Abstract
Our previous case–control study suggested that equol, a metabolite of isoflavone, has a preventive effect on prostate cancer. To examine the prostate cancer risk based on isoflavone intake and equol production, we carried out a phase II, randomized, double‐blind, placebo‐controlled trial of oral isoflavone (60 mg/day) for 12 months. The inclusion criteria were Japanese men between 50 and 75 years of age, a serum prostate‐specific antigen level of 2.5–10.0 ng/mL, and a single, negative prostate biopsy within 12 months prior to enrollment. The study included 158 men in eight Japanese centers. Their median age was 66.0 years, and the numbers of equol producers and non‐producers were 76 (48%) and 82 (52%), respectively. The majority of adverse events were mild or moderate in severity, and the scheduled intake of tablets was completed by 153 patients (96.8%). The prostate‐specific antigen value showed no significant difference before and after treatment. Of the 89 patients evaluated by central pathological review, the incidence of biopsy‐detectable prostate cancer in the isoflavone and placebo groups showed no significant difference (21.4%vs 34.0%, P = 0.140). However, for the 53 patients aged 65 years or more, the incidence of cancer in the isoflavone group was significantly lower than that in the placebo group (28.0%vs 57.1%, P = 0.031). These results support the value of isoflavone for prostate cancer risk reduction. A large‐scale phase III randomized study of isoflavone tablets in men with different hereditary factors and living environments is warranted. Registered with the UMIN Clinical Trials Registry (UMIN‐CTR) for clinical trials in Japan (C000000446). (Cancer Sci 2012; 103: 125–130)
Prostate cancer is the second most common cancer in men and the third most common cause of male cancer death worldwide.( 1 ) The incidence of clinical cancer in Japan is low, however, the incidence of total clinical and latent prostate cancer is the same between Japanese and American populations.( 2 ) Diet is thought to play an important role in the progression from microscopic to clinical cancer.( 1 , 3 ) Fat and calcium have been reported to be risk factors for prostate cancer. Conversely, lycopene, selenium, soy isoflavone, and vitamin E were reported to be preventive factors.( 4 , 5 , 6 ) However, the SELECT (Selenium and Vitamin E Cancer Prevention Trial) study,( 7 ) a recent large‐scale, double‐blind study, was unable to show a preventive effect for selenium or vitamin E on prostate cancer.
Basic research, including epidemiologic studies, suggested that soy isoflavone exerts an anticarcinogenic effect on prostate cancer.( 8 , 9 ) Our case–control study of the serum isoflavone levels in patients with prostate cancer and healthy volunteers( 8 ) found that some individuals were able to degrade daidzein into equol (equol producers) whereas others were not (non‐producers).
In another case–control study involving residents in Japan, Korea, and the USA,( 9 ) we found that the percentage of equol producers in patients with prostate cancer was significantly lower than in the healthy controls (30.3%vs 49.5%; P = 0.013). The percentage of equol producers in patients and controls was 29% and 46% in Japan (P = 0.004) and 30% and 59% in Korea (P = 0.001), respectively. The serum isoflavone level was markedly lower and the percentage of equol producers was also lower (17% for patients and 14% for controls) for Americans as compared to the Japanese and Koreans.
In another study,( 10 ) we carried out an age‐stratified dietary survey of soybean food consumption and measured the serum isoflavone levels in healthy Japanese and Korean men. The daily intake of genistein and daidzein in the teenage group was significantly lower than in the age group ≥30 years (P < 0.05). In the Japanese cohort, the proportion of equol producers in the teenage group was only 10%, which was significantly the lowest among all age‐strata. Those results suggest that equol or equol‐producing ability may be deeply involved in prostate cancer risk, and decreased intake of isoflavones in the young generation may lead to an increase in the prostate cancer incidence in Japan and Korea.
Recently, we clarified the mechanism of biodegradation from daidzein into equol by two kinds of intestinal bacteria.( 11 ) As a strategy for chemoprevention of prostate cancer, clinical intervention by changing equol non‐producers to producers, as well as by ingesting equol‐containing supplements, are anticipated.
Considering this background, we carried out a phase II, randomized, double‐blind, placebo‐controlled trial of oral isoflavone (60 mg/day) for 12 months.
Patients and Methods
Patients. This was a phase II, randomized, double‐blind, placebo‐controlled trial of isoflavone (60 mg/day), given orally for 12 months. The inclusion criteria were Japanese men between 50 and 75 years of age, a serum PSA level of 2.5–10.0 ng/mL (50–60 years) or 3.0–10.0 ng/mL (>60 years), and a single, negative prostate biopsy (6–12 cores) within 12 months prior to enrollment. Men with HG‐PIN or ASAP in the baseline biopsy, or a history of prostate cancer, were excluded from the study. None of the patients were using a steroidal or non‐steroidal anti‐androgen. The protocol was approved by the Institutional Review Board of each study site. The study was carried out in accordance with the Helsinki Declaration, and all participants signed informed consent forms.
Study design. The ingredients in the soy isoflavone tablet are shown in Table 1. Eligible patients were randomized to receive 10 isoflavone tablets (isoflavone 6 mg/tablet, totally 60 mg/day) or 10 placebo tablets for 12 months. Six‐core transrectal ultrasound‐guided biopsies were planned to be carried out at 12 months. The investigator was allowed to carry out “protocol‐independent” (for‐cause) biopsies whenever deemed clinically necessary. Protocol‐mandated biopsies and for‐cause biopsies were to be submitted for confirmation by central pathological review (University of Kyushu, Fukuoka, Japan). However, central pathological review was not the essential requirement for registration. No biopsy samples were collected at baseline. The negative biopsy prior to enrolment was confirmed by local pathological review only.
Table 1.
Ingredients of soy isoflavone tablet given to study participants for 12 months
| Component | mg/10 tablets (%) |
|---|---|
| Daidzin | 19.1 (31.9) |
| Genistin | 3.5 (5.8) |
| Glycitin | 10.4 (17.3) |
| Malonyl daidzin | 8.1 (13.5) |
| Malonyl genistin | 2.2 (3.7) |
| Malonyl glycitin | 3.4 (5.7) |
| Acetyl daidzin | 7.3 (12.2) |
| Acetyl genistin | 1.9 (3.2) |
| Acetyl glycitin | 3.6 (6.0) |
| Daidzein | 0.2 (0.3) |
| Genistein | 0.1 (0.1) |
| Glycitein | 0.2 (0.3) |
| Total | 60.0 (100.0) |
Ten isoflavone tablets (6 mg/tablet, totally 60 mg/day) are divided twice.
Because this study was planned as a pilot phase II study of the large‐scale clinical trial, the primary endpoints were the tolerability of the soy isoflavone tablet and the changes in PSA and sex hormones including testosterone, DHT, SHBG, and estradiol. The secondary endpoints were the incidences of biopsy‐detectable prostate cancer, HG‐PIN, and ASAP at 12 months.
Measurement of serum isoflavone levels. Blood samples were drawn at 0, 3, and 12 months (serum isoflavones and sex hormones) or 0, 3, 6, 9, and 12 months (PSA). They were drawn before breakfast, and the sera were separated and stored at −10°C or less. The frozen samples were transported to the laboratory of SRL (Tokyo, Japan). The details of the measurement of serum isoflavone levels have been described elsewhere.( 8 ) The assayed isoflavones were genistein, daidzein, and equol. Equol producers were defined as having a baseline serum equol concentration above the lower limit of detection of the present assay system, that is, 0.5 ng/mL.( 8 )
Statistical analyses. The efficacy analysis population consisted of patients who ingested the study medication for 12 months, in accordance with the study protocol. The safety analysis population included all randomized patients.
Statistical analyses were carried out using Wilcoxon’s test (non‐parametric), the chi‐square‐test and Fisher’s exact test. For the individual changes of laboratory tests we used the paired t‐test. A P‐value of <0.05 was defined as representing a statistically significant difference. Data were analyzed using SAS version 8 software (SAS Institute, Cary, NC, USA).
Results
Baseline patient characteristics. The study enrolled 158 men in eight Japanese centers. Their median age at the first blood collection was 66.0 years (range, 50–75 years). The number of equol producers and non‐producers was 76 (48%) and 82 (52%), respectively. These patients were randomized into an isoflavone group (n = 78) and a placebo group (n = 80).
Table 2 shows data on the baseline patient characteristics. These baseline characteristics, age, family history of prostate cancer, total PSA, prostate volume, and equol production, were generally similar in each treatment group to those of the overall study population.
Table 2.
Baseline patient characteristics
| Total (n = 158) | Isoflavone (n = 78) | Placebo (n = 80) | P‐value | |
|---|---|---|---|---|
| Age (years) | ||||
| Median | 66.0 | 66.5 | 65.0 | 0.974† |
| Range | 50.0–75.0 | 52.0–75.0 | 50.0–75.0 | |
| Family history, no. (%) | 3 (2) | 1 (1) | 2 (3) | 0.873‡ |
| Total PSA (ng/mL) | ||||
| Median | 5.75 | 5.83 | 5.73 | 0.784† |
| Range | 2.76–10.20 | 2.76–9.77 | 3.00–10.20 | |
| Prostate volume (mL) | ||||
| Median | 37.7 | 37.6 | 37.6 | 0.349† |
| Range | 0.5–93.5 | 12.3–93.5 | 0.5–84.0 | |
| Equol production, no. (%) | ||||
| Producer | 76 (48) | 38 (49) | 38 (48) | 0.502‡ |
| Non‐producer | 82 (52) | 40 (51) | 42 (53) | |
Non‐producers, individuals unable to degrade daidzein into equol; producers, individuals able to degrade daidzein into equol. †Wilcoxon’s test; ‡Fisher’s exact test.
Safety and tolerability. The planned intake of tablets was completed in 153 of 158 patients (96.8%). Of the five patients who did not complete the treatment course, three decided for themselves to quit taking the tablets. The other two patients had grade 3 adverse events: one in the isoflavone group suffered iliac artery stenosis, and the other in the placebo group suffered ileus. However, the majority of adverse events were mild in severity. The completion rates in the isoflavone and placebo group were 96.2% (75/78) and 97.5% (78/80), respectively. No significant changes in laboratory data were observed during the study (data not shown).
Serum isoflavone levels. 1, 2 show the median serum levels of daidzein and equol, stratified by treatment and baseline equol production. Daidzein was significantly increased in the isoflavone groups, and its level was lower in the isoflavone/producer group compared with the isoflavone/non‐producer group (Fig. 1).
Figure 1.
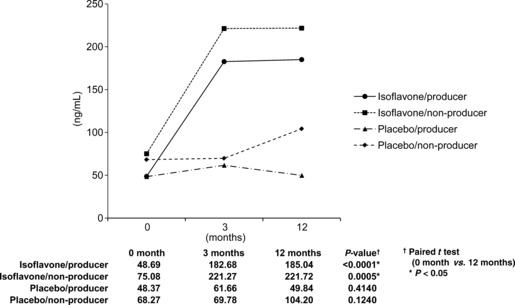
Median serum levels of daidzein in Japanese men given oral isoflavone (60 mg/day) or placebo for 12 months (n = 158). Daidzein was significantly increased in the isoflavone groups, and its level was lower in individuals who could also degrade daidzein into equol (isoflavone/producer group) compared with those who could not (isoflavone/non‐producer group).
Figure 2.
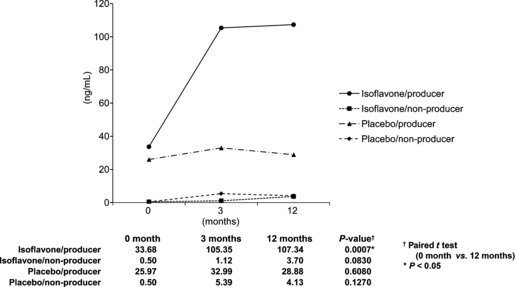
Median serum levels of equol in Japanese men given oral isoflavone (60 mg/day) or placebo for 12 months (n = 158). Individuals who could degrade daidzein into equol (equol producers), who received isoflavone, greatly increased equol production and showed the highest equol level. Equol producers given the placebo showed no change in the serum equol level from before treatment. The two groups of non‐producers (those unable to degrade daidzein into equol), treated with either isoflavone or placebo, showed the lowest equol levels.
In Figure 2, equol producers who received isoflavone greatly increased equol production and showed the highest equol level. Equol producers given the placebo showed no change in the serum equol level from before treatment. The two groups of non‐producers, given either isoflavone or placebo, showed the lowest equol levels.
Prostate‐specific antigen and sex hormones. The serum PSA value showed no significant change during the study (Fig. 3). There were also no differences among the treatment groups.
Figure 3.
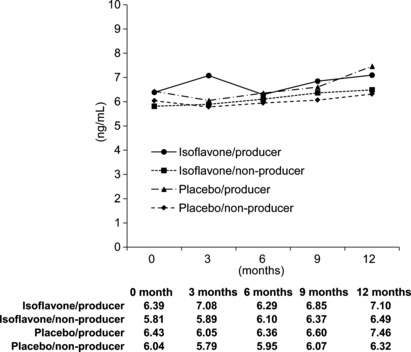
Serum prostate‐specific antigen (PSA) levels in Japanese men given oral isoflavone (60 mg/day) or placebo for 12 months (n = 158). The PSA value showed no significant change during the study. There were also no differences among the treatment groups. Non‐producers, individuals unable to degrade daidzein into equol; producers, individuals able to degrade daidzein into equol.
The interval changes in testosterone, DHT, and SHBG were not significant. In the non‐producer groups, estradiol decreased significantly, independent of treatment (Table 3).
Table 3.
Changes in hormone levels in Japanese men given oral isoflavone (60 mg/day) or placebo for 12 months (n = 158)
| 0 month (pre) | 3 months | 12 months | P‐value† | |
|---|---|---|---|---|
| Testosterone | ||||
| Isoflavone | 5.45 | 5.12 | 5.31 | 0.131 |
| Producer | 5.33 | 4.92 | 5.28 | 0.405 |
| Non‐producer | 5.57 | 5.33 | 5.34 | 0.200 |
| Placebo | 5.12 | 5.36 | 5.07 | 0.286 |
| Producer | 5.25 | 5.22 | 5.19 | 0.508 |
| Non‐producer | 5.01 | 5.48 | 4.99 | 0.411 |
| DHT | ||||
| Isoflavone | 0.94 | 0.95 | 1.01 | 0.533 |
| Producer | 0.92 | 0.89 | 1.00 | 0.708 |
| Non‐producer | 0.96 | 1.01 | 1.02 | 0.619 |
| Placebo | 0.91 | 0.99 | 0.98 | 0.337 |
| Producer | 1.02 | 1.09 | 1.04 | 0.869 |
| Non‐producer | 0.83 | 0.91 | 0.94 | 0.207 |
| Estradiol | ||||
| Isoflavone | 28.56 | 27.41 | 26.53 | 0.027* |
| Producer | 27.44 | 26.39 | 26.29 | 0.416 |
| Non‐producer | 29.59 | 28.46 | 26.76 | 0.023* |
| Placebo | 27.20 | 26.65 | 25.27 | 0.0002* |
| Producer | 26.71 | 26.97 | 25.70 | 0.122 |
| Non‐producer | 27.61 | 26.36 | 24.94 | 0.007* |
| SHBG | ||||
| Isoflavone | 52.00 | 55.43 | 55.97 | 0.064 |
| Producer | 52.76 | 52.70 | 57.00 | 0.261 |
| Non‐producer | 51.31 | 58.17 | 54.95 | 0.138 |
| Placebo | 50.11 | 48.82 | 51.71 | 0.321 |
| Producer | 53.98 | 50.11 | 53.48 | 0.511 |
| Non‐producer | 46.89 | 47.64 | 50.32 | 0.468 |
DHT, dihydrotestosterone; non‐producers, individuals unable to degrade daidzein into equol; producers, individuals able to degrade daidzein into equol. SHBG, sex hormone binding protein. †Paired t‐test (0 vs 12 M); *P < 0.05.
Pathologic endpoints. Of the 153 patients who completed the planned intake of tablets, 121 underwent needle biopsy of the prostate at 12 months. The remaining 32 patients did not agree to undergo the needle biopsy.
Of the 121 patients, 89, consisting of 42 in the isoflavone group and 47 in the placebo group, were evaluated by central pathological review, and 112 patients, consisting of 55 in the isoflavone group and 57 in the placebo group, were evaluated by local pathological review (Table 4). The specimens for 23 of the 112 patients were not approved to be sent to the central pathology laboratory by the Institutional Review Board. Thus, 89 were evaluated by both central and local pathological reviews. There were no significant differences between the results of central pathology and local pathology (data not shown). The following discussion focuses on the central pathology results.
Table 4.
Numbers and proportions of men with prostate cancer and high‐grade prostatic intraepithelial neoplasia (HG‐PIN) who participated in this study (n = 158)
| Central pathology | Local pathology | |||||
|---|---|---|---|---|---|---|
| Isoflavone (n = 42) | Placebo (n = 47) | P‐value† | Isoflavone (n = 55) | Placebo (n = 57) | P‐value† | |
| No. of patients with tumors | 9/42 (21.4%) | 16/47 (34.0%) | 0.140 | 8/55 (14.6%) | 14/57 (24.6%) | 0.137 |
| Age (years) | ||||||
| <64 | 2/17 (11.8%) | 0/19 (0.0%) | 0.220 | 2/27 (7.4%) | 0/26 (0.0%) | 0.255 |
| ≥65 | 7/25 (28.0%) | 16/28 (57.1%) | 0.031* | 7/31 (22.6%) | 14/31 (47.1%) | 0.035* |
| Equol production | ||||||
| Producer | 5/22 (22.7%) | 8/22 (36.4%) | 0.255 | 5/29 (17.2%) | 6/19 (24.0%) | 0.390 |
| Non‐producer | 4/20 (20.0%) | 8/25 (32.0%) | 0.288 | 3/26 (11.5%) | 8/32 (25.0%) | 0.168 |
| No. of positive cores | ||||||
| 1 | 6 | 11 | 0.713 | 5 | 10 | 0.510 |
| 2–4 | 3 | 5 | (1 vs 2–4) | 3 | 4 | (1 vs 2–4) |
| Gleason score | ||||||
| 5 | 0 | 0 | 0 | 1 | ||
| 6 | 5 | 12 | 4 | 8 | ||
| 7 | 3 | 4 | 3 | 2 | ||
| 8 | 0 | 0 | 1 | 3 | ||
| 9 | 1 | 0 | 0 | 0 | ||
| 5–6 | 5/9 (55.6%) | 12/16 (75.0%) | 0.287 | 4/8 (50.0%) | 9/14 (64.3%) | 0.416 |
| 7–9 | 4/9 (44.4%) | 4/16 (25.0%) | (G5–6 vs 7–9) | 4/8 (50.0%) | 5/14 (35.7%) | (G5–6 vs 7–9) |
| HG‐PIN | 2/42 (4.8%) | 8/47 (17.0%) | 0.660 | NE | NE | NE |
The Gleason score is the sum of the two most common histological patterns. NE, not evaluated; non‐producers, individuals unable to degrade daidzein into equol; producers, individuals able to degrade daidzein into equol. †Fisher’s exact test; *P < 0.05.
The incidence of biopsy‐detectable prostate cancer in the isoflavone and placebo groups was 21.4% (9/42) and 34.0% (16/47), respectively. These incidences of cancer detection were not statistically significantly different. However, for the patient stratum aged 65 years or more, the incidence of cancer in the isoflavone group was significantly lower than that in the placebo group (28.0% [7/25] vs 57.1% [16/28], P = 0.031).
The incidence of a Gleason score of 6 or less and HG‐PIN were numerically lower in the isoflavone group compared with the placebo group, but the difference was not statistically significant.
Table 5 presents the central pathology results for prostate cancer incidence based on isoflavone intake and equol production. The incidence of prostate cancer in the isoflavone group was significantly lower than that in the placebo group for the patient stratum aged 65 years or more and equol non‐production.
Table 5.
Numbers and proportions of men with prostate cancer and high‐grade prostatic intraepithelial neoplasia (HG‐PIN), based on an isoflavone intake administration and equol production (central pathology)
| Equal producer | Equal non‐producer | |||||
|---|---|---|---|---|---|---|
| Isoflavone (n = 22) | Placebo (n = 22) | P‐value† | Isoflavone (n = 20) | Placebo (n = 25) | P‐value† | |
| No. of patients with tumors | 5/22 (22.7%) | 8/22 (36.4%) | 0.255 | 4/20 (20.0%) | 8/25 (32.0%) | 0.288 |
| Age (years) | ||||||
| <64 | 1/9 (11.1%) | 0/6 (0.0%) | 0.600 | 1/8 (12.5%) | 0/13 (0.0%) | 0.381 |
| ≥65 | 4/13 (30.8%) | 8/16 (50.0%) | 0.293 | 3/12 (25.0%) | 8/12 (66.7%) | 0.049* |
| No. of positive cores | ||||||
| 1 | 4 | 7 | 0.641 | 2 | 2 | 0.727 |
| 2–4 | 1 | 1 | (1 vs 2–4) | 4 | 4 | (1 vs 2–4) |
| Gleason score | ||||||
| 6 | 2 | 6 | 3 | 6 | ||
| 7 | 2 | 2 | 1 | 2 | ||
| 8 | 0 | 0 | 0 | 0 | ||
| 9 | 1 | 1 | 0 | 0 | ||
| 5–6 | 2/5 (40.0%) | 6/8 (75.0%) | 0.250 | 3/4 (75.0%) | 6/8 (75.0%) | 0.764 |
| 7–9 | 3/5 (60.0%) | 2/8 (25.0%) | (G6 vs 7–9) | 1/4 (25.0%) | 2/8 (25.0%) | (G6 vs 7–9) |
| HG‐PIN | 1/22 (4.6%) | 4/22 (18.2%) | 0.172 | 1/20 (5.0%) | 4/25 (16.0%) | 0.251 |
The Gleason score is the sum of the two most common histological patterns. †Fisher’s exact test; *P < 0.05.
Discussion
Because of the high incidence of microscopic prostate cancer and the long latency period from microscopic lesions to clinical disease, development of strategies for reducing the risk of prostate cancer is a reasonable and promising approach. Clinical research has been carried out regarding the preventive effect of isoflavones on prostate cancer, including dietary supplement intervention trials for prostate cancer patients.( 12 , 13 ) In those studies, patients were given isoflavone‐containing drugs and the serum PSA was examined as a surrogate marker. Kumar et al. ( 12 ) gave a soy isoflavone beverage for 12 weeks to men with prostate cancer on watchful waiting, randomizing 59 patients to a soy group and a placebo group. Serum total PSA decreased or was unchanged in 69% of the subjects in the isoflavone‐treated group compared to 55% in the placebo group. Schröder et al. ( 13 ) gave an isoflavone‐containing drug to men with prostate cancer with increasing PSA after primary treatment. Forty‐two patients were examined in a placebo‐controlled, double‐blind, crossover study that involved 10 weeks of intervention, followed by a 4‐week washout period prior to crossover. Although no statistically significant difference was found in either total (P = 0.076) or free (P = 0.988) PSA between the two groups, the free PSA doubling‐time was significantly increased in the supplement group compared with the control group: 1150 vs 445 days (2.6‐fold, P = 0.041). These studies establish the need to further explore the effects of prolonged and consistent soy consumption.
In the present study, pure isoflavone was administered to 158 patients over a comparatively long period of 12 months. In an interventional study, if some patients more aggressively consume foods containing isoflavones, such as tofu, miso, and natto, the influence on intervention was supposed. In the present study, however, the plasma isoflavone concentrations in the isoflavone groups were clearly higher than in the placebo control groups. Therefore, such influence to the results is able to be excluded.
The incidence of prostate cancer was lower in the isoflavone group, but not significantly. However, for the patient stratum aged 65 years or more, the incidence of cancer in the isoflavone group was significantly lower than that in the placebo group (28.0%vs 57.1%, P = 0.031). The reason why a significant difference was not shown in the total patient cohort might be related to the fact that the incidence of prostate cancer was small.
One of the primary endpoints, the effect on PSA level, was not proven. However, one of the secondary endpoints, the incidence of biopsy‐detectable prostate cancer, was confirmed to have been significantly reduced in the group aged ≥65 years. Serum PSA is well‐established as a biomarker of prostate cancer, but it is not specific to neoplasia, and the data do not suggest that the level is related directly to the extent of neoplastic progression.
The incidence of Gleason scores of 6 or less and HG‐PIN were numerically lower in the isoflavone group compared with the placebo group, but not significantly. Based on this result, isoflavone may suppress small and low‐grade cancers. This may be one of the reasons why an effect of isoflavone on the serum PSA level was unable to be proven.
We are also interested in prostate cancer risk reduction based on isoflavone administration and equol production. Unfortunately, because of the insufficient number of enrolled men, we were unable to analyze for a relationship between isoflavone intake and equol production.
Although intake of isoflavone suppressed the incidence of prostate cancer, the hormonal data did not show significant changes. These results suggest that isoflavone exerts a cancer chemoprevention effect through an action other than hormonal. In published reports, effects such as apoptosis induction, tyrosine kinase inhibition, anti‐angiogenic action, anti‐oxygenation, and antipromotion have been reported for isoflavones based on in vitro and in vivo studies.( 14 , 15 ) In the non‐producer groups, estradiol decreased significantly, independent of treatment. We are not able to definitely interpret the phenomenon. Estrogen may decrease if the body weight tends to increase in non‐producer groups. In this study, the change in body weight was not measured.
This study was carried out in Japanese patients. Isoflavone intake showed an effect on prostate cancer, even though Japanese ordinarily ingest considerable isoflavone in daily life. It can be expected that this effect would become even greater in Europe and America, who ordinarily ingest little isoflavone. A global study including Europe and America is recommended.
In the PCPT( 16 ) and REDUCE( 17 ) studies, which used 5AR inhibitors, the incidence of prostate cancer was reduced by 25%.( 18 ) However, this treatment strategy is high in cost and leads to complications such as sexual dysfunction.( 18 ) Therefore, its suitability can be considered to be limited to men at very high risk of prostate cancer. However, foods and supplements including isoflavones would be suitable for men in general, because of their safety, low cost, and high feasibility. In addition, isoflavones are effective not only against prostate cancer but also cardiovascular diseases, osteoporosis, and hyperlipidemia.
The isoflavone tablets used in the present study showed no specific safety problems and were well tolerated, with 96.2% (75/78) of the patients in the isoflavone group completing the treatment regimen. Equol binds specifically with 5a‐DHT and sequesters it from the androgen receptor.( 19 ) The end result is similar to that achieved with 5AR inhibitors. We are able to obtain a similar effect to 5AR inhibitors, and safely, using isoflavone.
In conclusion, the incidence of prostate cancer in the isoflavone group was lower than that in the placebo group. In addition, the safety and tolerability of isoflavone intake were proven. These results support the value of isoflavone treatment for prostate cancer risk reduction. A large‐scale phase III randomized study, preferably international, that takes into account different hereditary factors and living environments is warranted.
Disclosure Statement
The authors have no conflicts of interest.
Abbreviations
- 5AR
5‐α‐reductase
- ASAP
atypical small acinar proliferation
- DHT
dihydrotestosterone
- HG‐PIN
high‐grade prostatic intraepithelial neoplasia
- PSA
prostate‐specific antigen
- SHBG
sex hormone binding protein
Acknowledgments
The authors wish to acknowledge the contribution of Dr Kentaro Kuroiwa (Department of Urology, Kyushu University, Fukuoka, Japan) for central pathology. This study was supported by the a grant‐in‐Aid for Scientific Research on Priority Area; Cancer A04, Ministry of Education, Science, Sports and Culture, Japan (Chief investigator; Hideyuki Akaza). The authors thank the cooperative researchers, Wataru Obara, Akira Yokomizo, Atsushi Mizokami, Daisaku Hirano, Kiyohide Fujimoto, Hisamitsu Ide, and Yuichiro Kurimura. We express our thanks to Fuji Oil Company (Osaka, Japan) for kindly providing the isoflavone tablets. [Correction added after online publication 2 December 2011: Acknowledgement added for Dr Kentaro Kuroiwa.]
References
- 1. Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin 2005; 55: 74–108. [DOI] [PubMed] [Google Scholar]
- 2. Yatani R, Chigusa I, Akazaki K, Stemmermann GN, Welsh RA, Correa P. Geographic pathology of latent prostatic carcinoma. Int J Cancer 1982; 29: 611–6. [DOI] [PubMed] [Google Scholar]
- 3. Coleman MP, Esteve J, Damiecki P, Arslon A, Renard H. Trends in Cancer Incidence and Mortality. IARC Scientific Publication No. 121. Lyon: International Association for Research on Cancer, 1993. [DOI] [PubMed] [Google Scholar]
- 4. Nagata Y, Sonoda T, Mori M et al. Dietary isoflavones may protect against prostate cancer in Japanese men. J Nutr 2007; 137: 1974–9. [DOI] [PubMed] [Google Scholar]
- 5. Kelloff GJ, Crowell JA, Steele VE et al. Progress in cancer chemoprevention: development of diet‐derived chemopreventive agents. J Nutr 2000; 130(2S Suppl): 467S–71S. [DOI] [PubMed] [Google Scholar]
- 6. Venkateswaran V, Klotz LH. Diet and prostate cancer: mechanisms of action and implications for chemoprevention. Nat Rev Urol 2010; 7: 442–53. [DOI] [PubMed] [Google Scholar]
- 7. Lippman SM, Klein EA, Goodman PJ et al. Effect of selenium and vitamin E on risk of prostate cancer and other cancers: the Selenium and Vitamin E Cancer Prevention Trial (SELECT). JAMA 2009; 301: 39–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Akaza H, Miyanaga N, Takashima N et al. Is daidzein non‐metabolizer a high risk for prostate cancer? A case–controlled study of serum soybean isoflavone concentration. Jpn J Clin Oncol 2002; 32: 296–300. [DOI] [PubMed] [Google Scholar]
- 9. Akaza H, Miyanaga N, Takashima N et al. Comparisons of percent equol producers between prostate cancer patients and controls: case–controlled studies of isoflavones in Japanese, Korean and American residents. Jpn J Clin Oncol 2004; 34: 86–9. [DOI] [PubMed] [Google Scholar]
- 10. Fujimoto K, Tanaka M, Hirao Y et al. Age‐stratified serum levels of isoflavones and proportion of equol producers in Japanese and Korean healthy men. Prostate Cancer Prostatic Dis 2008; 11: 252–7. [DOI] [PubMed] [Google Scholar]
- 11. Tsuji H, Moriyama K, Nomoto K, Miyanaga N, Akaza H. Isolation and characterization of the equol‐producing bacterium Slackia sp. strain NATTS. Arch Microbiol 2010; 192: 279–87. [DOI] [PubMed] [Google Scholar]
- 12. Kumar NB, Cantor A, Allen K et al. The specific role of isoflavones in reducing prostate cancer risk. Prostate 2004; 59: 141–7. [DOI] [PubMed] [Google Scholar]
- 13. Schröder FH, Roobol MJ, Boevé ER et al. Randomized, double‐blind, placebo‐controlled crossover study in men with prostate cancer and rising PSA: effectiveness of a dietary supplement. Eur Urol 2005; 48: 922–30. [DOI] [PubMed] [Google Scholar]
- 14. Mäkelä S, Poutanen M, Kostian ML et al. Inhibition of 17beta‐hydroxysteroid oxidoreductase by flavonoids in breast and prostate cancer cells. Proc Soc Exp Biol Med 1998; 217: 310–6. [DOI] [PubMed] [Google Scholar]
- 15. Peterson G, Barnes S. Genistein and biochanin A inhibit the growth of human prostate cancer cells but not epidermal growth factor receptor tyrosine autophosphorylation. Prostate 1993; 22: 335–45. [DOI] [PubMed] [Google Scholar]
- 16. Thompson IM, Goodman PJ, Tangen CM et al. The influence of finasteride on the development of prostate cancer. N Engl J Med 2003; 349: 215–24. [DOI] [PubMed] [Google Scholar]
- 17. Andriole GL, Bostwick DG, Brawley OW et al. Effect of dutasteride on the risk of prostate cancer. N Engl J Med 2010; 362: 1192–202. [DOI] [PubMed] [Google Scholar]
- 18. Wilt TJ, Macdonald R, Hagerty K et al. 5‐α‐Reductase inhibitors for prostate cancer chemoprevention: an updated Cochrane systematic review. BJU Int 2010; 106: 1444–51. [DOI] [PubMed] [Google Scholar]
- 19. Lund TD, Munson DJ, Haldy ME, Setchell KD, Lephart ED, Handa RJ. Equol is a novel anti‐androgen that inhibits prostate growth and hormone feedback. Biol Reprod 2004; 70: 1188–95. [DOI] [PubMed] [Google Scholar]


