Abstract
Respiratory syncytial virus (RSV) is the leading cause of acute lower respiratory tract infections in neonates, infants, and children worldwide. The virus is estimated to infect 97% of this population in the United States by the age of 2 years, leading to hospitalization for severe lower respiratory tract disease in 2–3% of infants younger than age 6 months. Two preventive options, prenatal administration of a maternal vaccine and administration of a long-acting monoclonal antibody to the infant, are now available for the prevention of RSV-associated lower respiratory tract infection in infants in the United States. The U.S. Food and Drug Administration (FDA) has approved and the Centers for Disease Control and Prevention (CDC) has recommended a new maternal vaccination, RSVPreF, to be administered between 32 0/7 and 36 6/7 weeks of gestation to reduce the risk of RSV-associated lower respiratory tract infection in infants in the first 6 months of life. The monoclonal antibody nirsevimab was approved by the FDA and recommended by the CDC for prevention of RSV-associated lower respiratory tract infection in infants younger than age 8 months who are born during or entering their first RSV season and for infants and children aged 8–19 months who are at high risk for RSV-associated lower respiratory tract infection and entering their second RSV season. Either maternal vaccination during pregnancy or monoclonal antibody administration to the infant is recommended to prevent RSV-associated lower respiratory tract infection among infants, but both are not needed for most infants. Given that the availability of these products may vary as these recommendations are implemented, it is important that obstetricians and other prenatal practitioners have the information they need to counsel their pregnant patients about both options. We review the safety and efficacy of these products, current recommendations for their use, and relative advantages and disadvantages of both newly approved options for the prevention of RSV-associated lower respiratory tract infection in infants to assist obstetricians and other prenatal practitioners in their counseling of pregnant patients.
The Centers for Disease Control and Prevention (CDC) recently released recommendations for two new products for the prevention of respiratory syncytial virus (RSV)–associated lower respiratory tract infection in infants, including an RSV vaccine, Abrysvo (RSVPreF), to be given from 32 0/7 to 36 6/7 weeks of gestation in pregnancy and a long-acting monoclonal antibody, Beyfortus (nirsevimab), to be given to infants after birth during the delivery hospitalization or in the outpatient setting.1–3 The CDC recommends that U.S. clinicians who care for pregnant individuals discuss the relative advantages and disadvantages of both RSVPreF and nirsevimab when counseling pregnant patients.4 Here, we provide an overview of RSV disease, the two U.S. options available for prevention of RSV-associated lower respiratory tract infection in infants, and their safety, efficacy, advantages and disadvantages.
RESPIRATORY SYNCYTIAL VIRUS INFECTION
Respiratory syncytial virus is an enveloped, non-segmented, negative-strand RNA virus from the Pneumoviridae family.5,6 The virus infects nearly all children by their second year of life and is associated with significant morbidity and mortality for those at high risk for severe disease.7 In the United States, RSV infection is the leading cause of infant hospitalization, with 2–3% of infants younger than age 6 months requiring hospitalization for RSV infection annually.6,8–10 Each year, RSV infection is associated with 58,000–80,000 hospitalizations and 100–300 deaths among U.S. children younger than age 5 years.8,10–13 Risk factors for severe disease include young age, preterm birth (especially before 29 weeks of gestation), congenital heart disease, chronic lung disease of prematurity, immunodeficiency, and neurologic and neuromuscular conditions.6 However, most infants and young children hospitalized for RSV infection have no underlying medical conditions; in one study, 79% of children younger than age 2 years hospitalized with RSV infection had no documented underlying conditions.14
Infection with RSV frequently results in mild upper respiratory infection, but 20–30% of children develop lower respiratory tract infection, including bronchiolitis and pneumonia, with the first infection.15 Treatment options for RSV infection are limited to supportive therapies.6 Infants and children who require hospitalization may need a combination of intravenous rehydration, supplemental oxygen, noninvasive respiratory support, intensive care, or invasive mechanical ventilation.16
A large burden of RSV infection is seen in the outpatient setting. One prospective, multicenter study of 5,067 children younger than age 5 years during the 2000–2004 RSV seasons found that RSV infection was associated with 1 in 13 primary care visits and 1 in 38 visits to the emergency department.8 Infants with RSV-associated lower respiratory tract infection are more likely to develop asthma later in life, although it is unknown whether this association is causal or whether infants who will later develop asthma are prone to having lower respiratory tract infection symptoms, including wheezing, when infected with RSV.6
Although infants and young children are the focus of the new preventive options in this article, other populations can also be affected by RSV infection and experience complications. Older adults and adults with certain underlying medical conditions are at increased risk for hospitalization and death with RSV infection.17,18 Limited information is available on the effects of RSV infection during pregnancy; however, evidence suggests that the burden of severe RSV disease in pregnancy is low and pregnancy is not known to be a risk factor for severe RSV disease.19–22
Respiratory syncytial virus is transmitted through close contact with an infected individual through respiratory droplets (typically less than 6 feet of distance) or by self-inoculation associated with fomites or contaminated surfaces.6,15,16 Infected individuals may spread the virus to close contacts with transmission minimized by proper hand hygiene and cleaning of commonly touched surfaces.6,16
Respiratory syncytial virus infections occur during annual epidemics in the United States, with the season usually beginning in fall, peaking in winter, and ending in spring; RSV epidemics follow a typical geographic pattern, beginning in the southeast and moving north and west in the continental United States.23 This pattern was altered during the coronavirus disease 2019 (COVID-19) pandemic because of nonpharmaceutical interventions (eg, masking, reduced social gathering), with low levels of circulation during 2020–2021 and an earlier and longer season during 2021–2022; however, data from the 2022–2023 season (Fig. 1) and early data from the 2023–2024 season suggest that seasonal patterns are returning to those seen in prepandemic years.4,23
Fig. 1.
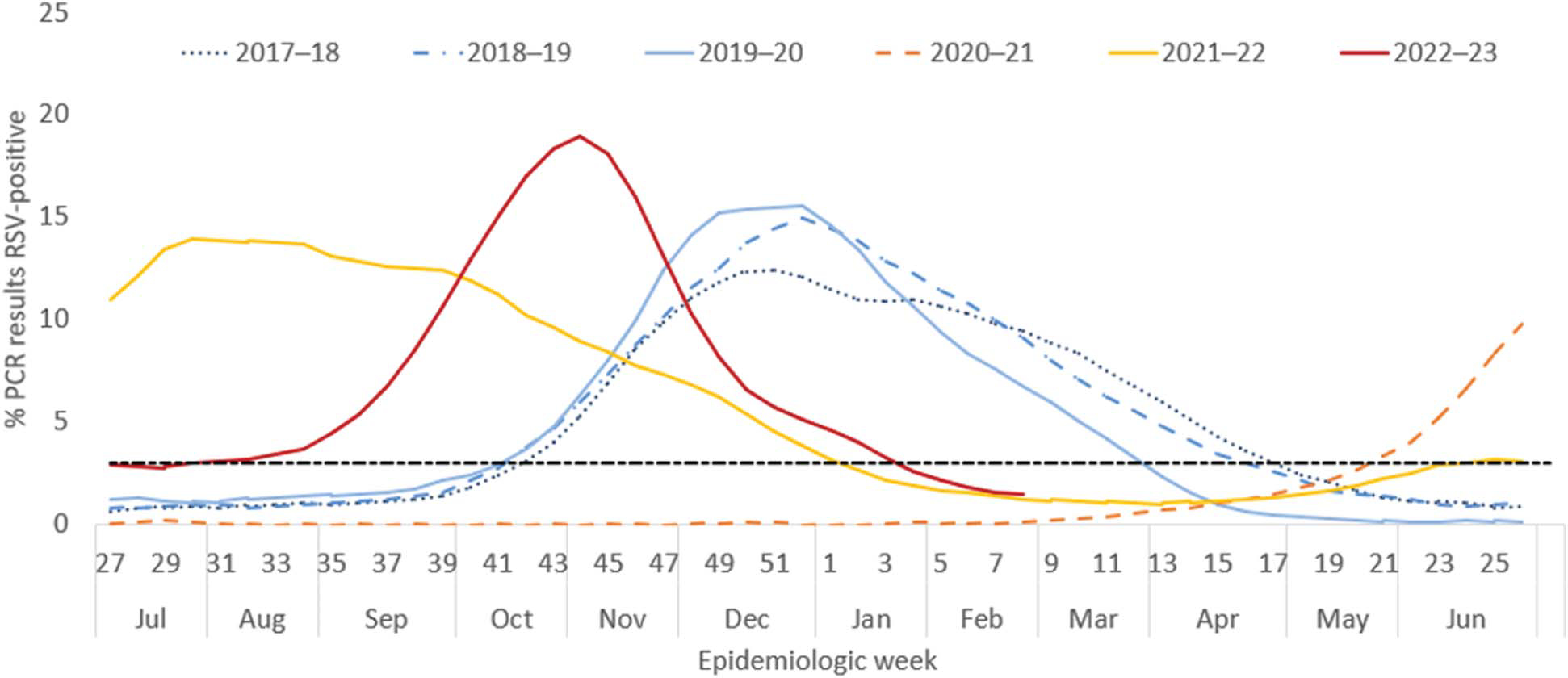
Percentage* of polymerase chain reaction (PCR) test results positive for respiratory syncytial virus (RSV), by epidemiologic week, National Respiratory and Enteric Virus Surveillance System, United States, July 2017–February 2023. The black dotted line represents the threshold for a seasonal epidemic (3% RSV-positive laboratory PCR results). *Three-week centered moving averages of percentage of RSV-positive PCR results nationwide. Modified from Hamid S, Winn A, Parikh R, Jones JM, McMorrow M, Prill MM, et al. Seasonality of respiratory syncytial virus—United States, 2017–2023. MMWR Morb Mortal Wkly Rep 2023;72: 355–61. doi: 10.15585/mmwr.mm7214a1
Debessai. Respiratory Syncytial Virus Prevention for Obstetricians. Obstet Gynecol 2024.
SAFETY AND EFFICACY OF MATERNAL RSVPreF VACCINE
In August 2023, the U.S. Food and Drug Administration (FDA) approved RSV-PreF, the first and only vaccine for use in pregnant individuals from 32 0/7 to 36 6/7 weeks of gestation for the prevention of RSV-associated lower respiratory tract infection in infants up to age 6 months.1 The vaccine contains RSV recombinant stabilized prefusion F proteins (a protein on the surface of RSV that facilitates entry into host respiratory epithelial cells), producing a strong neutralizing antibody response in vaccine recipients.24,25 Other recombinant protein vaccines include certain influenza and hepatitis B vaccines.26 Previous studies have shown that effective transplacental transfer of maternal antibodies occurs with administration of other maternal vaccines during pregnancy (eg, anti–severe acute respiratory syndrome coronavirus 2 [SARS-CoV-2] antibodies after COVID-19 vaccination).27–31 It is believed that at least 14 days are needed between maternal RSV vaccination and delivery to confer infant protection.32
In a randomized controlled clinical trial that included more than 7,000 pregnant people, including 3,682 who received the vaccine at 24–36 weeks of gestation and 3,676 who received a placebo, vaccine efficacy for severe medically attended RSV-associated lower respiratory tract infection in the infant was reported at 81.1% (99.5% CI, 40.6–96.3%) from 0 to 90 days of life, with six affected infants in the vaccine group and 33 in the placebo group, and 69.4% (99.5% CI, 44.3–84.1%) from 0 to 180 days of life (Table 1), with 19 affected infants in the vaccine group and 62 in the placebo group.32 Severe medically attended RSV-associated lower respiratory tract infection was defined as that occurring in infants found to be positive for RSV infection, with very fast breathing (respiratory rate 70 breaths/minute or higher in those younger than 2 months, 60 breaths/minute or higher in infants aged 2–12 months, 50 breaths/minute or higher in children aged 12–24 months), oxygen saturation below 93%, use of high-flow nasal cannula or mechanical ventilation, admission to intensive care unit for 4 or more hours, or lack of response or unconsciousness.32 Vaccine efficacy against RSV-associated hospitalization was 67.7% (99.17% CI, 15.9–89.5%) at 0–90 days of life and 56.8% (99.17% CI, 10.1–80.7%) at 0–180 days of life.32
Table 1.
Respiratory Syncytial Virus Vaccine (RSVPreF) Efficacy
| Trial Dosing Interval (24–36 wk of Gestation) VE [% (CI)] |
Approved Dosing Interval (32–36 wk of Gestation) VE* [% (CI)] |
|||
|---|---|---|---|---|
| Outcome | 0–90 d After Birth | 0–180 d After Birth | 0–90 d After Birth | 0–180 d After Birth |
|
| ||||
| Severe medically attended RSV- associated LRTI in infants† | 81.1 (40.6–96.3)‡ | 69.4 (44.3–84.1)§ | 91.1 (38.8–99.8) | 76.5 (41.3–92.1) |
| Hospitalization for RSV-associated LRTI∥ | 67.7 (15.9–89.5)¶ | 56.8 (10.1–80.7)¶ | Not reported | 48.2 (−22.9 to 79.6) |
| Medically attended RSV-associated LRTI# | 57.1 (14.7–79.8)‡ | 51.3 (29.4–66.8)§ | 34.7 (−34.6 to 69.3) | 57.3 (29.8–74.7) |
VE, vaccine efficacy; RSV, respiratory syncytial virus; LRTI, lower respiratory tract infection.
The VE CI is 95%.
A medically attended visit for a respiratory tract infection (defined as at least one of the following: nasal discharge for 24 hours or more; difficulty breathing, labored breathing, or rapid breathing; cough; inability to feed because of respiratory symptoms; apnea; other respiratory symptom of concern) and an RSV-positive test result for an infant with one of the following: increased respiratory rate (70 breaths/minute or higher in those younger than 2 months old, 60 breaths/minute or higher in infants 2–12 months old, 50 breaths/minute or higher in children 12–24 months old), oxygen saturation less than 93%, use of high-flow nasal cannula or mechanical ventilation, admission to intensive care unit for 4 or more hours, or lack of response or unconsciousness.
The CI is 99.5%.
The VE CI is 97.58%.
A respiratory tract infection (as defined above) attributable to RSV that results in hospitalization.
The VE CI is 99.17%.
A medically attended visit for a respiratory tract infection (as defined above) and an RSV-positive test result for an infant with one of the following: increased respiratory rate (60 breaths/minute or higher in those younger than 2 months old, 50 breaths/minute or higher in infants 2–12 months old, 40 breaths/minute or higher in children 12–24 months old), oxygen saturation less than 95%, or chest wall indrawing.
During the clinical trial with doses given from 24 0/7 to 36 0/7 weeks of gestation (the trial gestational age window), an imbalance in preterm births was noted, with a rate of 5.7% (95% CI, 4.9–6.5%) among vaccine recipients compared with 4.7% (95% CI, 4.1–5.5%) among those who received the placebo; however, this finding was not statistically significant, and no causal relationship has been identified. The incidence of preeclampsia was 1.8% (95% CI, 1.4–2.3%) in the vaccine group and 1.4% (95% CI, 1.1–1.9%) in the placebo group; this difference was also not statistically significant.1,32 In the trial population (24–36 weeks of gestation), more than half of preterm births occurred more than 30 days after maternal vaccination (121 preterm births [60%] in the vaccine group and 98 preterm births [58%] in the placebo group).33 Of the preterm births, most occurred at 33 or more weeks of gestation (194 [97%] in the vaccine group and 161 [95%] in the placebo group).33 Adverse effects of the vaccine were minimal, with most commonly reported local and systemic adverse reactions in pregnant individuals (10% or higher) being injection site pain (40.6%), headache (31.0%), muscle pain (26.5%), and nausea (20.0%).34,35 The FDA is requiring monitoring and postmarketing studies to assess the signals of preterm birth and hypertensive disorders of pregnancy. Practitioners can report adverse events to the CDC’s Vaccine Adverse Event Reporting System (https://www.cdc.gov/vaccinesafety/ensuringsafety/monitoring/vaers/index.html).1 In addition, V-safe is a CDC program that allows vaccinated individuals to share their experiences after vaccination; clinicians are encouraged to communicate or share printed materials with vaccinated individuals about the opportunity to provide health information to V-safe.
A subgroup analysis showed that vaccination at 32–36 weeks of gestation (the FDA-approved gestational age window) was also efficacious, with a vaccine efficacy of 76.5% (95% CI, 41.3–92.1%) in severe medically attended RSV-associated severe lower respiratory tract infection in infants during 0–180 days after birth.1,32,33 In this subanalysis limited to doses given during the approved gestational age window, preterm births occurred among 4.2% (95% CI, 3.3–5.3%) of vaccine recipients compared with 3.7% (95% CI, 2.8–4.7%) of those who received the placebo. Again, the imbalance in preterm birth was not a statistically significant difference.32,33 Most preterm births in the subanalysis group were late preterm, most frequently at 36 weeks of gestation (49 preterm births [72%] in the vaccine group and 35 preterm births [59%] in the placebo group).33
The vaccine was approved for use in pregnant individuals at 32 0/7–36 6/7 weeks of gestation to avoid any potential risk of preterm birth before 32 weeks of gestation and to reduce the potential risk for any preterm birth, given the preterm imbalance observed in the clinical trial. To ensure protection of infants during the RSV season, maternal vaccination is recommended beginning 1–2 months before the expected start of the RSV season and continuing through 2–3 months before the expected end of the season. Therefore, for most of the continental United States, maternal vaccination is recommended in September through January.2,36 In areas where RSV seasonality differs from most of the continental United States such as Florida, Alaska, and Hawaii, clinicians should follow local, state, or territorial guidance for seasonal timing of maternal vaccination.2,33
RSVPreF is supplied in a kit with a prefilled syringe and sterile water diluent component for reconstitution, which should be stored at 2–8°C until reconstituted. Once reconstituted, it should be used immediately or kept at room temperature (15–30°C) and used within 4 hours.34 The shelf life of the antigen and diluent is determined by the manufacturer.34,37
Although only one vaccine, RSVPreF, is approved for use during pregnancy, two vaccines, Abrysvo (Pfizer) and Arexvy (GSK), are available for prevention of RSV-associated lower respiratory tract infection in adults aged 60 years and older, a group at substantial risk of RSV-associated morbidity and mortality. A phase 3 clinical trial of a nonadjuvanted version of the GSK vaccine was halted because of a safety signal of increased risk of preterm birth that was statistically significant and associated with neonatal deaths (although the signal for neonatal deaths was not statistically significant).29 Both Abrysvo and Arexvy were shown to reduce the risk for RSV-associated lower respiratory tract infection disease in older adults.34,38 Six cases of inflammatory neurologic events (eg, Guillain–Barre syndrome) were reported after receipt of these RSV vaccines in more than 20,255 adults and 17,922 adults aged 60 years and older in the Pfizer and GSK clinical trials, respectively.18,38 It is unknown whether these events are the result of chance or whether RSV vaccine could increase the risk for inflammatory neurologic events; it is notable that no such events were reported in pregnant people in the Abrysvo trials.32
SAFETY AND EFFICACY OF NIRSEVIMAB
In July 2023, nirsevimab, a new long-acting monoclonal antibody, was approved by the FDA for the prevention of RSV-associated lower respiratory tract infection in infants and children younger than age 24 months.2 Nirsevimab is given as a single intramuscular injection and is expected to provide protection for at least 5 months.3 For infants born shortly before or during the RSV season, nirsevimab administration is recommended within 1 week of birth, and for infants younger than age 8 months born outside the RSV season, nirsevimab administration is recommended shortly before the RSV season. Nirsevimab was shown to reduce medically attended RSV-associated lower respiratory tract infection incidence within 150 days (5 months) after injection by 79.0% (95% CI, 68.5–86.1%) compared with placebo (pooled efficacy from multiple studies including phase 2b and phase 3 trials of preterm and term infants), with an efficacy of 80.6% (95% CI, 62.3–90.1%) for the prevention of RSV-associated lower respiratory tract infection with hospitalization and 90.0% (95% CI, 16.4–98.8%) for RSV-associated intensive care unit admission (Table 2).2,39,40 Data from RSVPreF and nirsevimab clinical trials should not be directly compared because of variations in study design, including differences in outcomes and observational periods. Most common adverse reactions were rash (0.9%) and injection site reactions (0.3%).39 The CDC recommends that nirsevimab should be given to infants younger than 8 months entering their first RSV season with unknown maternal RSV vaccination status or if no maternal RSV vaccine was given 14 days or more before birth.2 Nirsevimab is also recommended for infants and children aged 8–19 months who are at increased risk for severe RSV illness and entering their second RSV season, regardless of maternal vaccination status.2,3,16
Table 2.
Monoclonal Antibody (Nirsevimab) Efficacy
| Outcome | Efficacy 0–150 d After Administration [% (95% Cl)] |
|---|---|
|
| |
| RSV-associated ICU admission* | 90.0 (16.4–98.8) |
| Hospitalization for RSV-associated LRTI† | 80.6 (62.3–90.1) |
| Medically attended RSV-associated LRTI‡ | 79.0 (68.5–86.1) |
RSV, respiratory syncytial virus;ICU, intensive care unit; LRTI, lower respiratory tract infection.
Intensive care unit admission for an LRTI associated with a positive RSV polymerase chain reaction (PCR) test result. LRTI was defined as one or more documented physical examination findings localizing to the lower respiratory tract (rhonchi, rales, crackles, or wheeze), one or more clinical sign of severity (increased respiratory rate [age younger than 2 months, 60 breaths/minute or more;age 2–6 months, 50 breaths/minute or more;age more than 6 months to 2 years, 40 breaths/minute or more];hypoxemia [on room air]: oxygen saturation below 95% at altitudes of 1,800 m or less or below 92% at altitudes more than 1,800 m;acute hypoxic or ventilatory failure;new-onset apnea;nasal flaring;retractions [intercostal, subcostal, or supraventricular retractions];grunting;dehydration attributable to respiratory distress).
A medically attended visit for a respiratory tract infection (defined as at least one of the following: nasal discharge for 24 hours or more; difficulty breathing, labored breathing, or rapid breathing;cough;inability to feed because of respiratory symptoms;apnea;other respiratory symptom of concern) and an RSV-positive test result for an infant with one of the following indicators of a severe LRTI: increased respiratory rate (70 breaths/minute or higher in infants younger than age 2 months, 60 breaths/minute or higher in infants aged 2–12 months, 50 breaths/minute or higher in children aged 12–24 months), oxygen saturation less than 93%, use of high-flow nasal cannula or mechanical ventilation, admission to the ICU for 4 or more hours, or lack of response or unconsciousness.
A medically attended visit for a respiratory tract infection (as defined above) and an RSV-positive test result for an infant with one of the following indicators of an LRTI: increased respiratory rate (60 breaths/minute or higher in infants younger than age2 months, 50 breaths/ minute or higher in infants aged 2–12 months, 40 breaths/minute or higher in children aged 12–24 months), oxygen saturation less than 95%, or chest wall indrawing
For the 2023–2024 season, the manufacturer reports a limited supply of nirsevimab, particularly the 100-mg dose for infants weighing 5 kg or more. The CDC recommends prioritizing available nirsevimab 100-mg doses for infants at the highest risk for severe RSV disease: young infants (younger than 6 months) and infants with underlying conditions that place them at highest risk for severe RSV disease.41 Recommendations for the 50-mg dose of nirsevimab (for infants weighing less than 5 kg) remain unchanged.3,41 The nirsevimab supply concerns should be discussed when counseling pregnant people to ensure that they have the opportunity to determine whether nirsevimab is available when making decisions about the RSVPreF vaccine during pregnancy.
DISCUSSION OF OPTIONS
The CDC recommends that all infants be protected against RSV-associated lower respiratory tract infection with either RSVPreF (maternal vaccine) or nirsevimab (monoclonal antibody). Use of both products is not needed for most infants, and availability of these products may vary, warranting an understanding of local supplies when these options are offered. Both products should be discussed with patients during the prenatal period, ideally well before 32 weeks of gestation, so that families can have ample time to make informed decisions about their choice of product for the prevention of RSV-associated lower respiratory tract infection in infants. There are no data directly comparing the safety and efficacy of nirsevimab and RSVPreF, and there is no preferential recommendation of one option over the other. Advantages and disadvantages to be discussed with patients are included in Table 3. Many organizations, including the American College of Obstetricians and Gynecologists; the Society for Maternal-Fetal Medicine; the American Academy of Pediatrics; the American Academy of Family Physicians; the American College of Nurse-Midwives; the National Association of Nurse Practitioners in Women’s Health; and the Association of Women’s Health, Obstetric and Neonatal Nurses, endorsed the CDC’s recommendations.42–44
Table 3.
Considerations for Counseling Pregnant Individuals Regarding Options for Prevention of Respiratory Syncytial Virus in Infants
| RSVPreF Maternal Vaccine | Monoclonal Antibody, Nirsevimab | |
|---|---|---|
|
| ||
| Relative advantages | Provides immediate protection after birth (if maternal vaccination occurred 14 d or more before birth). | Protection may last longer than maternal vaccination. |
| Maternal vaccination results in a polyclonal response, which might be more resistant to potential virus mutations than a monoclonal product. | Direct antibody transfer to infant rather than passive transfer of maternal antibodies. | |
| No potential risk for adverse pregnancy outcomes. | ||
| Relative disadvantages | Potential risk of preterm birth and hypertensive disorders of pregnancy. | Availability may be limited during the 2023–2024 RSV season. |
| Reduced antibody transfer if delivery of neonate is less than 14 d after maternal immunization or maternal immunocompromised state (eg, inadequately treated maternal HIV infection). | Requires infant injection. | |
HIV, human immunodeficiency virus; RSV, respiratory syncytial virus.
Modified from Fleming-Dutra KE, Jones JM, Roper LE, Prill MM, Ortega-Sanchez IR, Moulia DL, et al. Use of the Pfizer respiratory syncytial virus vaccine during pregnancy for the prevention of respiratory syncytial virus–associated lower respiratory tract disease in infants: recommendations of the advisory committee on immunization practices—United States, 2023. MMWR Morb Mortal Wkly Rep 2023;72:1115–22.33
Discussion of the two new options must consider patient factors and clinical circumstances. Efficacy of the maternal vaccine depends on the timing of administration during pregnancy, an adequate immune response, and effective transplacental transfer of maternal antibodies to the fetus. If delivery is planned within 14 days, vaccination could result in inadequate development and passage of antibodies to the fetus, and nirsevimab, if available, would be the better option for the infant.
Nirsevimab may be also considered for infants born to vaccinated mothers in some rare circumstances.2,33 Examples would include infants born to a pregnant person who lacks the ability to mount an adequate immune response to vaccination or who has a condition associated with reduced transplacental antibody transfer (eg, hypergammaglobulinemia).2,33,45 In addition, infants who might have experienced a loss of maternal antibodies (eg, cardiopulmonary bypass, extracorporeal membrane oxygenation) or those who are at substantially increased risk of severe RSV disease (eg, hemodynamically significant congenital heart disease or neonatal intensive care admission requiring oxygen at hospital discharge) may also require nirsevimab after birth despite maternal vaccination.2,33 Patients with specific questions should be provided with additional resources, including MotherToBaby (https://mothertobaby.org/fact-sheets/respiratory-syncytial-virus-rsv/), an organization dedicated to providing information about exposures during pregnancy that provides a patient call-in center.46,47 Of note, RSVPreF can be co-administered with other vaccines, including influenza, COVID-19, and tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap), although Tdap is recommended to be given earlier in pregnancy (27–36 weeks of gestation).48
CONSIDERATIONS FOR ENSURING ACCESS
Barriers to health care exist and might limit access to these preventive options. As with many new products, it will take time for these products to be stocked in all settings across the country. As of November 10, 2023, RSVPreF has a list price of about $295 per dose (vs $46–52 for Tdap), and nirsevimab has a list price of up to $495.49,50 RSVPreF is included in the Vaccines for Children Program for eligible pregnant people younger than age 19 years, including those who have Medicaid insurance, who are American Indian or Alaska Native, or who are uninsured or underinsured.51 As with all CDC-recommended vaccines, RSVPreF will be covered for pregnant individuals aged 19 years and older who are full beneficiaries of Medicaid under the Inflation Reduction Act.52,53 In addition, in accordance with the current federal policy, private insurers are required to cover RSVPreF beginning about 12 months after the date of the CDC’s recommendation. However, coverage may vary by insurer, and patients with private insurance will need to check with their insurance provider regarding coverage.
Health care facilities and pharmacies have already begun to stock RSV vaccines, which is why it is important that all vaccinators are aware that RSVPreF is the only RSV vaccine that should be given to pregnant people.34,38 Some state policies and laws require a health care professional prescription for pregnant individuals, so it is important that prenatal practitioners either administer RSVPreF or proactively provide prescriptions to patients who are considering this vaccination. Finally, it is important that all vaccinators document vaccination in the medical record and immunization registry and provide patients with documentation of RSVPreF receipt, through patient portals, in hard copy, or by photographing the documentation on patients’ personal devices, to assist birthing hospitals and pediatric health care practitioners in knowing whether an infant is eligible for nirsevimab.49
Nirsevimab will be made available to infants through the Vaccines for Children Program and will be covered without cost sharing by the patient.51,54 However, the high costs of both RSVPreF and nirsevimab may present barriers for stocking these products in health care settings because clinics, offices, and hospitals may need to cover the upfront costs. Obstetric and pediatric practitioners can advocate within their health care systems to ensure availability of these products to avoid worsening existing health inequities.49 Because mistrust and concern regarding vaccination in the community could also limit access, building trust in the patient–physician relationship, using educational materials, and leveraging community efforts and partnerships may aid in increasing uptake of these preventive options.
CONCLUSION
Respiratory syncytial virus is a leading cause of hospitalization of infants in the United States. New options are available to protect infants from the severe complications of RSV infection: a maternal RSV vaccine, RSVPreF, and a postnatal monoclonal antibody, nirsevimab. All infants are recommended to be protected against RSV-associated lower respiratory tract infection by one of these options, but both are not needed for most infants; therefore, this choice will need to be made by the pregnant person during the prenatal period. Obstetricians will want to familiarize themselves with RSV, these two new options, and their availability to appropriately counsel patients before the vaccination timeframe during pregnancy. It will be helpful to review RSV immunizations (https://cdc.gov/vaccines/vpd/rsv/index.html) for information on how to counsel patients on these options.55 These newly approved products hold great promise for reducing the risk of RSV-associated hospitalizations and deaths in infants and children.
Supplementary Material
Acknowledgments
The authors acknowledge Katherine Fleming-Dutra, MD, for her review and contributions to ensure alignment with CDC recommendations; Naima Joseph, MD, MPH, for her review and contributions in collaboration with the Society for Maternal-Fetal Medicine; Sarah Carroll, MPH, for her review and contributions in collaboration with the American College of Obstetricians and Gynecologists; and Romeo Galang, MD, MPH, for his contributions to implementation considerations.
Footnotes
Financial Disclosure
Sonja Rasmussen reported that she serves on scientific advisory committees for several pregnancy registries, including registries for Wakix (Harmony), Sunosi (Axsome), Nurtec (Biohaven, recently acquired by Pfizer), and Myfembree (Myovant Sciences). The other authors did not report any potential conflicts of interest.
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the CDC.
Each author has confirmed compliance with the journal’s requirements for authorship.
REFERENCES
- 1.U.S. Food and Drug Administration. FDA approves first vaccine for pregnant individuals to prevent RSV in infants. Accessed August 21, 2023. https://fda.gov/news-events/press-announcements/fda-approves-first-vaccine-pregnant-individuals-prevent-rsv-infants
- 2.Jones JM, Fleming-Dutra KE, Prill MM, Roper LE, Brooks O, Sánchez PJ, et al. Use of nirsevimab for the prevention of respiratory syncytial virus disease among infants and young children: recommendations of the Advisory Committee on Immunization Practices—United States, 2023. MMWR Morb Mortal Wkly Rep 2023;72:920–5. doi: 10.15585/mmwr.mm7234a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.American Academy of Pediatrics. ACIP and AAP recommendations for nirsevimab. Accessed October 18, 2023. https://publications.aap.org/redbook/resources/25379/ACIP-and-AAP-Recommendations-for-Nirsevimab?autologincheck=redirected
- 4.Centers for Disease Control and Prevention. Increased respiratory syncytial virus (RSV) activity in parts of the southeastern United States: new prevention tools available to protect patients. Accessed October 31, 2023. https://emergency.cdc.gov/han/2023/han00498.asp [Google Scholar]
- 5.World Health Organization. Respiratory syncytial virus (RSV) disease. 2019. Accessed October 18, 2023. https://who.int/teams/health-product-policy-and-standards/standards-and-specifications/vaccine-standardization/respiratory-syncytial-virus-disease
- 6.Committee on Infectious Diseases American Academy of Pediatrics, Kimberlin DW, Barnett ED, Lynfield R, Sawyer MH. Respiratory syncytial virus: Red Book: 2021–2024 report of the Committee on Infectious Diseases. Accessed September 25, 2023. https://publications.aap.org/redbook/book/347/chapter/5755493/Respiratory-Syncytial-Virus
- 7.Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child 1986;140:543–6. doi: 10.1001/archpedi.1986.021402000530268 [DOI] [PubMed] [Google Scholar]
- 8.Hall CB, Weinberg GA, Iwane MK, Blumkin AK, Edwards KM, Staat MA, et al. The burden of respiratory syncytial virus infection in young children. N Eng J Med 2009;360:588–98. doi: 10.1056/NEJMoa0804877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Langley GF, Anderson LJ. Epidemiology and prevention of respiratory syncytial virus infections among infants and young children. Pediatr Infect Dis J 2011;30:510–7. doi: 10.1097/INF.0b013e3182184ae7 [DOI] [PubMed] [Google Scholar]
- 10.Suh M, Movva N, Jiang X, Bylsma LC, Reichert H, Fryzek JP, et al. Respiratory syncytial virus is the leading cause of United States infant hospitalizations, 2009–2019: a study of the National (Nationwide) Inpatient Sample. J Infect Dis 2022;226(suppl 2):S154–63. doi: 10.1093/infdis/jiac120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McLaughlin JM, Khan F, Schmitt H-J, Agosti Y, Jodar L, Simões EAF, et al. Respiratory syncytial virus–associated hospitalization rates among US infants: a systematic review and meta-analysis. J Infect Dis 2020;225:1100–11. doi: 10.1093/infdis/jiaa752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hansen CL, Chaves SS, Demont C, Viboud C. Mortality associated with influenza and respiratory syncytial virus in the US, 1999–2018. JAMA Netw Open 2022;5:e220527. doi: 10.1001/jamanetworkopen.2022.0527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thompson WW, Shay DK, Weintraub E, Brammer L, Cox N, Anderson LJ, et al. Mortality associated with influenza and respiratory syncytial virus in the United States. JAMA 2003;289:179–86. doi: 10.1001/jama.289.2.179 [DOI] [PubMed] [Google Scholar]
- 14.Hall CB, Weinberg GA, Blumkin AK, Edwards KM, Staat MA, Schultz AF, et al. Respiratory syncytial virus–associated hospitalizations among children less than 24 months of age. Pediatrics 2013;132:e341–8. doi: 10.1542/peds.2013-0303 [DOI] [PubMed] [Google Scholar]
- 15.Borchers AT, Chang C, Gershwin ME, Gershwin LJ. Respiratory syncytial virus–a comprehensive review. Clin Rev Allerg Immunol 2013;45:331–79. doi: 10.1007/s12016-013-8368-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. Respiratory syncytial virus—for healthcare providers. Accessed October 18, 2023. https://cdc.gov/rsv/clinical/index.html
- 17.Melgar M, Roper LE, Talbot HK, Long SS, Kotton CN, Havers FP. Use of respiratory syncytial virus vaccines in older adults: recommendations of the Advisory Committee on Immunization Practices—United States 2023. MMWR Morb Mortal Wkly Rep 2023;23:793–801. doi: 10.15585/mmwr.mm7229a4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Melgar M, Britton A. Updated evidence to recommendation framework: respiratory syncytial virus (RSV) in adults [presentation slides]. Accessed October 11, 2023. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-06-21-23/06-RSV-Adults-Melgar-508.pdf
- 19.Hause AM, Avadhanula V, Maccato ML, Pinell PM, Bond N, Santarcangelo P, et al. A cross-sectional surveillance study of the frequency and etiology of acute respiratory illness among pregnant women. J Infect Dis 2018;218:528–35. doi: 10.1093/infdis/jiy167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hause AM, Avadhanula V, Maccato ML, Pinell PM, Bond N, Santarcangelo P, et al. Clinical characteristics and outcomes of respiratory syncytial virus infection in pregnant women. Vaccine 2019;37:3464–71. doi: 10.1016/j.vaccine.2019.04.098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fleming-Dutra KE. RSV epidemiology and disease burden in infants from birth through 6 months of age. Accessed October 18, 2023. https://fda.gov/media/168259/download
- 22.Milucky J, Patel K, Taylor CA, Kirley PD, Alden NB, Yousey-Hindes K, et al. Outcomes of pregnant women hospitalized with RSV infection, RSV-NET, 2014–2018. Accessed October 31, 2023. https://resvinet.org/wp-content/uploads/2023/02/RSVVW23-Abstract-Booklet.pdf
- 23.Hamid S, Winn A, Parikh R, Jones JM, McMorrow M, Prill MM, et al. Seasonality of respiratory syncytial virus—United States, 2017–2023. MMWR Morb Mortal Wkly Rep 2023;72:355–61. doi: 10.15585/mmwr.mm7214a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jenco M FDA approves RSV vaccine for pregnant people to protect infants. Accessed October 11, 2023. https://publications.aap.org/aapnews/news/25580/FDA-approves-RSV-vaccine-for-pregnant-people-to
- 25.Qiu X, Xu S, Lu Y, Luo Z, Yan Y, Wang C, et al. Development of mRNA vaccines against respiratory syncytial virus (RSV). Cytokine Growth Factor Rev 2022;68:37–53. doi: 10.1016/j.cytogfr.2022.10.001 [DOI] [PubMed] [Google Scholar]
- 26.Pollet J, Chen WH, Strych U. Recombinant protein vaccines, a proven approach against coronavirus pandemics. Adv Drug Deliv Rev 2021;170:71–82. doi: 10.1016/j.addr.2021.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chu HY, Tielsch J, Katz J, Magaret AS, Khatry S, LeClerq SC, et al. Transplacental transfer of maternal respiratory syncytial virus (RSV) antibody and protection against RSV disease in infants in rural Nepal. J Clin Virol 2017;95:90–5. doi: 10.1016/j.jcv.2017.08.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Englund J, Glezen WP, Piedra PA. Maternal immunization against viral disease. Vaccine 1998;16:1456–63. doi: 10.1016/s0264-410x(98)00108-x [DOI] [PubMed] [Google Scholar]
- 29.Fleming-Dutra KE. Evidence to recommendations framework updates Pfizer maternal RSVpreF vaccine [presentation slides]. Accessed October 1, 2023. https://www.cdc.gov/vaccines/acip/meetings/downloads/slides-2023-09-22/06-Mat-Peds-Fleming-Dutra-508.pdf
- 30.Munoz FM, Posavad CM, Richardson BA, Badell ML, Bunge KE, Mulligan MJ, et al. COVID-19 booster vaccination during pregnancy enhances maternal binding and neutralizing antibody responses and transplacental antibody transfer to the newborn. Vaccine 2023;41:5296–303. doi: 10.1101/2022.06.13.22276354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palmeira P, Quinello C, Silveira-Lessa AL, Zago CA, Carneiro-Sampaio M. IgG placental transfer in healthy and pathological pregnancies. Clin Dev Immunol 2012;2012:985646. doi: 10.1155/2012/985646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kampmann B, Madhi SA, Munjal I, Simoes EAF, Pahud BA, Llapur C, et al. Bivalent prefusion F vaccine in pregnancy to prevent RSV illness in infants. N Engl J Med 2023;388:1451–64. doi: 10.1056/NEJMoa2216480 [DOI] [PubMed] [Google Scholar]
- 33.Fleming-Dutra KE, Jones JM, Roper LE, Prill MM, Ortega-Sanchez IR, Moulia DL, et al. Use of the Pfizer respiratory syncytial virus vaccine during pregnancy for the prevention of respiratory syncytial virus–associated lower respiratory tract disease in infants: recommendations of the Advisory Committee On Immunization Practices—United States, 2023. MMWR Morb Mortal Wkly Rep 2023;72:1115–22. doi: 10.15585/mmwr.mm7241e1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.U.S. Food and Drug Administration. Abrysvo prescribing information. Accessed October 18, 2023. https://fda.gov/vaccines-blood-biologics/abrysvo
- 35.Madhi SA, Polack FP, Piedra PA, Munoz FM, Trenholme AA, Simoes EAF, et al. Respiratory syncytial virus vaccination during pregnancy and effects in infants. N Engl J Med 2020;383:426–39. doi: 10.1056/NEJMoa1908380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Centers for Disease Control and Prevention. Vaccinating pregnant patients: pregnancy and whooping cough. Accessed October 11, 2023. https://cdc.gov/pertussis/pregnant/hcp/pregnant-patients.html [Google Scholar]
- 37.U.S. Food and Drug Administration. Abrysvo approval letter. Accessed November 11, 2023. https://fda.gov/media/168890/download
- 38.Arexvy prescribing information. Accessed October 18, 2023. https://gskpro.com/content/dam/global/hcpportal/en_US/Prescribing_Information/Arexvy/pdf/AREXVY.PDF
- 39.Griffin MP, Yuan Y, Takas T, Domachowske JB, Madhi SA, Manzoni P, et al. Single-dose nirsevimab for prevention of RSV in preterm infants. N Engl J Med 2020;383:415–25. doi: 10.1056/NEJMoa1913556 [DOI] [PubMed] [Google Scholar]
- 40.Muller WJ, Madhi SA, Seoane Nuñez B, Baca Cots M, Bosheva M, Dagan R, et al. Nirsevimab for prevention of RSV in term and late-preterm infants. N Engl J Med 2023;388:1533–4. doi: 10.1056/NEJMc2214773 [DOI] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. Limited availability of nirsevimab in the United States: interim CDC recommendations to protect infants from respiratory syncytial virus (RSV) during the 2023–2024 respiratory virus season. Accessed October 31, 2023. https://emergency.cdc.gov/han/2023/han00499.asp
- 42.Society for Maternal-Fetal Medicine. RSV vaccination in pregnancy. Accessed October 11, 2023. https://s3.amazonaws.com/cdn.smfm.org/media/4196/SMFM_Statement_RSV_September_2023.pdf
- 43.American College of Obstetricians and Gynecologists. Maternal respiratory syncytial virus vaccination. Accessed October 11, 2023. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2023/09/maternal-respiratory-syncytial-virus-vaccination
- 44.Maternal Immunization Task Force. Obstetric care professionals recommend RSV vaccine for pregnant individuals. Accessed October 18, 2023. https://acog.org/-/media/project/acog/acogorg/files/pdfs/news/rsv-joint-statement-2023.pdf
- 45.Atwell JE, Lutz CS, Sparrow EG, Feikin DR. Biological factors that may impair transplacental transfer of RSV antibodies: implications for maternal immunization policy and research priorities for low- and middle-income countries. Vaccine 2022;40:4361–70. doi: 10.1016/j.vaccine.2022.06.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.MotherToBaby. MotherToBaby respiratory syncytial virus (RSV) vaccine (AbrysvoTM) fact sheet. Accessed October 11, 2023. https://mothertobaby.org/fact-sheets/respiratory-syncytial-virus-rsv-vaccine-abrysvo/ [Google Scholar]
- 47.Centers for Disease Control and Prevention. Respiratory syncytial virus (RSV) vaccine information statement. Accessed October 11, 2023. https://cdc.gov/vaccines/hcp/vis/vis-statements/rsv.html
- 48.Centers for Disease Control and Prevention. Healthcare providers: RSV vaccination for pregnant people: vaccine and preventable diseases. Accessed October 11, 2023. https://cdc.gov/vaccines/vpd/rsv/hcp/pregnant-people.html
- 49.Peacock G Maternal RSV vaccine implementation considerations [slide presentation]. Accessed October 11, 2023. https://cdc.gov/vaccines/acip/meetings/downloads/slides-2023-09-22/08-mat-peds-peacock-508.pdf [Google Scholar]
- 50.Drugs.com. Abrysvo prices, coupons and patient assistance programs. Accessed October 10, 2023. https://drugs.com/price-guide/abrysvo
- 51.Centers for Disease Control and Prevention. Advisory Committee on Immunization Practices Vaccines for Children Program. Accessed October 11, 2023. https://cdc.gov/vaccines/programs/vfc/downloads/resolutions/rsv-resolution-508.pdf
- 52.Centers for Medicare & Medicaid Services. Anniversary of the Inflation Reduction Act: update on CMS implementation. Accessed October 10, 2023. https://cms.gov/newsroom/fact-sheets/anniversary-inflation-reduction-act-update-cms-implementation#:~:text=The%20Inflation%20Reduction%20Act%20requires%20state%20Medicaid%20programs%20to%20cover,later%20than%20October%201%2C%202023
- 53.O’Leary ST, Riley LE, Lindley MC, Allison MA, Crane LA, Hurley LP, et al. Immunization practices of U.S. obstetrician/-gynecologists for pregnant patients. Am Prev Med 2018;54:205–13. doi: 10.1016/j.amepre.2018.10.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Coverage of preventive health services. 42 U.S. Code § 300gg–13. [Google Scholar]
- 55.Centers for Disease Control and PreventionRespiratory syncytial virus (RSV) immunizations. Accessed November 12, 2023. https://cdc.gov/vaccines/vpd/rsv/index.html
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


