Abstract
Study Purpose:
Continuous renal replacement therapy (CRRT) is used for supportive management of acute kidney injury (AKI) and disorders of fluid balance (FB). Little is known about the predictors of successful liberation in children and young adults. We aimed to identify the factors associated with successful CRRT liberation.
Methods:
The Worldwide Exploration of Renal Replacement Outcomes Collaborative in Kidney Disease study is an international multicenter retrospective study (32 centers, 7 nations) conducted from 2015-2021 in children and young adults (aged 0-25 years) treated with CRRT for AKI or FB disorders. Patients with previous dialysis dependence, tandem extracorporeal membrane oxygenation use, died within the first 72 hours of CRRT initiation, and those who never had liberation attempted were excluded. Patients were categorized based on first liberation attempt: reinstituted (resumption of any dialysis within 72 hours) versus success (no receipt of dialysis for ≥72 hours). Multivariable logistic regression was used to identify factors associated with successful CRRT liberation.
Results:
A total of 622 patients were included: 287 (46%) had CRRT reinstituted and 335 (54%) were successfully liberated. After adjusting for sepsis at admission and illness severity parameters, several factors were associated with successful liberation, including higher VIS (vasoactive-inotropic score) at CRRT initiation (OR 1.35 [1.12-1.63]), higher PELOD-2 (pediatric logistic organ dysfunction-2) score at CRRT initiation (OR 1.71 [1.24-2.35]), higher urine output prior to CRRT initiation (OR 1.15 [1.001-1.32]), and shorter CRRT duration (OR 0.19 [0.12-0.28]).
Conclusion:
Inability to liberate from CRRT was common in this multinational retrospective study. Modifiable and non-modifiable factors were associated with successful liberation. These results may inform the design of future clinical trials to optimize likelihood of CRRT liberation success.
Keywords: CRRT, dialysis, pediatrics
Tweet:
Liberation of CRRT in children and young adults is influenced by modifiable and non-modifiable factors.
Introduction
Acute kidney injury (AKI) affects 20-25% of critically ill children and young adults and has no effective treatment options1. In patients of all ages, when AKI and excessive positive fluid balance (FB) (i.e., pathologic fluid balance) become severe enough, continuous renal replacement therapy (CRRT) is initiated to remove toxins and solutes, regulate fluid balance, maintain acid base status, control electrolytes, and allow for provision of nutrition and life sustaining medications, including antibiotics. Although a life-saving technology, extracorporeal CRRT is resource intensive, necessitates vascular access and anticoagulation, may limit early mobilization therapies2 and affects medication pharmacokinetics3. Furthermore, more than half of pediatric patients historically requiring CRRT do not survive, while others may not recover kidney function4,5. Thus, a better understanding of the clinical trajectory of children and young adults requiring CRRT is needed to improve outcomes and inform decision making.
Despite the widespread use of CRRT, little is known about which factors portend a greater success for liberation. Understanding these factors may allow optimization of outcomes. In patients who fail a CRRT liberation attempt, reinstitution of CRRT may result in hemodynamic changes, electrolyte and acid/base derangements, the negative consequences of further fluid accumulation, and additional blood product transfusions. Conversely, waiting longer than necessary for liberation increases the potential for higher hospital morbidity, healthcare costs, and delayed or impaired renal recovery6,7. Among pediatric patients, only urine output (UOP) peri-CRRT discontinuation attempt has been reported to be associated with successful liberation8. There is a paucity of data to guide clinicians on when to trial CRRT liberation, including what factors might contribute to CRRT reinstitution.
Given these knowledge gaps we queried a multinational database of pediatric patients who required CRRT (Worldwide Exploration of Renal Replacement Outcomes Collaborative in Kidney Disease; WE-ROCK)9; (Presented at the 28th International Advances in Critical Care Nephrology AKI&CRRT Conference; February 202310,11). We aimed to 1) identify the clinical factors that predict successful liberation; 2) evaluate the association between duration of CRRT and liberation; 3) determine the association between liberation status and mortality; and 4) investigate the association between liberation and morbidity (ventilator free days and ICU free days). We hypothesized that clinical factors available at the time of CRRT initiation and potential liberation could predict success. Additional hypotheses included that longer CRRT duration would be associated with a lower probability of liberation success, and that successful liberation would be associated with lower morbidity and mortality.
Methods:
Study Population:
The Worldwide Exploration of Renal Replacement Outcomes Collaborative in Kidney Disease (WE-ROCK) is an international registry of 996 patients from 32 centers and 7 nations who received CRRT from 2015-2021, with the majority (n = 932, 94%) of patients from 2018-2021. Patients were included if they were between 0-25 years of age and required CRRT due to AKI or FB disorders. As young adults with chronic diseases or comorbidities starting in early childhood are frequently admitted to pediatric institutions throughout early adulthood, we included those up to age 25 to capture the full spectrum of CRRT delivered in children’s hospitals. Patients with end-stage kidney disease (ESKD) who were dialysis dependent, had concurrent extracorporeal membrane oxygenation use, or those who received CRRT for a different primary indication (i.e., acute liver failure, intoxications, and inborn errors of metabolism) were excluded. Additional exclusion criteria were severe congenital anomalies of the kidney and urinary tracts with a high probability to progress to ESKD and chronic kidney disease with expected dialysis dependence due to natural disease course. For this analysis, we also excluded patients in whom liberation was not attempted during the first 28 days of CRRT or who died within the first 72 hours of CRRT. Detailed methods have been previously published12. All contributing centers had approval from their local institutional review board with a waiver of informed consent. Data sharing agreements were instituted between each site and the lead site.
Data Collection:
In brief, retrospective chart review was performed and data was entered into a central Research Electronic Data Capture (REDCap) database. Patient baseline and admission characteristics (admission category, comorbidities, and presence of sepsis) were obtained. Illness severity data at ICU admission: pediatric risk of mortality III (PRISM-III) score13 and highest values within 24 hours prior to CRRT initiation (vasoactive-inotrope [VIS], Pediatric logistic organ dysfunction score [PELOD-2 score]14, and cumulative fluid balance percent [%cFB]) were obtained. %cFB at CRRT initiation was defined as (100 x [total intake-total output at CRRT initiation]/ICU admission weight)15. Additional measurements of cumulative fluid balance including evaluation on day 3 of CRRT therapy (total intake and total output since day of ICU admission through day 3). Day 3 was chosen as this represents the 25th percentile of CRRT duration for the entire cohort, allowing us to capture a majority of patients still receiving therapy. UOP prior to CRRT initiation, time to CRRT initiation from ICU admission, CRRT dose, and daily %FB were recorded. A sub-analysis was performed on the subset of patients who received a loop diuretic within the 24 hours preceding CRRT liberation attempt.
Liberation Definitions:
Patients were categorized into two liberation categories based on the first liberation attempt during the first 28 days of therapy. A threshold of 72 hours was pre-specified based on prior evidence that delineated status at 72 hours after first CRRT liberation attempt16,17. Further, this timepoint was felt to be short enough to likely avoid other confounding events that occur in critically ill patients.
Liberated: patients had no receipt of CRRT or other dialysis modality for ≥72 hours after discontinuing CRRT.
Reinstituted: patients resumed CRRT or another dialysis modality within 72 hours after discontinuing CRRT.
Outcomes
The primary outcome was successful CRRT liberation. Secondary outcomes included duration of CRRT, mortality rates at ICU discharge, hospital discharge, and 90-day, 28-day ICU-free days, and 28-day ventilator free days. Ventilator and ICU free days for patients who died was 0.
Statistical Analysis:
Patient demographic and clinical characteristics were described using medians with interquartile ranges (IQR) for continuous variables and frequencies with percentages for categorical variables. Wilcoxon rank sum tests and chi-square tests were used to test for differences in continuous and categorical variables, respectively, according to liberation status.
A multivariable logistic regression model was used to estimate adjusted odds ratios (aOR) and 95% confidence intervals (CI) for successful liberation according to covariates of interest. For continuous predictors, odds ratios were presented for a comparison of the 75th versus 25th percentile (i.e., IQR odds ratios). A priori relevant covariates were selected based on consensus of the WE-ROCK investigators and existing literature4,5,18-20. Robust standard errors were obtained using the Huber-White method to correct for the clustering of patients within hospitals. To allow for potential non-linear associations, CRRT duration was modeled using restricted cubic spline terms with default four knots (5th, 35th, 65th, and 95th percentiles) as recommended by Harrell21. The final number of knots was determined based on the model Akaike Information Criterion. Predicted probabilities of liberation success by CRRT duration were obtained via an inverse logit transformation of the log odds. Patients who died within 72 hours of CRRT initiation were excluded from analyses. There was no imputation performed for missing data. All analyses included complete cases only and we expect any potential impact of missing data on the observed association to be minimal as the overall proportion was below 5% for the primary analysis.
Two sub-analyses were performed. First, we evaluated patients who received a loop diuretic within 24 hours preceding the CRRT liberation attempt using similar methods. UOP for these patients was recorded for 6 hours following loop diuretic administration and included in the multivariable logistic regression model. UOP at CRRT initiation and UOP at CRRT liberation attempt were strongly correlated (Pearson’s r =0.67), so were not included in the same model to avoid multicollinearity. Second, an alternative measure of cumulative fluid balance through CRRT day 3 was evaluated in the subset of patients who were still receiving CRRT at this timepoint. Two-sided p-values <0.05 were considered statistically significant. All statistical analyses were performed using R version 4.3.1 statistical software (https://www.r-project.org/). The logistic regression models were fit using the lrm function in the R package rms (version 6.7.1)22. The robust Huber-White variance was calculated using the robcov function in the rms package.
Results
Demographics of the Cohort:
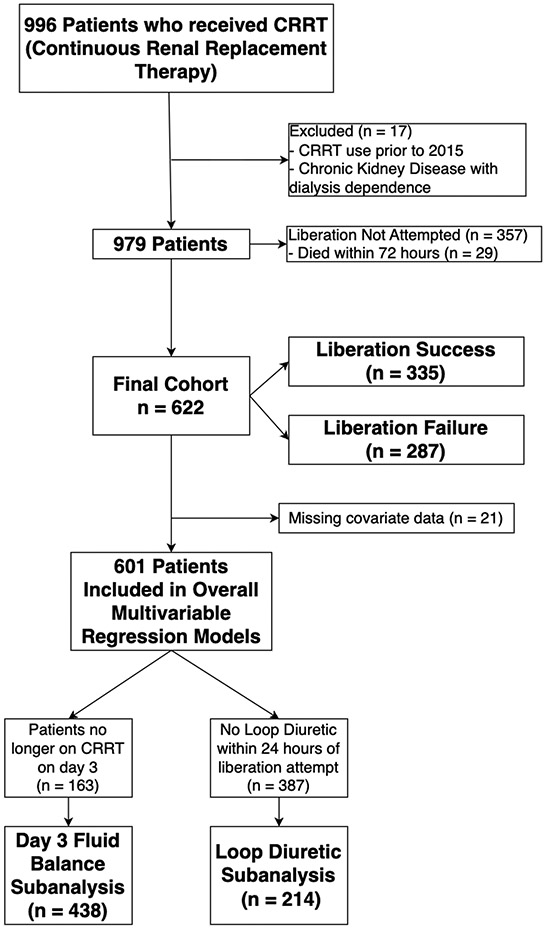
Nine hundred ninety-six patients were included in the registry. After exclusions for use of CRRT prior to 2015 or missing outcome data (n=17), 979 patients remained. Liberation was not attempted during the first 28 days of therapy in 357 (36%) patients. Therefore, 622 patients were included in the final analysis (Figure 1). The median age of the cohort was 9 years (IQR 2, 14.9) and 46% were female (Table 1). There were no underlying comorbidities in 23% of patients; the most common comorbidities were cardiac (19%), oncologic (20%), and gastrointestinal (19%) (Supplemental Table 1). The most common admission category was shock/infection/trauma (38%), and 262 (42%) had sepsis at ICU admission. The overall cohort had high illness severity estimates, with a median PRISM-III score of 14 (IQR 9, 18) at ICU admission and median PELOD-2 score at CRRT initiation of 6 (IQR 4, 8). Additional patient demographic and clinical characteristics are summarized in Table 1 and Supplemental Table 1.
Figure 1:
Consort Diagram of the Included Patients. There were 996 patients in the initial registry, of which 17 were removed as CRRT (Continuous renal replacement therapy) was performed prior to 2015 or the patients had a diagnosis of CKD (chronic kidney disease with dialysis dependence). In 357 patients there was no attempt at CRRT liberation or patients died within 72 hours after CRRT initiation and were not included in the analysis. This resulted in a final cohort of 622 patients. There was missing covariate data for 21 patients, and thus 601 patients were included in overall multivariable regression models. Subanalyses were performed based on patient characteristics.
Table 1.
Patient Characteristics
| Variable | Overall N = 622 |
Reinstituted N = 287 |
Liberated N = 335 |
p-value | |
|---|---|---|---|---|---|
| Patient Baseline Characteristics | |||||
| Age (years) | 9.0 (2.0, 14.9) | 10.8 (2.5, 15.2) | 6.2 (1.8, 14.4) | 0.04 | |
| Gender (female) | 286 (46%) | 141 (49%) | 145 (43%) | 0.2 | |
| Primary Comorbidities: None | 146 (23%) | 62 (22%) | 84 (25%) | 0.4 | |
| Primary Comorbidities: Renal/Urologic | 64 (10%) | 41 (14%) | 23 (6.9%) | 0.004 | |
| Primary Comorbidities: Immunologic | 78 (13%) | 47 (16%) | 31 (9.3%) | 0.011 | |
| Admission Characteristics | |||||
| Admission weight (kg) | 28.9 (12.7, 58.2) | 35.3 (13.8, 60.0) | 23.8 (11.5, 52.6) | 0.006 | |
| Admission Category | 0.3 | ||||
| Shock/Infection/Major Trauma | 234 (38%) | 101 (35%) | 133 (40%) | ||
| Respiratory Failure | 98 (16%) | 49 (17%) | 49 (15%) | ||
| Other | 152 (24%) | 79 (27%) | 73 (22%) | ||
| Illness Severity Parameters | |||||
| Sepsis at ICU Admission1 | 262 (42%) | 129 (45%) | 133 (40%) | 0.2 | |
| PELOD-22 Score at CRRT Initiation | 6 (4, 8) | 6 (3.5, 8) | 6 (4, 8) | 0.11 | |
| PELOD-2 Score at CRRT Liberation Attempt | 5 (3, 7) | 5 (2.5, 7) | 5 (3, 7) | 0.15 | |
| VIS3 at CRRT Initiation | 3 (0,15) | 0 (0, 11.2) | 5 (0, 20) | <0.001 | |
| VIS at CRRT Liberation Attempt | 0 (0, 3) | 0 (0, 2) | 0 (0, 3.3) | 0.2 | |
| PRISM-III4 Score at ICU Admission | 14 (9, 18) | 13 (9, 18) | 14 (10, 18) | 0.3 | |
| Time from ICU admission to CRRT initiation (days) | 2 (1, 5) | 2 (1, 5) | 2 (1, 4.5) | 0.6 | |
| Fluid Balance Parameters | |||||
| Urine output 24 h prior to CRRT initiation (ml/kg/h) | 0.5 (0.1, 1.3) | 0.4 (0.4, 1.11) | 0.5 (0.2, 1.5) | 0.08 | |
| Urine output 6h prior to CRRT liberation attempt (ml/kg/h) with loop diuretic | 0.5 (0.1, 1.5) N = 214 |
0.4 (0.1, 1.2) N = 107 |
0.70 (0.1, 2.1) N = 107 |
0.08 | |
| Time to first day of negative Fluid Balance (FB5) | 0.022 | ||||
| Day 0 | 243 (39%) | 113 (40%) | 130 (39%) | ||
| Day 1 | 211 (34%) | 106 (37%) | 105 (31%) | ||
| Fluid Balance Never Negative | 66 (11%) | 16 (5.6%) | 50 (15%) | <0.001 | |
| Cumulative Fluid Balance Percent (%cFB6) from ICU admission to CRRT initiation | 7.2 (2.1, 16.8) | 7.4 (1.9, 17.3) | 7.0 (2.2, 16.8) | 0.8 | |
| %cFB from ICU admission to CRRT day 3 | −21.5 (−71.1, 25.3) N =456 |
−20.3 (−73.4, 21.0) N = 223 |
−23.4 (−70.4, 29.7) N =233 |
0.8 | |
| % daily fluid balance day 4 (ml/kg) | −3.4 (−23.7, 8.8) N=373 |
−1.2 (−18.1, 8.4) N=183 |
−8.1 (−29.8, 8.7) N=190 |
0.02 | |
| % daily fluid balance day 5 (ml/kg) | −3.6 (−19.3, 12.3) N=304 |
0.1 (−16.2, 15.8) N=162 |
−6.4 (−21.9, 6.6) N=142 |
0.03 | |
Categorical variables are presented as n (%) and Continuous variables as median (IQR); P-values calculated using chi-square test or Wilcoxon rank sum test.
ICU: Intensive Care Unit
PELOD-2: Pediatric logistic organ dysfunction score
VIS: Vasoactive-inotrope score
PRISM-III: Pediatric risk of mortality III score
FB: Fluid Balance
%cFB: Cumulative fluid balance percent
Demographic Differences between the Liberated and Reinstituted Groups:
Of the 622 patients who had a CRRT liberation attempt, 53.8% (n=335) were successful (Figure 1). Comparisons between liberated and reinstituted groups are summarized in Table 1 and Supplemental Table 1. Liberated patients were younger than those for whom CRRT was reinstituted (6.2 vs. 10.8 years, p=0.04) and had a lower admission weight (23.8 kg vs. 35.3 kg; p= 0.006). Immunologic comorbidity and renal/urologic comorbidities were less common among those who successfully liberated.
Illness Severity Parameters:
There was no difference in PELOD-2 score at CRRT initiation or at time of CRRT liberation attempt, or in the incidence of sepsis between groups (Table 1). The liberated group had higher VIS (median 5; IQR 0, 20) at CRRT initiation compared to the reinstituted group (median 0; IQR 0, 11.2; p < 0.001), but there was no difference in VIS at time of CRRT liberation attempt. The liberated group had higher indexed UOP in the 24 hours prior to CRRT initiation (median 0.5; IQR 0.2, 1.5) vs the reinstituted group (median 0.4; IQR 04, 1.1), p = 0.08. A subset of patients (n = 214) who received a loop diuretic within 24 hours prior to CRRT liberation attempt had UOP recorded. Among this subset, the liberated group had higher indexed UOP in the 6 hours preceding CRRT liberation attempt (median 0.70; IQR 0.1, 2.1 vs. median 0.4; IQR 0.1, 1.2), although this difference was not significant (p= 0.08).
Fluid Balance and CRRT prescriptions:
Median %cFB from ICU admission to CRRT initiation was 7.2% (IQR 2.1, 16.8) and the median time from ICU admission to CRRT initiation was 2 days (IQR 1, 5) (Table 1). In the 456 (73%) patients still on CRRT at day 3, median %cFB from ICU admission to CRRT day 3 was −21.5% (IQR −71.1, 25.3), and there was no difference between the liberated and reinstituted groups (p=0.8). There were differences in time to first day of negative fluid balance between groups, with a higher proportion of patients achieving negative fluid balance on day 1 in the reinstituted group (37%) compared to the liberated group (31%), p=0.03. In addition, 15% of liberated patients (n= 50) never achieved a net negative fluid balance as compared to 5.6% (n=16) of the reinstituted patients (p < 0.001). The liberated group had a more negative daily %FB on day 4 (median −8.1; IQR −29.8, 8.7 vs. median −1.2; IQR −18.1, 8.4; p 0.02) and day 5 (median −6.4; IQR −21.9, 6.6 vs. median 0.1; IQR −16.2, 15.8; p= 0.03) compared to the reinstituted group, Supplemental Table 2. Prescribed CRRT dose was significantly higher in liberated patients on day 0 (median 43.1 dose/kg; IQR 31.4, 60 vs. median 38.1; IQR 28.4, 55.0; p=0.01) and day 1 (median 43.9 dose/kg; IQR 32, 60.1 vs. median 39.0 dose/kg; IQR 28.8, 54.2; p = 0.004), Supplemental Table 2.
Outcomes Based on Liberation Patterns
Table 2 shows patient outcomes based on liberation patterns. Mortality at all time points was higher in the reinstituted group, with an overall ICU mortality of 15% compared to 6.9% in the liberated group (p = 0.002). There was no difference in days to death after liberation attempt between groups. The liberated group had more ventilator free days (median 28, IQR 16, 28) compared to reinstituted group (median 23, IQR 5, 28), p=0.005. Additionally, the liberated group had more ICU free days (median 8, IQR 0, 17) compared to reinstituted group (median 0, IQR 0, 12), p <0.001. The majority of patients who were reinstituted were transitioned to intermittent hemodialysis (58%) while 39% were reinitiated on CRRT. Patients who reinstituted had a significantly higher rate of RRT dependence at 90 days as compared to those who successfully liberated (24% vs. 1.3%, p<0.001).
Table 2.
Patient Outcomes based on Liberation Success or Reinstitution
| Variable | Overall N = 622 |
Reinstituted N = 287 |
Liberated N = 335 |
p-value |
|---|---|---|---|---|
| CRRT1 duration (days) | 5 (3, 12) | 9 (4, 17) | 4 (2, 7) | <0.001 |
| ICU2 Mortality | 65 (10%) | 42 (15%) | 23 (6.9%) | 0.002 |
| Hospital Mortality | 79 (13%) | 48 (17%) | 31 (9.3%) | 0.008 |
| 90 Day Mortality | 76 (12%) | 46 (16%) | 30 (9%) | 0.01 |
| Time to Death (days after liberation attempt) | 28.5 (17, 51) N = 76 |
28.0 (19, 50.3) N = 46 |
30 (15.5, 50) N = 30 |
0.9 |
| 28-day ventilator-free days | 26 (11.8, 28) | 23 (5, 28) | 28 (16, 28) | 0.005 |
| 28-day ICU-free days | 5 (0, 15) | 0 (0, 12) | 8 (0, 17) | <0.001 |
| RRT3 dependence at 90 days | 61 (11%) N = 545 |
57 (24%) N = 240 |
4 (1.3%) N = 325 |
<0.001 |
Categorical variables are presented as n (%) and Continuous variables as median (interquartile range); P-values calculated using chi-square test or Wilcoxon rank sum test.
CRRT: Continuous renal replacement therapy
ICU: Intensive Care Unit
RRT: Renal Replacement Therapy
Multivariable Analysis for Associations with Liberation Success
Supplemental Table 3 shows all variables included in the multivariable regression model and Table 3 shows which variables were significantly associated with liberation success. Higher VIS at CRRT initiation (IQR OR 1.35; 95% CI 1.12-1.63), lower VIS at liberation attempt (IQR OR 0.99; 95% CI 0.98-0.99), and higher urine output prior to CRRT initiation (IQR OR 1.15; 95% CI 1.001-1.32) were associated with an increased odds of successful liberation. Increased odds of successful liberation were also seen in patients with higher PELOD-2 score at CRRT initiation (IQR OR 1.71; 95% CI 1.24-2.35). Earlier time to initiate CRRT trended towards increased odds of successful liberation (IQR OR 0.95; 95% CI 0.9-1.000) but was not significant. Having an underlying renal/urologic comorbidity was inversely associated with successful liberation (aOR: 0.39; 95% CI 0.20 – 0.76). Included renal/urologic comorbidities are summarized in supplemental table 4.
Table 3.
Multivariable regression model predicting successful CRRT liberation (n = 601).
| Significant Predictor Variables | Reference | Contrast | OR (95%CI) |
|---|---|---|---|
| Primary Comorbidities: Renal/Urologic | No | Yes | 0.38 (0.20-0.75) |
| Vasopressor-Inotrope Score at CRRT1 Initiation | 0.0 | 20.0 | 1.35 (1.12-1.63) |
| Vasopressor-Inotrope Score at Liberation Attempt | 0.0 | 3.6 | 0.99 (0.98-0.999) |
| PELOD-22 Score at CRRT Initiation | 4.0 | 9.0 | 1.71 (1.24 - 2.35) |
| Urine output (24h prior to CRRT Initiation) (ml/kg/h) | 0.1 | 1.2 | 1.15 (1.001-1.32) |
| CRRT duration (days) | 3.0 | 14.0 | 0.19 (0.12-0.28) |
Odds ratio (OR) and 95% confidence intervals (CI) obtained by logistic regression. The model accounts for the nesting of patients within hospitals via the Huber-While cluster sandwich estimator of variance. ORs for continuous predictors scaled to reflect the interquartile range odds ratio (i.e., reference = 25th percentile, contrast = 75th percentile). Other variables adjusted for include the following: weight, presence of no comorbidities, cardiac comorbidity, oncologic comorbidity, immunologic comorbidity, sepsis at ICU3 admission, PELOD-2 score at crrt liberation attempt, % fluid balance (ICU admit to CRRT initiation), calculated CRRT dose, time from ICU admission to CRRT initiation. 21 (3.4%) out of 622 patients were not included in the analysis due to missing covariate data.
CRRT: Continuous Renal Replacement Therapy
PELOD-2: Pediatric logistic organ dysfunction score
ICU: Intensive Care Unit
Diuretic Challenge Sub-Analysis
For the subset of patients who received a diuretic challenge prior to liberation attempt, multivariable analysis was conducted and adjusted for comorbidities, VIS, PELOD-2 score, %cFB at CRRT initiation, and UOP prior to CRRT liberation attempt. After adjusting for these variables, higher UOP prior to CRRT liberation was associated with a higher odds of successful liberation (IQR OR 1.32; 95% CI 1.08-1.61), Supplemental Table 5.
Day 3 Cumulative Fluid Balance Sub-Analysis
For the subset of patients who remained on CRRT on day 3, multivariable analysis was conducted to determine if there was an association between this variable and liberation success (Supplemental Table 6). Higher VIS at CRRT initiation, higher PELOD-2 at CRRT initiation, and higher UOP at CRRT initiation remained significantly associated with successful liberation. There was no association between day 3 %cFB and liberation status (p = 0.371).
Association between CRRT duration and probability of successful liberation
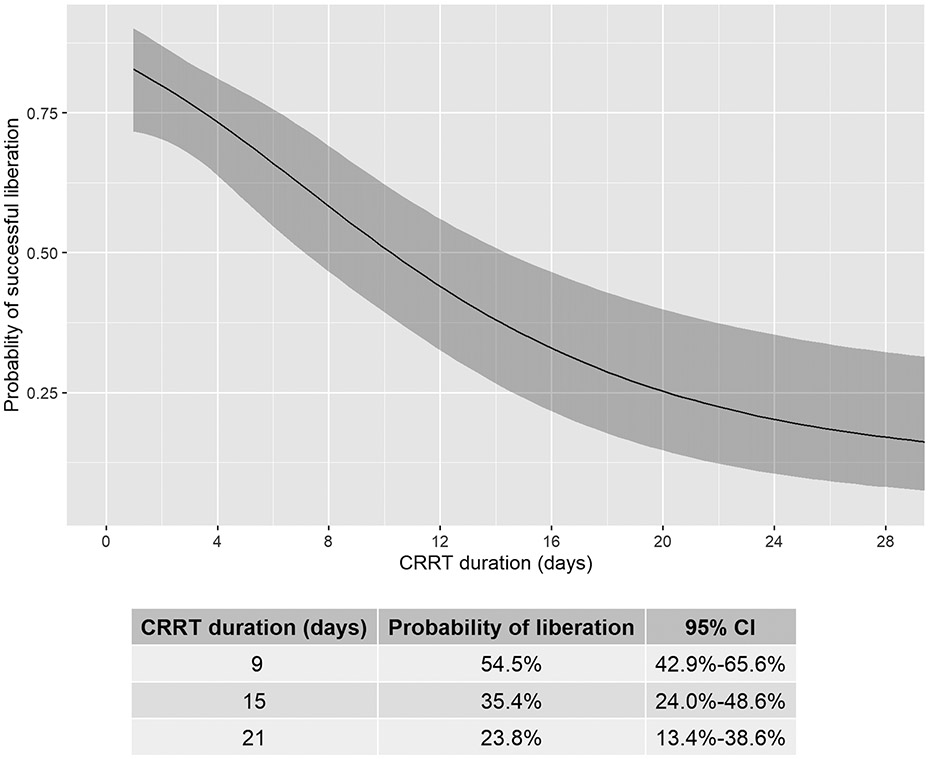
Figure 2 shows the nonlinear relationship between CRRT duration and successful liberation. The probability of successful liberation declined as CRRT duration increased. On day 9, the probability of successful liberation was 0.55 (95% CI 0.43, 0.66) and declined by day 15 to 0.35 (95% CI 0.24, 0.49) and day 21 to 0.24 (95% CI 0.13, 0.39).
Figure 2.
Predicted probability of successful CRRT (Continuous renal replacement therapy) liberation as a function of duration of CRRT days from multivariate logistic regression, adjusted for a priori relevant covariates (Supplemental Table 3) at the most frequent or median level. CRRT duration was modeled with restricted cubic splines (4 knots) to allow for potential non-linear association with successful liberation. CRRT duration (days) is significantly associated with decreased probabilities of successful liberation (p< 0.001). Shaded area denotes 95% confidence intervals.
Discussion:
In this retrospective, multinational cohort study, we provide a comprehensive evaluation of factors associated with successful liberation from CRRT in critically ill pediatric and young adult patients. Our key findings identified higher VIS and higher PELOD-2 score at CRRT initiation as predictive of successful CRRT liberation. Additionally, higher UOP at CRRT initiation, and higher UOP with diuretic administration at time of CRRT liberation attempt was predictive of successful CRRT liberation. Furthermore, we show that patients who required RRT reinstitution had worse outcomes (higher mortality, higher RRT dependence at 90 days, fewer ventilator free days and fewer ICU free days). To our knowledge, this is the first large, multicenter study in pediatric patients to identify these factors predicting CRRT successful liberation.
In evaluating patient characteristics, we show that higher VIS and higher PELOD-2 at CRRT initiation and lower VIS at liberation attempt were associated with successful CRRT liberation. Prior studies did not identify these findings, and even had trends showing that lower VIS at CRRT initiation and at CRRT liberation attempt were associated with liberation success23,24.The newly identified association between higher VIS and PELOD-2 and increased liberation success is likely multifactorial and the proposed explanations are purely speculative. In evaluating potential differences in underlying disease pathophysiology as a potential explanation for these findings we did not find different rates of sepsis or cardiac comorbidities. However, a higher VIS and a higher PELOD-2 score may reflect a greater degree of hemodynamic compromise or shock, which is potentially more reversible and thus associated with higher rates of liberation success. Another potential explanation is that clinicians may be more inclined to institute CRRT for volume management in patients that are sicker for controlled fluid removal and less inclined to undertake prolonged diuretic trials. Notably, at time of CRRT liberation attempt, the PELOD-2 score was no longer different while a lower VIS was associated with higher rates of liberation success, adding credence to the reversibility of hemodynamic compromise in these patients. In the future, clinical practice guidelines and trial development may use improvement in VIS and illness severity scores as potential indicators for timing of liberation attempts.
While intuitively straight-forward, the current study highlights the importance of urine output at multiple timepoints before and during CRRT for successful liberation. The current study evaluated UOP at CRRT initiation and at the time of CRRT liberation attempt (in patients who received a loop diuretic). We found that UOP prior to CRRT initiation was associated with CRRT liberation success. Further, our data supports the findings from a single-center pediatric study which identified that higher UOP in the period immediately preceding CRRT discontinuation to predict liberation success23. Preservation of UOP (prior to CRRT initiation, during CRRT, and after CRRT liberation attempt) has been identified as a positive predictor of successful CRRT liberation in adult critically ill patients16,17,25,26. These UOP findings point to the preservation of kidney function which may be potentially mediated through the maintenance of an adequate renal perfusion pressure and gentle fluid removal strategies to avoid dialy-trauma27-29. We speculate that renal perfusion pressures were optimized in patients with a higher VIS resulting in higher mean arterial pressure goals and higher UOP.
This study also details that the degree of pathologic positive %cFB at CRRT initiation is lower than prior reports. Prior evidence detailed the negative impacts of pathologic positive %cFB at CRRT initiation5 with the lowest survival rates seen in patients with high FB and electrolyte abnormalities20. Following that publication, a smaller single-center study showed a trend towards a less positive fluid balance at CRRT initiation over the years30. The majority of our patients were from 2018-2021 and thus demonstrates relative improvement in overall %cFB at CRRT initiation. This can potentially be explained by increased physician awareness of the deleterious effects of fluid accumulation.
There is also increasing awareness on the potential harms of rapid fluid removal during dialysis, which causes “dialy-trauma” and is associated with poor renal recovery31-36. In our study, we demonstrate that patients who required CRRT reinstitution had earlier time to negative fluid balance. It was not until day 4 of CRRT therapy that the liberated patients had more fluid removal than the reinstituted patients. While CRRT prescribed dose was significantly higher on day 0 and 1 in patients who ultimately liberated from CRRT, we were unable to evaluate the delivered dose in this cohort, leading to caution in interpreting this finding. Aggressive fluid removal was recently shown in adults to be associated with higher mortality rates28, and lower rates of renal recovery and RRT liberation29. The rate and volume of fluid removed during CRRT is currently being studied prospectively in adults (NCT05306964). Further investigation into this association and the potential underlying mechanisms, including severity of illness, fluid balance at CRRT initiation, rates of fluid removal, and timing of CRRT initiation are warranted.
In the current study we sought to evaluate the optimal timing of CRRT initiation and its impact on liberation outcomes. While not statistically significant, patients who initiated CRRT earlier trended towards successful liberation. When this is interpreted in the context of the finding that the liberated group had a longer time to negative fluid balance, it suggests that early CRRT initiation may allow for slower, more physiologic fluid removal and avoidance of dialy-trauma. Earlier initiation may have led to faster improvement of their underlying disease state, potentially through safer administration of nephrotoxic medications, earlier provision of nutrition, and slower fluid removal.
Our study adds to the literature showing the association between failure to liberate from CRRT and the associated morbidity and mortality. The mortality rates in both groups were high, but nearly double in patients who had CRRT reinstituted. There are likely many factors that contribute to mortality and future studies from this cohort will delve into this further. Taken together with the previously described findings above, the current study highlights the importance of successful liberation from CRRT on patient outcomes. This should be taken as a call to arms to systematically study CRRT as the complex procedure it is. The evaluation of initiation parameters, treatment decisions (dose, fluid removal, etc.), and liberation predictors are complex and intertwined. The current study takes the important first steps by showing the CRRT duration and severity of illness, are associated with successful CRRT liberation. These will be the building blocks for protocol and trial development evaluating liberation strategies.
Our study has many strengths. This was a very large heterogeneous cohort that included critically ill patients from multiple institutions from numerous countries, and therefore our data is likely generalizable. We included several clinical factors that have the potential to aid in development of risk tools for clinical decision support for when to consider liberation. Finally, we show that a prior modifiable risk factor (%cFB at CRRT initiation), has been optimized as the majority of patients had <20% cFB at time of CRRT initiation5,37. In addition to these strengths there are some limitations. Only the initial attempt at CRRT liberation was documented and we therefore may have missed patients who failed the first liberation attempt but were able to successfully liberate afterwards. We also did not capture the specific indication for CRRT initiation or need for re-initiation. Additionally, we did not have access to blood pressure and central venous pressure measurements throughout the ICU admission and at CRRT liberation attempt. We evaluated the predictors of liberation in our sample as a whole. It is possible that the variables associated with successful liberation will be different when patients are categorized by distinct patient level and disease subtypes. We plan to evaluate these in future work using existing registry data. While we did include centers from many countries, all were tertiary or quaternary, potentially limiting generalizability to smaller centers and resource limited environments using other dialytic modalities such as peritoneal dialysis. As with all retrospective studies, we can only test for associations, not causality. Finally, only multivariable results using the complete data are reported and patients with missing data excluded from these analyses.
In conclusion, this is the largest study describing clinically relevant factors for successful CRRT liberation in a multinational cohort of critically ill pediatric patients. We identified factors that may guide clinicians as it relates to family counseling regarding conversion to more durable access and intermittent dialysis modalities. We also generate hypotheses that may inform future clinical research for timing of CRRT initiation, optimizing blood pressure targets and thus renal perfusion pressure, which impacts UOP.
Supplementary Material
Take-Home Message:
In a retrospective analysis of over 600 critically ill children and young adults supported with continuous renal replacement therapy (CRRT), illness severity parameters, duration of continuous renal replacement therapy influence successful liberation. Future prospective trials should focus on initiation parameters and liberation predictors of CRRT in critically ill patients.
Acknowledgements
T. Christine E. Alvarez MHI RN1, Elizabeth Bixler BS2, Erica Blender Brown MA, CRA3, Cheryl L Brown BS1, Ambra Burrell BA4, Anwesh Dash BS5, Jennifer L Ehrlich RN MHA6, Simrandeep Farma HBSc7, Kim Gahring RN BSN, CCRN8, Barbara Gales RN9, Madison R Hilgenkamp10, Sonal Jain MS11, Kate Kanwar BA MS4, Jennifer Lusk BSN RN, CCRN8, Christopher J. Meyer BA AA1, Katherine Plomaritas BSN RN12, Joshua Porter BS5, Jessica Potts BSN RN13, Alyssa Serratore BNurs, GDipNP(PIC), RN, MsC14, Elizabeth Schneider BS5, Vidushi Sinha BS5, PJ Strack RN,BSN,CCRN15, Sue Taylor RN16, Katherine Twombley MD3, Brynna Van Wyk MSN, ARNP CPNP6, Samantha Wallace MS17, Janet Wang BS5, Megan Woods BS5, Marcia Zinger RN18, Alison Zong BS5
1Cincinnati Children's Hospital Medical Center, Cincinnati, OH, USA
2Baylor College of Medicine, Texas Children's Hospital, Houston, TX, USA
3Medical University of South Carolina, Charleston, SC, USA
4Nationwide Children's Hospital, Columbus, OH, USA
5University of Tennessee Health Science Center College of Medicine, Memphis, TN, USA
6University of Iowa Stead Family Children's Hospital, Carver College of Medicine, Iowa City, IA, USA
7Hospital for Sick Children, Toronto, ON, Canada
8Children's Hospital Colorado, Aurora, CO, USA
9Mattel Children Hospital at UCLA, Los Angeles, CA, USA
10University of Nebraska Medical Center, Children's Hospital & Medical Center, Omaha, NE, USA
11Seattle Children's Hospital, Seattle, WA, USA
12University of Michigan, C.S. Mott Children's Hospital, Ann Arbor, MI, USA
13Children's of Alabama/University of Alabama at Birmingham, Birmingham, AL, USA
14Royal Children's Hospital, Melbourne, VIC, Australia
15Children's Mercy Hospital, Kansas City, MO, USA
16King's College Hospital, London, England
17Indiana University School of Medicine, Riley Hospital for Children, Indianapolis, IN, USA
18Cohen Children's Medical Center, New Hyde Park, NY, USA
Funding/Support:
REDCap at Cincinnati Children’s Hospital Medical Center is funded and supported by the Center for Clinical and Translational Science and Training grant support (UL1TR001425). The funding sources for this study had no role in the design, conduct, collection, management, analysis, and interpretation of the data, nor the preparation, review, or decision to submit the manuscript for publication.
WE ROCK Collaborative Author List
The following individuals served as collaborators and investigators for the WE-ROCK studies. They collaborated in protocol development and review, data analysis, and participated in drafting or review of the manuscript, and their names should be citable by PubMed.
Emily Ahern CPNP, DNP1, Ayse Akcan Arikan MD2, Issa Alhamoud MD3, Rashid Alobaidi MD, MSc4, Pilar Anton-Martin MD, PhD5, Shanthi S Balani MD6, Matthew Barhight MD, MS7, Abby Basalely MD, MS8, Amee M Bigelow MD, MS9, Gabriella Bottari MD10, Andrea Cappoli MD10, Eileen A Ciccia MD11, Michaela Collins BA12, Denise Colosimo MD13, Gerard Cortina MD14, Mihaela A Damian MD, MPH15, Sara De la Mata Navazo MD16, Gabrielle DeAbreu MD8, Akash Deep MD17, Kathy L Ding BS18, Kristin J Dolan MD2, Sarah N Fernandez Lafever MD, PhD16, Dana Y Fuhrman DO, MS19, Ben Gelbart MBBS20, Katja M Gist , DO MSc12, Stephen M Gorga MD, MSc21, Francesco Guzzi MD22, Isabella Guzzo MD10, Taiki Haga MD23
Elizabeth Harvey MD24, Denise C Hasson MD25, Taylor Hill-Horowitz BS8, Haleigh Inthavong BS, MS2, Catherine Joseph MD2, Ahmad Kaddourah MD, MS26, Aadil Kakajiwala MD, MSCI27, Aaron D Kessel MD, MS8, Sarah Korn DO28, Kelli A Krallman BSN, MS12, David M Kwiatkowski MD Msc29, Jasmine Lee MSc24, Laurance Lequier MD4, Tina Madani Kia BS4, Kenneth E Mah MD, MS15, Eleonora Marinari MD10, Susan D Martin MD30, Shina Menon MD31, Tahagod H Mohamed MD9, Catherine Morgan MD MSc4, Theresa A Mottes APRN7, Melissa A Muff-Luett MD32, Siva Namachivayam MBBS20, Tara M Neumayr MD11, Jennifer Nhan Md, MS27, Abigail O'Rourke MD8, Nicholas J Ollberding PhD12, Matthew G Pinto MD33, Dua Qutob MD26, Valeria Raggi MD10, Stephanie Reynaud MD34, Zaccaria Ricci MD13, Zachary A Rumlow DO3, María J Santiago Lozano MD, PhD16, Emily See MBBS20, David T Selewski MD, MSCR35, Carmela Serpe MSc, PhD10, Alyssa Serratore RN, MsC20, Ananya Shah BS18, Weiwen V Shih MD1,18, H Stella Shin MD36, Cara L Slagle MD12, Sonia Solomon DO33, Danielle E Soranno MD37, Rachana Srivastava MD38, Natalja L Stanski MD12, Michelle C Starr MD, MPH37, Erin K Stenson MD1,18, Amy E Strong MD, MSCE3, Susan A Taylor MSc17, Sameer V Thadani MD2, Amanda M Uber DO32, Brynna Van Wyk ARNP, MSN3, Tennille N Webb MD, MSPH39, Huaiyu Zang PhD12, Emily E Zangla DO6, Michael Zappitelli MD, MSc24
1Children's Hospital Colorado, University of Colorado School of Medicine, Aurora, CO, USA
2Baylor College of Medicine, Texas Children's Hospital, Houston, TX, USA
3University of Iowa Stead Family Children's Hospital, Carver College of Medicine, Iowa City, IA, USA
4Univeristy of Alberta, Edmonton, Canada
5Le Bonheur Children's Hospital, Memphis, TN, USA
6University of Minnesota, Minneapolis, MN, USA
7Ann and Robert H. Lurie Children's Hospital of Chicago, Chicago, IL, USA
8Cohen Children's Medical Center, Zucker School of Medicine, New Hyde Park, NY, USA
9Nationwide Children's Hospital, The Ohio State University College of Medicine, Columbus, OH, USA
10Bambino Gesù Children Hospital, IRCCS, Rome, Italy
11Washington University School of Medicine, St. Louis Children's Hospital, St. Louis, MO, USA
12Cincinnati Children's Hospital Medical Center; University of Cincinnati College of Medicine, Cincinnati, OH, USA
13Meyer Children's Hospital, IRCCS, Florence, Italy
14Medical University of Innsbruck, Innsbruck, Austria
15Stanford University School of Medicine, Palo Alto, CA, USA
16Gregorio Marañón University Hospital; School of Medicine, Madrid, Spain
17King's College Hospital, London, England
18University of Colorado, School of Medicine, Aurora, CO, USA
19University of Pittsburgh Medical Center Children's Hospital of Pittsburgh, Pittsburgh, PA, USA
20Royal Children's Hospital, University of Melbourne, Murdoch Children's Research Institute, Melbourne, Victoria, Australia
21University of Michigan Medical School, C.S. Mott Children's Hospital, Ann Arbor, MI, USA
22Santo Stefano Hospital, Prato, Italy
23 Osaka City General Hospital, Osaka, Japan
24Hospital for Sick Children, Toronto, Ontario, Canada
25NYU Langone Health, Hassenfeld Children’s Hospital, New York, NY, USA
26 Sidra Medicine and Weil Cornel Medicine, Qatar, Doha, Qatar
27Children's National Hospital, Washington, DC, USA
28Westchester Medical Center, Westchester, NY, USA
29Lucile Packard Children's Hospital, Palo Alto, CA, USA
30Golisano Children's Hospital at University of Rochester Medical Center, Rochester, NY, USA
31Seattle Children's Hospital, University of Washington, Seattle, WA, USA
32University of Nebraska Medical Center, Children's Hospital & Medical Center, Omaha, NE, USA
33Maria Fareri Children's Hospital at Westchester Medical Center, Valhalla, NY, USA
34Hopital Bicetre, APHP Université Paris-Saclay, Kremlin-Bicetre, Val de Marne, France
35Medical University of South Carolina, Charleston, SC, USA
36Children's Healthcare of Atlanta, Emory University, Atlanta, GA, USA
37Indiana University School of Medicine, Riley Hospital for Children, Indianapolis, IN, USA
38Mattel Children's Hospital at UCLA, Los Angeles, Ca, USA
39Children's of Alabama/University of Alabama at Birmingham, Birmingham, AL, USA
Footnotes
Disclosures: All authors declare no real or perceived conflicts of interest that could affect the study design, collection, analysis and interpretation of data, writing of the report, or the decision to submit for publication. For full disclosure, we provide here an additional list of other author’s commitments and funding sources that are not directly related to this study: Katja Gist is a consultant for Bioporto Diagnostics and Potrero Medical. No other disclosures were reported.
Data Availability:
De-identified summary data are available through the WE-ROCK collaborative. Data dictionaries, in addition to study protocol, the statistical analysis plan will be made available upon request. More information about the process and available data can be obtained by contacting the corresponding author (EKS). The data from the WE-ROCK collaborative will be made available to researchers who provide a methodologically sound proposal for use in achieving the goals of the approved proposal following an application process and execution of a data-use agreement as required by the Institutional Review Board at the Cincinnati Children’s Hospital Medical as part of the approval of this collaborative study.
References:
- 1.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL. Epidemiology of Acute Kidney Injury in Critically Ill Children and Young Adults. N Engl J Med. Jan 5 2017;376(1):11–20. doi: 10.1056/NEJMoa1611391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wieczorek B, Ascenzi J, Kim Y, et al. PICU Up!: Impact of a Quality Improvement Intervention to Promote Early Mobilization in Critically Ill Children. Pediatr Crit Care Med. Dec 2016;17(12):e559–e566. doi: 10.1097/pcc.0000000000000983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Li L, Li X, Xia Y, et al. Recommendation of Antimicrobial Dosing Optimization During Continuous Renal Replacement Therapy. Front Pharmacol. 2020;11:786. doi: 10.3389/fphar.2020.00786 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutherland SM, Alexander SR. Continuous renal replacement therapy in children. Pediatr Nephrol. Nov 2012;27(11):2007–2016. doi: 10.1007/s00467-011-2080-x [DOI] [PubMed] [Google Scholar]
- 5.Sutherland SM, Zappitelli M, Alexander SR, et al. Fluid overload and mortality in children receiving continuous renal replacement therapy: the prospective pediatric continuous renal replacement therapy registry. Am J Kidney Dis. Feb 2010;55(2):316–25. doi: 10.1053/j.ajkd.2009.10.048 [DOI] [PubMed] [Google Scholar]
- 6.Cerdá J, Liu KD, Cruz DN, et al. Promoting Kidney Function Recovery in Patients with AKI Requiring RRT. Clin J Am Soc Nephrol. Oct 7 2015;10(10):1859–67. doi: 10.2215/cjn.01170215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark EG, Bagshaw SM. Unnecessary renal replacement therapy for acute kidney injury is harmful for renal recovery. Semin Dial. Jan-Feb 2015;28(1):6–11. doi: 10.1111/sdi.12300 [DOI] [PubMed] [Google Scholar]
- 8.Wei EY, Vuong KT, Lee E, Liu L, Ingulli E, Coufal NG. Predictors of successful discontinuation of continuous kidney replacement therapy in a pediatric cohort. Pediatr Nephrol. Jul 2023;38(7):2221–2231. doi: 10.1007/s00467-022-05782-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Menon S, Krallman KA, Arikan AA, et al. Worldwide Exploration of Renal Replacement Outcomes Collaborative in Kidney Disease (WE-ROCK). Kidney Int Rep. Aug 2023;8(8):1542–1552. doi: 10.1016/j.ekir.2023.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stenson Erin K IA, Alobaidi Rashid, Bottari Gabriella, Fernandez Sarah N, Fuhrman Dana Y, Guzzi Francesco, Marinari Eleonora, Mohamed Tahagod, Morgan Catherine J, Mottes Theresa A, Neumayer Tara M, Ollberding Nicholas, Raggi Valleria, Ricci Zaccaria, See Emily, Stanski Natalja L, Zang Huiayu, Zangla Emily E, Gist Katja M, on behalf of WE-ROCK Investigators. . Effect of Continuous Renal Replacement Therapy Liberation Patterns on Outcomes: A Retrospective Analysis of the Worldwide Exploration of Renal Replacement Outcomes Collaborative in Kidney Disease (WE-ROCK). 28th International Advances in Critical Care Nephrology AKI&CRRT Conference. 2023; [Google Scholar]
- 11.Stenson Erin K IA, Alobaidi Rashid, Bottari Gabriella, Fernandez Sarah N, Fuhrman Dana Y, Guzzi Francesco, Marinari Eleonora, Mohamed Tahagod, Morgan Catherine J, Mottes Theresa A, Neumayer Tara M, Ollberding Nicholas, Raggi Valleria, Ricci Zaccaria, See Emily, Stanski Natalja L, Zang Huiayu, Zangla Emily E, Gist Katja M, on behalf of WE-ROCK Investigators. . Factors associated with Major Adverse Kidney Events at 90 Days among children requiring continuous renal replacement therapy: A retrospective analysis of the Worldwide Exploration of Renal Replacement Outcomes Collaborative in Kidney Disease (WE-ROCK). 28th International Advances in Critical Care Nephrology AKI&CRRT Conference. 2023; [Google Scholar]
- 12.Menon S, Krallman KA, Arikan AA, et al. Worldwide Exploration of Renal Replacement Outcomes Collaborative in Kidney Disease (WE-ROCK). Kidney International Reports. doi: 10.1016/j.ekir.2023.05.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pollack MM, Holubkov R, Funai T, et al. The Pediatric Risk of Mortality Score: Update 2015. Pediatr Crit Care Med. Jan 2016;17(1):2–9. doi: 10.1097/pcc.0000000000000558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leteurtre S, Duhamel A, Salleron J, Grandbastien B, Lacroix J, Leclerc F. PELOD-2: an update of the PEdiatric logistic organ dysfunction score. Crit Care Med. Jul 2013;41(7):1761–73. doi: 10.1097/CCM.0b013e31828a2bbd [DOI] [PubMed] [Google Scholar]
- 15.Alobaidi R, Morgan C, Basu RK, Stenson E, Featherstone R, Majumdar SR, Bagshaw SM. Association Between Fluid Balance and Outcomes in Critically Ill Children: A Systematic Review and Meta-analysis. JAMA pediatrics. Mar 1 2018;172(3):257–268. doi: 10.1001/jamapediatrics.2017.4540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu C, Peng Z, Dong Y, et al. Continuous Renal Replacement Therapy Liberation and Outcomes of Critically Ill Patients With Acute Kidney Injury. Mayo Clin Proc. Nov 2021;96(11):2757–2767. doi: 10.1016/j.mayocp.2021.05.031 [DOI] [PubMed] [Google Scholar]
- 17.Jeon J, Kim DH, Baeg SI, et al. Association between diuretics and successful discontinuation of continuous renal replacement therapy in critically ill patients with acute kidney injury. Crit Care. Oct 10 2018;22(1):255. doi: 10.1186/s13054-018-2192-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho AY, Yoon HJ, Lee KY, Sun IO. Clinical characteristics of sepsis-induced acute kidney injury in patients undergoing continuous renal replacement therapy. Ren Fail. Nov 2018;40(1):403–409. doi: 10.1080/0886022x.2018.1489288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldstein SL, Somers MJ, Baum MA, et al. Pediatric patients with multi-organ dysfunction syndrome receiving continuous renal replacement therapy. Kidney Int. Feb 2005;67(2):653–8. doi: 10.1111/j.1523-1755.2005.67121.x [DOI] [PubMed] [Google Scholar]
- 20.Symons JM, Chua AN, Somers MJ, et al. Demographic characteristics of pediatric continuous renal replacement therapy: a report of the prospective pediatric continuous renal replacement therapy registry. Clin J Am Soc Nephrol. Jul 2007;2(4):732–8. doi: 10.2215/cjn.03200906 [DOI] [PubMed] [Google Scholar]
- 21.Harrell FE. Regression Modeling Strategies: With Applications to Linear Models, Logistic and Ordinal Regression, and Survival Analysis (Springer Series in Statistics). 2nd Edition ed. 2015. [Google Scholar]
- 22.Harrell FJ. Regression Modeling Strategies. 2023, 2023. https://cran.r-project.org/web/packages/rms/index.html [Google Scholar]
- 23.Wei EY, Vuong KT, Lee E, Liu L, Ingulli E, Coufal NG. Predictors of successful discontinuation of continuous kidney replacement therapy in a pediatric cohort. Pediatr Nephrol. Oct 31 2022;doi: 10.1007/s00467-022-05782-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Liu C, Peng Z, Dong Y, Li Z, Andrijasevic NM, Albright RC Jr., Kashani KB. Predicting successful continuous renal replacement therapy liberation in critically ill patients with acute kidney injury. J Crit Care. Dec 2021;66:6–13. doi: 10.1016/j.jcrc.2021.07.020 [DOI] [PubMed] [Google Scholar]
- 25.Hansrivijit P, Yarlagadda K, Puthenpura MM, Ghahramani N, Thongprayoon C, Vaitla P, Cheungpasitporn W. A meta-analysis of clinical predictors for renal recovery and overall mortality in acute kidney injury requiring continuous renal replacement therapy. J Crit Care. Dec 2020;60:13–22. doi: 10.1016/j.jcrc.2020.07.012 [DOI] [PubMed] [Google Scholar]
- 26.Katulka RJ, Al Saadon A, Sebastianski M, et al. Determining the optimal time for liberation from renal replacement therapy in critically ill patients: a systematic review and meta-analysis (DOnE RRT). Crit Care. Feb 13 2020;24(1):50. doi: 10.1186/s13054-020-2751-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murugan R, Bellomo R, Palevsky PM, Kellum JA. Ultrafiltration in critically ill patients treated with kidney replacement therapy. Nature reviews Nephrology. Apr 2021;17(4):262–276. doi: 10.1038/s41581-020-00358-3 [DOI] [PubMed] [Google Scholar]
- 28.Murugan R, Kerti SJ, Chang CH, et al. Association of Net Ultrafiltration Rate With Mortality Among Critically Ill Adults With Acute Kidney Injury Receiving Continuous Venovenous Hemodiafiltration: A Secondary Analysis of the Randomized Evaluation of Normal vs Augmented Level (RENAL) of Renal Replacement Therapy Trial. JAMA network open. Jun 5 2019;2(6):e195418. doi: 10.1001/jamanetworkopen.2019.5418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Murugan R, Kerti SJ, Chang CH, et al. Association between Net Ultrafiltration Rate and Renal Recovery among Critically Ill Adults with Acute Kidney Injury Receiving Continuous Renal Replacement Therapy: An Observational Cohort Study. Blood Purif. 2022;51(5):397–409. doi: 10.1159/000517281 [DOI] [PubMed] [Google Scholar]
- 30.Riley AA, Watson M, Smith C, Guffey D, Minard CG, Currier H, Akcan Arikan A. Pediatric continuous renal replacement therapy: have practice changes changed outcomes? A large single-center ten-year retrospective evaluation. BMC Nephrol. Oct 19 2018;19(1):268. doi: 10.1186/s12882-018-1068-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pipili C, Vasileiadis I, Grapsa E, et al. Microcirculatory alterations during continuous renal replacement therapy in ICU: A novel view on the 'dialysis trauma' concept. Microvasc Res. Jan 2016;103:14–8. doi: 10.1016/j.mvr.2015.09.004 [DOI] [PubMed] [Google Scholar]
- 32.Maynar Moliner J, Honore PM, Sánchez-Izquierdo Riera JA, Herrera Gutiérrez M, Spapen HD. Handling continuous renal replacement therapy-related adverse effects in intensive care unit patients: the dialytrauma concept. Blood Purif. 2012;34(2):177–85. doi: 10.1159/000342064 [DOI] [PubMed] [Google Scholar]
- 33.van der Sande FM, Dekker MJ, Leunissen KML, Kooman JP. Novel Insights into the Pathogenesis and Prevention of Intradialytic Hypotension. Blood Purif. 2018;45(1-3):230–235. doi: 10.1159/000485160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Martin M, Bansal A, Perez L, Stenson EK, Kendrick J. Use of Relative Blood Volume Monitoring to Reduce Intradialytic Hypotension in Hospitalized Patients Receiving Dialysis. Kidney Int Rep. Sep 2022;7(9):2105–2107. doi: 10.1016/j.ekir.2022.06.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wald R, Beaubien-Souligny W, Chanchlani R, et al. Delivering optimal renal replacement therapy to critically ill patients with acute kidney injury. Intensive Care Med. Oct 2022;48(10):1368–1381. doi: 10.1007/s00134-022-06851-6 [DOI] [PubMed] [Google Scholar]
- 36.Wald R, Gaudry S, da Costa BR, et al. Initiation of continuous renal replacement therapy versus intermittent hemodialysis in critically ill patients with severe acute kidney injury: a secondary analysis of STARRT-AKI trial. Intensive Care Med. Nov 2023;49(11):1305–1316. doi: 10.1007/s00134-023-07211-8 [DOI] [PubMed] [Google Scholar]
- 37.Hayes LW, Oster RA, Tofil NM, Tolwani AJ. Outcomes of critically ill children requiring continuous renal replacement therapy. J Crit Care. Sep 2009;24(3):394–400. doi: 10.1016/j.jcrc.2008.12.017 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified summary data are available through the WE-ROCK collaborative. Data dictionaries, in addition to study protocol, the statistical analysis plan will be made available upon request. More information about the process and available data can be obtained by contacting the corresponding author (EKS). The data from the WE-ROCK collaborative will be made available to researchers who provide a methodologically sound proposal for use in achieving the goals of the approved proposal following an application process and execution of a data-use agreement as required by the Institutional Review Board at the Cincinnati Children’s Hospital Medical as part of the approval of this collaborative study.