1 Introduction
Mosquitoes transmit many life threatening parasitic and viral diseases including filariasis (Culex, Mansonia and Anopheles spp.), yellow fever (Aedes aegypti), dengue fever (Ae. aegypti) and malaria (Anopheles spp.), with malaria being the most important mosquito-borne disease. An estimated 216 million reported malaria cases resulted in 655,000 deaths worldwide in 2010 alone [1]. Various strategies have been tried to control mosquito populations in the last 100 years, including the use of chemical pesticides and biological control agents (entomophagous bacteria, fungi, viruses, parasites and predators) [2-6]. In addition to affecting human health and harmful effects on the environment, the indiscriminate use of chemical pesticides has led to development of pesticide resistance reducing the effectiveness of this control strategy [5]. Hence, new strategies to control insect disease-vector populations more effectively are under evaluation. To be effective, these strategies must be, 1) insect vector or pathogen specific, 2) robust or catalytic in mode of action, 3) stable over long periods of time, 4) simple and efficient to deliver and manage 5) have low cost, and 6) self-sustainable with little or no impact on non-target organisms.
One molecular strategy for vector and/or pathogen control that meets the first three requirements listed above is interference RNA (RNAi). RNAi elements inhibit the expression of specific or unique gene(s) of interest, are catalytic in action, and use molecular machinery that is evolutionarily and functionally conserved between many eukaryotes. Recently, several laboratories have demonstrated that injection of plasmids encoding dsRNAs or small interfering RNA (siRNA) elements into adult mosquitoes effectively silences specific target genes of interest [7,8]. Thus, delivery of RNAi elements to mosquitoes that could silence essential genes of interest has the potential to be an effective strategy for mosquito control.
To exploit RNAi technology for mosquito control there is a need for an effective, low cost and sustainable RNAi delivery system. Recently, it was demonstrated that functional RNAi elements can be transferred between organisms that belong to different taxonomic kingdoms. Nematodes have been effectively controlled by expressing nematode-specific siRNAs in bacteria [9] or plants upon which they feed [10,11]. Duplex dsRNA (bacteria) or siRNA (plant) when ingested by the nematode were processed and assembled into the nematode DICER/RISC complex which catalytically cleaved the targeted mRNA and silenced the gene of interest [10,11].
2 The idea
Under the present Grand Challenge Exploration grant (Round 3; 2009) we explored an RNAi strategy to control mosquitoes and prevent spread of mosquito-borne diseases. The strategy involved delivery of double-stranded RNA (dsRNA) elements to mosquito larvae using unicellular, transgenic algae as vectors. Significantly, microorganisms including microalgae are the primary source of nutrition for mosquito larvae in water bodies that serve as the breeding grounds for mosquitoes [12]. This strategy of delivering RNA elements to suppress essential target genes in mosquito larvae has many potential advantages over existing technologies including: 1) the RNA elements can be designed to be very specific, inhibiting expression only of gene(s) of interest, 2) only a small amount of dsRNA is required to initiate production of RNAi elements by the evolutionarily conserved gene silencing machinery, and 3) the delivery systems are self-replicating in environments where mosquito larvae grow. Most importantly, both mosquitoes and microalgae possess the machinery to generate RNAi molecules and to degrade target mRNAs [7, 8, 13]. Furthermore, the trans-kingdom transfer of RNA molecules to control gene expression has previously been demonstrated in transgenic plants expressing siRNAs that target essential nematode genes providing nematode resistance [10, 11].
To test our hypothesis, the model microalga, Chlamydomonas reinhardtii was selected as the vector for delivering dsRNA elements to mosquito larvae to target suppression of an essential mosquito gene; 3-hydroxykynurenine transaminase (3-HKT) by RNAi mediated transcriptional inactivation. 3-HKT catalyzes the transamination of 3-hydroxykynurenine (3-HK) to xanthurenic acid (XA) in the tryptophan catabolism pathway of mosquitoes [14]. The 3-HK molecule is a highly reactive intermediate that can autooxidize under normal physiological conditions resulting in the production of reactive oxygen species that can kill the insect [14]. Injection of μM amounts of 3-HK into grey flesh flies (Neobellieria bullata) is known to induce apoptosis of neurons leading to paralysis and death [15]. Hence, 3-HK levels are very tightly regulated by its rapid conversion into more stable downstream intermediates (e.g., XA) of tryptophan catabolism [14]. The 3-HKT gene was also selected as the target for siRNA-mediated gene silencing because the protein encoded by this gene has only been found in mosquitoes and not among any other organisms including other dipterans such as Drosophila. Moreover 3-HKT was an attractive target because it is expressed primarily during all active feeding stages (larvae and adults) of the mosquito life cycle [16]. The results presented here indicate that mosquito larvae can be killed or impaired in their development before they are competent to infect humans by targeting essential, species-specific, larval-stage genes for silencing.
3 Results
3.1 Chlamydomonas chloroplast transformation with 3HKT inverted repeat construct
Chlamydomonas chloroplast genome was selected for expression of duplex RNAs. The chloroplast genome of Chlamydomonas has been very well characterised and is not known to possess the RNAi processing machinery consisting of the DICER/RISC complex [17]. Hence, the dsRNAs expressed from the chloroplast genome are not expected to be processed into siRNAs in the chloroplast and would presumably be processed into siRNAs only by the mosquito DICER/RISC complex following ingestion. Importantly, there are also no known mechanisms for gene silencing in chloroplasts, thus increasing the probability of stable transgene expression. Moreover, the packaging of dsRNA in the plastid is expected to provide extra protection and prevent it from degradation during ingestion of algae by the larvae.
A 328 bp region of the Anopheles gambiae 3-HKT gene not showing identity of more than 13 contiguous bases to any sequence in NCBI database was selected to design the inverted DNA repeats that would generate the dsRNA which would then presumably be processed to siRNAs. Importantly, this region of the 3-HKT gene exhibits 70% identity with the Aedes and Culex 3-HKT genes, two species that transmit dengue, and filariasis respectively. An inverted repeat construct of 3-HKT gene fragment for expression in the chloroplast genome (Fig. 1a) was constructed and its expression was driven by the atpA gene promoter. Chloroplast transformation of the psbA (chloroplast encoded, photosystem II D1 subunit) deletion strain CC-4147 was achieved by homologous integration of the pCVAC108 vector into the chloroplast genome following particle bombardment. The integrating DNA also carried the psbA gene restoring photosynthesis to the psbA deletion strain. Integration of the 3HKT inverted repeat construct into the chloroplast genome was confirmed by PCR (Fig.1b).
Figure 1.
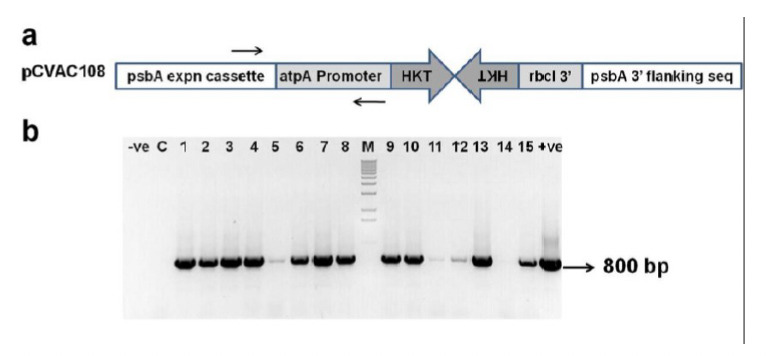
a: Design of the 3-HKT inverted repeat construct used for Chlamydomonas chloroplast transformation. The two black arrows indicate the binding sites of primers used for PCR confirmation of Chlamydomonas chloroplast transformants. b: PCR confirmation of Chlamydomonas chloroplast transformants. -ve: water control, C: non transgenic parent strain (CC-4147), 1-15: Chlamydomonas chloroplast transformants, +ve: plasmid control as template.
3.2 Transgenic Chlamydomonas expressing 3-HKT dsRNA are toxic to mosquito larvae
Chlamydomonas chloroplast transformants carrying 3-HKT inverted repeats were used to carry out bioassays on Anopheles stephensi larvae. Bioassays with algae as a feed were started on the first day after larval emergence. Transgenic Chlamydomonas clones were grown to log phase (OD750= 0.7 to 1.2) in Tris-acetate-phosphate (TAP) medium. In total, 12 independent chloroplast transformants were evaluated in the bioassays to test their lethality to mosquito larvae. It is noted that while the transforming DNA integrates into the chloroplast genome by homologous recombination, random integration of the transforming DNA into the nuclear genome is possible and may account for differential phenotypes in independent transgenics. Overall, more than 30% larval mortality was observed with 5 of the 12 independent clones tested, with highest mortality of 53% being observed with clone 108-2. Additional bioassays of mosquito larvae were then repeated with selected chloroplast transformants (clones 108-13 and 108-15). Larvae feeding on transgenics expressing 3-HKT dsRNA in the chloroplast genome had statistically significantly greater mortality (~40% larval mortality) than the control algae (~15% larval mortality) in every bioassay experiment tested (Fig. 2a).
Figure 2.
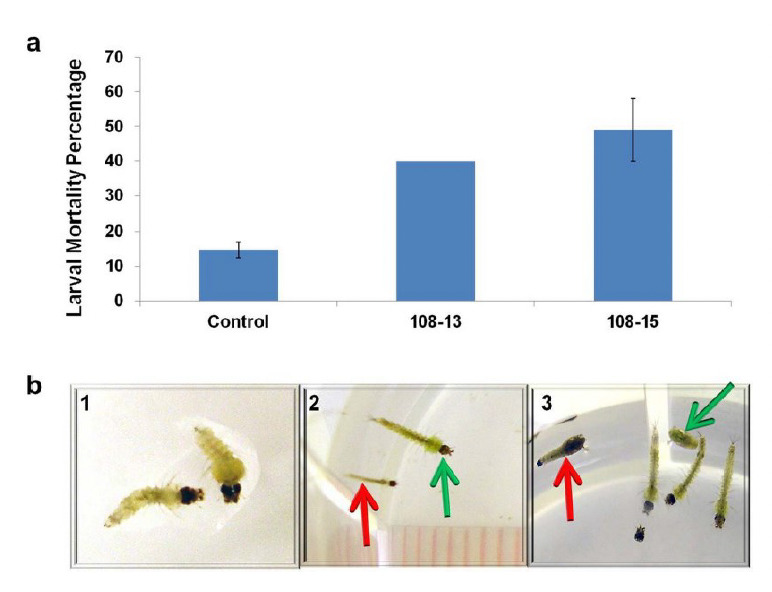
Effect of feeding Chlamydomonas chloroplast trans-formants expressing 3-HKT dsRNA on Anopheles stephensi larvae. a) An.stephensi larval mortality observed with Chlamydomonas chloroplast (CC-4147/pCVAC108) transformants expressing 3-HKT dsRNAs. Twenty (1st experiment) or 30 (2nd experiment) newly hatched An. stephensi larvae were reared on algae plus 1/3rd yeast+ sera micron (1/3rd amount of yeast+micron mixture fed to control larvae). Control larvae were reared on non-transgenic algae with a yeast+sera micron mixture diet. Data shown are the average of two experiments. For clone 108-13 results were identical (40% mortality) in both the experiments, hence there is no variation. b) Different phenotypes exhibited by An. stephensi larvae following feeding on transgenic algae producing 3-HKT dsRNA. 1) Dead larvae of An. stephensi reared on transgenic algae. 2) Comparison of a larva exhibiting growth inhibition (red arrow) upon feeding on transgenic algae to a normally developing larva (green arrow) on day 12. 3) Image of a dead pupa (red arrow) and a live pupa (green arrow) in an experimental well with transgenic algae.
The earliest stage in larval development at which mortality was observed among larvae reared on transgenic Chlamydomonas expressing 3-HKT dsRNA was the 2nd instar stage. Larvae reared on transgenic Chlamydomonas expressing 3-HKT dsRNA were also observed to die at the 3rd and 4th instars, as well as the pupal stage. Importantly, larvae that died at later developmental stages exhibited slower growth relative to larvae reared on nontransgenic Chlamydomonas and were smaller in size (Fig. 2b). It is important to note that the larvae with impaired growth would presumably be less fit and are expected to give rise to adults with reduced fitness, fecundity and fertility as reported with insects reared on diet supplemented with Bacillus thuringiensis δ–endotoxins [18].
3.3 Mosquito larvae reared on transgenic Chlamydomonas expressing 3-HKT dsRNA have significantly reduced levels of 3-HKT transcripts
Transcription of 3-HKT inverted repeat (IR) RNA among chloroplast transformants 108-13 and 108-15 was confirmed by RT-PCR. Both clones were found to transcribe the 3-HKT IR (Fig. 3) indicating that the dsRNA elements produced in the transgenic clones were likely responsible for the significantly higher mortality observed in the mosquito larvae feeding on transgenic Chlamydomonas compared to the non transgenic alga control.
Figure 3.
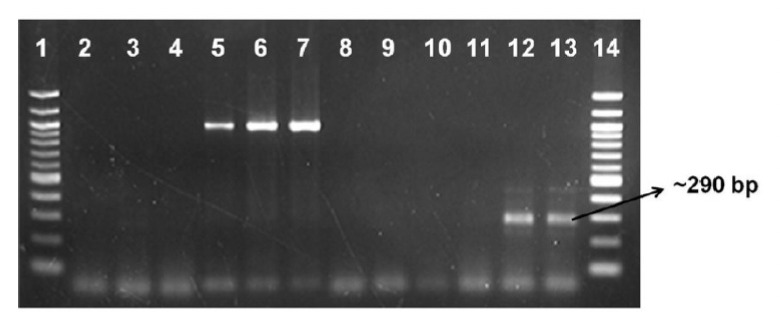
Confirmation of 3-HKT dsRNA transcription in Chlamydomonas chloroplast transformants. CC4147 chloroplast transformants 13 and 15 were tested for expression of 3-HKT dsRNA by RT-PCR. psbD, a chloroplast gene was used as control in the experiment. Absence of any band in the control PCR experiments for psbD (lanes 2 to 4) and 3-HKT (lanes 9 and 10) indicates that all the RNA samples were free of DNA contamination. Amplification of psbD in lanes 5 to 7 indicates that cDNA synthesis was successful and the absence of the 3-HKT band in CC4147 control (lane 11) was real as it lacks 3-HKT. Presence of the 3-HKT band in CC4147/pCVAC108 clones 13 and 15 confirms that these are transgenics and they transcribe the 3-HKT dsRNA. Loading pattern: 1:100 bp ladder, 2: Parent strain CC-4147 no RT, PCR with psbD primers, 3:108-13 no RT, PCR with psbD primers, 4: CC4147/pCVAC108-15 no RT, PCR with psbD primers , 5: Parent strain CC4147 cDNA, PCR with psbD primers, 6: CC4147/pCVAC108-13 cDNA, PCR with psbD primers, 7: CC4147/pCVAC108-15 cDNA, PCR with psbD primers, 8: Parent strain CC4147 no RT, PCR with 3-HKT primers, 9: CC4147/pCVAC108-13 no RT, PCR with 3-HKT primers, 10: CC4147/pCVAC108-15 no RT, PCR with 3-HKT primers, 11: Parent strain CC4147 cDNA, PCR with 3-HKT primers, 12: CC4147/pCVAC108-13 cDNA, PCR with 3-HKT primers,13: CC4147/pCVAC108-15 cDNA, PCR with 3-HKT primers, 14: 100 bp ladder.
To determine whether the steady-state transcript levels of 3-HKT were altered in larvae fed transgenic Chlamydomonas expressing 3-HKT dsRNA relative to larvae fed on nontransgenic algae, total RNA was extracted from larvae and pupae reared on the appropriate algae and 3-HKT transcript levels were quantified by real-time PCR and normalized to actin mRNA levels as an internal standard. The data were analyzed following single factor ANOVA. The surviving larvae reared on Chlamydomonas chloroplast transformants 108-13 and 108-15 showed significant (10-fold) reduction in 3-HKT transcript levels relative to larvae fed on nontransgenic Chlamydomonas used as a control (Fig. 4a). 3-HKT transcript levels were also significantly reduced in dead pupal samples that were reared on the 108-13 and 108-15 algal clones (Fig. 4b and 4c). The results indicate that the mortality observed among mosquito larvae reared on transgenic Chlamydomonas expressing 3-HKT dsRNA was due to reductions in 3-HKT steady-state levels consistent with RNAi-targeted degradation of the 3-HKT mRNA.
Figure 4.

Real time analysis to measure steady state levels of 3-HKT transcript among surviving/dead An. stephensi larvae and pupae reared on transgenic Chlamydomonas expressing 3-HKT dsRNA. In each experiment 3-HKT expression was compared and normalised to An. stephensi actin transcript levels. The data shown in each figure is the average of three biological replicates. Each biological replication involved five technical replications for each treatment and control. The mean ΔΔCT values from three biological replicates were subjected to analysis through single factor ANOVA. Asterisks indicate the treatments that had significantly lower 3-HKT transcript levels relative to the parental control, determined by single factor ANOVA (P<0.05). RNA extracted from larvae/pupae reared on nontransgenic Chlamydomonas was used as the control in all experiments. In all experiments A, B, C (as in 108-13A1) etc. refer to the wells from which the larvae/pupae were sourced for analysis and the numbers 1, 2 refer to the first or second larvae/pupae used for analysis from a single well. a) 3-HKT transcript levels observed among surviving larvae reared on clones 108-13 and 108-15. Except for larvae 108-15C1, which did not differ significantly from the control larvae, all other treatments showed significant reduction in 3-HKT transcript levels compared to the parental control. b) 3-HKT transcript levels observed among dead pupae from larvae reared on 108-13. c) 3-HKT transcript levels observed among dead pupae from larvae reared on the 108-15 transgenic Chlamydomonas clone.
4 Discussion
The recent discovery that functional RNAi elements can be transferred across genera orally has led to development of transgenic plants expressing dsRNA that target silencing of specific insect-pest genes [10,11]. Here, we report the first trans-kingdom delivery of an organellar-encoded double-stranded RNA to selectively inactivate an essential mosquito gene (3HKT).
Even though delivery of dsRNA through injection or feed has been shown to lead to gene silencing in mosquitoes [7,8,19,20], an RNAi based bio-insecticide cannot be developed without a proper delivery system that not only delivers the RNAi elements to mosquitoes but also must be self-replicating in the environment. An ideal delivery system would be one which can be easily genetically engineered for expression of dsRNAs targeting mosquito genes, simple to propagate and maintain, is part of the mosquito food chain, and can replicate in mosquito breeding grounds. The microalga Chlamydomonas meets many of these requirements and qualifies as an appropriate delivery system. Feeding of transgenic Chlamydomonas expressing dsRNA molecules complimentary to the 3HKT gene and expressed in the chloroplast was found to be an effective means to inhibit the growth of mosquito larvae and ultimately cause their death. The oral delivery of dsRNA elements through microalgae targeting the mosquito specific 3-HKT gene resulted in up to 50% larval mortality. Importantly, there was a statistically significant reduction in 3-HKT transcript levels observed in both surviving and dead larvae as well as pupae from larvae treated with transgenic expressing 3-HKT dsRNA relative to those fed wild-type algae when normalized on the basis of larval actin levels. These results indicate that targeted dsRNA elements delivered to larvae via microalgae is an effective means to inhibit 3-HKT expression, resulting in slower larval growth and significant larval mortality.
Other means of delivering dsRNA to inactivate essential mosquito genes including topical application has been shown to be effective in causing mortality of adult Aedes mosquitoes [21]. Topical application of dsRNAs targeting Aedes aegypti inhibitor of apoptosis protein 1 gene (AaeIAP1) resulted in up to 48% mortality [21], which is similar to the mortality rates observed in the present study. However, the significantly higher costs associated with synthesis and application of dsRNA make topical application an impractical approach at this time. Even though the present study observed only about 50% larval mortality in bioassays, the effectiveness of this strategy can potentially be enhanced by targeting two or more mosquito genes, simultaneously. Although Chlamydomonas was shown to be an effective vector for delivering functional dsRNA elements to mosquito larvae, the potency of this strategy could potentially be further improved by employing micro-algae that are the preferred food for mosquito larvae. Overall, these initial studies demonstrate for the first time that trans-kingdom of organellar-expressed dsRNA can effectively target suppression of essential mosquito larval genes effectively reducing mosquito populations before they reach maturity and the capability to transmit malaria in a species specific-manner.
5 Future perspectives
The next steps towards development of an RNAi based algal larvicide would involve evaluation of the efficacy of Chlamydomonas chloroplast transformants expressing 3-HKT dsRNA in controlling mosquito larvae in extensive laboratory trials. These trials would involve proper monitoring of mosquito larvae that survive feeding on transgenic Chlamydomonas and develop into adults. The surviving adults need to be evaluated for their development, fitness and their ability to reproduce as this data is very critical to know the overall effectiveness of RNAi strategy in controlling mosquito populations. Since the 3-HKT sequence used for making the IR constructs in this study also exhibited 70% identity with Aedes and Culex 3-HKT, the ability of Chlamydomonas chloroplast transformants developed in this study in controlling Aedes and Culex larvae should also be evaluated. Data from the trials on Aedes and Culex larvae would indicate the possibilities of developing a single RNAi based larvicide to control mosquito species belonging two or more genera. In addition, targeting of two or more mosquito genes simultaneously and delivery of functional dsRNA elements to mosquito larvae through microalgae/micro-organisms that are preferred food sources of mosquito larvae should be explored.
6 Acknowledgements
This research was supported by the Bill and Melinda Gates Foundation, Microalgal mediated eradication of malarial mosquito larvae to RTS.
Annex: Materials and Methods
Algal strains and cultural conditions
Chlamydomonas strain CC-4147 (FUD7 mt+) was obtained from the Chlamydomonas culture collection at Duke University, USA. Strains were grown mixotrophically in liquid or on solid tris acetate phosphate (TAP) or high salt (HS) medium [22] at 23° C under continuous white light (40 μE m-2s-1). Selection of chloroplast transformants using strain CC4147 (FUD7 mt+) was performed with high salt (HS) medium.
Vector Construction
For making 3HKT inverted repeat (IR) constructs, a 328 bp long fragment of Anopheles gambiae 3HKT coding sequence (GenBank accession number AM042695.1) representing the region from 952 to 1253 bp of the 3-HKT gene was amplified with primers HKTFwd2 (5’- ATGCTAAGCTTGCATGCATGAACCAAAACGTTATCACCATAC-3’) and HKTRev2 (5’- AAGATGGATCCGCTAGCATAATACCCACACGCCATG C-3’) and cloned into HindIII/BamHI sites of vector pBSKS, creating plasmid pCVAC88. To create 3HKT IR, 3HKT amplified with primers HKTFwd3 (5’- AGTCAGAGCTCCCATGGATGAACCAAAACGTTATCA CCATAC-3’) and HKTRev2 was cloned into SacI/XbaI sites of vector pCVAC88 creating plasmid pCVAC99. HKT inverted repeat from pCVAC99 was excised as a NcoI/SphI fragment and cloned into the same sites of vector pGatpA, creating plasmid pCVAC101. Finally, the 3HKT inverted repeat from pCVAC101 was excised as a XhoI/SphI fragment and cloned into XhoI/SphI sites of vector pBA155 creating plasmid pCVAC108.
Chlamydomonas chloroplast transformation
Chlamydomonas chloroplast transformation was performed following the protocol described by Ishikura et al. [23]. Briefly, the psbA deletion strain (CC-4147) of Chlamydomonas was grown in 100 mL of TAP liquid media. Cells were harvested in log phase (OD750=0.8 to 1.0) by centrifugation at 2900 g and resuspended in 2 mL of sterile HS medium. Approximately 300 μL of cells were spread in the center of HS agar plates. Gold particles (1 μm) (InBio Gold, Eltham, Victoria, Australia) coated with plasmid DNAs were shot into Chlamydomonas cells on the agar plate using a Bio-Rad PDS 1000He Biolistic gun (Bio-Rad, Hercules, CA, USA) at 1100 psi under vacuum. Following shooting, cells were plated onto HS agar plates for selection.
PCR analysis
Genomic DNA was extracted from putative transformants growing on selection medium using a modified xanthine mini prep method described by Dr. Steve Surzycki (http://www.chlamy.org/methods/dna.html). A half loop of algal cells were resuspended in 300 μL of xanthogenate buffer (12.5 mM potassium ethyl xanthogenate, 100 mM Tris-HCl pH 7.5, 80 mM EDTA pH 8.5, 700 mM NaCl) and incubated at 65o C water for 1.0 hour. Following incubation, the cell suspension was centrifuged for 10 minutes (~16,000 g) to collect the supernatant. The supernatant was transferred to a fresh micro-centrifuge tube and 2.5 volume of cold 95% ethanol (750 μL) was added. The solution was mixed well by inverting the tube several times allowing DNA to precipitate. The samples were then centrifuged for 5 min (16,000 g) to pellet the DNA. The DNA pellet was washed with 700 μL of cold 70% ethanol and centrifuged for 3.0 min. The ethanol was removed by decanting and the DNA pellet was dried using a speedvac to remove of any residual ethanol. The DNA pellet was then resuspended in 100 μL of sterile double distilled water and 2-5 μL of the DNA sample was used as a template for setting up a PCR.
Real Time PCR
Total cellular RNA was extracted from single mosquito larvae or pupae from different treatments using Nucleospin RNAII kit (Clonetech, Mountain view, CA, USA) following the manufacturer’s instructions. Contaminating genomic DNA was removed by treating RNA samples with DNAase I (Promega, Madison, WI USA) following the manufacturer’s instructions. RNA quality and concentration were measured using a Nanodrop (Thermo-scientific, Wilmington, DE, USA) spectrometer at 260 nm. 2.0 mg of total RNA was used for cDNA synthesis using the Quantas cDNA synthesis (Quantas, USA) following manufacturer’s instructions. Real-time quantitative RT-PCR was carried out using an ABI – Step One Plus (Applied Biosystems, Foster City, CA, USA) using PerfeCTaTM SYBR® Green FastMixTM (ROX dye) (Quanta Biosciences, Gaithersburg, MD, USA) according to the manufacturer’s instructions. The A. stephensi actin gene (GenBank accession no. AY820172.1) (ASActinRTFwd1 5’- GGTCGTAACCACCGGTATTG-3’ and ASAc-tinRTRev2 5’- GGTGGTGGTGAACGAGTAGC - 3’) was used as a reference gene/internal control and was amplified in parallel with the target 3-HKT gene (ASHKTRTFwd2 5’- TTTAGCCTGGAAACGCTGAC-3’ and ASHKTRTRev2 5’- TCGATTTCCCATTTGTCCAT-3’) allowing gene expression normalization and providing quantification. Reactions were carried out with 10 ng of cDNA. All the primers were designed using the Primer Express software following the manufacturer’s guidelines. Each experiment was carried out in three biological replicates. Each biological replication involved five technical replications for each treatment and control. The quantification of the relative transcript levels was performed using the comparative CT (threshold cycle) method [24]. The mean ΔΔCT values from three biological replicates were subjected to analysis through single factor ANOVA.
References
- 1.World Health Organization: World Malaria Report. 2011.
- 2.Chandrahas RK, Rajagopalan PK: Observations on mosquito breeding and the natural parasitism of larvae by a fungus Coelomomyces and a mermithid nematode Romanomermis in paddy fields in Pondicherry. Indian J. Med. Res. 1979;69:63–67. [PubMed] [Google Scholar]
- 3.Porter AG, Davidson E, Liu JW: Mosquitocidal toxins of bacilli and their genetic manipulation for effective biological control of mosquitoes. Microbiol. Rev. 1993;57:838–861. doi: 10.1128/mr.57.4.838-861.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Nam VS, Yen NT, Holynska M, Reid JW et al. National progress in dengue vector control in Vietnam: survey for Mesocyclops (Copepoda), Micronecta (Corixidae), and fish as biological control agents. Am. J. Trop. Med. Hyg. 2000;62:5–10. doi: 10.4269/ajtmh.2000.62.5. [DOI] [PubMed] [Google Scholar]
- 5.Poopathi S, Tyagi BK: The challenge of mosquito control strategies: from Primordial to Molecular Approaches. BMBR. 2006;1:51–65. [Google Scholar]
- 6.Moreira LA, Ormaetxe II, Jeffery JA, Lu G et al. A Wolbachia symbiont in Aedes aegypti limits infection with dengue, chikungunya, and Plasmodium. Cell. 2009;139:1268–1278. doi: 10.1016/j.cell.2009.11.042. [DOI] [PubMed] [Google Scholar]
- 7.Blandin S, Moita LF, Kocher T, Wilm M et al. Reverse genetics in the mosquito Anopheles gambiae: targeted disruption of the Defensin gene. EMBO Rep. 2002;3:852–856. doi: 10.1093/embo-reports/kvf180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Boisson B, Jacques JC, Choumet V, Martin E et al. Gene silencing in mosquito salivary glands by RNAi. FEBS Lett. 2006;580:1988–1992. doi: 10.1016/j.febslet.2006.02.069. [DOI] [PubMed] [Google Scholar]
- 9.Kamath RS, Martinez-Campos M, Zipperlen P, Frase AG et al. Effectiveness of specific RNA-mediated interference through ingested double stranded RNA in Caenorhabditis elegans. Genome Biol. 2001;2 doi: 10.1186/gb-2000-2-1-research0002. research0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang G, Allen R, Davis EL, Baum TJ et al. Engineering broad root-knot resistance in transgenic plants by RNAi silencing of a conserved and essential root-knot nematode parasitism gene. Proc. Natl. Acad. Sci. USA. 2006;103:14302–14306. doi: 10.1073/pnas.0604698103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yadav BC, Veluthambi K, Subramaniam K: Host-generated double stranded RNA induces RNAi in plant-parasitic nematodes and protects the host from infection. Mol. Biochem. Parasitol. 2006;148:219–22. doi: 10.1016/j.molbiopara.2006.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Merritt RW: Feeding behaviour, natural food, and nutritional relationships of larval mosquitoes. Ann. Rev. Entomol. 1992;37:349–376. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- 13.Schroda M: RNA silencing in Chlamydomonas: mechanisms and tools. Curr. Genet. 2006;49:69–84. doi: 10.1007/s00294-005-0042-1. [DOI] [PubMed] [Google Scholar]
- 14.Han Q, Beerntsen BT, Li J: The tryptophan oxidation pathway in mosquitoes with emphasis on xanthurenic acid bio-synthesis. J. Insect Physiol. 2007;53:254–263. doi: 10.1016/j.jinsphys.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cerstiaens A, Huybrechts J, Kotanen S, Lebeau I et al. Neurotoxic and neurobehavioral effects of kynurenines in adult insects. BBRC. 2003;312:1171–1177. doi: 10.1016/j.bbrc.2003.11.051. [DOI] [PubMed] [Google Scholar]
- 16.Rossi F, Lombardo F, Paglino A, Cassani C et al. Identification and biochemical characterization of the Anopheles gambiae 3-hydroxykynurenine transaminase. FEBS J. 2005;272:5653–5662. doi: 10.1111/j.1742-4658.2005.04961.x. [DOI] [PubMed] [Google Scholar]
- 17.Maul JE, Lilly JW, Cui L, dePamphilis CW et al. The Chlamydomonas reinhardtii plastid chromosome: Islands of genes in a sea of repeats. The Plant Cell. 2002;14:2659–2679. doi: 10.1105/tpc.006155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dulmage HT, Martinez E: The effects of continuous exposure to low concentrations of the δ-endotoxin of Bacillus thuringiensis on the development of the tobacco budworm, Heliothis virescens. J. Invertebr. Pathol. 1973;22:14–22. [Google Scholar]
- 19.Zhang X, Zhang J, Zhu KY: Chitosan/double-stranded RNA nanoparticle-mediated RNA interference to silence chitin synthase genes through larval feeding in the African malaria mosquito (Anopheles gambiae). Insect Mol. Biol. 2010;19:683–693. doi: 10.1111/j.1365-2583.2010.01029.x. [DOI] [PubMed] [Google Scholar]
- 20.Coy MR, Sanscrainte ND, Chalaire KC, Inberg A et al. Gene silencing in adult Aedes aegypti mosquitoes through oral delivery of double-stranded RNA. J. Appl. Entomol. 2012;10:741–748. [Google Scholar]
- 21.Pridgeon JW, Zhao L, Becnel JJ, Strickman DA et al. Topically applied AaIAP1 double-stranded RNA kills female adults of Aedes aegypti. J. Med. Entomol. 2008;45:414–420. doi: 10.1603/0022-2585(2008)45[414:taadrk]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 22.Harris EH: Chlamydomonas as a Model Organism. Annu. Rev. Plant Physiol. Plant Mol.Biol. 2001;52:363–406. doi: 10.1146/annurev.arplant.52.1.363. [DOI] [PubMed] [Google Scholar]
- 23.Ishikura K, Takaoka Y, Kato K, Sekine M et al. Expression of a foreign gene in Chlamydomonas reinhardtii chloroplast. J. Biosci. Bioeng. 1999;87:307–314. doi: 10.1016/s1389-1723(99)80037-1. [DOI] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD: Analysis of relative gene expression data using real-time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]


