Abstract
A limited period of chemotherapy during primary immunodeficiency virus infection might provide a long-term clinical benefit even if treatment is initiated at a time point when virus is already detectable in plasma. To evaluate this strategy, we infected rhesus macaques with the pathogenic simian/human immunodeficiency virus RT-SHIV and treated them with the antiretroviral drug (R)-9-(2-phosphonylmethoxypropyl)adenine (PMPA) for 8 weeks starting 7 or 14 days postinfection. PMPA treatment suppressed viral replication efficiently in all of the monkeys. After chemotherapy ended, virus replication rebounded and viral RNA in plasma reached levels comparable to that of the controls in four of the six monkeys. However, in the other two animals, virus loads peaked only moderately after withdrawal of the drug and then declined to low or even undetectable levels. These low levels of viremia remained stable for at least 31 weeks after cessation of therapy. At this time point, these two monkeys were challenged with SIV8980 to evaluate whether the host responses which were able to keep RT-SHIV replication under control were also sufficient to protect against infection with a highly pathogenic heterologous virus. Both monkeys proved to be protected against the heterologous virus. In one of the two animals, low levels of SIV8980 replication were detected. Thus, by chemotherapy during the acute phase of pathogenic virus replication, we could achieve not only persistent virus load suppression in two out of six monkeys but also protection from subsequent heterologous challenge. By this chemotherapeutic attenuation, the replication kinetics of attenuated viruses could be mimicked and a vaccination effect similar to that induced by live attenuated simian immunodeficiency virus vaccines was achieved.
Antiretroviral chemotherapy so far has concentrated on the treatment of established chronic human immunodeficiency virus (HIV) infection to prevent or delay disease progression. Combination chemotherapy using potent antiretroviral agents has led to significant advances in the clinical management of HIV disease (7). Postexposure prophylactic treatment, when initiated within hours or on the first day, was shown to largely prevent immunodeficiency virus infection in humans (6) and in the simian immunodeficiency virus (SIV) rhesus macaque model (27). Delay of treatment initiation until 2 to 3 days after inoculation with SIV did not prevent infection (25). However, early short-term antiretroviral therapy seemed to induce long-term clinical benefits even if infection was not prevented (29, 30). Emerging clinical evidence also suggests that early chemotherapeutic treatment may modify the balance between the immune system and virus replication in favor of the host by limiting virus infection to the extent that effective immune responses capable of controlling the infection may be developed (B. Walker, personal communication).
Further evidence for the existence of various levels of protection from infection or disease is coming from clinical observations, as well as from animal models. Certain human individuals, such as long-term nonprogressors, seem to develop an immune response capable of keeping the virus under long-lasting control (5, 21). Attenuated virus strains such as SIV defective in the gene nef replicate to substantial titers during the acute phase of infection in rhesus macaques but establish only low virus loads in the chronic state (10, 15). Beside having a reduced potential to cause disease, attenuated viruses have been demonstrated to induce immune responses capable of controling replication of pathogenic virus strains after challenge (reviewed in references 1, 9, and 14) even in the absence of sterilizing immunity (23). Thus, limited and transient infection may even induce protective immunity.
In the present study, we used the rhesus macaque model to evaluate whether a long-lasting beneficial effect of antiretroviral therapy could be obtained when treatment was initiated in the acute phase of primary virus infection, namely, 1 or 2 weeks after inoculation. By initiating treatment shortly prior to peak viremia, we attempted to imitate the growth curves of attenuated immunodeficiency virus vaccines. Therefore, we determined whether this strategy conferred not only control of the primary virus infection but also protection against challenge with a heterologous pathogenic SIV.
MATERIALS AND METHODS
Animals.
Rhesus monkeys (Macaca mulatta) of Indian origin were purpose bred at the Biomedical Primate Research Center. At the beginning of the study, their ages ranged from 2.0 to 2.5 years and their weights were between 2 and 3 kg. The monkeys were in good health; were negative by serology for SIV, simian T-lymphotropic virus type I, and simian retrovirus type D; and had not been used in previous experiments.
Viruses.
The chimeric simian/human immunodeficiency virus (SHIV) containing the HIV-1 HXBc2 gene for reverse transcriptase (RT) in the genomic background of SIVmac239 (RT-SHIV) (28) was used for primary infection, and pathogenic SIV strain 8980 (SIV8980) was used for challenge. SIV8980 is a highly virulent, serially in vivo-passaged strain derived from SIVsmΔB670 which has been used before as the challenge strain in a vaccine study (12). Both virus stocks had been grown in rhesus monkey peripheral blood mononuclear cells (PBMC) and had been titrated in vitro in C8166 cells and in vivo in rhesus monkeys after intravenous injection. The RT-SHIV stock contained 4.0 × 106 tissue culture-infective doses per ml and 3.2 × 105 50% monkey-infective doses (MID50) per ml; the SIV8980 stock contained 3.3 × 106 50% tissue culture-infective doses per ml and 2.5 × 104 MID50/ml.
Test compound.
The test compound (R)-9-(2-phosphonylmethoxypropyl)adenine (PMPA) (3, 4), a potent acyclic nucleoside phosphonate analog RT inhibitor, was provided by GILEAD Sciences Inc. (Foster City, Calif.). The compound was suspended in water to a concentration of 30 mg/ml with 0.1 M NaOH added to obtain a final pH of 7.0 and dissolve it. The solution was then filter sterilized and stored in aliquots at −20°C.
Infection, treatment, and monitoring.
Ten rhesus monkeys were infected intravenously on day 0 with 100 MID50 of the RT-SHIV stock. Six monkeys received PMPA for 57 days, two animals starting on day 7 (8PMPA and 86PMPA) and the other four animals starting on day 14 postinfection (p.i.) (1PMPA, 4PMPA, 25PMPA, and 28PMPA); four animals were mock treated with saline instead, two starting on day 7 (16NaCl and 84NaCl) and two starting on day 14 (19NaCl and 22NaCl) p.i. PMPA or saline was administered subcutaneously without sedation at a dose of 30 mg/kg of body weight per day once daily for 57 days. Four out of the 10 rhesus macaques (1PMPA, 4PMPA, 8PMPA, and 25PMPA) were inoculated 41 weeks after infection with RT-SHIV with 50 MID50 of cell-free, rhesus PBMC-grown SIV8980 by the intravenous route. Two naive monkeys were added as infection controls. For blood sample collection, monkeys were sedated with ketamine. Body weights and temperatures were measured before the start of the experiment and each time the animals were sedated for virus injection or blood sample collection. Behavior and clinical signs (including appetite) were observed twice daily during the whole experiment.
Determination of virus load.
Quantitative RNA PCR was used to estimate the virus load in plasma as described recently (24). In brief, RNA was extracted from plasma using guanidine isothiocyanate-mediated lysis, followed by propanol-2 precipitation. A known amount of internal standard RNA (IS-RNA) was added before the RNA extraction and was copurified to monitor the efficiency of purification. The RNA was reverse transcribed and amplified in a single reaction using recombinant Tth DNA polymerase and biotinylated primers. The IS-RNA was coamplified to monitor the amplification efficiency. The amplified fragments were denatured and hybridized to an immobilized capture probe in a microwell plate. They were detected by an avidin-enzyme conjugate-mediated colorimetric reaction. The amplified IS-RNA was hybridized to a different capture probe in separate microwells. The lower detection limit of this RT-PCR method was 40 RNA equivalents per ml of plasma.
DNA PCR was used to discriminate between RT-SHIV and SIV8980. DNA was extracted from purified PBMC using sodium dodecyl sulfate lysis and proteinase K digestion. For each PCR, 1 μg of sample DNA was used to which a known amount of internal standard was added to monitor the amplification efficiency. The procedure for detection of the amplified fragments was identical to the RNA PCR protocol. Discrimination between RT-SHIV and the challenge virus SIV8980 was achieved by restriction enzyme analysis of the DNA PCR products.
For quantitative virus isolation (QVI), twofold dilutions of purified rhesus PBMC were cocultured for 3 to 4 weeks with the CD4-positive lymphocyte cell line C8166; the cells were then scored for the presence of syncytia.
Measurement of immune responses.
Antibodies specific for SIV gp120 were determined in plasma using an enzyme-linked immunosorbent assay technique. In brief, plates were coated with the viral protein (National Institute of Biological Standards and Controls, Potters Bar, United Kingdom) and after incubation with the plasma to be analyzed, bound antibodies were detected by addition of a rabbit anti-rhesus immunoglobulin G-alkaline phosphatase conjugate, followed by chromogenic substrate.
For the analyses of antigen-specific lymphocyte proliferation, PBMC were stimulated in triplicate with different concentrations of SIV gp120. The negative control was medium alone, and the positive control was concanavalin A (5 μg/ml). The cells were cultured for 90 h; during the last 18 h, they were pulsed with 0.5 μCi of [3H]thymidine per 2 × 105 cells. Subsequently, the cultures were harvested on glass fiber filters and label uptake was determined by counting simultaneously in an open-well Packard Matrix Counter (Direct Beta Counter) with 96 counting tubes. Results were expressed as mean counts ± the standard deviation, and stimulation indices of triplicate determinations were calculated by dividing the mean counts of antigen-containing cultures by the mean counts of cultures without antigen. Stimulation indices higher than 2 were considered positive.
Fluorescence-activated cell sorter analyses.
Phenotyping of cells by flow cytometry was performed with fresh whole blood. The following monoclonal antibodies (Dako) were used for single-, double-, or triple-color staining essentially by following the manufacturer's instructions: A, IgFITC and IgPE (controls); B, CD29FITC, CD4PE, and CD8PerCP; C, CD3FITC, CD4PE, and CD8PerCP. Flow cytometry was performed on a FACsort using the CellQuest software (Becton Dickinson, Etten-Leur, The Netherlands), and 5,000 events in a gate set on mononuclear cells were analyzed per monoclonal antibody mixture.
Euthanasia and necropsy.
Animals were euthanized by pentobarbital overdose at the end of the observation period. If an animal showed clinical symptoms earlier during the course of the experiment, it was euthanized for ethical reasons. Criteria for euthanasia were therapy-resistant chronic or recurrent diarrhea, neurological symptoms, evidence of chronic wasting such as more than 10% loss of body weight together with persistently low CD4 counts or high viral loads, or any other poor condition of the animal as judged by clinical veterinarians. A complete necropsy was performed in which the abdominal and thoracic cavities and the skull were opened and internal organs were examined in situ. Necropsies were performed in a blinded fashion. Simian AIDS was diagnosed post mortem if monkeys had at least two of the following symptoms or pathological alterations: therapy-resistant diarrhea, opportunistic infections, a low percentage of CD4-positive cells (<10%), or a high viral RNA load (>106 copies/ml).
RESULTS
Virus load.
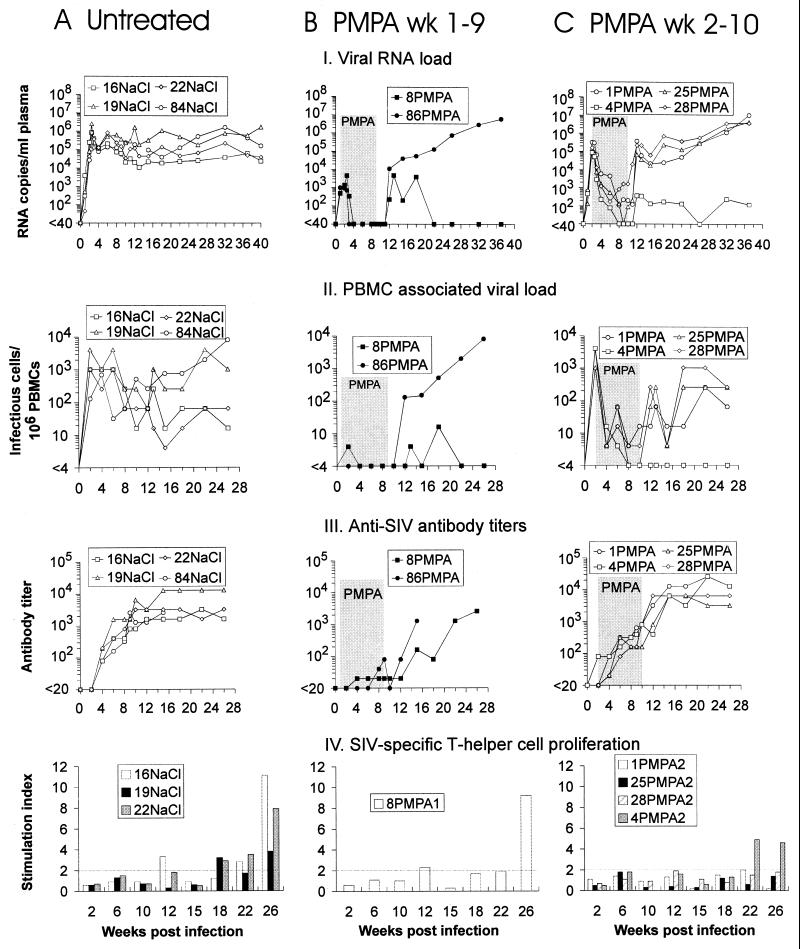
To investigate whether imitating the growth curves of live attenuated immunodeficiency virus vaccines by antiretroviral therapy during primary immunodeficiency virus infection would also lead to protective immune responses, 10 rhesus monkeys were infected with a SIV/HIV-1 hybrid virus (RT-SHIV) in which the RT of SIVmac is replaced with the HIV-1 enzyme (28). RT-SHIV inoculation was shown to induce high viral RNA levels (24) and AIDS-like symptoms and pathology in rhesus monkeys (28). Six RT-SHIV-infected monkeys were treated with the antiretroviral drug PMPA for 8 weeks starting either 1 (Fig. 1B) or 2 (Fig. 1C) weeks p.i.; four monkeys received saline for the same time period (Fig. 1A). Viremia was monitored in plasma by RNA PCR (Fig. 1, part I), and the PBMC-associated viral load was determined by QVI (Fig. 1, part II). Levels of viremia in the control animals reached up to 2 × 106 RNA copies per ml of plasma at day 17 and stayed high (>104 copies/ml) for 40 weeks. In the PMPA-treated animals, viral RNA was already detectable at day 7. Treatment initiation 1 or 2 weeks p.i. led to an approximately 400- or 6.5-fold reduction of mean peak titers, respectively. PMPA treatment suppressed viral RNA levels below the level of detection of 40 RNA copies/ml in four of the six treated monkeys. The other two monkeys (1PMPA and 28PMPA) showed low levels of virus replication while being still treated with the drug.
FIG. 1.
Viral load and antiviral immune responses in RT-SHIV-infected rhesus macaques treated with either saline (A) or PMPA on days 7 to 63 (B) or 14 to 70 (C) p.i. (treatment period shaded). Each monkey is identified by a one- or two-digit number, followed by the name of the substance used for treatment.
After termination of PMPA treatment, virus replication rebounded in all animals and numbers of viral RNA copies in plasma in four monkeys (1PMPA, 25PMPA, 28PMPA, and 86PMPA) reached set point levels (6 months p.i.) comparable to those of the controls. However, in two animals (4PMPA and 8PMPA), virus loads increased only moderately after treatment was ended and became low or even undetectable again shortly thereafter. Set point RNA levels in these monkeys were at least 1,000-fold lower than those in untreated control monkeys. These low levels of RT-SHIV viremia were maintained for 31 weeks after termination of PMPA treatment, the time point at which these monkeys were challenged.
Levels of cell-associated viremia as measured by QVI essentially paralleled those of plasma viremia; the sensitivity of this assay, however, is approximately 3 orders of magnitude lower than that of RNA PCR (Fig. 1, part II). For the two monkeys maintaining low viral RNA levels, no virus could be isolated at most time points after termination of chemotherapy.
Antiviral immune response.
To determine whether the reduction of viral load by antiretroviral therapy during primary infection would influence the humoral immune response, plasma antibody titers against SIV gp120 protein were measured by enzyme-linked immunosorbent assay. All saline-treated monkeys developed high antibody titers that started to become detectable at week 4 and peaked 8 to 10 weeks p.i. (Fig. 1A, part III). In all animals having received PMPA treatment starting 2 weeks p.i., significant antibody responses were detected from week 4 on and continued to increase slightly irrespective of drug treatment (Fig. 1C, part III). After PMPA treatment ended at week 10, the virus load rebound seemed to induce a further increase in SIV-specific antibody titers. Peak levels were reached 12 weeks after infection. Obviously, allowing virus replication for 2 weeks before the start of treatment was sufficient to induce a significant humoral immune response. The two animals having received PMPA treatment from day 7 on showed low (8PMPA) or even undetectable (86PMPA) SIV gp120-specific antibody levels during the first weeks p.i. (Fig. 1B, part III). After treatment ended at week 9 the virus load rebound was followed in these monkeys by an immediate increase in the humoral immune responses, which reached levels comparable to those of the other animals. Overall, antibody titers correlated with viral loads in the sense that high levels of viremia induced high humoral immune responses. However, the quantity of this immune response did not correlate with the capacity to keep the virus load low after chemotherapy ended.
Lymphoproliferative responses to SIV gp120 were not detectable during the period of chemotherapy but developed only relatively late after infection, namely, 12 to 22 weeks after infection (Fig. 1, part IV). Positive responses (stimulation indices, >2) were observed in all tested saline controls (16NaCl, 19NaCl, and 22NaCl). Remarkably, SIV-specific T-helper cell responses in the PMPA-treated group of monkeys (Fig. 1B and C, part IV) were only detectable in those two animals which were able to control virus replication after treatment ended, namely, 4PMPA and 8PMPA. Those PMPA-treated monkeys that established high virus loads after chemotherapy ended did not show a lymphoproliferative response to SIV gp120. However, since the mock-treated control monkeys had also developed SIV-specific T-helper cell responses and, nevertheless, showed high steady-state viremia, the proliferative response observed for animals 4PMPA and 8PMPA was not sufficient to explain suppression of virus replication in these monkeys.
Heterologous challenge with SIV8980.
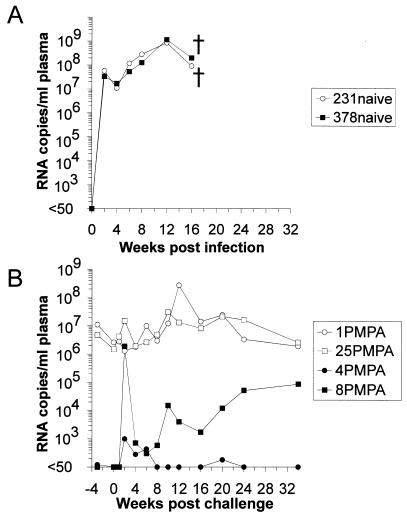
To evaluate whether imitating the growth curves of live attenuated immunodeficiency virus vaccines by antiretroviral therapy was sufficient to confer protection against infection with a heterologous virus, we challenged those two monkeys which had controlled RT-SHIV infection after treatment ended (4PMPA and 8PMPA) with the SIVsmΔB670 derived highly virulent SIV8980 strain. Intravenous inoculation of SIV8980 was performed 41 weeks after the initial infection with RT-SHIV. Two naive rhesus macaques (231naive and 378naive) were inoculated in parallel with SIV8980 as infection controls. Different degress of protection against the heterologous virus were observed in both previously RT-SHIV-infected monkeys. In one of them (8PMPA), the SIV8980 challenge virus was readily detectable (Table 1). In this animal, a transient increase in the number of RNA copies per milliliter of plasma up to >106 was observed upon challenge (Fig. 2B). However, the steady-state level of viremia established in this animal, predominantly due to SIV8980, was more than 104-fold lower than the peak viral loads measured in the two naive control monkeys (Fig. 2A). The other RT-SHIV-infected animal (4PMPA) showed only a minor increase in viral load after challenge, and SIV8980 could not be detected at any time point (Table 1). Monkey 4PMPA, which had been able to suppress RT-SHIV replication so far, was still able to control replication of this virus after challenge and showed undetectable or low numbers of RNA copies per milliliter of plasma until it was euthanized at week 75 p.i.
TABLE 1.
Discrimination of RT-SHIV and SIV8980 after challengea
FIG. 2.
Plasma viral RNA loads in naive or RT-SHIV-infected macaques after inoculation with SIV8980. Monkeys 231naive and 378naive served as challenge controls. Euthanasia of monkeys during the observation period is indicated by a cross.
As additional controls, two PMPA-treated monkeys which had high virus loads (1PMPA and 25PMPA) were inoculated with SIV8980 (Fig. 2B). In both animals, the SIV8980 challenge virus could not be detected (Table 1). However, since the plasma levels of RT-SHIV RNA in these two monkeys had been very high, it is possible that these animals were not protected by immunological mechanisms but that the large excess of ongoing RT-SHIV replication interfered with establishment of SIV8980 infection by competition.
Lymphocyte subsets.
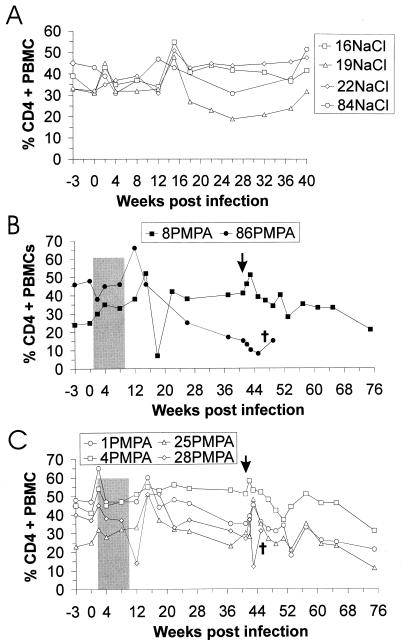
Fluorescence-activated cell sorter analysis of blood cell surface antigens was performed throughout the whole study. Percentages of CD3+ and CD3+ CD8+ lymphocytes on PBMC were essentially stable over the time span of the experiment; no effect of PMPA treatment or virus infection was detectable in any monkey (data not shown). The four RT-SHIV-infected, mock-treated animals showed no significant decrease in CD4+ T cells during the 40-week observation period (Fig. 3A). Two of the six PMPA-treated monkeys (28PMPA and 86PMPA) developed clinical symptoms during the experimental period (see below). Shortly before they were euthanized, the proportions of CD4-positive cells were reduced to 8 and 12% in monkeys 86PMPA (Fig. 3B) and 28PMPA (Fig. 3C), respectively. The percentages of CD4+ lymphocytes in PBMCs of the four challenged animals showed a tendency to decrease continuously during the 75-week observation period (Fig. 3B and C). The decrease in percentages of CD4-positive cells was most pronounced for monkeys 1PMPA and 25PMPA, which contained high steady-state virus loads. A transient increase in percentages of CD4+ T cells was obvious at weeks 15 and 43 in three out of the four challenged animals (1PMPA, 8PMPA, and 25PMPA). These increases were correlated in time and therefore may have been induced by the rebounding virus replication after PMPA treatment ended at week 9 or 10 and by the challenge with SIV8980 at week 41 p.i. For monkey 4PMPA, no detectable increase of CD4-positive cells around week 15 and only a minor rise at week 42 were observed. This animal was the one which had shown only a minor rebound in virus replication after chemotherapy ended and in which the SIV8980 challenge virus had not been detected. In the two naive control monkeys (231naive and 378naive), which rapidly developed simian AIDS after infection with SIV8980 (see below), CD4 counts were not reduced (data not shown). This is not unusual, since progression to AIDS with normal CD4 counts has been repeatedly observed in rapidly progressing SIV-infected macaques (13, 16, 17).
FIG. 3.
Percentage of CD4+ cells in PBMC from RT-SHIV-infected macaques treated with either saline (A) or PMPA (B and C). The time point at which monkeys 1PMPA, 4PMPA, 8PMPA, and 25PMPA were challenged is marked by a vertical arrow. Euthanasia of monkeys during the observation period is indicated by a cross. Treatment periods (shaded) and identification of monkeys are as described in the legend to Fig. 1.
Clinical observations and findings at necropsy.
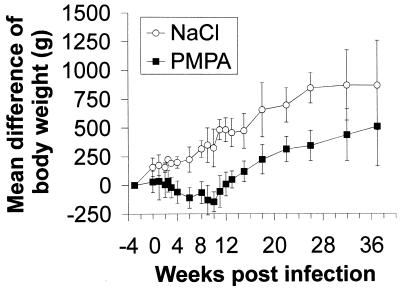
No abnormal behavior was observed at any time point for any monkey. No local reactions were detected during subcutaneous PMPA treatment. It was noticed that all PMPA-treated animals stopped gaining body weight during the period of drug treatment (Fig. 4). After termination of chemotherapy, the animals started to gain body weight again with kinetics similar to those of the saline-treated controls. This side effect of PMPA has been observed before, particularly when higher doses were used (26); the weight gain impairment is, however, clearly reversible after a treatment period of 8 weeks (Fig. 4). Body temperatures were stable between 38 and 40°C over the time period of the study.
FIG. 4.
Body weight differences at different times p.i. with RT-SHIV. The mean and the standard deviation of four mock-treated and six PMPA-treated monkeys are shown.
Two of the six PMPA-treated animals, 86PMPA and 28PMPA, which had high levels of RT-SHIV viremia after chemotherapy ended were euthanized 49 and 45 weeks p.i., respectively, because they showed chronic wasting (Table 2). At necropsy, evidence of an AIDS-like disease was obtained: both monkeys showed enlarged lymph nodes and follicular hyperplasia of the spleen; in 86PMPA, an abscess in the liver and a yellow, soft foreign body in the gall bladder neck were detected, which explains the observed jaundice. Pathomorphological analysis of both animals revealed an opportunistic infection represented by microsporidiosis of the biliary tract associated with hyperplastic inflammation.
TABLE 2.
Clinical symptoms and pathological alterationsa
| Monkey | Time of death (wpi/wpc) | Clinical symptom(s) | Pathology |
|---|---|---|---|
| 86PMPA | 49/NA | Diarrhea, jaundice, loss of body weight | Liver abscess, biliary microsporidiosis, diffuse peritonitis, enteropathy, ELN (++, mesenteric only), FH (+++) |
| 28PMPA | 45/NA | Diarrhea, loss of body weight | Diffuse peritonitis, enteropathy, biliary microsporidiosis, ELN (++), FH (+) |
| 1PMPA | 75/34 | Lymphadenopathy | ELN (++), FH (++) |
| 4PMPA | 75/34 | None | ELN (+), FH (+) |
| 8PMPA | 75/34 | None | ELN (++), FH (++) |
| 25PMPA | 75/34 | Lymphadenopathy, splenomegaly | ELN (++), FH (+++) |
| 231naive | NA/16 | Anemia, loss of body weight | ELN (mesenteric, +++; peripheral, +), biliary and pancreatic cryptosporidiosis, giant cell disease |
| 378naive | NA/14 | Anemia, diarrhea, loss of body weight | ELN (+), biliary cryptosporidiosis |
Two of the other four PMPA-treated and then challenged animals, 1PMPA and 25PMPA, showed lymphadenopathy toward the end of the observation period, and monkey 25PMPA, in addition, showed significant splenomegaly, which suggested progression to disease in these two highly viremic monkeys. At necropsy, monkey 1PMPA showed moderate lymph node enlargement and follicular hyperplasia of the spleen. Severe hyperplasia of the spleen and lymph node enlargement were found in 25PMPA as well (Table 2).
In the two animals with stable low virus loads, no clinical abnormalities were observed. However, at autopsy, monkey 8PMPA also showed evidence of immunodeficiency virus infection, i.e., moderate to severe follicular hyperplasia of the spleen and lymph node enlargement (Table 2); this animal was the RT-SHIV-infected one which could be superinfected with SIV8980. Remarkably, animal 4PMPA, which had a low level of RT-SHIV viremia and in which the SIV8980 challenge virus could not been detected, showed only minor abnormalities of the lymphatic tissues.
The two naive challenge control monkeys (378naive and 231naive) had to be euthanized 14 and 16 weeks, respectively, after infection with SIV8980. Clinically, severe loss of body weight and anemia and, in addition, diarrhea in animal 378naive were observed (Table 2). Findings at autopsy confirmed the clinical evidence of simian AIDS: histology revealed an opportunistic infection (cryptosporidiosis) of the gall bladder and/or pancreas in both animals and primary SIV-associated giant cell disease in the brain, lungs, and intestinal tract of in monkey 231naive. Both animals also showed enlargement of the lymph nodes.
DISCUSSION
Attempts to mimic the replication kinetics of live attenuated immunodeficiency viruses by short-term antiretroviral therapy of primary immunodeficiency virus infection led to persistent suppression of the viral load and to protection against subsequent challenge in two out of six RT-SHIV-infected macaques. A comparison of the results obtained for the monkeys maintaining low levels of viremia after cessation of treatment with those developing high viral loads revealed a similarity to effective versus noneffective live attenuated immunodeficiency virus vaccines. The degree of protection of live attenuated immunodeficiency virus vaccines seems to correlate with the amount of replication of the vaccine virus in the host (20, 32). Similarly, monkey 86PMPA, in which virus replication was most efficiently suppressed by PMPA treatment, developed high viral loads after cessation of treatment. This suggests that protective immune responses were not induced and is reminiscent of highly attenuated immunodeficiency viruses, which do not induce protection either (9). A slightly higher level of virus replication, as observed in monkey 8PMPA, led to establishment of reduced viral loads after cessation of therapy and after challenge. This monkey was protected from rapid progression to AIDS in the absence of sterilizing immunity. In monkey 4PMPA, the peak viral load during the acute phase of infection was similar to that induced by effective live attenuated SIV vaccines, and superinfection was not detectable after challenge. This suggests that the level of virus replication before drug-mediated suppression is crucial for the long-term outcome of infection and that a certain minimum level is needed to induce sufficient host immune responses. It is apparent that a balance must be reached, since higher levels of initial virus replication may proove to be immunosuppressive. The molecular cause of the attenuation of the vaccine virus does not seem to be important for protection, since chemotherapeutic attenuation of primary infection induced protection similar to that induced by genetically attenuated immunodeficiency virus vaccines.
Evidence for a beneficial effect of short-term postexposure prophylactic treatment on the course of immunodeficiency virus infection has been observed previously even if treatment did not prevent infection. In SIVmne-infected juvenile monkeys, PMPA treatment initiation 2 or 3 days after infection led to sustained viral load suppression in two out of four monkeys in each group (25). However, one out of four untreated control monkeys showed a similar attenuated course of infection. Treatment initiation 5 days after infection of newborn monkeys reduced viral loads and delayed disease progression (29). Similar to our study and in contrast to the study by Tsai et al. (25), virus replication was detectable prior to treatment initiation. Since vigorous virus replication during the first weeks after infection leads to rapid dissemination of the virus and might already block the development of protective antiviral immune responses, it was questionable whether a further delay in treatment initiation would also lead to long-term beneficial effects.
We initiated PMPA treatment 1 or 2 weeks p.i. At that time, viral RNA levels were between 5 × 102 and 3 × 105 copies/ml. In two of the six RT-SHIV-infected monkeys, viral RNA set point levels after termination of therapy were below 200 copies/ml. Since viral RNA levels in SIV-infected macaques were shown to be predictive of disease progression (24, 31), our findings suggest that short-term antiretroviral therapy during primary immunodeficiency virus infection can lead to long-term benefits, even if therapy is initiated after immunodeficiency virus infection can be diagnosed. Persistent viral load suppression after cessation of therapy suggests that the treatment can alter the balance between the immune system and virus replication in favor of the host. Further support for the hypothesis that attenuation of primary immunodeficiency virus infection by antiretroviral therapy could facilitate the development of protective immune responses comes from the challenge experiment. The two monkeys maintaining low viral loads after cessation of therapy were also protected from subsequent challenge with a highly pathogenic heterologous virus. The precise nature of the host responses controlling virus replication and mediating protection from infection and disease remains to be determined.
In our experiment, only intermediate levels of virus replication prior to initiation of therapy seemed to induce long-term clinical benefits. Lack of persistent viral load suppression after cessation of highly active antiretroviral therapy (HAART) in a small number of patients with primary HIV-1 infection (8, 22) suggests that at the time of diagnosis it was too late to achieve long-lasting beneficial effects. However, another patient treated early during primary HIV infection maintained a low viral load after cessation of therapy (19). Since primary HIV-1 infection is often asymptomatic and therefore not diagnosed, short-term antiretroviral therapy during primary infection might only be possible in a small number of patients. Mimicking of the replication kinetics of live attenuated vaccines might also be beneficial in a more widely applicable clinical situation if the immune system can indeed recover under HAART (2, 11, 18). In patients successfully treated with HAART for an extended period of time, the viral load usually rebounds to high levels after cessation of therapy. One could speculate that short-term omission of HAART in these patients until moderate viral loads have developed, followed by immediate reinitiation of HAART, might also potentiate protective immune responses capable of controlling virus replication if treatment is later discontinued. Mimicking of the replication kinetics of live attenuated immunodeficiency virus vaccines by antiretroviral therapy is an alternative strategy to long-term HAART which warrants further consideration.
ACKNOWLEDGMENTS
We thank E. De Clercq for helpful advice.
This project was supported by grants from the German Research Foundation (Ue45/1-1 and Ue45/3-1) and by the EU Centralized Facility Program for HIV-1 vaccine development (grants BM H4-CT95-0206 and BMH4-CT97-2067).
REFERENCES
- 1.Almond N M, Heeney J L. AIDS vaccine development in primate models. AIDS. 1998;12(Suppl. A):S133–S140. [PubMed] [Google Scholar]
- 2.Autran B, Carcelain G, Li T S, Blanc C, Mathez D, Tubiana R, Katlama C, Debre P, Leibowitch J. Positive effects of combined antiretroviral therapy on CD4+ T cell homeostasis and function in advanced HIV disease. Science. 1997;277:112–116. doi: 10.1126/science.277.5322.112. [DOI] [PubMed] [Google Scholar]
- 3.Balzarini J, Aquaro S, Perno C F, Witvrouw M, Holy A, De Clercq E. Activity of the (R)-enantiomers of 9-(2-phosphonylmethoxypropyl)-adenine and 9-(2-phosphonylmethoxypropyl)-2,6-diaminopurine against human immunodeficiency virus in different human cell systems. Biochem Biophys Res Commun. 1996;219:337–341. doi: 10.1006/bbrc.1996.0234. [DOI] [PubMed] [Google Scholar]
- 4.Balzarini J, Holy A, Jindrich J, Naesens L, Snoeck R, Schols D, De Clercq E. Differential antiherpesvirus and antiretrovirus effects of the (S) and (R) enantiomers of acyclic nucleoside phosphonates: potent and selective in vitro and in vivo antiretrovirus activities of (R)-9-(2-phosphonomethoxypropyl)-2,6-diaminopurine. Antimicrob Agents Chemother. 1993;37:332–338. doi: 10.1128/aac.37.2.332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cao Y, Qin L, Zhang L, Safrit J, Ho D D. Virologic and immunologic characterization of long-term survivors of human immunodeficiency virus type 1 infection. N Engl J Med. 1995;332:201–208. doi: 10.1056/NEJM199501263320401. [DOI] [PubMed] [Google Scholar]
- 6.Cardo D M, Culver D H, Ciesielski C A, Srivastava P U, Marcus R, Abiteboul D, Heptonstall J, Ippolito G, Lot F, McKibben P S, Bell D M. A case-control study of HIV seroconversion in health care workers after percutaneous exposure. Centers for Disease Control and Prevention Needlestick Surveillance Group. N Engl J Med. 1997;337:1485–1490. doi: 10.1056/NEJM199711203372101. [DOI] [PubMed] [Google Scholar]
- 7.Carpenter C C, Fischl M A, Hammer S M, Hirsch M S, Jacobsen D M, Katzenstein D A, Montaner J S, Richman D D, Saag M S, Schooley R T, Thompson M A, Vella S, Yeni P G, Volberding P A. Antiretroviral therapy for HIV infection in 1998: updated recommendations of the International AIDS Society-USA Panel. JAMA. 1998;280:78–86. doi: 10.1001/jama.280.1.78. [DOI] [PubMed] [Google Scholar]
- 8.Daar E S, Bai J, Hausner M A, Majchrowicz M, Tamaddon M, Giorgi J V. Acute HIV syndrome after discontinuation of antiretroviral therapy in a patient treated before seroconversion. Ann Intern Med. 1998;128:827–829. doi: 10.7326/0003-4819-128-10-199805150-00005. [DOI] [PubMed] [Google Scholar]
- 9.Desrosiers R C. Live attenuated vaccines: best hope to prevent HIV? HIV Adv Res Ther. 1996;3:3–9. [Google Scholar]
- 10.Desrosiers R C, Lifson J D, Gibbs J S, Czajak S C, Howe A Y, Arthur L O, Johnson R P. Identification of highly attenuated mutants of simian immunodeficiency virus. J Virol. 1998;72:1431–1437. doi: 10.1128/jvi.72.2.1431-1437.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Douek D C, McFarland R D, Keiser P H, Gage E A, Massey J M, Haynes B F, Polis M A, Haase A T, Feinberg M B, Sullivan J L, Jamieson B D, Zack J A, Picker L J, Koup R A. Changes in thymic function with age and during the treatment of HIV infection. Nature. 1998;396:690–695. doi: 10.1038/25374. [DOI] [PubMed] [Google Scholar]
- 12.Heeney J L, Holterman L, ten Haaft P, Dubbes R, Koornstra W, Teeuwsen V, Bourquin P, Norley S, Niphuis H. Vaccine protection and reduced virus load from heterologous macaque-propagated SIV challenge. AIDS Res Hum Retroviruses. 1994;10(Suppl. 2):S117–S121. [PubMed] [Google Scholar]
- 13.Hoch J, Lang S M, Weeger M, Stahl-Hennig C, Coulibaly C, Dittmer U, Hunsmann G, Fuchs D, Müller J, Sopper S, Fleckenstein B, Überla K T. vpr deletion mutant of simian immunodeficiency virus induces AIDS in rhesus monkeys. J Virol. 1995;69:4807–4813. doi: 10.1128/jvi.69.8.4807-4813.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson R P. Live attenuated AIDS vaccines: hazards and hopes. Nat Med. 1999;5:154–155. doi: 10.1038/5515. [DOI] [PubMed] [Google Scholar]
- 15.Kestler H W, Ringler D J, Mori K, Panicali D L, Sehgal P K, Daniel M D, Desrosiers R C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991;65:651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- 16.Lackner A A. Pathology of simian immunodeficiency virus induced disease. Curr Top Microbiol Immunol. 1994;188:35–64. doi: 10.1007/978-3-642-78536-8_3. [DOI] [PubMed] [Google Scholar]
- 17.Lewis M G, Bellah S, McKinnon K, Yalley-Ogunro J, Zack P M, Elkins W R, Desrosiers R C, Eddy G A. Titration and characterization of two rhesus derived SIVmac challenge stocks. AIDS Res Hum Retroviruses. 1994;10:213–220. doi: 10.1089/aid.1994.10.213. [DOI] [PubMed] [Google Scholar]
- 18.Li T S, Tubiana R, Katlama C, Calvez V, Ait M H, Autran B. Long-lasting recovery in CD4 T-cell function and viral-load reduction after highly active antiretroviral therapy in advanced HIV-1 disease. Lancet. 1998;351:1682–1686. doi: 10.1016/s0140-6736(97)10291-4. [DOI] [PubMed] [Google Scholar]
- 19.Lisziewicz J, Rosenberg E, Lieberman J, Jessen H, Lopalco L, Siliciano R, Walker B, Lori F. HIV control following discontinuation of antiretroviral therapy. N Engl J Med. 1999;340:1683–1684. doi: 10.1056/NEJM199905273402114. [DOI] [PubMed] [Google Scholar]
- 20.Lohman B L, McChesney M B, Miller C J, McGowan E, Joye S M, Van Rompay K K, Reay E, Antipa L, Pedersen N C, Marthas M L. A partially attenuated simian immunodeficiency virus induces host immunity that correlates with resistance to pathogenic virus challenge. J Virol. 1994;68:7021–7029. doi: 10.1128/jvi.68.11.7021-7029.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pantaleo G, Menzo S, Vaccarezza M, Graziosi C, Cohen O J, Demarest J F, Montefiori D, Orenstein J M, Fox C, Schrager L K. Studies in subjects with long-term nonprogressive human immunodeficiency virus infection. N Engl J Med. 1995;332:209–216. doi: 10.1056/NEJM199501263320402. [DOI] [PubMed] [Google Scholar]
- 22.Perrin L, Rakik A, Yerly S, Baumberger C, Kinloch-de L S, Pechere M, Hirschel B. Combined therapy with zidovudine and L-697,661 in primary HIV infection. AIDS. 1996;10:1233–1237. doi: 10.1097/00002030-199609000-00009. [DOI] [PubMed] [Google Scholar]
- 23.Shibata R, Siemon C, Czajak S C, Desrosiers R C, Martin M A. Live, attenuated simian immunodeficiency virus vaccines elicit potent resistance against a challenge with a human immunodeficiency virus type 1 chimeric virus. J Virol. 1997;71:8141–8148. doi: 10.1128/jvi.71.11.8141-8148.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.ten Haaft P, Verstrepen B, Überla K, Rosenwirth B, Heeney J. A pathogenic threshold of virus load defined in simian immunodeficiency virus- or simian-human immunodeficiency virus-infected macaques. J Virol. 1998;72:10281–10285. doi: 10.1128/jvi.72.12.10281-10285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tsai C-C, Emau P, Follis K E, Beck T W, Benveniste R E, Bischofberger N, Lifson J D, Morton W R. Effectiveness of postinoculation (R)-9-(2-phosphonylmethoxypropyl)adenine treatment for prevention of persistent simian immunodeficiency virus SIVmne infection depends critically on timing of initiation and duration of treatment. J Virol. 1998;72:4265–4273. doi: 10.1128/jvi.72.5.4265-4273.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tsai C C, Follis K E, Beck T W, Sabo A, Bischofberger N, Dailey P J. Effects of (R)-9-(2-phosphonylmethoxypropyl)adenine monotherapy on chronic SIV infection in macaques. AIDS Res Hum Retroviruses. 1997;13:707–712. doi: 10.1089/aid.1997.13.707. [DOI] [PubMed] [Google Scholar]
- 27.Tsai C C, Follis K E, Sabo A, Beck T W, Grant R F, Bischofberger N, Benveniste R E, Black R. Prevention of SIV infection in macaques by (R)-9-(2-phosphonylmethoxypropyl)adenine. Science. 1995;270:1197–1199. doi: 10.1126/science.270.5239.1197. [DOI] [PubMed] [Google Scholar]
- 28.Überla K, Stahl Hennig C, Böttiger D, Mätz-Rensing K, Kaup F J, Li J, Haseltine W A, Fleckenstein B, Hunsmann G, Öberg B, Sodroski J. Animal model for the therapy of acquired immunodeficiency syndrome with reverse transcriptase inhibitors. Proc Natl Acad Sci USA. 1995;92:8210–8214. doi: 10.1073/pnas.92.18.8210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Van Rompay K K A, Dailey P J, Tarara R P, Canfield D R, Aguirre N L, Cherrington J M, Lamy P D, Bischofberger N, Pedersen N C, Marthas M L. Early short-term 9-[2-(R)-(phosphonomethoxy)propyl]adenine treatment favorably alters the subsequent disease course in simian immunodeficiency virus-infected newborn Rhesus macaques. J Virol. 1999;73:2947–2955. doi: 10.1128/jvi.73.4.2947-2955.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Watson A, McClure J, Ranchalis J, Scheibel M, Schmidt A, Kennedy B, Morton W R, Haigwood N L, Hu S L. Early postinfection antiviral treatment reduces viral load and prevents CD4+ cell decline in HIV type 2-infected macaques. AIDS Res Hum Retroviruses. 1997;13:1375–1381. doi: 10.1089/aid.1997.13.1375. [DOI] [PubMed] [Google Scholar]
- 31.Watson A, Ranchalis J, Travis B, McClure J, Sutton W, Johnson P R, Hu S L, Haigwood N L. Plasma viremia in macaques infected with simian immunodeficiency virus: plasma viral load early in infection predicts survival. J Virol. 1997;71:284–290. doi: 10.1128/jvi.71.1.284-290.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wyand M S, Manson K H, Garcia-Moll M, Montefiori D, Desrosiers R C. Vaccine protection by a triple deletion mutant of simian immunodeficiency virus. J Virol. 1996;70:3724–3733. doi: 10.1128/jvi.70.6.3724-3733.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]