Abstract
Mitral valve prolapse (MVP) is the most common valve disease in the western world and recently emerged as a possible substrate for sudden cardiac death (SCD). It is estimated an annual risk of sudden cardiac death of 0.2 to 1.9% mostly caused by complex ventricular arrhythmias (VA). Several mechanisms have been recognized as potentially responsible for arrhythmia onset in MVP, resulting from the combination of morpho-functional abnormality of the mitral valve, structural substrates (regional myocardial hypertrophy, fibrosis, Purkinje fibers activity, inflammation), and mechanical stretch. Echocardiography plays a central role in MVP diagnosis and assessment of severity of regurgitation. Several abnormalities detectable by echocardiography can be prognostic for the occurrence of VA, from morphological alteration including leaflet redundancy and thickness, mitral annular dilatation, and mitral annulus disjunction (MAD), to motion abnormalities detectable with “Pickelhaube” sign. Additionally, speckle-tracking echocardiography may identify MVP patients at higher risk for VA by detection of increased mechanical dispersion. On the other hand, cardiac magnetic resonance (CMR) has the capability to provide a comprehensive risk stratification combining the identification of morphological and motion alteration with the detection of myocardial replacement and interstitial fibrosis, making CMR an ideal method for arrhythmia risk stratification in patients with MVP. Finally, recent studies have suggested a potential role in risk stratification of new techniques such as hybrid PET-MR and late contrast enhancement CT. The purpose of this review is to provide an overview of the mitral valve prolapse syndrome with a focus on the role of imaging in arrhythmic risk stratification.
Clinical relevance statement
Mitral valve prolapse is the most frequent valve condition potentially associated with arrhythmias. Imaging has a central role in the identification of anatomical, functional, mechanical, and structural alterations potentially associated with a higher risk of developing complex ventricular arrhythmia and sudden cardiac death.
Keywords: Mitral valve prolapse, Arrhythmogenic mitral valve prolapse, Cardiac imaging technique, Magnetic resonance, Computed tomography
Graphical Abstract
Introduction
Mitral valve prolapse (MVP) is the most common valve disease, affecting approximately 2–3% of the general population [1]. It is defined by the systolic displacement of one or both mitral valve leaflets into the left atrium (LA) > 2 mm above the plane of the mitral annulus in the sagittal view [1]. Two main etiologies have been described: the diffuse myxomatous degeneration also called Barlow disease, which may present as a genetic disorder, and the fibroelastic degeneration, due to an accelerated aging process [2]. Despite the lack of specific criteria to distinguish Barlow disease (BD) from fibroelastic deficiency (FED), some differences have been reported in imaging. Patients with BD usually show bileaflet MVP with multiscallops involvement, elongated and thick chordae at a low probability of rupture, higher prolapsed volume and height, and increased annular dimensions. Conversely, in FED, a single-leaflet prolapse with focal myxomatous changes in chordae, chordal thinning at a high probability of rupture is usually reported [2, 3].
MVP is associated with progressive mitral regurgitation (MR) with chronic volume overload causing cardiac remodeling with left ventricular (LV) eccentric hypertrophy and dysfunction [4, 5].
Therefore, MVP is more a cardiac disease than an isolated valve disease.
Although it is generally considered a benign condition, current research suggests that MVP is associated with complex ventricular arrhythmias (VA) and sudden cardiac death (SCD) [6].
The prevalence and relative risk of SCD in MVP remains unsettled, due to low incidence and potential confounding factors [7]. In the Veneto region cardiac pathology registry, among 650 SCDs of young adults, 7% were attributed to
MVP [8]. In a meta-analysis of the SCD autopsy series, 22.1% had an undetermined cause of death and MVP was present in 11.7% of cases [6].
Among unselected MVP patients, cohort studies suggest an annual SCD incidence below 1% and more likely around 0.1–0.4%, translating into very modest incremental risk due to MVP [6, 9–11].
Data derived from SCD and out-of-hospital cardiac arrest series suggests that young females may be at higher risk of malignant arrhythmias [8]. However, this is likely due to selection bias as arrhythmic mitral valve prolapse (AMVP) seems to equally affect both sexes [12]. Similarly, AMVP as a cause of SCD may be underestimated in older adults where competitive causes may erroneously be attributed [13], and these patients may actually be at higher risk due to degenerative MVP progression and development of arrhythmic substrata [14]. Chest pain, palpitations, and dyspnea are frequently reported. However, these symptoms are comparable among individuals with and without MVP [15] and between MVP patients with and without arrhythmias [12]. Conversely, syncope is reported in up to 35% of MVP patients with malignant arrhythmias and is infrequent in those without, suggesting consideration as an important red flag [15–17]. T wave inversion in the inferior and lateral leads is prevalent among AMVP patients (up to 65%) and predicts malignant arrhythmias [6]. Importantly, specificity remains low, T wave inversion being reported in 40% of unselected MVP patients [18]. Even if QT interval prolongation has been described in MVP [19] its pre-dictivity for malignant arrhythmias remains uncertain [3]. Finally, QRS fragmentation has been associated with malignant arrhythmias, although evidence remains low [20].
Recently, the EHRA expert consensus statement [3] defined the AMVP complex by the presence of (a) MVP (with or without mitral annular disjunction (MAD)), (b) ventricular arrhythmia (frequent (≥ 5% total PVC burden) or complex (NSVT, VT, VF)) and (c) the absence of any other well-defined arrhythmic substrate. The characteristics of AMVP patients are poorly defined, identification and management of these patients represent an unmet clinical need.
MVP as arrhythmogenic disease: mechanistic hypothesis
The postulated mechanism of sudden death in MVP has been life-threatening ventricular arrhythmias [21].
As shown in Fig. 1, the underlying pathophysiology of ventricular electrical instability in MVP patients involves the combination of myocardial fibrosis, premature ventricular contractions (PVCs), and transient modulators such as a hyperadrenergic state, hemodynamic conditions, and electrolyte imbalances [22].
Fig. 1.
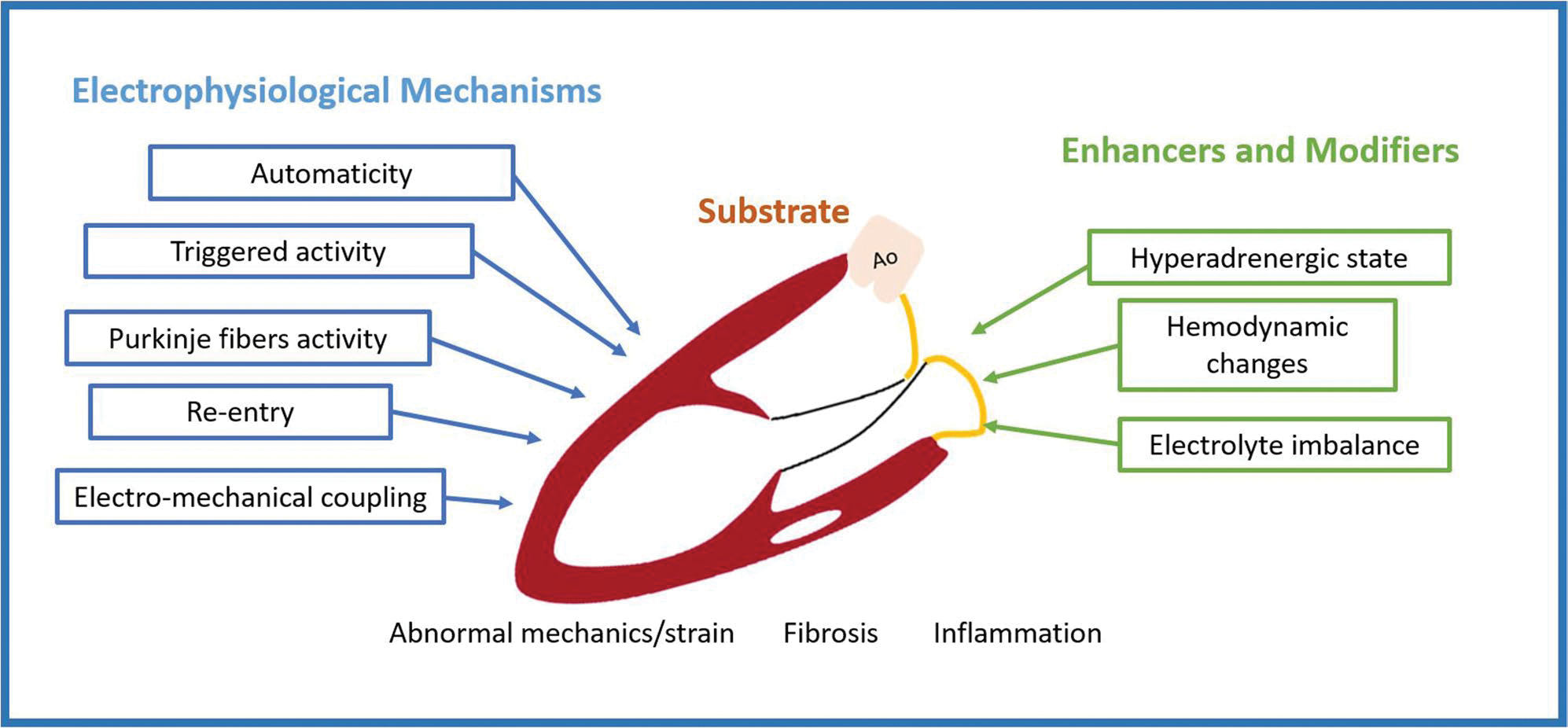
Pathophysiology of arrhythmogenic mitral valve prolapse
Regional myocardial fibrosis in the left ventricular inferobasal wall and in the papillary muscles has been initially recognized as a potential trigger of ventricular arrhythmia in an autoptic study from the Padua group [8]. These findings were further supported by subsequent autoptic studies [23–25].
Two types of myocardial fibrosis have been found in MVP patients: reactive interstitial fibrosis and replacement fibrosis [26] with a significant endocardial-to-epicardial gradient of cardiac fibrosis [24]. It has been postulated that myocardial fibrosis may result from chronic injury related to mechanical stress caused by papillary muscle (PM) systolic stretch, mitral annular disjunction, curling, and frictional contact between billowing mitral leaflets and chordae on the endocardium [9, 27]. The genesis of malignant arrhythmias in MVP probably recognizes the combination of the substrate (myocardial fibrosis) and the trigger (mechanical stretch) eliciting premature ventricular arrhythmias at risk for SCD [27] (Table 1).
Table 1.
Histopathological findings documenting myocardial remodeling In MVP and potential arrhythmogenic role of fibrosis In patients with isolate MVP and SCD
| Main author | Publication year | Number of enrolled patients | Patient characteristics | Tissue sampling technique | Histological technique | Main histological findings |
|---|---|---|---|---|---|---|
|
| ||||||
| Basso [8] | 2015 | 43 | SCD Complex ventricular arrhythmias (detected on 12-lead 24-h Holter monitoring) | Autopsy | Hematoxylin eosin, Weigert-van Gieson Heidenhain trichrome, Alcian-periodic acid—Schiff | •LV myocardium with increased endoperimysial and patchy replacement-type fibrosis at the level of PMs and adjacent free wall (100%) •Subendocardial-midmural layer distribution in the inferobasal wall under the posterior MV leaflet (88%) •Mean fibrous tissue percent area at the level of PMs: 30.5% •Mean fibrous tissue percent area at the level of the inferobasal wall: 33.1 % •Increased diameter cardiomyocytes with dysmorphic and dysmetric nuclei |
| Garbi [28] | 2018 | 68 | SCD | Autopsy | Hematoxylin eosin | •Fibrosis in 81% (11% involving the RV) •Focal, fine, interstitial fibrosis involving the inner subendocardium, trabeculae and PMs •Fibrosis confluent within the inner third of the LV basal posterior wall (extensive replacement into the trabeculae and posteromedial PM) •Hypertrophy and degenerative features of the myocytes with focal cytoplasmic vacuolation |
| Han [23] | 2020 | 70 | SCD | Autopsy | Hematoxylin eosin Connective tissue strain | Abnormal LV features in 79% of patients, including: •Fibrosis or scarring (predominantly interstitial and/ or perivascular) 85% fibrosis involving the subendocardial-midmural layer •Myocyte hypertrophy •Contraction band •PM fibrosis •PM calcification |
| Han [24] | 2021 | 17 | SCD | Autopsy | Masson’s trichrome | •Fibrosis in all regions (inner, middle, and outer thirds of the anterior, lateral, and posterior wall; LV third, middle third, and RV third of the interventricular septum) •Significantly higher levels of fibrosis in the lateral and posterior regions with a significant epicardial-to-endocardial gradient of fibrosis •Comparable fibrosis in the anterior wall and the interventricular septum •Comparable fibrosis in the lateral wall and the posterior wall |
| Morningstar [25] | 2021 | 6 | Severe mitral regurgitation secondary to MVP and indications for MV repair | Biopsy | Masson’s trichrome Collagen stain | •Fibrosis within the inferobasal myocardium; little evidence of fibrosis within the apex or Interventricular septum •Myocyte loss •Increased collagen I protein In fibrotic regions •Activated myofibroblasts and an Increase In CD206 + cells In the PM region |
LV, left ventricle; MV, mitral valve; MVP, mitral valve prolapse; PM, papillary muscle; RV, right ventricle; SCD, sudden cardiac death
Indeed, the presence of fibrosis in the LV wall and PM sets an arrhythmogenic substrate, increasing susceptibility to triggered activity or re-entry VA, which features a right bundle-branch block pattern or polymorphic complex morphology [29].
Acute myocardial stretch can also cause changes in myocyte electrophysiology, such as action potential duration shortening, decreased resting diastolic potential, and the development of early afterdepolarizations, affecting cellular excitation–contraction coupling [30].
Remarkably, the PM, particularly the distal Purkinje fibers, are prone to afterdepolarization and abnormal automaticity [9].
In fact, abnormal Purkinje signals have been observed preceding ventricular fibrillation-triggering PVCs in patients with prior cardiac arrest and bileaflet MVP [31].
The local inflammatory process may account for an additional substrate for VA in MVP. An overlap with noninfectious myocarditis was previously described in a patient with MVP and recurrent episodes of malignant VA triggered by PVCs [32].
Regional LV inflammation, mediated by macrophages, has been observed in the peripapillary myocardium of MVP patients [25], and it could be strictly related to the development of myocardial fibrosis. In fact, resident myocardial mast cells mediate pro and anti-fibrogenic signals [33] and endothelial cells producing pro-inflammatory molecules could be involved in recruiting lymphocytes and macrophages with fibrogenic potential, explaining the perivascular fibrosis in pathological specimens [34].
An abnormal autonomic function, particularly an increase in circulating catecholamine levels, has been reported to enhance myocardial tissue vulnerability to complex VA [35]. Activation of stretch receptors, induction of mechano-electric feedback, and unfavorable modulation of ion-channel and calcium handling, further contribute to MVP-related arrhythmogenesis [36].
Some studies based on endomyocardial biopsy [37, 38] have found increased right ventricular fibrosis in patients with malignant ventricular arrhythmias and MVP as occurs in patients with the biventricular cardiomyopathic process. Being based on right side puncture of the interventricular septum, their results were considered questionable.
However, the mutation of the sarcomeric protein filamin C (FLNC), already known for other cardiomyopathies, has been found to be associated with arrhythmogenic bileaflet forms of MVP [39] as the mutation of Dachsous1 gene (DCHS1), a member of the cadherin superfamily [3].
Altogether, the interplay between triggering PVCs, structural mitral valve abnormalities, and myocardial fibrosis and inflammation, creates the conditions for the development of SCD in susceptible MVP patients under transitory regulatory influences.
Echocardiographic predictors of arrhythmic MVP
Various echocardiographic signs have been associated with AMVP, from the severity of regurgitation to several morphologic alterations including MAD (Table 2).
Table 2.
Echocardiographic biomarkers of arrhythmic risk in MVP
| Parameter | Diagnostic criteria | Possible mechanism of arrhythmogenesis |
|---|---|---|
|
| ||
| Bileaflet prolapse | ≥ 2 mm atrial displacement of both MV leaflets | Higher tension on chordae tendineae and papillary muscles |
| MAD | Clear systolic separation between basal LV myocardium and posterior leaflet-atrial junction | Loss of mechanical annular function causing periannular fibrosis, basal ventricular stretching, and papillary muscles stretching causing calcium-dependent early-afterdepolarizations |
| Systolic curling | Excessive apical systolic motion of the posterior annulus and inward excursion of the adjacent posterobasal myocardium, visually assessed | Mechanical stretch of the basal ventricular wall and papillary muscles, myocardial hypertrophy, fibrosis |
| Pickelhaube sign | Spiked systolic high-velocity signal (≥ 16 cm/s) on the lateral mitral annulus by pulsed-wave tissue Doppler | Stretch-induced arrhythmias |
| Post-systolic shortening | Maximal segmental myocardial shortening after aortic valve closure at speckle tracking analysis | Stretch-induced arrhythmias, index of fibrosis |
| Double-peak strain pattern | Longitudinal strain pattern characterized by double peaks before and after end-systole | Stretch-induced arrhythmias, index of fibrosis |
| Mechanical dispersion | Standard deviation of time from onset of systole to peak longitudinal strain in all 18 segments | Index of fibrosis |
MAD, mitral annular disjunction; MV, mitral valve; MVP, mitral valve prolapse
Mitral regurgitation
Mitral regurgitation severity and severe valve degeneration have been found to be associated with higher mortality and SCD [48]. Severe degenerative mitral regurgitation is defined by a regurgitant volume ≥ 60 mL/beat and by an effective regurgitant orifice area ≥ 40 mm2. The mortality rate increases from 20 to 30 mm2 of effective regurgitant orifice area with a linear increase for larger values [31, 49].
Leaflet abnormalities
Bileaflet prolapse, thickening, and redundancy are frequent in patients with MVP. Echocardiography is more accurate than CMR for measuring leaflet thickness. Maximal thickness should be measured during diastole in long axis views, and a thickness ≥ 5 mm discriminates a classic MVP (Barlow’s disease) from non-classic MVP [50].
While some studies have shown a potential association between bileaflet prolapse and cardiac arrest [31], conflicting results exist in the literature, and this relationship has not been consistently reported [51, 52].
Mitral annular disjunction
Mitral annular disjunction (MAD) is defined as an abnormal systolic separation between the basal left ventricular myocardium (lower limit) and the hinge point of the posterior leaflet (upper limit), which is dislocated through the left atrial wall [53]. MAD evaluation is typically done using long-axis echocardiographic views (preferably parasternal long-axis view). Dynamic frame-by-frame evaluation of systolic images is required to spot excessive posterior leaflet tissue of a normally implanted annulus that could incorrectly be interpreted as MAD [3]. MAD is a common, but not exclusive, feature of patients with MVP [54]. MAD length > 8.5 mm has been associated with non-sustained ventricular tachycardia [55], even in patients without mitral regurgitation [56]. However, MAD has never been tested as an independent risk factor for SCD in large studies.
Motion abnormalities and strain
Systolic curling of the basal posterior and lateral LV walls, caused by excessive mobility of the leaflets and loss of mechanical annular function, is frequently associated with MVP and MAD. This systolic curling accounts for a mechanical stretch of the infero-basal wall and papillary muscles, leading to myocardial hypertrophy and scarring [56]. Similarly, the “Pickelhaube sign” [57] is a high-velocity (≥ 16 cm/s), spiked late-systolic signal recorded by tissue Doppler imaging (TDI) at the lateral annular level (Fig. 2). It has been related to the pulling of the posteromedial papillary muscle by the prolapsing leaflets causing the adjacent basal left ventricular wall curling movement toward the apex and thus the observed spiked configuration of the lateral annular velocities. This echocardiographic marker has been found to be overrepresented in patients with arrhythmic MVP, suggesting its role as a risk marker for malignant arrhythmias [58]. Additionally, speckle-tracking echocardiography allows to study MVP-related myocardial mechanics. Post-systolic deformation [59] double peak strain pattern (with two distinct peaks, one before and the second after end-systole) [41] and mechanical dispersion [56] are peculiar strain patterns observed in patients with MVP and associated with basal inferior-lateral fibrosis at CMR, suggesting pathophysiological links between MVP-related mechanical abnormalities and myocardial fibrosis, potentially serving as new imaging markers for increased arrhythmic risk [41].
Fig. 2.
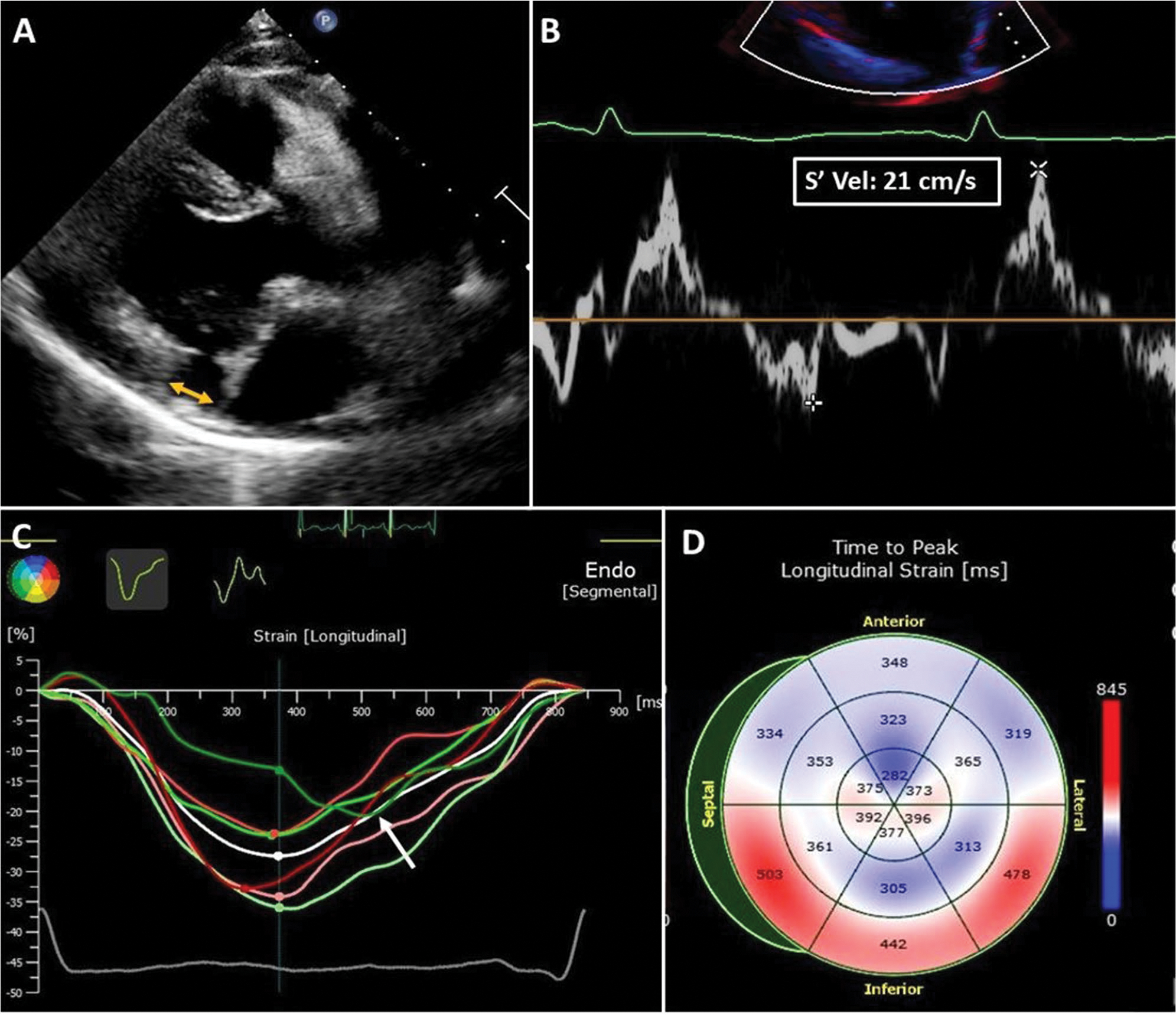
Echocardiographic biomarkers of arrhythmic MVP in a 35-year-old woman with MVP, moderate mitral regurgitation, and frequent premature ventricular contractions. A MAD. The yellow arrow shows the distance between the basal ventricular myocardium and the hinge point of the posterior leaflet in late systole. Bulging of the basal inferior-lateral myocardium, typical of the systolic curling, was also present in this patient. B Pickelhaube sign. A spiked late systolic high-velocity (21 cm/s) signal is recorded at the level of the lateral mitral annulus in a four-chamber view. C Speckle tracking imaging. Late post-systolic shortening (after aortic valve closure) of the basal inferior-lateral left ventricular wall (white arrow, green line). D Mechanical dispersion. Prolonged time-to-peak longitudinal strain is observed at the basal inferior, inferior-septal, and inferior-lateral walls and is related to myocardial periannular fibrosis
CMR biomarkers defining AMVP
CMR has the unique capability to characterize MVP not only in terms of morphological alteration and hemodynamic impact but also to evaluate the presence of tissue alterations as fibrosis, providing a comprehensive characterization of higher risk features [60–63] (Table 3).
Table 3.
CMR tissue analysis in arrhythmic MVP (some studies from the last 5 years)
| First author | Source-Year [ref] | Study | Pts MVP /MAD(with CMR) | Study population arrhythmias/events | CMR fibrosis Analysis | LGE/mapping analysis technique | CMR main findings |
|---|---|---|---|---|---|---|---|
|
| |||||||
| Guglielmo M. [40] | J Cardiovasc Magn Reson. 2023 | Retrospective | 42 MVP/42 MAD | 23 pts (55%) MAD-Complex VA | LGE, T1 map, ECV, FT | LGE Semi-quantitatively signal intensity > 5 SDs + quantitative assessment maps and CMR-FT | MAD-ComVA group LGE 78%; T1 and ECV increase; Regional changes in CMR-FT |
| Nagata Y. [41] | Circ Cardiovasc Imaging. 2023 | Prospective | 113MVP | Arrhythmic events in 36 with > 6-month follow-up | LGE | LGE was quantitatively measured using the full-width-at-half-maximal method | LGE 38% basal-midven-tricular inferior-lateral wall and papillary muscles |
| Chivulescu M. [42] | Europace 2022 | Prospective | 113 MVP and MAD | 16 pts with severe VA (aborted cardiac arrest or sustained ventricular tachycardia), 34 TWI | LGE, T1 map, ECV | Not specified for LGE (visual assessment)-quantitative assessment of mapping | LGE 48% (basal and papillary); Lateral diffuse fibrosis (ECV increase) was associated with VA, also in the absence of LGE |
| Constant Dit Beaufils AL. [43] | Circulation. 2021 | Prospective | 400 MVP, 206 MAD | Ventricular arrhythmias in 31% patients including 24% with frequent PVCs and 16% with non-sustained ventricular tachycardia/ life-threatening ventricular arrhythmia history | LGE | LGE was visually evaluated | LGE 28% (71 with basal inferolateral wall, 29 with papillary muscle) |
| Pavon A.G. [44] | J Cardiovasc Magn Reson 2021 | Retrospective | 30 MVP and MAD | Among patients with Holter, 87% had complex ventricular arrhythmia | LGE, T1 map, ECV | LGE was visually evaluated and its extent was semi-quantitatively reported | LGE 47%; ECV increase also in absence of LGE |
| Gatti M. [45] | Eur Radiol. 2021 | Retrospective | 52 bMVP | 20 complex VAs | LGE, FT | LGE Semi-quantitatively signal intensity > 5 SDs and CMR-FT | LGE 95% 85% LV wall 50% papillary muscle |
| Predella S [46] | Eur Radiol. 2019 | Retrospective | 34 MVP | 11 complex VA | LGE, T1 map, ECV | LGE visually evaluated. Quantitative analysis of nTl and ECV | 44% mainly in midwall myocardial LGE of the basal inferolateral wall. LGE involved 1.5 segments on average. Higher nT1 and ECV compared to the control group |
| Garbi M. [28] | Open Heart 2018 | Retrospective | 68 MVP | 68 SCD-Barlow disease | LGE;T1 map | Not specified for LGE (visual assessment)/quantitative assessment of mapping | LGE 41% papillary muscle Fibrosis; T1 mapping increase by 91% |
| Kitkungvan D. [47] | JACC 2018 | Prospective | 177 MVP | During the follow-up period underwent mitral valve surgery 72 pts with MVP. Arrhythmic events occurred In 8 patients | LGE | LGE visually evaluated and using a semiquantitative method | LGE 36.7% |
LGE, late gadolinium enhancement; ECV, extracellular volume; CMR, cardiovascular MR; FT, feature tracking; MAD, mitral anular disjunction; VA, ventricular arrhythmias; TWI, T-wave inversion; SCD, sudden cardiac death
Mitral annular disjunction
MAD is measured as the distance of the posterior annulus to the base of the left ventricle in end-systole preferably in the 3-chambers long axis view (Fig. 3) [64, 65]. The assessment of frame-by-frame systo-diastolic modification is required in order to distinguish real MAD from pseudo-MAD, the latter due to the systolic juxtaposition of the posterior leaflet on the atrial wall [66]. CMR is more accurate than echocardiography in detecting MAD, especially for small-length MAD [67]. The clinical significance of MAD is still debated: some authors suggested that MAD could be a variant of the normal annular architecture [66, 68], while others found MAD associated with complex VA [56, 65, 69–71], even in absence of MVP [65]. Several values of MAD length were found to be associated with complex VA: 3 mm [56], 4.8 mm [56], 10 ± 3 mm [54] and 8.5 mm [55]. However, despite the aforementioned debate, MAD is a frequent finding in advanced myxomatous degeneration [71] and in AMVP with LV fibrosis [45, 63], suggesting a potential role of MAD in scar development due to excessive mobility of the leaflets and mechanical stretch of the myocardium [9, 65, 72].
Fig. 3.
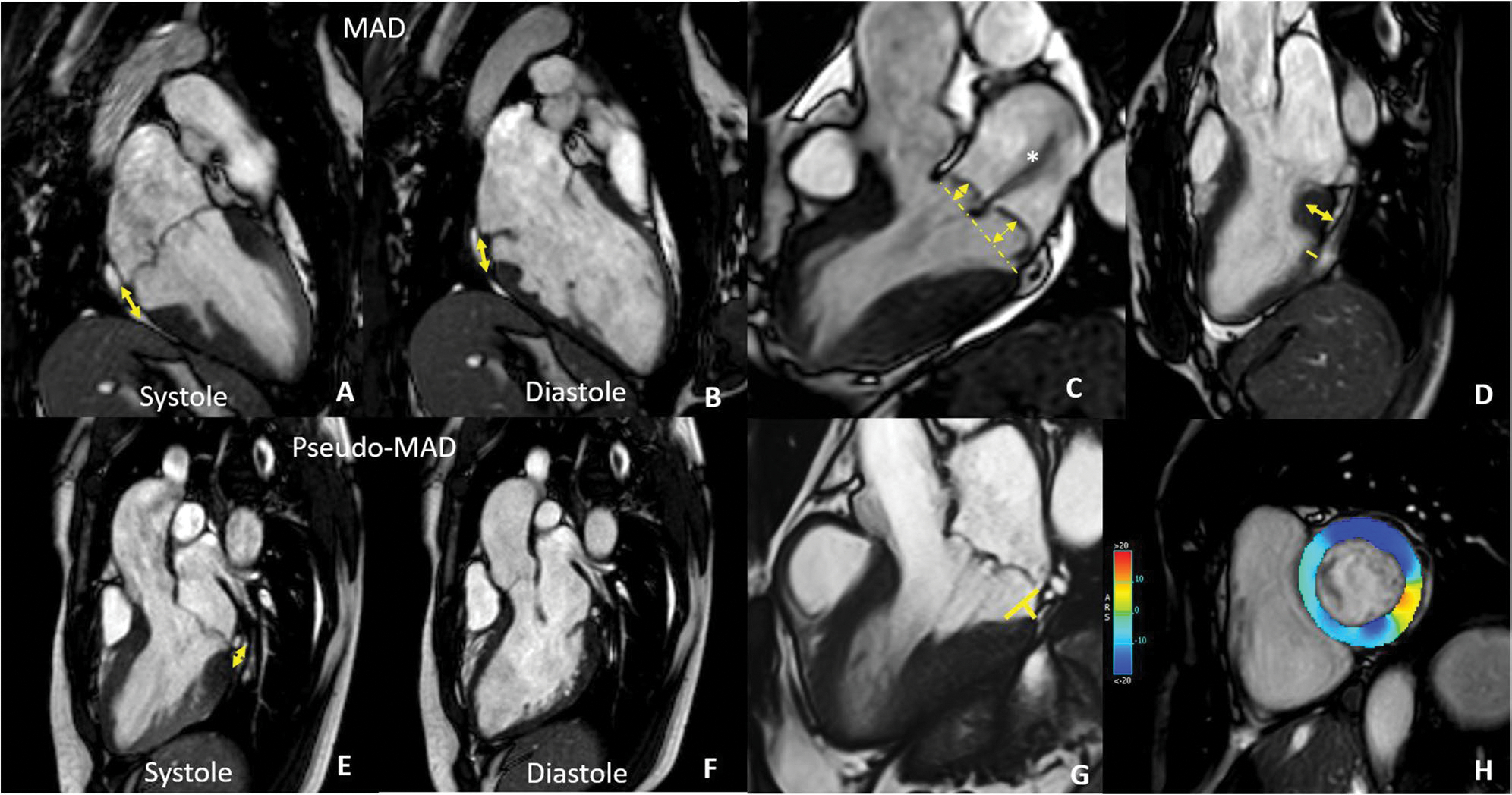
CMR findings in MVP. In A and B are reported systolic (A) and diastolic (B) frames of the same patient showing mitral annulus disjunction (double-headed arrow) that needs to be distinguished from pseudo-MAD (E and F) due to the juxtaposition of the posterior leaflet on the atrial wall in systole. C 3-chamber long axis showing severe bileaflet mitral valve prolapse with high prolapse volume and a huge jet of regurgitation (asterisks). D Basal LV hypertrophy with a ratio of LV thickness between basal and mid segments of the inferolateral wall > 1.5 at end-diastole. G Curling distance by tracing a line between the top of the LV I wall and the LA–MV leaflet junction, and from this line, a perpendicular line to the lower limit of the mitral annulus at end-systole. H GLS analysis showing contractility alteration of the inferolateral wall
Systolic curling of the postero‑basal left ventricle and papillary muscle anomalies
Although it is not clear whether MAD is a degenerative, congenital, or acquired structural abnormality, the association with the typical appearance of the posterolateral wall suggests a degenerative pathway that involves, in addition to the valve, the stretched ventricular wall [9, 56].
Indeed, the mid-basal lateral wall shows a disarray appearance with a relative increase of thickness in the basal segment compared to the mid one. In particular, a ratio > 1.5 between basal to midventricular lateral wall at end-diastole indicates basal LV hypertrophy. Basal LV hypertrophy is usually associated with systolic curling of the lateral and inferolateral basal wall, which is considered severe when ≥ 3.5 mm (Fig. 3) [70, 73–75]. CMR feature tracking analysis may have a role in risk stratification [40, 45, 76], being able to detect subtle alteration of wall deformation associated with scarring and arrhythmic substrate (Fig. 3).
Additionally, a recent study on a large population of arrhythmic patients noticed systolic hypointensity of both papillary muscles (Dark-Paps) in end-systolic cine images acquired early after gadolinium injection. This finding was associated with a higher prevalence of MVP and MAD, and to a higher risk of cardiac event [77]. This phenomenon is probably due to transitory perfusion defect of muscles during the peak of ventricle contraction [77] and could have a potential prognostic role.
Myocardial fibrosis: LGE and mapping
The prevalence of fibrosis in patients with MVP is not known; however, available clinical studies show an association between fibrosis and complex VA and SCD [45, 58, 78–80].
Myocardial fibrosis in MVP correlates with a worse outcome regardless of the severity of the prolapse [63] and of the regurgitant fraction [9, 80, 81]. The non-invasive standard for the assessment of myocardial fibrosis is CMR using LGE and mapping technique (Fig. 4).
Fig. 4.
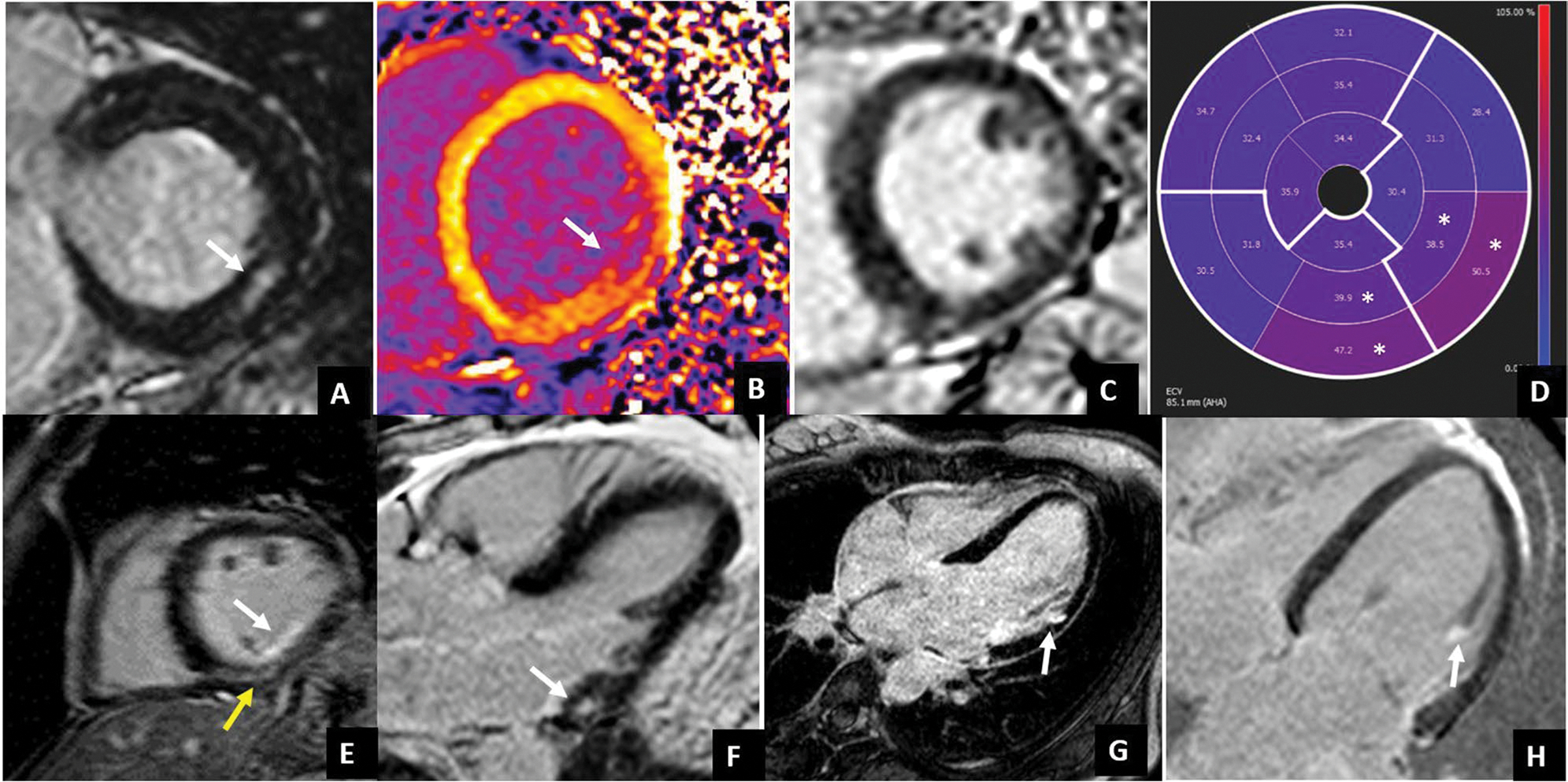
LGE patterns typically associated with arrhythmic MVP. LGE usually occurs at the level of the LV inferolateral wall. Different LGE patterns have been described: non-ischemic mid-wall LGE (white arrows in A and F, yellow arrow in E), subendocardial LGE (white arrows in E and G), papillary muscle LGE (with arrow in H). Interstitial fibrosis documented by native T1 mapping and ECV values has been found to be increased not only at the site of LGE (white arrow in native T1 map in B) but also in LGE negative patients (case example in C) with diffusely high ECV values which are higher in the inferior and inferolateral mid-basal wall (white asterisks)
LGE images showed replacement fibrosis to be more prevalent in patients with MVP than in patients with mitral regurgitation without prolapse [47]. It mainly involves the posteromedial papillary muscle and the inferior and lateral basal wall of the left ventricle, with non-ischemic or patchy appearance [63, 74] and less frequently with subendocardial pattern [45, 47].
LGE extent is associated with MAD length [65] and prolapse severity [8].
Beyond LGE, recent findings highlighted a possible role of interstitial fibrosis in arrhythmogenesis [8, 78, 82]. Native T1 and ECV allow the identification of fibrosis at an earlier stage if compared to LGE and result in their association with ventricular arrhythmias in MVP [24, 40, 44, 83]. Moreover, higher than normal native T1 and ECV values have been found in the mid-basal left ventricle inferolateral wall even in the absence of focal fibrosis [40, 44, 84]. Additionally, a diffuse global increase of native T1 [84] or a reduction of post-contrast T1 [82] has been found in MVP suggesting diffuse interstitial derangement with a larger amount of fibrosis occurring in the infero-lateral wall.
Characterization of inflammation in MVP Using 18F-FDG PET/MRI
While multiple clinical features have been identified as markers of increased risk, left ventricular replacement fibrosis appears to be a consistent feature of arrhythmic MVP [8, 9, 47, 85]. However, the majority of patients who experienced MVP-related sudden cardiac death have evidence of myocardial fibrosis, and ~ 25% of them do not have fibrosis [23, 47]. Within this patient group, subclinical myocardial inflammation [86] might explain why patients with no fibrosis can still experience ventricular arrhythmia. This relationship was recently reaffirmed by a study demonstrating histopathological evidence of regionalized left ventricular inflammation and activated myofibroblasts in preclinical models of MVP [25] (Table 4).
Table 4.
Inflammatory and fibrotic myocardial changes In arrhythmic mitral valve
| Regions Involved | • Papillary muscle • Basal subvalvular lateral and infero-lateral LV wall |
| Trigger to myocardial remodeling | • Repetitive traction resulting from the excess leaflet motion |
| Histological myocardial changes [47, 87] | • Protective stretch-stress response from the interstitial mechano-receptive resident myocardial fibroblasts and endothelial cells • Transformation into activated myofibroblasts • Expression of pro-fibrotic cytokines • Recruitment of inflammatory cells • Myocardial Fibrosis |
| Substrate for ventricular arrhythmias [88–92] | • Alteration of the myocardial architecture by fibrosis • Pro-arrhythmic effects from activated fibroblasts when coupled to adjacent cardiomyocytes • Left ventricular inflammation and activated myofibroblasts • Alterations in tissue conductance • Disruption of normal myocardial electrophysiology • Increased vulnerability to triggered activity due to premature ventricular contractions |
| Diagnosis [23, 47, 86, 93–96] | Myocardial Fibrosis: • LGE on the LV wall lateral - inferolateral wall or in the papillary muscle • Increased values of T1 and ECV in the same segments Subclinical myocardial inflammation: • Increased FDG uptake on the LV wall lateral - inferolateral wall |
Positron emission tomography, using 18F-fluorodeoxyglucose ([18F]FDG PET), identifies areas of increased glucose uptake. [18F]FDG uptake is a recognized marker of inflammation whereby inflammatory cells show increased metabolic activity compared to surrounding tissue [93]. Additionally, integrated [18F]FDG PET and magnetic resonance imaging (Hybrid PET/MRI) allow for the simultaneous detection and quantification of cardiac anatomy, function, and fibrosis with CMR, as well as providing metabolic information with PET [94]. Hybrid PET/MRI has been helpful in the diagnosis and prognostication of other inflammatory cardiac conditions (e.g., sarcoidosis, myocarditis) [97] In the MVP setting, a previous study has shown that patients with significant mitral regurgitation due to degenerative mitral valve disease and a history of ventricular ectopy could have occult inflammation in addition to myocardial fibrosis and that this inflammation could be detected by Hybrid PET/MRI imaging [95]. This study showed that 85% of patients exhibited focal or focal-on-diffuse uptake of [18F]FDG, while 70% exhibited both focal uptake of [18F]FDG and LGE (Fig. 5). Typical distribution (basal to mid inferolateral) was more commonly seen in those patients with single leaflet (posterior) prolapse while atypical distribution (patchy, involving multiple segments including anterior, apical, and basal inferolateral) was more commonly observed in those with bi-leaflet MVP. Another study performed in patients with MVP and a history of ventricular ectopy but only mild to moderate mitral regurgitation showed that focal [18F]FDG uptake and LGE were present in 75% of patients [96]. Taken together, these results suggest that patients with MVP and different degrees of regurgitation have evidence of myocardial inflammation that could be detected, quantified, and precisely localized within the myocardium by means of hybrid PET/MRI.
Fig. 5.
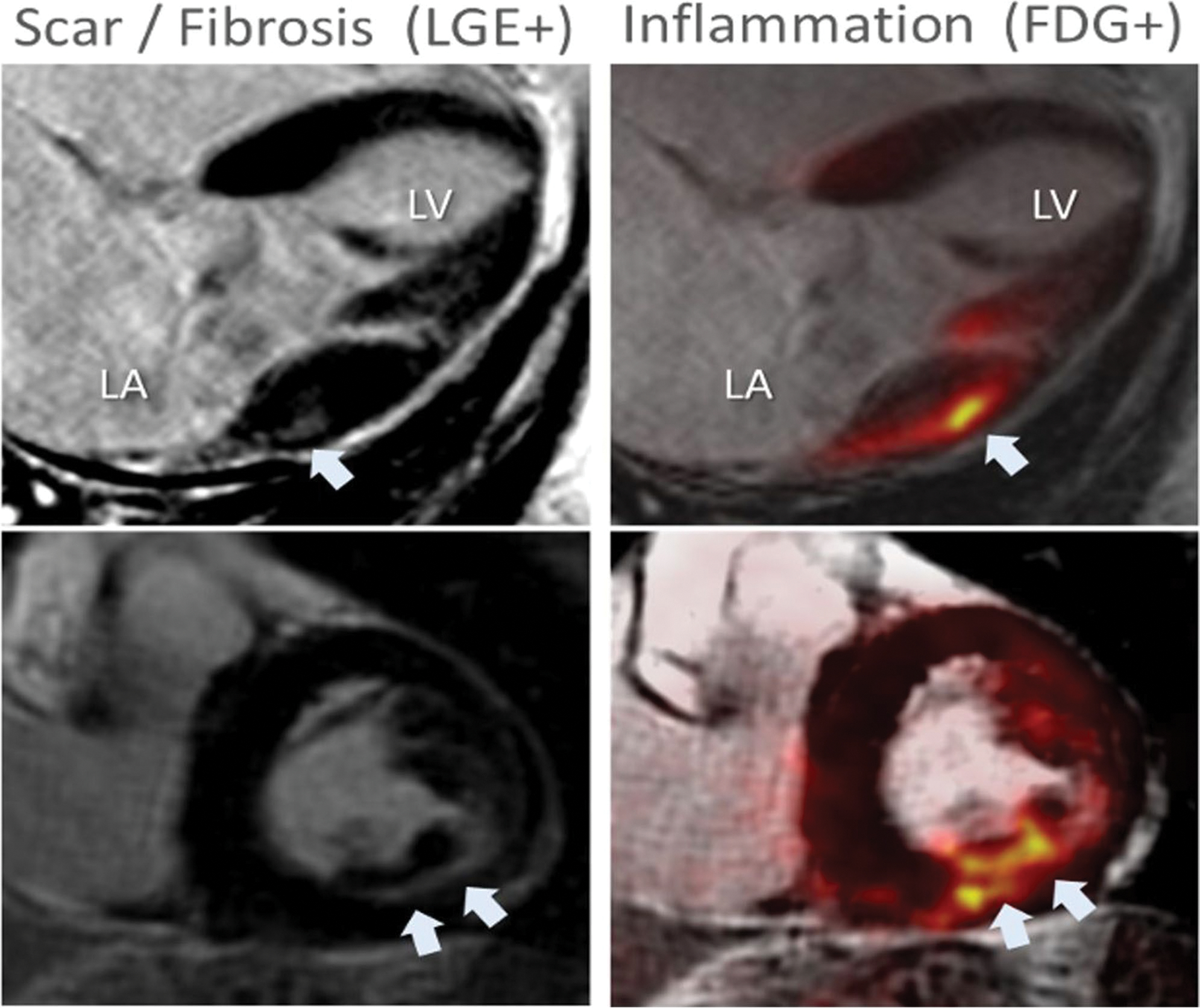
Hybrid [18F]FDG PET/MRI in a patient with MVP. An example of concordant [18F]FDG uptake and LGE in an asymptomatic patient with chronic severe degenerative mitral regurgitation and absent left ventricular remodeling (LVEF 60%, LVESD 38 mm). White arrowheads indicate areas of either LGE or FDG uptake. LA indicates left atrium; LV, left ventricle
Cardiac computed tomography in arrhythmic MVP
Cardiac CT has been demonstrated to be able to provide a comprehensive evaluation of MV complex and morphological abnormalities with an accurate assessment of the annulus anatomy, leaflet excursion and thickness [98], MAD presence and length [99, 100], and valve dynamic changes over the cardiac cycle [5, 101]. Despite this anatomical advantages, CT is rarely used for MVP risk stratification. This is mainly related to its limited capability to assess myocardial fibrosis. However, recent studies have suggested the possibility of detecting myocardial scar in CT regardless of transmural involvement and underlying cardiomyopathy [102–104] and also to quantifying ECV [104] similarly to CMR and with good agreement [104, 105].
This would be important, considering that CT is commonly used for planning percutaneous interventions and before surgery [66, 106]. Hence, the combined evaluation of scar and ECV may improve risk stratification as recently demonstrated for aortic stenosis [107]. Additionally, hybrid PET-CT may combine anatomical with metabolic information [108] providing information about anatomy, scars, and inflammation. However, few data are currently available [32] (Fig. 6).
Fig. 6.
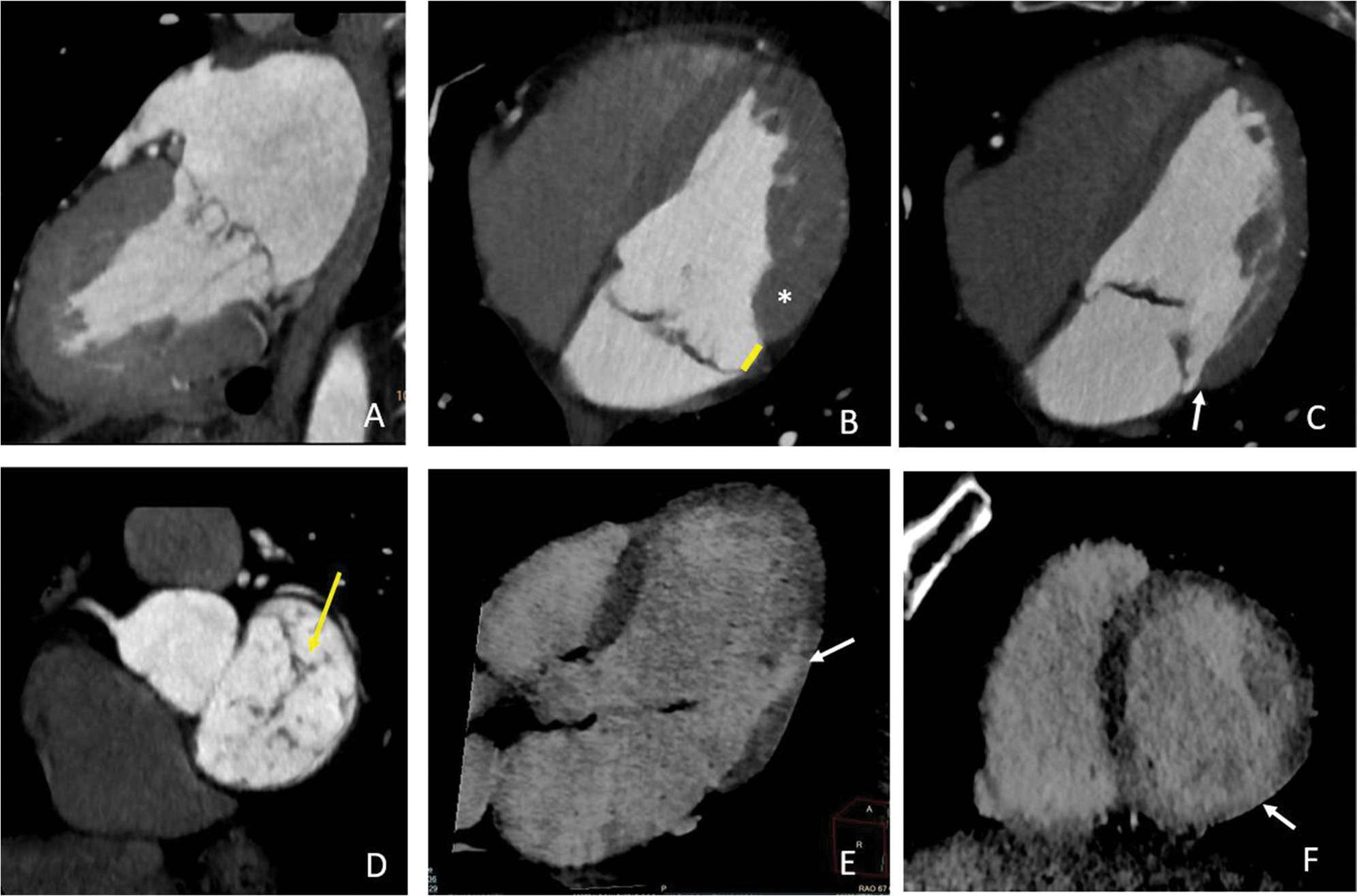
Cardiac CT in a patient with arrhythmic MVP. CT images show a bileaflet multiscallop mitral valve prolapse (A) in a patient with MAD recognizable in systole (arrow in B) and diastole (arrow in C), with curling of the inferolateral mid-basal wall (white asterisks in B) and mild regurgitation due to a small coaptation defect (arrow in D). Late contrast enhancement 3-chambers long axis (E) and 2-chambers short axis (F) documented a small area of late enhancement in the mid inferolateral wall close to the posterior papillary muscle insertion point (arrows in E and F)
Conclusion
MVP can pose a risk of complex VA and SCD. The hypothesized mechanism for arrhythmia onset in MVP is complex and multifactorial, mainly related to myocardial anatomical, mechanical, and structural alterations. Imaging techniques, such as echocardiography and CMR, play a crucial role in the identification of high-risk imaging biomarkers, providing valuable insights into risk stratification and potential preventive measures for susceptible patients. Hybrid PET/MR and cardiac CT may play a role in selected patients but available data are limited. Further research is needed to better understand the pathophysiology of arrhythmic MVP and to optimize risk prediction and management strategies.
Key Points.
Mitral valve prolapse is a common valve disease potentially associated with complex ventricular arrhythmia and sudden cardiac death.
The mechanism of arrhythmogenesis in mitral valve prolapse is complex and multifactorial, due to the interplay among multiple conditions including valve morphological alteration, mechanical stretch, myocardial structure remodeling with fbrosis, and infammation.
Cardiac imaging, especially echocardiography and cardiac magnetic resonance, is crucial in the identifcation of several features associated with the potential risk of serious cardiac events. In particular, cardiac magnetic resonance has the advantage of being able to detect myocardial fbrosis which is currently the strongest prognosticator.
Funding
The authors state that this work has not received any funding.
Abbreviations
- AMVP
Arrhythmic mitral valve prolapse
- BD
Barlow’s disease
- CMR
Cardiovascular MR
- cVA
Complex ventricular arrhythmia
- FED
Fibroelastic deficiency
- MAD
Mitral annular disjunction
- MVP
Mitral valve prolapse
- NSVT
Non-sustained ventricular tachycardia
- PVC
Premature ventricular contraction
- SCD
Sudden cardiac death
- VF
Ventricular fibrillation
- VT
Ventricular tachycardia
Footnotes
Conflict of interest
Marco Francone is European Radiology’s section editor of Cardiac in the Scientific editorial board. He has not taken part in the review or selection process of this article.
The authors of this manuscript declare no relationships with any companies, whose products or services may be related to the subject matter of the article. Anna Palmisano is a member of the European Radiology Experimental scientific editorial board.
Guarantor
The scientific guarantor of this publication is Antonio Esposito.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was not required for this study because it is a review article.
Ethical approval
Institutional Review Board approval was not required because it is a review article.
Study subjects or cohorts overlap
No original data are included in the review.
Methodology
• narrative review
References
- 1.Delling FN, Vasan RS (2014) Epidemiology and pathophysiology of mitral valve prolapse. Circulation. 10.1161/CIRCULATIONAHA.113.006702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adabifirouzjaei F, Hsiao A, DeMaria AN (2022) Mitral valve prolapse—the role of cardiac imaging modalities. Structural Heart. 10.1016/j.shj.2022.100024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sabbag A, Essayagh B, Barrera JDR et al. (2022) EHRA expert consensus statement on arrhythmic mitral valve prolapse and mitral annular disjunction complex in collaboration with the ESC Council on valvular heart disease and the European Association of Cardiovascular Imaging endorsed cby the Heart Rhythm Society, by the Asia Pacific Heart Rhythm Society, and by the Latin American Heart Rhythm Society. EP Europace. 10.1093/europace/euac125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Parwani P, Avierinos J-F, Levine RA, Delling FN (2017) Mitral valve prolapse: multimodality imaging and genetic insights. Prog Cardiovasc Dis. 10.1016/j.pcad.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Palmisano A, Nicoletti V, Colantoni C et al. (2021) Dynamic changes of mitral valve annulus geometry at preprocedural CT: relationship with functional classes of regurgitation. Eur Radiol Exp. 10.1186/s41747-021-00231- [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nalliah CJ, Mahajan R, Elliott AD et al. (2019) Mitral valve prolapse and sudden cardiac death: a systematic review and meta-analysis. Heart. 10.1136/heartjnl-2017-312932 [DOI] [PubMed] [Google Scholar]
- 7.Tseng ZH, Olgin JE, Vittinghoff E et al. (2018) Prospective countywide surveillance and autopsy characterization of sudden cardiac death. Circulation. 10.1161/CIRCULATIONAHA.117.033427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Basso C, Perazzolo Marra M, Rizzo S et al. (2015) Arrhythmic mitral valve prolapse and sudden cardiac death. Circulation. 10.1161/CIRCULATIONAHA.115.016291 [DOI] [PubMed] [Google Scholar]
- 9.Basso C, Iliceto S, Thiene G, Perazzolo Marra M (2019) Mitral valve prolapse, ventricular arrhythmias, and sudden death. Circulation. 10.1161/CIRCULATIONAHA.118.034075 [DOI] [PubMed] [Google Scholar]
- 10.Düren DR, Becker AE, Dunning AJ (1988) Long-term follow-up of idiopathic mitral valve prolapse in 300 patients: a prospective study. J Am Coll Cardiol. 10.1016/0735-1097(88)90164-7 [DOI] [PubMed] [Google Scholar]
- 11.Nishimura RA, McGoon MD, Shub C et al. (1985) Echocardiographically documented mitral-valve prolapse. N Engl J Med. 10.1056/NEJM198511213132101 [DOI] [PubMed] [Google Scholar]
- 12.Aabel EW, Chivulescu M, Lie ØH et al. (2023) Ventricular arrhythmias in arrhythmic mitral valve syndrome—a prospective continuous long-term cardiac monitoring study. EP Europace. 10.1093/europace/euac182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Virani SS, Alonso A, Aparicio HJ et al. (2021) Heart disease and stroke statistics—2021 Update. Circulation. 10.1161/CIR.0000000000000950 [DOI] [PubMed] [Google Scholar]
- 14.Grigioni F, Enriquez-Sarano M, Ling LH et al. (1999) Sudden death in mitral regurgitation due to flail leaflet. J Am Coll Cardiol. 10.1016/S0735-1097(99)00474-X [DOI] [PubMed] [Google Scholar]
- 15.Savage DD, Devereux RB, Garrison RJ et al. (1983) Mitral valve prolapse in the general population. 2. Clinical features: the Framingham Study. Am Heart J 106(3):577–581. 10.1016/0002-8703(83)90705-6 [DOI] [PubMed] [Google Scholar]
- 16.Han H, Ha FJ, Teh AW et al. (2018) Mitral valve prolapse and sudden cardiac death: a systematic review. J Am Heart Assoc. 10.1161/JAHA.118.010584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freed LA, Levy D, Levine RA et al. (1999) Prevalence and Clinical Outcome of Mitral-Valve Prolapse. N Engl J Med. 10.1056/NEJM199907013410101 [DOI] [PubMed] [Google Scholar]
- 18.Bhutto ZR, Barron JT, Liebson PR et al. (1992) Electrocardiographic abnormalities in mitral valve prolapse. Am J Cardiol. 10.1016/0002-9149(92)91287-E [DOI] [PubMed] [Google Scholar]
- 19.Bekheit SG, Ali AA, Deglin SM, Jain AC (1982) Analysis of QT interval in patients with idiopathic mitral valve prolapse. Chest. 10.1378/chest.81.5.620 [DOI] [PubMed] [Google Scholar]
- 20.Kaya Ü, Eren H (2020) Fragmented QRS may be associated with complex ventricular arrhythmias in mitral valve prolapse. Minerva Cardioangiol 68(6):577–585. 10.23736/S0026-4725.20.05123-3 [DOI] [PubMed] [Google Scholar]
- 21.Basso C, Calabrese F, Corrado D, Thiene G (2001) Postmortem diagnosis in sudden cardiac death victims: macroscopic, microscopic and molecular fndings. Cardiovasc Res. 10.1016/S0008-6363(01)00261-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller MA, Dukkipati SR, Turagam M et al. (2018) Arrhythmic mitral valve prolapse. J Am Coll Cardiol. 10.1016/j.jacc.2018.09.048 [DOI] [PubMed] [Google Scholar]
- 23.Han H, Parsons SA, Teh AW et al. (2020) Characteristic histopathological fndings and cardiac arrest rhythm in isolated mitral valve prolapse and sudden cardiac death. J Am Heart Assoc. 10.1161/JAHA.119.015587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Han H-C, Parsons SA, Curl CL et al. (2021) Systematic quantifcation of histologic ventricular fbrosis in isolated mitral valve prolapse and sudden cardiac death. Heart Rhythm. 10.1016/j.hrthm.2020.12.021 [DOI] [PubMed] [Google Scholar]
- 25.Morningstar JE, Gensemer C, Moore R et al. (2021) Mitral Valve Prolapse Induces Regionalized Myocardial Fibrosis. J Am Heart Assoc. 10.1161/JAHA.121.022332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hinderer S, Schenke-Layland K (2019) Cardiac fbrosis – a short review of causes and therapeutic strategies. Adv Drug Deliv Rev. 10.1016/j.addr.2019.05.011 [DOI] [PubMed] [Google Scholar]
- 27.Rizzo S, Perazzolo Marra M, De Gaspari M, Basso C (2021) The missing pieces in the puzzle of arrhythmic mitral valve prolapse: papillary muscles, mitral annulus dysjunction, and myocardial scarring. Heart Rhythm. 10.1016/j.hrthm.2021.01.004 [DOI] [PubMed] [Google Scholar]
- 28.Garbi M, Lancellotti P, Sheppard MN (2018) Mitral valve and left ventricular features in malignant mitral valve prolapse. Open Heart. 10.1136/openhrt-2018-000925 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.SantoroBIASE FLDI, HRANITZKY P et al. (2014) Ventricular fbrillation triggered by PVCs from papillary muscles: clinical features and ablation. J Cardiovasc Electrophysiol. 10.1111/jce.12478 [DOI] [PubMed] [Google Scholar]
- 30.Alhede C, Higuchi S, Hadjis A et al. (2022) Premature ventricular contractions are presaged by a mechanically abnormal sinus beat. JACC Clin Electrophysiol. 10.1016/j.jacep.2022.05.005 [DOI] [PubMed] [Google Scholar]
- 31.Sriram CS, Syed FF, Ferguson ME et al. (2013) Malignant bileafet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J Am Coll Cardiol. 10.1016/j.jacc.2013.02.060 [DOI] [PubMed] [Google Scholar]
- 32.Villatore A, Sala S, Stella S et al. (2021) Autoimmune myocarditis and arrhythmogenic mitral valve prolapse: an unexpected overlap syndrome. J Cardiovasc Dev Dis. 10.3390/jcdd8110151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jiang W, Xiong Y, Li X, Yang Y (2021) Cardiac fbrosis: cellular efectors, molecular pathways, and exosomal roles. Front Cardiovasc Med. 10.3389/fcvm.2021.715258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Salvador AM, Nevers T, Velázquez F et al. (2016) Intercellular adhesion molecule 1 regulates left ventricular leukocyte infltration, cardiac remodeling, and function in pressure overload–induced heart failure. J Am Heart Assoc. 10.1161/JAHA.115.003126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Śniez̊ek-Maciejewska M, Dubiel JP, Piwowarska W et al. (1992) Ventricular arrhythmias and the autonomic tone in patients with mitral valve prolapse. Clin Cardiol. 10.1002/clc.4960151029 [DOI] [PubMed] [Google Scholar]
- 36.Myles RC, Wang L, Kang C et al. (2012) Local β-adrenergic stimulation overcomes source-sink mismatch to generate focal arrhythmia. Circ Res. 10.1161/CIRCRESAHA.111.262345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mason JW, Koch FH, Billingham ME, Winkle RA (1978) Cardiac biopsy evidence for a cardiomyopathy associated with symptomatic mitral valve prolapse. Am J Cardiol. 10.1016/0002-9149(78)90623-9 [DOI] [PubMed] [Google Scholar]
- 38.La VL, Ometto R, Centofante P et al. (1998) Arrhythmic profle, ventricular function, and histomorphometric fndings in patients with idiopathic ventricular tachycardia and mitral valve prolapse: clinical and prognostic evaluation. Clin Cardiol. 10.1002/clc.4960211007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bains S, Tester DJ, Asirvatham SJ et al. (2019) A novel truncating variant in FLNC-encoded flamin C may serve as a proarrhythmic genetic substrate for arrhythmogenic bileafet mitral valve prolapse syndrome. Mayo Clin Proc. 10.1016/j.mayocp.2018.11.028 [DOI] [PubMed] [Google Scholar]
- 40.Guglielmo M, Arangalage D, Bonino MA et al. (2023) Additional value of cardiac magnetic resonance feature tracking parameters for the evaluation of the arrhythmic risk in patients with mitral valve prolapse. J Cardiovasc Magn Reson. 10.1186/s12968-023-00944-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nagata Y, Bertrand PB, Baliyan V et al. (2023) Abnormal mechanics relate to myocardial fbrosis and ventricular arrhythmias in patients with mitral valve prolapse. Circ Cardiovasc Imaging. 10.1161/CIRCIMAGING.122.014963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chivulescu M, Aabel EW, Gjertsen E et al. (2022) Electrical markers and arrhythmic risk associated with myocardial fbrosis in mitral valve prolapse. Europace. 10.1093/europace/euac017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Constant Dit Beaufls AL, Huttin O, Jobbe-Duval A et al. (2021) Replacement myocardial fbrosis in patients with mitral valve prolapse: relation to mitral regurgitation, Ventricular Remodeling, and Arrhythmia. Circulation. 10.1161/CIRCULATIONAHA.120.050214 [DOI] [PubMed] [Google Scholar]
- 44.Pavon AG, Arangalage D, Pascale P et al. (2021) Myocardial extracellular volume by T1 mapping: a new marker of arrhythmia in mitral valve prolapse. J Cardiovasc Magn Reson. 10.1186/s12968-021-00797-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gatti M, Palmisano A, Esposito A et al. (2021) Feature tracking myocardial strain analysis in patients with bileafet mitral valve prolapse: relationship with LGE and arrhythmias. Eur Radiol. 10.1007/s00330-021-07876-z [DOI] [PubMed] [Google Scholar]
- 46.Pradella S, Grazzini G, Brandani M et al. (2019) Cardiac magnetic resonance in patients with mitral valve prolapse: focus on late gadolinium enhancement and T1 mapping. Eur Radiol. 10.1007/s00330-018-5634-5 [DOI] [PubMed] [Google Scholar]
- 47.Kitkungvan D, Nabi F, Kim RJ et al. (2018) Myocardial fbrosis in patients with primary mitral regurgitation with and without prolapse. J Am Coll Cardiol. 10.1016/j.jacc.2018.06.048 [DOI] [PubMed] [Google Scholar]
- 48.Bennett S, Tafuro J, Duckett S et al. (2022) Defnition, prevalence, and clinical signifcance of mitral annular disjunction in diferent patient cohorts: a systematic review. Echocardiography. 10.1111/echo.15299 [DOI] [PubMed] [Google Scholar]
- 49.Lancellotti P, Pibarot P, Chambers J et al. (2022) Multi-modality imaging assessment of native valvular regurgitation: an EACVI and ESC council of valvular heart disease position paper. Eur Heart J Cardiovasc Imaging. 10.1093/ehjci/jeab253 [DOI] [PubMed] [Google Scholar]
- 50.Zoghbi WA, Adams D, Bonow RO et al. (2017) Recommendations for noninvasive evaluation of native valvular regurgitation. J Am Soc Echocardiogr. 10.1016/j.echo.2017.01.007 [DOI] [PubMed] [Google Scholar]
- 51.Nordhues BD, Siontis KC, Scott CG et al. (2016) Bileafet mitral valve prolapse and risk of ventricular dysrhythmias and death. J Cardiovasc Electrophysiol. 10.1111/jce.12914 [DOI] [PubMed] [Google Scholar]
- 52.Avierinos J-F, Gersh BJ, Melton LJ et al. (2002) Natural history of asymptomatic mitral valve prolapse in the community. Circulation. 10.1161/01.CIR.0000028933.34260.09 [DOI] [PubMed] [Google Scholar]
- 53.Hutchins GM, Moore GW, Skoog DK (1986) The association of foppy mitral valve with disjunction of the mitral annulus fbrosus. N Engl J Med. 10.1056/NEJM198602273140902 [DOI] [PubMed] [Google Scholar]
- 54.Eriksson MJ, Bitkover CY, Omran AS et al. (2005) Mitral annular disjunction in advanced myxomatous mitral valve disease: echocardiographic detection and surgical correction. J Am Soc Echocardiogr. 10.1016/j.echo.2005.06.013 [DOI] [PubMed] [Google Scholar]
- 55.Carmo P, Andrade MJ, Aguiar C et al. (2010) Mitral annular disjunction in myxomatous mitral valve disease: a relevant abnormality recognizable by transthoracic echocardiography. Cardiovasc Ultrasound. 10.1186/1476-7120-8-53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Perazzolo Marra M, Basso C, De Lazzari M et al. (2016) Morphofunctional abnormalities of mitral annulus and arrhythmic mitral valve prolapse. Circ Cardiovasc Imaging. 10.1161/CIRCIMAGING.116.005030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Muthukumar L, Rahman F, Jan MF et al. (2017) The Pickelhaube sign . JACC Cardiovasc Imaging. 10.1016/j.jcmg.2016.09.016 [DOI] [PubMed] [Google Scholar]
- 58.Slavich M, Palmisano A, Pannone L et al. (2019) Hidden danger behind the prolapse. Circ Cardiovasc Imaging. 10.1161/CIRCIMAGING.119.009639 [DOI] [PubMed] [Google Scholar]
- 59.Huttin O, Pierre S, Venner C et al. (2016) Interactions between mitral valve and left ventricle analysed by 2D speckle tracking in patients with mitral valve prolapse: one more piece to the puzzle. Eur Heart J Cardiovasc Imaging. 10.1093/ehjci/jew075 [DOI] [PubMed] [Google Scholar]
- 60.Essayagh B, Sabbag A, Antoine C et al. (2021) The mitral annular disjunction of mitral valve prolapse. JACC Cardiovasc Imaging. 10.1016/j.jcmg.2021.04.029 [DOI] [PubMed] [Google Scholar]
- 61.Tong J, Yew M, Huang W, Yong QW (2022) The dance of death: cardiac arrest, mitral and tricuspid valve prolapses, and biannular disjunctions. CASE (Phila). 10.1016/j.case.2021.11.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tayal B, Delling FN, Malahfi M, Shah DJ (2021) Cardiac imaging for risk assessment of malignant ventricular arrhythmias in patients with mitral valve prolapse. Front Cardiovasc Med. 10.3389/fcvm.2021.574446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Figliozzi S, Georgiopoulos G, Lopes PM et al. (2023) Myocardial fbrosis at cardiac MRI helps predict adverse clinical outcome in patients with mitral valve prolapse. Radiology. 10.1148/radiol.220454 [DOI] [PubMed] [Google Scholar]
- 64.Zugwitz D, Fung K, Aung N et al. (2022) Mitral annular disjunction assessed using CMR imaging. JACC Cardiovasc Imaging. 10.1016/j.jcmg.2022.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Dejgaard LA, Skjølsvik ET, Lie ØH et al. (2018) The mitral annulus disjunction arrhythmic syndrome. J Am Coll Cardiol. 10.1016/j.jacc.2018.07.070 [DOI] [PubMed] [Google Scholar]
- 66.Faletra FF, Leo LA, Paiocchi VL et al. (2022) Morphology of mitral annular disjunction in mitral valve prolapse. J Am Soc Echocardiogr. 10.1016/j.echo.2021.09.002 [DOI] [PubMed] [Google Scholar]
- 67.Mantegazza V, Volpato V, Gripari P et al. (2021) Multimodality imaging assessment of mitral annular disjunction in mitral valve prolapse. Heart. 10.1136/heartjnl-2020-317330 [DOI] [PubMed] [Google Scholar]
- 68.Angelini A, Ho SY, Anderson RH et al. (1988) A histological study of the atrioventricular junction in hearts with normal and prolapsed leafets of the mitral valve. Heart. 10.1136/hrt.59.6.712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu S, Siegel RJ (2022) Mitral annular disjunction: a case series and review of the literature. Front Cardiovasc Med. 10.3389/fcvm.2022.976066 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Groeneveld SA, Kirkels FP, Cramer MJ et al. (2022) Prevalence of mitral annulus disjunction and mitral valve prolapse in patients with idiopathic ventricular fbrillation. J Am Heart Assoc. 10.1161/JAHA.121.025364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Essayagh B, Iacuzio L, Civaia F et al. (2019) Usefulness of 3-Tesla cardiac magnetic resonance to detect mitral annular disjunction in patients with mitral valve prolapse. Am J Cardiol. 10.1016/j.amjcard.2019.08.047 [DOI] [PubMed] [Google Scholar]
- 72.Basso C, Perazzolo Marra M (2018) Mitral annulus disjunction. J Am Coll Cardiol. 10.1016/j.jacc.2018.07.069 [DOI] [PubMed] [Google Scholar]
- 73.Putnam AJ, Kebed K, Mor-Avi V et al. (2020) Prevalence of mitral annular disjunction in patients with mitral valve prolapse and severe regurgitation. Int J Cardiovasc Imaging 36(7):1363–1370. 10.1007/s10554-020-01818-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kulkarni AA, Chudgar PD, Burkule NJ, Kamat NV (2022) Mitral annulus disjunction and arrhythmic mitral valve prolapse: emerging role of cardiac magnetic resonance imaging in the workup. Indian J Radiol Imaging 32(4):576–581. 10.1055/s-0042-1754357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haugaa K (2021) Improving the imaging diagnosis of mitral annular disjunction. Heart. 10.1136/heartjnl-2020-317667 [DOI] [PubMed] [Google Scholar]
- 76.Romero Daza A, Chokshi A, Pardo P et al. (2021) Mitral valve prolapse morphofunctional features by cardiovascular magnetic resonance: more than just a valvular disease. J Cardiovasc Magn Reson. 10.1186/s12968-021-00800-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Aquaro GD, De Gori C, Grilli G et al. (2023) Dark papillary muscles sign: a novel prognostic marker for cardiac magnetic resonance. Eur Radiol. 10.1007/s00330-023-09400-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dieterlen M-T, Klaeske K, Spampinato R et al. (2023) Histopathological insights into mitral valve prolapse-induced fbrosis. Front Cardiovasc Med. 10.3389/fcvm.2023.1057986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Pradella S, Grazzini G, Miele V (2020) Mitral valve prolapse imaging: the role of tissue characterization. Quant Imaging Med Surg. 10.21037/qims-2020-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Niu Z, Chan V, Mesana T, Ruel M (2016) The evolution of mitral valve prolapse: insights from the Framingham Heart Study. J Thorac Dis. 10.21037/jtd.2016.07.58 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Thamman R, Gupta N, Doshi R et al. (2020) Mitral annular disjunction: Wolf in sheep’s clothing? Echocardiography. 10.1111/echo.14898 [DOI] [PubMed] [Google Scholar]
- 82.Bui AH, Roujol S, Foppa M et al. (2017) Difuse myocardial fbrosis in patients with mitral valve prolapse and ventricular arrhythmia. Heart. 10.1136/heartjnl-2016-309303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Fulton BL, Liang JJ, Enriquez A et al. (2018) Imaging characteristics of papillary muscle site of origin of ventricular arrhythmias in patients with mitral valve prolapse. J Cardiovasc Electrophysiol. 10.1111/jce.13374 [DOI] [PubMed] [Google Scholar]
- 84.Guglielmo M, Fusini L, Muscogiuri G et al. (2021) T1 mapping and cardiac magnetic resonance feature tracking in mitral valve prolapse. Eur Radiol. 10.1007/s00330-020-07140-w [DOI] [PubMed] [Google Scholar]
- 85.Hourdain J, Clavel MA, Deharo J-C et al. (2018) Common phenotype in patients with mitral valve prolapse who experienced sudden cardiac death. Circulation. 10.1161/CIRCULATIONAHA.118.033488 [DOI] [PubMed] [Google Scholar]
- 86.Kim AJ, Xu N, Umeyama K et al. (2020) Defciency of circulating monocytes ameliorates the progression of myxomatous valve degeneration in marfan syndrome. Circulation. 10.1161/CIRCULATIONAHA.119.042391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Mewton N, Liu CY, Croisille P et al. (2011) Assessment of myocardial fbrosis with cardiovascular magnetic resonance. J Am Coll Cardiol. 10.1016/j.jacc.2010.11.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yue L, Xie J, Nattel S (2011) Molecular determinants of cardiac fbroblast electrical function and therapeutic implications for atrial fbrillation. Cardiovasc Res. 10.1093/cvr/cvq329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Sridhar S, Vandersickel N, Panflov AV (2017) Efect of myocyte-fbroblast coupling on the onset of pathological dynamics in a model of ventricular tissue. Sci Rep. 10.1038/srep40985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wynn TA, Ramalingam TR (2012) Mechanisms of fbrosis: therapeutic translation for fbrotic disease. Nat Med. 10.1038/nm.2807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Travers JG, Kamal FA, Robbins J et al. (2016) Cardiac Fibrosis. Circ Res. 10.1161/CIRCRESAHA.115.306565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Piek A, de Boer RA, Silljé HHW (2016) The fbrosis-cell death axis in heart failure. Heart Fail Rev. 10.1007/s10741-016-9536-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Nensa F, Bamberg F, Rischpler C et al. (2018) Hybrid cardiac imaging using PET/MRI: a joint position statement by the European Society of Cardiovascular Radiology (ESCR) and the European Association of Nuclear Medicine (EANM). Eur Radiol. 10.1007/s00330-017-5008-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nazir MS, Ismail TF, Reyes E et al. (2018) Hybrid positron emission tomography–magnetic resonance of the heart: current state of the art and future applications. Eur Heart J Cardiovasc Imaging. 10.1093/ehjci/jey090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Miller MA, Adams DH, Pandis D et al. (2020) Hybrid positron emission tomography/magnetic resonance imaging in arrhythmic mitral valve prolapse. JAMA Cardiol. 10.1001/jamacardio.2020.1555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Miller MA, Devesa A, Robson PM et al. (2023) Arrhythmic mitral valve prolapse with only mild or moderate mitral regurgitation. JACC Clin Electrophysiol. 10.1016/j.jacep.2023.04.011 [DOI] [PubMed] [Google Scholar]
- 97.Dweck MR, Abgral R, Trivieri MG et al. (2018) Hybrid magnetic resonance imaging and positron emission tomography with fuorodeoxyglucose to diagnose active cardiac sarcoidosis. JACC Cardiovasc Imaging. 10.1016/j.jcmg.2017.02.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Feuchtner GM, Alkadhi H, Karlo C et al. (2010) Cardiac CT angiography for the diagnosis of mitral valve prolapse: comparison with echocardiography <sup/>. Radiology. 10.1148/radiol.2541090393 [DOI] [PubMed] [Google Scholar]
- 99.Tsianaka T, Matziris I, Kobe A et al. (2021) Mitral annular disjunction in patients with severe aortic stenosis: extent and reproducibility of measurements with computed tomography. Eur J Radiol Open. 10.1016/j.ejro.2021.100335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Toh H, Mori S, Izawa Y et al. (2021) Prevalence and extent of mitral annular disjunction in structurally normal hearts: comprehensive 3D analysis using cardiac computed tomography. Eur Heart J Cardiovasc Imaging. 10.1093/ehjci/jeab022 [DOI] [PubMed] [Google Scholar]
- 101.Koo HJ, Kang J-W, Oh SY et al. (2019) Cardiac computed tomography for the localization of mitral valve prolapse: scallop-by-scallop comparisons with echocardiography and intraoperative fndings. Eur Heart J Cardiovasc Imaging. 10.1093/ehjci/jey139 [DOI] [PubMed] [Google Scholar]
- 102.Esposito A, Palmisano A, Barbera M et al. (2019) Cardiac computed tomography in troponin-positive chest pain. JACC Cardiovasc Imaging. 10.1016/j.jcmg.2018.08.013 [DOI] [PubMed] [Google Scholar]
- 103.Vignale D, Palmisano A, Colantoni C et al. (2023) Toward a One-Stop Shop CT Protocol in Acute Chest Pain Syndrome. Radiology. 10.1148/radiol.220844 [DOI] [PubMed] [Google Scholar]
- 104.Palmisano A, Vignale D, Tadic M et al. (2022) Myocardial late contrast enhancement CT in troponin-positive acute chest pain syndrome. Radiology. 10.1148/radiol.211288 [DOI] [PubMed] [Google Scholar]
- 105.Palmisano A, Vignale D, Benedetti G et al. (2020) Late iodine enhancement cardiac computed tomography for detection of myocardial scars: impact of experience in the clinical practice. Radiol Med. 10.1007/s11547-019-01108-7 [DOI] [PubMed] [Google Scholar]
- 106.Piroli F, Boccellino A, Ingallina G et al. (2023) Feasibility and reliability of comprehensive three-dimensional transoesophageal echocardiography screening process for transcatheter mitral valve replacement. Eur Heart J Cardiovasc Imaging. 10.1093/ehjci/jead015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vignale D, Palmisano A, Gnasso C et al. (2023) Extracellular volume fraction (ECV) derived from pre-operative computed tomography predicts prognosis in patients undergoing transcatheter aortic valve implantation (TAVI). Eur Heart J Cardiovasc Imaging. 10.1093/ehjci/jead040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Palmisano A, Vignale D, Peretto G et al. (2021) Hybrid FDG-PET/MR or FDG-PET/CT to detect disease activity in patients with persisting arrhythmias after myocarditis. JACC Cardiovasc Imaging. 10.1016/j.jcmg.2020.03.009 [DOI] [PubMed] [Google Scholar]


