Abstract
Background:
The saliva and salivary glands of ticks possess a wide range of immuno-pharmacologically active molecules that effectively modulate the activity of enzymes, antibodies, and amines that have a role in different biological processes. Derived components from saliva and salivary glands of hard ticks Ixodidae have been characterized as potential natural sources for discovering promising anti-cancer drug candidates.
Methods:
The anti-cancer activity of salivary gland extracts (SGEs) from Hyalomma anatolicum, Hyalomma dromedarii, Hyalomma marginatum, and Hyalomma schulzei was assessed. MTT assays and flow cytometry were done on the HT-29 colorectal cancer cell line to evaluate the anti-viability and proliferative inhibition.
Results:
Based on the MTT assay results, the SGEs from Hy. dromedarii had the highest and lowest substantial anti-viability effects on the HT-29 cancer cell and human foreskin fibroblast (HFF) normal cell, respectively. The cytometric assessment revealed a significant increase in the apoptosis and necrosis ratio of the HT-29 cancer cells after treatment with Hy. dromedarii SGEs.
Conclusion:
The results demonstrated that Hy. dromedarii SGEs have significant anti-proliferative, anti-viability, and apoptotic potential. The result of this study suggests that Hy. dromedarii SGEs is an appropriate candidate for further investigations to identify and purify the mechanisms and molecules involved in the anti-cancer activity of the SGEs.
Keywords: Tick, Hyalomma, Salivary gland extract, Anti-cancer, HT-29 cell line
Introduction
Cancer is the main cause of death and an important obstacle to increasing life expectancy in any country (1). Based on the World Health Organization (WHO) estimation for 2019, cancer rates as the first or second cause of mortality before the age of 70 in 112 out of 183 countries and also the third or fourth in another 23 countries (2). More than 1.9 million new cases of colorectal cancer and 935,000 deaths are estimated for 2020, which is about one in 10 cancer cases and deaths. Colorectal cancer ranks third in incidence and second in mortality (3). Conventional cancer treatments include surgery, radiation therapy, and chemotherapy. Despite technological advances, most cancers are still incurable, and many patients who undergo cancer treatment experience side effects and relapse after a period of remission. Therefore, any new effective and safe therapeutic agent will be in high demand (4).
Natural products can help pharmacists and clinicians develop novel medications and treat chronic and degenerative illnesses. These compounds can be found in plants and other living organisms. Recent advances in the development of arthropods’ natural products as potential novel medicines indicate that arthropods and their compounds are an excellent option to achieve this goal (5–11). Among arthropods, ticks have received attention in biomedicine and treating complicated diseases (12–14). The ticks’ saliva contains rich and potential chemical sources of neurotoxins, terpenoids, saponins, serpins, and carbohydrates (15–19). Moreover, it also provides a broad range of proteins and peptides to facilitate the production of the first line of defense against pathogens. It exhibits cytolysis, vasodilator, anticoagulant, anti-inflammatory, and immunosuppressive activities (20–23). The levels of non-protein substances and secreted proteins in tick saliva are effective in controlling host response mechanisms. These compounds activate the immune system with varying levels of regulatory activity of chemicals that affect cell signaling, such as enzymes, antibodies, vascular amines, molecular adhesion, cytokines, and chemokines, or directly affect target cells. Some peptides have been shown to suppress gene and protein expression, while others cause membrane lysis, apoptosis, or cell cycle arrest (24–29). This study aims to investigate the inhibitory effects of tick salivary gland extracts (SGEs) on cancer cell viability and proliferation.
Materials and Methods
Preparation of samples
Tick specimens were collected from different provinces of Iran, including East Azerbaijan, West Azerbaijan, Gilan, Isfahan, Qom, and Tehran, in favorable seasons from May to December 2021. Adult ticks were collected by fine-tipped angled forceps from the ear, mammary glands, under the tail, and the rest of the body of the appropriate host, including sheep and camels, following the standard method (30–31). The engorged and semi-engorged male and female ticks were used in this work. The specimens were preserved in the tubes, and relative information, including the code, date, gender, place of collection, and host type, was recorded. All tubes were surrounded with a wet towel, placed in a cold box containing an ice pack, and then transferred to the Vector Biology Laboratory at the School of Public Health, Tehran University of Medical Sciences. Ticks were identified using the identification keys of Hoogstraal (32) and Walker (33).
Preparation of the salivary gland extracts (SGEs)
In total, 700 males and females of the genus Hyalomma, including Hy. anatolicum, Hy. dromedarii, Hy. marginatum, and Hy. schulzei, were dissected under cold, sterile Dulbecco’s PBS with a pH of 7.2. The SGEs of each species were divided into males and females, pooled, and frozen at −80 °C. Before the assays, pools of SGEs were homogenized and centrifuged at 18,000g at 4 °C for 30 min. Supernatants were pooled, and the soluble protein concentration of SGEs was determined using the bicinchoninic acid assay (34). The SGEs were lyophilized for further study.
Cell culture
The present study was conducted on the HT-29, a human colorectal adenocarcinoma cell line. The cell number IBRC C10097 was provided by the Iranian Biological Resource Center. The HT-29 cells were seeded for primary cultures at a density of 80,000 cells/cm2 (T-25 cm2 flask). The cells were grown in DMEM medium supplemented with 10% FBS, four mM L-glutamine, and 100 units/ml penicillin and streptomycin. It was incubated in a humidified atmosphere at 37 °C with 5% CO2. The cells were detached with 0.25% trypsin and 0.02% EDTA for 3 min at 37 °C, spun at 300–1,000 g for 5–10 min, and resuspended in a complete medium. In this method, the cells were passaged three times per week. Also, human foreskin fibroblast (HFF) was used as a normal cell; the cell number RSCB0564 was provided by the Stem Cell Bank of Royan ATMPTDC and maintained in DMEM (high glucose with 10% FBS under culture conditions similar to HT-29).
Cell viability assay
The effects of Hy. anatolicum, Hy. marginatum, Hy. dromedarii, and Hy. schulzei SGEs on the viability of HT-29 and HFF cells were assessed using the MTT assay (35). In this assay, mitochondrial succinate dehydrogenase catalyzes the enzymatic conversion of 3-[4,5-dimethylthiazole-2-yl]-2,5-diphenyltetrazolium bromide (MTT) to purple formazan dye. The dye produced is proportional to the number of metabolically active cells. In this study, the cells were seeded into a 96-well microplate at 104 cells per well density. Each well was filled with 100 μL of medium and cultured at 37 °C for 24 hours before being replaced with fresh media containing SGEs at various concentrations (60, 30, 15, and 7.5 μg/mL). Each experiment was repeated at least three times. Morphological changes were checked using an optical microscope. After the indicated time, 12 μL MTT (5 mg/mL) was added, and the cells were incubated for 4 h. After that time, the wells were removed entirely, and 100 μL of DMSO was added to solubilize the formazan crystals. The absorbance was determined spectrophotometrically at 570 nm on an Elx800 UV universal microplate reader (BioTek Co., Winooski, VT, U.S.). Control cells were those that had only been incubated with the medium. The half-maximal inhibitory concentrations (IC50 values) were calculated by fitting the survival curve from dose-dependent response data.
Evaluation of cell death by flow cytometry
The HT-29 cells were treated with each species’ SGEs at the IC50 concentrations in a 12-well microplate at a density of 3 × 104 cells per well for 24 h and then washed with phosphate-buffered saline (PBS; 500 μL/well). To evaluate apoptosis and necrosis, at least 105 cells must be harvested and incubated with 2 mM Annexin-V and 10 mM of PI in PBS for 30 min at room temperature in the dark. The untreated cells served as a negative control. A flow cytometer immediately analyzed the cells (FACS Calibur, Becton Dickinson Co., San Jose, CA, U.S.). Data from three independent experiments were analyzed using FlowJo software (36).
Statistical methods
The data were described using the mean ± SD, or frequency (%). The chi-squared, or Fisher’s exact, test was used to evaluate the relationship between categorical data. A twoway analysis of variance (ANOVA) was performed to compare genders and treatments, considering the interaction between gender and treatments. For pairwise comparisons (post hoc analysis), Tukey was used to improve the power of the comparisons. P-values less than 0.05 were considered significant. All of the analysis was done with SPSS version 26 software.
Results
The frequency of Hyalomma species in the study areas
A total of 3,299 hard ticks of the genus Hyalomma in four species, including Hy. anatolicum, Hy. dromedarii, Hy. schulzei, and Hy. marginatum were collected (Table 1). Hyalomma anatolicum was discovered in all sampling areas, including Tehran, Qom, Isfahan, Gilan, East Azerbaijan, and West Azerbaijan. Hyalomma dromedarii and Hy. schulzei were found at the highest rates in Tehran and Qom, while Hy. marginatum was collected in these two provinces, followed by Isfahan and Gilan. Hyalomma anatolicum, Hy. marginatum, and Hy. dromedarii were the dominant species during the sampling, while Hy. schulzei was collected late in the summer and during the autumn.
Table 1.
Data on Hyalomma ticks sampling and collection sites established in Iranian provinces using a collection technique derived from captured hosts from May 1 to December 30, 2021
| Species | Ticks (No.) | Location (Province) | Coordinates | Months | |
|---|---|---|---|---|---|
|
| |||||
| Male | Female | ||||
| Hy. dromedarii | 40 | 25 | Tehran | 35.7117°N 51.4070°E | July to December |
| Hy. schulzei | 148 | 55 | |||
| Hy. marginatum | 185 | 60 | |||
| Hy. anatolicum | 50 | 25 | |||
| Hy. dromedarii | 490 | 320 | Qom | 34.6456°N 50.8798°E | July to December |
| Hy. schulzei | 220 | 60 | |||
| Hy. marginatum | 266 | 85 | |||
| Hy. anatolicum | 272 | 78 | |||
| Hy. anatolicum | 60 | 95 | Isfahan | 32.6577°N 51.6692°E | June and July |
| Hy. marginatum | 100 | 40 | |||
| Hy. anatolicum | 30 | 140 | Gilan | 37°26′N 49°33′E | May, June, July, and August |
| Hy. marginatum | 120 | 40 | |||
| Hy. dromedarii | 20 | 130 | |||
| Hy. anatolicum | 85 | 20 | East Azerbaijan | 38.0766°N 46.2800°E | July and August |
| Hy. anatolicum | 30 | 10 | West Azerbaijan | 37.5528°N 45.0759°E | June |
| Total | 2116 | 1183 | |||
The protein concentration of salivary gland extracts (SGEs)
A standard curve was obtained to determine protein concentration. Table 2 displays the protein concentration of male and female Hyalomma species’ SGEs. Generally, the protein concentration of female ticks’ SGEs was lower than that of males. The highest and lowest protein concentrations were calculated for Hy. dromedarii and Hy. schulzei SGEs, respectively.
Table 2.
Comparison of the protein concentrations of salivary gland extracts (SGEs) and the IC50 (inhibitory concentrations) values of SGEs from Hyalomma species on the viability of human colorectal adenocarcinoma cell line (HT-29)
| Species | Protein Concentration (mg/mL) | IC50 value (μg/mL) | ||
|---|---|---|---|---|
|
| ||||
| Male | Female | Male | Female | |
| Hy. dromedarii | 11.48 | 8.36 | 7.70 | 9.89 |
| Hy. anatolicum | 10.38 | 8.04 | 7.84 | 14.13 |
| Hy. marginatum | 8.93 | 5.93 | 11.03 | 60.20 |
| Hy. schulzei | 6.75 | 5.81 | 13.44 | 67.07 |
The effect of salivary gland extracts (SGEs) on cell viability
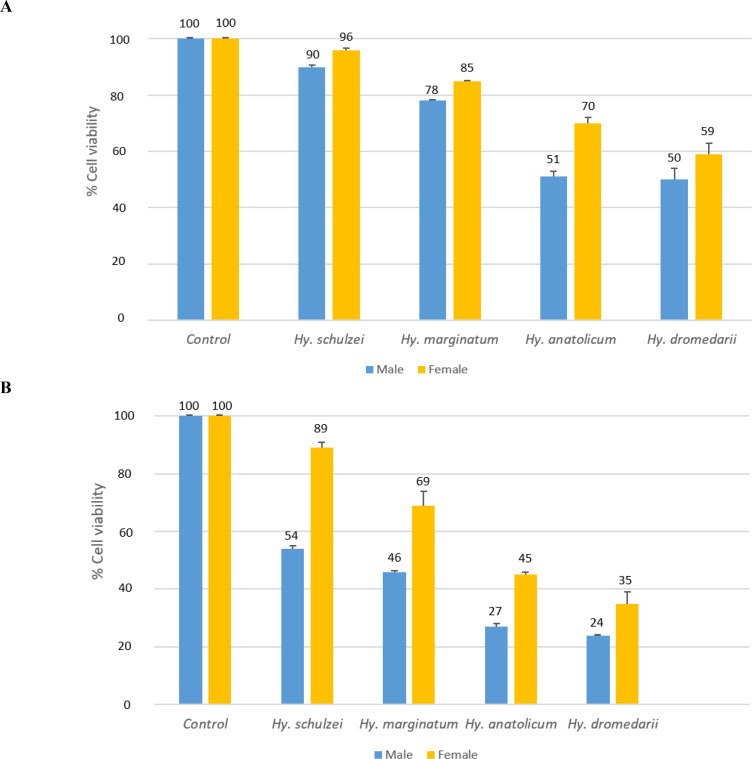
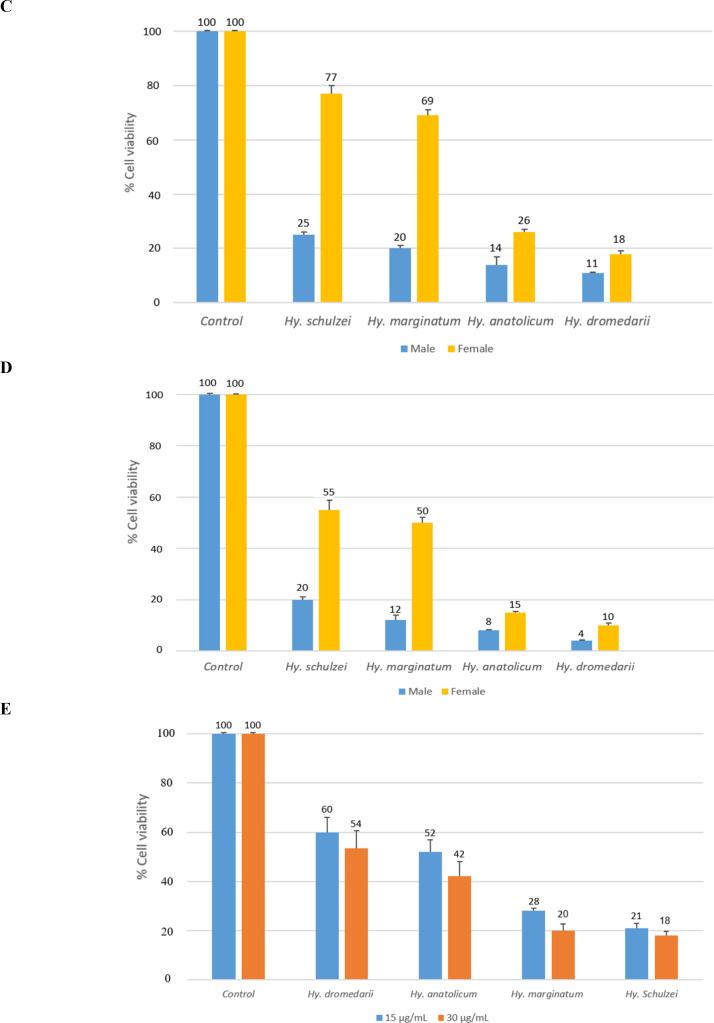
The mean of HT-29 cancer cell viability was compared to four SGE concentrations: 60, 30, 15, and 7.5 μg/mL. In all doses, the mean cell viability in Hy. schulzei was significantly higher than in all other treatments (P< 0.001). The cells with the lowest mean cell viability were referred to as Hy. dromedarii (P< 0.001). Mean cell viability at dose 7.5 was high for all treatments, and dose 60 was lower than the other doses (Fig. 1). Males had significantly lower mean cell viability than females across all doses (P< 0.001). In all doses, the IC50 values (Table 2) in females were higher than in males. The lowest IC50 value was referred to as Hy. dromedarii. There was a dramatic increase in the IC50 value in Hy. marginatum and Hy. schulzei. The cells exhibited more sensitivity after exposure to male SGEs; therefore, male ticks’ SGEs were selected for further investigations. In the case of the HFF as a normal cell, SGEs of Hy. dromedarii and Hy. schulzei had the highest and lowest means of cell viability among male Hyalomma species’ SGEs in doses of 30 and 15 μg/mL, respectively (P= 0.004).
Fig. 1.
The viability (mean ± SD) of human colorectal adenocarcinoma cell line (HT-29) after 24 h of treatment with salivary gland extracts (SGEs) from male and female Hyalomma species at various concentrations, A: 7.5, B: 15, C: 30, D: 60 (μg/mL), and (E) normal human foreskin fibroblast (HFF) cells after 24 h of treatment with 15 and 30 μg/mL of male Hyalomma species’ salivary gland extracts. In each graph, the negative control refers to untreated cells.
Cell death, necrosis, and apoptosis
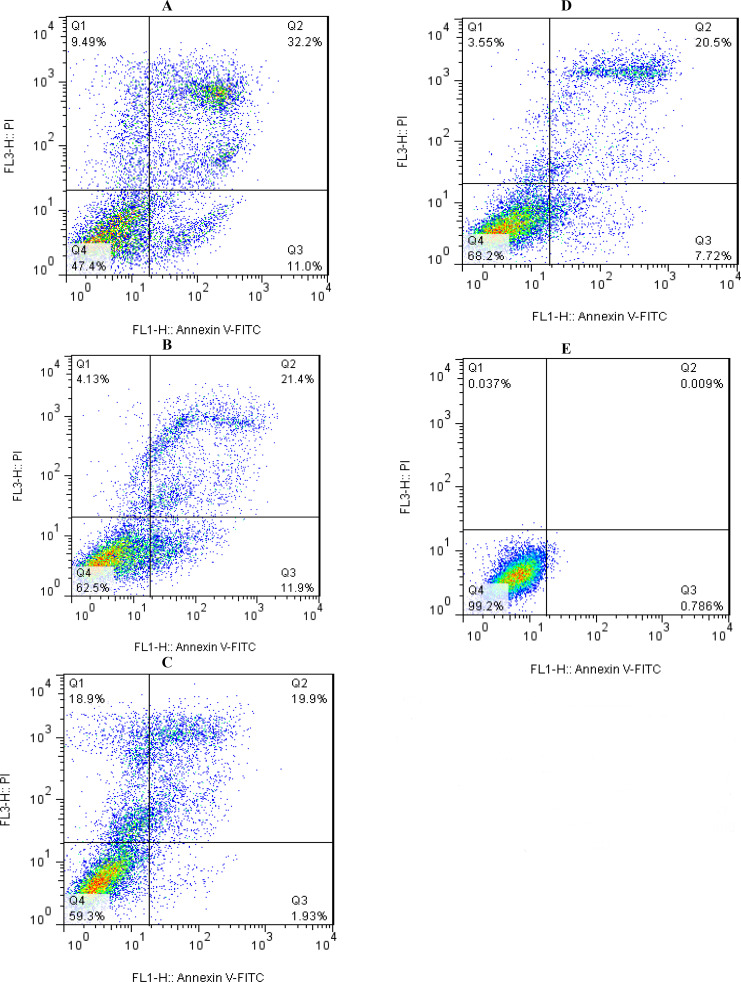
A flow cytometric analysis using the IC50 values revealed HT-29 cells’ apoptosis and necrosis after 24h of treatment with male Hyalomma species’ SGEs compared to untreated control cells (Fig. 2). The highest and lowest rates of late apoptosis were 32.2% and 19.9%, respectively, in Hy. dromedarii and Hy. anatolicum. Hyalomma marginatum and Hy. anatolicum had the highest and lowest percentages of early apoptosis, at 11.9% and 1.93%, respectively. Moreover, following an exposure of Hy. anatolicum and Hy. schulzei SGEs, the cells presented necrosis, with the highest value being 18.9% and the lowest value being 3.55%, respectively.
Fig. 2.
Annexin V/PI double-staining assay of human colorectal adenocarcinoma cell line (HT-29) treated with male Hyalomma species’ salivary gland extracts of (A) Hy. dromedarii, (B) Hy. marginatum, (C) Hy. schulzei, and (D) Hy. anatolicum, compared with (E) the negative control group (untreated cells). Dot plots display the portions of HT-29 cells undergoing (Q1) necrosis, (Q2) late apoptosis, (Q3) early apoptosis, and (Q4) live cells
Discussion
Saliva is an essential biofluid secreted by the tick salivary glands, which modulates host defenses and facilitates the flow of blood to assure adequate feeding. Saliva and salivary gland extracts from Ixodidae were characterized as potential natural sources for the discovery of promising anti-cancer drug candidates (37). We investigated the effects of SGEs from Hy. anatolicum, Hy. marginatum, and Hy. schulzei on a cancer cell for the first time. Other researchers have done extra studies on Hy. dromedarii SGEs. Interestingly, our data demonstrate that Hy. dromedarii SGEs exhibit a higher anti-viability, anti-proliferative, and apoptotic potential on colorectal cancer cells among all of the Hyalomma species’ SGEs. Sousa et al. (38) found that the saliva of Ixodidae contains proteinaceous molecules and potential anti-cancer properties. In the present research, according to the calculated concentration of protein, the protein concentration of Hy. dromedarii SGEs was higher than that of SGEs from other species. Our study is consistent with the suggestion that cancer cell cytotoxicity is influenced by the protein level (39–41). The anti-cancer effect of Hy. dromedarii SGEs on U87 glioblastoma cells was investigated earlier (42). Hyalomma dromedarii SGEs exerted an anti-viability and anti-proliferative effect on the glioblastoma cell. These findings make possible the characterization and development of novel molecules involved in the critical stages of tumor progression. Surprisingly, the viability of cancer cells largely decreased in a dose-dependent manner after seventy-two hours of treatment with Hy. dromedarii SGEs, with IC50 values of 98.68 and 84.76 μg/mL, respectively. The mean of HT-29 colorectal cancer cell viability in the current study was 0.04 at a concentration of 60 μg/mL after 24h of treatment with Hy. dromedarii SGEs and the estimated IC50 value was 7.7 μg/mL. This outcome is consistent with the earlier research (42). Though, our results go beyond the previous. Similar to Bensaoud’s study (42), Hy. dromedarii was collected from camels in our study. Also, we collected Hy. schulzei from camels. The anti-viability and apoptotic effects of Hy. schulzei SGEs on HT-29 cancer cells were significantly lower than those of Hy. dromedarii SGEs. Hyalomma anatolicum and Hy. marginatum were collected from sheep, and the anti-viability and apoptotic effects of SGEs derived from these species were significantly different. The results revealed that the effects of SGEs from Hy. anatolicum are approximately similar to those of Hy. dromedarii. Our investigation demonstrated that the anti-cancer effects of SGEs were not related to the host’s type. The means of cancer cell viability after treatment with Hy. dromedarii and Hy. anatolicum SGEs were similar to each other, but Hy. anatolicum SGEs had the lowest apoptotic effect among all of the species’ SGEs. Hyalomma schulzei and Hy. marginatum SGEs had the lowest anti-cancer effects. Surprisingly, their anti-viability effects on normal cells were higher than all of the species’ SGEs. The findings of Hy. schulzei and Hy. marginatum were opposite to those of Hy. dromedarii SGEs. It is noteworthy that in our study, a higher inhibitory effect on cancer cells was achieved after exposure to Hy. dromedarii SGEs compared to SGEs from the other species. Also, that was higher than the inhibitory effect of saliva from the other genera of Ixodidae in previous studies (43). According to our findings, after being exposed to Hy. dromedarii SGEs, 32.2 percent of HT-29 cancer cells induced late apoptosis. Previous studies have been conducted by utilizing laboratory ticks. Our results show that ticks from the field have a significant inhibitory effect on cancer cells. Previous studies have confirmed that salivary and salivary gland extracts from various genera and species of Ixodidae exhibit cytotoxicity against cancer cells, but not against non-cancer cells (44). In our study, SGEs derived from Hy. dromedarii yielded similar patterns of results. It supports the idea that the cytotoxic activity of the saliva and salivary gland extracts from some tick species is limited to cancer cells. Further studies are required to determine the relationship between molecular and functional properties of protein fractions and significant differences in the anti-viability of Hy. dromedarii, Hy. marginatum, and Hy. schulzei SGEs on normal and cancer cells. Our data illustrate that cancer cells exhibited significantly less sensitivity to female ticks’ SGEs. Comparing our findings to previous research (45, 46), we suggest that male ticks are promising candidates for investigating the anti-cancer effect of saliva and salivary gland extracts in future studies. Hyalomma species were collected from different regions of Iran. The species’ SGE qualities and quantities along with their anti-cancer properties, were not related to the collection sites, and they were strikingly similar. These findings suggest new avenues for investigating and developing the anti-cancer effect of SGEs and the molecules involved in the key steps of cancer progression from ticks in Iran.
Conclusion
The findings suggest that Hy. dromedarii SGEs are acceptable towards the recognition of new therapeutic compounds and effective molecules which could be further used in the development and management of colorectal cancer. However, thorough research is required to identify and purify the mechanisms and molecules involved in the inhibitory effect of Hy. dromedarii SGEs.
Acknowledgements
Thanks to the Department of Vector Biology and Control of Diseases, School of Public Health, Tehran University of Medical Sciences. Miss Tahoora Tavassoli deserves special recognition for her assistance and for allowing us to use her vehicle to collect samples across the country. This project has been financially supported by Tehran University of Medical Sciences and Health Services grant No. 9511260001.
Footnotes
Ethical consideration
The university research review committee revised the research proposal according to the rule and regulations. Accordingly, the study was approved by the Ethics Committee of Tehran University of Medical Sciences (ID: IR.TUMS. VCR.REC. 805).
Conflict of interest statement
The authors declare there is no conflict of interests.
References
- 1.Bray F, Laversanne M, Weiderpass E, Soerjomataram I. (2021) The ever-increasing importance of cancer as a leading cause of premature death worldwide. Cancer. 127(16): 3029–3030. [DOI] [PubMed] [Google Scholar]
- 2.By cause GD. (2020) By country and by region. 2000–2019 World Health Organization. [Google Scholar]
- 3.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. (2021) Global cancer statistics 2020: Globocan estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 71(3): 209–249. [DOI] [PubMed] [Google Scholar]
- 4.Seyed MA. (2019) A comprehensive review on Phyllanthus derived natural products as potential chemotherapeutic and immunomodulators for a wide range of human diseases. Biocatal Agric Biotechnol. 17: 529–537. [Google Scholar]
- 5.Arokiyaraj C, Tamilarasan K, Manikandan R, Janarthanan S. (2022) Purification and structural characterization of lectin with antibacterial and anticancer properties from grubs of hide beetle, Dermestes frischii. Int J Biol Macromol. 203: 312–332. [DOI] [PubMed] [Google Scholar]
- 6.Cardoso P, Barton PS, Birkhofer K, Chichorro F, Deacon C, Fartmann T, Fukushima CS, Gaigher R, Habel JC, Hallmann CA. (2020) Scientists’ warning to humanity on insect extinctions. Biol Conserv. 242: 108426. [Google Scholar]
- 7.Dioguardi M, Caloro GA, Laino L, Alovisi M, Sovereto D, Crincoli V, Aiuto R, Dioguardi A, Delillo A, Troiano G. (2020) Therapeutic anticancer uses of the active principles of “Rhopalurus junceus” Venom. Biomedicines. 8: 382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ebenebe C, Okweche S, Okore O, Okpoko V, Amobi M, Eze J. N, Ezenyilimba B, Okonkwo M. (2021) Arthropods in cosmetics, pharmaceuticals, and medicine. Intech. 96159 [Google Scholar]
- 9.Hmmati SA, Tabin S. (2022) Insect protease inhibitors; promising inhibitory compounds against SARS-CoV-2 main protease. Comput Biol Med. 142: 105228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lozano-Trujillo LA, Garzon-Perdomo DK, Vargas AC, De los reyes LM, Avila-Rodriguez MF, Gay OT, Turner LF. (2021) Cytotoxic effects of blue scorpion venom (Rhopalurus junceus) in a glioblastoma cell line model. Curr Pharm Biotechnol. 22(5): 636–645. [DOI] [PubMed] [Google Scholar]
- 11.Saadoun JH, Sogari G, Bernini V, Camorali C, Rossi F, Neviani E, Lazzi C. (2022) A critical review of intrinsic and extrinsic antimicrobial properties of insects. Trends Food Sci. 122: 40–48. [Google Scholar]
- 12.Bartikova P, Kazimirova M, Stibraniova I. (2020) Ticks and the effects of their saliva on growth factors involved in skin wound healing. J Venom Res. 10: 45–52. [PMC free article] [PubMed] [Google Scholar]
- 13.Nascimento TG, Vieira PS, Cogo SC, Diasnetipanyj MF, Franca ND, Camara DA, Porcacchia AS, Mendonca RZ, Morenoamaral AN, Sa PL. (2019) Antitumoral effects of Amblyomma sculptum Berlese saliva in neuroblastoma cell lines involve cytoskeletal deconstruction and cell cycle arrest. Rev Bras Parasitol Vet. 28(1): 126–133. [DOI] [PubMed] [Google Scholar]
- 14.Sousa AC, Oliveira CJ, Szabo MP, SILVA MJ. (2018) Anti-neoplastic activity of Amblyomma sculptum, Amblyomma parvum, and Rhipicephalus sanguineus tick saliva on breast tumor cell lines. Toxicon. 148: 165–171. [DOI] [PubMed] [Google Scholar]
- 15.Azmiera N, Krasilnikova A, Sahudin S, ALTalib H, Heo C. (2022) Antimicrobial peptides isolated from insects and their potential applications. J Asia-Pac Entomol. 101892. [Google Scholar]
- 16.Meyer-Rochow VB. (2017) Therapeutic arthropods and other, largely terrestrial, folk-medicinally important invertebrates: a comparative survey and review. J Ethnobiol Ethnomed. 13: 1–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moretta A, Scieuzo C, Petrone AM, Salvia R, Manniello MD, Franco A, Lucchetti D, Vassallo A, Vogel H, Sgmbato A. (2021) Antimicrobial peptides: A new hope in biomedical and pharmaceutical fields. Front Cell Infect Microbiol. 11: 668632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mylonakis E, Podsialowski L, Muhammed M, Vilcinskas A. (2016) Diversity, evolution and medical applications of insect antimicrobial peptides. Philos Trans R Soc Lond B Biol Sci. 371: 20150290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Varunrajan V, Deepa B. (2016) Prospecting arthropod biomolecules for medicinal and therapeutic use: Recent breakthroughs. Int J Trop Insect Sci. 978–981. [Google Scholar]
- 20.Bakshi M, Kim TK, Porter L, Mwangi W, Mulenga A. (2019) Amblyomma americanum ticks utilizes countervailing pro and anti-inflammatory proteins to evade host defense. PLOS Pathogens. 15(11): e1008128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bonnet S, Kazimirova M, Richardson J, Simo L. (2018) Tick saliva and its role in pathogen transmission. Skin arthropod vectors. Elsevier. 5: 121–191. [Google Scholar]
- 22.Pereira MC, Nodari EF, De Abreu MR, Paiatto LN, Simioni PU, Camargo-Mathias MI. (2021) Rhipicephalus sanguineus salivary gland extract as a source of immunomodulatory molecules. Exp Appl Acarol. 83(3): 387–398. [DOI] [PubMed] [Google Scholar]
- 23.Aounallah H, Fessel MR, Goldfeder MB, Carvalho E, Bensaoud C, Chudzinski-Tavassi AM, Bouattour A, Mghirbi Y, Faria F. (2021) rDromaserpin: a novel anti-hemostatic serpin, from the salivary glands of the hard tick Hyalomma dromedarii. Toxins. 13(12): 913–937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bensaoud C, Aounallah H, Sciani JM, Faria F, Chudzinski-Tavassi AM. (2019) Proteomic informed by transcriptomic for salivary glands components of the camel tick Hyalomma dromedarii. BMC Genomics. 20(1): 675–687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Braunger K, Ahn J, Jore MM, Johnson S, Tang TT, Pedersen DV, Andersen GR, Lea SM. (2022) Structure and function of a family of tick-derived complement inhibitors targeting properdin. Nat Commun. 13: 317–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chmelar J, Kotal J, Kovarikova A, Kotsyfakis M. (2019) The use of tick salivary proteins as novel therapeutics. Front Physiol. 10: 812–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seabrooks L, HU L. (2017) Insects: an underrepresented resource for the discovery of biologically active natural products. Acta Pharm Sin B. 7(4): 409–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stibraniova I, Bartikova P, Holikova V, Kazimirova M. (2019) Deciphering biological processes at the tick-host interface opens new strategies for treatment of human diseases. Front Physiol. 10: 830–851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tang J, Fang Y, Han Y, Bai X, Yan X, Zhang Y, Lai R, Zhang Z. (2015) YY-39, a tick anti-thrombosis peptide containing RGD domain. Peptides. 68: 99–104. [DOI] [PubMed] [Google Scholar]
- 30.Espada C, Cummins H, Gonzales JA, Notto L, Gaff HD. (2021) A comparison of tick collection materials and methods in Southeastern Virginia. J Med Entomol. 58(2): 692–698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Salomon J, Hamer SA, Swei A. (2020) A beginner’s guide to collecting questing hard ticks (Acari: Ixodidae): a standardized tick dragging protocol. J Insect Sci. 20 (6): 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hoogstraal H. (1956) African ixodoidea, Department of the Navy, Bureau of Medicine and Surgery.
- 33.Walker AR. (2003) Ticks of domestic animals in Africa: a guide to identification of species, Bioscience Reports Edinburgh.
- 34.Smith PE, Krohn RI, Hermanson GT, Mallia AK, Gartner FH. (1985) Measurement of protein using bicinchoninic acid. Anal Biochem. 150 (1): 76–85. [DOI] [PubMed] [Google Scholar]
- 35.Mosmann T. (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J Immunol Methods. 65(1–2): 55–63. [DOI] [PubMed] [Google Scholar]
- 36.Aounallah H, Bensaoud C, Mghirbi Y, Faria F, Chmelar JI, Kotsyfakis M. (2020) Tick salivary compounds for targeted immunomodulatory therapy. Front Immunol. 11: 583845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chudzinski-Tavassi AM, Moris KL, Moris, Pacheco MT, Pasqualoto KF. (2016) Tick salivary gland as potential natural source for the discovery of promising antitumor drug candidates. Biomed Pharmacother. 77: 14–19. [DOI] [PubMed] [Google Scholar]
- 38.Sousa AC, Szabo MP, Oliveira CJ, Silva MJ. (2015) Exploring the anti-tumoral effects of tick saliva and derived components. Toxicon. 102: 69–73. [DOI] [PubMed] [Google Scholar]
- 39.Stappenbeck TS, Miyoshi H. (2009) The role of stromal stem cells in tissue regeneration and wound repair. Science. 324 (5935): 1666–1669. [DOI] [PubMed] [Google Scholar]
- 40.Deslouches B, DI YP. (2017) Antimicrobial peptides with selective antitumor mechanisms: prospect for anticancer applications. Oncotarget. 8(28): 46635–46651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ziaja M, Dziedzic A, Szafraniec K. (2020) Cecropins in cancer therapies-where we have been? Eur J Pharmacol. 882: 173317. [DOI] [PubMed] [Google Scholar]
- 42.Bensaoud C, Abdelkafi-Koubaa Z, Ben Mabrouk H. (2017) Hyalomma dromedarii (Acari: Ixodidae) salivary gland extract inhibits angiogenesis and exhibits in vitro antitumor effects. J Med Entomol. 54(6): 1476–1482. [DOI] [PubMed] [Google Scholar]
- 43.De Sa Jounior PL, Camara DA, Sciani JM, Porcacchia AS, Fonseca PM, Mendonca RZ, Elifo-Esposito S, Simons SM. (2019) Antiproliferative and antiangiogenic effect of Amblyomma sculptum (Acari: Ixodidae) crude saliva in endothelial cells in vitro. Biomed Pharmacother. 110: 353–361. [DOI] [PubMed] [Google Scholar]
- 44.Simons MS, De Sa Junior PL, Faria F. (2011) The action of Amblyomma cajennense tick saliva in compounds of the hemostatic system and cytotoxicity in tumor cell lines. Biomed Pharmacother. 65(6): 443–450. [DOI] [PubMed] [Google Scholar]
- 45.Deslouches B, Steckbeck JD, Craigo JK, Doi Y, Burns JL, Montelaro RC. (2015) Engineered cationic antimicrobial peptides to overcome multidrug resistance by ESKAPE pathogens. Antimicrob Agents Chemother. 59(2): 1329–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Destoumieux-Garzon D, Rosa RD, Schmitt P, Barreto C, Vidal-Dupiol J, Mitta G, Gueguen Y, Bachere E. (2016) Antimicrobial peptides in marine invertebrate health and disease. Philos Trans R Soc Lond B Biol Sci. 371(1695): 20150300. [DOI] [PMC free article] [PubMed] [Google Scholar]