Abstract
Anaplasma platys (Apl), ‘Candidatus Mycoplasma haemominutum’ (CMh), Bartonella henselae (Bh) and Bartonella koehlerae (Bk) were confirmed by polymerase chain reaction (PCR) amplification and DNA sequencing in a cat diagnosed with multiple myeloma. Other inconsistently documented hematologic abnormalities included anemia, thrombocytopenia, eosinophilia and hypoglycemia. Persistent Apl infection was confirmed for the first time in a North American cat by sequencing three bacterial genes (16S rRNA, p44 and GroEL) in peripheral blood samples collected 100 days apart. Following doxycycline treatment for Apl, multiple myeloma was diagnosed based upon a monoclonal gammopathy and splenic plasmacytosis, and the cat was treated with melphalan, chlorambucil and prednisolone. Apl DNA was not amplified from post-treatment blood samples and the hyperglobulinemia resolved temporarily following chemotherapy. Retrospective PCR analysis of stored DNA extracts identified CMh, Bk and Bh infections. Retrospective PCR for antigen receptor rearrangements (PARR) of splenic aspirates did not confirm B- or T-cell clonality. Co-infection with multiple vector-borne pathogens should be a diagnostic consideration in cats with chronic hypergammaglobulinemia, monoclonal gammopathy and splenic plasmacytosis.
Case Report
In December 2011, a 14-year-old male castrated American domestic shorthair cat was initially examined at the North Carolina State University – College of Veterinary Medicine (NCSU–CVM) for persistent hyperglobulinemia and treated for multiple myeloma at another veterinary referral hospital. Historically, the owner reported no clinical signs; however, at 6, 8 and 24 months prior to presentation the cat’s previous medical history included mild mitral and tricuspid insufficiency; mild renal azotemia, stage 2 under the International Renal Interest Society guidelines (peak serum creatinine 2 mg/dl, reference interval (RI) 1.0–2.1 mg/dl; blood urea nitrogen 22 mg/dl, RI 15–37 mg/dl; and urine specific gravity 1.016, RI 1.035–1.060); and bilateral elbow and hip osteoarthritis. The cat had no history of travel after moving to North Carolina in 2001. It was fed a commercial renal diet, housed indoors with daily outdoor access and received topical Frontline (Merial) and Revolution (Pfizer) once a month. Medications and supplements included 6 mg/kg gabapentin twice daily, Dasuquin (Nutramax Laboratories) (two capsules once a day) and Welactin fish oil nutraceutical (Nutramax Laboratories) (once a day).
Hyperglobulinemia was initially documented in March 2011 and remained elevated, above the RI, throughout the 2 year monitoring of this case, except in October 2012, where it was at the high end of the RI (Table 1). In November 2011, a serum protein electrophoresis (SPE) scan performed by Antech Diagnostics identified a gammopathy characterized by a monoclonal spike arising from a broad polyclonal band in the gamma region (3.77 g/dl) (Figure 1). Proteinuria was absent as determined by a urine protein-to-creatinine ratio of 0.1 (<0.5 normal). Thoracic radiographs were unremarkable, with no evidence of osteolytic lesions. Abdominal ultrasound did not identify structural abnormalities. Feline immunodeficiency virus/feline leukemia virus SNAP test combo (IDEXX Laboratories) was negative. On 22 December 2011, serum and ethylenediamine tetra acetic acid (EDTA)-anti-coagulated, aseptically collected whole blood were submitted to the Intracellular Pathogens Research Laboratory for Bartonella serology and Bartonella alpha-proteobacteria growth medium (BAPGM) diagnostic platform.1–3 By indirect immunofluorescent antibody testing, the cat was seronegative to Bh, Bk and Bartonella vinsonii subspecies berkhoffii (Bvb) antigens. Bartonella species were neither amplified nor isolated from the blood using BAPGM enrichment blood culture. Following negative Bartonella species results, a previously described 16S ribosomal RNA PCR assay to detect Anaplasma or Ehrlichia species infection was performed.4,5 A 331 base pair (bp) portion of the 16S rRNA gene was amplified, sequenced and analyzed in the National Center for Biotechnology Information (NCBI) GenBank nucleotide database identifying Apl with 100% coverage and 100% identity to Apl 16S rRNA gene (accession number JX112780.1). Prior to initiating a 30 day course of doxycycline, two additional EDTA–whole blood samples were collected on 1 April 2012 and 6 April 2012, for repeat Anaplasma and Ehrlichia PCR, and attempted blood culture isolation of Apl using DH82 canine monocytoid cell line, respectively. While there were no obvious clinical abnormalities, a blood sample collected on 2 April 2012 showed thrombocytopenia, neutrophilia, eosinophilia, hyperproteinemia, hyperglobulinemia and hypoglycemia (Table 1).
Table 1.
Summary of hematological values for a cat diagnosed with multiple myeloma and infected with Anaplasma platys, Bartonella henselae, Bartonella koehlerae and ‘Candidatus Mycoplasma haemominutum’. Dates of treatments are indicated as follows: (1) doxycycline; (2) melphalan and prednisolone, and (3) melphalan discontinued, started chlorambucil. Values outside of the reference interval (RI) are in bold. The two dates measured by Antech Diagnostics are indicated. All Antech Diagnostics laboratory values are within the RI except those values shown with their respective RIs
| 1 | 2 | 3 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| RI NCSU–CVM |
12/3/11 Antech |
20/4/11 | 2/8/11 | 22/11/11 Antech |
2/4/12 | 25/5/12 | 12/7/12 | 7/8/12 | 5/9/12 | 19/9/12 | 30/10/12 | 7/1/13 | 11/4/13 | |
| PCV (%) | 32–48 | 39.4 | 38.4 | NA | 34.4 | 34 | 28.3 | 30 | 27 | 27 | 33 | 24.9 | 29 | 31 |
| RBC (x106/μl) | 6.91–10.49 | 8.6 | 8.26 | NA | 7.52 | 7.10 | 6.26 | 6.56 | 5.71 | 5.91 | 6.76 | 5.29 | 6.25 | 6.25 |
| PLT (x103/μl) | 198–434 |
95
RI: 200–500 |
274 | NA | 361 | 54 * | clmp | 222 | clmp | clmp | clmp | 242 | 254 | 254 |
| WBC (x 103/μl) | 4.28–14.3 | 8.4 | 8.11 | NA | 9.0 | 10.73 | 10.90 | 0.49 | 0.89 | 9.66 | 6.54 | 6.18 | 7.22 | 7.40 |
| Neut (x 103/µl) | 2.773–6.975 | 6.3 | 6.569 | NA | 6.570 | 7.833 | 8.284 | 0.284 | 0.392 | 5.893 | 5.821 | 5.129 | 5.920 | 5.846 |
| Lymph (x 103/µl) | 0.415–4.996 | 1.092 | 0.406 | NA | 1.710 | 0.858 | 0.981 | 0.167 | 0.427 | 0.773 | 0.065 | 0.433 | 0.217 | 0.296 |
| Eosin (x 103/µl) | 0.118–0.879 | 0.840 | 0.568 | NA | 0.450 | 1.288 | 0.872 | NR | NR | 1.932 | 0.458 | 0.309 | 0.794 | 0.888 |
| BUN (mg/dl) | 15–37 | 33 | 22 | 22 | 22 | 25 | 23 | 23 | 26 | NA | 24 | 21 | 22 | 26 |
| Cr (mg/dl) | 0.7–1.9 | 2.4 | 1.5 | 2.0 | 2.0 | 1.8 | 1.8 | 1.5 | 1.8 | NA | 2.0 | 1.7 | 1.6 | 1.8 |
| Glucose (mg/dl) | 70–182 | 82 | 88 | 79 | 79 | 58 | 57 | 93 | 64 | NA | 81 | 83 | 68 | 86 |
| Albumin (g/dl) | 2.9–4.0 | 3.5 | 2.9 | 2.7 | 3.1 | 2.6 | 2.2 | 2.7 | 2.7 | NA | 3.0 | 2.7 | 2.8 | 3.1 |
| Globulin (g/dl) | 2.9–4.8 | 5.1 | 4.9 | 5.7 |
6.8
RI: 2.3–5.3 |
7.1 | 7.7 | 7.4 | 6.3 | NA | 5.5 | 4.8 | 5.5 | 5.0 |
| TP (g/dl) | 6.4–8.2 | 8.6 | 7.9 | 8.4 |
9.3
RI: 5.2–8.8 |
9.7 | 9.9 | 10.1 | 9.0 | 8.5 | 8.5 | 7.5 | 8.3 | 8.2 |
| Gamma (g/dl) | NA | NA | NA | NA |
3.77
†
RI: 0.5–1.9 |
NA | NA | NA | NA | NA | NA | NA | NA | NA |
NCSU-CVM = North Carolina State University – College of Veterinary Medicine; PCV = packed cell volume; RBC = red blood cells; PLT = platelets; WBC = white blood cells; Neut = neutrophils; Lymph = lymphocytes; Eosin = eosinophils; BUN = blood urea nitrogen; Cr = creatinine; TP = total protein; Gamma = gamma protein fraction; NA = not available; clmp = clumping; NR = not reported
Results confirmed by manual evaluation of smear
Monoclonal gammopathy protein electrophoresis
Figure 1.
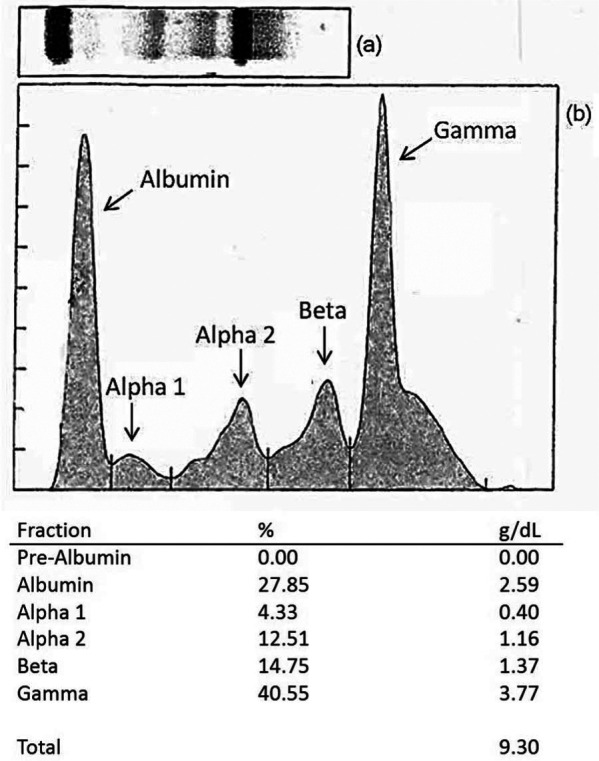
Protein immunoblot (a) and serum protein electrophoresis (b) from a cat with hyperglobulinemia, splenic plasmacytosis and subsequently diagnosed with Anaplasma platys, ‘Candidatus Mycoplasma haemominutum’, Bartonella henselae San Antonio 2 (SA2) and Bartonella koehlerae infections
To confirm persistent Apl infection, genomic DNA was extracted from EDTA–whole blood and leukocyte-rich fractions from both April 2012 blood samples. Primers targeting alternative Apl genes p44 (BA Qurollo, unpublished data) and GroEL (RG Maggi, unpublished data) were used to test for Apl. EDTA–whole blood from a blood donor cat housed at NCSU–CVM served as DNA extraction and PCR controls. Apl 16S rRNA, p44 and GroEL gene segments were amplified and sequenced from both the December 2011 and 1 April 2012 whole blood samples, but not from 6 April 2012 whole blood or leukocyte-rich fractions (Table 2). Controls remained negative. PCR products were sequenced directly (GENEWIZ) and alignments made with NCBI GenBank sequences. Sequence identities for the partial p44 and GroEL genes are as follows, all with 100% coverage: p44, 99% similar (535/537 bp) to Apl partial p44 (accession number GQ868750.1); GroEL, 99% similar (416/420 bp) to Apl partial GroEL (accession number EU516386.1). Each of the three Apl gene sequences obtained from the December 2011 and 1 April 2012 specimens were identical. Apl was never successfully cultured from this cat’s blood using canine DH82 cells.
Table 2.
Polymerase chain reaction (PCR) results for cat infected with Anaplasma platys (Apl), ‘Candidatus Mycoplasma haemominutum’ (‘CMh’), Bartonella henselae San Antonio 2 (BhSA2) and Bartonella koehlerae (Bk)
| Sample | Collection date | PCR results |
|||||
|---|---|---|---|---|---|---|---|
|
Ehrlichia/Anaplasma
|
Apl
|
Apl
|
Mycoplasma
|
Bartonella
|
Bk species-specific |
||
| (16S rRNA) | (p44) | (GroEL) | (16S rRNA) | (16–23S ITS) | (16–23S ITS) | ||
| Wb | 22 December 2011 | Apl | Apl | Apl | ‘CMh’ | Neg | Neg |
| Wb | 1 April 2012 | Apl | Apl | Apl | ‘CMh’ | Neg | Bk * |
| Lc | 1 April 2012 | Neg | Neg | Neg | NA | NA | NA |
| Wb | 6 April 2012 | Neg | Neg | Neg | ‘CMh’ | Neg | Neg |
| Lc | 6 April 2012 | Neg | Neg | Neg | ‘CMh’ | Neg | Bk* |
| Wb | 25 May 2012 | Neg | Neg | Neg | ‘CMh’ | BhSA2* | Neg |
| Wb | 1 July 2012 | Neg | Neg | Neg | ‘CMh’ | BhSA2 † | Neg |
| Wb | 1 January 2013 | Neg | Neg | Neg | ‘CMh’ | Neg | Neg |
| Wb | 1 April 2013 | Neg | Neg | Neg | ‘CMh’ | Neg | Neg |
All amplicons were sequenced to confirm genus and species identity
ITS = intergenic transcribed spacer; Wb = whole blood; Lc = leukocyte-rich fractions; Neg = negative; NA = not available
Bartonella amplicon from blood
Bartonella amplicon from Bartonella alpha-proteobacteria growth medium enrichment blood culture
Following PCR confirmation of a persistent Apl infection, doxycycline (5 mg/kg PO twice daily) was administered for 1 month. A post-treatment EDTAsample (25 May 2012) was PCR-negative for Apl using three gene targets (16S rRNA, p44 and GroEL). Hematological abnormalities included anemia, neutrophilia, hyperglobulinmia, hyperproteinemia, hypoglycemia and hypoalbuminemia, with eosinophils at the upper end of the RI (Table 1). Owing to platelet clumping, an automated count was not obtained; however, platelet numbers appeared adequate on direct blood smear. In June 2012, splenic aspirates identified a marked plasma cell proliferation with presumptive diagnosis of multiple myeloma. Chemotherapy was initiated with melphalan at 2 mg PO once daily for 5 days and then repeated every 3 weeks. Prednisolone was administered at 2.5 mg PO once daily for 10 days followed by every other day for 60 days. In July 2012 the melphalan dosage was decreased to 2 mg PO once daily for 3 days and eventually discontinued owing to profound neutropenia. At this time, platelet numbers were normal; however, hyperglobulinemia, hyperproteinemia and mild anemia persisted (Table 1). Owing to neutropenia, chemotherapeutics were changed to chlorambucil at 2 mg PO every other day, leading to remission of the myeloma. The cat remained negative for Apl by PCR. Four months after completion of chemotherapy (October 2012) normal serum globulins and protein were documented; however, in January 2013 and April 2013, the cat was hyperglobulinemic again. Complete blood counts between April 2012 and April 2013 revealed a persistent mild anemia, with eosinophilia documented in April 2012, September 2012 and April 2013 (Table 1).
Retrospective examinations of Giemsa-stained peripheral blood smears made from pre-doxycycline samples collected in April 2012 did not reveal platelet inclusion-bodies consistent with Apl infection. However, epicellular organisms consistent with a hemotropic Mycoplasma species were visualized on erythrocyte surfaces. This observation prompted further retrospective PCR testing of all available DNA extracts using previously described 16S rRNA PCR for hemotropic Mycoplasma species. 6 Based upon DNA sequencing, both pre- and post-doxycycline treatment samples were positive for ‘CMh’ (Table 2). Controls remained negative for Mycoplasma species DNA.
Based on negative Bartonella serology and PCR obtained from the December 2011 blood sample, additional Bartonella testing was not initially performed. Following documentation of splenic plasmacytosis in June 2012, Bartonella bacteremia was re-examined with retrospective EDTA–whole blood samples using PCR and the BAPGM diagnostic platform (Table 2).1–3 Bk DNA was amplified from April 2012 whole blood and leukocyte-rich fractions; no enrichment cultures were performed on samples collected in April 2012. Bh San Antonio 2 DNA (BhSA2) was amplified from the pre-enrichment May 2012 blood sample. From the July 2012 blood sample, BhSA2 DNA was amplified from 7 and 14 day BAPGM enrichment cultures; however, no isolates were obtained. One out of three DNA extractions from the negative control blood donor cat contained Bk DNA, using Bk-specific primers. The canine negative control DNA extractions were PCR-negative for Bk. All controls were negative for BhSA2. Subsequently, EDTA blood samples collected in January 2013 and April 2013 were PCR-negative for Apl and Bartonella species; however, ‘CMh’ persisted (Table 2). The cat remained seronegative to all Bartonella species antigens tested.
PARR diagnostics have been reported in cats with B cell neoplasias, so retrospective PARR was performed by NCSU–CVM Clinical Immunology Laboratory 7 using cells from a splenic cytology smear generated in June 2012. The splenic plasmacytocis did not confirm B or T cell clonality. While the cat discussed in this report remains free from obvious clinical signs, hematological and biochemical parameters continue to be monitored.
Between March 2011 and May 2012, the geriatric cat discussed in this report developed a chronic hyperglobulinemia (5.1–7.7 g/dl), monoclonal gammopathy and splenic plasmacytosis and was ultimately treated for multiple myeloma. Based upon molecular evidence of Apl infection before initiating cancer chemotherapy, the cat was treated for anaplasmosis. After initiation of chemotherapy, molecular evidence of concurrent infection with ‘CMh’, Bh and Bk was documented over the next few months. Despite the identification of multiple blood-borne pathogens and repeated documentation of mild anemia, hyperglobulinemia, mild hypoalbuminemia, eosinophilia and mild hypoglycemia (Table 1), this cat remained outwardly healthy.
In cats, monoclonal gammopathies have been reported in association with feline infectious peritonitis, FIV, lymphosarcoma and myeloma-related disorders (MRD) such as multiple myeloma, extramedullary plasmacytoma and monoclonal gammopathy of undetermined significance.8–14 Persistent infection with intracellular vector-borne organisms, including Anaplasma phagocytophilum, Ehrlichia canis, Leishmania infantum and Bh have been related to polyclonal and monoclonal gammopathies in dogs, and therapeutic elimination of E canis has resulted in resolution of monoclonal gammopathies.15–21 One cat, with unknown clinical history, was characterized with a monoclonal gammopathy after hyperglobulinemia was further defined using SPE in 20 Bartonella seroreactive cats. 22 Although rare in cats, the most frequently reported cause of monoclonal gammopathies are MRD, which comprise various plasma cell neoplasms.8,10,13,14 Clinical signs in cats with MRD commonly include lethargy, decreased appetite, vomiting, diarrhea and weight loss, all of which were absent from the cat discussed in this report.8,13,14 In addition to illness, extramedullary involvement such as abdominal organomegaly and/or plasma cell infiltration of the spleen and liver are common in cats with MRD, whereas osteolytic lesions are less common. 13 One study evaluating 16 cats with multiple myeloma found the most common ultrasonographic abnormalities were seen in the spleen and liver, and splenic aspirates were always diagnostic for plasma cell infiltration. 14 The cat in our study had a monoclonal gammopathy with cytological documentation of splenic plasmacytosis with no evidence of osteolytic lesions.
An important, but unanswered, question for the cat discussed in this report was whether the monoclonal gammopathy and splenic plasmacytosis were related to persistent infection with multiple intravascular pathogens, or whether MRD contributed to immunosuppression, facilitating the co-infections. When the globulins had not decreased a month after doxycycline treatment for the Apl infection, and splenic aspirates identified plasmacytosis, the owner elected to treat for multiple myeloma. Documentation of infection with Bh, Bk and ‘CMh’ was obtained retrospectively and after the decision to treat the cat for multiple myeloma. A recent case report describes a dog with serological evidence supporting multiple concurrent infections that subsequently developed multiple myeloma despite targeted therapy for each infection. 20 In that dog, A phagocytophilum and E canis antibody titers were markedly elevated for more than 6 years prior to presentation, and remained high following treatment for and resolution of the dog’s multiple myeloma. Plasmacytosis or monoclonal gammopathy have not been reported in association with Apl or ‘CMh’, but have been reported in association with Bartonella species infection in animals and humans.15,23–26 Hypergammaglobulinemia and plasma cell infiltrations were documented in a cat with recurrent osteomyelitis due to Bvb genotype II, and Whittemore et al 22 showed a significant association (odds ratio = 11.9, P <0.0001) between hyperglobulinemia and serological evidence of Bartonella species exposure in privately owned cats. 25 From a comparative medicine standpoint, monoclonal and biclonal gammopathies have been reported in two immunocompetent human patients infected with Bh, and a recent report from our laboratory documented Bh infection in a human patient with hypergammaglobulinemia, splenomegaly and polyclonal splenic plasmacytosis.23,26 Neither patient with gammopathies had evidence supporting other known causes of a plasma cell dyscrasia, and antibiotic eradication of the Bh infection resulted in the prompt disappearance of their respective gammopathies. 23 Alternatively, the cat discussed in this report may have developed co-infections due to immunosuppression from MRD. Complications seen in canine and feline multiple myeloma patients include immunosupression,8,27 and human patients with multiple myeloma have numerical and functional abnormalities in mature B cells, dendritic cells, T cells, natural killer cells and non-myeloma IgA, IgG and IgM.28–30
PARR did not confirm B or T cell clonality in splenic cytology smear cells from the cat in this case. Most plasma cell neoplasms are monoclonal proliferations of mature B cells; however, the use of molecular diagnostics in diagnosing multiple myeloma or plasmacytosis has been limited in veterinary medicine. 31 A study using PARR to identify clonality in feline B cell neoplasias demonstrated monoclonal results in 15/22 cats with B cell neoplasias, with 2/2 previously diagnosed myelomas and 3/3 plasmacytomas. 7 It is important to note, however, that feline B cell PARR is approximately 60% sensitive in determining B cell clonality and detects from 1 to 10% of tumor cells, so if only a small neoplastic population of B-cells is among a large normal population, the results may be negative. 7
Bartonella antibody titers in this cat remained below detection despite elevated globulins and PCR documentation of infection. A subset of bacteremic animals and humans demonstrate an attenuated antibody response despite chronic, persistent infection with Bartonella species.32,33 It is also possible that the excess immunoglobulin detected in this cat was functionally impaired. In one study, a monoclonal antibody generated against listeria–lysin O (LLO) and administered in large doses to mice was protective against the intracellular pathogen Listeria monocytogenes; however, anti-LLO antibodies titers were low negative in the serum. 34 Another study evaluating different doses of IgG subclasses and pathogen inoculum showed administration of large amounts of antibody resulted in reduced or no immune protection against Cryptococcus neoformans infections in mice. 35 Because abnormal production of immunoglobulins can cause immunosuppression, ineffective antibodies may have played a role in persistent infection of multiple organisms in this case.
While the documentation of Bh after Bk may represent a sequential infection, it is likely that this cat was co-infected with both pathogens in April 2012. Bk bacteremia was documented shortly before, whereas Bh bacteremia was documented after the cat completed cancer chemotherapy. As previously reviewed, immunosuppression appears to increase the number of Bartonella organisms in systemic circulation, thereby facilitating detection by PCR or enrichment blood cultures using BAPGM. 36 Amplification of BhSA2 DNA after chemotherapy may have been related to chemotherapy-induced immune suppression or translocation of bacteria from intracellular niches into systemic circulation. It is not unexpected that only one of two organisms within the same genus would be PCR amplified at a given point in time, particularly if the bacterial load varied greatly between the two species. 2 Furthermore, a recurring mild eosinophilia (0.985; RI 0.118–0.879 × 103 cells/μl) and high normal globulins (4.9; RI 2.4–4.9 g/dl), first detected almost 5 years before presentation, lends support for a persistent infection with one or more Bartonella species. Eosinophilia was documented in experimentally infected cats with persistent Bh bacteremia, despite the absence of fleas and intestinal parasites. 37 The cat in this report had a regularly documented mild hypoglycemia that cannot be explained; however, it is noteworthy that Whittemore et al 22 showed a decreased glucose concentration in cats was associated with an increased risk of seropositivity to Bartonella species. It remains unclear to what extent the Bartonella species infections attributed to abnormalities seen in this cat. Limited studies have been reported to document the pathogenicity of Bk in cats, but cats experimentally infected with Bk did not show obvious clinical disease. 38
To our knowledge, this is the first report identifying natural Apl infection in a cat from North America. In the only other published case of feline Apl infection, anorexia, lethargy, anuria and constipation were the presenting signs in a cat from Brazil. 39 Originally described in dogs, Apl causes canine infectious cyclic thrombocytopenia. 40 Although Apl can cause persistent infections in dogs and, potentially, cats, the organism is not considered highly pathogenic and thus rarely implicated as an important canine vector-borne pathogen in North America. Experimentally, however, co-infection and sequential infection with Apl and E canis potentiated the severity of thrombocytopenia when compared with infection caused by Apl or E canis alone. 4 Apl infection in the cat from this report was initially detected in December 2011; however, it is impossible to determine when it was first infected. Thrombocytopenia was first documented in March 2011 and again in April 2012 with confirmation by manual examination of the smear. Because this cat was co-infected with other bacteria and diagnosed with an MRD, it is unclear whether Apl infection can cause a cyclic thrombocytopenia in cats.
‘CMh’ DNA was PCR amplified retrospectively from all available blood samples obtained from the cat in this report. It remains unclear if persistent infection with ‘CMh’ contributed to any of the hematological (anemia, thrombocytopenia, eosinophilia) or serum biochemical (hypoalbuminemia, hyperglobulinemia, hypoglycemia) abnormalities documented in this cat (Table 1). Experimental infections of cats with ‘CMh’ rarely cause obvious clinical or hematologic abnormalities, unlike Mycoplasma hemofelis, which can cause severe anemia in cats.41,42 However, Reynolds and Lappin 43 retrospectively evaluated medical records from a group of 21 clinically ill cats naturally infected with ‘CMh’ and found 13 to be anemic, with six having no other identifiable cause for their anemia. Co-infection with other intracellular pathogens (Apl, Bh and Bk), complicates the association of infection with ‘CMh’ to the disease pathogenesis. A mild, chronic anemia (not classified as regenerative or non-regenerative) was noted at several time points before and after the animal’s treatments. This could be contributed to chronic inflammatory disease, ‘CMh’ infection, multiple myeloma or a combination of these entities.
Conclusions
We report an intriguing case of a cat with a monoclonal gammopathy and splenic plasmacytosis that was concurrently infected with three vector-borne pathogens (Apl, ‘CMh’ and Bk), and was either concurrently or sequentially infected with a fourth organism (Bh). Following treatment with doxycycline, Apl DNA could no longer be amplified from the cat’s blood. Chemotherapy for multiple myeloma decreased globulins; however, by January 2013 globulins had increased slightly and remained elevated in April 2013 (Table 1). Persistent ‘CMh’ infection was documented in blood samples from January and April 2013. Multiple, documented blood-borne infections combined with an atypical clinical presentation for multiple myeloma and splenic plasmacytosis highlight the following possibilities: (1) chronic intravascular infection with one or more pathogens may perhaps mimic or play a role in MRD and (2) MRD in cats may significantly alter the immune system, facilitating chronic infection with multiple pathogens. In either situation, this case emphasizes the importance of performing comprehensive diagnostic testing for vector-borne pathogens in cats with persistent hematologic abnormalities and protracted hyperglobulinemia.
Acknowledgments
We thank clinical pathology technician, Tillie Laws, for review of blood smears, Tonya Lee for editorial assistance, Andrea Thomson for blood collection and preparation, Dr Steve Suter for PARR diagnostics, and Drs Wendy Simpson, Jaimie Tarigo and Angela Kozicki who contributed to the care of this cat over the study period.
Footnotes
Dr Barbara Qurollo’s fellowship in Vector Borne Disease Research at the College of Veterinary Medicine, North Carolina State University is supported by Idexx Laboratories.
Funding: Research testing of this cat was supported, in part, by the Sigmon Trust.
Accepted: 12 December 2013
References
- 1. Duncan AW, Maggi RG, Breitschwerdt EB. A combined approach for the enhanced detection and isolation of Bartonella species in dog blood samples: pre-enrichment culture followed by PCR and subculture onto agar plates. J Microbiol Meth 2007; 69: 273–281. [DOI] [PubMed] [Google Scholar]
- 2. Maggi RG, Duncan AW, Breitschwerdt EB. Novel chemically modified liquid medium that will support the growth of seven Bartonella species. J Clin Microbiol 2005; 43: 2651–2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Breitschwerdt EB, Maggi RG, Mozayeni BR, et al. PCR amplification of Bartonella koehlerae from human blood and enrichment blood cultures. Parasit Vectors 2010; 3: 76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gaunt S, Beall M, Stillman B, et al. Experimental infection and co-infection of dogs with Anaplasma platys and Ehrlichia canis: hematologic, serologic and molecular findings. Parasit Vectors 2010; 3: 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Eddlestone SM, Dinz PPVP, Neer TM, et al. Doxycycline clearance of experimentally induced chronic Ehrlichia canis infection in dogs. J Vet Intern Med 2007; 21: 1237–1242. [DOI] [PubMed] [Google Scholar]
- 6. Varanat M, Maggi RG, Linder KE, et al. Molecular prevalence of Bartonella, Babesia, and Hemotropic Mycoplasma sp. in dogs with splenic disease. J Vet Intern Med 2011; 25: 1284–1291. [DOI] [PubMed] [Google Scholar]
- 7. Werner JA, Woo JC, Vernau W, et al. Characterization of feline immunoglobulin heavy chain variable region genes for the molecular diagnosis of B-cell neoplasia. Vet Pathol 2005; 42: 596–607. [DOI] [PubMed] [Google Scholar]
- 8. Hanna F. Multiple myelomas in cats. J Feline Med Surg 2005; 7: 275–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rosenberg MP, Hohenhaus AE, Matus RE. Monoclonal gammopathy and lymphoma in a cat infected with feline immunodeficiency virus. J Am Anim Hosp Assoc 1991; 27: 335–337. [Google Scholar]
- 10. Mellor PJ, Haugland S, Smith KC, et al. Histopathologic, immunohistochemical, and cytologic analysis of feline myeloma-related disorders: further evidence for primary extramedullary development in the cat. Vet Pathol 2008; 45: 159–173. [DOI] [PubMed] [Google Scholar]
- 11. Bienzle D, Silverstein DC, Chaffin K. Multiple myeloma in cats: variable presentation with different immunoglobulin isotypes in two cats. Vet Pathol 2000; 37: 364–369. [DOI] [PubMed] [Google Scholar]
- 12. Taylor SS, Tappin SW, Dodkin SJ, et al. Serum protein electrophoresis in 155 cats. J Feline Med Surg 2010; 12: 643–653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mellor PJ, Haugland S, Murphy S, et al. Myeloma-related disorders in cats commonly present as extramedullary neoplasms in contrast to myeloma in human patients: 24 cases with clinical follow up. J Vet Intern Med 2006; 20: 1376–1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Patel RT, Caceres A, French AF, et al. Multiple myeloma in 16 cats: a retrospective study. Vet Clin Pathol 2005; 34: 341–352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tabar MD, Maggi RG, Altet L, et al. Gammopathy in a Spanish dog infected with Bartonella henselae. J Small Anim Pract 2011; 52: 209–212. [DOI] [PubMed] [Google Scholar]
- 16. Breitschwerdt EB, Woody BJ, Zerbe CA, et al. Monoclonal gammopathy associated with naturally occurring canine ehrlichiosis. J Vet Intern Med 1987; 1: 2–9. [DOI] [PubMed] [Google Scholar]
- 17. Font A, Closa JM, Mascort J. Monoclonal gammopathy in a dog with visceral leishmaniasis. J Vet Intern Med 1994; 8: 233–235. [DOI] [PubMed] [Google Scholar]
- 18. Giraudel JM, Pagès JP, Guelfi JF. Monoclonal gammopathies in the dog: a retrospective study of 18 cases (1986–1999) and literature review. J Am Anim Hosp Assoc 2002; 38: 135–147. [DOI] [PubMed] [Google Scholar]
- 19. Perille AL, Matus RE. Canine ehrlichiosis in six dogs with persistently increased antibody titers. J Vet Intern Med 1991; 5: 195–198. [DOI] [PubMed] [Google Scholar]
- 20. Giegy C, Riond B, Bley CR, et al. Multiple myeloma in a dog with multiple concurrent infectious diseases and persistent polyclonal gammopathy. Vet Clin Pathol 2013; 42: 47–54. [DOI] [PubMed] [Google Scholar]
- 21. Ng S, Lim S, Daub A, et al. Benign monoclonal gammopathy in a dog exhibiting a low antibody titer to Ehrlichia canis. J Clin Immunol Immunopathol Res 2010; 2: 9–14. [Google Scholar]
- 22. Whittemore JC, Hawley JR, Radecki SV, et al. Bartonella species antibodies and hyperglobulinemia in privately owned cats. J Vet Intern Med 2012; 26: 639–644. [DOI] [PubMed] [Google Scholar]
- 23. Krause R, Auner HW, Daxböck F, et al. Monoclonal and biclonal gammopathy in two patients infected with Bartonella henselae. Ann Hemotol 2003; 82: 455–457. [DOI] [PubMed] [Google Scholar]
- 24. Se’ve P, Turner R, Stankovic K, et al. Transient monoclonal gammopathy in a patient with Bartonella quintana endocarditis. Am J Hematol 2006; 81: 115–117. [DOI] [PubMed] [Google Scholar]
- 25. Varanat M, Travis A, Lee W, et al. Recurrent osteomyelitis in a cat due to infection with Bartonella vinsonii subsp. berkhoffii genotype II. J Vet Intern Med 2009; 23: 1273–1277. [DOI] [PubMed] [Google Scholar]
- 26. Balakrishnan N, Jawanda JS, Miller MB, et al. Bartonella henselae infection in a man with hypergammaglobulinemia, splenomegaly and polyclonal plasmacytosis. J Med Microbiol 2013; 62: 338–341. [DOI] [PubMed] [Google Scholar]
- 27. Hammer AS, Couto CG. Complications of multiple myeloma. J Am Anim Hosp Assoc 1994; 30: 9–14. [Google Scholar]
- 28. Martin-Ayuso M, Almeida J, Perez-Andres M, et al. Peripheral blood dendritic cell subsets from patients with monoclonal gammopathies show an abnormal distribution and are functionally impaired. Oncologist 2008; 13: 82–92. [DOI] [PubMed] [Google Scholar]
- 29. Pilarski LM, Andrews EJ, Mant MJ, et al. Humoral immune deficiciency in multiple myeloma patients due to compromised B-cell function. J Clin Immunol 1986; 6: 491–501. [DOI] [PubMed] [Google Scholar]
- 30. Schutt P, Brandhorst D, Stellberg W, et al. Immune parameters in multiple myeloma patients: influence of treatment and correlation with opportunistic infections. Leuk Lymphoma 2006; 47: 1570–1582. [DOI] [PubMed] [Google Scholar]
- 31. Burnett RC, Vernau W, Modiano JF, et al. Diagnosis of canine lymphoid neoplasia using clonal rearrangements of antigen receptor genes. Vet Pathol 2003; 40: 32–41. [DOI] [PubMed] [Google Scholar]
- 32. Perez C, Maggi RG, Dinz PPV, et al. Molecular and serological diagnosis of Bartonella infection in 61 dogs from the United States. J Vet Intern Med 2011; 25: 805–810. [DOI] [PubMed] [Google Scholar]
- 33. Koehler JE, Sanchez MA, Tye S, et al. Prevalence of Bartonella infection among human immunodeficiency virus–infected patients with fever. Clin Infect Dis 2003; 37: 559–566. [DOI] [PubMed] [Google Scholar]
- 34. Edelson BT, Cossart P, Unanue ER. Cutting edge: paradigm revisited: antibody provides resistance to Listeria infection. J Immunol 1999; 163: 4087–4090. [PubMed] [Google Scholar]
- 35. Taborda CP, Rivera J, Zaragoza O, et al. More is not necessarily better: prozone-like effects in passive immunization with immunoglobulin G. J Immunol 2003; 170: 3621–3630. [DOI] [PubMed] [Google Scholar]
- 36. Breitschwerdt EB, Maggi RG, Chomel BB, et al. Bartonellosis: an emerging infectious disease of zoonotic importance to animals and human beings. J Vet Emerg Crit Care 2010; 20: 8–30. [DOI] [PubMed] [Google Scholar]
- 37. Kordick DL, Talmage TB, Kwangok S, et al. Clinical and pathologic evaluation of chronic Bartonella henselae or Bartonella clarridgeiae infection in cats. J Clin Microbiol 1999; 37: 1536–1547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Yamamoto K, Chomel BB, Kasten RW, et al. Experimental infection of domestic cats with Bartonella koehlerae and comparison of protein and DNA profiles with those of other Bartonella species infecting felines. J Clin Microbiol 2002; 40: 466–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Lima MLF, Soares PT, Ramos CAN, et al. Molecular detection of Anaplasma platys in a naturally-infected cat in Brazil. Braz J Microbiol 2010; 41: 381–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Harvey JW, Simpson CF, Gaskin JM. Cyclic thrombocytopenia induced by a Rickettsia-like agent in dogs. J Infect Dis 1978; 137: 182–188. [DOI] [PubMed] [Google Scholar]
- 41. Tasker S, Peters IR, Papasouliotis K, et al. Description of outcomes of experimental infection with feline haemoplasmas: copy numbers, haematology, Coombs’ testing and blood glucose concentrations. Vet Microbiol 2009; 139: 323–332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tasker S, Caney SMA, Day MJ, et al. Effect of chronic FIV infection, and efficacy of marbofloxacin treatment, on ‘Candidatus Mycoplasma haemominutum’ infection. Microbes Infect 2006; 8: 653–661. [DOI] [PubMed] [Google Scholar]
- 43. Reynolds CA, Lappin MR. ‘Candidatus Mycoplasma haemominutum’ infections in 21 client-owned cats. J Am Anim Hosp Assoc 2007; 43: 249–257. [DOI] [PubMed] [Google Scholar]


