell extracts containing capsid proteins and in a purified system in which capsids are formed from purified proteins (24–26, 42). Studies with such systems show that mature, icosahedral capsids can be assembled from the major capsid protein (VP5), the two triplex proteins (VP19C and VP23), and a scaffolding protein (e.g., pre-VP22a). Further, the mature capsid is found to be assembled by way of a spherical, more fragile intermediate called the procapsid. Apart from a minor difference in the scaffolding protein (pre-VP22a is not cleaved in the procapsid [24]), the procapsid has the same protein composition as the mature capsid formed in vitro. The procapsid differs, however, in important respects: (i) the procapsid is spherical in overall morphology whereas the mature capsid is icosahedral; (ii) the procapsid is a more porous structure, having holes between capsomers that are sealed in the mature capsid; (iii) many procapsid hexons are oval in cross section whereas those of the mature capsid are hexagonal (46); and (iv) procapsids are disassembled after incubation at 4°C whereas mature capsids are unaffected. Procapsids transform into mature capsids in a process in which the shell undergoes the structural changes outlined above, and the scaffold disengages from the surface shell and is either expelled or retracted into a smaller core structure. In vivo, this transformation is dependent on the presence and activity of the maturational protease (5, 12, 30, 34).
Formation of capsids in vitro by way of the procapsid intermediate suggests that the same intermediate may be found in vivo, and Rixon and McNab have recently provided evidence this is the case (34). Electron micrographs of cells infected with HSV-1 ts1201 showed that the capsids accumulating at the nonpermissive temperature (NPT) were spherical like procapsids and sensitive to disassembly when the cells were incubated at 0°C. To clarify the properties of procapsids present in infected cells, we isolated them by antibody precipitation from infected cell lysates and examined them by electron microscopy, sensitivity to disruption at 0°C, and sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis. Experiments were carried out with an HSV-1 mutant (m100) in which procapsids are expected to accumulate due to the absence of the maturational protease (12). The results as described below show that procapsids isolated from infected cells are essentially the same particles as procapsids assembled in vitro.
MATERIALS AND METHODS
Cells and viruses.
m100 and tsProt.A viruses were isolated by Min Gao and his colleagues, who generously provided both viruses for this study (12). Previously described procedures were used for growing m100 on the complementing cell line, BMS-MG22 (12). Stock virus titers were in the range of 5 × 108 to 1 × 109 PFU/ml. Wild-type HSV-1 (strain 17MP) and tsProt.A were propagated on monolayer cultures of BHK or Vero cells that were grown in minimal essential medium containing 10% fetal calf serum and antibiotics. HSV-1 A and B capsids were isolated as previously described (27) from infected BHK cells.
Procapsid isolation.
m100 procapsids were isolated from BHK cells that were infected in 150-cm2 plastic flasks at a multiplicity of infection of 10 and incubated for 15 h at 37°C, during which time cells became detached from the substrate. All subsequent steps were carried out at room temperature (∼21°C). Cells from one to two flasks (∼2 × 108 cells) were harvested by low-speed centrifugation, suspended in 1 ml of phosphate-buffered saline (PBS), and lysed by sonication in a probe sonicator (Ultrasonics, Inc., model W-375; setting 3; two cycles of ∼8 s each). Protease inhibitors (aprotinin, leupeptin, and Pefabloc; 1/10 volume of a stock solution prepared by dissolving 1 tablet of Boehringer Mannheim Complete, Mini in 1 ml of PBS) were then added, and sedimentable material was removed by centrifugation for 6 min at 16,000 × g. Procapsids were then precipitated from the lysate by addition of 50 μl of VP5-specific monoclonal antibody (MAb) 6F10 (4 mg/ml) (24, 39) followed by incubation for 5 min at room temperature. Precipitates were harvested by centrifugation for 2 min at 16,000 × g, resuspended in 200 μl of PBS, and subjected to two further cycles of antibody precipitation as described above. Most preparations yielded 100 to 200 μl of procapsids at a concentration of ∼1 mg/ml, and these were used for structural and biochemical analyses as described below.
Cryoelectron microscopy and image reconstruction.
Immunoprecipitated procapsids were prepared for cryoelectron microscopy by adsorption onto a thin carbon film (supported on a thick holey carbon film) and freezing as previously described (25, 54). The same procedures were used for A capsids except that specimens were not immunoprecipitated. Micrographs were recorded on a Philips CM200-FEG electron microscope at a magnification of ×38,000, using minimal electron dose methods producing radiation levels of ∼8 electrons/Å2.
Three-dimensional reconstructions of procapsids and A capsids were computed beginning with micrographs that were selected for analysis by visual appraisal (e.g., to assess density of particles and contrast) and by optical diffraction to assess the state of defocus and resolution. For m100 procapsids, two micrographs whose first contrast transfer function (CTF) zeros were 1/29.7 Å−1 and 1/24.7 Å−1 were scanned at 17 μm/pixel on a Perkin-Elmer 1010MG microdensitometer, yielding an effective pixel size of ∼4.6 Å. CTF correction consisted of simple phase flipping (48). A total of 410 images were processed as previously described (46). For tsProt.A procapsids, 160 images from 13 micrographs (with CTF zeros between 1/22 and 1/26 Å−1) were analyzed to a resolution of 28 Å after scanning at 25 μm/pixel, yielding an effective pixel size of ∼6.9 Å. For A capsids, 1,488 images from 14 micrographs (with CTFs zeros between 1/18 and 1/25 Å−1) were analyzed to 18-Å resolution. Three-dimensional structures were solved by the polar Fourier transform method (1), with our earlier reconstructions (46) as starting models. After iterative cycles of refinement of orientation angles and origins, images with the highest correlation coefficients were selected and a density map was calculated (11). Calculations were performed with 338, 89, and 907 images for m100 procapsids, tsProt.A procapsids, and A capsids, respectively. The resolution of the resulting reconstructions was 18 Å for m100 procapsids and A capsids and 28 Å for tsProt.A procapsids as assessed by the FRC3D criterion (6).
SDS-polyacrylamide gel electrophoresis and Western immunoblotting.
Previously described procedures were used for SDS-polyacrylamide gel electrophoresis followed by Coomassie blue staining (23). Stained bands were determined quantitatively by scanning the gel in a flatbed scanner followed by densitometric analysis using ImageQuant software. Specimens to be examined by SDS-polyacrylamide gel electrophoresis and Western immunoblotting were precipitated with 10% trichloroacetic acid, resuspended in loading buffer (200 mM Tris-HCl, 100 mM dithiothreitol, 2% SDS, 10% glycerol [pH 8.8]), boiled for 3 min, and separated by electrophoresis on 4 to 20% polyacrylamide gradient gels. Proteins were electrophoretically transferred to nitrocellulose membranes, washed twice in Tris-buffered saline (20 mM Tris-HCl, 500 mM NaCl [pH 7.5]), blocked for 60 min in blocking buffer (Tris-buffered saline plus 0.2% nonfat dry milk), and processed by using an Immunostar chemiluminescence detection kit (Bio-Rad) as directed by the manufacturer. Primary antibodies were diluted in blocking buffer plus 0.1% Tween 20 and added to blots for 2 h at the following dilutions: VP5, MAb 13-183 (Advanced Biotechnologies Inc.), 1:1,000; VP23, MAb 1D2 (see below), 1:2,000; UL26.5 gene products, MAb MCA406 (Serotec, Inc.), 1:10,000; VP19C, rabbit polyclonal antibody NC2 (49), 1:10,000; VP26, rabbit polyclonal antibody R31/B3 (22), 1:500; and actin, mouse monoclonal clone C4 (ICN Pharmaceuticals), 1:500. Blots were then treated for 2 h with alkaline phosphatase-conjugated secondary antibodies, either goat anti-mouse or goat anti-rabbit immunoglobulin G (BioRad) (1:4,000 dilution) and developed with an Immunostar chemiluminescence detection kit. Labeled bands were detected by exposing blots to Kodak XAR-5 film.
VP23-specific MAb 1D2.
A mouse hybridoma cell line secreting MAb 1D2 was isolated by the procedures described previously for immunization of BALB/c mice and for fusion of spleen cells with Sp2/O-Ag14 myeloma cells (24). The immunogen was VP23 that had been purified biochemically from lysates of Sf9 cells expressing VP23 as a result of infection with a recombinant baculovirus (BAC-UL18) encoding VP23 (45). Beginning with the cell lysate, purification was accomplished by ammonium sulfate precipitation followed by cation-exchange chromatography as described previously for purification of triplexes (25). Hybridoma cell lines were screened by enzyme-linked immunosorbent assay (9) using VP23-containing Sf9 cell extracts as antigen. The antibody secreted by hybridoma cell line 1D2 was found to be specific for VP23 by enzyme-linked immunosorbent assay and by Western immunoblot assays. Previously described procedures were used for growing the 1D2 hybridoma cells as an ascites and for purifying the antibody (immunoglobulin G1 subclass) from the ascites fluid (10). Experiments were performed beginning with a stock solution of antibody whose concentration was 2.8 mg/ml.
Electron microscopy.
Procapsid-containing precipitates were prepared for electron microscopy by fixation, embedding in Epon 812, and thin sectioning as described previously (20, 24). Negative staining with 1% uranyl acetate was carried out as described by Thomas et al. (44). All electron micrographs of thin-sectioned or negatively stained specimens were recorded on a JEOL 100CS transmission electron microscope operated at 80 keV.
RESULTS
Efforts to isolate procapsids from HSV-1-infected cells are expected to be influenced by the fact that the procapsid is an assembly intermediate present only transiently during formation of the more stable, mature capsid (24). Procapsids are therefore expected in lower abundance than mature capsids. To address this problem, our studies were carried out with a protease-deficient HSV-1 mutant because cells infected with protease-defective viruses are found to accumulate large-cored B capsids (12, 30), structures that we interpret to be procapsids. Also, experience with capsid assembly in cell extracts has indicated that while the protease is not required for production of mature, icosahedral capsids, its presence significantly enhances conversion of the procapsid to the mature form (26).
m100 was considered an appropriate protease-deficient mutant for this study because while the protease domain of its parent gene is lacking, the scaffolding protein region (i.e., the UL26.5 gene) is present and functions normally (12). To isolate procapsids, cells were infected with m100 virus and lysed by sonication, and the lysates were treated with VP5-specific MAb 6F10, a treatment that precipitates procapsids (24). Precipitated procapsids were then examined by electron microscopy, SDS-polyacrylamide gel electrophoresis and tests to evaluate their sensitivity to disassembly at 0°C.
Electron microscopy.
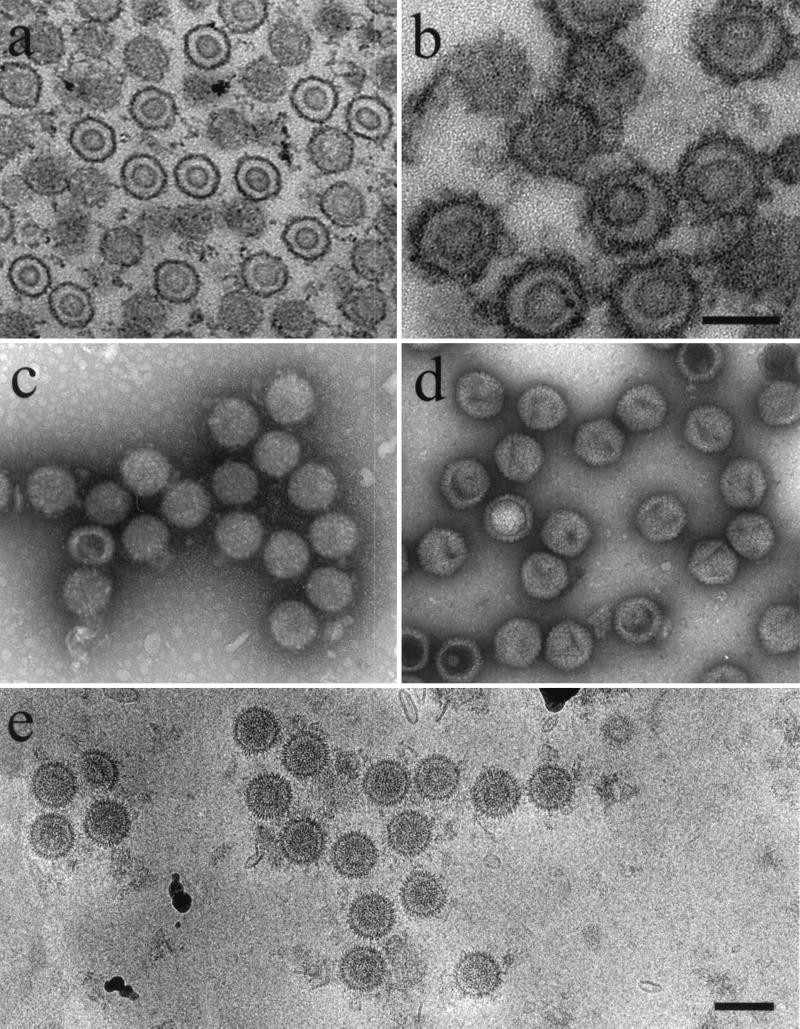
Figure 1b shows a thin-section electron micrograph of a procapsid-containing precipitate. In such preparations, m100 procapsids were found to be round or slightly irregular in overall shape, with distinct shell and core layers. The images of precipitated procapsids (Fig. 1b) were similar to those of procapsids seen in infected cell nuclei (Fig. 1a) (12, 30), suggesting that the isolation process did not markedly alter procapsid morphology.
FIG. 1.
Electron microscopy of HSV-1 procapsids. (a) Thin section showing procapsids in the nucleus of a BHK cell infected for 15 h with HSV-1 mutant m100; (b) thin section preparation of m100 procapsids after isolation by antibody precipitation; (c) m100 procapsids after negative staining with uranyl acetate; (d) negatively stained HSV-1 B-capsids; (e) m100 procapsids preserved in the frozen hydrated state. Note that m100 procapsids are round in profile (c and e) and consist of distinct shell and core layers. All micrographs are shown at the same magnification (bar = 1,500 Å) except for panel b (bar = 1,000 Å).
In negatively stained preparations (Fig. 1c), nearly all m100 procapsids were seen to be uniform in structure and round in profile, suggesting that they are spherical in shape. The round profile contrasts with that of HSV-1 B capsids (Fig. 1d), where distinct angles, indicative of a polyhedral morphology, are evident. Capsomers could be seen in most images of negatively stained procapsids (Fig. 1c). Micrographs of both thin-sectioned and negatively stained specimens indicated that most procapsids were intact. It was rare to see fragments of procapsids such as might arise from incomplete procapsid assembly or from fragmentation of complete procapsids (Fig. 1a to c).
Images of procapsids preserved in the frozen hydrated state also indicated that they have a uniform, spherical morphology with distinct shell and core layers (Fig. 1e). The measured procapsid diameter in such images was 124 ± 3 nm (n = 46), and capsomers could be seen in most individual procapsids (e.g., second procapsid from the right in Fig. 1e).
Three-dimensional reconstruction.
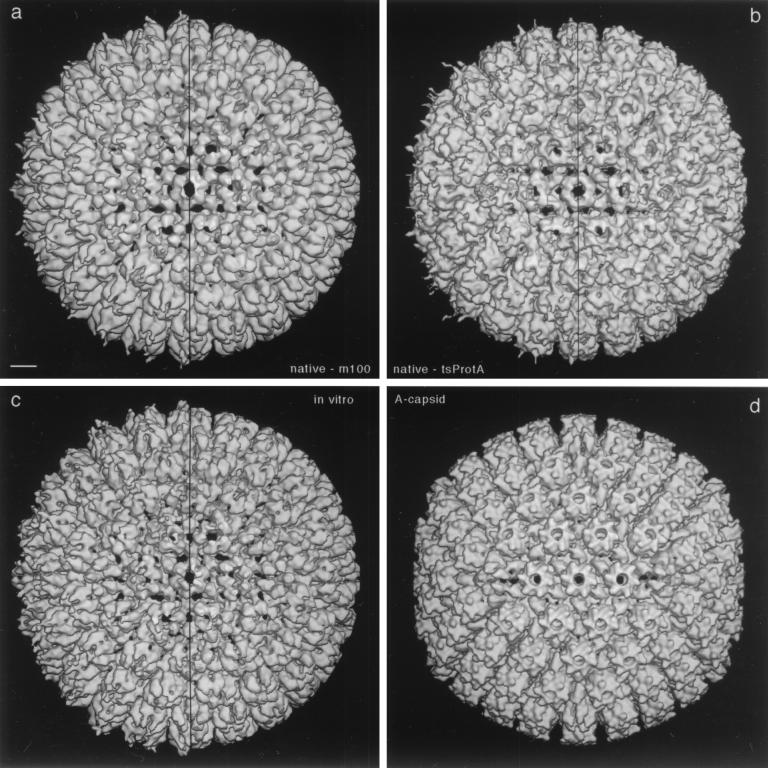
Images of cryopreserved specimens such as those shown in Fig. 1e were used to compute a three-dimensional reconstruction of the m100 procapsid. A total of 338 procapsid images were included in the reconstruction which was computed by the polar Fourier transform method as previously described (1, 46) to a resolution of 18 Å. A surface-shaded view of the reconstruction is shown in Fig. 2a viewed along an axis of twofold symmetry. The vertical line down the center of the image divides it into halves in which irregular density associated with antibodies bound to the tips of the capsomers has been computationally removed in the right half of the image. The irregularity of this density may be assigned to both partial occupancy and flexibility of the antibodies about their points of attachment to the capsid.
FIG. 2.
Three-dimensional reconstructions of HSV-1 procapsids from three different sources (a to c) compared to the mature capsid (d). (a) Native procapsids isolated from BHK cells infected with the mutant m100; (b) native procapsids isolated from BHK cells infected at the NPT temperature with tsProt.A; (c) procapsids assembled in vitro from lysates of Sf9 cells containing HSV-1 capsid proteins (reproduced from reference 46); (d) native A capsids isolated from BHK cells infected with wild-type HSV-1. All reconstructions are shown in surface-shaded representations as viewed along the icosahedral twofold axis of symmetry. To facilitate comparison, the reconstructions shown in panels a and d were restricted to 25-Å resolution, which is similar to the values for the reconstructions in panels b (∼28-Å resolution) and c (∼27-Å resolution). To isolate and concentrate the fragile procapsids for cryoelectron microscopy, they were precipitated with MAb 6F10 as described in Materials and Methods (24, 39). In consequence, some antibody-associated density is present around their peripheries in these density maps. The maps are represented both with this density present (left half of each panel) and with it computationally removed (right half) by setting the density to background outside a radius of ∼640 Å. Note that the three procapsids are essentially identical in structure. Bar = 100 Å.
To examine the m100 procapsid reconstruction, we find it helpful to start with the hexon at the center of the image (i.e., the hexon on the icosahedral twofold axis). Moving laterally to the right, one encounters a P hexon with the oval morphology characteristic of procapsid hexons (Fig. 3) (46). Further to the right is a penton, identifiable because it has five rather than six subunits. Proceeding upward and to the left, one sees a row of three hexons followed by a penton near 12 o'clock. A face hexon (C hexon [40]) can be identified in the angle created by the excursion described above.
FIG. 3.
Diagram showing the salient features of the T = 16 surface lattice of herpesvirus capsids. Pentons (capsomers with five nearest neighbors) are at each of the 12 vertices. There are three classes of hexons (capsomers with six nearest neighbors) distinguished according to their lattice sites. A peripentonal hexon (P hexon) is adjacent to a penton; E hexons are at the middle of each edge and sit on icosahedral twofold axes; and three C hexons are found at the center of each facet (adapted from reference 40). At each trigonal site (a site of local or global threefold symmetry, surrounded by three capsomers) is a triplex (15, 41). These heterotrimeric complexes are indicated by small triangles. Triplexes may be subclassified according to their positions on the surface lattice (52).
For comparison with the m100 procapsid, Fig. 2 shows a reconstruction of the HSV-1 A capsid (Fig. 2d) and the reconstruction described earlier of the procapsids assembled in vitro from extracts of insect cells containing HSV-1 proteins (Fig. 2c) (24). All reconstructions are viewed along the icosahedral twofold axis. Comparison of the three structures shows that whereas the A capsid is icosahedral in overall shape, the two procapsids are spherical with no evidence of angularity. Examination of capsomer morphology also emphasizes the similarity of m100 and in vitro procapsids. For example, the P hexon is found to be oval in the two procapsid reconstructions (Fig. 2a and c), whereas it is hexagonal in the A capsid (Fig. 2d). The same comparison is shown at higher magnification in Fig. 4a (m100 procapsid) and b (A capsid).
FIG. 4.
The structural changes that accompany HSV-1 capsid maturation are characterized by comparing the hexons (a and b) and pentons (c and d) of the m100 procapsid (a and c) and the mature capsid (A capsid; b and d). The outer surfaces are shown in each case. Panels a and b are centered on the E hexon which resides at each twofold axis and includes portions of the surrounding P and C hexons (for nomenclature, see the legend to Fig. 3). In the left halves of panels a and c, the less ordered density associated with the immunoprecipitating 6F10 antibodies is present, whereas it has been computationally removed in the right halves. The resolution is 18 Å. Bar = 100 Å.
Comparison of the reconstructions shows that the porous nature of procapsids assembled in vitro is also seen in m100 procapsids. For example, whereas a set of six small holes (black spots) surrounds the twofold axis hexon (E hexon) in both procapsid reconstructions, all are sealed in the A capsid (compare Fig. 2a and c with 2d and Fig. 4a with 4b). Similarly, holes surrounding the pentons in both procapsids are not seen in A-capsid pentons (compare Fig. 4c and d).
tsProt.A procapsids.
Procapsids were also isolated from cells infected with HSV-1 mutant tsProt.A. This mutant, constructed to contain the same amino acid changes found in ts1201 (12, 30), lacks function of the UL26-encoded protease when grown at the NPT (39°C), and so procapsids are expected to accumulate (5, 12, 30, 34). To isolate tsProt.A procapsids, therefore, cells were infected with tsProt.A at the NPT, and procapsids were harvested by antibody precipitation as described above for cells infected with m100. Electron microscopy of such procapsids supports the view that their structure is that of the procapsid and distinct from the mature capsid. For example, in both negatively stained and frozen hydrated preparations, tsProt.A procapsids appear round rather than angular in profile, suggesting the spherical procapsid morphology (data not shown).
A three-dimensional reconstruction computed from cryomicrographs of tsProt.A procapsids showed that they have the same basic structural features as in vitro and m100 procapsids. These include a spherical morphology, porous shell, and asymmetric hexons—oval in the case of P and E hexons and triangular in the case of C hexons (compare Fig. 2b with 2a and c). Like m100 procapsids, tsProt.A procapsids were found to be distinct in these features from mature capsids which are polyhedral and closed and have symmetric hexons (compare Fig. 2b with 2d). Although close comparison of the tsProt.A and m100 procapsids suggests subtle differences, at the present state of analysis these may be attributed to the lower resolution of the tsProt.A reconstruction (28 Å vs. 18 Å, and 89 particles vs. 338 particles) and perhaps to a slight difference in incipient maturation (see Discussion).
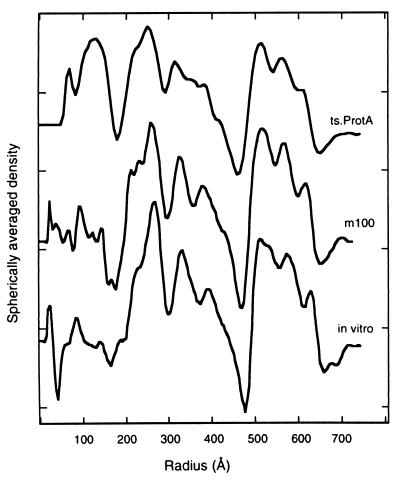
Spherical internal scaffolds are present in both m100 and tsProt.A procapsids, as in procapsids assembled in vitro (25, 46). In cryomicrographs (e.g., Fig. 1e), this feature appears as an inner ring of projected density. In the reconstructed density maps, there is very little contrast to differentiate local features within the capsid shell (data not shown), and such a density distribution could, in principle, arise from icosahedral averaging of a nonsymmetric structure. Thus, the question of whether the scaffold is icosahedrally symmetric remains unsettled. Nevertheless, the radial distribution of density through the procapsid, including the scaffold, may be examined by calculating spherically averaged radial density profiles from the corresponding three-dimensional density maps, and these are shown in Fig. 5.
FIG. 5.
Radial density profiles of HSV-1 procapsids from three sources: native procapsids isolated from BHK cells infected at the NPT with tsProt.A (top curve), native procapsids isolated from BHK cells infected with m100 (middle), and procapsids assembled in vitro from lysates of Sf9 cells containing HSV-1 capsid proteins (bottom) (46). The profiles were calculated by spherical averaging of the corresponding three-dimensional density maps. The main features of the surface shell (three peaks between radii of 480 and 650 Å) and the scaffold (three peaks between radii of 200 and 460 Å) are well conserved. However, the tsProt.A procapsids are the only ones to show a significant density peak inside the scaffold shell suggested to correspond to the HSV-1 UL26 gene product, the virus protease.
The profiles can be considered to consist of three regions: (i) a region from a radial distance of ∼480 to 640 Å corresponding to the procapsid shell, (ii) a scaffold region from ∼180 to 480 Å, and (iii) an inner region inside a radius of 180 Å. The density profiles were found to be quite similar for the three procapsid reconstructions, particularly in the shell layer, which was found to have almost exactly the same thickness and radial distance from the center in the three reconstructions. The radius of the external procapsid edge, for example, differed by less than 20 Å among the three procapsids. Three peaks of density were found in the shell region of each procapsid type. The scaffold region was also similar among the three reconstructions, showing three major peaks of density. Among the three procapsid types, the major difference was prominent density in the inner region of tsProt.A procapsids (radial distance of ∼100 to 160 Å) that was absent in the profiles of in vitro and m100 procapsids.
Procapsid maturation.
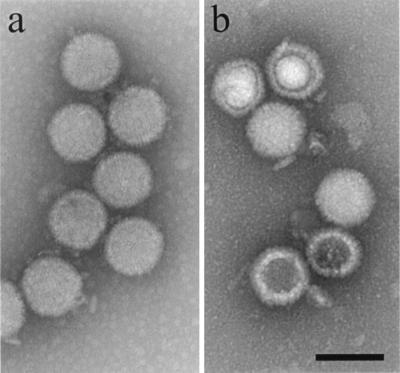
Both m100 and tsProt.A procapsids were tested for the ability to transform in vitro into the mature, icosahedral capsid morphology. Tests were carried out with procapsids that were suspended in PBS at a concentration of approximately 0.5 mg/ml, incubated at room temperature (21°C), and tested by electron microscopy for their conversion to the mature capsid morphology. High proportions (greater than 80%) of both m100 and tsProt.A procapsids were found to be transformed in most experiments. Figure 6, for example, shows the results obtained with tsProt.A procapsids before (Fig. 6a) and after (Fig. 6b) incubation for 72 h at 21°C. Note that capsids were round in profile before incubation but angular afterward. Prolonged incubation at 21°C was required for procapsids to be transformed. It was rare to see any evidence of procapsid transformation prior to approximately 48 h of incubation, but most procapsids were converted by 60 to 72 h.
FIG. 6.
tsProt.A procapsids before (a) and after (b) incubation at 21°C for 72 h. Procapsids were isolated by antibody precipitation as described in Materials and Methods from BHK cells infected for 15 h at 39°C (the NPT). Note that before incubation (a) procapsids are round in profile, indicating they have not angularized, while after incubation (b) capsid profiles show angles indicating they have the mature, icosahedral structure. Bar = 1,500 Å.
Cold sensitivity.
The sensitivity of m100 and tsProt.A procapsids to dissociation following incubation at 0°C was tested by electron microscopy of negatively stained specimens. Procapsids found to be intact after incubation at room temperature (21°C) or 0°C were counted as a measure of structural integrity. The results showed a marked decrease in the number of both m100 and tsProt.A procapsids present after incubation at 0°C, suggesting they were dissociated at the cold temperature. Representative results for m100 procapsids are shown in Table 1. No comparable decrease in the number of B capsids was observed after similar incubation at 0°C. Material found in micrographs of dissociated procapsids could not be interpreted to suggest any clear structural relationship to the parent procapsid (data not shown).
TABLE 1.
Effect of incubation at 0°C on the integrity of m100 procapsids and wild-type HSV-1 B-capsidsa
| Incubation for 30 min at (°C): | Mean no./electron microscope field ± SD (n)
|
|
|---|---|---|
| m100 procapsids | HSV-1 B capsids | |
| 21 | 120 ± 18 (3) | 178 ± 30 (n = 3) |
| 0 | 4 ± 3 (4) | 199 ± 45 (n = 4) |
The procedures described in Materials and Methods were used to isolate procapsids from BHK cells infected for 18 h with HSV-1 m100 virus. The procapsid-containing precipitate was resuspended in 100 μl of PBS and divided into two aliquots, which were incubated at room temperature (21°C) or 0°C. Samples were then applied to electron microscope grids, negatively stained with 1% uranyl acetate, and photographed in the electron microscope. Procapsids were counted on electron microscope negatives which were recorded at a magnification of ×8,300. The same analysis was performed with wild-type HSV-1 B capsids which were prepared from infected BHK cells as previously described (27).
Protein composition.
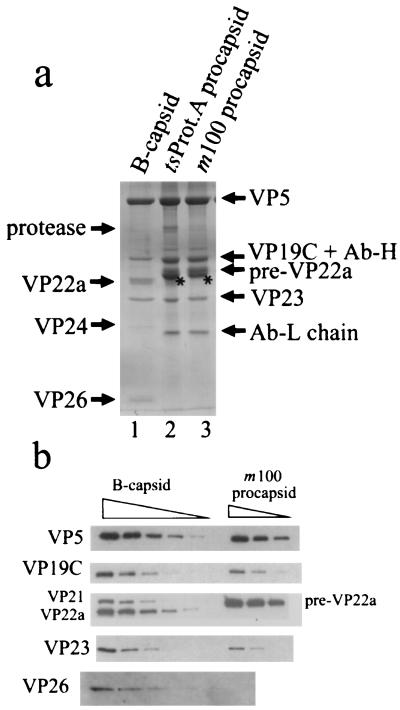
The protein composition of procapsids was determined by SDS-polyacrylamide gel electrophoresis followed by staining with Coomassie blue. The results shown in Fig. 7a demonstrated that both m100 and tsProt.A procapsids contain the major capsid protein (VP5), the two triplex proteins (VP19C and VP23), and the scaffolding protein, pre-VP22a. Only the uncleaved form of the scaffolding protein (i.e., pre-VP22a) was detected in m100 procapsids. In tsProt.A procapsids most of the scaffolding protein was uncleaved, but a trace of the cleaved form, VP22a, was observed. In addition to the proteins mentioned above, tsProt.A procapsids contained a small amount of a protein migrating with an apparent molecular mass of ∼66 kDa (labeled “protease” in Fig. 7a) and staining with a MAb (MCA406) specific for the UL26 and UL26.5 gene products (data not shown). We infer that this protein corresponds to the full-length UL26 gene product. No VP24 was found associated with tsProt.A procapsids. As expected, no 66-kDa protein was detected in m100 procapsids. Neither m100 nor tsProt.A procapsids were found to contain VP26 (Fig. 7a), a 12-kDa polypeptide found at the hexon tips in mature capsids (3, 53).
FIG. 7.
Protein composition of HSV-1 procapsids. SDS-polyacrylamide gel electrophoresis of m100 and tsProt.A procapsids was followed by staining with Coomassie blue (a) and Western immunoblotting with specific antibodies (b). Similar analyses of HSV-1 B-capsids are shown for comparison. The starred bands in lanes 2 and 3 of panel a were identified as actin as described in the text. The positions of the HSV-1 protease (UL26 gene product) and the antibody heavy and light chains (Ab-H and Ab-L) are indicated in panel a. Specimens in panel b were loaded for electrophoresis in twofold dilutions. The amount of sample loaded in the VP26 row was twofold greater than that loaded in other rows. Note that procapsid bands identified as VP5, VP19C, pre-VP22a, and VP23 were stained with specific antibodies, and VP26 was not detected in either m100 or tsProt.A procapsids.
In addition to the capsid proteins described above, stained gels of both m100 and tsProt.A procapsid proteins were found to contain a previously unrecognized protein migrating slightly more rapidly than pre-VP22a (starred band in Fig. 7a). In Western immunoblots, this protein stained positively with a MAb (clone C4; ICN Pharmaceuticals) specific for actin (data not shown). We assume therefore that the protein corresponds to actin and that it is derived from the BHK cells from which procapsids were isolated.
Antibodies specific for HSV-1 capsid proteins were used in Western immunoblotting to confirm the identities of the m100 procapsid proteins described above. Bands identified as VP5, VP19C, pre-VP22a, and VP23 were found to react with specific antibodies as shown in Fig. 7b. No VP26 was detected by immunoblot analysis of m100 procapsids, however, despite the fact that it was detected in control HSV-1 B capsids (Fig. 7) and in m100-infected cell extracts (data not shown).
Stained gels such as that shown in Fig. 7a were used to make quantitative measurements of the m100 procapsid protein composition. Protein amounts were determined by densitometric scanning of stained gels, and copy numbers were estimated on the assumption that procapsids contain 960 copies of VP5, the number present in mature capsids (15, 27). Analysis was affected by the fact that during electrophoresis, VP19C migrates coincidently with the heavy chain of the antibody used to precipitate procapsids. No quantitative measurement could therefore be made for VP19C. Pre-VP22a and actin bands were closely spaced, but resolution was sufficient to permit them to be determined separately. Protein copy numbers determined for pre-VP22a and VP23 were 1,918 ± 170 (n = 4) and 730 ± 308 (n = 4), respectively. For comparison, the VP23 copy number in mature capsids is 640 (15, 33, 41). Although no quantitative determination of VP19C could be obtained from Coomassie blue-stained gels, comparison of the intensities of the Western immunoblot signals confirmed that VP19C, and the other capsid shell proteins, were present at comparable levels in all capsid types (Fig. 7b).
The scaffolding protein content of m100 procapsids was also determined beginning with the three-dimensional reconstruction shown in Fig. 2a. The mass in the scaffold region of the radial density profile was calculated by an appropriately weighted integral of the density above background in the scaffold region (between radii of 180 and 480 Å) and calibrated against the corresponding integral for the surface shell, which was taken to be 180.9 MDa (Table 2). Taking into account the molecular weight of pre-VP22a, the calculations yielded a copy number of 1,866 to 2,070 scaffolding protein molecules per procapsid depending on where the baseline of the radial density profile was set. Similar values were obtained earlier by radial integration of the reconstructions computed for procapsids assembled in vitro (Table 2). The range of values obtained for m100 procapsids is in satisfactory agreement with the value, 1,918 ± 170 pre-VP22a molecules/m100 procapsid (see above), determined from gel electrophoresis of procapsid proteins.
TABLE 2.
Procapsid scaffolding protein content measured from three-dimensional reconstructions
| Procapsid source | Scaffold mass (MDa)a | Scaffold protein copy no.b | Reference |
|---|---|---|---|
| Assembled in vitro in cell extracts | 66.4–76.7 | 1,967–2,272 | 46 |
| Assembled in vitro from purified proteins | 75.8 | 1,902 | 25 |
| Isolated from m100- infected cells | 63.0–69.9 | 1,866–2,070 | This study |
Determined by integration of radial electron density profiles such as those shown in Fig. 4. Ranges of values are shown for reconstructions in which there was uncertainty regarding the baseline in the radial density profile. The scaffold mass was calibrated relative to the shell mass, which was assumed to be 180.9 MDa (i.e., 960, 320, and 640 copies of VP5, VP19C, and VP23, respectively).
Calculated by assuming the following molecular weights: for preVP22a, 33,760 (cell extract and m100 procapsids); for pUL80.5-H, 39,855 (purified protein procapsids).
DISCUSSION
MAb 6F10 was used initially to isolate procapsids from lysates of m100-infected cells because it was found to be effective in precipitating procapsids formed in vitro. 6F10 also precipitates capsids with the mature morphology such as A and B capsids, but it appears to be particularly efficient in precipitating procapsids. Attempts were made to isolate m100 and tsProt.A procapsids by sucrose density gradient centrifugation, but these efforts met with only limited success. Since antibody precipitation showed procapsids were present in infected cell lysates, we assume the procedures used for sucrose gradient isolation resulted in procapsid maturation, degradation, aggregation or disassembly.
Procapsids assembled in vivo and in vitro are structurally indistinguishable.
Electron micrographs of m100 (Fig. 1) and tsProt.A (data not shown) procapsids show structures with round profiles suggesting that they have the spherical morphology described earlier for procapsids assembled in vitro (24). It was rare to see capsids with angles in precipitates from m100 or tsProt.A-infected cells. The very high proportion of procapsids compared to polyhedral capsids present in lysates of m100- and tsProt.A-infected cells supports the view that procapsids are the predominant capsid type that accumulates in infected cells lacking activity of the maturational protease (5, 7, 12, 24, 30, 34).
The three-dimensional reconstructions of m100 and tsProt.A procapsids (Fig. 2a and b) revealed a wealth of structural information not present in images of negatively stained or thin-sectioned specimens. Of particular interest is the marked similarity of the m100 procapsid structure with that of procapsids assembled in vitro from cell extracts (Fig. 2c). In the shell layer particularly, the m100 and in vitro procapsid structures were found to be identical in even the subtlest features seen at the resolution of the current reconstructions (compare Fig. 2a and c). Such features include the structures of the hexons, the pentons, the triplexes, and holes through the capsid shell. There can be little doubt therefore that the m100 procapsid is the structural homolog of procapsids assembled in vitro. The homology is further emphasized by the cold sensitivity of m100 procapsids (Table 1), a defining property of procapsids assembled in vitro (24, 25).
The three-dimensional reconstruction of tsProt.A procapsids shows they have the same basic structure as m100 and in vitro procapsids. Such small differences as are seen between the respective density maps (e.g., a slightly more symmetrical hexon morphology in tsProt.A procapsids) may reflect either differing resolution or very early steps of maturation in tsProt.A procapsids. Such early maturation steps could be promoted by expression of a low level of protease function during procapsid isolation. The structure of the tsProt.A procapsid is of particular significance because it is known that tsProt.A procapsids can mature into infectious virions in vivo (5, 30, 34). Thus, maturability in vivo is a property of procapsids with the structure defined here for m100 and tsProt.A procapsids.
Procapsid protein composition.
The predominant protein components of m100 and tsProt.A procapsids were found to be the same as those of procapsids assembled in vitro, namely, VP5, VP19C, VP23, and pre-VP22a. Apart from the presence of the protease in tsProt.A procapsids (Fig. 7a), in vivo procapsids did not contain any major protein species observable by Coomassie blue staining that was not also present in in vitro procapsids. The above observation was unexpected since in vivo procapsids have the potential to mature into virions and might therefore contain proteins involved in DNA processing and packaging not found in procapsids formed in vitro. Since small amounts of DNA processing and packaging proteins, detectable in Western immunoblots, are found associated with intracellular mature capsids (e.g., B capsids [21, 36, 43, 51]), we have initiated immunoblot studies to attempt to detect them in procapsids derived from m100- and tsProt.A-infected cells.
VP26 is a small (12-kDa) protein located at the distal tips of HSV-1 capsid hexons (3, 46, 53). One VP26 molecule is bound to each VP5 in all 150 hexons, and so there are a total of 900 VP26 molecules in a mature capsid. Studies with an HSV-1 mutant lacking the gene (UL35) encoding VP26 have demonstrated that VP26 potentiates HSV-1 growth in neural cells in vivo but is not required for virus replication in cell culture (8). The protein analyses reported here consistently failed to detect VP26 in either m100 or tsProt.A procapsids despite the fact that the same methods revealed its presence in B capsids (Fig. 7) and in the cells from which m100 and tsProt.A procapsids were isolated (data not shown). We conclude that VP26 is not a component of the HSV-1 procapsid. Since it is present in virions and in mature capsids, it must be added after procapsids are formed. The function of VP26 in capsids must be expressed in a process (e.g., DNA packaging or addition of tegument) that occurs as the mature capsid is formed or thereafter.
SDS-polyacrylamide gel analysis of m100 and tsProt.A procapsid precipitates demonstrated the presence of a band corresponding to cellular actin (starred band in Fig. 7a), a protein not seen in procapsids precipitated from Sf9 cell extracts (24). Two further observations relate to interpretation of the presence of actin. First, analysis of 6F10 immune precipitates from uninfected BHK cells showed the same actin band (data not shown). Second, no evidence of procapsid-associated actin was observed in electron micrographs of procapsids or in the three-dimensional reconstructions (Fig. 2a and b). We suggest therefore that actin in procapsid precipitates may result from a cross-reaction of MAb 6F10 with BHK cell actin or with a protein bound to actin. As indicated above, the cross-reacting form of actin is expected to be present in BHK but not Sf9 cells.
Scaffold structure: tentative localization of the protease and nature of procapsid building blocks.
The three-dimensional reconstructions of m100 and tsProt.A procapsids showed no strongly contrasted features in the core region (data not shown). The same result was obtained earlier with procapsids assembled in vitro (24, 46). As we would expect such features to be visible at the resolution now reached (18 Å), we conclude it is unlikely that the scaffold has highly ordered, icosahedrally symmetric features as the shell does. The absence of icosahedral ordering in the core is consistent with the suggestion made earlier (46) that the scaffold is organized as a protein micelle in which individual scaffold molecules are highly extended and arranged radially with their C termini attached to the shell and N termini extending inward toward the procapsid center.
More revealing information about the structure of the core was obtained from the radial density profiles shown in Fig. 5. In the region corresponding to the scaffolding protein (i.e., between radial distances of 180 and 480 Å), procapsids assembled in vitro showed three peaks of density interpreted earlier to correspond to three condensed scaffolding protein domains separated by flexible linkers (46). The same three peaks of density are readily identifiable in the profiles of m100 and tsProt.A procapsids (Fig. 4), suggesting that the basic arrangement of the scaffold is the same in the three procapsid types.
In the innermost region of the core, at a radial distance of less than 180 Å, the density profile computed for tsProt.A procapsids shows a large peak of density that is absent in m100 and in vitro procapsids (Fig. 5). This peak suggests itself as the location of the maturational protease (UL26 gene product), as the protease is found in tsProt.A but not in m100 or in vitro procapsids (Fig. 7a). Further studies, perhaps with mutants containing deletions in the UL26 gene, should be carried out to confirm this presumptive location for the protease.
The scaffolding protein content of m100 procapsids was determined quantitatively from stained SDS-polyacrylamide gels and from integration of the scaffolding protein region in the three-dimensional reconstruction. The values obtained, 1,918 ± 170 and 1,866 to 2,070 copies per procapsid respectively, are in good agreement with each other and with the scaffolding protein content of procapsids assembled in vitro (Table 2). Compared to other virus procapsids, the amount of m100 procapsid scaffolding protein appears high. For example, the molar ratio of scaffolding to major capsid protein in m100 procapsids is approximately 2.0 (i.e., 1,918 ± 170 scaffold:960 VP5 molecules), while the comparable values for the procapsids of phages P22, φ29, and T4 are 0.71 (300 scaffold:420 capsid), 0.9 (180 scaffold:200 capsid), and 0.67 (640 gp22 plus gp21:960 major capsid), respectively (2, 18, 31). The greater number of scaffolding protein molecules in HSV-1 procapsids may be necessitated by the larger major capsid protein (VP5 molecular weight of ∼150,000, compared with 47,500, for instance, for the phage P22 coat protein, gp5) or perhaps by the greater diameter of the HSV-1 procapsid (∼126 nm, compared with 58 nm for the P22 phage procapsid [29]). For example, despite the greater diameter of the HSV-1 procapsid compared to that of phage P22, the amounts of scaffolding protein per unit of volume are similar in the two, calculated values being 0.21 and 0.15 kDa/nm3 for HSV-1 and P22, respectively (assuming that HSV-1 and P22 procapsid cavities have diameters of 950 and 450 Å).
The molar ratio of approximately 1 VP5:2 scaffolding molecules observed in the m100 procapsid may provide a clue about the nature of the subunit(s) used in procapsid growth. Since the procapsid could be formed from subunits with the same molecular proportions as the completed structure, assembly units with a 1:2 VP5:scaffolding protein molar ratio are suggested. It is relevant to note therefore that sucrose gradient studies of the VP5-scaffolding protein interaction revealed complexes containing 1 VP5 plus 2 scaffold molecules and 2 VP5 plus 4 scaffold molecules (25). Both complexes are attractive as potential procapsid assembly units.
The pre-VP22a content of m100 procapsids (i.e., 1,918 ± 170 copies per procapsid) was found to be significantly higher than the content determined earlier of VP22a in HSV-1 B-capsids (1,153 ± 169 copies per capsid [27]). This observation suggests some of the scaffolding protein is lost from the procapsid as it matures to form the B capsid.
Procapsid maturation.
Isolation of procapsids from m100- and tsProt.A-infected cells as described here suggests that procapsids may function in vivo in the same way they do in vitro, as precursors to the mature capsid form. As an overall measure of the ability of m100 and tsProt.A procapsids to undergo further development, they were tested in vitro for the ability to be transformed into the icosahedral capsid morphology. Tests showed that both procapsid types were able to mature (Fig. 6), with a high proportion being transformed in each case. We interpret the results to support the view that procapsids function as assembly intermediates in vivo. The time required for transformation at 21°C, however, was longer than is expected in infected cells. For example, 60 to 72 h were required for procapsid transformation in vitro whereas in extracts maturation was complete in 8 h (24), and in infected cells the same process requires less than 3 h (5). We conclude that while m100 and tsProt.A procapsids are capable of maturation in vitro, the rate of transformation in vivo is enhanced by some factor(s) not present in our in vitro incubations. Absence of the protease may account for at least part of the delay in the case of m100 procapsids. With tsProt.A procapsids, the time required for thermoreversion of the protease may contribute to the observed delay. Packaging of virus DNA in vivo may also be involved in promoting the transformation from the procapsid to the mature capsid structure.
It is now clear that the overall pathway of HSV-1 capsid formation has important similarities to that observed in dsDNA bacteriophage such as P22, T4, φ29, and λ (4, 13, 31). For instance in dsDNA phage, capsid formation involves a scaffolding protein, and it proceeds by way of a spherical, more fragile procapsid intermediate. DNA is packaged into an empty phage capsid, and in most cases where the process has been well studied, packaging is found to be initiated with the procapsid whose shell matures into the icosahedral form before packaging is completed (14, 16, 17, 19, 37; but see reference 32). If HSV-1 DNA packaging also conforms to the phage model, then in the future it may be productive to examine the role of HSV-1 procapsids in initiation of DNA encapsidation.
ACKNOWLEDGMENTS
We thank David Burkwall, Oneida Mason, Min Gao, David Belnap, Geoffrey Williams, and James Conway for help and advice during the course of this study. We are also most grateful to Gary Cohen and Richard Courtney for providing antibodies specific for HSV-1 capsid proteins.
This work was supported in part by NIH grants AI41644 and AI37549 and by NSF grant MCB 9904879.
REFERENCES
- 1.Baker T S, Cheng R H. A model-based approach for determining orientations of biological molecules imaged by cryoelectron microscopy. J Struct Biol. 1996;116:120–130. doi: 10.1006/jsbi.1996.0020. [DOI] [PubMed] [Google Scholar]
- 2.Black L W, Showe M K, Steven A C. Morphogenesis of the T4 head. In: Karam J D, editor. Bacteriophage T4. Washington, D.C.: ASM Press; 1994. pp. 218–258. [Google Scholar]
- 3.Booy F P, Trus B L, Newcomb W W, Brown J C, Conway J F, Steven A C. Finding a needle in a haystack: detection of a small protein (the 12-kDa VP26) in a large complex (the 200-MDa capsid of herpes simplex virus) Proc Natl Acad Sci USA. 1994;91:5652–5656. doi: 10.1073/pnas.91.12.5652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Casjens S, Hendrix R. Control mechanisms in dsDNA bacteriophage assembly. In: Calendar R, editor. The bacteriophages. New York, N.Y: Plenum Press; 1988. pp. 15–91. [Google Scholar]
- 5.Church G A, Wilson D W. Study of herpes simplex virus maturation during a synchronous wave of assembly. J Virol. 1997;71:3603–3612. doi: 10.1128/jvi.71.5.3603-3612.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conway J F, Trus B L, Booy F P, Newcomb W W, Brown J C, Steven A C. Visualization of three-dimensional density maps reconstructed from cryo-electron micrographs of viral capsids. J Struct Biol. 1996;116:200–208. doi: 10.1006/jsbi.1996.0031. [DOI] [PubMed] [Google Scholar]
- 7.Dasgupta A, Wilson D W. ATP depletion blocks herpes simplex virus DNA packaging and capsid maturation. J Virol. 1999;73:2006–2015. doi: 10.1128/jvi.73.3.2006-2015.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desai P, DeLuca N A, Person S. Herpes simplex virus type 1 VP26 is not essential for replication in cell culture but influences production of infectious virus in the nervous system of infected mice. Virology. 1998;247:115–124. doi: 10.1006/viro.1998.9230. [DOI] [PubMed] [Google Scholar]
- 9.Engvall E, Perlman P. Enzyme-linked immunosorbent assay (ELISA): quantitative assay for immunoglobulin. Immunochemistry. 1971;8:871–874. doi: 10.1016/0019-2791(71)90454-x. [DOI] [PubMed] [Google Scholar]
- 10.Ey P L, Prowse S J, Jenkin C R. Isolation of pure IgG1, IgG2a and IgG2b immunoglobulins from mouse serum using protein A-sepharose. Immunochemistry. 1978;15:429–436. doi: 10.1016/0161-5890(78)90070-6. [DOI] [PubMed] [Google Scholar]
- 11.Fuller S D, Butcher S J, Cheng R H, Baker T S. Three-dimensional reconstruction of icosahedral particles—the uncommon line. J Struct Biol. 1996;116:48–55. doi: 10.1006/jsbi.1996.0009. [DOI] [PubMed] [Google Scholar]
- 12.Gao M, Matusick-Kumar L, Hurlburt W, DiTusa S F, Newcomb W W, Brown J C, McCann III P J, Deckman I, Colonno R J. The protease of herpes simplex virus type 1 is essential for functional capsid formation and viral growth. J Virol. 1994;68:3702–3712. doi: 10.1128/jvi.68.6.3702-3712.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hendrix R W. Shape determination in virus assembly: the bacteriophage example. In: Casjens S, editor. Virus structure and assembly. Boston, Mass: Jones and Bartlett Publishers, Inc.; 1985. pp. 169–203. [Google Scholar]
- 14.Hohn B, Hohn T. Activity of empty, headlike particles for packaging of DNA in bacteriophage lambda in vitro. Proc Natl Acad Sci USA. 1974;71:2372–2376. doi: 10.1073/pnas.71.6.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Homa F L, Brown J C. Capsid assembly and DNA packaging in herpes simplex virus. Rev Med Virol. 1997;7:107–122. doi: 10.1002/(sici)1099-1654(199707)7:2<107::aid-rmv191>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 16.Jardine P J, Coombs D H. Capsid expansion follows the initiation of DNA packaging in bacteriophage T4. J Mol Biol. 1998;284:661–672. doi: 10.1006/jmbi.1998.2179. [DOI] [PubMed] [Google Scholar]
- 17.Jardine P J, McCormick M C, Lutze-Wallace C, Coombs D H. The bacteriophage T4 DNA packaging apparatus targets the unexpanded prohead. J Mol Biol. 1998;284:647–659. doi: 10.1006/jmbi.1998.2178. [DOI] [PubMed] [Google Scholar]
- 18.Lee C S, Guo P. In vitro assembly of infectious virions of double-stranded DNA phage φ29 from cloned gene products and synthetic nucleic acids. J Virol. 1995;69:5018–5023. doi: 10.1128/jvi.69.8.5018-5023.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Masker W E, Serwer P. DNA packaging in vitro by an isolated bacteriophage T7 procapsid. J Virol. 1982;43:1138–1142. doi: 10.1128/jvi.43.3.1138-1142.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Matusick-Kumar L, Hurlburt W, Weinheimer S P, Newcomb W W, Brown J C, Gao M. Phenotype of the herpes simplex virus type-1 protease substrate ICP35 mutant virus. J Virol. 1994;68:5384–5394. doi: 10.1128/jvi.68.9.5384-5394.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McNab A R, Desai P, Person S, Roof L L, Thomsen D R, Newcomb W W, Brown J C, Homa F L. The product of the herpes simplex virus type 1 UL25 gene is required for encapsidation but not for cleavage of replicated viral DNA. J Virol. 1998;72:1060–1070. doi: 10.1128/jvi.72.2.1060-1070.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McNabb D S, Courtney R J. Identification and characterization of the herpes simplex virus type 1 virion protein encoded by the UL35 open reading frame. J Virol. 1992;66:2653–2663. doi: 10.1128/jvi.66.5.2653-2663.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Newcomb W W, Brown J C. Use of Ar+ plasma etching to localize structural proteins in the capsid of herpes simplex virus type 1. J Virol. 1989;63:4697–4702. doi: 10.1128/jvi.63.11.4697-4702.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Newcomb W W, Homa F L, Thomsen D R, Booy F P, Trus B L, Steven A C, Spencer J V, Brown J C. Assembly of the herpes simplex virus capsid: characterization of intermediates observed during cell-free capsid assembly. J Mol Biol. 1996;263:432–446. doi: 10.1006/jmbi.1996.0587. [DOI] [PubMed] [Google Scholar]
- 25.Newcomb W W, Homa F L, Thomsen D R, Trus B L, Cheng N, Steven A C, Booy F P, Brown J C. Assembly of the herpes simplex virus procapsid from purified components and identification of small complexes containing the major capsid and scaffolding proteins. J Virol. 1999;73:4239–4250. doi: 10.1128/jvi.73.5.4239-4250.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newcomb W W, Homa F L, Thomsen D R, Ye Z, Brown J C. Cell-free assembly of the herpes simplex virus capsid. J Virol. 1994;68:6059–6063. doi: 10.1128/jvi.68.9.6059-6063.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Newcomb W W, Trus B L, Booy F P, Steven A C, Wall J S, Brown J C. Structure of the herpes simplex virus capsid: molecular composition of the pentons and the triplexes. J Mol Biol. 1993;232:499–511. doi: 10.1006/jmbi.1993.1406. [DOI] [PubMed] [Google Scholar]
- 28.Poteete A R, Jarvik V, Botstein D. Encapsulation of phage P22 DNA in vitro. Virology. 1979;95:550–564. doi: 10.1016/0042-6822(79)90508-7. [DOI] [PubMed] [Google Scholar]
- 29.Prasad B V V, Prevelige P E, Marietta E, Chen R O, Thomas D, King J, Chiu W. Three-dimensional transformation of capsids associated with genome packaging in a bacterial virus. J Mol Biol. 1993;231:65–74. doi: 10.1006/jmbi.1993.1257. [DOI] [PubMed] [Google Scholar]
- 30.Preston V G, Coates J A V, Rixon F J. Identification and characterization of a herpes simplex virus gene product required for encapsidation of virus DNA. J Virol. 1983;45:1056–1064. doi: 10.1128/jvi.45.3.1056-1064.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prevelige P E, King J. Assembly of bacteriophage P22: a model for ds-DNA virus assembly. Prog Med Virol. 1993;40:206–221. [PubMed] [Google Scholar]
- 32.Rao V B, Black L W. DNA packaging of bacteriophage T4 proheads in vitro; evidence that prohead expansion is not coupled to DNA packaging. J Mol Biol. 1985;185:565–578. doi: 10.1016/0022-2836(85)90072-5. [DOI] [PubMed] [Google Scholar]
- 33.Rixon F J. Structure and assembly of herpesviruses. Semin Virol. 1993;4:135–144. [Google Scholar]
- 34.Rixon F J, McNab D. Packaging-competent capsids of a herpes simplex virus temperature-sensitive mutant have properties similar to those of in vitro-assembled procapsids. J Virol. 1999;73:5714–5721. doi: 10.1128/jvi.73.7.5714-5721.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Roizman B, Sears A E. Herpes simplex viruses and their replication. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Roizman B, Straus S E, editors. Fields virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2231–2295. [Google Scholar]
- 36.Salmon B, Baines J D. Herpes simplex virus DNA cleavage and packaging: association of multiple forms of UL15-encoded proteins with B capsids requires at least the UL6, UL17, and UL28 genes. J Virol. 1998;72:3045–3050. doi: 10.1128/jvi.72.4.3045-3050.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shibata H, Fujisawa H, Minagawa T. Characterization of the bacteriophage T3 DNA packaging reaction in vitro in a defined system. J Mol Biol. 1987;196:845–851. doi: 10.1016/0022-2836(87)90409-8. [DOI] [PubMed] [Google Scholar]
- 38.Sodeik B, Ebersold M W, Helenius A. Microtubule-mediated transport of incoming herpes simplex virus 1 capsids to the nucleus. J Cell Biol. 1997;136:1007–1021. doi: 10.1083/jcb.136.5.1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Spencer J V, Trus B L, Booy F P, Steven A C, Newcomb W W, Brown J C. Structure of the herpes simplex virus capsid: peptide A862-H880 of the major capsid protein is displayed on the rim of the capsomer protrusions. Virology. 1997;228:229–235. doi: 10.1006/viro.1996.8392. [DOI] [PubMed] [Google Scholar]
- 40.Steven A C, Roberts C R, Hay J, Bisher M E, Pun T, Trus B L. Hexavalent capsomers of herpes simplex virus type 2: symmetry, shape, dimensions, and oligomeric status. J Virol. 1986;57:578–584. doi: 10.1128/jvi.57.2.578-584.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steven A C, Spear P G. Herpesvirus capsid assembly and envelopment. In: Burnett R, Chiu W, Garcea R, editors. Structural biology of viruses. New York, N.Y: Oxford University Press; 1996. pp. 312–351. [Google Scholar]
- 42.Tatman J D, Preston V G, Nicholson P, Elliott R M, Rixon F J. Assembly of herpes simplex virus type 1 capsids using a panel of recombinant baculoviruses. J Gen Virol. 1994;75:1101–1113. doi: 10.1099/0022-1317-75-5-1101. [DOI] [PubMed] [Google Scholar]
- 43.Taus N S, Baines J D. Herpes simplex virus 1 DNA cleavage/packaging: the UL28 gene encodes a minor component of B capsids. Virology. 1998;252:443–449. doi: 10.1006/viro.1998.9475. [DOI] [PubMed] [Google Scholar]
- 44.Thomas D, Newcomb W W, Brown J C, Wall J S, Hainfeld J F, Trus B L, Steven A C. Mass and molecular composition of vesicular stomatitis virus: a scanning transmission electron microscopy analysis. J Virol. 1985;54:598–607. doi: 10.1128/jvi.54.2.598-607.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomsen D R, Roof L L, Homa F L. Assembly of herpes simplex virus (HSV) intermediate capsids in insect cells infected with recombinant baculoviruses expressing HSV capsid proteins. J Virol. 1994;68:2442–2457. doi: 10.1128/jvi.68.4.2442-2457.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Trus B L, Homa F L, Booy F P, Newcomb W W, Thomsen D R, Cheng N, Brown J C, Steven A C. Herpes simplex virus capsids assembled in insect cells infected with recombinant baculoviruses: structural authenticity and localization of VP26. J Virol. 1995;69:7362–7366. doi: 10.1128/jvi.69.11.7362-7366.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trus B L, Booy F P, Newcomb W W, Brown J C, Homa F L, Thomsen D R, Steven A C. The herpes simplex virus procapsid: structure, conformational changes upon maturation, and roles of the triplex proteins VP19C and VP23 in assembly. J Mol Biol. 1996;263:447–462. doi: 10.1016/s0022-2836(96)80018-0. [DOI] [PubMed] [Google Scholar]
- 48.Trus B L, Roden R B, Greenstone H L, Vrhel M, Schiller J T, Booy F P. Novel structural features of bovine papillomavirus capsid revealed by a three-dimensional reconstruction to 9 Å resolution. Nat Struct Biol. 1997;4:413–420. doi: 10.1038/nsb0597-413. [DOI] [PubMed] [Google Scholar]
- 49.Vernon S, Ponce de Leon M, Cohen G, Eisenberg R, Rubin B. Morphological components of herpesvirus. III. Localization of herpes simplex virus type 1 nucleocapsid polypeptides by immune electron microscopy. J Gen Virol. 1981;54:39–46. doi: 10.1099/0022-1317-54-1-39. [DOI] [PubMed] [Google Scholar]
- 50.Whitley R J. Herpes simplex viruses. In: Fields B N, Knipe D M, Howley P M, Chanock R M, Melnick J L, Monath T P, Strauss S E, Roizman B, editors. Fields' virology. Philadelphia, Pa: Lippincott-Raven; 1996. pp. 2297–2342. [Google Scholar]
- 51.Yu D, Weller S K. Herpes simplex virus type 1 cleavage and packaging proteins UL15 and UL28 are associated with B but not C capsids during packaging. J Virol. 1998;72:7428–7439. doi: 10.1128/jvi.72.9.7428-7439.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou Z H, Prasad B V V, Jakana J, Rixon F J, Chiu W. Protein subunit structures in the herpes simplex virus capsid determined from 400-kV spot-scan electron cryomicroscopy. J Mol Biol. 1994;242:456–469. doi: 10.1006/jmbi.1994.1594. [DOI] [PubMed] [Google Scholar]
- 53.Zhou Z H, He J, Jakana J, Tatman J D, Rixon F J, Chiu W. Assembly of VP26 in herpes simplex virus-1 inferred from structures of wild-type and recombinant capsids. Nat Struct Biol. 1995;2:1026–1030. doi: 10.1038/nsb1195-1026. [DOI] [PubMed] [Google Scholar]
- 54.Zlotnick A, Cheng N, Conway J F, Booy F P, Steven A C, Stahl S J, Wingfield P T. Dimorphism of hepatitis B virus capsids is strongly influenced by the C-terminus of the capsid protein. Biochemistry. 1996;35:7412–7421. doi: 10.1021/bi9604800. [DOI] [PubMed] [Google Scholar]