Abstract
The aim of this study is to evaluate the correlation between multidetector row helical computed tomography (MDCT) findings and the histopathological characteristics of patients with invasive ductal carcinoma. We retrospectively reviewed MDCT findings and the corresponding histopathological features of 442 women with invasive ductal carcinoma. We received informed consent from the patients and the protocol was approved by the Ethics Committee at Tohoku University. The median age was 53 years (26–89 years). We examined the MDCT findings based on mass shape classified into well, moderate, poorly and scattered demarcated shapes, the enhancement pattern classified into homogenous, heterogeneous, rim and poor, and mass density classified into high, intermediate or low. We subsequently compared these radiological findings with the histological characteristics and clinical outcome. Poorly demarcated types were higher in ER+/HER2− (P = 0.008), while the well‐demarcated type was higher in ER−/HER2− and ER−/HER2+ (P < 0.001 and P = 0.010). Rim pattern was higher in ER−/HER2− (P < 0.001). Intermediate or low density was higher in ER−/HER2− (P < 0.001, respectively). Further analysis based on histological grade, mitotic counts and lymphovascular invasion demonstrated that the well‐demarcated shape was higher in grade 2 and 3 (P = 0.006 and P < 0.001, respectively), and rim pattern was observed in grade 3 (P < 0.001). Regarding mitotic counts, poorly and scattered demarcated shapes were observed in score 1 (P = 0.008 and P = 0.014), while well‐demarcated shape and rim enhancement were observed in score 3 (P < 0.001, respectively). Lymphovascular invasion correlated with a moderate demarcated shape (P = 0.029). Regarding recurrence rates, there were statistically significant differences between well and moderate, poorly or scattered demarcated shapes (P = 0.007, 0.028 and 0.035, respectively). These proposed MDCT diagnostic criteria based on biological characteristics contribute to more accurately predicting the biological behavior of breast cancer patients. (Cancer Sci 2012; 103: 67–72)
Histological tumor type, grade, lymphovascular invasion and molecular markers such as estrogen receptor (ER) and human epidermal growth factor receptor 2 (HER2) status are standard prognostic indicators in breast cancer patients.( 1 , 2 , 3 ) This histological information can contribute to an optimal selection of treatment strategy including endocrine therapy, chemotherapy and targeted therapy in individual patients with breast cancer.( 4 , 5 ) Several previous studies examined the correlation between radiological findings and the corresponding histopathological characteristics.( 6 , 7 , 8 , 9 , 10 , 11 ) A number of independent studies demonstrated that spiculated periphery masses in mammography were associated with a good outcome, whereas well‐defined masses were associated with triple‐negative breast cancer cases.( 7 , 8 ) In addition, a spiculated margin of breast cancer on high spatial resolution dynamic magnetic resonance imaging (MRI) was reported to be able to predict a lower histological grade.( 9 ) As for ultrasonography, a poorly circumscribed margin, abrupt boundary and a hypoechoic or complex echo pattern were reported to be more frequent in grade 3 than in grade 1–2 invasive cancer cases.( 10 ) Therefore, an accurate correlation of radiological findings with their corresponding histopathological features is considered one of the most important in imaging evaluation of breast malignancies, especially with reference to the study of biological features of the patients.
The recent development of multidetector row helical computed tomography (MDCT) has markedly improved the resolution that can be achieved in CT scanning, allowing the entire breast to be scanned in thin slices.( 12 , 13 ) This instrument can detect much smaller lesions and provide more detailed information regarding the extent of breast cancer infiltration because of faster scanning, a wider area of scan coverage and higher resolution of the volume data than single helical CT.( 12 , 13 ) However, to the best of our knowledge, no studies have reported on the correlation between these MDCT findings and the corresponding biological characteristics in individual patients with breast cancer. Therefore, in the present study, we evaluated the MDCT findings and compared the histological characteristics including ER, HER2 status, histological grade, mitotic counts of the cancer cells and lymphovascular invasion in a retrospective manner.
Materials and Methods
Patients. We retrospectively reviewed the MDCT findings and histopathological features of 442 invasive ductal carcinoma of the breast for which surgery was performed in Tohoku University Hospital in Sendai Japan from January 2005 to June 2010. We received informed consent from all patients and the protocol for the present study was approved by the Ethics Committee at Tohoku University Graduate School of Medicine. The median age of the patients was 53 years (range 26–89 years). Table 1 summarizes the patient characteristics.
Table 1.
Patient characteristics
| Patient age, median (range) (years) | 53 (26–89) |
| Hormone receptor and HER2 expression | |
| ER+/HER2− | 327 |
| ER+/HER2+ | 29 |
| ER−/HER2− | 55 |
| ER−/HER2+ | 31 |
| Histological grade | |
| HG1 | 149 |
| HG2 | 213 |
| HG3 | 80 |
| Mitotic counts of carcinoma cells | |
| Score 1 | 298 |
| Score 2 | 72 |
| Score 3 | 72 |
| Lymphovascular invasion | |
| Ly+ | 220 |
| Ly− | 222 |
ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; HG, histological grade; Ly, lymphovascular invasion; +, positive; −, negative.
Imaging devices and breast tissue specimens. The MDCT evaluations were performed using a 16‐row detector CT system (Somatom Sensation Cardiac; Siemens Medical Solutions, Erlangen, Germany). A total of 2 mL/kg of nonionic iodine contrast materials (300 mg I/mL) was injected at a rate of 2.0 mL/s. The CT data acquisition started 60 s after commencing the injection of contrast medium. We used an X‐ray tube modulation system (CARE Dose 4D; Siemens Medical Solutions). The X‐ray tube voltages were 80, 100 or 120 kV and the quality reference tube current‐time product was set at 120 mAs. The CT was performed in a craniocaudal direction with a section thickness of 0.75 mm and a table feed of 12 mm per rotation, resulting in a pitch factor of 1. The gantry rotation time was 0.5 s. Transverse CT images were reconstructed using a section thickness and increment of 1 mm each. The additional reconstruction was achieved by targeting the relevant side of the breast with the same thickness and increment. All data were sent to the Advantage Workstation v4.1/4.2 (GE Healthcare, Milwaukee, WI, USA).
The slides of the cases were stained with hematoxylin–eosin (HE) and immunohistochemical antibody for ER and HER2. Surgical specimens had been fixed in 10% formaldehyde solution and cut into serial 5‐mm‐thick slices, embedded in paraffin, cut into 4‐μm‐thick sections, and placed on the glue‐coated glass slides. We used the avidin–streptavidin immunoperoxidase method using the clone 6F11 antibody (Ventana, Tucson, AZ, USA) in an automated immunostainer (Benchmark System; Ventana). A standardized immunohistochemistry kit (HercepTest for Immunoenzymatic Staining; Dako, Copenhagen, Denmark) was used for HER2 staining.
Imaging and histopathological analyses. Two experienced breast physicians and one experienced radiologist independently evaluated the MDCT findings of all cases examined in the present study. These three investigators were blinded to the histopathological diagnosis and the clinical outcome of the patients. If there were discrepancies between the investigators, they reached a final decision using consensus evaluations from eight experienced breast physicians and radiologists. We recorded tumor shape, enhancement pattern and density. Table 2 summarizes the definition of MDCT findings. Tumor shape was defined on the basis of gross tumor configuration from stellate to circumscribed, and tentatively classified into a well‐demarcated shape including an oval mass shape and circumscribed periphery, a poorly demarcated shape including an irregular mass shape and spiculated periphery, a moderate demarcated shape having a mixed contour and a scattered demarcated shape (Fig. 1). The enhancement pattern was tentatively classified into homogenous, heterogenous, rim and poor enhancement (Fig. 1). Density of the lesion after injection of contrast media was subsequently compared with that of normal mammary gland and tentatively classified into high and intermediate or low (Fig. 1).
Table 2.
Definition of MDCT findings
| Definition | |
|---|---|
| Tumor shape | |
| Well demarcated | Oval mass shape and circumscribed periphery |
| Moderate demarcated | Mixed contour of well and poorly demarcated shape |
| Poorly demarcated | Irregular mass shape and spiculated periphery |
| Scattered demarcated | Lesion with multiple spotted foci |
| Enhancement pattern | |
| Homogenous | Diffuse and monotonous enhancement pattern |
| Heterogenous | Mosaic pattern |
| Rim | Enhancement only in periphery of the tumor |
| Poor | Weak enhancement |
| Density | |
| High | High density compared with the normal mammary gland |
| Intermediate or low | Equal or low density compared with the normal mammary gland |
MDCT, multidetector row helical computed tomography.
Figure 1.
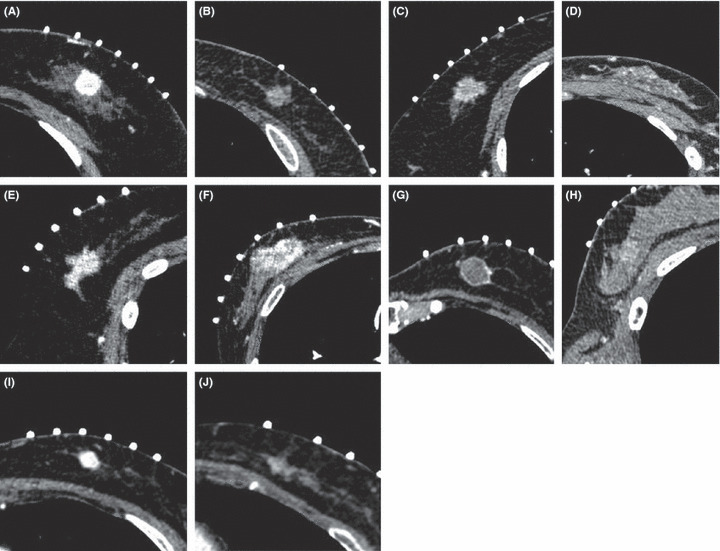
Representative images of tumor shape, enhancement pattern and density according to multidetector row helical computed tomography findings. (A) Well‐demarcated shape, (B) moderate demarcated shape, (C) poorly demarcated shape, (D) scattered demarcated shape, (E) homogenous enhancement pattern, (F) heterogenous pattern, (G) rim pattern, (H) poor enhancement, (I) high density and (J) intermediate or low density.
Two of the experienced pathologists independently evaluated the surgical pathology specimens, respectively. Histopathological evaluation was based on the World Health Organization histological classification of tumors of the breast and Rosen’s Breast Pathology.( 14 , 15 ) Estrogen receptor was determined by nuclear staining graded from 0 to 8 using the Allred score, and positive was grade 3 or more.( 16 ) With regard to HER2 evaluation, membranous staining was graded as follows: score 0–1+, 2+ and 3+.( 17 ) Scoring of 2+ cases were added in the other examination of fluorescence in situ hybridization (FISH) that was used to calculate the gene copy ratio of HER2‐to‐CEP17 (PathVysion HER2 DNA Probe kit; Abbott, Chicago, IL, USA). Positive was defined as either HER2 : CEP17 signal ratio (FISH score) >2.2.( 17 ) Histological grades and mitotic counts were assessed according to the criteria of Elston and Ellis.( 2 ) We also identified the presence or absence of lymphovascular invasion according to Rosen’s Breast Pathology.( 15 )
We examined the MDCT findings including tumor shape, enhancement pattern and density with the histopathological characteristics including ER, HER2 status, histological grade, mitotic counts and lymphovascular invasion. In addition, we examined the correlation between MDCT findings and clinical outcome including recurrence rate and recurrence‐free survival of the patients.
Statistical analysis. To compare the MDCT findings with the histopathological findings, multivariate statistics were used. All analyses were performed with the use of statistical software (SPSS, version 10.0; SPSS, Chicago, IL, USA), with P < 0.05 indicating a significant difference.
Results
Comparison of MDCT findings with ER and HER2 status. Table 3 summarizes the results of the numbers and ratios of each MDCT findings according to ER and HER2 status. There were statistically higher cases of moderate and poorly in the ER+/HER2− group (P = 0.014 and P = 0.008, respectively), well in the ER−/HER2− group (P < 0.001) and well in the ER−/HER2+ group (P = 0.010). However, there were statistically lower ratios of well‐demarcated shape compared with other tumor shapes in the ER+/HER2− group (P < 0.001) and of poorly in the ER−/HER2− group (P = 0.004). Regarding enhancement patterns and density, there were statistically higher cases demonstrating rim pattern and/or intermediate and low density in the ER−/HER2− group (P < 0.001, respectively). However, there were statistically lower ratios of rim in the ER+/HER2− group (P < 0.001) and of homogenous pattern in the ER−/HER2− group (P = 0.014), and of high density in the ER−/HER2− group (P < 0.001) (Table 3).
Table 3.
Comparison of MDCT findings with ER and HER2 status
| ER+/HER2− | ER+/HER2+ | ER−/HER2− | ER−/HER2+ | |
|---|---|---|---|---|
| Tumor shape | ||||
| Well | 23 | 5 | 24* | 9* |
| Moderate | 169* | 12 | 20 | 13 |
| Poorly | 110* | 8 | 8 | 8 |
| Scattered | 25 | 4 | 5 | 1 |
| Enhancement pattern | ||||
| Homogenous | 142 | 10 | 14 | 14 |
| Heterogenous | 162 | 16 | 26 | 15 |
| Rim | 8 | 2 | 12* | 2 |
| Poor | 15 | 1 | 3 | 0 |
| Density | ||||
| High | 198 | 21 | 20 | 22 |
| Intermediate/low | 129 | 8 | 35* | 9 |
*Higher ratio P < 0.05. ER, estrogen receptor; HER2, human epidermal growth factor receptor 2; MDCT, multidetector row helical computed tomography; +, positive; −, negative.
Comparison of MDCT findings with histological grades. Table 4 summarizes the results of the numbers and ratios of each MDCT finding according to histological grade. As for mass shape, there was a statistically higher ratio of well in the grade 3 group (P < 0.001) and poorly in the grade 1 group (P = 0.012), whereas lower ratios of well in the grade 1 and grade 2 groups (P < 0.001 and P = 0.006) and moderate and poorly in the grade 3 group (P = 0.010 and P < 0.001). Regarding the enhancement pattern, there were statistically higher ratios of homogenous in the grade 1 group (P = 0.041) and of rim pattern in the grade 3 group (P < 0.001). However, there was a statistically lower ratio of homogenous in the grade 3 group (P = 0.020) and of rim pattern in the grade 1 group (P < 0.001). There was no statistical significance in the correlation between histological grade and density (Table 4).
Table 4.
Comparison of MDCT findings with histological grade
| Grade 1 | Grade 2 | Grade 3 | |
|---|---|---|---|
| Tumor shape | |||
| Well | 3 | 19 | 38* |
| Moderate | 75 | 109 | 28 |
| Poorly | 61* | 72 | 13 |
| Scattered | 10 | 13 | 1 |
| Enhancement pattern | |||
| Homogenous | 72* | 88 | 24 |
| Heterogenous | 76 | 103 | 35 |
| Rim | 1 | 13 | 19* |
| Poor | 0 | 9 | 2 |
| Density | |||
| High | 92 | 127 | 48 |
| Intermediate/low | 57 | 86 | 32 |
*Higher ratio P < 0.05. MDCT, multidetector row helical computed tomography.
Comparison of MDCT findings with mitotic counts. Table 5 summarizes the results of the numbers and ratios of each MDCT finding according to mitotic counts. There were statistically higher ratios of poorly in the score 1 group (P = 0.008), well in the score 3 group (P < 0.001) and scattered in the score 1 group (P = 0.014), whereas lower ratios of well‐demarcated shape in the score 1 group (P = 0.015) and poorly demarcated shape in the score 3 group (P = 0.007). As for the enhancement pattern, there were higher ratios of rim pattern in the score 2 and score 3 groups (P = 0.004 and P < 0.001, respectively) and poor pattern in the score 1 group (P = 0.027), whereas a lower ratio of rim pattern in the score 1 group (P < 0.001). There was no statistical significance in the correlation between mitotic counts and density (Table 5).
Table 5.
Comparison of MDCT findings with mitotic counts
| Score 1 | Score 2 | Score 3 | |
|---|---|---|---|
| Tumor shape | |||
| Well | 27 | 15 | 28* |
| Moderate | 142 | 33 | 31 |
| Poorly | 103* | 22 | 9 |
| Scattered | 28* | 2 | 2 |
| Enhancement pattern | |||
| Homogenous | 127 | 26 | 21 |
| Heterogenous | 147 | 31 | 31 |
| Rim | 4 | 14* | 16* |
| Poor | 22* | 1 | 2 |
| Density | |||
| High | 183 | 46 | 38 |
| Intermediate/low | 115 | 26 | 34 |
*Higher ratio P < 0.05. MDCT, multidetector row helical computed tomography.
Comparison of MDCT findings with lymphovascular invasion. Table 6 summarizes the results of the numbers and ratios of each MDCT finding according to lymphovascular invasion. There was a statistically higher ratio of lymphovascular invasion in the moderate demarcated shape group (P = 0.029). However, there were statistically lower ratios of lymphovascular invasion in the scattered and/or poor enhancement pattern (P < 0.001 and P = 0.037) (Table 6).
Table 6.
Comparison of MDCT findings with lymphovascular invasion
| Ly+ | Ly− | |
|---|---|---|
| Tumor shape | ||
| Well | 36 | 26 |
| Moderate | 113* | 91 |
| Poorly | 62 | 72 |
| Scattered | 9 | 33 |
| Enhancement pattern | ||
| Homogenous | 82 | 92 |
| Heterogenous | 110 | 99 |
| Rim | 19 | 11 |
| Poor | 9 | 20 |
| Density | ||
| High | 139 | 128 |
| Intermediate/low | 81 | 94 |
*Higher ratio P < 0.05. Ly, lymphovascular invasion; MDCT, multidetector row helical computed tomography; +, positive; −, negative.
Comparison of MDCT findings with clinical outcome of the patients. Disease recurrence rates according to tumor shape were 25.0% in the well‐demarcated shape, 6.7% in the moderate demarcated shape, 8.8% in the poorly demarcated shape and 0% in the scattered demarcated shape. There were statistically significant differences between well and moderate, poorly or scattered demarcated shapes (P = 0.007, 0.028 and 0.035, respectively). Recurrence rates according to enhancement pattern were 5.7% in homogenous, 10.6% in heterogenous, 20.0% in rim and 9.1% in the rim enhancement pattern. There were no statistically significant differences according to enhancement patterns. Recurrence rates according to density were 9.0% in high and 8.9% in intermediate or low density and there were no statistically significant differences between these two groups. Figure 2 shows the recurrence‐free survival in relation to tumor shape and enhancement patterns according to the Kaplan–Meier method. There were statistically significant differences between the well‐demarcated shape and the moderate or scattered demarcated shape (P = 0.024 and 0.038, respectively).
Figure 2.
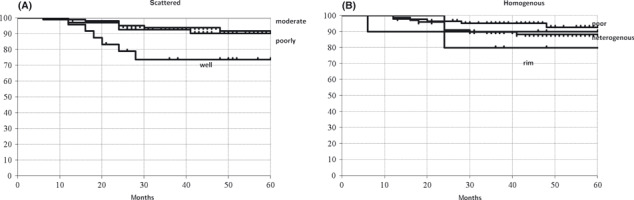
Recurrence‐free survival according to multidetector row helical computed tomography findings using the Kaplan–Meier method. (A) Tumor shape and (B) enhancement pattern.
Discussion
Breast cancer is a disease associated with heterogeneous outcomes and numerous studies have been reported regarding the establishment of prognostic factors of individual patients. Traditional prognostic factors that were correlated with the overall survival of patients include histological grade, mitotic counts and lymphovascular invasion of individual cases.( 2 , 18 ) Several molecular prognostic factors including ER and HER2 status have over the past few years been clearly demonstrated to contribute significantly to the management and subsequent prognosis of patients with breast cancer.( 18 , 19 ) Therefore, an accurate correlation of radiological findings with their corresponding histopathological features is considered most important in the radiological evaluation of patients with breast cancer. In addition, the recognition of how many radiological features obtained might actually reflect the prognosis of individual patients is considered markedly important in gaining insight into how the tumor proliferates and into potentially determining which tumors can be managed with aggressive adjuvant treatment. The purpose of the present study is therefore to evaluate the correlation of MDCT findings including tumor shape, enhancement pattern and density with ER expression, HER2 status, histological grade, mitotic counts and lymphovascular invasion in Japanese patients with breast cancer.
Several previous studies evaluated the correlation between radiological findings including mammography, ultrasonography and MRI and histopathological characteristics of individual patients.( 7 , 8 , 9 , 10 , 11 , 20 ) For mammography, a number of independent study groups demonstrated that spiculated periphery masses were significantly associated with a good outcome for patients,( 7 , 8 ) but well‐defined masses were associated with triple‐negative breast cancer.( 7 , 8 ) Previous studies also compared the ultrasonographic findings of ER‐negative/HER2‐negative cancers with those of ER‐negative/HER2‐positive cancers and concluded that ER‐negative/HER2‐positive breast cancers were likely to demonstrate spiculated margins and to be associated with calcifications.( 10 ) In addition, ER‐negative/HER2‐negative breast cancers were more likely to show as smooth or circumscribed masses.( 10 ) A spiculated margin in MRI findings did demonstrate an association with positive ER expression and negative HER2 status, and high‐grade malignant breast cancers circumscribed margins.( 9 , 20 )
Results of the present study did demonstrate that there was a higher incidence of increased ratios of ER+/HER2− type, lower histological grade, lower mitotic counts and a lower ratio of lymphovascular invasion in poorly demarcated masses, whereas the presence of higher ratios of triple‐negative type, higher histological grade, higher mitotic counts and lymphovascular invasion were all demonstrated to be associated with well‐demarcated masses in the present study. In addition, these results all suggest that poorly demarcated breast cancer was associated with a good clinical outcome, and well‐demarcated shape of carcinoma cells was associated with an adverse clinical outcome and negative ER status in carcinoma cells. Results of the present study were similar to those of previously reported studies regarding the correlation between mammography or MRI findings and intrinsic subtype or histological grade of cases.( 7 , 8 , 9 , 10 , 11 , 20 ) Inoue et al. ( 21 ) reported the correlation between MDCT findings and the ratio of malignant cases but they did not necessarily focus on the histopathological characteristics of the cases examined. Therefore, to the best of our knowledge, this is the first study to examine the correlation between MDCT findings and the corresponding histopathological features. However, the mechanisms of the correlation between intrinsic subtype or histological grade and the growth pattern of carcinoma cells have not been elucidated. Therefore, further studies are required to investigate the potential mechanisms of correlation between biological characteristics and the growth pattern of carcinoma cells.
Results of the present study also demonstrated that there were statistically significant correlations of rim enhancement patterns with triple‐negative breast cancer and higher histological grade. Rim enhancement was one of the important morphological signs in predicting the worse clinical outcomes. These results all suggest that high angiogenesis might be present in the peripheral lesion of the masses and central necrosis and fibrosis. A previous MRI study also demonstrated the correlation between rim enhancement and large tumor size, higher histological grade or negative hormone receptor status of cases.( 22 ) However, this is the first study examining the correlation between rim enhancement in MDCT findings and biological characteristics of breast malignancies. In general, rim enhancement is more frequently noted in rapidly growing breast carcinomas.
In addition, we also examined the recurrence ratio and recurrence‐free survival of patients according to MDCT findings. Results of the present study demonstrated that a well‐demarcated shape was associated with a significantly higher recurrence ratio than other groups (P = 0.007, 0.028 and 0.035, respectively). A similar tendency was also detected in the rim enhancement pattern but the difference did not reach statistical significance. Therefore, MDCT findings of well‐demarcated shape and rim enhancement pattern can become one of the predictors of a worse clinical outcome for patients.
We noted significant differences in the MDCT features of different primary breast cancer types in the present study. Stratifying the MDCT features according to phenotypes reveals distinct differences among cancer subtypes. However, the present study was retrospective and examined in a single institute. Therefore, it is probable that further investigation of not only Japanese women but also other Asian women will confirm the new MDCT criteria. Biological and histopathological differences may result in the imaging differences that might help us better understand breast cancer development. These proposed MDCT diagnostic criteria based on biological characteristics might provide a more accurate prediction of biological behavior of breast malignancies when radiologists evaluate the findings of MDCT.
Disclosure Statement
The authors have no conflict of interest.
Acknowledgments
The authors thank Medical Technician Yayoi Takahashi, for her excellent technical assistance for immunohistochemical staining. This work was supported in part by a Grant‐in‐Aid from Kurokawa Cancer Research Foundation.
References
- 1. Carter CL, Allen C, Henson DE et al. Relation of tumour size, lymph node status, and survival in 24,740 breast cancer cases. Cancer 1989; 63: 181–7. [DOI] [PubMed] [Google Scholar]
- 2. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histopathological grade in breast cancer: experience from a large study with long‐term follow‐up. Histopathology 1991; 19: 403–10. [DOI] [PubMed] [Google Scholar]
- 3. Lee AHS, Pinder SE, Macmillan RD et al. Prognostic value of lymph vascular invasion in women with lymph node negative invasive breast carcinoma. Eur J Cancer 2006; 42: 357–62. [DOI] [PubMed] [Google Scholar]
- 4. Bauer KR, Brown M, Cress RD et al. Descriptive analysis of estrogen receptor (ER)‐negative, progesterone receptor (PR)‐negative, and HER2‐negative invasive breast cancer, the so‐called triple‐negative phenotype: a population‐based study from the California cancer Registry. Cancer 2007; 109: 1721–8. [DOI] [PubMed] [Google Scholar]
- 5. Tamaki K, Sasano H, Ishida T et al. Comparison of core needle biopsy (CNB) and surgical specimens for accurate preoperative evaluation of ER, PgR and HER2 status of breast cancer patients. Cancer Sci 2010; 101: 2074–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Tamaki K, Sasano H, Ishida T et al. The correlation between ultrasonographic findings and pathologic features in breast disorders. Jpn J Clin Oncol 2010; 40: 905–12. [DOI] [PubMed] [Google Scholar]
- 7. Luck AA, Evans AJ, James JJ et al. Breast carcinoma with basal phenotype: mammographic findings. AJR Am J Roentgenol 2008; 191: 346–51. [DOI] [PubMed] [Google Scholar]
- 8. Evans AJ, Pinder SE, James JJ et al. Is mammographic spiculation an independent, good prognostic factor in screening detected invasive breast cancer? AJR Am J Roentgenol 2006; 187: 1377–80. [DOI] [PubMed] [Google Scholar]
- 9. Lee SH, Cho N, Kim SJ et al. Correlation between high resolution dynamic MR features and prognostic factors in breast cancer. Korean J Radiol 2008; 9: 10–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kim SH, Seo BK, Lee J et al. Correlation of ultrasound findings with histology, tumor grade, and biological markers in breast cancer. Acta Oncol 2008; 47: 1531–8. [DOI] [PubMed] [Google Scholar]
- 11. Amano G, Ohuchi N, Ishibashi T et al. Correlation of three‐dimensional magnetic resonance imaging with precise histopathological map concerning carcinoma extension in the breast. Breast Cancer Res Treat 2000; 60: 43–55. [DOI] [PubMed] [Google Scholar]
- 12. Takase K, Furuta A, Harada N et al. Assessing the extent of breast cancer using multidetector row helical computed tomography. J Comput Assist Tomogr 2006; 30: 479–85. [DOI] [PubMed] [Google Scholar]
- 13. Harada‐Shoji N, Yamada T, Ishida T et al. Usefulness of lesion image mapping with multidetector‐row helical computed tomography using a dedicated skin marker in breast‐conserving surgery. Eur Radiol 2009; 19: 868–74. [DOI] [PubMed] [Google Scholar]
- 14. Tavassoli FA, Devilee P. World Health Organization Classification of Tumors. Tumor of the Breast and Females Gental Organs. Lyon: IARC Press, 2003. [Google Scholar]
- 15. Rosen PP. Rosen’s Breast Pathology, 3rd edn. Philadelphia, PA, USA: Lippncott Williams & Wilkins, 2009. [Google Scholar]
- 16. Allred DC, Harvey JM, Berardo M, Clark GM. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 1998; 11: 155–68. [PubMed] [Google Scholar]
- 17. Wolff AC, Hammond MH, Schwartz JN et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol 2007; 25: 118–45. [DOI] [PubMed] [Google Scholar]
- 18. Goldhirsch A, Ingle JN, Gelber RD et al. Thresholds for therapies: highlights of the St Gallen International Expert Consensus on the primary therapy of early breast cancer 2009. Ann Oncol 2009; 20: 1319–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Jalava P, Kuopio T, Juntti‐Patinen L et al. Ki67 immunohistochemistry: a valuable marker in prognostication but with a risk of misclassification: proliferation subgroups formed based on Ki67 immunoreactivity and standardized mitotic index. Histopathology 2006; 48: 674–82. [DOI] [PubMed] [Google Scholar]
- 20. Uematsu T, Kasami M, Yuen S. Triple‐negative breast cancer: correlation between MR imaging and pathologic findings. Radiology 2009; 250: 638–47. [DOI] [PubMed] [Google Scholar]
- 21. Inoue M, Sano T, Watai R et al. Dynamic multidetector CT of breast tumors: diagnostic features and comparison with conventional techniques. AJR Am J Roentgenol 2003; 181: 679–86. [DOI] [PubMed] [Google Scholar]
- 22. Jeh SK, Kim SH, Kim HS et al. Correlation of the apparent diffusion coefficient value and dynamic magnetic resonance imaging findings with prognostic factors in invasive ductal carcinoma. J Magn Reson Imaging 2011; 33: 102–9. [DOI] [PubMed] [Google Scholar]


