Abstract
A 4-year-old female spayed domestic longhair cat was referred for dyspnea. Further diagnostics revealed severe pleural effusion and a peritoneopericardial diaphragmatic hernia (PPDH). Following surgical correction of the PPDH the pleural effusion persisted. Re-check echocardiogram 4 weeks after initial evaluation revealed leftward deviation of the interventricular septum and interatrial septum occurring with inspiration. There were also exaggerated phasic changes in trans-tricuspid flow velocities suggestive of constrictive pericardial disease. Cardiac catheterization was performed and revealed elevated pressures in the right atrium and right ventricle. Constrictive pericarditis (CP) and epicarditis was confirmed at surgery, where subtotal pericardiectomy was performed with epicardial decortication. The cat continued to develop recurrent pleural effusion after surgery, although the volume and frequency of recurrence slowed over time. This is the first reported case of CP following PPDH repair in a cat.
Case Report
A 4-year-old 5.7 kg female spayed domestic longhair cat was referred for evaluation of dyspnea of 4 days duration. Physical examination at the regular veterinarian revealed muffled heart and lung sounds bilaterally. Thoracic radiographs performed by the referring veterinarian identified severe pleural effusion and an enlarged cardiac silhouette. Ultrasound-guided thoracocentesis removed 250 ml of serosanguineous fluid from the right hemithorax and 70 ml of serosanguineous fluid from the left hemithorax. Cytological analysis of the pleural effusion revealed a total protein of 3.4 g/dl, 5130 white blood cells/μl and 320,000 red blood cells/μl. It was assessed as being consistent with a lymphocytic-rich modified transudate. Complete blood count and serum biochemistry chemistry profile were unremarkable. The cat was started on furosemide (1.1 mg/kg PO q12h) and benazepril (0.5 mg/kg PO q24h) and was referred for cardiac evaluation.
On presentation, the cat was eupneic with decreased airway sounds in the ventral lung fields bilaterally. Heart rate was 200 beats/min with a regular rhythm and no auscultable heart murmur. Femoral pulses were moderate in quality, and synchronous and jugular venous distension was evident. Two-view thoracic radiographs revealed an enlarged cardiac silhouette (vertebral heart score = 8.5) with suspicion of cranial displacement of the liver. The caudal right pericardium was confluent with the ventral cupula of the diaphragm on the ventro-dorsal projection. On echocardiographic examination, the liver was identified adjacent and caudal to the left ventricle. The cardiac dimensions and systolic function of the left ventricle were within normal range. The cat was diagnosed with a peritoneopericardial diaphragmatic hernia (PPDH) and the pleural effusion from right-sided congestive heart failure. Owing to the finding of a PPDH, surgical correction was performed and a 15 cm ventral midline incision was made from the xiphoid extending caudally. The falciform fat was removed with cautery with a single ligature of 3-0 PDS placed at the cranial attachment. The abdomen was explored and approximately 30% of the liver and the gall bladder was cranially displaced into the pericardium through a connection between the two structures. The structures were reduced and a focal area of hemorrhage was addressed with a 3-0 PDS ligature. The pericardium was opened by resecting a 1 × 2 cm section and the mediastinum was broken down. A 10 French thoracostomy tube was placed in the right hemithorax for closure. A moderate amount of serosanguineous fluid was removed from the thorax and the pericardium was left open. The diaphragm was closed with 3-0 PDS horizontal mattress sutures. A 2 cm cystic structure was removed from the tip of a liver lobe with a strangulating suture. The abdomen was flushed with saline and checked for hemorrhage; none was noted. Air was evacuated from the thorax until negative pressure was achieved, and the thoracostomy tube was removed. The stoma site was closed with a single 3-0 nylon cruciate suture. The body wall was closed with 3-0 PDS simple continuous sutures. The subcutaneous tissues were closed with 4-0 PDS simple continuous sutures. The skin was closed with 3-0 nylon cruciate sutures.
Despite surgical correction of the PPDH, the cat developed recurrent pleural effusion in the days after the surgery and required regular thoracocentesis every 3–5 days, removing approximately 40–60 ml/kg of effusion during each thoracocentesis. Another cytological analysis of the effusion was performed and was again assessed as being consistent with a lymphocyte-rich modified transudate. Triglyceride (41 mg/dl, reference interval: 25–160 mg/dl) and cholesterol levels of the effusion (26 mg/dl, reference interval: 75–220 mg/dl) were compared with the peripheral blood triglyceride (39 mg/dl, reference interval: 26–160 mg/dl) and cholesterol (26 mg/dl, reference interval: 75–220 mg/dl) values, and were not consistent with a chylous effusion. Aerobic culture of the pleural fluid was negative. A thoracic computed tomography (CT) scan was performed, which identified mild right atrial dilation, and distension of the caudal vena cava and hepatic vessels, but was otherwise unremarkable. No abnormalities in the pericardium were noted.
Owing to the recurrent accumulation of pleural effusion, as well as the distension of the caudal vena cava and hepatic veins, another echocardiogram was performed approximately 4 weeks after the initial echocardiogram. Cardiac chamber dimensions and wall thickness were within normal limits. Scant pericardial effusion and a marked amount of pleural effusion was identified. Leftward deviation of the interventricular septum (IVS) and interatrial septum (IAS) occurred with inspiration. There was also discordant phasic variation of trans-tricuspid flow (Figure 1) with maximal diastolic trans-tricuspid flow velocities subjectively assessed as occurring after onset of inspiration. The pericardium was irregularly thickened, measuring approximately 1.5 mm. Based on the echocardiographic findings, constrictive pericarditis (CP) was suspected.
Figure 1.
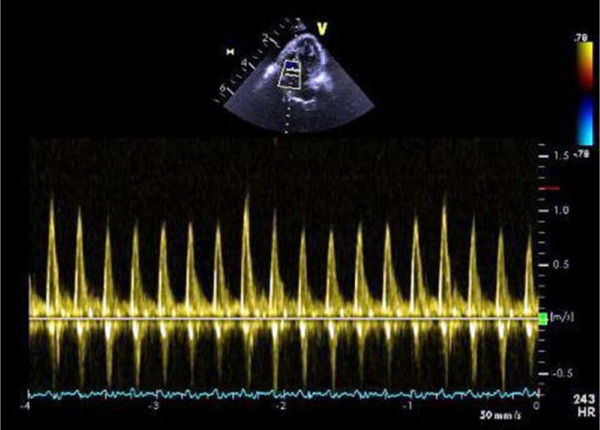
Trans-tricuspid flow showing inflow variation subjectively assessed as being associated with phases of respiration
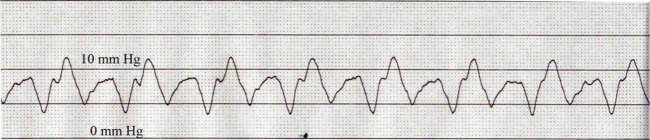
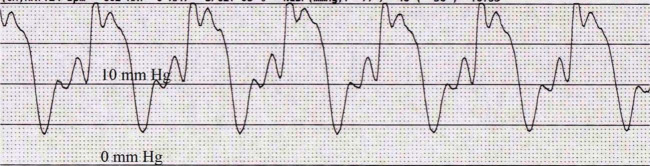
Following the echocardiogram, a right heart catheterization was performed. The cat was sedated with butorphanol (0.2 mg/kg IV) and midazolam (0.2 mg/kg IV), induced with propofol (3 mg/kg IV to effect), and intubated. The left jugular vein was isolated via a vascular cut-down and a 5 French × 13 cm vascular introducer sheath was inserted. Utilizing fluoroscopy, a 4 French × 60 cm Berman catheter was advanced through the right heart chambers. A pressure transducer was flushed and zeroed, and attached to the catheter for direct cardiac pressure measurement. The mean pressure of in the right atrium (RA) was 6 mmHg (reference interval: <5 mmHg) 1 and right ventricular (RV) systolic and diastolic pressure measured 24/10 mmHg (reference interval: <20/5 mmHg) (Figures 2 and 3). 1
Figure 2.
Right atrial pressure waveform with a mean pressure of approximately 6 mmHg. The paper speed is 50 mm/s
Figure 3.
Right ventricular pressure waveform identifying a systolic pressure of 24 mmHg and end-diastolic pressure of 10 mmHg. The paper speed is 50 mm/s
A lateral thoracotomy was performed; the pericardium was thickened and there was copious gray scar tissue covering the epicardial surface. A subtotal pericardiectomy removing approximately 70% of the pericardium and epicardial decortication was performed. Once the pericardium and epicardium were surgically removed the heart noticeably expanded intraoperatively. A portion of the pericardium was submitted for histopathology, bacterial and fungal culture, as well as feline infectious peritonitis (FIP) polymerase chain reaction (PCR). Chronic fibrosing pericarditis and epicarditis was identified on histology with no evidence of neoplastic or infectious causes. Bacterial and fungal cultures, as well as FIP PCR, were negative. In the weeks after surgery, the cat’s pleural effusion persisted and required an upward titration of diuretic therapy. The cat has remained on aggressive diuretic therapy for 10 months after the procedure (furosemide 5.5 mg/kg PO q8h), as well as benazepril (0.5 mg/kg PO q24h), prednisone (0.5 mg/kg PO q24h) and potassium supplementation (164.2 mg/kg PO q12h). While the appearance of the pleural effusion has remained the same, the volume of pleural effusion and frequency of thoracocentesis necessary to control the dyspnea has gradually declined over the subsequent 11 months to approximately 20 ml/kg of effusion removed every 3–4 weeks.
CP is characterized by the encasement of the heart by a dense and rigid pericardial tissue, leading to restriction of diastolic filling and signs of right-sided congestive heart failure. 2 In human medicine, the most common causes are idiopathic, post-surgery or post-radiation therapy.3,4 In dogs, CP is most commonly identified as an idiopathic disease or associated with infection.5,6 There is one case report in the veterinary literature of a cat with idiopathic constrictive pericarditis. 7 To our knowledge, this is the first case of CP following surgical repair of a PPDH.
In the normal heart, with inspiration, there is a fall in both the intra-thoracic and intra-cardiac pressures but the driving pressure from the lungs into the left side of the heart remains constant.8–13 With CP, the fibrotic and rigid pericardium limits this transmission of decreased intra-thoracic pressure to the left side of the heart. 2 Thus, with inspiration, the left ventricle (LV) becomes under-filled as the normal driving force from the lungs to the left heart is reduced.3,4 This under-filling of left ventricle is further exacerbated owing to ventricular inter-dependence.8–13 If the LV is under-filled at inspiration, there is a reciprocal increase in filling in the RV. However, the RV cannot expand owing to the diseased pericardium, and the IVS shifts towards the left side.8–13 This is reflected in the intermittent phasic leftward deviation of the IVS and IAS identified with inspiration. Assessing the pattern of flow velocities across the mitral and tricuspid valves is used to evaluate ventricular filling, and such respiratory variations are evident by increased trans-tricuspid flow on inspiration and decreased trans-tricuspid flow on expiration.8–13 These abnormalities are considered relatively sensitive and specific criteria for diagnosis of CP in humans.10–12
In early diastole the ventricles fill abnormally rapidly because of markedly elevated atrial pressures and accentuated early diastolic ventricular suction. This causes the rapid ‘X’ and ‘Y’ descents in the RA pressure waveform, which produces what is classically described as an ‘M’ or ‘W’ waveform.8 –13 During early-to-mid diastole, ventricular filling abruptly ceases when the intra-cardiac volume reaches the limit set by the stiff pericardium. As a result, almost all ventricular filling occurs in early diastole leading to the ‘square root sign’ of CP, which is also called the ‘dip and plateau’ configuration in the ventricular pressure waveform.8–13 In humans it is preferred to perform simultaneous left and right heart catheterizations to demonstrate near equalization of pressures in all four cardiac chambers;3,4 however, this was not performed in the case described here.
Pericardiectomy is considered the treatment for choice for CP and, usually, most human patients do not require an epicardectomy.11,12 If the whole pericardium is not removed, it is possible for the remaining pericardium to cause localized constriction, which may explain the persistent pleural effusion in the cat reported here.11,12 Furthermore, symptomatic relief and normalization of cardiac pressure may take weeks to months after surgery,11,12 which may also explain the cat’s persistent requirement for diuretics to control the recurrent pleural effusion. Ideally, another right heart catheterization would have been performed to re-evaluate the right sided pressures after pericardiectomy; however, this was not performed.
PPDH is the most common congenital cardiac defect diagnosed in cats older than 2 years of age.14,15 To our knowledge this is the first case of CP reported after surgical correction of PPDH in cat. In human medicine, postoperative CP is an important, but relatively uncommon, cause of CP in cardiac surgery patients, with a reported incidence of 0.2%.16,17 The pathophysiology of post-surgery CP is unclear and is likely multifactorial as it has been hypothesized that pooling of blood into the pericardial cavity may represent a stimulus for ongoing pericardial irritation and development of tight adhesions.17,18
Pleural effusion is a rare initial finding and post operative complication of PPDH repair in cats, and in some cases persists after surgical repair, as in the cat here.14,15 The pathophysiology of pleural effusion development in cats with PPDH has not be identified, but in a dog with PPDH and chylothorax it was postulated to be secondary to compression of the right heart by the abdominal viscera; 19 this was the initial hypothesis for the development of pleural effusion in the cat here. It is possible that another disease was causing the pleural effusion, which consequently developed into CP, although extensive testing, including multiple echocardiograms, thoracic CT, pleural fluid cytology, bacterial and fungal cultures, FIP PCR and pericardial biopsy, failed to identify any underlying causes or concurrent diseases. Furthermore, recurrent pleural effusion is relatively common in cats from idiopathic or underlying cardiac disease; therefore, if chronic recurrent pleural effusion caused CP, we would expect CP to be identified more frequently in cats. We cannot rule out the possibility that CP was present initially, although there was no evidence of it on the initial echocardiogram and the pericardial thickness at surgery was normal. However, echocardiography is not 100% sensitive for CP in humans,10 –12 and humans with CP can have normal pericardial thickness. 20 Regardless of the underlying cause of the pleural effusion, the results of this case report, as well as the limited information regarding this rare presentation of in PPDH in cats,14,15 suggests the effusion frequently persists even after repair of the PPDH and will require continued medical management.
Conclusions
We report development of CP following PPDH repair in a cat. The treatment of choice is pericardiectomy; however, long-term management may still require medical management for chronic right-sided heart failure.
Footnotes
The authors do not have any potential conflicts of interest to declare.
Funding: The authors received no specific grant from any funding agency in the public, commercial or not-for-profit sectors for the preparation of this case report.
Accepted: 22 November 2013
References
- 1. Boon JA. Evaluation of size, function and hemodynamics. In: Boon JA. (ed). Veterinary echocardiography . Ames: Blackwell Publishing, 2011, pp 153–266. [Google Scholar]
- 2. Osterberg L, Vagelos R, Atwood JE. Case presentation and review: constrictive pericarditis. West J Med 1998; 169(4): 232–239. [PMC free article] [PubMed] [Google Scholar]
- 3. Ling LH, Oh JK, Schaff HV, et al. Constrictive pericarditis in the modern era: evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation 1999; 100: 1380–1386. [DOI] [PubMed] [Google Scholar]
- 4. Bertog SC, Thambidorai SK, Parakh K, et al. Constrictive pericarditis: etiology and cause-specific survival after pericardiectomy. J Am Coll Cardiol 2004; 43: 1445–1452. [DOI] [PubMed] [Google Scholar]
- 5. Heinritz CK, Gilson SD, Soderstrom MJ, et al. Subtotal pericardiectomy and epicardial excision for treatment of coccidioidomycosis-induced effusive-constrictive pericarditis in dogs: 17 cases (1999–2003). J Am Vet Med Assoc 2005; 227: 435–440. [DOI] [PubMed] [Google Scholar]
- 6. Thomas WP, Reed JR, Bauer G, et al. Constrictive pericardial disease in the dog. J Am Vet Med Assoc 1984; 184: 546–553. [PubMed] [Google Scholar]
- 7. Thomason JD, Radlinsky MG, Rapoport G, et al. Doppler echocardiographic diagnosis and surgical therapy of constrictive pericarditis in a cat. J Feline Med Surg 2012; 14: 276–279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sohn DW. Constrictive pericarditis as a never ending story: what’s new? Korean Circ J 2012; 42: 143–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghavdiel AA, Gholampour M, Kyavar M, et al. Constrictive pericarditis treated by surgery. Tex Heart Inst J 2012; 39: 199–205. [PMC free article] [PubMed] [Google Scholar]
- 10. Sorajja P. Invasive hemodynamics of constrictive pericarditis, restrictive cardiomyopathy and cardiac tamponade. Cardiol Clin 2011; 29: 191–199. [DOI] [PubMed] [Google Scholar]
- 11. Asher CR, Klein AL. Diastolic heart failure: restrictive cardiomyopathy, constrictive pericarditis, and cardiac tamponade: clinical and echocardiographic evaluation. Cardiol Rev 2002; 10: 218–229. [DOI] [PubMed] [Google Scholar]
- 12. Schwefer M, Aschenbach R, Heidemann J, et al. Constrictive pericarditis, still a diagnostic challenge: comprehensive review of clinical management. Eur J Cardiothorac Surg 2009; 36: 502–510. [DOI] [PubMed] [Google Scholar]
- 13. Matyal R, Skubas NJ, Shernan SK, et al. Perioperative assessment of diastolic dysfunction. Anesth Analg 2011; 113: 449–472. [DOI] [PubMed] [Google Scholar]
- 14. Reimer SB, Kyles AE, Filipowicz DE, et al. Long-term outcome of cats treated conservatively or surgically for peritoneopericardial diaphragmatic hernia: 66 cases (1987–2002). J Am Vet Med Assoc 2004: 224: 728–732. [DOI] [PubMed] [Google Scholar]
- 15. Burns CG, Bergh MS, McLoughlin MA. Surgical and nonsurgical treatment of peritoneopericardial diaphragmatic hernia in dogs and cats: 58 cases (1999–2008). J Am Vet Med Assoc 2013; 242: 643–650. [DOI] [PubMed] [Google Scholar]
- 16. Kutcher MA, King SB, III, Alimurung BN. Constrictive pericarditis as a complication of cardiac surgery: recognition of an entity. Am J Cardiol 1982; 50: 742–748. [DOI] [PubMed] [Google Scholar]
- 17. Ribeiro R, Sapsford R, Evans T, et al. Constrictive pericarditis as a complication of coronary artery bypass surgery. Br Heart J 1984; 51: 205–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Dardas P, Tsikaderis D, Ioannides E, et al. Constrictive pericarditis after coronary artery bypass surgery as a cause of unexplained dyspnea: a report of five cases. Clin Cardiol 1998; 21: 691–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Schmiedt CW, Washabaugh KF, Rao DB, et al. Chylothorax associated with congenital peritoneopericardial diaphragmatic hernia in a dog. J Am Anim Hosp Assoc 2009; 45: 134–137. [DOI] [PubMed] [Google Scholar]
- 20. Talreja DR, Edwards WD, Danielson GK, et al. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation 2003; 108: 1852–1857. [DOI] [PubMed] [Google Scholar]