Abstract
This Lilliput article provides a literature overview on ecological effects of the plant microbiome with a focus on practical application in forestry, agriculture and urban greenspace under the spectre of climate change. After an overview of the mostly bacterial microbiome of the model plant Arabidopsis thaliana, worldwide data from forests reveal ecological differentiation with respect to major guilds of predominantly fungal plant root symbionts. The plant‐microbiome association forms a new holobiont, an integrated unit for ecological adaptation and evolutionary selection. Researchers explored the impact of the microbiome on the capacity of plants to adapt to changing climate conditions. They investigated the impact of the microbiome in reforestation programs, after wildfire, drought, salination and pollution events in forestry, grasslands and agriculture. With increasing temperatures plant populations migrate to higher latitudes and higher altitudes. Ecological studies compared the dispersal capacity of plant seeds with that of soil microbes and the response of soil and root microbes to experimental heating of soils. These studies described a succession of microbiome associations and the kinetics of a release of stored soil carbon into the atmosphere enhancing global warming. Scientists explored the impact of synthetic microbial communities (SynComs) on rice productivity or tea quality; of whole soil addition in grassland restoration; or single fungal inoculation in maize fields. Meta‐analyses of fungal inoculation showed overall a positive effect, but also a wide variation in effect sizes. Climate change will be particularly prominent in urban areas (“urban heat islands”) where more than half of the world population is living. Urban landscape architecture will thus have an important impact on human health and studies started to explore the contribution of the microbiome from urban greenspace to ecosystem services.
This Lilliput article provides a literature overview on ecological effects of the plant microbiome with a focus on practical application in forestry, agriculture and urban greenspace under the spectre of climate change. Picture credit: Rahul Mahadev Shelake, Dibyajyoti Pramanik and Jae‐Yean Kim. doi:10.3390/microorganisms7080269.
THE PLANT WORLD IS ON THE MOVE
Tempora mutantur (the times are changing) is a medieval Latin adage which then claims that we are also changing with time. While this is a typical but somewhat trivial human experience, the adage got a new meaning with climate change and its impact on the biosphere. A modern version of this adage could thus read: the times are changing and the ecosystems change, too. For example, between 1964 and 2004 researchers investigated the northern hardwood/boreal forest ecotone (transition area between two biological communities) in the Green Mountains of Vermont /USA. Using aerial photographs and satellite imagery, they documented an about 100‐m upslope shift of this ecotone. In the 700–800 m above sea level transition zone, they observed an increase of hardwood and a decline of boreal trees. There also was a regional climate change during this period with a 1.1°C increase in annual temperature (Beckage et al., 2008).
Comparable data were reported for 171 forest plant species across six mountain ranges in Western Europe. The evaluation was based on large scale floristic surveys conducted between 1905 and 2005. Between 1965 and 2005 the yearly mean surface temperatures had also increased by 1°C. The researchers determined the optimum elevation, i.e. the altitude at the peak point of the maximum probability for the presence of a given plant species. They observed a significant upward shift in species optimum elevation, averaging a 29‐m increase per decade (Lenoir et al., 2008). Another group of researchers conducted a meta‐analysis of distribution data for 764 animal and plant species and calculated a shift to higher elevations with a median rate of 11 m per decade, and to higher latitudes with a median rate of 17 km per decade. Notably, in this meta‐analysis the distances moved by species were the greatest in study areas showing the highest levels of local warming. There were great differences in the rate of movement between individual species, suggesting that a number of additional factors influenced the migration rate (Chen et al., 2011). Climate change signals on organismal migration are not easy to identify since non‐climate effects will also influence local biological changes. Researchers looked for systematic trends when analysing distribution data for 1700 species. They detected diagnostic fingerprints for climate change migration in 279 species. Global meta‐analyses documented significant range shifts of about 6 km per decade towards the poles or 6 meters per decade upward in altitude. During the study period spring advanced by 2.3 days per decade (Parmesan & Yohe, 2003). Climate change has also caused a northward expansion of boreal forests which replaced tundra, leading to a decrease of surface albedo and altered subsurface carbon stocks, with positive feedback on global warming. The northward migration of forests was notably better correlated with the retreat of sea ice cover than with temperature increase (Dial et al., 2024).
Further data indicate that the plant world is on the move. Using the Global Forest Biodiversity Initiative database, scientists determined that 5% of forest plots screened worldwide were invaded with 250 non‐native tree species (mostly by Robinia pseudoacacia, Pinus sylvestris, Maclura pomifera, Picea abies and Ailanthus altissima). The report identified anthropogenic factors shaping the invasion pattern (e.g. proximity of ports), but also an influence of abiotic factors such as mean annual temperature and level of precipitation. The authors of this study proposed that increased temperature impacts on the resource availability in the topsoil by changing the belowground microorganism composition (Delavaux et al., 2023). Such a hypothesis raises a number of questions. Trees cannot migrate, only their seeds can be dispersed. However, the seeds must fall literally on fertile ground and if the plant microbiome also matters for survival, trees (and generally plants) need not only appropriate abiotic soil conditions, but also appropriate microbiological helpers for taking a foothold.
MICROBIOME INSIGHTS FROM THE MODEL PLANT ARABIDOPSIS THALIANA
Bacterial microbiome
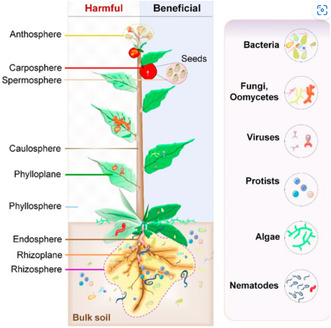
Substantial research has been conducted on the microbiome associated with the model plant Arabidopsis thaliana, a member of the mustard family (Brassicaceae). By sequencing 16S rRNA PCR amplicons, researchers working in Cologne / Germany investigated the bacterial community composition in soil, the rhizosphere and the root of two Arabidopsis ecotypes. The soil yielded Acidobacteria, Proteobacteria and Actinobacteria as major constituents. The rhizosphere, i.e. soil particles firmly attached to roots, showed some shifts in relative abundance (e.g. an increase in Planctomycetes), but still resembled that of the soil compartment. The root compartment comprises bacteria living in intercellular spaces of roots as endophytes and showed a distinct microbial composition. Before analysis, the roots were depleted from soil particles and epiphytes growing on their surface by washes and sonication. The endophytes in the root were derived from the microbiota reservoir present in the natural soil but passed a highly selective gating process. Proteobacteria, Actinobacteria and Bacteroidetes were the dominating phyla, with Betaproteobacteria, Streptomycetaceae and Flavobacteriaceae, respectively, representing over‐represented families. These are copiotrophic bacteria, i.e. bacteria that only compete successfully when organic resources are abundant as occurs in the root zone. The host plant genotype had only a small effect on the root endophyte microbiome (Bulgarelli et al., 2012). Researchers working in the US conducted a similar study also analysing soil, rhizosphere and endophyte samples from A. thaliana. Despite the difference between soil types from two different continents, the root‐associated bacteria from Arabidopsis were comparable in Germany and the US. The endophyte microbiome of Arabidopsis in the US was dominated by Actinobacteria, Proteobacteria and Firmicutes. Within Proteobacteria, two alphaproteobacterial families, Rhizobiaceae and Methylobacteriaceae, and two gammaproteobacterial families, Pseudomonadaceae and Moraxellaceae, dominated the endophyte microbiome (Lundberg et al., 2012).
Prokaryotic‐eukaryotic microbiome interactions
Arabidopsis and other Brassicaceae are not well colonized by arbuscular mycorrhizal fungi which justified the concentration on the bacterial microbiome in these pioneering studies. A few years later, scientists from Germany extended the microbiome sequencing efforts around the roots of Arabidopsis to the sequencing of both 16S rRNA and internal transcribed spacer regions of rRNA from eukaryotic microorganisms. They observed a gradual decrease of microbial diversity from bulk soil over the rhizosphere to the root interior displaying 730, 450 and 180 operational taxonomic units (OTUs), respectively. They identified 96 bacterial, 24 fungal (prominently: Leotiomycetes, Sordariomycetes) and 1 oomycetal (Phytium) OTUs as core microbiome of Arabidopsis from two different countries. Fungi and oomycetes (fungus‐like eukaryotic microorganisms within the Stramenopiles) constitute distinct kingdoms of life (Mycota and Chromista). Microbial network analysis revealed mostly negative correlations between bacterial and fungal OTUs and identified the bacterial genera Variovorax and Acidovorax as keystone taxa driving the fungal‐bacterial balance in Arabidopsis. To prove this point, the researchers derived a culture collection of root‐derived microbes and constituted synthetic microbiomes. Inoculation experiments with synthetic microbiomes consisting of fungal or oomycetal isolates had a strong detrimental effect on plant growth and survival rate when compared to microbe‐free control plants. Adding bacterial isolates to either the fungal or the oomycete isolates rescued the growth to that of microbe‐free plants. Notably, combining bacterial, fungal and oomycete isolates achieved a 125% increase in plant biomass over the microbe‐free control plants. Fungal‐bacterial interaction screening revealed Variovorax and Acidovorax strains as promising biocontrol microbes against a detrimental growth effect of fungal or oomycete on plants (Durán et al., 2018).
Towards synthetic microbiomes
Researchers isolated 5800 individual colonies from root and 2100 colonies from leaf samples of Arabidopsis growing 500 km apart. All root‐associated bacteria were recruited from the surrounding soil biome and more than half of the root‐associated bacteria closely resembled leaf‐associated bacteria. More than 400 bacterial genomes were sequenced. Leaf bacteria were enriched for the “carbohydrate metabolism” gene category reflecting the complex organic carbons on leaves (polysaccharides and cuticular waxes) whereas the gene content in root‐associated bacteria mirrored the simple carbon compounds produced by root exudation (sugars, amino acids, aliphatic acids and aromatic compounds). Synthetic communities consisting of root and soil, as well as leaf bacteria, were capable of ectopic colonization of both leaves and roots, suggesting that the leaf microbiota establishment benefits from both air‐ and soil‐borne bacterial inoculations (Bai et al., 2015).
Plant immunity effects on the microbiome
Root microbiomes have predictable compositions across individuals of a given species, suggesting a control of the microbiome by the plant. In addition, plants possess an immune system which distinguishes “self” from “non‐self” and provides a gating process that allows the colonization of the roots by potentially beneficial microbes and defends roots against invasion by pathogenic microbes. It is frequently observed in biology that mutants offer crucial insights into fundamental processes. Salicylic acid (SA), jasmonic acid, and gaseous ethylene mediate localized and systemic plant immune responses. Mutants impaired in SA synthesis and signalling are hypersusceptible to some biotrophic pathogens (microbes that exploit plant‐produced nutrients without killing cells). Mutants impaired in jasmonic acid and ethylene synthesis and signalling are hypersusceptible to herbivorous insects and necrotrophic pathogens (microorganisms that kill cells to obtain nutrients; Haney & Ausubel, 2015). US researchers investigated mutants which constitutively produce and accumulate SA, as well as mutants that either accumulate less SA or cannot respond to it. They observed that SA influences the microbial community structure of the root: the former mutants showed an increased abundance of α‐ and β‐Proteobacteria, whereas the latter mutants showed an increase of γ‐Proteobacteria. Elimination of all three defence phytohormone signalling pathways resulted in even more pronounced root microbiome composition changes which affected the survival of the plant in soil (Lebeis et al., 2015). Notably, also mixing the three plant immune mediators as chemicals in pot experiments affected root microbial community composition in specific ways (Carvalhais et al., 2014) An interesting twist was the fact that some root bacteria such as Streptomyces can grow in vitro with SA as sole carbon source (Lebeis et al., 2015).
A four‐gene mutant in Arabidopsis involved in pattern‐triggered immunity and a vesicle‐trafficking pathway caused not only leaf chlorosis, but also a major shift in the leaf endophyte microbiome. A consortium of US and Chinese researchers observed a reduced bacterial diversity and conversion of a Firmicutes‐rich community to a Proteobacteria‐rich community in the leaves of these mutant plants. More importantly, wildtype Arabidopsis inoculated with the leaf microbiome of the mutant plants developed necrosis and chlorosis and a reduced biomass. This observation proves that an unbalanced (“dysbiotic”) microbiome is sufficient to confer a negative health effect to wild‐type plants. Five Proteobacteria isolates were individually able to cause leaf damage. Further underlining the disease‐causing role of a dysbiotic microbiome was the observation that a 4‐gene mutant grown under sterile conditions was healthy (Chen et al., 2020).
Microbiome effects on plant growth
An international consortium of plant scientists inoculated axenic Arabidopsis with a synthetic consortium of 185 root bacterial isolates under variable abiotic conditions of salinity, temperature, phosphate concentrations and pH in pot experiments with sterilized soil. Twelve days later they determined the root microbiome composition and did a statistical deconstruction of the microbiome into four modules of co‐occurring bacteria. Module A was characterized by an enrichment of γ‐Proteobacteria, module B contained mainly low‐abundance Firmicutes, and modules C and D mainly α‐Proteobacteria and Actinobacteria, respectively. Seedlings inoculated with modules C and D showed a strong primary root growth inhibition (RGI) that was attenuated to normal growth by addition of module A bacteria. In mono‐associations 34 taxonomically diverse strains across all four modules induced strong RGI. This observation confirms that not only plants control the association of roots with soil microbes, but microbes also determine important plant phenotypes. The researchers then screened microbe‐microbe interactions on plant phenotype expression and observed 18 strains from module A that suppressed the RGI trait, which was induced by strains of modules B, C or D (e.g. Arthrobacter). All RGI‐attenuating strains belonged to the genus Variovorax. By a combined genomics and transcriptome analysis, the researchers identified an auxin‐degradation operon in Variovorax that was mechanistically involved in the suppression of RGI phenotype induced by Arthrobacter (Finkel et al., 2020).
LOOKING BEYOND ARABIDOPSIS
Plant microbiome diversity
The plant species specificity of the associated microbiome points to a huge plant microbiome diversity. In fact, leaves (the phyllosphere) of plants represent one of the most common habitats for terrestrial microorganisms. Early estimates based on 16S rRNA sequencing for three tree species from tropical coastal rainforests in Brazil yielded between 100 and 700 leaf‐associated bacterial species per plant species with a 0.5% interspecies overlap. Since the Brazilian forests comprise about 20,000 vascular plant species, the total bacterial species count on the leaves of Brazilian forests might exceeded 2 million microbial species; a similar amount of bacterial species was estimated to live in the oceans and twice as much bacterial species might be found in soil (Lambais et al., 2006).
Fungal root symbioses in trees
The major guilds of plant root symbionts are represented by arbuscular mycorrhizal fungi (AMF), ectomycorrhizal fungi (EMF), ericoid mycorrhizal fungi and nitrogen‐fixing bacteria (N‐fixers). N‐fixers (Rhizobia and Actinobacteria) convert atmospheric N2 to nitrogen usable for plants. EMF evolved from multiple lineages of saprotrophic ancestors and demand like N‐fixers more plant photosynthate than AMF. AMF constitute a symbiosis between plants and an ancient phylum of fungi, the Glomeromycota, where the fungi improve the supply of water and nutrients to the host plant. This relationship evolved 450 million years ago with the colonization of land by plants. The plant provides in exchange fixed carbon to the fungus. Fungal hyphae penetrate plant cell walls, where they form highly branched structures known as arbuscules. EMF are derived from the Basidiomycota and Ascomycota phyla. In contrast to AMF, EMF do not penetrate the plant cell walls. EMF form a fine network on the roots of about 2% of plant species while AMF associate with 80% of plant species. However, EMF is associated with 60% of tree species, including birch, beech, willow and pine. A large consortium of scientists developed a map of tree root symbioses at the global scale based on the Global Forest Biodiversity database (Steidinger et al., 2019). The researchers described a generalized pattern of successions in the Earth forest biomes with a transition from low‐latitude arbuscular mycorrhizal through N‐fixer to high‐latitude ectomycorrhizal ecosystems. Ericoid mycorrhizal fungi‐associated trees were rare. The warm tropical broadleaf forests without seasons, dominated by AMF symbiosis (>75% vs. 8% for EMF trees), occurred between 25° N and 25° S latitude. Trees displaying symbiosis with N‐fixers reach a peak in abundance in the arid zone at around 30° N or 30° S. Moving further north another transition zone occurs at 50° N separating mixed AMF and EMF temperate forests from EMF‐dominated boreal forests further north. Regional variations in climate and soil pH (for N‐fixers) were the primary factors that influenced the relative dominance of each guild at the global scale. The slow decomposition of leaf litter at high latitudes favours EMF owing to their increased capacity to liberate organic nutrients from litter which was also chemically more resistant to decomposition. Their climate model for 2070 predicts a decline of EMF trees along the boreal–temperate ecotone. A “mycorrhizal tree map” study in the US showed strongly EMF dominated forests along the mountainous western regions, strongly AMF dominated forests surrounding desert regions and modestly dominated EMF forests along the Atlantic coast which transit into modestly AMF forests when going further inland. Nitrogen deposition and fire suppression by humans, enforced by climate change, have increased AMF tree dominance during the past three decades in the eastern US (Jo et al., 2019). Ericoid mycorrhizal plants, by contrast, are primarily understory shrubs in forests (Ward et al., 2022). The role of widespread tree root‐associated fungi, such as recently described dark septate endophytes in shaping the root microbiome in European forests is currently under investigation (Netherway et al., 2024).
THE PLANT‐MICROBIOME HOLOBIONT CONCEPT
The intimate two‐way relationship between plants and their associated microbiome led to the concept of treating them together as a single super‐organismal unit in ecology and evolution analyses. The plant‐microbiome unit has been termed a “holobiont”, an intimately integrated unit of ecological adaptation and evolutionary selection of organisms belonging to very different taxonomical species. Notably, the rapid adaptation of microbes to altered abiotic conditions could provide plants an important asset by serving as an effective buffer against environmental change. Indeed, in a multigeneration drought experiment, the genetic and evolutionary responses of plants to drought were relatively weak while plant fitness was strongly associated with the rapid response of the soil microbial community structure, as evidenced by moisture manipulation experiments. When faced with environmental change, plants may as a short‐term response (before long‐term plant evolution proper could set in) not be limited to migration strategies, they may also benefit from association with microbes that are capable to respond rapidly to environmental change (Lau & Lennon, 2012). In addition, the dynamic nature of the microbial associations with the plant host could compensate for the limitation of the sessile lifestyle of plants. Indeed, molecular work with synthetic bacterial communities in Arabidopsis have shown that master transcriptional regulators of phosphate stress response also directly repress defence responses allowing an association with new useful microbes to enhance plant performance: apparently nutritional needs trumped over defence needs (Castrillo et al., 2017). The researchers suggested that synthetic bacterial communities could be deployed to enhance plant performance in agriculture under stressed conditions.
Phenotypic plasticity
The phenotypic plasticity of plants can now be addressed by genetic analyses. Chinese researchers analysed the agronomically relevant phenotypes of 827 foxtail millet (Setaria italica) cultivars. They conducted plant genome‐wide association studies (GWAS), microbiome‐wide association studies (MWAS) and microbial genome‐wide association studies (mGWAS). They observed that the rhizosphere microbiota composition of foxtail millet is mainly driven by variations in plant genes related to immunity, metabolite secretion, hormone signalling and nutrient uptake. They defined a plant genotype‐microbiome interaction network that contributed to plant phenotype plasticity. They detected that host genetic variations and the microbiome affected the plant phenotypes independently. Host genetics shaped the composition of the root microbiome which in turn shaped several agronomically relevant traits of foxtail millet. Notably, most of the variations in specific leaf, inflorescence and stem traits were predicted by rhizosphere microbiome alone, documenting an important effect of the rhizosphere microbiome on plant phenotypes. The authors suggest that precision microbiome engineering will be crucial for efficient and sustainable agriculture in the future (Wang et al., 2022).
The Montreal garden experiment
Demonstrating the influence of the plant microbiome on host plant fitness and ecosystem functions is an even more complex task. Canadian researchers treated the microbiome as an extended genotype of host plants and conducted a tree biodiversity–ecosystem function experiment with 620 trees from 19 species in a field near Montreal. One to 12 tree species were grown together for 5 years in 4 × 4 m experimental plots. Plant species richness was the strongest determinant of plant community productivity. Host species identity played the dominant role in determining leaf microbial community structure. The diversity of bacterial communities on tree leaves explained significant amounts of variation in plant community productivity. Removing that link from the structural equation model yielded an unstable model. The researchers warned that this leaf microbiome‐plant productivity association is not a proof of causality which would necessitate to manipulate leaf bacterial diversity experimentally and observe the productivity effect after intervention (Laforest‐Lapointe et al., 2017).
Microbiome and plant domestication
Another approach to demonstrate the close relationship between plants and their associated microbiome is to follow this association through domestication events. An international consortium of scientists followed two bean species (Phaseolus vulgaris and P. lunatus) which were independently domesticated in Mesoamerica and the Andean region, respectively. They identified mineral content of the seeds as a trait which differentiated domesticated from wildtype beans. The seed microbiome did not differ between 70 different plant genotypes of domesticated beans, but it differed clearly between domesticated and wildtype beans. The reduced Ca2+ seed content, characteristic for domesticated P. vulgaris, was associated with the dominance of Proteobacteria, particularly Pseudomonaceae, in the seed microbiome. The data support the hypothesis that changes in plant traits selected during domestication also have driven changes in microbiota composition (Soldan et al., 2024) compatible with the concept of a plant‐microbe co‐evolution and that the plant‐microbiome holobiont represents a unit of selection.
FIELD TRIALS TESTING THE ROLE OF THE MICROBIOME FOR PLANT ADAPTATION AND MIGRATION
Extinction
Not only human migration which causes a lot of contemporary political discussions, but also plant and animal migration and non‐native species invasion will be hot topics for discussions among biologists in the decades to come. Climate change will push species outside of their evolved tolerance zones, creating a leading edge of expansion towards areas where the conditions become less stressful than in the currently inhabited climate zone (think for example about plants or animals migrating to higher latitudes or higher altitudes to escape heat waves) and a trailing edge of retraction where the conditions become unbearable for them leading to their extinction. Species must thus either migrate to track optimal climates for them or adapt and acclimate to the changed conditions. If they stay put without adapting, they may face extinction. These are not small numbers of organisms that are affected by extinction: It was estimated that about a fifth of species will suffer extinction over the next century. Some biologists speak already of a sixth human‐caused Anthropocene mass extinction event (What is mass extinction and are we facing a sixth one? | Natural History Museum (nhm.ac.uk); Ceballos & Ehrlich, 2023).
A US tree seedling field trial
The plant‐microbiome axis under the threat of extinction by climate change was investigated in an interesting study. US plant pathologists explored the impact of plant‐associated soil microbes on the capacity of plants to adapt to changing climate conditions. They set up a sophisticated field experiment across two US states, Wisconsin and Illinois, the former showing a marked gradient of chilling winter temperatures and the latter a gradient of aridity. Live soil was collected from six sites in each state. Seedlings collected from both Wisconsin and Illinois sites were germinated in sterile conditions and then grown for 8 weeks with live field soil prior to transplantation into a field site at the cold edge of Wisconsin or the arid edge of Illinois, respectively. Natural rainfall was also experimentally manipulated. Seedling survival in the field was tested over a 3‐year growth period at northern and southern sites and the fungal communities on the surviving seedlings were determined. At the northern site, seedling survival was‐ as predicted for a microbially induced cold tolerance‐ increased when both arbuscular (AMF) and ectomycorrhizal (EMF) trees were pre‐inoculated with microbial communities sourced from soils of colder areas. However, the difference in survival between the soil sources disappeared when conducted under rain restriction conditions. At the southern site, seedling survival was strongly promoted by pre‐inoculation with microbial communities from more arid sites, but only under rainfall restrictions and not for EMF trees. Apparently, soil transfer can attenuate winter cold stress or summer drought stress, but not a combined temperature/precipitation stress. The fungal communities on surviving seedlings reflected the initial microbial inoculum sources and correlated with seedling survival probabilities. Some inoculated fungi persisted over the 3‐year observation period. The main conclusion of this report was that the ability to enhance plant‐host tolerance to climate change is predictable by the climatic history of the transplanted microbial community (Allsup et al., 2023).
Adaptation speed
Evolutionary ecologists have determined that the evolutionary rate was consistently lower in tree and shrubs than in related herbaceous plants and this hold true when compared across five major angiosperm clades. The researchers attributed the slower rate of molecular evolution (which must however not necessarily be associated with a slower phenotypic evolution in trees) with their longer generation times (Smith & Donoghue, 2008). Since the generation time of microbes is much shorter and their population size in soil much greater than that of the plants, one would anticipate a much higher evolution rate for plant‐associated microbes. In addition, dynamic changes in the composition of the microbiome provides an additional path for rapid ecological adaptability to plants.
An Austrian grassland drought trial
In that context, a field experiment in a mountain grassland in central Austria compared 10 years of recurrent, experimental drought events with a single drought event to ambient control conditions (Canarini et al., 2021). Experimental drought consisted of complete experimental exclusion of precipitation for two consecutive months. The Austrian researchers found a large shift in the community composition of bacteria, archaea and fungi, when exposed to 10 years of recurrent drought events, but not for a single drought event. Even after 5 years of drought, microbiome changes were not observed. Apparently also microbiome changes need several years for adapting to new environmental conditions. No changes in edaphic factors such as soil pH or soil carbon (C) content were seen over the observation period. However, microbiomes changed significantly after 10 years of successive drought. On the bacterial side Actinobacteria increased and Proteobacteria decreased; on the fungal side Ascomycota increased and Basidiomycota decreased. AMF showed a decrease in biomass and composition. Acclimatization to drought in bacteria was demonstrated by osmolyte production, synthesis of capsules (that retain water), decreased energy consumption and spore formation. Apparently, plant fitness was mediated by rapid responses of the soil microbial community to drought. The environmental stress created a kind of ecological memory in the soil microbiome. In a follow‐up study using stable isotope probing in this Austrian grassland, the researchers observed that drought caused more than 90% of bacterial and archaeal taxa to stop dividing (Metze et al., 2023). Under drought conditions, growing taxa accounted for only 4% of the total community as compared to 35% in control communities. Drought‐tolerant bacteria belonged mostly to the Streptomyces genus of Actinobacteria. Pre‐exposure of the grassland to future climate conditions (+3°C temperature, increased CO2) alleviated drought effects on microbial growth since under such conditions 9% of the microbial taxa were growing.
Microbiome dispersal capacities
Migration under the pressure of climate change is another important escape lane for stressed organisms to avoid extinction. What is known about the relative dispersal rate of plants and soil microbes underlying their migration capacity? In that context, another Austrian study compared the dispersal of about 100 plant species, 15,000 bacterial OTUs and 11,000 fungal OTUs in a glacier forefield from the Alps (Junker et al., 2021). Community assembly is the result of several filters, first of a dispersal filter (how far organisms are propagated physically by wind and water), followed by an environmental filter (abiotic factors such as nutrient availability, pH, etc. which determine whether the dispersed organism finds a suitable new habitat for colonization) and an interaction filter (biotic factors, i.e. competition or cooperation of the invading with the native species at the new site). In this Austrian study, plant communities contained the strongest spatial signal (i.e. were spatially the most restricted), followed by microbes colonizing the soil, while leaf‐associated bacterial and fungal communities were relatively independent of spatial distance. The researchers concluded that the differences in community assembly processes can be attributed to the size of the organisms' propagules because plant seeds are larger than fungal spores which are larger than bacterial cells. Since leaf microbes are more exposed to wind and rain than soil microbes, soil microbes disperse less well than leaf microbes. Since for microbes a few mm might fundamentally change their living conditions in soil and on plants, the environmental and interaction filters might represent greater barriers for microbes than for plants, partially offsetting the dispersal advantage of microbes.
ENVIRONMENTAL APPLICATIONS
Reforestation
Plant‐microbiome effects are not any longer questions of fundamental ecology, but became practical concerns with respect to climate change mitigation strategies. For example, the World Economic Forum's One Trillion Trees Initiative, the United Nations Decade on Ecosystem Restoration, or the US One Trillion Trees Interagency Council call for massive global reforestation. The goal is severalfold. It was calculated that reforestation could sequester an average of 6 metric tons of CO2 per hectare per year. Since in the US alone tens of millions of hectares are potentially available for reforestation, a substantial amount of emitted greenhouse gas could be bound in tree biomass. There are potential beneficial effects beyond CO2 fixation: Reforestation could also increase evapotranspiration, stabilize soils, protect water sources and create wildlife habitats. Recent data have however indicated that the benefit of forestation has been overestimated. Forestation decreases surface albedo (the reflectance of solar radiation), leading to an increased warming by solar irradiation. Forestation causes increased biogenic organic emissions from trees, leading to an increased greenhouse gas emission of methane and ozone as well as more aerosol scattering. Together these effects could offset an estimated one third of the expected CO2 removal by forestation (Weber et al., 2024).
US forest specialists calculated the cost of reforestation for 30 billion trees on 26 million hectares of land with about US$ 33 billion (Fargione et al., 2021). Cost is not the only limiting factor: current tree nursery production levels would have to double without however compromising the seedling quality. Seed collections must be created that allow the out‐planting of seedlings appropriate for the climate and soil conditions in the areas to be reforested. In view of the experience of the Wisconsin tree seedling trial, inoculation with appropriate soil microbiomes should be considered to increase the survival and vigour of the seedlings. The increasing wildfire rates particularly in Northern America and Australia also call for restoration measures. However, in the US tree plantation accounts for just a third of reforestation efforts, most is left to the lengthy process of natural regeneration. Forestation of mining areas, landfills, polluted areas, and of poor soils should likewise be considered, raising interest in the root microbiome implications for reforestation.
Wildfire
Detailed molecular data are available from wildfire effects on soil microbiomes in burned conifer forests from Colorado and Wyoming in the US (Nelson et al., 2022). One year after the fire, the researchers have determined the soil microbiome by metagenome sequencing at different depth of the topsoil and after different fire severity. On the bacterial side, Actinobacteria (specifically the genera Arthrobacter and Blastococcus) dominated the microbiome in burned surface layers of soils. Gene counts indicated that spore formation is a trait supporting survival and post‐fire colonization in burned soil. Increases in trehalose and glycine betaine synthesis genes also supported microbial viability under low soil moisture conditions after fire. Severe fire selected for rapidly growing Actinobacteria that are able to process pyrogenic aromatic organic matter and dead bacteria (necromass). Numerous viruses were detected in the soil metagenomes from wildfire areas; most of them were tailed phages. By matching CRISPR spacers to protospacers in bacterial genomes, the researchers revealed that abundant phages targeted abundant and transcriptionally active bacteria, representing a viral shunt for soil carbon cycling. Within fungi, a known pyrophilous (fire‐loving) Ascomycete from the class Leotiomycetes increased to prominence. This fungus can degrade aromatic compounds. Notable were also decreases of Acidobacteria and Verrucomicrobia and the loss of nitrogen fixation genes. EMF showed a dramatic 99% decrease in burned soil. The authors concluded that this has important implications for the re‐establishment of Pinus contorta, the dominant tree species in the affected forests in Colorado, which depends on the EMF Cenoccum geophilum, which could not be detected in burned soil.
A study from Portugal demonstrated that nursery inoculation with EMF of Pinus pinaster saplings resulted in a 1.5 higher stem height when planted in a reforested burned area compared to uninoculated saplings. Several species of the nursery‐inoculated fungal cocktail were detected in the field 5 years after plantation of the saplings, demonstrating persistence of the inoculated fungi in the environment (Franco et al., 2014). The EMF inoculation is not a new technique. Nearly 50 years ago US researchers demonstrated that several pine tree species inoculated in the nursery with a single isolated fungal species, Pisolithus tinctorius, showed a significant height and weight increase 2 years after plantation over seedlings that were colonized with the natural nursery ectomycorrhiza (Marx et al., 1977). A recent survey of forests across 15 European countries that used molecular data for EMF detection and that controlled for climate and soil factors, also revealed that specific EMF species were correlated with increased tree growth. The strongest effect was shown by Russula, which promoted growth in both broad leaf and needle trees, and Atheliaceae and Cenoccum, whose growth stimulating effect was limited to needle trees. Tree growth promotion was positively correlated with energy and nutrient metabolism genes in fungi and negatively correlated with organic nitrogen (N) cycling genes in fungi. Stand‐level tree growth rates were tripled in association with fast‐growth EMF communities and had thus comparable effects as temperature and precipitation on tree growth (Anthony et al., 2022).
Salinity
Wildfire is not the only climate change threat to plant life and the associated soil microbiome. There are several other causes for soil degradation. Each year, around 1–2% of fertile soils are being degraded worldwide as a consequence of salinity increases. About 20% of irrigated land is currently already affected by salinization. Most affected areas are arid and semi‐arid regions of the world, but also coastal regions such as the Netherlands or Bangladesh suffer from rising seawater levels (Coban et al., 2022).
Bangladeshi researchers conducted a meta‐analysis of the literature for reports that compared AMF inoculated and non‐inoculated plants grown under salinity stress, where physiological parameters were measured and statistical effect sizes were reported. Overall, 97 publications documented growth data for 50 plant species. Generally, mycorrhizal colonization significantly increased plant shoot and root dry biomass compared to uninoculated plants and the effect size increased with increasing salinity. As the amount of available data were sufficiently large, the researchers could differentiate the impact of a number of factors on the shoot biomass. With respect to AMF, Funneliformis showed the greatest effects. With respect to plant hosts, Fabaceae plants demonstrated the clearest AMF‐induced effect sizes with increasing salt levels. Effects on dicots were higher than for monocots. With respect to photosynthesis types, C3 but not C4 plants showed a prominent effect. Shrubs showed more effects than grasses and trees; perennials more than annuals, legumes more than non‐legumes. AMF effects needed long salinity exposure times to get manifested. Salinity induces both osmotic and ion stress in plants. Initially, water scarcity in the root zone is the major stress, followed in a second phase by toxicity effects of excess Na+ and Cl− ions accumulation in the cytoplasm. The inoculation of AMF consistently increases shoot K+ and decreases shoot Na+ concentrations (Dastogeer et al., 2020).
About a third of irrigated land is already affected by salinization which initially reduces the agricultural productivity and finally makes the land unusable for farming. Halophytes such as Suaeda altissima, a succulent halophyte of the Chenopodiaceae, can grow on high salt soils in coastal ecosystems. It accumulates salt which is transported from the root into the fleshy leaves. This property could be exploited for bioremediation and Suaeda has been proposed as a biological tool for soil desalination. Chinese researchers identified Halomonas, Arthrobacter, Marmoricola as key microorganisms in the Suaeda rhizosphere that played a vital role in promoting nitrogen cycling and improving plant salinity tolerance, demonstrating the importance of microbes for this process (Wang et al., 2024).
Also desert plants can efficiently cope with multiple stress factors such as nutrient deficiency, drought and salinity. Indigofera argentea is a perennial legume subshrub from desert regions. However, Indigofera could not grow on sterilized desert soil. Adding Rhizobium, a bacterial N‐fixer, isolated from the desert soil rescued the growth of Indigofera. A consortium of Dutch and Chinese scientists developed a bacterial cocktail (“SynCom”) derived from the desert plant Indigofera consisting of 15 or 5 bacterial strains which promoted the growth of the cash crop tomato under salt stress. The researchers demonstrated by transcription analysis that 4 days post salt application, the SynCom cocktail mediated an early salt stress response in both the root and shoot tissue of tomatoes. They argued that cocktails are more likely than single externally applied strains to compete with the resident soil microbiome for tomato colonization (Schmitz et al., 2022).
Pollution
Soil can also become contaminated by chemicals from agricultural and industrial activities, dumping waste and urbanization. The size of polluted areas can be quite substantial, for example China has categorized 16% of all its soils as polluted (Coban et al., 2022). Heavy metals in soil cannot be broken down, but only chemically reduced or oxidized. Phytoextraction is one approach explored for removing heavy metal from contaminated soil. The principle is to grow a plant on contaminated fields that resist the toxic effect of the heavy metals and is able to extract heavy metals from soil and accumulate the metal in aerial parts of the plant. The plant is then removed from the field and discarded, leaving a field with a reduced heavy metal content. Phytoextraction is a low‐tech and low‐cost measure to decontaminate fields. However, there is a drawback: many plants cannot grow well on contaminated fields because heavy metals are toxic to plants, thus impeding growth. There are exceptions: Solanum nigrum (the black nightshade) is a fast growing, high biomass, cadmium hyperaccumulator plant that can grow on metal‐contaminated fields. Chinese researchers demonstrated in pot studies that S. nigrum inoculated with a Pseudomonas endophyte showed in comparison with uninoculated plants not only a 16% higher shoot biomass, but also a 47% higher cadmium and 16% higher zinc and copper concentrations in the aerial parts of the plant. In addition the soil microbial biomass increased by 39% in pots planted with Pseudomonas‐inoculated S. nigrum compared to uninoculated plants (Chen et al., 2014).
SOIL WARMING AND MICROBIOME: A POSITIVE FEEDBACK LOOP
For the global carbon cycle, tropical forests play a large role since they represent two‐thirds of the terrestrial plant biomass and a substantial fraction of global soil carbon. What will be the effect of global warming on the soil carbon stocks?
Panama plots
A detailed study was conducted in semideciduous, moist lowland tropical forest on volcanic soil in Panama. Over 2 years, plots of 20 m2 were electrically heated to a depth of 1.2 m. The temperature increased by 4°C compared to adjacent control plots, while moisture was not affected. The researchers measured in heated plots a 55% increased soil CO2 emission. The emission (respiration) was mostly derived from heterotrophic, soil‐derived and autotrophic, root‐derived sources. The increased CO2 emission was maintained over the 2‐year study period indicating no attenuation by nutrient limitation. The hydrolytic enzyme activity of the soil remained unaffected by the heating but the microbial biomass carbon increased, suggesting a microbial growth in response to greater organic‐matter turnover in the soil. Global warming will thus add further CO2 to the atmosphere creating a positive feedback, until the soil carbon content will become limiting. Over the 2‐year period, the scientists measure a 13% decrease in total soil carbon stock (Nottingham et al., 2020). In follow‐up experiments, these researchers documented a decline in diversity of both the bacterial and the fungal soil microbiome in the heated plots. Bacteria showed a loss of abundant taxa while fungi lost mostly rare taxa. Warming thus caused a shift in soil community composition. Warming decreased the relative abundance of Bacteroidetes (non‐spore‐forming degraders of polysaccharides), but increased the abundance of the more stress‐tolerant Firmicutes (many can form endospores) and thermophilic Actinobacteria such as Thermoleophilia. Within respect to fungi, warming increased AMF, particularly Glomerales, and thermotolerant saprophytic and pathogenic species from Ascomycota. Overall, the soil microbiome showed a shift to more thermo‐tolerant taxa (Nottingham et al., 2022).
North American forests
Researcher imposed a 4°C soil temperature increase by posing heating rods in soils from North American coniferous temperate forests. This intervention increased soil respiration by 35% (Hicks Pries et al., 2017). Soil respiration did not acclimate to warming nor became it substrate‐limited. Forty per cent of the warming response occurred at a soil depth below 15 cm. Radiocarbon data indicated that respiration was dominated by carbon fixed within the past 50 years. Compared with bulk carbon residence times on the order of 1000 years in soils, this observation suggests decomposition of relatively young and thus not particularly recalcitrant soil carbon. Overall, CO2 emission from warming subsoil of temperate forests was estimated to 30% of the anthropogenic emission.
Conducting the soil warming over a longer 26‐year periods provided insights into the longer‐term dynamic of soil‐microbe responses to warming (Melillo et al., 2017). Plots in an experimental forest consisting of red maple and black oak trees growing in Massachusetts were heated by 5°C with buried heating cables. Controls consisted of buried cables without heating or no cable intervention. The researchers observed transient increases of soil respiration rates; two thirds were due to soil microbial respiration, only one third to root respiration. Overall, 17% of the soil carbon was metabolized. The researchers distinguished three phases. Phase I covered the first decade and showed a significantly increased CO2 emission from the heated soil accompanied by a soil carbon loss which led to a microbial biomass loss. Phase II showed no additional CO2 emission from the heated compared to the control plots and persisted over 6 years. During this phase, the microbial soil community showed a reorganization: fungal biomarkers decreased, Gram‐positive bacteria, and particularly Actinobacteria increased. Phase III occupied the next 5 years with another period of increased CO2 emission in heated over unheated plots accompanied by another reorganization of the soil microbiome to an oligotrophic community with a reduced microbial biomass. Recalcitrant substrates such as lignin became important carbon sources. Reduction in lignin abundance led to another reduction of microbial biomass.
The question of CO2 emission from the soil in a warming world is the greatest unknown in climate projections. In the most recent Earth system models, soils will change from representing CO2 sinks to becoming CO2 sources with the warming trend. This will create a positive feedback for climate change and complicate further climate mitigation efforts (Ren et al., 2024).
AGRICULTURAL APPLICATIONS
The green revolution
During the Green revolution in the 1960s the emphasis for agricultural innovation was to increase food production to assure food security for a growing world population. The Green Revolution has fulfilled its promises – where it not for political conflicts and wars, farmers would be able to feed a now substantially greater world population. For example, since 1990 China has more than doubled its crop production. As elsewhere, this remarkable development has however been accompanied by undesired side effects: groundwater levels have dropped, agricultural greenhouse gas (GHG) emissions have increased, the current crop production level could only be achieved with a heavy use of fertilizers and pesticides, leading to pollution problems.
Sustainability
The key word for the 21th century farming systems is now sustainability. China is an important test case since it represents a fifth of the world population and of crop production and a quarter of the worldwide use of fertilizers. Scientists calculated that reductions in resource use and pollution, while maintaining the same level of food production can be achieved if geographical crop switching is done. Shifting away from maize towards soybean, sugar beet and rice cultivation in the Northeast Plain would reduce the overuse of fertilizers and pesticides and preventing black‐soil degradation. For the Yangtze River Plain reducing rapeseed and rice and increasing wheat and maize cultivation would achieve reductions in GHG emission. In the North China Plain, increases in soybean, rapeseed and rice production and decrease in wheat, maize, and cotton cultivation could alleviating regional water scarcity and excessive fertilizer use (Xie et al., 2023). Before such dramatic geographical changes in crop productions are executed, one might want to test whether the soil microbiome supports the shifted crop plants.
Synthetic bacterial cocktails for rice
Microbiome engineering for quality or quantity improvement of economically important crops is not science fiction as demonstrated with two examples.
Two major Asian cultivated rice types, indica and japonica, have emerged displaying distinct genotypes and phenotypes. Chinese researchers investigated the root microbiome of 68 indica and 27 japonica varieties when grown in the field. They observed that the two varieties recruited distinct root microbiomes: indica varieties showed a higher proportion and diversity of nitrogen cycle‐related bacteria than japonica varieties. This difference was correlated with a single nucleotide polymorphism in the plant protein NRT1.1B, a nitrate transporter and sensor. In nrt1.1Bb mutant indica rice, three key genes involved in the ammonification process were significantly reduced in the root microbiome. These observations explain the known better nitrogen use efficiency of indica over japonica varieties. The researchers went a step further and isolated and cultivated bacteria from the roots of both indica and japonica rice and selected 16 indica‐enriched and 3 japonica‐enriched OTUs for synthetic cocktails. In pot experiments with organic nitrogen supply, the 16‐member indica‐derived bacterial cocktail stimulated root length and plant height of rice seedlings significantly over that of germ‐free plants, or plants treated with a heat‐killed indica cocktail or a live cocktail with unrelated soil bacteria. The 3‐member japonica cocktail showed only an intermediate growth‐promoting effect. These experiments demonstrate not only the two‐way interaction between plants and root microbiome for an economically important plant phenotype, but could pave the way for technologies to modulate the root microbiota in order to increase crop productivity and sustainability (Zhang et al., 2019).
Synthetic bacterial cocktails for tea
Another report demonstrated that also a crucial trait of tea plants is conferred by the root microbiome. The content of theanine, a non‐protein amino acid present in tea leaves, but synthesized in the roots is a critical determinant of tea quality. For its synthesis tea plants needs bioavailable forms of nitrogen, such as ammonium salts or nitrates. Chinese researchers sorted 17 germplasms of tea into low‐ and high‐level theanine producer plants. When analysing the corresponding root microbiomes, they observed an enrichment of Comamonadaceae, Caulobacteraceae, Burkholderiaceae, Rhizobiaceae, Haliangiaceae, and Nitrosomonadaceae in high theanine content tea varieties. At the functional level, these bacteria are enriched for ammonia synthesis capacity. Indeed, tea cultivars with high theanine content also exhibited higher levels of ammonia in their roots. Then the researchers isolated bacterial strains from the roots of high‐theanine plants and constituted a synthetic cocktail containing 21 strains. When this cocktail was applied to low‐theanine tea varieties, inoculated seedlings showed higher root growth, increased ammonia content in the roots and a boosted theanine content. Control experiments demonstrated that the 21 bacterial strain cocktail also conferred enhanced root growth to nitrogen‐starved Arabidopsis. This effect was not observed with a 16‐strain cocktail, which lacked bacteria with nitrogen providing functions. The authors concluded that their research offers avenues for augmenting tea plant quality by modulating the associated root bacteria (Xin et al., 2024).
Synthetic microbial communities (SynComs) are time‐consuming to constitute because a large number of microbial isolates have to be cultivated with different media and mixed into a balanced cocktail. Since these cocktails are valuable research tools to explore the two‐way relationships between plants and their microbiome, a protocol was developed to produce isolates at large scale and freezing them as stocks for mixing by different research groups (Parnell et al., 2024).
Soil inoculation to restore grassland
For technical and cost reasons, inoculation of fields with soil samples instead of SymComs might have a greater practical potential. The use of soil transfers has been demonstrated in grassland restoration experiments. Grasslands cover about 40% of the earth's surface, half of it is rated as degraded. Researchers from The Netherlands demonstrated in a 6‐year field experiment that a soil inoculum not only promoted ecosystem restoration on ex‐arable land but that the origin of the donor soil from grassland or heathland steered the recipient land to plant communities resembling that of the plant community of the donor soil. The inoculum consisted of 1–2.5 L of donor soil spread per m2 recipient soil area. When the topsoil of the recipient land was removed up to the mineral layer, an even better result of restoration was achieved, but represented extra work (Wubs et al., 2016). A 3‐year experiment with a soil inoculum from two different grassland donor sites to a degraded grassland in China confirmed the steering of both the microbe and plant community of the recipient sites into different directions. Inoculation with upland meadow soil induced a more complex microbial network in the recipient soil than an inoculum from meadow steppe soil. However, larger amounts of soil ranging from 10 to 50 litre per m2 were applied to the degraded recipient grassland from which 5 cm of the topsoil was also removed (Han et al., 2022).
Fungal inoculations: Meta‐analyses
A multifactor meta‐analysis evaluated two thousand field and laboratory mycorrhizal inoculation studies. Plant response to mycorrhizal inoculation showed wide variation, but the average plant response was positive (Hoeksema et al., 2010). Plant responses to mycorrhizal inoculation were substantially lower with N‐fertilization, regardless of mycorrhiza type while P‐fertilization was consistently unimportant. C4 grasses exhibited greater positive responses to fungal inoculation than C3 grasses. Plants with N‐fixing bacterial symbionts consistently exhibited only small responses to inoculation while non‐N fixing woody plants showed a positive response. The plant response was substantially lower when inoculated with single AMF species, compared to multiple fungal species or a whole soil inoculum. The response was even higher when non‐mycorrhizal microbes were added. In contrast, the fungal genus used in the inoculum did not emerge as an important predictor variable; most inoculated fungi belonged to the genera Glomus, Pisolithus and Laccaria. A more recent meta‐analysis confirmed and extended the conclusions: Inoculation with symbionts from different guilds (e.g. mycorrhizal fungi and rhizobia bacteria) yielded strongly positive effects, while inoculation with symbionts from the same guild yielded frequently not a greater growth than the best individual symbiont alone. Overall, there were substantial differences in effect size between individual studies (Magnoli & Bever, 2023).
Fungal inoculation on Swiss maize fields
Substantial variation in effect size was also observed when using a single native AMF, Rhizoglomus irregulare, on 54 maize fields over three years in Northern Switzerland (Lutz et al., 2023). The effect was assessed as the percentage change in maize biomass in AMF‐inoculated compared to control plots. The biomass change varied from weak growth inhibition (−12%) to substantial growth promotion (+40%). Only a quarter of the fields showed a significant effect, defined as a 12% growth promotion. This effect is however much higher than achieved by plant breeding (+1%) and corresponds to that of biofertilizer use. The researchers analysed 38 soil chemistry parameters and the soil microbiome by sequencing. They found a high relative abundance of Ascomycota, followed by Mortierellomycota and Basidiomycota. The inoculum AMF achieved no or very low establishment in a quarter of the treated fields. The degree of establishment was not associated with the growth effect. A statistical model identified 10 predictors of growth outcome: growth correlated with 4 soil chemistry parameters (magnesium, ammonium, mineralized N, and microbial biomass carbon); 3 fungal OTUs were associated with growth promotion, while 3 other fungal OTUs were associated with growth inhibition. In fields with growth promotion, the introduced AMF reduced the relative abundance of several plant pathogenic fungi, such as Olpidium and Cladosporium. The researchers concluded that AMF inoculation might protect plant roots from attack by soil‐borne pathogens by various mechanisms (nutrient uptake, systemic resistance, root alteration). As a single AMF strain might not fulfil all these tasks, the authors recommended cocktails of strains or AMF inoculum rotation to improve soil health.
Green deal dilemma
AMF inoculation is not the only approach to improve soil health. In the Farm to Fork (F2F) Strategy under the “European Green Deal” of the EU, organic farming is proposed to increase from currently 9% to 25% of agriculturally used land by 2030. Organic farming (OF), in contrast to conventional farming (CF), does not use synthetic fertilizers and pesticides, but plant residues or livestock manure to enhance soil fertility. OF reduces soil erosion, groundwater pollution, and should foster soil biodiversity and increase farmer income compared to CF (Azarbad, 2022). However, there is a problem. A meta‐analysis of 316 OF and CF yield comparisons for 34 crop species showed that OF yields are typically 25% lower than CF yields. The extent of yield reduction is context dependent. For fruits it is negligible, for oilseed crops it is small, while for cereals and vegetables it is substantial. Yield reduction in OF is larger in annual than in perennial plants, greater in non‐legumes than in legumes. Barley and wheat yield reductions by OF are larger than those for maize. The researchers investigated confounding factors and detected that soil pH, N addition and irrigation affected the extent of yield reduction. OF vs. CF performance was −35% under irrigated conditions, but only −17% under rain‐fed condition (Seufert et al., 2012), which was interpreted as evidence for a better water‐holding capacity of OF soils, raising the question whether specific soil microbiome characteristics could allow farming to better cope with droughts. The authors of this study, dealing with an ideologically charged subject, warned that lower yields in OF could require the need for more land to produce the same amount of food as CF, resulting in more widespread deforestation and biodiversity loss, and could thus undermine the ecological benefit of OF. At the end, in addition to solid scientific data on the subject, a decision must also weigh ecological benefits against economic and political constraints (Meyfroidt et al., 2022; Wittwer et al., 2021).
Farming intensity and soil microbiome
Another study from Switzerland investigated the impact of different farming systems, namely of CF, no‐till farming, and OF on wheat root fungal communities across 60 farmlands. No difference in relative abundance of various orders of wheat root fungal communities or in alpha diversity indices was observed between the three farming types. However, OF harboured a much more complex fungal network with significantly higher connectivity than conventional and no‐till farming systems. The abundance of keystone taxa was the highest under OF conditions (Banerjee et al., 2019).
These researchers then asked whether intensively managed agricultural areas across Europe suffer a loss of diversity in the soil microbiome compared to less managed grasslands. They investigated 150 arable and 60 grassland sites from Sweden to Spain. Arable land showed consistently a lower fungal diversity than grasslands. Arable lands had 17% fewer fungal OTUs than grasslands and rare fungal OTUs were particularly affected. Agricultural intensity, determined by tillage intensity and the application of chemical fertilizers and pesticides, was negatively associated with overall fungal OTUs richness. The researchers concluded that intensive agriculture is associated with the loss of soil microbial biodiversity and a strong homogenizing effect on fungal communities, manifested by a reduction of rare taxa. AMF of the order Glomerales were more abundant in grasslands, while plant pathogens were more abundant in arable lands (Banerjee et al., 2024).
URBAN GREENSPACE
Urban heat islands
Forestry and agriculture are not the only sectors which depend on soil ecological services, less well investigated is the effect of soil conditions on urban greenspace. This neglect is not justified. According to UN data, 53% of the world population lived in 2018 in cities and it is projected that this percentage will increase to 68% by 2050 (https://population.un.org/wup/Publications/Files/WUP2018‐Report.pdf). Urban settings are therefore the determining environment for the majority of the human world population for most of their life. Climate change will cause increasing temperatures and heat spell events. Due to the urban heat‐island effect, temperature increases will be particularly high in cities. On a yearly average, urban areas are 2.9°C warmer than the non‐urban fringe (Imhoff et al., 2010). The health‐compromising effect of heat will thus be particularly high in cities. Therefore, urban forests and grass lawns serve not only as recreation areas, but have important health and ecological functions by dampening heat spells and air pollution, regulating water budgets and sequestering greenhouse gases. Climate change will not only increase air temperatures, but also increase soil temperatures. Researchers measured a mean yearly aboveground temperature increase of 2°C for a city in China compared to the surrounding rural area. In the summer, the urban–rural soil temperature difference even reached 3°C (Shi et al., 2012).
Urban greenspaces and soil biodiversity
A large consortium of researchers representing many different scientific branches conducted a standardized survey of urban greenspaces from 56 municipalities across six continents. In a first analysis, the researchers compared the urban greenspace soil microbiome in public parks and large residential gardens with that of adjacent natural ecosystems (Delgado‐Baquerizo et al., 2021). They observed globally a greater similarity among the urban greenspaces than among the adjacent natural sites. Urban greenspaces represented important hot spots for the local (alpha) diversity of some bacterial groups, particularly for fast‐growing Gammaproteobacteria and Bacteroidetes that could take advantage of fertilization and irrigation practices, manifested by higher N and P cycling. The higher bacterial diversity was associated with a higher soil pH in urban greenspace. Urban greenspaces showed a higher proportion of plant and human (Mycobacterium, Listeria, Vibrio) pathogens in the soil. Metagenome sequencing suggested that urban greenspace might be associated with higher greenhouse gas emissions due to denitrification and methanogenesis by soil microorganisms. In a follow‐up analysis these researchers investigated plants (by census) and soil biodiversity (by metagenome sequencing) and correlated these data with 18 surrogates of ecosystem functions (including microbe‐driven carbon pools, water regulation, nutrient cycling, plant–soil mutualism, organic matter decomposition, plant productivity, pathogen control) (Fan et al., 2023). They documented that the diversity of soil bacteria, fungi, protists and invertebrates correlated positively and significantly with multiple ecosystem functions. Invertebrates and fungal diversity showed the greatest, bacterial diversity a lesser impact, while plant diversity, somewhat surprisingly, showed no correlation with ecosystem services. This does not mean, however, that plants as such had no ecological role, but this role did not increase with plant species diversity. The abundant and not the rare soil taxa were associated with ecosystem services. Management practices played a major role: mowing was positively associated with ecological multifunctionality, while fertilization and irrigation suppressed soil biodiversity. Metagenome sequencing allowed association studies of gene functions with ecological services. Soil microbial genes related to methane, nitrogen, phosphate and sulfur metabolism were positively correlated with ecological multifunctionality.
The authors noted that plants in urban greenspaces are often non‐indigenous species, are selected for horticultural value rather than improvement of the soil microbiome. One might therefore expect to observe a difference between native and non‐native plants in ecosystem services. Ecologists from Germany addressed this question when studying 20 dry grassland plots in Berlin for plant species richness and its effect on soil multifunctionality and soil organic carbon content. The average number of plant species were 28 per plot, 21 were native and 7 non‐native plants. Native and non‐native plant species richness had different effect sizes on ecological multifunctionality with native plants having higher effects (Schittko et al., 2022). Creating forests in urban areas is complicated by habitat fragmentation, invasive species, and degraded soils. Clearing first exotic shrubs and vines from sites destined for reforestation of urban parks in New York, followed by planting native trees and shrubs has shown beneficial effects (Simmons et al., 2016). Compost amendment increased the growth of tree saplings in an urban forest restoration in New York City (Oldfield et al., 2015). Industrial compost (which is heated and therefore is re‐colonized by microbes during maturation) has protective effects from pathogens but this effect is batch‐dependent. Compost amendments, interplanting with shrubs, and enriched planted tree species composition increased not only the basal area of the tree saplings by 20%, but also the soil microbial biomass and water holding capacity of soils (Ward et al., 2021).
OUTLOOK
Achieving food security for a growing world population without increasing the surface of the arable land which would result in further biodiversity loss, represents an important challenge for the future. Optimizing yield on conventionally farmed arable surface comes with a heavy ecological price as the use of chemical fertilizers increases greenhouse gas emissions and synthetic crop protection products take an environmental toll.
Developing a new generation of synthetic agrochemicals with a sustainable profile and features suitable for integrated pest management is anything but easy, triggering increasing research interest into microbiological approaches to stimulate plant health and productivity, particularly under the spectre of global climate change (Singh & Trivedi, 2017). For agrochemicals the research profile has been shifted substantially from efficiency and potency to sustainability, low toxicity and integrated solutions due to restrictions for its registration in developed countries. The breeders put much hope in Green gene technology. However, soil microbiologists warn that plant growth‐promoting microbes, biofertilizers and biopesticides should not be regarded as an alternative to agrochemicals, but as a complement which could confer environmental benefits (Batista & Singh, 2021). The idea is not new: N‐fixing Rhizobium was patented as bioinoculant more than 100 years ago. AMF inoculants were subsequently introduced to increase P uptake and stimulate plant growth. The agricultural biologicals market including bioinoculants is estimated to have a value of about 13 billion US$ in 2023 and is expected to grow at a double‐digit growth rate in the next years (https://www.fortunebusinessinsights.com/industry‐reports/agricultural‐biologicals‐market‐100411). The market is only moderately consolidated and bears interesting opportunities for established companies as well as for a wide array of emerging ones. Governmental decisions such as the EU Green Deal are likely to cause a relative increase in biopesticides and biofertilizers use in the future.
Biofertilizers such as Bradyrhizobium are already used at large scale in Brazil and Argentina on soybean fields, and Azospirillum for corn, wheat, rice and pastures in Brazil. Trichoderma is the most widely used bio‐fungicide in India. Inconsistent results with bioinoculants are still a major reason for the preference of farmers particularly from the developed world for chemical products. Current efforts explore the introduction of bioinoculants by seed treatment, foliar spray or by soil application; researchers use synthetic communities or build applications based on the concepts of a core microbiome associated with healthy plants or with a focus on “hub” microbes. Probiotic microbes also entered the discussion to further the health of plants as well as prebiotics (e.g. chitin amendments) to enhance the activity of probiotic microbes. Some emphasis is given to seed or seedling “biopriming” which involves controlled hydration along with inoculation of biological agents triggering pre‐germination metabolic activities (Singh et al., 2023). Although promising effects have been described in controlled glasshouse experiments, effects of bioinoculants on plant growth and productivity under field conditions were much less consistent suggesting the impact of confounding factors. As in the case of targeted modification of the human microbiome by bioinoculants, the plant field should avoid unfounded hype and overoptimism with respect to rapid field applications due to many remaining knowledge gaps. However, the link of plant microbiome and bioinoculants with the health of plants seems more straightforward than the impact of the human microbiome and the use of probiotics and prebiotics to ameliorate human health. A first step towards a more reproducible effect achievable with bioinoculants for plants is a standardization of the commercial products, the development of reliable bioassays and the regulation of product release by state authorities (Salomon et al., 2022).
AUTHOR CONTRIBUTIONS
Felix Brüssow: Investigation; writing – original draft. Friederike Bruessow: Writing – original draft; investigation. Harald Brüssow: Conceptualization; investigation; writing – original draft.
CONFLICT OF INTEREST STATEMENT
The authors have no conflict of interest to report.
ACKNOWLEDGEMENTS
We thank Christopher Blake for reading the manuscript.
Brüssow, F. , Bruessow, F. & Brüssow, H. (2024) The role of the plant microbiome for forestry, agriculture and urban greenspace in times of environmental change. Microbial Biotechnology, 17, e14482. Available from: 10.1111/1751-7915.14482
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.
REFERENCES
- Allsup, C.M. , George, I. & Lankau, R.A. (2023) Shifting microbial communities can enhance tree tolerance to changing climates. Science, 380(6647), 835–840. Available from: 10.1126/science.adf2027 [DOI] [PubMed] [Google Scholar]
- Anthony, M.A. , Crowther, T.W. , van der Linde, S. , Suz, L.M. , Bidartondo, M.I. , Cox, F. et al. (2022) Forest tree growth is linked to mycorrhizal fungal composition and function across Europe. The ISME Journal, 16(5), 1327–1336. Available from: 10.1038/s41396-021-01159-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azarbad, H. (2022) Conventional vs. organic agriculture‐which one promotes better yields and microbial resilience in rapidly changing climates? Frontiers in Microbiology, 13, 903500. Available from: 10.3389/fmicb.2022.903500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai, Y. , Müller, D.B. , Srinivas, G. , Garrido‐Oter, R. , Potthoff, E. , Rott, M. et al. (2015) Functional overlap of the Arabidopsis leaf and root microbiota. Nature, 528(7582), 364–369. Available from: 10.1038/nature16192 [DOI] [PubMed] [Google Scholar]
- Banerjee, S. , Walder, F. , Büchi, L. , Meyer, M. , Held, A.Y. , Gattinger, A. et al. (2019) Agricultural intensification reduces microbial network complexity and the abundance of keystone taxa in roots. The ISME Journal, 13(7), 1722–1736. Available from: 10.1038/s41396-019-0383-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banerjee, S. , Zhao, C. , Garland, G. , Edlinger, A. , García‐Palacios, P. , Romdhane, S. et al. (2024) Biotic homogenization, lower soil fungal diversity and fewer rare taxa in arable soils across Europe. Nature Communications, 15(1), 327. Available from: 10.1038/s41467-023-44073-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Batista, B.D. & Singh, B.K. (2021) Realities and hopes in the application of microbial tools in agriculture. Microbial Biotechnology, 14(4), 1258–1268. Available from: 10.1111/1751-7915.13866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckage, B. , Osborne, B. , Gavin, D.G. , Pucko, C. , Siccama, T. & Perkins, T. (2008) A rapid upward shift of a forest ecotone during 40 years of warming in the Green Mountains of Vermont. Proceedings of the National Academy of Sciences of the United States of America, 105(11), 4197–4202. Available from: 10.1073/pnas.0708921105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bulgarelli, D. , Rott, M. , Schlaeppi, K. , Loren, V. , van Themaat, E. , Ahmadinejad, N. et al. (2012) Revealing structure and assembly cues for Arabidopsis root‐inhabiting bacterial microbiota. Nature, 488(7409), 91–95. Available from: 10.1038/nature11336 [DOI] [PubMed] [Google Scholar]
- Canarini, A. , Schmidt, H. , Fuchslueger, L. , Martin, V. , Herbold, C.W. , Zezula, D. et al. (2021) Ecological memory of recurrent drought modifies soil processes via changes in soil microbial community. Nature Communications, 12(1), 5308. Available from: 10.1038/s41467-021-25675-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalhais, L.C. , Dennis, P.G. & Schenk, P.M. (2014) Plant defence inducers rapidly influence the diversity of bacterial communities in a potting mix. Applied Soil Ecology, 84, 1–5. [Google Scholar]
- Castrillo, G. , Teixeira, P.J. , Paredes, S.H. , Law, T.F. , de Lorenzo, L. , Feltcher, M.E. et al. (2017) Root microbiota drive direct integration of phosphate stress and immunity. Nature, 543(7646), 513–518. Available from: 10.1038/nature21417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceballos, G. & Ehrlich, P.R. (2023) Mutilation of the tree of life via mass extinction of animal genera. Proceedings of the National Academy of Sciences of the United States of America, 120(39), e2306987120. Available from: 10.1073/pnas.2306987120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, I.C. , Hill, J.K. , Ohlemüller, R. , Roy, D.B. & Thomas, C.D. (2011) Rapid range shifts of species associated with high levels of climate warming. Science, 333(6045), 1024–1026. Available from: 10.1126/science.1206432 [DOI] [PubMed] [Google Scholar]
- Chen, L. , Luo, S. , Li, X. , Wan, Y. , Chen, J. & Liu, S. (2014) Interaction of Cd‐hyperaccumulator Solanum nigrum L. and functional endophyte pseudomonas sp. Lk9 on soil heavy metals uptake. Soil Biology and Biochemistry, 68, 300–308. [Google Scholar]
- Chen, T. , Nomura, K. , Wang, X. , Sohrabi, R. , Xu, J. , Yao, L. et al. (2020) A plant genetic network for preventing dysbiosis in the phyllosphere. Nature, 580(7805), 653–657. Available from: 10.1038/s41586-020-2185-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coban, O. , De Deyn, G.B. & van der Ploeg, M. (2022) Soil microbiota as game‐changers in restoration of degraded lands. Science, 375(6584), abe0725. Available from: 10.1126/science.abe0725 [DOI] [PubMed] [Google Scholar]
- Dastogeer, K.M.G. , Zahan, M.I. , Tahjib‐Ul‐Arif, M. , Akter, M.A. & Okazaki, S. (2020) Plant salinity tolerance conferred by arbuscular mycorrhizal fungi and associated mechanisms: a meta‐analysis. Frontiers in Plant Science, 9(11), 588550. Available from: 10.3389/fpls.2020.588550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delavaux, C.S. , Crowther, T.W. , Zohner, C.M. , Robmann, N.M. , Lauber, T. , van den Hoogen, J. et al. (2023) Native diversity buffers against severity of non‐native tree invasions. Nature, 621(7980), 773–781. Available from: 10.1038/s41586-023-06440-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delgado‐Baquerizo, M. , Eldridge, D.J. , Liu, Y.R. , Sokoya, B. , Wang, J.T. , Hu, H.W. et al. (2021) Global homogenization of the structure and function in the soil microbiome of urban greenspaces. Science Advances, 7(28), eabg5809. Available from: 10.1126/sciadv.abg5809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dial, R.J. , Maher, C.T. , Hewitt, R.E. , Wockenfuss, A.M. , Wong, R.E. , Crawford, D.J. et al. (2024) Arctic sea ice retreat fuels boreal forest advance. Science, 383(6685), 877–884. Available from: 10.1126/science.adh2339 [DOI] [PubMed] [Google Scholar]
- Durán, P. , Thiergart, T. , Garrido‐Oter, R. , Agler, M. , Kemen, E. , Schulze‐Lefert, P. et al. (2018) Microbial Interkingdom interactions in roots promote Arabidopsis survival. Cell, 175(4), 973–983.e14. Available from: 10.1016/j.cell.2018.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan, K. , Chu, H. , Eldridge, D.J. , Gaitan, J.J. , Liu, Y.R. , Sokoya, B. et al. (2023) Soil biodiversity supports the delivery of multiple ecosystem functions in urban greenspaces. Nature Ecology and Evolution, 7(1), 113–126. Available from: 10.1038/s41559-022-01935-4 [DOI] [PubMed] [Google Scholar]
- Fargione, J. , Haase, D.L. , Burney, O.T. , Kildisheva, O.A. , Edge, G. , Cook‐Patton, S.C. et al. (2021) Challenges to the reforestation pipeline in the United States. Frontiers in Forests and Global Change, 4, 629198. [Google Scholar]
- Finkel, O.M. , Salas‐González, I. , Castrillo, G. , Conway, J.M. , Law, T.F. , Teixeira, P.J.P.L. et al. (2020) A single bacterial genus maintains root growth in a complex microbiome. Nature, 587(7832), 103–108. Available from: 10.1038/s41586-020-2778-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco, A.R. , Sousa, N.R. , Ramos, M.A. , Oliveira, R.S. & Castro, P.M. (2014) Diversity and persistence of ectomycorrhizal fungi and their effect on nursery‐inoculated Pinus pinaster in a post‐fire plantation in northern Portugal. Microbial Ecology, 68(4), 761–772. Available from: 10.1007/s00248-014-0447-9 [DOI] [PubMed] [Google Scholar]
- Han, X. , Li, Y. , Li, Y. , Du, X. , Li, B. , Li, Q. et al. (2022) Soil inoculum identity and rate jointly steer microbiomes and plant communities in the field. ISME Communications, 2(1), 59. Available from: 10.1038/s43705-022-00144-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haney, C.H. & Ausubel, F.M. (2015) Plant microbiome blueprints. Science, 349(6250), 788–789. Available from: 10.1126/science.aad0092 [DOI] [PubMed] [Google Scholar]
- Hicks Pries, C.E. , Castanha, C. , Porras, R.C. & Torn, M.S. (2017) The whole‐soil carbon flux in response to warming. Science, 355(6332), 1420–1423. Available from: 10.1126/science.aal1319 [DOI] [PubMed] [Google Scholar]
- Hoeksema, J.D. , Chaudhary, V.B. , Gehring, C.A. , Johnson, N.C. , Karst, J. , Koide, R.T. et al. (2010) A meta‐analysis of context‐dependency in plant response to inoculation with mycorrhizal fungi. Ecology Letters, 13(3), 394–407. Available from: 10.1111/j.1461-0248.2009.01430.x [DOI] [PubMed] [Google Scholar]
- Imhoff, M.L. , Zhang, P. , Wolfe, R.E. & Bounoua, L. (2010) Remote sensing of the urban heat Island effect across biomes in the continental USA. Remote Sensing of Environment, 114(3), 504–513. [Google Scholar]
- Jo, I. , Fei, S. , Oswalt, C.M. , Domke, G.M. & Phillips, R.P. (2019) Shifts in dominant tree mycorrhizal associations in response to anthropogenic impacts. Science Advances, 5(4), eaav6358. Available from: 10.1126/sciadv.aav6358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junker, R.R. , He, X. , Otto, J.C. , Ruiz‐Hernández, V. & Hanusch, M. (2021) Divergent assembly processes? A comparison of the plant and soil microbiome with plant communities in a glacier forefield. FEMS Microbiology Ecology, 97(10), fiab135. Available from: 10.1093/femsec/fiab135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laforest‐Lapointe, I. , Paquette, A. , Messier, C. & Kembel, S.W. (2017) Leaf bacterial diversity mediates plant diversity and ecosystem function relationships. Nature, 546(7656), 145–147. Available from: 10.1038/nature22399 [DOI] [PubMed] [Google Scholar]
- Lambais, M.R. , Crowley, D.E. , Cury, J.C. , Büll, R.C. & Rodrigues, R.R. (2006) Bacterial diversity in tree canopies of the Atlantic forest. Science, 312(5782), 1917. Available from: 10.1126/science.1124696 [DOI] [PubMed] [Google Scholar]
- Lau, J.A. & Lennon, J.T. (2012) Rapid responses of soil microorganisms improve plant fitness in novel environments. Proceedings of the National Academy of Sciences of the United States of America, 109(35), 14058–14062. Available from: 10.1073/pnas.1202319109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebeis, S.L. , Paredes, S.H. , Lundberg, D.S. , Breakfield, N. , Gehring, J. , McDonald, M. et al. (2015) Salicylic acid modulates colonization of the root microbiome by specific bacterial taxa. Science, 349(6250), 860–864. Available from: 10.1126/science.aaa8764 [DOI] [PubMed] [Google Scholar]
- Lenoir, J. , Gégout, J.C. , Marquet, P.A. , de Ruffray, P. & Brisse, H. (2008) A significant upward shift in plant species optimum elevation during the 20th century. Science, 320(5884), 1768–1771. Available from: 10.1126/science.1156831 [DOI] [PubMed] [Google Scholar]
- Lundberg, D.S. , Lebeis, S.L. , Paredes, S.H. , Yourstone, S. , Gehring, J. , Malfatti, S. et al. (2012) Defining the core Arabidopsis thaliana root microbiome. Nature, 488(7409), 86–90. Available from: 10.1038/nature11237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutz, S. , Bodenhausen, N. , Hess, J. , Valzano‐Held, A. , Waelchli, J. , Deslandes‐Hérold, G. et al. (2023) Soil microbiome indicators can predict crop growth response to large‐scale inoculation with arbuscular mycorrhizal fungi. Nature Microbiology, 8(12), 2277–2289. Available from: 10.1038/s41564-023-01520-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magnoli, S.M. & Bever, J.D. (2023) Plant productivity response to inter‐ and intra‐symbiont diversity: mechanisms, manifestations and meta‐analyses. Ecology Letters, 26(9), 1614–1628. Available from: 10.1111/ele.14274 [DOI] [PubMed] [Google Scholar]
- Marx, D.H. , Bryan, W.C. & Cordell, C.E. (1977) Survival and growth of pine seedlings with Pisolithus Ectomycorrhizae after two years on reforestation sites in North Carolina and Florida. Forest Science, 23, 363–373. [Google Scholar]
- Melillo, J.M. , Frey, S.D. , DeAngelis, K.M. , Werner, W.J. , Bernard, M.J. , Bowles, F.P. et al. (2017) Long‐term pattern and magnitude of soil carbon feedback to the climate system in a warming world. Science, 358(6359), 101–105. Available from: 10.1126/science.aan2874 [DOI] [PubMed] [Google Scholar]
- Metze, D. , Schnecker, J. , Canarini, A. , Fuchslueger, L. , Koch, B.J. , Stone, B.W. et al. (2023) Microbial growth under drought is confined to distinct taxa and modified by potential future climate conditions. Nature Communications, 14(1), 5895. Available from: 10.1038/s41467-023-41524-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyfroidt, P. , de Bremond, A. , Ryan, C.M. , Archer, E. , Aspinall, R. , Chhabra, A. et al. (2022) Ten facts about land systems for sustainability. Proceedings of the National Academy of Sciences of the United States of America, 119(7), e2109217118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson, A.R. , Narrowe, A.B. , Rhoades, C.C. , Fegel, T.S. , Daly, R.A. , Roth, H.K. et al. (2022) Wildfire‐dependent changes in soil microbiome diversity and function. Nature Microbiology, 7(9), 1419–1430. Available from: 10.1038/s41564-022-01203-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netherway, T. , Bengtsson, J. , Buegger, F. , Fritscher, J. , Oja, J. , Pritsch, K. et al. (2024) Pervasive associations between dark septate endophytic fungi with tree root and soil microbiomes across Europe. Nature Communications, 15(1), 159. Available from: 10.1038/s41467-023-44172-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nottingham, A.T. , Meir, P. , Velasquez, E. & Turner, B.L. (2020) Soil carbon loss by experimental warming in a tropical forest. Nature, 584(7820), 234–237. Available from: 10.1038/s41586-020-2566-4 [DOI] [PubMed] [Google Scholar]
- Nottingham, A.T. , Scott, J.J. , Saltonstall, K. , Broders, K. , Montero‐Sanchez, M. , Püspök, J. et al. (2022) Microbial diversity declines in warmed tropical soil and respiration rise exceed predictions as communities adapt. Nature Microbiology, 7(10), 1650–1660. Available from: 10.1038/s41564-022-01200-1 [DOI] [PubMed] [Google Scholar]
- Oldfield, E.E. , Felson, A.J. , Novem Auyeung, D.S. , Crowther, T.W. , Sonti, N.F. , Harada, Y. et al. (2015) Growing the urban forest: tree performance in response to biotic and abiotic land management. Restoration Ecology, 23(5), 707–718. [Google Scholar]
- Parmesan, C. & Yohe, G. (2003) A globally coherent fingerprint of climate change impacts across natural systems. Nature, 421(6918), 37–42. Available from: 10.1038/nature01286 [DOI] [PubMed] [Google Scholar]
- Parnell, J.J. , Vintila, S. , Tang, C. , Wagner, M.R. & Kleiner, M. (2024) Evaluation of ready‐to‐use freezer stocks of a synthetic microbial community for maize root colonization. Microbiology Spectrum, 12(1), e0240123. Available from: 10.1128/spectrum.02401-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren, S. , Wang, T. , Guenet, B. , Liu, D. , Cao, Y. , Ding, J. et al. (2024) Projected soil carbon loss with warming in constrained earth system models. Nature Communications, 15(1), 102. Available from: 10.1038/s41467-023-44433-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salomon, M.J. , Watts‐Williams, S.J. , McLaughlin, M.J. , Bücking, H. , Singh, B.K. , Hutter, I. et al. (2022) Establishing a quality management framework for commercial inoculants containing arbuscular mycorrhizal fungi. iScience, 25(7), 104636. Available from: 10.1016/j.isci.2022.104636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schittko, C. , Onandia, G. , Bernard‐Verdier, M. , Heger, T. , Jeschke, J.M. , Kowarik, I. et al. (2022) Biodiversity maintains soil multifunctionality and soil organic carbon in novel urban ecosystems. Journal of Ecology, 110, 916–934. [Google Scholar]
- Schmitz, L. , Yan, Z. , Schneijderberg, M. , de Roij, M. , Pijnenburg, R. , Zheng, Q. et al. (2022) Synthetic bacterial community derived from a desert rhizosphere confers salt stress resilience to tomato in the presence of a soil microbiome. The ISME Journal, 16(8), 1907–1920. Available from: 10.1038/s41396-022-01238-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seufert, V. , Ramankutty, N. & Foley, J.A. (2012) Comparing the yields of organic and conventional agriculture. Nature, 485(7397), 229–232. [DOI] [PubMed] [Google Scholar]
- Shi, B. , Tang, C.‐S. , Gao, L. , Liu, C. & Wang, B.‐J. (2012) Observation and analysis of the urban heat Island effect on soil in Nanjing, China. Environmental Earth Sciences, 67(1), 215–229. [Google Scholar]
- Simmons, B.L. , Hallett, R.A. , Falxa Sonti, N. , Auyeung, D.S.N. & Lu, J.W.T. (2016) Long‐term outcomes of forest restoration in an urban park. Restoration Ecology, 24(1), 109–118. [Google Scholar]
- Singh, B.K. & Trivedi, P. (2017) Microbiome and the future for food and nutrient security. Microbial Biotechnology, 10(1), 50–53. Available from: 10.1111/1751-7915.12592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh, P. , Vaishnav, A. , Liu, H. , Xiong, C. , Singh, H.B. & Singh, B.K. (2023) Seed biopriming for sustainable agriculture and ecosystem restoration. Microbial Biotechnology, 16(12), 2212–2222. Available from: 10.1111/1751-7915.14322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, S.A. & Donoghue, M.J. (2008) Rates of molecular evolution are linked to life history in flowering plants. Science, 322(5898), 86–89. Available from: 10.1126/science.1163197 [DOI] [PubMed] [Google Scholar]
- Soldan, R. , Fusi, M. , Cardinale, M. , Homma, F. , Santos, L.G. , Wenzl, P. et al. (2024) Consistent effects of independent domestication events on the plant microbiota. Current Biology, 34(3), 557–567.e4. Available from: 10.1016/j.cub.2023.12.056 [DOI] [PubMed] [Google Scholar]
- Steidinger, B.S. , Crowther, T.W. , Liang, J. , Van Nuland, M.E. , Werner, G.D.A. , Reich, P.B. et al. (2019) Climatic controls of decomposition drive the global biogeography of forest‐tree symbioses. Nature, 569(7756), 404–408. Available from: 10.1038/s41586-019-1128-0 [DOI] [PubMed] [Google Scholar]
- Wang, Q. , He, D. , Zhang, X. , Cheng, Y. , Sun, Y. & Zhu, J. (2024) Insight into bacterial and archaeal community structure of Suaeda altissima and Suaeda dendroides rhizosphere in response to different salinity level. Microbiology Spectrum, 12(1), e0164923. Available from: 10.1128/spectrum.01649-23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, Y. , Wang, X. , Sun, S. , Jin, C. , Su, J. , Wei, J. et al. (2022) GWAS, MWAS and mGWAS provide insights into precision agriculture based on genotype‐dependent microbial effects in foxtail millet. Nature Communications, 13(1), 5913. Available from: 10.1038/s41467-022-33238-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward, E.B. , Doroski, D.A. , Felson, A.J. , Hallett, R.A. , Oldfield, E.E. , Kuebbing, S.E. et al. (2021) Positive long‐term impacts of restoration on soils in an experimental urban forest. Ecological Applications, 31(5), e02336. Available from: 10.1002/eap.2336 [DOI] [PubMed] [Google Scholar]
- Ward, E.B. , Duguid, M.C. , Kuebbing, S.E. , Lendemer, J.C. & Bradford, M.A. (2022) The functional role of ericoid mycorrhizal plants and fungi on carbon and nitrogen dynamics in forests. The New Phytologist, 235(5), 1701–1718. Available from: 10.1111/nph.18307 [DOI] [PubMed] [Google Scholar]
- Weber, J. , King, J.A. , Abraham, N.L. , Grosvenor, D.P. , Smith, C.J. , Shin, Y.M. et al. (2024) Chemistry‐albedo feedbacks offset up to a third of forestation's CO2 removal benefits. Science, 383(6685), 860–864. Available from: 10.1126/science.adg6196 [DOI] [PubMed] [Google Scholar]
- Wittwer, R.A. , Bender, S.F. , Hartman, K. , Hydbom, S. , Lima, R.A.A. , Loaiza, V. et al. (2021) Organic and conservation agriculture promote ecosystem multifunctionality. Science Advances, 7(34), eabg6995. Available from: 10.1126/sciadv.abg6995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wubs, E.R. , van der Putten, W.H. , Bosch, M. & Bezemer, T.M. (2016) Soil inoculation steers restoration of terrestrial ecosystems. Nature Plants, 2, 16107. Available from: 10.1038/nplants.2016.107 [DOI] [PubMed] [Google Scholar]
- Xie, W. , Zhu, A. , Ali, T. , Zhang, Z. , Chen, X. , Wu, F. et al. (2023) Crop switching can enhance environmental sustainability and farmer incomes in China. Nature, 616(7956), 300–305. Available from: 10.1038/s41586-023-05799-x [DOI] [PubMed] [Google Scholar]
- Xin, W. , Zhang, J. , Yu, Y. , Tian, Y. , Li, H. , Chen, X. et al. (2024) Root microbiota of tea plants regulate nitrogen homeostasis and theanine synthesis to influence tea quality. Current Biology, 34(4), 868–880.e6. Available from: 10.1016/j.cub.2024.01.044 [DOI] [PubMed] [Google Scholar]
- Zhang, J. , Liu, Y.X. , Zhang, N. , Hu, B. , Jin, T. , Xu, H. et al. (2019) NRT1.1B is associated with root microbiota composition and nitrogen use in field‐grown rice. Nature Biotechnology, 37(6), 676–684. Available from: 10.1038/s41587-019-0104-4 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.


