Abstract
Limitations in the current clinical management of critical-sized osseous defects have driven the need for multifunctional bone constructs. The ideal bone scaffold should possess advanced microarchitecture, well-defined pore interconnectivity, and supply biological signals, which actively guide and control tissue regeneration while simultaneously preventing post-implantation complications. Here, a natural medicine-based localized drug delivery from 3D printed scaffold is presented, which offers controlled release of curcumin, piperine from nano-sized polymeric micelles, and burst release of antibacterial carvacrol from the coating endowing the scaffold with their distinct, individual biological properties. This functionalized scaffold exhibits improved osteoblast (hFOB) cell attachment, 4-folds higher hFOB proliferation, and 73% increased hFOB differentiation while simultaneously providing cytotoxicity towards osteosarcoma cells with 61% lesser viability compared to control. In vitro, early tube formation (p < 0.001) indicates that the scaffolds can modulate the endothelial cellular network, critical for faster wound healing. The scaffold also exhibits 94% enhanced antibacterial efficacy (p < 0.001) against gram-positive Staphylococcus aureus, the main causative bacteria for osteomyelitis. Together, the multifunctional scaffolds provide controlled delivery of natural biomolecules from the nano-sized micelle-loaded 3D printed matrix for significant improvement in osteoblast proliferation, endothelial formation, osteosarcoma, and bacterial inhibition, guiding better bone regeneration for post-traumatic defect repair.
Keywords: Curcumin, Micelle, 3D printing, Bone tissue engineering, Multifunctional bone Scaffold
1. Introduction
With an annual incidence rate of 4.4 cases per million in the US, osteosarcoma represents less than 1% of all diagnosed cancers reported in adults. However, it is the third most prevalent malignancy among children and adolescents after lymphoma and brain tumors [1]. Limb salvaging surgery, the mainstay of osteosarcoma treatment, enhances the risk of metastatic recurrence when the tumor has not been resected completely. Tumor resection surgery also poses significant challenges to the patients since it leaves critical-sized defects that bone cannot heal by [2,3]. Therefore, removing residual tumor cells and simultaneous restoration of bone defects necessitates designing and fabricating biomaterials with well-defined architecture and enhanced biological performance.
The increased risk of adverse effects with chemotherapeutic drugs has led researchers to look for viable, safer alternatives. In this context, curcumin, the active constituent of turmeric (Curcuma longa), has made remarkable strides in the past few decades due to its potential in various therapeutic and medicinal applications [4-6]. Recent reports have demonstrated curcumin’s efficacy as a chemopreventive agent for various malignancies, including breast, lung, prostate, colorectal, and pancreatic carcinoma [7-10]. Given its dietary role, it is also well-tolerated among humans at dosages of up to 12 g daily, making it comparatively safer than synthetic chemopreventive agents that may cause severe and long-term side effects [11]. Curcumin not only enhances osteoblast cell proliferation but also promotes early-stage osteogenesis [12]. Recent studies show that curcumin release upregulates bone osteocalcin or gamma-carboxy glutamic acid-containing protein (BGLAP) expression, a bone protein solely secreted by osteoblast cells [13,14]. Curcumin also upregulates the RUNX2 expression, an early osteogenic differentiation marker, indicating enhanced differentiation of mesenchymal stem cells into osteoblasts [15,16]. ALP, membrane-bound glycoprotein, which plays a crucial role in bone mineralization by providing phosphate concentration at the osteoblast cell surface [17-19], is also upregulated in the presence of curcumin. Recent studies also indicate its capability to influence bone remodeling and regeneration processes [20-23]. Despite its broad pharmacological activity and safety, its clinical translation has been hindered due to its inherent instability, poor bioavailability, low aqueous solubility, rapid metabolism, and fast elimination [24]. Polymeric micelle has been reported to enhance the therapeutic efficacy of curcumin by improving its aqueous formulation and bioavailability, prolonging systemic circulation, and enhancing cellular uptake [25-28]. Another strategy that has attracted significant attention and has been suggested for improving curcumin’s bioavailability involves co-administration of curcumin with a ‘bioenhancer,’ piperine [29]. Piperine, a naturally sourced polyphenol from black pepper, enhances curcumin’s absorption by inhibiting the metabolizing enzymes and bypassing the first-pass metabolism [30]. It is reported that, when combined with piperine, curcumin’s bioavailability was increased by 2000% in humans, decreasing the elimination half-life and clearance of curcumin [31]. Piperine, similar to curcumin, also protects against oxidative damage and reduces cancer incidences by down-regulating the NF-κB pathway [32,33].
One of the challenges in the bone tissue engineering field is the need for more vascularization within synthetic bone grafts to promote sufficient engraftment and integration with the surrounding host tissue [34]. Bone is a highly vascularized tissue, yet current tissue engineering strategies have yet to actively or specifically investigate the formation of blood vessels and the arrangement of a vascular network within the engineered tissue. Angiogenesis, or the development of blood vessels, is an essential process for bone regeneration and wound healing since the vascular network ensures a constant supply of oxygen, nutrients, growth factors, hormones, cytokines, and osteoblast/osteoclast precursors, removes waste products, as well as forms a communicative network between the bone graft and host tissue. While the designed porous architecture of patient-specific, 3D printed bone grafts allows the formation of new blood vessels through the porous channels [35,36], without biochemical cues, prolonged maintenance of those vasculatures is almost unfeasible.
Another concern associated with the surgical implantation of synthetic bone grafts is the elevated risk of secondary infections at the surgical site. Methicillin-resistant Staphylococcus aureus comprises up to 50% of all periprosthetic joint infections caused after orthopedic surgery. The prevalence of joint infection following surgery is steadily increasing, resulting in higher incidences of implant failure, revision surgery, and administration of a full spectrum of antibiotics. Despite contemporary clinical measures, osteomyelitis treatment faces a recurring challenge due to antibiotic-resistant bacteria, which created a recent surge of interest in plant-based antimicrobial agents. Carvacrol, an essential oil, has demonstrated antimicrobial properties, and the mechanism relies on the disruption of the bacterial cytoplasmic membrane, which results in the leakage of essential intracellular components and reduction in ATP synthesis that leads to changes in other energy-dependent cell processes, inhibition of enzyme synthesis and subsequent cell death [37-39]. It has been observed in recent studies that curcumin also demonstrates potential anti-bacterial efficacy against Staphylococcus aureus [40].
The standard delivery method of natural medicinal compounds has been via oral administration [41]. However, in the recent past, 3D-printed scaffolds have been utilized as an excellent drug carrier to achieve on-site localized delivery systems [42-44]. One of the substantial advantages of using 3D printing technology for manufacturing implants is that it can be patient-specific and defect-specific [45,46]. Therefore, considering the biological significance of natural medicinal compounds, curcumin, piperine, and carvacrol, it might be an efficient strategy to encapsulate them within nano-sized micelle, which could be adopted to modify and functionalize 3D printed bone scaffolds with multipronged therapeutic properties, including effective osteosarcoma prevention, bone cell proliferation, antibacterial protection, and endothelial cell formation. The 3D printed TCP scaffold with designed porosity has been fabricated by binder-jetting, followed by incorporating curcumin-piperine-carvacrol loaded micelle to mimic bone tissue’s structural and functional complexity. Natural medicinal compounds loaded with multifunctional 3DP scaffolds with multifunctional properties that can address critical-sized defect repair challenges have yet to be studied. The objective of this study includes (i) fabricating a designed 3D printed scaffold that will allow the controlled release of multiple biomolecules with different biological properties and (ii) investigating if the effect of biomolecule delivery from 3D printed scaffold could improve the regenerative potential of the conventional scaffold and reduces the risk of implant-related infection. To meet these objectives, the scaffold has been evaluated for sustained release of the drugs, followed by a detailed investigation of in vitro chemopreventive, osteogenic, angiogenic, and antibacterial efficacy.
2. Materials and methods
2.1. Fabrication of 3D printed scaffold
ß-TCP ceramics are synthesized using the solid-state synthesis method. 1 M calcium carbonate (CaCO3) and 2 M calcium phosphate dibasic (CaHPO4) are ball milled for 2 h to obtain homogeneous mixing. The powder is calcined at 1050 °C for 24 h followed by sieving and ball-milling with 2:3 W/V ethanol (200 proof, Decon Labs, PA) and 5:1 ball and powder ratio. After 6 h, the solvent is completely evaporated at 60 °C to obtain ß-TCP powder. The synthesized ß-TCP powder is sieved and used for fabricating 3D printed parts using the binder jet printer (ProMetal®, ExOne LLC, PA, USA). CAD is implemented to design a 3 mm diameter and 5 mm height cylindrical scaffold in which 400 μm interconnected square-shaped 3D pores penetrate orthogonally towards X, Y, and Z directions [31]. Once the print is finished, the build bed is placed at 175 °C for 90 min to remove the binder. After curing, the green parts are obtained from the build bed, and depowderization is performed by air-blowing to carefully remove the loosely adhered powder that might block the pores. Finally, the scaffolds are sintered at 1250 °C in a conventional muffle furnace for 2 h. In order to characterize the formation of ß-TCP, the powder is pressed at 4.5 psi using hydraulic press (Buehler inc.) and are then gold coated for 4 min and then elemental analysis is performed using Energy dispersive X-Ray spectroscopy (EDAX, FEI Sirion). The phase purity of synthesized TCP is analysed using X-Ray diffraction (Siemens D500 Diffractometer) in the 2θ range of 20–60 degrees, step size of 0.05 at 30 mA and 35 kV is used.
2.2. Drug preparation and loading
2.2.1. Preparation for characterization of curcumin-piperine encapsulated micelle
Curcumin 10 mg and piperine (Sigma Aldrich, St. Louis, Missouri, USA) 10 mg are dissolved in 5 mL of ethanol. 300 mg of Pluronic F127 (Sigma Aldrich, St. Louis, Missouri, USA) is dissolved in 10 mL of ethanol. Drug and polymer solutions are mixed and stirred for 30 min at 37 °C. Next, ethanol is evaporated under reduced pressure at 45 °C until a clear gel-like matrix is formed. The temperature is increased to 60 °C, and 50 mL of water is added to the mixture under vigorous stirring until polymeric micelles containing curcumin and piperine are formed. The resulting solutions are filtered with a 0.45 μm pore size filter to remove unencapsulated or aggregated drug particles. A clear solution of polymeric micelles containing curcumin and piperine is obtained.
The micelle morphology is characterized utilizing transmission electron microscopy (TEM FEI T20) with the negative stain method. Briefly, 2 μl of 1:100 diluted micelle solution is applied on a copper grid coated with carbon film and left for 3–5 min. The excess solution is removed from the edge of the grid using a filter paper. Next, the grid is stained with 2 μl of 0.5% uranyl acetate and drawn off using a filter paper. After overnight drying at room temperature, TEM is carried out.
Curcumin is dissolved in 1% DMSO to stabilize the compound, and the characteristic peak is observed in a UV-Vis spectrometer (BioTek, US) over a range of 350–550 nm wavelength. Once the optical density is determined for bare curcumin in DMSO, the same for micelle-formulated curcumin is measured in water.
The polymeric micelles dissolved in water are lyophilized, and powdered micelles are obtained. The solid powders and powdered curcumin are then analyzed using Fourier transform infrared (FT-IR) spectra to look for functionalization and stability. Each spectrum represented an average of 50 scans and at a resolution of 1 cm−1 over a frequency region of 450–4000 cm−1. DLS characterization of micelle encapsulated curcumin is carried out using ZS Xplorer (Malvern Panalytical) with an in-range parameter of 96.65%. Water is used as dispersant and a detector angle of 173° and a temperature of 25 °C is maintained.
2.2.2. Drug loading in 3D printed scaffold
Following drug-loading procedure is carried out in sterile hood under aseptic condition. 150 μl of aqueous micelle solution containing 30 μg of curcumin and 30 μg of piperine is pipetted on top of the autoclave-sterilized scaffold surface. Finally, 100 μl of 0.25 mM carvacrol (Sigma Aldrich, St. Louis, Missouri, USA) in ethanol solution is added on top of the micelle-loaded scaffold. Drug-loaded scaffolds are left overnight in the dark at room temperature to ensure solvent removal.
2.3. In vitro drug release kinetics from micelle
In vitro drug release study is performed at pH 7.4 phosphate buffer and pH 5.0 acetate buffer (n = 3) to imitate the physiological pH and the acidic environment right after surgery, respectively. The scaffolds are kept in glass vials, and 1 mL of buffer solution is added to immerse the scaffold completely. The vials are placed in an orbital shaker at 37 °C and 150 rpm. After specific time points, the buffer media is collected and replaced with fresh pH media. 200 μl of the collected buffer is pipetted in a 96-well plate, and the absorbances of each well are measured at 427, 345, and 278 nm wavelength for curcumin, piperine, and carvacrol, respectively. The absorbances are measured using a Biotek Synergy 2 SLFPTAD microplate reader (Biotek, Winooski, VT, USA). Drug concentration is determined using a standard curve and plotted against time.
2.4. In vitro osteoblast and osteosarcoma cell culture
Before cell culture, the samples are sterilized by using an autoclave (Tuttnauer 3870 M, USA) at 121 °C for 60 min and all procedures are carried out in aseptic conditions inside a sterile hood.
2.4.1. Osteoblast cell culture
Passage 6 human osteoblast cell line (hFOB, PromoCell GmbH, Germany) is used for this study. The cells are cultured in a 75 cm2 flask, and upon reaching 85–90% confluency, they are detached from the bottom of the flask using 0.25% trypsin EDTA solution (ATCC, USA). 2 × 106 cells are seeded on each sample kept in 24 well plates, followed by the addition of 1 mL of osteoblast growth medium (PromoCell GmbH, Germany). The osteoblast growth medium is changed every 2 days during the entire cell culture study. Well plates are kept in an incubator at 37 °C in a 5% CO2 environment as recommended by PromoCell for this cell line.
2.4.2. Osteosarcoma cell culture
Passage 6 human osteosarcoma cell line (MG-63) (ATCC, USA) is used for this study. The cells are cultured in a 75 cm2 flask, and upon reaching 85–90% confluency, they are detached from the bottom of the flask using 0.25% trypsin EDTA solution (ATCC, USA). 2.5 × 104 cells are seeded on each sterilized sample placed in 24 well plates followed by the addition of 1 mL of cell culture media, that is eagles minimum essential medium (EMEM) (ATCC, USA). The well plates are placed in an incubator kept at 37 °C and 5% CO2 environment. Cell culture media is replaced every 2–3 days during the study.
2.4.3. Cell morphology
FESEM is carried out to characterize the cellular morphology on the sample surface after 3,7, and 11 days of cell culture. At first, SEM fixative is prepared by adding 2% paraformaldehyde/2% glutaraldehyde in 0.1 M phosphate buffer. After specific time points, cell media is aspirated from the wells, and the samples are transferred to a new 24 well plates for morphological characterization. SEM fixative is added into the wells to cover the samples overnight at 4 °C. After washing the samples with 0.1 M phosphate buffer, post-fixation is carried out by adding 2% osmium tetroxide (OSO4) on the samples and kept at room temperature for 1 h. Then, a series of ethanol (30%, 50%, 70%, 95%, and 100%) is used to dehydrate the samples, followed by hexamethyldisilane (HMDS) drying. Samples are kept at room temperature for overnight drying. Non-conductive ceramic scaffolds and coatings are gold coated with a thickness of 10–15 nm thickness using a sputter coater (Technics Hummer V Sputter Coater-Gold, USA). FESEM (Apreo VolumeScope™, USA) is utilized to observe the morphology of the gold-coated samples.
2.4.4. MTT cell viability assay
MTT (3-(4,5-dimethylthiazol-2-yl)– 2,5-diphenyl tetrazolium bromide) (Sigma, St. Louis, MO) assay is used to evaluate hFOB and MG-63 cell viability after 3,7 and 11 days of cell culture. Before the assay, MTT solution is prepared by dissolving 5 mg of MTT in 1 mL of sterile-filtered phosphate-buffered saline (PBS), and MTT solubilizer is prepared by adding 10% Triton X-100 in acidic isopropanol (0.1 N HCl). After specific time points, the cell media are aspirated from each sample-containing well, and 100 μl of MTT solution is added on top of each sample, followed by a quick addition of 900 μl of cell medium. The samples are kept in the incubator for 2 h. After 2 h of incubation, the MTT and cell media solution from each well are aspirated, and 600 μl of MTT solubilizer is added to dissolve the purple formazan crystals. After thoroughly mixing the formazan crystals by pipetting up and down the solubilizer, 100 μl is transferred into a 96-well plate and analysed for UV–vis absorbance at 570 nm using the microplate spectrophotometer (BioTek, US).
2.4.5. Alkaline phosphatase (ALP) osteoblast cell differentiation assay
Osteoblastic differentiation is carried out using alkaline phosphatase assay (SensoLyte® pNPP Alkaline Phosphatase Assay Kit, AnaSpec, CA, USA). Briefly, the samples are washed twice using a 1X assay buffer followed by the addition and thorough mixing of 0.2% Triton X-100 diluted in 1X assay buffer to scrape off the adherent cells. The cell suspension is collected and incubated at 4 °C for 10 min under constant agitation. Then, they are centrifuged at 2500 g for 10 min at 4 °C. 50 μl of the resultant supernatant is added into a 96 well plate followed by the addition of 50 μl of pNPP substrate. The reagents are gently mixed for 30 s and incubated for 45 min at room temperature. Finally, 50 μl of stop solution is added to each well. The plate is shaken on a plate shaker for 1 min before measuring the absorbance at 405 nm. The ALP/cell has been expressed by normalizing with the viable cell number from MTT assay (OD405/OD570).
2.4.6. ALP staining
After 7 days of culture the samples loaded with the osteoblast cells are fixed in 3.7% paraformaldehyde in PBS solution. The fixation is carried out for 10 mins. Subsequent washing of the samples are carried out for 5 mins 3 times using PBS. The cells are permeabilized using with 0.1% Triton X-100 (in PBS) for 4 min at room temperature. Samples are washed in TBST solution and incubated in TBST-Bovine serum albumin blocking reagent. Then, the samples are stained using primary antibody against alkaline phosphatase (ALP) (Sigma, St. Louis, MO) (1:100 dilution) and incubated at 4 °C overnight. The samples are then incubated for 1 h with secondary antibody, goat-anti mouse (GAM) Oregon green (Molecular Probes, Eugene, OR) (1:100 dilution). Samples are washed with TBST followed by 5 min washing with PBS. The samples are mounted on glass cover slips with DAPI and are kept at 4 °C. Leica SP-5 confocal laser fluorescent microscope is used to image the ALP stain at 488 and 588 nm.
2.5. In vitro tube formation assay by human umbilical vein endothelial cells (HUVEC)
All 3/5 mm 3D printed TCP scaffolds are autoclave sterilized (Tuttnauer 3870 M, USA) at 121 °C for 60 min, and the following procedures are carried out in aseptic conditions inside sterile hood. Primary umbilical vein endothelial cells, normal, human, pooled (HUVEC) ATCC® PCS-100–013™ are used for this study. HUVEC at an initial seeding density of 3300 cells/cm2 is cultured in a flask with 15 mL vascular cell basal media (ATCC PCS-100–030), supplemented with endothelial cell growth kit-VEGF (ATCC PCS-100–041). Matrigel (Corning, USA) is thawed overnight in ice and handled with pre-chilled pipet tips. It is diluted in the ice-cold complete growth medium (ATCC, USA) at a 1:1 ratio, and 500 μl of diluted Matrigel is added in the lower chamber of 12-transwell plates (Corning, USA). 7.5 × 104 HUVECs (passage 2, ATCC, USA) are seeded onto Matrigel. Autoclave-sterilized scaffolds are drug-loaded in a sterile hood and kept in the transwell inserts with 500 μl of complete growth media added into it. The plates are kept in a humid incubator at 37 °C with a 5% CO2 environment. After 3, 6, 12, and 24 h, the 24 well-plates are imaged on an inverted microscope to observe tube formation [47]. The quantification is carried out using the Angiogenesis Analyzer tool from ImageJ software.
2.6. Anti-bacterial study
3D printed TCP scaffolds are autoclave sterilized (Tuttnauer 3870 M, USA) at 121 °C for 60 min and drug loading is carried out inside the sterile hood. 400 μg of carvacrol and 400 μg of micellar curcumin have been added on top of the scaffolds using pipette. The freeze-dried Staphylococcus aureus (Carolina Biological Supply company, Burlington, NC, USA) is rehydrated using the rehydrating media consisting of tryptic soy as per company’s instruction protocol. The bacterial suspension is kept for 48 h in optimum condition (37 °C) for activation. The bacterial suspension is then checked for optical density values in order to attain 106 bacterial colony forming units (CFUs). The bacteria suspension is diluted in 3 batches to 1:10, 1:100 and 1:1000 using serial dilution process. 100 μl of this suspension is transferred to 96-well plate and optical density is measured at 600 nm wavelength. Upon receiving the specific optical density, it is compared with the McFarland standard and bacteria colony is measured. Based on the bacteria count, further dilution is carried out so that 100 μl of suspension contains 106 CFUs of bacteria. 106 CFUs of bacteria are then further diluted to obtain 102 CFU of bacterial colonies. 102 CFU of bacteria is added on top of each sample followed by the addition of 1 mL of broth media (CRITERION™ Tryptic Soy Broth #2, Hardy Diagnostics) as recommended by the company for specific bacteria. The samples are kept in 24-well plate at optimum environment (37 °C, 90% humidity) in the incubator. After specific time point (24 h and 48 h), the samples are taken out and bacterial suspension and samples are moved to glass vials. They are vortexed for 10 s and 50 μl of suspension is plated on agar plate. The agar plates are kept in an incubator at optimum environment (37 °C, 90% humidity) for 24 h. Then these plates are photographed, and bacterial colonies are counted [48].
| [1] |
| [2] |
In Equation (1), is average colony count for each plate, is dilution factor, which is 6 for this study, and 1 is the volume of the bacterial suspension added on each samples and here 1 = 200. To further analyse the bacterial cell morphology and bacterial growth, SEM and live/dead staining are performed following the protocol mentioned above in Section 2.4.3. The samples (n = 3) are incubated with 400 μl of 3 μM of calcein AM (BioLegend, CA) and propidium iodide (Fisher Scientific, MA) for 30 min at 37 °C. The imaging is performed using confocal laser scanning microscope (Leica SP-5) and absorbance detection is carried out at 535 nm and 620 nm wavelength respectively for calcein AM and propidium iodide.
2.7. RT- qPCR study
RT-qPCR is performed following human mesenchymal stem cells (hMSCs) and HUVEC culture for 7 and 3 days, respectively. Once the culture is completed, mRNA extraction is performed. Extraction of mRNA includes cell lysis, RNA binding to the columns, serial washing of the extraction, and finally, elution of RNA. The extraction of total RNA is performed with Aurum TM Total RNA Mini Kit (Bio-Rad, USA). Once the mRNA is extracted, the RNA is quantified using a nanodrop. Once the extraction and calculation are complete, the mRNA is used to synthesize cDNA using reverse transcription kit (BioRad, USA). 4 μl of iScript reaction mix is mixed with 1 μl of the iScript reverse transcript enzyme. The amplification solution is made using 1 μl of cDNA solution, 1 μl of primer, 10 μl of 2x SSO advanced supermix solution, and 8 μl of nuclease-free water. The steps include AC transcription, which is carried out for 2 mins at 95 °C; denaturation at 45 C for 5 s, which consists of 40 cycles; and the final step is annealing at 60 °C for 30 s which includes 40 cycles. Finally quantitative RT-PCR (qRT-PCR) is performed to detect expression of Runt-related transcription factor 2 (Runx2), osteocalcin, also known as bone gamma-carboxy glutamic acid-containing protein (BGLAP), Mammalian target of rapamycin (mTOR) and alkaline phosphatase (ALPL) with SsoAdvanced Universal SYBRs Green Supermix (Bio-Rad, USA), following the manufacturer’s instruction. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) is used for the internal control, and the relative gene expression is analyzed by the two-fold gene expression method.
2.8. Statistical analyses
All statistical analyses are performed using student’s t test or two-way ANOVA with a Bonferroni post-hoc analysis in GraphPad Prism 8 software (CA, USA). All data are representative of three biological replicates (n = 3) and each biological replicate has three technical replicates. The data are normally distributed by the Shapiro–Wilk test and are represented as mean± Std. P value ≤ 0.05 is considered as statistically significant.
3. Results
3.1. Morphology and characterization of the 3D printed scaffold and micelle as nano-scale drug delivery vehicle
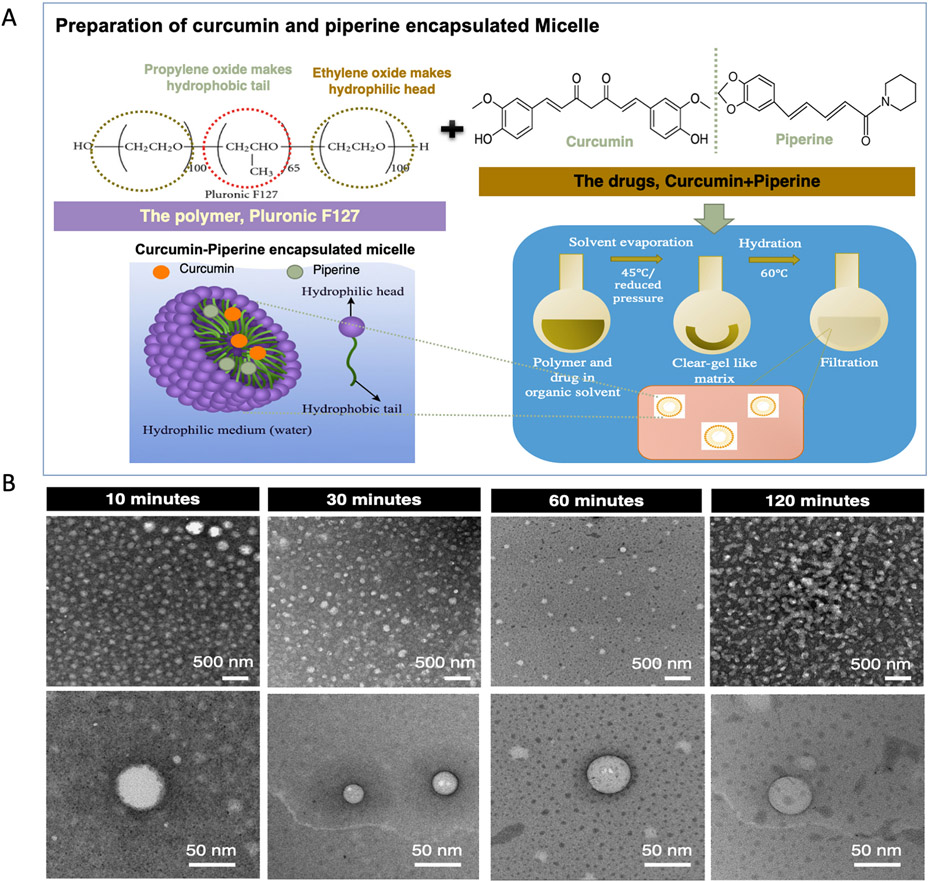
Fig. 1A shows the schematic representation featuring the micelle formation procedure and Fig. 1B depicts the TEM micrographs of curcumin-piperine loaded micelles in aqueous solutions at various stages of development. The figure exhibits a clear distinction of morphology with a gradual change of appearance within different processing times. 10 min and 30 min of micelle formation time is inefficient for the optimum micelle formation, which is confirmed by non-distinct vesicle shell, and 120 min formation time resulted in disrupted vesicle due to unstable micelle structure. 60 min processing time showed the most stabilized micelle morphology with the formation of regular, spherical shaped micelle. The high-resolution image reveals that the drug-loaded polymeric micelles are homogeneously dispersed in pure water without forming agglomeration and had an average diameter of 47 nm.
Fig. 1.
Synthesis of drug encapsulated nano-sized micelle. (A) Pluronic F127 polymer self-assemble in aqueous solution to form spherical micelles with a hydrophobic propylene oxide (PPO) centre surrounded by hydrophilic polyethylene oxide (PEO) shell. During micelle formation, the hydrophobic drugs, curcumin and piperine get entrapped within the PPO core, while hydrated outer shell ensures the solubility of the drug in an aqueous environment, enhancing the bioavailability of the drug. (B) Transmission Electron Microscopy (TEM) showing the micelle structure: Micelle formation is optimized by tailoring the mixing time to achieve critical micelle concentration. 30 min and 60 min mixing time resulted uniform and homogeneous formation of micelle with a size of 47 ± 5 nm, whereas 10 min resulted in a non-distinct vesicle wall, which is attributed to the inadequate time for micelle formation. 120 min mixing time resulted in ruptured micelle wall due to the disruption in the structure.
A simple schematic in Fig. 2a represents the drug loading procedure in 3D printed porous TCP scaffold. As shown in Fig. 2b, the CAD designed scaffolds exhibited a highly open porous structure with interconnected porous walls. After obtaining the non-load bearing porous TCP scaffolds by the 3DP binder jetting process, the porous microarchitecture of the sintered TCP scaffolds is assessed by FESEM, as shown in Fig. 2c. Due to volume shrinkage during sintering, the 400 μm designed pore size is reduced to 347 ± 7 μm pores in the sintered scaffold. The inherent microporosity of the scaffold is also observed in high magnification images, as shown in Fig. 2d and Fig. 2e, indicating the presence of heterogeneous residual pores with an average size of 5–20 μm. The samples’ porosity (volume fraction percent) is observed to be 62 ± 4% post sintering. The compressive strength of scaffold obtained is calculated 12.3 MPa. The EDS mapping shown in Fig. 2f-g illustrates a homogeneous distribution of the elements. Fig. 2g confirms the formation of TCP as the peaks of Ca, P, and O are observed. The synthesized powder shows that there is no presence of carbon peaks after washing, depicting that the carbonate salts present in precursor powder are dissolved during the washing process. Fig. 2h-i represents the successful encapsulation of curcumin in micelle using UV-Vis and FTIR respectively. A sharp peak in the observance depicts that the encapsulation has taken place successfully, which might be due to a non-polar environment. On the other hand, curcumin showed a broader peak showing the polarity of the environment [49]. FT-IR results also show a peak of 1512 cm−1, indicating stretching vibration of the conjugated carbonyl group. The stretching vibration of carbon double bond in micellar curcumin is shown at 1581 cm−1 depicting the stability of curcumin in a micellar environment [50]. Fig. 2j shows the XRD peaks depicting the formation of β-TCP. The peaks observed are at (1 0 10), (2 1 4), (0 2 10), (2 2 0) and (2 0 20) confirms the formation of β-TCP. DLS results in Fig. 2k shows the formation of micelles, the diameter of the micelles calculated are observed to be at 101 nm. The polydispersity index obtained is 0.22. The peak one mean by intensity is observed to be 132.6 nm and the peak two mean by intensity is observed to be 25.62 nm. The average diameter that has been observed in TEM is 47 nm which is in between 25.62 nm and 132.6 nm.
Fig. 2.
Morphology and drug-loading in 3D printed scaffolds (a) Schematic of drug loading in scaffold showing curcumin-piperine encapsulated micelle is pipetted on 3D printed TCP scaffold, followed by the incorporation of carvacrol as a coating. (b-e) 3D printed TCP scaffold showing the presence of hierarchical porous architecture with 400 μm designed macropores and 5–20 μm residual micropores, which are crucial for enhanced bone ingrowth. (f-g) EDS mapping of elements showing Ca (blue), O (green) and P (Red) suggesting uniform distribution of the elements. Ca, P and O peaks are obtained showing that there is negligible carbonate salt present due to the absence of carbon peak in EDS. (h) UV-Vis spectroscopy data showing a shift in the absorbance and sharper peak in case of micelle-encapsulated curcumin, whereas broad peak and lesser optical density in free curcumin due to presence of non-polar environment in curcumin encapsulated micelle. (i) FTIR results showing a peak shift in 1510–1512, 1581 and 1286–1282 cm−1 depicting stability of curcumin in micellar environment. (j) XRD peaks showing the presence of β-TCP after sintering at 1250 °C. (k) DLS of micelle encapsulated curcumin showing the average micelle diameter being 101 nm.
3.2. In vitro release kinetics for curcumin, piperine, and carvacrol from micelle loaded 3D printed scaffold
After incorporating the drug encapsulated micelle within the porous 3DP bone scaffolds, a release study is carried out to ensure a controlled and sustained release of the entrapped drug from the porous TCP scaffold. Fig. 3(a-b) shows the cumulative release of curcumin, and piperine, and carvacrol from micelle-loaded 3D printed scaffold in different conditions. Fig. 3(c-e) demonstrate drug release in the first 24 h. The release study is conducted in two different release media, phosphate buffer (pH 7.4) to represent the physiological environment of the body, and acetate buffer (pH 5.0) mimics the acidic tumor microenvironment as well as the post-surgical microenvironment. The cumulative percentage release for all the drugs is higher in case of pH 5.0, due to faster degradation of the scaffold, showing a release percentage of about 75.6%, 92.1%, and 97.0% for curcumin, carvacrol, and piperine, respectively. Micelle slowed down the burst release at pH 7.4, exhibiting a much-delayed release with about 79.7%, 89.6%, and 84.7% release for curcumin, piperine, and carvacrol, respectively.
Fig. 3.
In vitro release kinetics from micelle-loaded 3D printed scaffold and schematic representation of drug release mechanism from 3D printed scaffold. (a) In vitro release kinetics of micelle loaded drugs in physiological pH (pH 7.4) showing a controlled release of curcumin, piperine, and carvacrol with about 79.7%, 89.6%, and 84.7% drug release, respectively in 60 days. (b) In vitro release kinetics of micelle loaded drugs in pH 5.0 showing a significantly higher cumulative release percentage of about 75.6%, 97.0%, 92.1% at pH 5.0 for curcumin, piperine, and carvacrol, respectively within 6 days. The burst release of drugs at pH 5.0 indicates rapid delivery of chemopreventive biomolecules, curcumin and piperine, and antibacterial drug, carvacrol in acidic tumor microenvironment and right after the surgery, whereas controlled release at pH 7.4 indicates sustained drug delivery to provide localized prevention of osteosarcoma cell growth, anti-bacterial protection for secondary infection and subsequent bone regeneration at the defect site for prolonged period. (c-e) The release of drugs (curcumin, piperine and carvacrol) from 3D printed scaffold at initial 24 h are plotted to show the distinct effect of pH on their release.
3.3. Effect of micelle loaded 3D printed scaffold on in vitro osteoblast (hFOB) proliferation and differentiation
To ensure the micelle loaded scaffold does not induce adverse effects and promotes osteoblast cell proliferation, hFOB cells are cultured on the scaffold surface, and cell viability, proliferation, and differentiation are assessed by morphological characterization, MTT assay, and ALP assay, as shown in Fig. 4(a-b), respectively. In terms of cell viability, 3DP scaffolds with micelle showed significantly improved osteoblast cell viability at day 3 and 7 with a stark four-folds increase on day 11. The normalized ALP expression also demonstrated significant cellular differentiation at day 11 in the presence of micelle-loaded 3DP scaffold, compared to the control. FESEM characterization in Fig. 4(c) exhibits abundant cellular growth, filopodial processes with no signs of cytotoxicity in the micelle-loaded scaffold.
Fig. 4.
In vitro osteoblast (hFOB) proliferation by 3D printed TCP scaffold (a) MTT cell viability assay showing micelle loaded 3D printed scaffold with statistically significant difference in hFOB proliferation at day 3,7, 11, compared to the control. At day 11, micelle loaded scaffolds show a 4-fold increase in hFOB cell viability compared to the control scaffold. (b) ALP osteoblast differentiation assay showing enhanced hFOB cellular differentiation in the presence of micelle at day 11, compared to control [* * denotes p value< 0.001]. (c) Morphological characterization by FESEM showing firm hFOB attachment, presence of filopodial processes and abundant proliferation in all the samples, suggesting no cytotoxicity of the drug towards the healthy bone cell. (d) FESEM micrograph showing osteoblast cell attachment differentiating from hMSCs after 7-day time point. Samples loaded with micelle encapsulated curcumin showed healthier morphology. (e) ALP staining images showing that there are more number of healthy osteoblast cells in the drug loaded samples as compared to control sample. (f) 2-fold gene expression obtained from RT-qPCR showing upregulation of genes related to osteogenesis in presence of micelle encapsulated curcumin.
hMSCs were cultured for 7 days on scaffold surface to evaluate osteogenic differentiation by micelle loaded scaffold. After 7 days of culture, SEM images in Fig. 4(d) shows osteoblast-like cellular phenotype in both control and micelle loaded TCP scaffolds, however later has more distinct, well-spread morphology. Fig. 4(e) shows higher ALP positive cells in curcumin-piperine encapsulated micelle loaded 3D printed TCP scaffolds than the control samples after 7 days of culture. This suggest curcumin-piperine encapsulated micelle promote early osteogenic differentiation. RT-qPCR is performed to evaluate micelle-loaded 3DP scaffold’s efficacy on osteoblast cell differentiation at 7 days, as shown in Fig. 4(f). Micelle loaded 3DP TCP showed almost 2-fold increase in BGLAP or osteocalcin, 3.5 folds increase in ALPL and 2.5 folds increase in RUNX2, compared to control scaffold. Upregulation of these bone differentiation marker genes further validate our finding that that micelle loaded 3D printed scaffold accelerate in vitro osteogenic differentiation.
3.4. Effect of micelle-loaded 3D printed scaffold on in vitro cytotoxicity study against human osteosarcoma cells (MG-63)
To evaluate the clinical efficacy of the scaffold with regard to osteosarcoma chemoprevention, MG-63 cells are cultured and assessed for cell viability and proliferation after 3, 7, and 11 days, as shown in Fig. 5(a). Due to their inherent biocompatibility and non-toxicity, control scaffolds showed a gradual increase in osteosarcoma cell viability with increasing time. Micelle loaded scaffold did not show any signs of chemoprevention at day 3, as indicated by MTT assay. However, after 7 and 11, it showed less than 70% of cell viability compared to the control, suggesting its cytotoxicity towards the osteosarcoma cell line, as shown in Fig. 5(b). Although it is challenging to visualize cellular attachment in the porous scaffold, FESEM images in Fig. 5(c) showed the presence of osteosarcoma cells with abundant cytoplasmic processes that help to anchor the surrounding scaffold surface demonstrating their firm attachment and growth in control samples. However, micelle-loaded scaffolds did not show any signs of cellular proliferation on the scaffold surface at day 3,7, and 11, which implies the in vitro chemopreventive potential of the drug-loaded micelle.
Fig. 5.
In vitro cytotoxicity study against human osteosarcoma cells (MG-63). (a) MTT cytotoxicity assay showing no inhibitory effect on osteosarcoma cells in presence of micelle at day 3, however 2- and 3.5-folds lower osteosarcoma cell viability at day 7 and day 11, respectively [* * denotes p value< 0.001]. (b) % cytotoxicity denoting lower than 70% cellular viability at day 7 and 11 suggesting chemopreventive potential of the scaffold loaded with micelle. (c) FESEM images at day 3, 7 and 11 days of cell culture showing the presence of osteosarcoma cell with typical fibroblast-like morphology in control samples, whereas very few osteosarcoma cells in micelle-loaded 3D printed TCP.
3.5. In vitro tube formation assay by Human Endothelial Vein Endothelial Cells (HUVEC)
Tube formation assay is a principle, rapid in vitro measurement for angiogenesis. To investigate the in vitro angiogenic effect of curcumin and curcumin-piperine encapsulated micelle loaded 3D printed TCP on HUVEC, cells are seeded on Matrigel in the lower chamber with the control 3D printed TCP, and drug-loaded 3D printed TCP scaffolds in transwell inserts, depicted in Fig. 6A. As shown in Fig. 6B, curcumin and curcumin encapsulated micelles significantly stimulated tube formation at as early as 3 h in HUVECs grown on Matrigel, compared to the control 3D printed TCP scaffolds. While maximum tube formation is reached at 6 h for all the compositions, by 12 h, the HUVECs start to undergo apoptosis, and the tubes begin to disconnect. At 24 h, most of the branches are disrupted, leaving some cells attached to Matrigel.
Fig. 6.
In vitro tube formation assay by Human Endothelial Vein Endothelial Cells (HUVEC) (A) Schematic diagram of the endothelial cell assay on Matrigel (B) Light microscopy images showing tube formation by HUVEC after 3, 6, 12 and 24 h on Matrigel with control, curcumin and micelle loaded 3D printed TCP scaffolds in transwell inserts. Curcumin and curcumin encapsulated micelles significantly stimulated tube formation at as early as 3 h, compared to the control 3D printed TCP scaffolds, which showed few tubes and minimal network among the branches suggesting enhanced and accelerated early tubular network formation ability by curcumin encapsulated micelle loaded 3D printed TCP scaffold. Maximum tube formation is reached at 6 h for all the compositions, by 12 h, the HUVECs starts to undergo apoptosis and the tubes begin to dissociate, which is a usual phenomenon for HUVEC cell.
Significant features of tube formation such as mesh, nodes, junction, branch, and master segment, as shown in Fig. 7A, were quantified for control, curcumin and curcumin encapsulated micelles loaded 3D printed TCP at 3 and 6 h. 3D printed TCP scaffolds showed comparatively fewer nodes, branches, and master segments within the tubular network as shown in Fig. 7B-G. The total master segment length and total branch length are also significantly higher in the presence of curcumin than the control. Fig. 7H shows the SEM micrographs of HUVEC cells cultured on 3D printed scaffold surface for 3 days. The samples loaded with micellar curcumin shows distinct, healthy cellular morphology, while control samples showed irregular cell structure. RT-qPCR is carried out following a 3 day’s HUVEC culture to analyze mTOR expression, a gene that assists in blood vessel formation. Micelle loaded 3DP TCP showed 2.5 folds upregulation in MTOR expression, as shown in Fig. 7I.
Fig. 7.
Quantitative analysis of HUVEC tube formation assay by Angiogenesis Analyzer for ImageJ software (A) A representative image of tube formation showing presence of nodes, meshes, junction, branches and master segment in different colour codes (B-G) Number of nodes, meshes, master segment, branches and total master segment length, total branching length, total segments length and total length are calculated for control (3D printed scaffold), curcumin loaded 3D printed scaffold and curcumin-piperine loaded micelle incorporated 3D printed scaffold at 3 h and 6 h of HUVEC cell culture [* represents p < 0.05, * * represents p < 0.001]. Statistically significant enhancement of nodes, branches and master segments have been observed in presence of curcumin and drug loaded micelle at both 3 and 6 h, indicating their stabilizing effect towards HUVEC formed capillary-like tube structure. (H) FESEM micrographs showing HUVEC cell morphology after a period of 3 days of study. Samples loaded with micelle encapsulated curcumin showed healthier cell morphology. (I) 2-fold gene expression study showing mTOR expression after 3 days. Presence of micelle encapsulated curcumin upregulated the mTOR expression which in turn enhanced cell proliferation.
3.6. In vitro antibacterial efficacy of the micelle-encapsulated scaffold against Staphylococcus aureus
To assure clinical success and enhance the longevity of the implant, it is important to make sure that the implant does not induce any bacterial infection, which is one of the major reasons for implant failure. In this study, anti-bacterial effects of curcumin and carvacrol release from the 3D printed discs are assessed for 24 and 48 h utilizing Staphylococcus aureus, a gram-positive pathogenic bacterium. Fig. 8A demonstrated the schematic representation of bacterial culture. Fig. 8B-C depicts the representative images and quantification of bacterial colony formation on the agar plate after culturing and incubating them on the sample surface for 24 and 48 h. Higher number of bacterial colony formation can be observed on the control agar plates control (3DP TCP scaffolds) than the treatment plates (3DP TCP loaded with drugs). The bacterial colony counting is performed from the agar plate and it is observed that micellar curcumin loaded 3DP TCP showed 21 ± 3% and carvacrol loaded with carvacrol showed 71 ± 6% antibacterial efficacy after 24 h. However, the strongest antibacterial effect with 98 ± 1% efficacy against Staphylococcus aureus is observed with 3DP TCP loaded with micellar curcumin and carvacrol after 24 h of incubation. Similar results are obtained after 48 h of incubation, where, 23 ± 2%, 73 ± 3% and 94 ± 4% antibacterial efficacy are observed in micellar curcumin loaded 3DP TCP scaffolds, carvacrol loaded 3DP TCP and micellar curcumin and carvacrol loaded 3DP TCP scaffolds, respectively. Further characterization using FESEM and live-dead staining are carried out to support the antibacterial data. Fig. 8D shows the representative FESEM images of the 3DP TCP scaffolds surface with lesser number of bacterial colony present on scaffolds that has been treated with micellar curcumin, carvacrol and micellar curcumin+carvacrol. Live/dead-stained confocal images shown in Fig. 8E are in accordance with the FESEM images where vast majority of live cells stained in green calcein AM stain can be observed in control 3DP TCP scaffolds. Scaffolds loaded with micellar curcumin, carvacrol and micellar curcumin+carvacrol exhibited larger number of dead bacterial colony stained with red propidium iodide.
Fig. 8.
In vitro anti-bacterial efficacy against Staphylococcus aureus (A) Schematic diagram of the antibacterial study. (B-C) Agar plates and related quantification showing the presence of Staphylococcus aureus colonies in control 3DP TCP, after 24 and 48 h of incubation. Lesser number of bacterial colonies are observed in agar plates associated with scaffolds loaded with micellar curcumin (21 ± 3% and around 23 ± 2% efficacy at 24 and 48 h respectively). Further reduction in bacterial colonies are seen with scaffolds loaded with carvacrol with 71 ± 6% and 73 ± 3% antibacterial efficacy after 24 and 38 h. Highest antibacterial efficacy are exhibited with scaffolds loaded with micellar curcumin+carvacrol with 98 ± 1% and 94 ± 4% antibacterial efficacy after 24 and 48 h (** p < 0.001). (D) SEM images depicting higher Staphylococcus aureus colonies in control 3DP TCP, whereas most reduction in the bacterial colony are observed in 3DP TCP loaded with micellar curcumin and carvacrol. (E) Live/dead confocal imaging showing greater live Staphylococcus aureus colonies in control 3DP TCP. Scaffolds loaded with micelle encapsulated curcumin and carvacrol exhibited higher presence dead bacterial colonies in red stain and least number of live bacteria in green. (F) Schematic depicting the mechanism of bactericidal effects of curcumin and carvacrol.
4. Discussion
The past decade has witnessed a tremendous development in 3D printing technology, allowing the design and fabrication of patient-specific tissue scaffolds that closely mimic the complex hierarchical structure of osseous tissue [4]. However, the development of functionalized scaffolds for highly localized, targeted drug delivery, which has become an essential requirement for complex bone disorders such as bone malignancy or implant-related bacterial infection, is still in its infancy. In this study, a micelle-loaded multi-drug delivery approach has been developed to overcome these fundamental obstacles by improving the biological function of the conventional 3DP scaffolds. The three natural medicinal compounds, curcumin, piperine, and carvacrol, are each responsible for specific biological functions are encapsulated within the polymeric micelle.
The functionalized scaffold is constructed stepwise by optimizing the micelle formation and subsequent integration of drug-loaded polymeric micelle into the 3D printed TCP scaffold. The TEM images shows the formation of a stable, homogeneous, spherical structure with no aggregation. DLS confirms the homogenous size distribution of the micelle obtained after encapsulation. During the formation of micelle, the inner hydrophobic core houses the poorly soluble curcumin, piperine, and carvacrol. The outer hydrophilic shell ensures the stability and solubility of micelle in an aqueous environment, enhancing the bioavailability of the encapsulated drugs. [18-20,42,43-45]. FTIR show the presence of characteristic peaks in micelle-encapsulated curcumin, portraying the stability of curcumin in a micellar environment. The biodegradable TCP scaffolds with high porosity, pore interconnectivity, and uniform pore distribution are fabricated via a binder-jetting process. EDS elemental mapping suggest successful formation of TCP, illustrated by the absence of carbon peaks and the presence of Ca, P, and O peaks confirming the absence of precursor carbonate salt. Micelle-encapsulated curcumin shows a sharper peak than bare curcumin depicting the non-polar environment created by micelle encapsulation. XRD further confirms that β-TCP phase is retained after sintering at 1250 °C. These 3DP scaffolds with an interpenetrating network of 400 μm sized macropores serve as a template to provide functional and structural support to the newly formed bone tissue by allowing cell and ion transport, bone ingrowth, and formation of blood vessels. Besides, the presence of 5–20 μm residual micropores benefits the scaffold by increasing the surface area for protein absorption and osteoblast attachment, thereby inducing the natural tissue regeneration process and development [44, 45]. Together, the combination of designed macropores and inherent micropores favour the osteogenesis and osseointegration, facilitating the interlocking between the scaffolds and the surrounding host bone.
Based on the in vitro release studies, it is evident that the nano-sized polymeric micelle serves as a unique drug carrier as it enables the release of hydrophobic drugs from the 3D printed TCP scaffold in both acidic and physiological microenvironments. A burst release of drugs is demonstrated in the acidic release media within the first 5 days, resulting in cumulative release of 75.6%, 92.1% and 97% for curcumin, piperine, and carvacrol, respectively. With the rapid release of chemopreventive and antibacterial drugs, any residual tumor cells in the local acidic microenvironment could be eradicated, and the risk of implant-related infection might be potentially minimized. Local antibiotic delivery with a high burst release is highly desirable for tissue engineering scaffolds. It can respond to the elevated risk of infection from the bacteria that have been introduced during the implantation procedure [40]. Interestingly, these drugs showed a much slower release pattern in the phosphate buffer or normal physiological pH, demonstrating a cumulative release for 79.7% and 89.6% for curcumin and piperine, respectively, that continued over 60 days. The burst release of the drugs in acidic pH might be based on either a higher degradation rate of the TCP substrate or the pH sensitivity of the micelle, which accelerated the degradation and subsequent release of the encapsulated drugs [41]. Besides, the slower release of the antibacterial drug carvacrol, which exhibited a cumulative release of 84.7% for 60 days, helps to combat the bacteria that are introduced systematically after implantation or surgery. The slower release of the drugs in the physiological environment can be easily attributed to the hydrophobic core of the micelle, which strongly restricts the migration of the drugs into the release medium. The release kinetics are fitted with the Korsmeyer-Peppas model, which indicates a diffusion-controlled drug release mechanism.
On account of the chemopreventive efficacy of curcumin and piperine, the nano-sized micelle-loaded scaffold displays an excellent performance for in vitro osteosarcoma inhibition. Curcumin’s chemopreventive effect is well-established with its ability to target multiple molecular pathways related to tumorigenesis, among which inhibition of the transcription factor NFκB (nuclear factor κB) is considered one of the most plausible pathways explaining the anti-tumor effects of curcumin [7,8,51,52]. The presence of piperine, another chemopreventive biomolecule, further accelerates the in vitro anticancer potential of the scaffold, confirming the effectiveness of combination chemopreventive therapy over a single-agent approach. Piperine’s anticancer potential has also been demonstrated in many types of cancer cells through its influence on the activation of apoptotic signalling and cell cycle arrest, which subsequently inhibits the proliferation and disrupts tumor cell survival [24,25,53].
To demonstrate the potential of the micelle-loaded 3DP TCP scaffold for bone tissue engineering application, in vitro osteoblast cell culture is carried out. It has previously been noted that curcumin can increase the OPG/RANKL ratio, and thereby promoting osteoblast proliferation and differentiation [54,55]. Recent reports from other groups have also indicated bone regenerating ability by sustained release of curcumin in in vivo tumor-bearing mice model [13]. Additionally, a recent study also reported enhanced osteoblast differentiation ability demonstrated by piperine via the upregulation of RUNX2 expression [56]. After conducting the osteoblast proliferation and differentiation study in a low load bearing 3D printed TCP scaffold, we were interested to see if the efficacy of curcumin-piperine micelle system can be translated to a load-bearing implant as well. To gain additional evidence of the in vitro cytocompatibility and osteogenic efficacy of the micelle in a load-bearing metal implant, hydroxyapatite (HA) coated Ti implant is utilized as a substrate (see supplementary section, Fig. S1). Ti implants with HA coating are used for weight-bearing applications, where porous biodegradable ceramic scaffolds cannot be used due to their poor mechanical properties [57]. It was assuring to observe similar result in a micelle-loaded HA-coated Ti implants that did not show any cytotoxicity towards the human fetal osteoblast cells (hFOB), indicative of excellent cytocompatibility. Other than demonstrating potential to proliferate osteoblast cells, micelle loaded TCP scaffold upregulated different osteogenic differentiation gene expressions including BGLAP, ALPL, and RUNX2, which further suggest the scaffold’s ability for osteogenic differentiation.
Bone formation is closely associated with vascularization or angiogenesis, together which maintain the skeletal integrity. Therefore, current bone grafts that provide only structural and mechanical support to repair bone defects face significant challenges due to insufficient nutrient supply, rejection, and inability to integrate with the surrounding bone tissue. This study sought to fabricate a functional scaffold by integrating 3D printed TCP scaffold and natural medicinal compounds, promoting early and accelerated vascularization. To investigate the in vitro angiogenic effect of micelle-loaded 3D printed TCP scaffold, a real-time simulation of angiogenesis in tube formation assay is carried out, where HUVEC is seeded on Matrigel and stimulated by curcumin’s release from micelle loaded scaffolds placed in a transwell insert. The angiogenesis analysis ImageJ tool allowed to quantify node (pixel having at least three neighbours), the junction (multi-intersection nodes forming bifurcation or branches in the angiogenic structures), mesh (area enclosed between two junctions), master segment (portions of an angiogenic capillary connecting two junctions, which are exclusively linked with other capillaries), and branches (angiogenic tube linked with one junction and one extremity). Total master segment length determines the ability to form branches, and total length includes all recognizable capillaries, including all segments and branches [58,59]. Controlled release of micellar curcumin from 3D printed TCP scaffold resulted in enhanced and early formation of a tubular network in the Matrigel. The complexity of the tubular network, in terms of the number of nodes, meshes, branches, master segments, is much higher in the case of curcumin and micelle loaded 3D printed TCP scaffold than the control. Overall increased total master segment length and total length indicate that controlled release of curcumin promoted HUVEC to build a complex capillary network with a significantly greater number of connections between tubes. Study found that curcumin augments the expression for various pro-angiogenesis factors such as VEGF, MMP-2 and TGF-β, which induces blood vessel formation with increased micro-vessel density [60,61]. In this study, enhanced angiogenesis in the presence of micellar curcumin can be attributed to upregulation of mTOR gene expression by amino acids in presence of curcumin, which assists in blood vessel regeneration [62,63]. Collectively, these reports indicate curcumin’s bidirectional action on angiogenesis, which might also explain the enhanced and accelerated early tubular network formation by curcumin encapsulated micelle-loaded 3D printed TCP scaffold at 3 h.
Bacterial infections are the major cause of implant failure and emerging to be one of the biggest concerns in the tissue engineering field. To demonstrate the antibacterial efficacy of the micelle loaded 3D printed TCP, the scaffolds are challenged with Staphylococcus aureus, a gram-positive microorganism, which is the most commonly occurring bacterial strain, constituting about 34% of total incidences of implant-related infection [64]. Since systemic and localized antibody treatment is losing its efficacy due to the ever-increasing crisis of antibiotic-resistance, surface functionalization of implants with dopants or other natural compounds have been used to avoid the spread of antibiotic-resistant bacteria [61]. Therefore, carvacrol is chosen due to its stronger inhibitory action against gram-positive bacteria compared to the gram-negative which are seen far less frequently in implant-related infection [65]. Moreover, the gram-positive bacteria are more susceptible to the phenolic components of essential oil, such as carvacrol, which is also a phenolic monoterpenoid, than gram-negative bacteria [66]. Carvacrol, being hydrophobic, interferes with the lipid bilayer of the bacterial membrane and aligns itself within the hydrocarbon chains causing disruption and destabilization of membrane architecture, which leads to the formation of ion channels for essential cellular components and ions to leave the cytoplasm [67,68]. Cell division in most bacteria depends on the function of an essential protein called Filamenting temperature-sensitive mutant Z(FtsZ). FtsZ forms a ring like structure by polymerizing at the future division site called Z-ring. Curcumin binds to FtsZ proteins, in turn inhibiting the assembly of FtsZ with protofilaments. This, in turn, suppresses the formation of Z-ring, which assist in bacterial cell growth leading to inhibition of cytokinesis and bacterial proliferation, as shown in Fig. 8F [69].
This work successfully incorporates naturally sourced biomolecules with chemopreventive, osteogenic and antibacterial properties, onto a porous interconnected 3D printed TCP scaffold to fabricate an advanced, functionalized bone substitute with improved biological function and stepwise therapeutic approach based on in vitro bone regeneration, enhanced angiogenesis, prevention of osteosarcoma and bacterial viability. The result of this study opens many new avenues for future exploration. Particularly, the controlled release of natural biomolecules could cause significant changes in osteogenic and angiogenic properties in vitro is intriguing and suggests that follow up in vivo animal study is required to further understand the mechanism. Further, combining different natural medicinal compounds to understand their chemopreventive, osteogenic and antibacterial potential in a 3D printed scaffold is unique and can be scalable for larger animal models.
5. Summary
A multifunctional 3D printed bone scaffold incorporating curcumin and piperine in the micelle, and carvacrol as a coating showed sequential drug release properties facilitated by in vitro bone-cell proliferating, chemopreventive and antibacterial properties for efficient localized treatment of osteosarcoma. In vitro release kinetics study demonstrates a burst release of the loaded drug in the acidic tumor microenvironment with 75.6%, 92.1%, and 97.0% release by curcumin, piperine, and carvacrol, within 6 days respectively, and a continuous sustained release by the same with 79.7%, 89.6%, and 84.7% after 60 days in the physiological environment. In vitro cytotoxicity assessment at days 3, 7, and 11 exhibit excellent anti- osteosarcoma efficacies with less than 70% cellular proliferation after day 7 and day 11 by the micelle-loaded scaffold suggesting their chemopreventive potential. Intriguingly, the scaffold does not possess any toxicity towards the osteoblast cells; in fact, the presence of micelle endows the scaffolds with a unique osteogenic property favouring the proliferation, attachment, and differentiation of the cells with a 4-fold enhancement in cell viability after 11 days. A similar in vitro osteogenic effect is observed on days 3, 7, and 11, particularly at day 11; 30% enhanced hFOB cell viability is noted along with a 33% increase in ALP activity. Owing to carvacrol and curcumin’s anti-bacterial property, the scaffolds showed an increased anti-bacterial efficacy of 98% and 94% respectively for 24 h and 48 h, suggesting its potential to prevent implant-related infection. Additionally, the scaffolds provide promising activity towards in vitro tubular formation and modulation of endothelial vascular network suggesting its potential for promoted angiogenesis, crucial for wound healing. Taken together, carvacrol coated and curcumin-piperine encapsulated micelle is incorporated within a 3D printed porous scaffold with designed architecture to provide localized prevention of osteosarcoma cell growth, antibacterial protection from secondary infection, and subsequent bone regeneration at the defect site for new-generation osteosarcoma treatment.
Supplementary Material
Acknowledgments
Authors would like to acknowledge financial support from the National Institute of Dental and Craniofacial Research (NIDCR) of the NIH under Award number R01 DE029204–01 and National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) of the National Institutes of Health (NIH) under Award number R01 AR066361. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute of Health.
Footnotes
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: Ujjayan Majumdar reports financial support was provided by National Institutes of Health. The authors declare no conflict of interest. This content is solely authors’ responsibility and does not necessarily represent the official views of the National Institutes of Health.
CRediT authorship contribution statement
Susmita Bose: Conceptualization, Methodology, Visualization, Writing – original draft preparation, Supervision, Funding acquisition, Project administration. Naboneeta Sarkar: Conceptualization, Methodology, Visualization, Writing – original draft preparation. Ujjayan Majumdar: Conceptualization, Methodology, Visualization, Validation.
Data and materials availability
Data will be made available upon request.
Appendix A. Supporting information
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.colsurfb.2023.113563.
Data availability
No data was used for the research described in the article.
References
- [1].Durfee RA, Mohammed M, Luu HH, Review of osteosarcoma and current management, Rheumatol. Ther 3 (2) (2016) 221–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Anderson ME, Update on survival in osteosarcoma, Orthop. Clin 47 (1) (2016) 283–292. Jan 1. [DOI] [PubMed] [Google Scholar]
- [3].Harrison DJ, Geller DS, Gill JD, Lewis VO, Gorlick R, Current and future therapeutic approaches for osteosarcoma, Expert Rev. Anticancer Ther 18 (1) (2018) 39–50. Jan 2. [DOI] [PubMed] [Google Scholar]
- [4].Ak T, Gülçin İ, Antioxidant and radical scavenging properties of curcumin, Chem. -Biol. Interact 174 (1) (2008) 27–37. Jul 10. [DOI] [PubMed] [Google Scholar]
- [5].Sarkar N, Bose S, Controlled delivery of curcumin and vitamin K2 from hydroxyapatite-coated titanium implant for enhanced in vitro chemoprevention, osteogenesis, and in vivo osseointegration, ACS Appl. Mater. Interfaces 12 (12) (2020) 13644–13656. Feb 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Shishodia S, Sethi G, Aggarwal BB, Curcumin: getting back to the roots, Ann. N. Y. Acad. Sci 1056 (1) (2005) 206–217. [DOI] [PubMed] [Google Scholar]
- [7].Pillai GR, Srivastava AS, Hassanein TI, Chauhan DP, Carrier E, Induction of apoptosis in human lung cancer cells by curcumin, Cancer Lett. 208 (2) (2004) 163–170. May 28. [DOI] [PubMed] [Google Scholar]
- [8].Sarkar N, Bose S, Liposome-encapsulated curcumin-loaded 3D printed scaffold for bone tissue engineering, ACS Appl. Mater. Interfaces 11 (19) (2019) 17184–17192. Mar 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Dorai T, Cao YC, Dorai B, Buttyan R, Katz AE, Therapeutic potential of curcumin in human prostate cancer. III. Curcumin inhibits proliferation, induces apoptosis, and inhibits angiogenesis of LNCaP prostate cancer cells in vivo, prostate 47 (4) (2001) 293–303. Jun 1. [DOI] [PubMed] [Google Scholar]
- [10].Wang Y, Yu J, Cui R, Lin J, Ding X, Curcumin in treating breast cancer: A review, J. Lab. Autom 21 (6) (2016) 723–731. [DOI] [PubMed] [Google Scholar]
- [11].Dhillon N, Aggarwal BB, Newman RA, Wolff RA, Kunnumakkara AB, Abbruzzese JL, Ng CS, Badmaev V, Kurzrock R, Phase II trial of curcumin in patients with advanced pancreatic cancer, Clin. Cancer Res 14 (14) (2008) 4491–4499. Jul 15. [DOI] [PubMed] [Google Scholar]
- [12].Son HE, Kim EJ, Jang WG, Curcumin induces osteoblast differentiation through mild-endoplasmic reticulum stress-mediated such as BMP2 on osteoblast cells, Life Sci. 193 (2018) 34–39. Jan 15. [DOI] [PubMed] [Google Scholar]
- [13].Hosseini S, Naderi-Manesh H, Vali H, Eslaminejad MB, Sayahpour FA, Sheibani S, Faghihi S, Contribution of osteocalcin-mimetic peptide enhances osteogenic activity and extracellular matrix mineralization of human osteoblast-like cells, Colloids Surf. B: Biointerfaces 173 (2019) 662–671. Jan 1. [DOI] [PubMed] [Google Scholar]
- [14].Chen Z, Xue J, Shen T, Mu S, Fu Q, Curcumin alleviates glucocorticoid-induced osteoporosis through the regulation of the Wnt signaling pathway, Int. J. Mol. Med 37 (2) (2016) 329–338. Feb 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Komori T, Regulation of osteoblast differentiation by Runx2. InOsteoimmunology: Interactions of the Immune and skeletal systems II, Springer US,, 2010, pp. 43–49. [DOI] [PubMed] [Google Scholar]
- [16].Li X, Chen Y, Mao Y, Dai P, Sun X, Zhang X, Cheng H, Wang Y, Banda I, Wu G, Ma J, Curcumin protects osteoblasts from oxidative stress-induced dysfunction via GSK3β-Nrf2 signaling pathway, Front. Bioeng. Biotechnol 8 (2020), 625. Jun 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Samiei M, Abedi A, Sharifi S, Maleki Dizaj S, Early osteogenic differentiation stimulation of dental pulp stem cells by calcitriol and curcumin, Stem Cells Int. 2021 (2021). May 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Liu W, Zhang L, Xuan K, Hu C, Liu S, Liao L, Li B, Jin F, Shi S, Jin Y, Alpl prevents bone ageing sensitivity by specifically regulating senescence and differentiation in mesenchymal stem cells, Bone Res. 6 (1) (2018), 27. Sep 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Gu Q, Cai Y, Huang C, Shi Q, Yang H, Curcumin increases rat mesenchymal stem cell osteoblast differentiation but inhibits adipocyte differentiation, Pharmacogn. Mag 8 (31) (2012) 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Chen S, Liang H, Ji Y, Kou H, Zhang C, Shang G, Shang C, Song Z, Yang L, Liu L, Wang Y, Curcumin modulates the crosstalk between macrophages and bone mesenchymal stem cells to ameliorate osteogenesis, Front. Cell Dev. Biol 9 (9) (2021) 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Chen B, Liang Y, Zhang J, Bai L, Xu M, Han Q, Han X, Xiu J, Li M, Zhou X, Guo B, Synergistic enhancement of tendon-to-bone healing via anti-inflammatory and pro-differentiation effects caused by sustained release of Mg2+/curcumin from injectable self-healing hydrogels, Theranostics 11 (12) (2021) 5911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Tan B, Wu Y, Wu Y, Shi K, Han R, Li Y, Qian Z, Liao J, Curcumin-Microsphere/IR820 Hybrid Bifunctional Hydrogels for In Situ Osteosarcoma Chemo-co-Thermal Therapy and Bone Reconstruction, ACS Appl. Mater. Interfaces (2021). Jun 30. [DOI] [PubMed] [Google Scholar]
- [23].Bose S, Sarkar N, Banerjee D, Effects of PCL PEG and PLGA polymers on curcumin release from calcium phosphate matrix for in vitro and in vivo bone regeneration, Mater. Today Chem 8 (2018) 110–120. Jun 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Anand P, Kunnumakkara AB, Newman RA, Aggarwal BB, Bioavailability of curcumin: problems and promises, Mol. Pharm 4 (6) (2007) 807–818. Dec 3. [DOI] [PubMed] [Google Scholar]
- [25].Bansal SS, Goel M, Aqil F, Vadhanam MV, Gupta RC, Advanced drug delivery systems of curcumin for cancer chemoprevention, Cancer Prev. Res 4 (8) (2011) 1158–1171. Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Kazunori K, Masayuki Y, Teruo O, Yasuhisa S, Block copolymer micelles as vehicles for drug delivery, J. Control. Release 24 (1–3) (1993) 119–132. May 1. [Google Scholar]
- [27].Rapoport N, Physical stimuli-responsive polymeric micelles for anti-cancer drug delivery, Prog. Polym. Sci 32 (8–9) (2007) 962–990. Aug 1. [Google Scholar]
- [28].Sahu A, Kasoju N, Goswami P, Bora U, Encapsulation of curcumin in Pluronic block copolymer micelles for drug delivery applications, J. Biomater. Appl 25 (6) (2011) 619–639. [DOI] [PubMed] [Google Scholar]
- [29].Liu W, Zhai Y, Heng X, Che FY, Chen W, Sun D, Zhai G, Oral bioavailability of curcumin: problems and advancements, J. Drug Target 24 (8) (2016) 694–702. Sep 13. [DOI] [PubMed] [Google Scholar]
- [30].Majeed M, Badmaev V, Rajendran R, U.S. Patent No. 5,744,161, U.S. Patent and Trademark Office,, Washington, DC, 1998. [Google Scholar]
- [31].Shoba G, Joy D, Joseph T, Majeed M, Rajendran R, Srinivas PS, Influence of piperine on the pharmacokinetics of curcumin in animals and human volunteers, Planta Med. 64 (04) (1998) 353–356. [DOI] [PubMed] [Google Scholar]
- [32].Kumar S, Singhal V, Roshan R, Sharma A, Rembhotkar GW, Ghosh B, Piperine inhibits TNF-α induced adhesion of neutrophils to endothelial monolayer through suppression of NF-κB and IκB kinase activation, Eur. J. Pharmacol 575 (1–3) (2007) 177–186. Dec 1. [DOI] [PubMed] [Google Scholar]
- [33].Mujumdar AM, Dhuley JN, Deshmukh VK, Raman PH, Naik SR, Anti-inflammatory activity of piperine, Jpn. J. Med. Sci. Biol 43 (3) (1990) 95–100. [DOI] [PubMed] [Google Scholar]
- [34].Lei D, Yang Y, Liu Z, Yang B, Gong W, Chen S, Wang S, Sun L, Song B, Xuan H, Mo X, 3D printing of biomimetic vasculature for tissue regeneration, Mater. Horiz 6 (6) (2019) 1197–1206. [Google Scholar]
- [35].Fielding G, Bose S, SiO2 and ZnO dopants in three-dimensionally printed tricalcium phosphate bone tissue engineering scaffolds enhance osteogenesis and angiogenesis in vivo, Acta Biomater. 9 (11) (2013) 9137–9148. Nov 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Novosel EC, Kleinhans C, Kluger PJ, Vascularization is the key challenge in tissue engineering, Adv. Drug Deliv. Rev 63 (4–5) (2011) 300–311. Apr 30. [DOI] [PubMed] [Google Scholar]
- [37].Nostro A, Papalia T, Antimicrobial activity of carvacrol: current progress and future prospectives, Recent Pat. anti-Infect. Drug Discov. 7 (1) (2012) 28–35. Apr 1. [DOI] [PubMed] [Google Scholar]
- [38].Ben Arfa A, Combes S, Preziosi-Belloy L, Gontard N, Chalier P, Antimicrobial activity of carvacrol related to its chemical structure, Lett. Appl. Microbiol 43 (2) (2006) 149–154. [DOI] [PubMed] [Google Scholar]
- [39].Heras C, Jiménez-Holguín J, Doadrio AL, Vallet-Regí M, Sánchez-Salcedo S, Salinas AJ, Multifunctional antibiotic-and zinc-containing mesoporous bioactive glass scaffolds to fight bone infection, Acta Biomater. 114 (2020) 395–406. Sep 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Teow SY, Liew K, Ali SA, Khoo AS, Peh SC, Antibacterial action of curcumin against Staphylococcus aureus: a brief review, J. Trop. Med 2016 (2016) (Oct). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sahu Abhishek, et al. , “Encapsulation of curcumin in pluronic block copolymer micelles for drug delivery applications.”, J. Biomater. Appl vol. 25 (6) (2011) 619–639. [DOI] [PubMed] [Google Scholar]
- [42].Subbiah Vivek, “The next Generation of Evidence-Based Medicine.”, Nat. Med. vol 29 (1) (2023) 49–58. [DOI] [PubMed] [Google Scholar]
- [43].Naboneeta Sarkar, Bose Susmita, “Liposome-Encapsulated Curcumin-Loaded 3D Printed Scaffold for Bone Tissue Engineering.”, ACS Appl. Mater. Interfaces vol 11 (19) (2019) 17184–17192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Jo Yongdeok, Sarkar Naboneeta, Bose Susmita, “In Vitro Biological Evaluation of Epigallocatechin Gallate (EGCG) Release from Three-Dimensional Printed (3DP) Calcium Phosphate Bone Scaffolds.”, J. Mater. Chem. B, Dec. (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Bose Susmita, Yongdeok Majumdar Jo, Amit Ujjayan Bandyopadhyay, in: Narayan Roger J. (Ed.), Binder Jet Additive Manufacturing of Biomaterials.” Additive Manufacturing in Biomedical Applications, ASM International, 2022, pp. 77–91. [Google Scholar]
- [46].Ke Dongxu, Bose Susmita, “Effects of Pore Distribution and Chemistry on Physical, Mechanical, and Biological Properties of Tricalcium Phosphate Scaffolds by Binder-Jet 3D Printing.”, Addit. Manuf. vol 22 (2018) 111–117. [Google Scholar]
- [47].Deng Y, Jiang C, Li C, Li T, Peng M, Wang J, Dai K, 3D printed scaffolds of calcium silicate-doped β-TCP synergize with co-cultured endothelial and stromal cells to promote vascularization and bone formation, Sci. Rep 7 (1) (2017) 1–4. Jul 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Liu R, Tang Y, Zeng L, Zhao Y, Ma Z, Sun Z, Xiang L, Ren L, Yang K, In vitro and in vivo studies of anti-bacterial copper-bearing titanium alloy for dental application, Dent. Mater 34 (8) (2018) 1112–1126. Aug 1. [DOI] [PubMed] [Google Scholar]
- [49].Leung Mandy H.M., et al. , “Encapsulation of curcumin in cationic micelles suppresses alkaline hydrolysis.”, Langmuir vol. 24 (11) (2008) 5672–5675. [DOI] [PubMed] [Google Scholar]
- [50].Sahu Abhishek, et al. , Encapsulation of curcumin in pluronic block copolymer micelles for drug delivery applications, J. Biomater. Appl vol. 25 (6) (2011) 619–639, 10.1177/0885328209357110. [DOI] [PubMed] [Google Scholar]
- [51].Aggarwal BB, Kumar A, Bharti AC, Anticancer potential of curcumin: preclinical and clinical studies, Anticancer Res. 23 (1/A) (2003) 363–398. Jan 1. [PubMed] [Google Scholar]
- [52].Singh S, Khar A, Biological effects of curcumin and its role in cancer chemoprevention and therapy, Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. -Anti-Cancer Agents) 6 (3) (2006) 259–270. [DOI] [PubMed] [Google Scholar]
- [53].Rather RA, Bhagat M, Cancer chemoprevention and piperine: molecular mechanisms and therapeutic opportunities, Front. Cell Dev. Biol 6 (2018), 10. Feb 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Chang R, Sun L, Webster TJ, Selective cytotoxicity of curcumin on osteosarcoma cells compared to healthy osteoblasts, Int. J. Nanomed 9 (2014) 461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Hussan F, Ibraheem NG, Kamarudin TA, Shuid AN, Soelaiman IN, Othman F, Curcumin protects against ovariectomy-induced bone changes in rat model, Evid. -Based Complement. Altern. Med 2012 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Kim DY, Kim EJ, Jang WG, Piperine induces osteoblast differentiation through AMPK-dependent Runx2 expression, Biochem. Biophys. Res. Commun 495 (1) (2018) 1497–1502. Jan 1. [DOI] [PubMed] [Google Scholar]
- [57].Vu AA, Robertson SF, Ke D, Bandyopadhyay A, Bose S, Mechanical and biological properties of ZnO, SiO2, and Ag2O doped plasma sprayed hydroxyapatite coating for orthopaedic and dental applications, Acta Biomater. 92 (2019) 325–335. Jul 1. [DOI] [PubMed] [Google Scholar]
- [58].Carpentier G, Berndt S, Ferratge S, Rasband W, Cuendet M, Uzan G, Albanese P, Angiogenesis Analyzer for imageJ—A comparative morphometric analysis of “endothelial tube formation Assay” and “fibrin Bead Assay”, Sci. Rep 10 (1) (2020) 1–3. Jul 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].DeCicco-Skinner KL, Henry GH, Cataisson C, Tabib T, Gwilliam JC, Watson NJ, Bullwinkle EM, Falkenburg L, O’Neill RC, Morin A, Wiest JS, Endothelial cell tube formation assay for the in vitro study of angiogenesis, J. Vis. Exp.: JoVE (91) (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Wang TY, Chen JX, Effects of curcumin on vessel formation insight into the pro- and antiangiogenesis of curcumin, Evid. -Based Complement. Altern. Med 2019 (2019). Jun 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Sharma AV, Ganguly K, Paul S, Maulik N, Swarnakar S, Curcumin heals indomethacin-induced gastric ulceration by stimulation of angiogenesis and restitution of collagen fibers via VEGF and MMP-2 mediated signaling, Antioxid. Redox Signal 16 (4) (2012) 351–362. Feb 15. [DOI] [PubMed] [Google Scholar]
- [62].Ahmed-Farid Omar A.H., et al. , Beneficial Effects of Curcumin Nano-Emulsion on Spermatogenesis and Reproductive Performance in Male Rats under Protein Deficient Diet Model: Enhancement of Sperm Motility, Conservancy of Testicular Tissue Integrity, Cell Energy and Seminal Plasma Amino Acids Content, J. Biomed. Sci. vol 24 (1) (2017), 66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wang Ruxia, et al. , ‘L-Arginine Enhances Protein Synthesis by Phosphorylating mTOR (Thr 2446) in a Nitric Oxide-Dependent Manner in C2C12 Cells’ (PubMed Central), Oxid. Med. Cell. Longev. vol 2018 (2018), 7569127, 10.1155/2018/7569127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Caroline Jordan S, et al. , ‘Nonconformity of biofilm formation in vivo and in vitro based on staphylococcus aureus accessory gene regulator status’, Sci. Rep vol. 12 (1) (2022), 1251 10.1038/s41598-022-05382-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Nandi Samit Kumar, et al. , Silver nanoparticle deposited implants to treat osteomyelitis, J. Biomed. Mater. Res. Part B: Appl. Biomater. vol 106 (3) (2018) 1073–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Guarda A, Rubilar JF, Miltz J, Galotto MJ, The antimicrobial activity of microencapsulated thymol and carvacrol, Int. J. Food Microbiol 146 (2) (2011) 144–150. [DOI] [PubMed] [Google Scholar]
- [67].Magi G, Marini E, Facinelli B, Antimicrobial activity of essential oils and carvacrol, and synergy of carvacrol and erythromycin, against clinical, erythromycin-resistant Group A Streptococci, Front. Microbiol 6 (2015) 165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Cacciatore I, Di Giulio M, Fornasari E, Di Stefano A, Cerasa LS, Marinelli L, Turkez H, Di Campli E, Di Bartolomeo S, Robuffo I, Cellini L, Carvacrol codrugs: a new approach in the antimicrobial plan, PLoS One 10 (4) (2015), e0120937. Apr 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Xiao J, Goley ED, Redefining the roles of the FtsZ-ring in bacterial cytokinesis, Curr. Opin. Microbiol 34 (2016) 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
No data was used for the research described in the article.