Abstract
Chiral 1,2,3-triazoles are highly attractive motifs in various fields. However, achieving catalytic asymmetric click reactions of azides and alkynes for chiral triazole synthesis remains a significant challenge, mainly due to the limited catalytic systems and substrate scope. Herein, we report an enantioselective azidation/click cascade reaction of N-propargyl-β-ketoamides with a readily available and potent azido transfer reagent via copper catalysis, which affords a variety of chiral 1,2,3-triazoles with up to 99% yield and 95% ee under mild conditions. Notably, chiral 1,5-disubstituted triazoles that have not been accessed by previous asymmetric click reactions are also prepared with good functional group tolerance.
Subject terms: Asymmetric synthesis, Catalyst synthesis, Synthetic chemistry methodology
Catalytic asymmetric click reactions of azides and alkynes for chiral triazole synthesis remains a challenge, due to the limited catalytic systems and substrate scope. Herein, the authors report the enantioselective azidation/click cascade reaction of N-propargyl-β-ketoamides via copper catalysis, affording a variety of chiral 1,2,3-triazoles.
Introduction
1,2,3-Triazoles play a crucial role in drug discovery, biorthogonal chemistry, synthetic chemistry, and materials sciences1–8. Since being simultaneously reported by the groups of Sharpless9,10 and Meldal11, copper-catalyzed azide-alkyne cycloaddition, also known as click reaction12–19, has emerged as a highly efficient and biocompatible strategy for synthesizing 1,2,3-triazoles. Despite the well-explored racemic version, the synthesis of chiral triazoles remains challenging mainly due to no sp3 stereogenic center in the triazole skeleton and the linear geometry of the azide and alkyne20,21. In this context, Zhou22–26, Fossey27–29, Topczewski30,31, and others32–39 have employed strategies such as (dynamic) kinetic resolution or desymmetrization to achieve a series of enantioselective copper-catalyzed click reaction of azides and terminal alkynes, which generated enantioenriched 1,4-disubstituted triazoles regioselectively (Fig. 1a). Notably, Zhou and co-workers used 1-iodoalkynes as special internal alkynes to prepare the corresponding chiral 1,4,5-trisubstituted triazoles24. In 2021, Topczewski et al. discovered enantioselective nickel-catalyzed click reaction with internal alkynes for dynamic kinetic resolution of allylic azides40, which gave the expected 1,4,5-trisubstituted triazoles with up to 86% ee (Fig. 1b). Recently, significant progress has been made in the atroposelective click reaction of β-naphthol-derived internal alkynes with azides, independently disclosed by Li41, Xu42, and Cui43,44. In these protocols, a range of axially chiral 1,4,5-trisubstituted triazoles were prepared by employing precious rhodium or iridium catalyst (Fig. 1c). Although current methods could provide diverse access to chiral 1,4-disubstituted and 1,4,5-trisubstituted triazoles, there were a few kind of asymmetric click reactions and no reports on efficient strategy for synthesis of chiral 1,5-disubstituted triazoles, which are core units of various bio-active molecules45–50. Therefore, exploring a kind of efficient catalytic system to achieve asymmetric click reaction especially for synthesizing chiral 1,5-disubstituted triazoles, is still highly desirable.
Fig. 1. Strategies for catalytic asymmetric synthesis of chiral 1,2,3-triazoles.
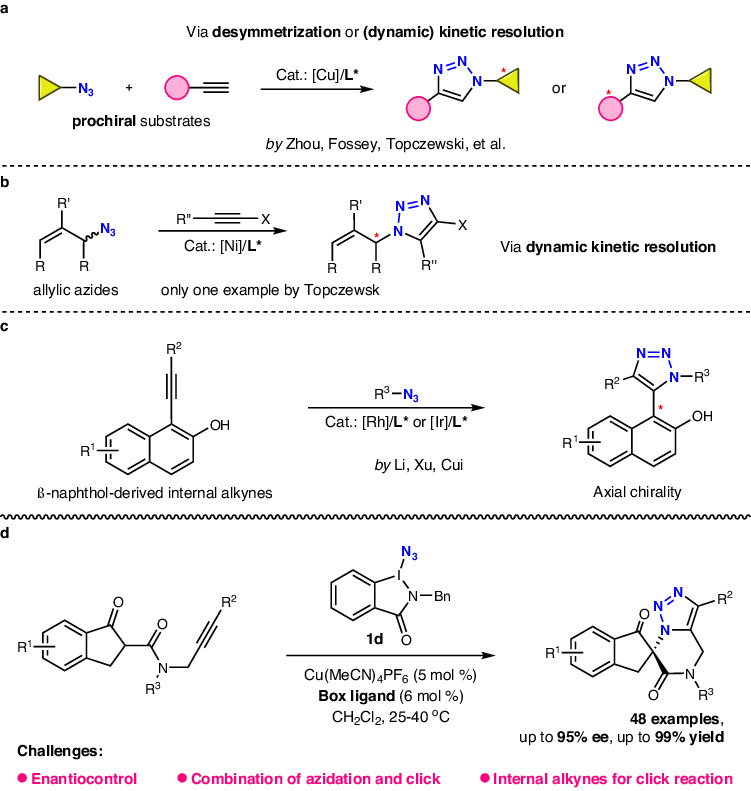
a Synthesis of chiral 1,4-disubstituted triazoles by enantioselective Cu-catalyzed Click reaction. b Synthesis of chiral 1,4,5-trisubstituted triazoles by enantioselective Ni-catalyzed Click reaction. c Synthesis of chiral 1,4,5-trisubstituted triazoles by atroposelective Rh/Ir-catalyzed Click reaction. d This work: synthesis of chiral 1,5-disubstituted triazoles (unexplored) and 1,4,5-trisubstituted triazoles by enantioselective Cu-catalyzed azidation/Click cascade. Cat. catalyst, L ligand, Cu copper, Ni nickel, Rh rhodium, Ir iridium.
Organic azides, as one of the key starting materials for click reactions, have been extensively studied for their asymmetric synthesis51–59. Among these, hypervalent iodine-based azidating reagents were often selected as the azido sources60–66 due to their advantages of mild properties and high selectivity. In previous research, benziodazolone-based azidating reagents were identified as efficient azido transfer reagents that could significantly improve reaction outcomes by adjusting the substituents64. In continuation of our interest in hypervalent iodine chemistry64–69, we report herein an efficient enantioselective copper-catalyzed azidation/click cascade reaction of N-propargyl-β-ketoamides and azidobenziodazolone 1d. This method demonstrates broad substrate scope, easy operation, and mild reaction conditions. Importantly, this work represents catalytic asymmetric example of combining azidation and click reactions to construct chiral triazoles, particularly chiral 1,5-disubstituted triazoles (Fig. 1d).
Results
We studied the model reaction of N-propargyl-β-ketoamide 2a with azidobenziodoxolone (1a) in the presence of the catalyst prepared in situ from 10 mol % of Cu(MeCN)4PF6 and 12 mol % of Box ligand to optimize the reaction conditions (Table 1, see the details of optimization in Supplementary Tables 1–4). When the reaction was carried out in 1,2-dichloroethane at room temperature, the desired chiral triazole 3a was observed. Screening a series of Box ligands revealed that using L8 as the optimal ligand afforded 3a in 62% yield with 92% ee (Table 1, entries 1−10). After evaluating various solvents, it was found that dichloromethane could improve the yield and ee value of 3a (70% yield, 94% ee; Table 1, entry 11). A range of azidating reagents were subsequently tested. In comparison to 1a and azidodimethylbenziodoxole (1b), azidobenziodazolones (1c−1g) were more suitable for the reaction, yielding product 3a with higher yields even at a reduced catalyst loading of 5 mol % (Table 1, entries 11−17). These results implied that the substituent on the nitrogen atom could significantly influence the reaction efficiency, with N-benzyl-azidobenziodazolone (1d) identified as the optimal azido source, affording product 3a in 99% yield with 94% ee (Table 1, entry 14).
Table 1.
Optimization of the reaction conditionsa
 | ||||||
|---|---|---|---|---|---|---|
| Entry | L | “N3” | Solvent | Time (h) | Yield (%) | ee (%) |
| 1 | L1 | 1a | DCE | 24 | 35 | 14 |
| 2 | L2 | 1a | DCE | 24 | 28 | 12 |
| 3 | L3 | 1a | DCE | 24 | 50 | 23 |
| 4 | L4 | 1a | DCE | 24 | 37 | 75 |
| 5 | L5 | 1a | DCE | 24 | 44 | 44 |
| 6 | L6 | 1a | DCE | 24 | 40 | 82 |
| 7 | L7 | 1a | DCE | 24 | 45 | 80 |
| 8 | L8 | 1a | DCE | 24 | 62 | 92 |
| 9 | L9 | 1a | DCE | 24 | 47 | 33 |
| 10 | L10 | 1a | DCE | 24 | 52 | 31 |
| 11 | L8 | 1a | DCM | 24 | 70 | 94 |
| 12b | L8 | 1b | DCM | 24 | 30 | 27 |
| 13c | L8 | 1c | DCM | 48 | 80 | 90 |
| 14c | L8 | 1d | DCM | 48 | 99 | 94 |
| 15c | L8 | 1e | DCM | 48 | 78 | 94 |
| 16c | L8 | 1f | DCM | 48 | 95 | 93 |
| 17c | L8 | 1g | DCM | 48 | 93 | 93 |
DCE: 1,2-dichloroethane, DCM: dichloromethane, L: Ligand; N3: high iodine azide reagent; 1a: azidobenziodoxolone, 1b: azidodimethylbenziodoxole; 1c–g: azidobenziodazolones.
aReaction conditions: 2a (0.1 mmol), 1a (0.15 mmol), Cu(MeCN)4PF6 (10 mol%), L (12 mol %), solvent (2 mL), 25 °C; isolated yields; ee values were determined by HPLC analysis.
b1b was used in place of 1a.
cThe designated azidating reagent was used in place of 1a; 5 mol % of Cu(MeCN)4PF6 and 6 mol% of L8 were used instead.
With the optimized reaction conditions in hand, we evaluated the scope of the substrates bearing terminal alkyne unit (Fig. 2). When R2 was a benzyl group, all of the substrates were compatible with the reaction conditions to deliver the desired chiral 1,5-disubstituted triazoles 3 in high yields with high to excellent ee values. For example, the substrates bearing diverse substituents at the different sites of the aryl ring, including electron-withdrawing groups (5-Br, 4-Br, 6-Br, 7-Br, 5-Cl, 6-F, 6-CN, 6-CF3), electron-donating groups (4-OMe, 5-OMe, 6-OMe, 7-OMe, 5-Me) and electron-neutral group (H) were well tolerated under the conditions, affording products 3a−3n in 75−99% yields and 85−95% ees. Moreover, substrate possessing 4,5-di-OMe on the aryl ring and naphthalene-derived substrate were also successfully employed in the process, generating 3o (81% yield, 80% ee) and 3p (93% yield, 91% ee), respectively. Subsequently, the influence of the N-substituent (R2) was investigated. Groups such as 1-naphthylmethyl, 1,1-diphenylmethyl, and even methyl were tolerated in this protocol, leading to the formation of the corresponding products 3q−3s in 80−98% yields with 88−95% ees, while tert-butyl amide only gave the product 3t with 78% ee.
Fig. 2. Scope for the synthesis of chiral 1,5-disubstituted triazoles.
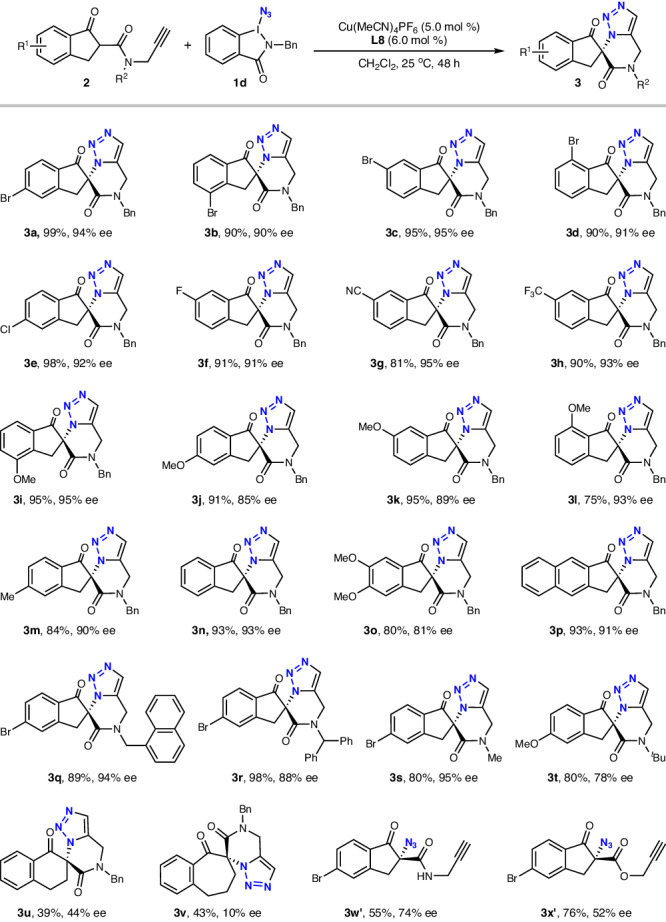
Reaction conditions: 2 (0.1 mmol), 1d (0.15 mmol), Cu(MeCN)4PF6 (5 mol%), L8 (6 mol%), CH2Cl2 (2 mL), 25 °C; isolated yields; ee values were determined by HPLC analysis.
When six-membered ring β-ketoamide 2u and seven-membered ring β-ketoamide 2v were used as substrates, the corresponding product 3u and 3v were obtained with poor ee values, respectively. Unprotected N-propargyl-β-ketoamide 2w and O-propargyl-β-ketoester 2x were not compatible with the reaction, only generating the corresponding azide 3w’ and 3x’, respectively. These results showed the limitations of substrate scope.
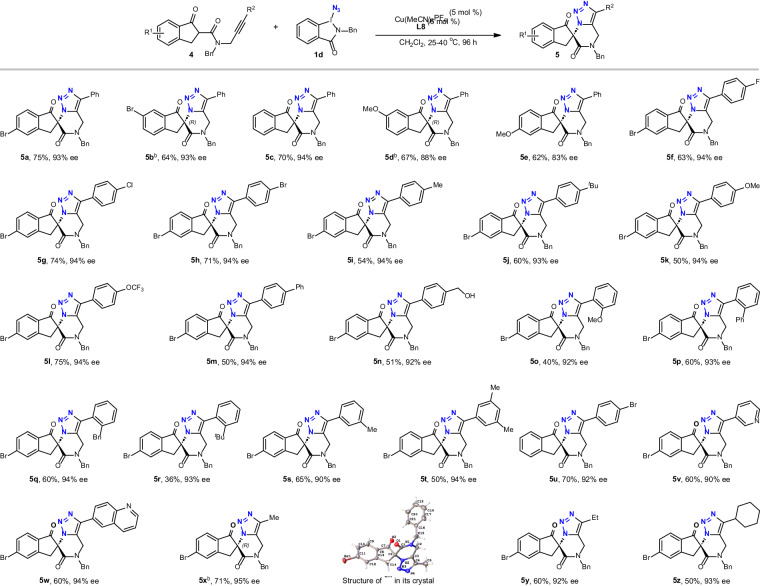
As is well known, copper-catalyzed click reactions are traditionally limited to terminal alkynes14,17,18, except for a few special examples24,70,71. After investigating the scope of substrates bearing terminal alkyne units, we explored the possibilities for the reaction of internal alkynes (Fig. 3). Using 4a as the substrate, the cycloaddition step indeed did not proceed completely under the standard conditions mentioned above. Fortunately, the expected 1,4,5-trisubstituted triazoles 5a could be prepared in 75% yield and 93% ee just by simply raising the temperature to 40 °C and extending the reaction time to 96 h. Substrates with different groups (R1) in the backbone got the desired products 5b−5e in 62−70% yields with 83−94% ees.
Fig. 3. Scope for the synthesis of chiral 1,4,5-trisubstituted triazoles.
Reaction conditions: 4 (0.1 mmol), 1d (0.15 mmol), Cu(MeCN)4PF6 (5 mol %), L8 (6 mol %), CH2Cl2 (2 mL); the mixture was stirred at 25 °C for 48 h, then turned to 40 °C for more 48 h; isolated yields; ee values were determined by HPLC analysis. bThe absolute configuration was established to be R by the single-crystal X-ray structure analysis.
When the substituents (R2) of the triple bond were aryl rings with diverse functional groups in different positions, the corresponding products 5f−5u could be formed in 36−75% yields with 90−94% ees. To our delight, the protocol was also applicable to the substrates with heteroaryl framworks such as 3-pyridyl and 6-quinolyl, affording the expected products 5v (60% yield, 90% ee) and 5w (60% yield, 94% ee), respectively. Furthermore, substrates with alkyl units such as methyl, ethyl and cyclohexyl in the triple bond could be converted to the corresponding products 5x−5z with excellent enantiocontrol. Notably, the absolute configuration of enantiopure 5b, 5d, and 5x was established to be R by single-crystal X-ray structure analysis (for details, see Supplementary Notes). The stereochemical assignment of all other products was also made by analogy.
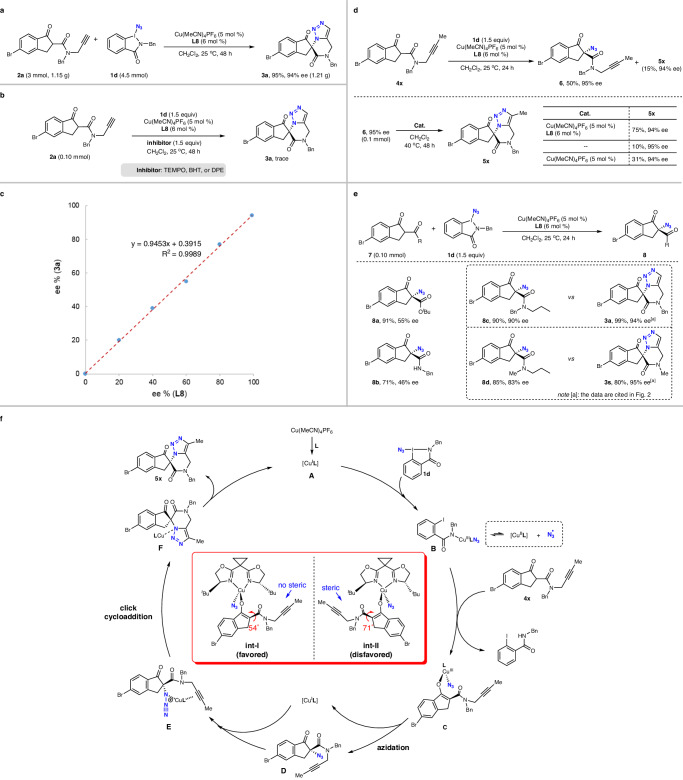
To demonstrate the synthetic utility of this protocol, a gram scale reaction was conducted with 2a on a 3 mmol scale (1.15 g) under standard conditions, affording the desired product 3a (1.21 g) in 95% yield without loss of enantioselectivity (Fig. 4a).
Fig. 4. Gram-scale reaction and mechanistic studies.
a Gram-scale reaction. b Radical inhibition experiments. c Nonlinear effect of catalyst. d Investigation of intermediate azide. e The role of triple bond in enantiocontrol. f Proposed mechanism. TEMPO: 2,2,6,6-tetramethylpiperidoxyl, BHT: butylated hydroxytoluene, DPE: 1,1-diphenylethylene.
A series of control experiments were conducted to gain a preliminary understanding of the reaction mechanism. The reaction was inhibited completely by adding radical scavengers such as 2,2,6,6-tetramethyl-1-piperidinyloxy (TEMPO), 2,6-di-tert-butyl-4-methylphenol (BHT), or 1,1-diphenylethene (DPE) (Fig. 4b). These results revealed that the reaction pathway probably involves a radical process. A linear correlation was observed between the ee values of L8 and the enantioenrichment of the corresponding products (Fig. 4c). The absence of a nonlinear effect in this reaction suggested that a single catalyst is likely involved in the enantiodetermining transition state72. To confirm that the reaction is performed sequentially in the steps of azidation and cycloaddition, we terminated the reaction of 4x with 1d to get the intermediate azide 6 with 95% ee, which could be converted to the desired product 5x in 75% yield with 94% ee under standard reaction conditions. However, the cycloaddition step did not proceed effectively under conditions without a catalyst or with only Cu(MeCN)4PF6 as the catalyst (Fig. 4d). These results indicated that the Cu/L8 catalyst probably involved in both steps of the reaction, with the azidation being the enantio-determining step. To verify the role of the triple bond in enantiocontrol, several control compounds were chosen to perform the asymmetric azidation reaction (Fig. 4e). Ketoester 7a and ketoamide 7b, both with no propargyl group, got the corresponding products 8a−8b with moderate ee values. Compounds 7c and 7d, using propyl instead of propargyl, could get the azidating products 8c−8d with reduced ee values in comparison with those of the analogous triazoles (3a and 3q), respectively. These data implied that the triple bond in the substrate might behave as a directing group in enantiocontrol of azidation step.
To gain insights into the mechanisms of azido transfer and the origin of the enantioselectivity induced by the Box ligand, we carried out DFT calculations to propose a catalytic model (Fig. 4f). The bidentate ligand L8, azido anion, and a carbonyl group of substrate coordinate to the Cu(III) center to form a complex with a distorted tetrahedral coordination geometry as the intermediate. The favored conformation of the intermediate (int-I) exhibits an energy that is 8.34 kcal/mol lower than that of the other side (int-II). This discrepancy is primarily attributed to the steric hindrance caused by the carbonyl group of substrate and the tert-butyl group of ligand L8. Such hindrance leads to an increase in the angle between the exocyclic amide carbonyl and the five-membered ring from 54° (int-I) to 71° (int-II). The enlargement of this angle results in a deterioration of conjugation, which not only elevates the energy level but also makes the enol double bond more reactive due to the loss of conjugation. Additionally, the enlargement of the angle causes the groups attached to the amide, such as the alkynyl group in this case, to rotate towards the backside of the α-carbon, thereby obstructing the approach of azide.
Even though the exact mechanism remains unclear, a tentative catalytic mechanism was proposed according to the experimental results and previous related reports (Fig. 4f)52,73,74. Initially, copper ion coordinates with Box ligand to get the chiral catalyst A, which activates azidobenziodazolone 1d to afford azido-Cu(III) species B73,74. Intermediate B exhibits radical properties and can dissociate into an azido radical. Intermediate B then reacts with substrate 4x to get intermediate C, where copper probably coordinates with both a carbonyl group of the substrate and an azido anion. Intermediate C, in its favored conformation int-I, undergoes stereoselective reductive elimination to afford the chiral intermediate azide D. Copper species would coordinate with both the azido group and triple bond in D to render intermediate E, which probably proceeds the click cycloaddition to form intermediate F75,76. Finally, F releases product 5x along with the regeneration of catalyst A.
Discussion
In conclusion, we have developed an enantioselective copper-catalyzed azidation/click cascade reaction of N-propargyl-β-ketoamides using our previously developed azidobenziodazolone as the efficient azido source. This protocol afforded an efficient and mild route to synthesize chiral triazoles, especially chiral 1,5-disubstituted triazoles, which have not been obtained via reported asymmetric click reactions. Moreover, high functional group tolerance, scalability in synthesis, and easily operated reaction conditions further demonstrated the synthetic utility of this strategy. Initial mechanistic studies revealed that the copper/Box catalyst participates in both azidation and click steps. Further mechanistic investigation and expansion of the azidation/click cascade strategy to access more chiral triazoles are ongoing in our laboratory.
Methods
General procedure for the synthesis of racemic 3a–3v
To a mixture of Cu(MeCN)4PF6 (0.005 mmol, 5 mol %), substrates 2a–2v (0.10 mmol), 1d (0.15 mmol) in dry dichloromethane (2 mL) under nitrogen atmosphere, the reaction system was stirred at 25 °C for 48 h. The disappearance of substrates 2a–2v was monitored by TLC, indicating complete consumption. Finally, the crude product was purified by silica gel flash chromatography to afford the desired product rac-3a–rac-3v.
General procedure for the synthesis of racemic 5a–5z
To a mixture of Cu(MeCN)4PF6 (0.005 mmol, 5 mol %), substrates 4a–4z (0.10 mmol), 1d (0.15 mmol) in dry dichloromethane (2 mL) under nitrogen atmosphere, the reaction system was stirred at 40 °C for 48 h. The disappearance of substrates 4a–4z was monitored by TLC, indicating complete consumption. Finally, the crude product was purified by silica gel flash chromatography to afford the desired products rac-5a–rac-5z.
General procedure for the synthesis of chiral 3a–3v
After stirring a mixture of Cu(MeCN)4PF6 (0.005 mmol, 5 mol %) and L8 (0.006 mmol, 6 mol %) in dry dichloromethane (1 mL) at 25 °C under nitrogen atmosphere for 1.5 h, substrates 2a–2v (0.10 mmol) and 1d (0.15 mmol) in dry dichloromethane (1 mL) were added. The reaction mixture was then stirred at 25 °C under a nitrogen atmosphere. After 48 h, the substrates 2a–2v disappeared completely (monitored by TLC). Finally, the crude product was purified by silica gel flash chromatography to afford the desired products 3a–3v.
General procedure for the synthesis of chiral 5a-5z
After stirring a mixture of Cu(MeCN)4PF6 (0.005 mmol, 5 mol %) and L8 (0.006 mmol, 6 mol %) in dry dichloromethane (1 mL) at 25 °C under nitrogen atmosphere for 1.5 h, substrates 4a–4z (0.10 mmol) and 1d (0.15 mmol) in dry dichloromethane (1 mL) were added. The reaction mixture was stirred at 25 °C under a nitrogen atmosphere. After 48 h, when the substrates 4a–4z disappeared (monitored by TLC), the reaction mixture was stirred at 40 °C for an additional 48 h. Finally, the crude product was purified by silica gel flash chromatography to afford the desired product 5a–5z.
Supplementary information
Source data
Acknowledgements
This paper is in memory of Professor Lixin Dai. We are grateful for the financial support from the Shuguang program (20SG44) from Shanghai Education Development Foundation and Shanghai Municipal Education Commission, the National Natural Science Foundation of China (22371187), the Natural Science Foundation of Shanghai (22ZR1445200), the Chinese Education Ministry Key Laboratory and International Joint Laboratory on Resource Chemistry, the “111” Innovation and Talent Recruitment Base on Photochemical and Energy Materials (D18020), and the Shanghai Engineering Research Center of Green Energy Chemical Engineering (18DZ2254200).
Author contributions
Q.-H.D. conceived and directed the project. L.-F.J. and S.-H.W. conducted most of the experiments, including the synthesis of the hypervalent iodine azidating reagents and substrates. Y.-X.J., H.-X.M., J.-J.H., Y.-B.B., D.-Y.K., Y.-F.C., and X.C. synthesized some substrates. L.-F.J. performed the computational studies. L.-F.J. drafted the Supporting Information. Q.-H.D. prepared the manuscript and revised the Supporting Information.
Peer review
Peer review information
Nature Communications thanks Subhendu Bag and the other anonymous reviewer(s) for their contribution to the peer review of this work. A peer review file is available.
Data availability
The authors declare that the data supporting the findings of this study, including synthetic procedures, characterization data, further details of computational studies and NMR spectra, are available within the article and the Supplementary Information file, or from the corresponding author upon request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC2327853 (for 5b), CCDC2327852 (for 5d), and CCDC2327851 (for 5x). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-024-49313-x.
References
- 1.Singh A, et al. Rational utilization of 1,2,3-triazole scaffold in anti-MRSA drug development: design strategies, structural insights and pharmacological outcomes. J. Mol. Struct. 2024;1295:136557. doi: 10.1016/j.molstruc.2023.136557. [DOI] [Google Scholar]
- 2.Zhao S, Liu J, Lv Z, Zhang G, Xu Z. Recent updates on 1,2,3-triazole-containing hybrids with in vivo therapeutic potential against cancers: a mini-review. Eur. J. Med. Chem. 2023;251:115254. doi: 10.1016/j.ejmech.2023.115254. [DOI] [PubMed] [Google Scholar]
- 3.Joseph MC, Swarts AJ, Mapolie SF. Transition metal complexes of click-derived 1,2,3-triazoles as catalysts in various transformations: an overview and recent developments. Coord. Chem. Rev. 2023;493:215317. doi: 10.1016/j.ccr.2023.215317. [DOI] [Google Scholar]
- 4.Agouram N. 1,2,3-triazole derivatives as antiviral agents. Med. Chem. Res. 2023;32:2458–2472. doi: 10.1007/s00044-023-03154-3. [DOI] [Google Scholar]
- 5.Bezerra Morais PA, et al. Triazole: a new perspective in medicinal chemistry and material science. Curr. Org. Chem. 2022;26:1691–1702. doi: 10.2174/1385272827666221213145147. [DOI] [Google Scholar]
- 6.Akter M, Rupa K, Anbarasan P. 1,2,3-Triazole and its analogues: new surrogates for diazo compounds. Chem. Rev. 2022;122:13108–13205. doi: 10.1021/acs.chemrev.1c00991. [DOI] [PubMed] [Google Scholar]
- 7.Wang X, Zhang X, Ding S. 1,2,3-Triazole-based sequence-defined oligomers and polymers. Polym. Chem. 2021;12:2668–2688. doi: 10.1039/D1PY00123J. [DOI] [Google Scholar]
- 8.Usachev BI. Chemistry of fluoroalkyl-substituted 1,2,3-triazoles. J. Fluorine Chem. 2018;210:6–45. doi: 10.1016/j.jfluchem.2018.02.012. [DOI] [Google Scholar]
- 9.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise Huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew. Chem. Int. Ed. 2002;41:2596–2599. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 10.Kolb HC, Finn MG, Sharpless KB. Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed. 2001;40:2004–2021. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Tornøe CW, Christensen C, Meldal M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(I)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J. Org. Chem. 2002;67:3057–3064. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 12.Zhao R, Zhu J, Jiang X, Bai R. Click chemistry-aided drug discovery: A retrospective and prospective outlook. Eur. J. Med. Chem. 2024;264:116037. doi: 10.1016/j.ejmech.2023.116037. [DOI] [PubMed] [Google Scholar]
- 13.Tashrifi Z, Khanaposhtani MM, Bahadorikhalili S, Larijani B, Mahdavi M. Intramolecular click cycloaddition reactions: synthesis of 1,2,3-triazoles. Curr. Org. Synth. 2024;21:166–194. doi: 10.2174/1570179420666230407103320. [DOI] [PubMed] [Google Scholar]
- 14.Leyva E, Rubén Rodríguez-Gutiérrez I, Moctezuma E, Noriega S. Mechanisms, copper catalysts, and ligands involved in the synthesis of 1,2,3-triazoles using click chemistry. Curr. Org. Chem. 2022;26:2098–2121. doi: 10.2174/1385272827666230201103825. [DOI] [Google Scholar]
- 15.Porte K, Riomet M, Figliola C, Audisio D, Taran F. Click and bio-orthogonal reactions with mesoionic compounds. Chem. Rev. 2021;121:6718–6743. doi: 10.1021/acs.chemrev.0c00806. [DOI] [PubMed] [Google Scholar]
- 16.Fantoni NZ, El-Sagheer AH, Brown T. A Hitchhiker’s guide to click-chemistry with nucleic acids. Chem. Rev. 2021;121:7122–7154. doi: 10.1021/acs.chemrev.0c00928. [DOI] [PubMed] [Google Scholar]
- 17.Agrahari AK, et al. Cu(I)-catalyzed click chemistry in glycoscience and their diverse applications. Chem. Rev. 2021;121:7638–7956. doi: 10.1021/acs.chemrev.0c00920. [DOI] [PubMed] [Google Scholar]
- 18.Tiwari VK, et al. Cu-catalyzed click reaction in carbohydrate chemistry. Chem. Rev. 2016;116:3086–3240. doi: 10.1021/acs.chemrev.5b00408. [DOI] [PubMed] [Google Scholar]
- 19.Wang C, Ikhlef D, Kahlal S, Saillard JY, Astruc D. Metal-catalyzed azide-alkyne “click” reactions: mechanistic overview and recent trends. Coord. Chem. Rev. 2016;316:1–20. doi: 10.1016/j.ccr.2016.02.010. [DOI] [Google Scholar]
- 20.Qin C-Q, Zhao C, Chen G-S, Liu Y-L. Catalytic enantioselective azide–alkyne cycloaddition chemistry opens up new prospects for chiral triazole syntheses. ACS Catal. 2023;13:6301–6311. doi: 10.1021/acscatal.3c00911. [DOI] [Google Scholar]
- 21.Brittain WDG, Buckley BR, Fossey JS. Asymmetric copper-catalyzed azide-alkyne cycloadditions. ACS Catal. 2016;6:3629–3636. doi: 10.1021/acscatal.6b00996. [DOI] [Google Scholar]
- 22.Gong Y, et al. Sulfonyl-PYBOX ligands enable kinetic resolution of α-tertiary azides by CuAAC. Angew. Chem. Int. Ed. 2023;62:e202301470. doi: 10.1002/anie.202301470. [DOI] [PubMed] [Google Scholar]
- 23.Liao K, Gong Y, Zhu R-Y, Wang C, Zhou F, Zhou J. Highly enantioselective CuAAC of functional tertiary alcohols featuring an ethynyl group and their kinetic resolution. Angew. Chem. Int. Ed. 2021;60:8488–8493. doi: 10.1002/anie.202016286. [DOI] [PubMed] [Google Scholar]
- 24.Wang C, Zhu R-Y, Liao K, Zhou F, Zhou J. Enantioselective Cu(I)-catalyzed cycloaddition of prochiral diazides with terminal or 1-iodoalkynes. Org. Lett. 2020;22:1270–1274. doi: 10.1021/acs.orglett.9b04522. [DOI] [PubMed] [Google Scholar]
- 25.Zhu R-Y, Chen L, Hu X-S, Zhou F, Zhou J. Enantioselective synthesis of P-chiral tertiary phosphine oxides with an ethynyl group via Cu(I)-catalyzed azide–alkyne cycloaddition. Chem. Sci. 2020;11:97–106. doi: 10.1039/C9SC04938J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhou F, et al. Asymmetric copper(I)-catalyzed azide–alkyne cycloaddition to quaternary oxindoles. J. Am. Chem. Soc. 2013;135:10994–10997. doi: 10.1021/ja4066656. [DOI] [PubMed] [Google Scholar]
- 27.Brittain WDG, et al. Coetaneous catalytic kinetic resolution of alkynes and azides through asymmetric triazole formation. Sci. Rep. 2019;9:15086. doi: 10.1038/s41598-019-50940-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Brittain WDG, et al. The Bull–James assembly as a chiral auxiliary and shift reagent in kinetic resolution of alkyne amines by the CuAAC reaction. Org. Biomol. Chem. 2016;14:10778–10782. doi: 10.1039/C6OB01623E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brittain WDG, Buckley BR, Fossey JS. Kinetic resolution of alkyne-substituted quaternary oxindoles via copper catalysed azide–alkyne cycloadditions. Chem. Commun. 2015;51:17217–17220. doi: 10.1039/C5CC04886A. [DOI] [PubMed] [Google Scholar]
- 30.Liu E-C, Topczewski JJ. Enantioselective copper catalyzed alkyne–azide cycloaddition by dynamic kinetic resolution. J. Am. Chem. Soc. 2019;141:5135–5138. doi: 10.1021/jacs.9b01091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alexander JR, Ott AA, Liu E-C, Topczewski JJ. Kinetic resolution of cyclic secondary azides, using an enantioselective copper-catalyzed azide–alkyne cycloaddition. Org. Lett. 2019;21:4355–4358. doi: 10.1021/acs.orglett.9b01556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zu B, Guo Y-H, He C. Catalytic enantioselective construction of chiroptical boron-stereogenic compounds. J. Am. Chem. Soc. 2021;143:16302–16310. doi: 10.1021/jacs.1c08482. [DOI] [PubMed] [Google Scholar]
- 33.Wright AJ, Hughes DL, Page PCB, Stephenson GR. Induction of planar chirality using asymmetric click chemistry by a novel desymmetrisation of 1,3-bisalkynyl ferrocenes. Eur. J. Org. Chem. 2019;2019:7218–7222. doi: 10.1002/ejoc.201901192. [DOI] [Google Scholar]
- 34.Chen M-Y, et al. Catalytic asymmetric Huisgen alkyne–azide cycloaddition of bisalkynes by copper(I) nanoparticles. ChemCatChem. 2018;10:280–286. doi: 10.1002/cctc.201701336. [DOI] [Google Scholar]
- 35.Song T, et al. Enantioselective copper-catalyzed azide–alkyne click cycloaddition to desymmetrization of maleimide-based bis(alkynes) Chemistry. 2015;21:554–558. doi: 10.1002/chem.201405420. [DOI] [PubMed] [Google Scholar]
- 36.Osako T, Uozumi Y. Mechanistic insights into copper-catalyzed azide-alkyne cycloaddition (CuAAC): observation of asymmetric amplification. Synlett. 2015;26:1475–1479. doi: 10.1055/s-0034-1380534. [DOI] [Google Scholar]
- 37.Osako T, Uozumi Y. Enantioposition-selective copper-catalyzed azide-alkyne cycloaddition for construction of chiral biaryl derivatives. Org. Lett. 2014;16:5866–5869. doi: 10.1021/ol502778j. [DOI] [PubMed] [Google Scholar]
- 38.Stephenson GR, et al. An Investigation of the asymmetric Huisgen ‘click’ reaction. Synlett. 2013;24:2723–2729. doi: 10.1055/s-0033-1340152. [DOI] [Google Scholar]
- 39.Meng J-C, Fokin VV, Finn MG. Kinetic resolution by copper-catalyzed azide-alkyne cycloaddition. Tetrahedron Lett. 2005;46:4543–4546. doi: 10.1016/j.tetlet.2005.05.019. [DOI] [Google Scholar]
- 40.Liu E-C, Topczewski JJ. Enantioselective nickel-catalyzed alkyne-azide cycloaddition by dynamic kinetic resolution. J. Am. Chem. Soc. 2021;143:5308–5313. doi: 10.1021/jacs.1c01354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guo W-T, et al. Enantioselective Rh-catalyzed azide-internal-alkyne cycloaddition for the construction of axially chiral 1,2,3-triazoles. J. Am. Chem. Soc. 2022;144:6981–6991. doi: 10.1021/jacs.2c01985. [DOI] [PubMed] [Google Scholar]
- 42.Zhang X, et al. Asymmetric azide-alkyne cycloaddition with Ir(I)/squaramide cooperative catalysis: atroposelective synthesis of axially chiral aryltriazoles. J. Am. Chem. Soc. 2022;144:6200–6207. doi: 10.1021/jacs.2c02563. [DOI] [PubMed] [Google Scholar]
- 43.Chen R, Zeng L, Lai Z, Cui S. Iridium-catalyzed hydroxyl-enabled cycloaddition of azides and alkynes. Adv. Synth. Catal. 2019;361:989–994. doi: 10.1002/adsc.201801410. [DOI] [Google Scholar]
- 44.Zeng L, Li J, Cui S. Rhodium‐catalyzed atroposelective click cycloaddition of azides and alkynes. Angew. Chem. Int. Ed. 2022;61:e202205037. doi: 10.1002/anie.202205037. [DOI] [PubMed] [Google Scholar]
- 45.Ruck RT, et al. Harnessing the power of catalysis for the synthesis of CRTH2 antagonist MK-1029. Org. Process Res. Dev. 2022;26:648–656. doi: 10.1021/acs.oprd.1c00128. [DOI] [Google Scholar]
- 46.Santhanam V, Pant P, Jayaram B, Ramesh NG. Design, synthesis and glycosidase inhibition studies of novel triazole fused iminocyclitol-δ-lactams. Org. Biomol. Chem. 2019;17:1130–1140. doi: 10.1039/C8OB03084G. [DOI] [PubMed] [Google Scholar]
- 47.Oehlrich D, et al. Evaluation of a series of β-secretase 1 inhibitors containing novel heteroaryl-fused-piperazine amidine warheads. ACS Med. Chem. Lett. 2019;10:1159–1165. doi: 10.1021/acsmedchemlett.9b00181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kayet A, Datta D, Das A, Dasgupta S, Pathak T. 1,5-Disubstituted 1,2,3-triazole linked disaccharides: metal-free syntheses and screening of a new class of ribonuclease A inhibitors. Bioorg. Med. Chem. 2018;26:455–462. doi: 10.1016/j.bmc.2017.12.004. [DOI] [PubMed] [Google Scholar]
- 49.Sirivolu VR, et al. Clicking 3′-azidothymidine into novel potent inhibitors of human immunodeficiency virus. J. Med. Chem. 2013;56:8765–8780. doi: 10.1021/jm401232v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miyakoshi H, et al. 1,2,3-Triazole-containing uracil derivatives with excellent pharmacokinetics as a novel class of potent human deoxyuridine triphosphatase inhibitors. J. Med. Chem. 2012;55:6427–6437. doi: 10.1021/jm3004174. [DOI] [PubMed] [Google Scholar]
- 51.Sivaguru P, Ning Y, Bi X. New strategies for the synthesis of aliphatic azides. Chem. Rev. 2021;121:4253–4307. doi: 10.1021/acs.chemrev.0c01124. [DOI] [PubMed] [Google Scholar]
- 52.Ge L, Chiou M-F, Li Y, Bao H. Radical azidation as a means of constructing C(sp3)-N3 bonds. Green Synth. Catal. 2020;1:86–120. doi: 10.1016/j.gresc.2020.07.001. [DOI] [Google Scholar]
- 53.Ding P-G, Hu X-S, Zhou F, Zhou J. Catalytic enantioselective synthesis of α-chiral azides. Org. Chem. Front. 2018;5:1542–1559. doi: 10.1039/C8QO00138C. [DOI] [Google Scholar]
- 54.Guo W, Jiang F, Li S, Sun J. Organocatalytic asymmetric azidation of sulfoxonium ylides: mild synthesis of enantioenriched α-azido ketones bearing a labile tertiary stereocenter. Chem. Sci. 2022;13:11648–11655. doi: 10.1039/D2SC03552A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang J, et al. Asymmetric azidation under hydrogen bonding phase-transfer catalysis: a combined experimental and computational study. J. Am. Chem. Soc. 2022;144:4572–4584. doi: 10.1021/jacs.1c13434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ge L, et al. Iron-catalysed asymmetric carboazidation of styrenes. Nat. Catal. 2021;4:28–35. doi: 10.1038/s41929-020-00551-4. [DOI] [Google Scholar]
- 57.Lv D, et al. Iron-catalyzed radical asymmetric aminoazidation and diazidation of styrenes. Angew. Chem. Int. Ed. 2021;60:12455–12460. doi: 10.1002/anie.202017175. [DOI] [PubMed] [Google Scholar]
- 58.Wu L, et al. Anionic bisoxazoline ligands enable copper-catalyzed asymmetric radical azidation of acrylamides. Angew. Chem. Int. Ed. 2021;60:6997–7001. doi: 10.1002/anie.202015083. [DOI] [PubMed] [Google Scholar]
- 59.Uyanik M, Sahara N, Tsukahara M, Hattori Y, Ishihara K. Chemo- and enantioselective oxidative α-azidation of carbonyl compounds. Angew. Chem. Int. Ed. 2020;59:17110–17117. doi: 10.1002/anie.202007552. [DOI] [PubMed] [Google Scholar]
- 60.Simonet-Davin R, Waser J. Azidation with hypervalent iodine reagents. Synthesis. 2022;55:1652–1661. [Google Scholar]
- 61.Mironova IA, Kirsch SF, Zhdankin VV, Yoshimura A, Yusubov MS. Hypervalent iodine-mediated azidation reactions. Eur. J. Org. Chem. 2022;2022:e202200754. doi: 10.1002/ejoc.202200754. [DOI] [Google Scholar]
- 62.Cao M, Wang H, Ma Y, Tung C-H, Liu L. Site- and enantioselective manganese-catalyzed benzylic C–H azidation of indolines. J. Am. Chem. Soc. 2022;144:15383–15390. doi: 10.1021/jacs.2c07089. [DOI] [PubMed] [Google Scholar]
- 63.Deng Q-H, Bleith T, Wadepohl H, Gade LH. Enantioselective iron-catalyzed azidation of β-keto esters and oxindoles. J. Am. Chem. Soc. 2013;135:5356–5359. doi: 10.1021/ja402082p. [DOI] [PubMed] [Google Scholar]
- 64.Chen Y-X, et al. Azidobenziodazolones as azido sources for the enantioselective copper-catalyzed azidation of N-unprotected 3-trifluoromethylated oxindoles. Org. Lett. 2023;25:2739–2744. doi: 10.1021/acs.orglett.3c01005. [DOI] [PubMed] [Google Scholar]
- 65.Lin C-Z, et al. Enantioselective synthesis of 3a-azido-pyrroloindolines by copper-catalyzed asymmetric dearomative azidation of tryptamines. Chem. Commun. 2023;59:7831–7834. doi: 10.1039/D3CC01438J. [DOI] [PubMed] [Google Scholar]
- 66.Wang C-J, et al. Enantioselective copper-catalyzed electrophilic dearomative azidation of β-naphthols. Org. Lett. 2019;21:7315–7319. doi: 10.1021/acs.orglett.9b02604. [DOI] [PubMed] [Google Scholar]
- 67.Cheng X, et al. Simple and versatile nitrooxylation: Noncyclic hypervalent iodine nitrooxylating reagent. Angew. Chem. Int. Ed. 2023;62:e202302521. doi: 10.1002/anie.202302521. [DOI] [PubMed] [Google Scholar]
- 68.Li B, et al. Zinc-catalyzed asymmetric nitrooxylation of β-keto esters/amides with a benziodoxole-derived nitrooxy transfer reagent. Org. Chem. Front. 2020;7:3509–3514. doi: 10.1039/D0QO01022G. [DOI] [Google Scholar]
- 69.Li Z-R, et al. Iron-catalyzed trifluoromethylation of vinylcyclopropanes: facile synthesis of CF3-containing dihydronaphthalene derivatives. Org. Chem. Front. 2016;3:934–938. doi: 10.1039/C6QO00166A. [DOI] [Google Scholar]
- 70.Yamada M, et al. Antimony–lithium exchange reaction: synthesis of 1,4,5-trisubstituted-1,2,3-triazoles by triazolyllithium with electrophiles. J. Organomet. Chem. 2017;834:83–87. doi: 10.1016/j.jorganchem.2017.02.019. [DOI] [Google Scholar]
- 71.Hein JE, Tripp JC, Krasnova LB, Sharpless KB, Fokin VV. Copper(I)-catalyzed cycloaddition of organic azides and 1-iodoalkynes. Angew. Chem. Int. Ed. 2009;48:8018–8021. doi: 10.1002/anie.200903558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Satyanarayana T, Abraham S, Kagan HB. Nonlinear effects in asymmetric catalysis. Angew. Chem. Int. Ed. 2009;48:456–494. doi: 10.1002/anie.200705241. [DOI] [PubMed] [Google Scholar]
- 73.Shen K, Wang Q. Copper-catalyzed alkene aminoazidation as a rapid entry to 1,2-diamines and installation of an azide reporter onto azahetereocycles. J. Am. Chem. Soc. 2017;139:13110–13116. doi: 10.1021/jacs.7b06852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Huang L, Lin J-S, Tan B, Liu X-Y. Alkene trifluoromethylation-initiated remote α-azidation of carbonyl compounds toward trifluoromethyl γ-lactam and spirobenzofuranone-lactam. ACS Catal. 2015;5:2826–2831. doi: 10.1021/acscatal.5b00311. [DOI] [Google Scholar]
- 75.Himo F, et al. Copper(I)-catalyzed synthesis of azoles. DFT study predicts unprecedented reactivity and intermediates. J. Am. Chem. Soc. 2005;127:210–216. doi: 10.1021/ja0471525. [DOI] [PubMed] [Google Scholar]
- 76.Zhang L, et al. Ruthenium-catalyzed cycloaddition of alkynes and organic azides. J. Am. Chem. Soc. 2005;127:15998–15999. doi: 10.1021/ja054114s. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The authors declare that the data supporting the findings of this study, including synthetic procedures, characterization data, further details of computational studies and NMR spectra, are available within the article and the Supplementary Information file, or from the corresponding author upon request. The X-ray crystallographic coordinates for structures reported in this study have been deposited at the Cambridge Crystallographic Data Centre (CCDC) under deposition numbers CCDC2327853 (for 5b), CCDC2327852 (for 5d), and CCDC2327851 (for 5x). These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via https://www.ccdc.cam.ac.uk/structures/. Source data are provided with this paper.