Abstract
Background
Acetaminophen is the only analgesic considered safe for use throughout pregnancy. Recent studies suggest that use during pregnancy may be associated with poorer neurodevelopmental outcomes in children, but few have examined language development.
Methods
The Illinois Kids Development Study is a prospective birth cohort in east-central Illinois. Between December 2013 and March 2020, 532 newborns were enrolled and had exposure data available. Participants reported the number of times they took acetaminophen six times across pregnancy. Language data were collected at 26.5–28.5 months using the MacArthur-Bates Communicative Development Inventories (CDI; n = 298), and 36–38 months using the Speech and Language Assessment Scale (SLAS; n = 254).
Results
Taking more acetaminophen during the second or third trimester was associated with marginally smaller vocabularies and shorter utterance length (M3L) at 26.5–28.5 months. More acetaminophen use during the third trimester was also associated with increased odds of M3L scores ≤25th percentile in male children. More use during the second or third trimester was associated with lower SLAS scores at 36–38 months. Third trimester use was specifically related to lower SLAS scores in male children.
Conclusions
Higher prenatal acetaminophen use during pregnancy may be associated with poorer early language development.
Impact
Taking more acetaminophen during pregnancy, particularly during the second and third trimesters, was associated with poorer scores on measures of language development when children were 26.5–28.5 and 36–38 months of age.
Only male children had lower scores in analyses stratified by child sex.
To our knowledge, this is the first study that has used a standardized measure of language development to assess the potential impact of prenatal exposure to acetaminophen on language development.
This study adds to the growing body of literature suggesting that the potential impact of acetaminophen use during pregnancy on fetal neurodevelopment should be carefully evaluated.
Introduction
Acetaminophen (paracetamol) is the most common drug ingredient in the United States and only analgesic considered safe to use throughout pregnancy.1 Both the American College of Obstetricians and Gynecologists2 and Society for Maternal-Fetal Medicine3 recommend acetaminophen as the first-line pharmacological intervention for pain and/or fever during pregnancy as other analgesics have been linked to problems in both the pregnant individual and fetus.3 Studies indicate 50–65% of pregnant women in North America and Europe report taking acetaminophen, or a medication containing it, at least once during pregnancy.1,4–6 Little is known about the safety of acetaminophen use during pregnancy due to the lack of clinical trials in pregnant women and very few reported cases of adverse effects in the developing child;1,7,8 however, acetaminophen can cross the placenta,9–11 and there has been increased interest in examining whether acetaminophen use during pregnancy could be related to child health outcomes. While some studies report no relationship between prenatal acetaminophen exposure and child health and development,12–15 other studies in animals and humans suggest it may be related to poorer neurodevelopmental outcomes.8,16–30 Multiple epidemiological studies indicate that prenatal acetaminophen exposure is associated with motor delays,4,31,32 attention problems,6,33–41 and behavioral problems.4,34,38,42–45 Early language development is predictive of later IQ, reading ability, and school success,46,47 yet only a few studies have investigated whether there is a relationship between prenatal acetaminophen use and language development. In the Swedish Environmental, Longitudinal, Mother and child, Asthma and Allergy Study, acetaminophen use early in pregnancy was related to delayed language development in female children at 30 months.48 In the Norwegian Mother and Child Cohort study, acetaminophen use during pregnancy was associated with poorer scores on the communication scale from the Ages and Stages Questionnaire (ASQ) at both 18-months31 and 3 years.4,49
The few studies that have assessed the association between prenatal acetaminophen use and language development have relied upon self-report measures of acetaminophen collected 1–3 times during pregnancy, leaving ample room for inaccuracies in memory and not allowing for accurate evaluation of whether the timing of exposure is important. Further research is needed to better-understand the potential for prenatal acetaminophen exposure to impact early language development. In the Illinois Kids Development Study (IKIDS) cohort, pregnant people were recruited early in pregnancy, and participants were interviewed about acetaminophen use in six discrete periods of pregnancy, leaving less time between interviews and allowing for a more accurate evaluation of acetaminophen use during each trimester. In the present study, the association of prenatal acetaminophen use with language development was evaluated at 26.5–28.5 months using the MacArthur-Bates Communicative Development Inventories: Words and Sentences (CDI)50 and at 36–38 months using the Speech and Language Assessment Scale (SLAS).51
Methods
Study cohort
IKIDS is a prospective pregnancy and birth cohort in east-central Illinois, United States originally designed to evaluate the relationship of gestational exposure to phthalates and phenols with neurodevelopment.52 Participants whose children were included in this analysis were recruited between December 2013 and March 2020 at two local obstetric clinics and gave birth at two local hospitals. Clinics gave brochures regarding the study to patients at their first prenatal visit. Patients completed a reply card indicating their interest in being contacted about participation. Interested individuals were contacted via telephone to receive more information about participation and determine eligibility. Individuals were eligible to participate if they were: <15 weeks of gestation; fluent in English; 18–40 years old; not carrying multiples; willing to provide a fasting blood sample and five urine samples throughout pregnancy; did not have a child already participating in IKIDS; resided within a 30-minute drive of the University of Illinois campus; their pregnancy had not been classified as high-risk by their doctor for a reason other than advanced maternal age; and they planned to remain in the area until the child’s first birthday. Those who chose to participate were enrolled at 8–14 weeks of gestation and provided written informed consent. Demographics, pregnancy and health history, pregnancy symptoms, medication use, and lifestyle factors were obtained by interview shortly after enrollment and updated throughout the pregnancy. Written informed consent was also obtained at each age that language development data were collected. IKIDS was approved and overseen by the University of Illinois Urbana-Champaign Institutional Review Board.
Acetaminophen use during pregnancy
At approximately 10–14, 16–18, 22–24, 28–30, and 34–36 weeks of gestation, and within 24 hours of the child’s birth, participants were interviewed about their medication use. At the first interview (10–14 weeks), participants were asked to list all medications they had used beginning at their estimated conception date through the time of the interview as well as their reason for use (indication), and frequency of use. At subsequent interviews, participants were asked to recall the same information for the period between their last interview and the current one. From these data, medications containing acetaminophen as an active ingredient were identified. Using reported frequency and dates of use, the number of times participants took acetaminophen during the first, second, and third trimesters was calculated. Cumulative use was the number of times acetaminophen was taken across all three trimesters.
Language measures
Language data included in these analyses were collected between December 2016 and August 2022. When children reached 26.5–28.5 and 36–38 months of age, caregivers were asked to participate in follow-ups of the study child. Those who agreed were mailed a packet of questionnaires which included the CDI50 at 26.5–28.5 months, and the SLAS51 at 36–38 months.
The CDI is a parental report form which provides measures of the child’s expressive vocabulary, language complexity, and mean length of the longest three utterances (M3L) standardized by sex and age. To measure expressive vocabulary, caregivers indicate which words their child says from a checklist of 680 words. For language complexity, caregivers are asked to indicate which in each pair of 37 sentences sounds more like how their child talks. Caregivers also provide three examples of their child’s longest sentences which are broken down into morphemes (smallest unit of meaning) to calculate M3L. Raw scores were calculated and normalized to percentile scores based on the child’s age and sex. Analyses were conducted using raw scores in generalized linear regression models and percentile scores categorized as above or ≤25th percentile in logistic regression models to calculate odds ratios (ORs).
The SLAS is a short questionnaire with Likert scale questions which evaluate five areas of language use: assertiveness, responsiveness, semantics, syntax, and articulation.51,53,54 It has been found to be reliable and correlated with other language development measures, including the CDI. In the initial study evaluating the SLAS, five items pertaining to comprehension, cultural awareness, and speaking too loudly or softly were found to be unreliable. Therefore, subsequent studies have excluded these items.51,53,54 Talkativeness is based on one question; thus, most studies have also excluded it.53,54 Because each of the scores for the five scales consist of only two or three questions, only the total score, calculated using the 13 items with high inter-rater reliability from these scales, was used in this study.
Covariates
Acetaminophen formulation and indication were collected only for those who reported taking acetaminophen, thus were not included in models. The following sociodemographic factors were considered for inclusion as covariates in multivariable and logistic regression models based on a priori knowledge and using directed acyclic graphs (Supplementary Fig. S1): parental education, maternal age, parity, household income, tobacco smoking and alcohol use during the first trimester, whether the participant’s native language was English, and child age at assessment and number of older siblings. Additionally, mean perceived stress score (PSS) and Edinburgh Postnatal Depression Scale (EPDS) scores averaged from the following life stages: prenatal, infancy, early childhood, and individual scores at the time of each assessment, were considered. Maternal verbal IQ and birth weight were not considered due to the high amount of missingness (Table 1). Correlations of potential covariates with both exposure and outcome variables were explored, and covariates were included in models when they were correlated with exposure and at least one outcome. In all models using continuous outcomes, child sex was included as a potential modifier. Mean PSS and EPDS scores during pregnancy were included for both the CDI and SLAS. Child age at the time of assessment and maternal parity and education were also included in the CDI models.
Table 1.
Parental characteristics for all IKIDS participants with an infant enrolled and had exposure data available (n = 532), those who provided language data at 26.5–28.5 months (CDI; n = 298), and those who provided language data at 36–38 months (SLAS; n = 254) by whether they reported taking acetaminophen during pregnancy or not.
| Participant Characteristics | Participants with an infant enrolled in study & exposure data available | Participants who completed the CDI when their child was 26.5–28.5 months | Participants who completed the SLAS when their child was 36–38 months | |||
|---|---|---|---|---|---|---|
| Participant did not take acetaminophen during pregnancy | Participant took acetaminophen during pregnancy | Participant did not take acetaminophen during pregnancy | Participant took acetaminophen during pregnancy | Participant did not take acetaminophen during pregnancy | Participant took acetaminophen during pregnancy | |
| (n = 155) | (n = 377) | (n = 90) | (n = 208) | (n = 80) | (n = 174) | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| Maternal race & ethnicity | ||||||
| White, Non-Hispanic | 121 (78.1) | 324 (85.9) | 71 (78.9) | 182 (87.5) | 67 (83.8) | 156 (89.6) |
| Other | 34 (21.9) | 51 (13.5) | 19 (21.1) | 26 (12.5) | 13 (16.2) | 17 (9.8) |
| Unknown/Missing | 0 (0.0) | 2 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.6) |
| Other parent’s race & ethnicity | ||||||
| White, Non-Hispanic | 118 (76.1) | 318 (84.4) | 72 (80.0) | 185 (88.9) | 65 (81.2) | 155 (89.1) |
| Other | 37 (23.9) | 57 (15.1) | 18 (20.0) | 23 (11.1) | 15 (18.8) | 18 (10.3) |
| Unknown/Missing | 0 (0.0) | 2 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.6) |
| Maternal marital status | ||||||
| Married/Living as married | 143 (92.3) | 351 (93.1) | 84 (93.3) | 198 (95.2) | 77 (96.2) | 166 (95.4) |
| Separated/Divorced/ Widowed/Single | 12 (7.7) | 26 (6.9) | 6 (6.7) | 10 (4.8) | 3 (3.8) | 8 (4.6) |
| Maternal education | ||||||
| < Bachelor’s degree | 25 (16.1) | 78 (20.7) | 7 (7.8) | 27 (13.0) | 4 (5.0) | 22 (12.6) |
| ≥ Bachelor’s degree | 130 (83.9) | 299 (79.3) | 83 (92.2) | 181 (87.0) | 76 (95.0) | 152 (87.4) |
| Other parent’s education | ||||||
| < Bachelor’s degree | 41 (26.5) | 116 (30.8) | 16 (17.8) | 45 (21.6) | 14 (17.5) | 41 (23.6) |
| ≥ Bachelor’s degree | 113 (72.9) | 259 (68.7) | 74 (82.2) | 163 (78.4) | 66 (82.5) | 133 (76.4) |
| Unknown/Missing | 1 (0.7) | 2 (0.5) | ||||
| Maternal parity | ||||||
| 0 | 75 (48.4) | 198 (52.5) | 47 (52.2) | 119 (57.2) | 41 (51.2) | 94 (54.0) |
| ≥1 | 80 (51.6) | 178 (47.2) | 43 (47.8) | 89 (42.8) | 39 (48.8) | 79 (45.4) |
| Missing | 0 (0.0) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.6) |
| Annual household income | ||||||
| $0–$49,999 | 33 (21.3) | 70 (18.6) | 17 (18.9) | 29 (13.9) | 11 (13.8) | 25 (14.4) |
| $50,000–$99,999 | 72 (46.4) | 181 (48.0) | 41 (45.5) | 96 (46.2) | 37 (46.2) | 85 (48.8) |
| ≥$100,000 | 48 (31.0) | 124 (32.9) | 32 (35.6) | 83 (39.9) | 31 (38.8) | 64 (36.8) |
| Unknown/Missing | 2 (1.3) | 2 (0.5) | 0 (0.0) | 0 (0.0) | 1 (1.2) | 0 (0.0) |
| Maternal health insurance status | ||||||
| Insured | 153 (98.7) | 376 (99.7) | 90 (100.0) | 208 (100.0) | 80 (100.0) | 174 (100.0) |
| Uninsured | 2 (1.3) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Maternal tobacco smoking during 1st trimester | ||||||
| Yes | 7 (4.5) | 18 (4.8) | 3 (3.3) | 4 (1.9) | 3 (3.8) | 8 (4.6) |
| No | 139 (89.7) | 333 (88.3) | 84 (93.3) | 191 (91.8) | 72 (90.0) | 151 (86.8) |
| Unknown/Missing | 9 (5.8) | 26 (6.9) | 3 (3.3) | 13 (6.3) | 5 (6.2) | 15 (8.6) |
| Maternal alcohol during 1st trimester consumption | ||||||
| Yes | 116 (74.8) | 278 (73.7) | 68 (75.6) | 153 (73.6) | 59 (73.8) | 130 (74.7) |
| No | 39 (25.2) | 98 (26.0) | 22 (24.4) | 55 (26.4) | 21 (26.2) | 44 (25.3) |
| Unknown/Missing | 0 (0.0) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Gestational weeks 0–4 | ||||||
| None | 96 (61.9) | 222 (58.9) | 55 (61.1) | 121 (58.2) | 49 (61.3) | 98 (56.3) |
| <4 drinks/week | 46 (29.7) | 124 (32.9) | 27 (30.0) | 72 (34.6) | 24 (30.0) | 59 (33.9) |
| 4–10 drinks/week | 8 (5.2) | 25 (6.6) | 7 (7.8) | 12 (5.8) | 9 (11.3) | 14 (8.0) |
| 11–21 drinks/week | 1 (0.7) | 5 (1.3) | 1 (1.1) | 3 (1.4) | 1 (1.3) | 3 (1.7) |
| Unknown/Missing | 0 (0.0) | 1 (0.3) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Gestational weeks 5–8 | ||||||
| None | 151 (97.4) | 364 (96.6) | 87 (96.7) | 200 (96.2) | 77 (96.3) | 166 (95.4) |
| <4 drinks/week | 3 (1.9) | 6 (1.6) | 2 (2.2) | 5 (2.4) | 2 (2.5) | 3 (1.7) |
| Unknown/Missing | 1 (0.6) | 7 (1.9) | 1 (1.1) | 3 (1.4) | 1 (1.3) | 5 (2.9) |
| Gestational weeks 9–14 | ||||||
| None | 152 (98.1) | 370 (98.1) | 88 (97.8) | 204 (98.1) | 79 (98.8) | 171 (98.3) |
| <4 drinks/week | 3 (1.9) | 3 (0.8) | 2 (2.2) | 2 (1.0) | 1 (1.3) | 1 (0.6) |
| Unknown/Missing | 0 (0.0) | 4 (1.1) | 0 (0.0) | 2 (1.0) | 0 (0.0) | 2 (1.1) |
| Maternal non-native English speaker | ||||||
| Yes | 17 (11.0) | 10 (2.7) | 11 (12.2) | 9 (4.3) | 8 (10.0) | 8 (4.6) |
| No | 138 (89.0) | 367 (97.3) | 79 (87.8) | 199 (95.7) | 72 (90.0) | 166 (95.4) |
| Maternal age (years) at baseline | 30.82 (4.06) | 30.16 (4.13) | 31.21 (3.99) | 30.55 (3.77) | 30.99 (3.79) | 30.55 (3.68) |
| Maternal verbal IQ (PPVTa standardized score) | 107.66 (12.92)d | 107.96 (10.64)e | 108.26 (12.99)g | 108.21 (10.19)h | 109.61 (11.30)i | 109.24 (10.59)j |
| Mean maternal stress (PSS-10b) score during pregnancy | 10.61 (5.64) | 11.33 (5.69) | 10.26 (5.46) | 10.82 (5.29) | 9.82 (5.18) | 10.63 (5.47) |
| Mean maternal depression (EPDSc) score during pregnancy | 3.94 (3.05) | 4.31 (3.38) | 3.89 (2.83) | 3.96 (3.15) | 3.62 (2.76) | 3.96 (3.17) |
aPPVT-IV: Peabody Picture Vocabulary Test - Fourth Edition.
bPSS-10: Perceived Stress Scale.
cEPDS: Edinburgh Postnatal Depression Scale
dPPVT unable to be collected due to missed visit or COVID-19 n = 121.
ePPVT unable to be collected due to missed visit or COVID-19 n = 47.
fPPVT unable to be collected due to missed visit or COVID-19 n = 2.
gPPVT unable to be collected due to missed visit or COVID-19 n = 18.
hPPVT unable to be collected due to missed visit or COVID-19 n = 38.
iPPVT unable to be collected due to missed visit or COVID-19 n = 13.
jPPVT unable to be collected due to missed visit or COVID-19 n = 22.
Statistical approach
Multivariable generalized linear regression models were used to evaluate the relationship between the number times acetaminophen was taken during each trimester and throughout pregnancy, and each continuous outcome at 26.5–28.5 months (vocabulary size, M3L, and complexity) and 36–38 months (total SLAS score). Because language development is sexually dimorphic, with females developing language earlier and faster than males,55 sex-by-exposure interactions were also examined, and when the interaction p-value was <0.10, results were also stratified by child sex.
Additionally, the raw CDI scores were converted to percentile scores and categorized as ≤25th or >25th percentile for complementary analyses using multivariable logistic regression. This analysis allowed for characterization of the odds of a child having scores on the low end of the normal distribution (≤25th percentile) for their sex and age with increasing prenatal acetaminophen exposure. Child sex and age were not included in these models because they are accounted for when raw scores are converted to percentile scores; however, results were stratified by child sex.
Sensitivity analyses were used to evaluate the potential impact of several other variables on the associations. These included: maternal alcohol use (Supplementary Table S1), smoking, whether the mother’s native language was English, marital status, postnatal PSS and EPDS scores (average scores during infancy for the CDI, overall average scores for the SLAS, and scores collected at the same time the outcomes were collected), and the other parent’s education. Other sensitivity analyses were conducted excluding participants for various reasons, including: potential leverage points (Cook’s D > 0.04) in multivariable linear regression; observations with high leverage (extreme values on a predictor variable), defined as greater than 0.3, in logistic regression models; children outside of the defined age range at the time the CDI was collected (n = 41); and participants who reported taking psychotropics56 (CDI n = 37; SLAS n = 31) or other analgesics3 (n = 49). An additional sensitivity analysis was performed including only children who had data available at both ages (n = 212). All statistical analyses were conducted using SAS software, Version 9.4 of the SAS System for Windows (Copyright © 2013, SAS Institute Inc., Cary, NC).
Results
Participation and demographics
By January 2023, 688 pregnant women had been enrolled in the IKIDS cohort, and 153 withdrew or became ineligible before or at the time of birth, resulting in 535 infants born and enrolled in the study (Fig. 1). Three children did not have exposure data available. Demographic information for the 532 participants with an infant enrolled in IKIDS and exposure data available was largely similar to those who completed the CDI at 26.5–28.5 months (n = 298) or the SLAS at 36–38 months (n = 254), although more were white, non-Hispanic, had attained at least a bachelor’s degree, gave birth vaginally, and had lower mean PSS and EPDS scores during pregnancy in each subsample (Tables 1–2). Participants who reported taking acetaminophen during pregnancy generally did not differ from those who did not (Supplementary Table S2). However, more participants who took acetaminophen were white, non-Hispanic and spoke English as their native language compared to participants who did not take acetaminophen.
Fig. 1. Participant recruitment and inclusion in present analyses.
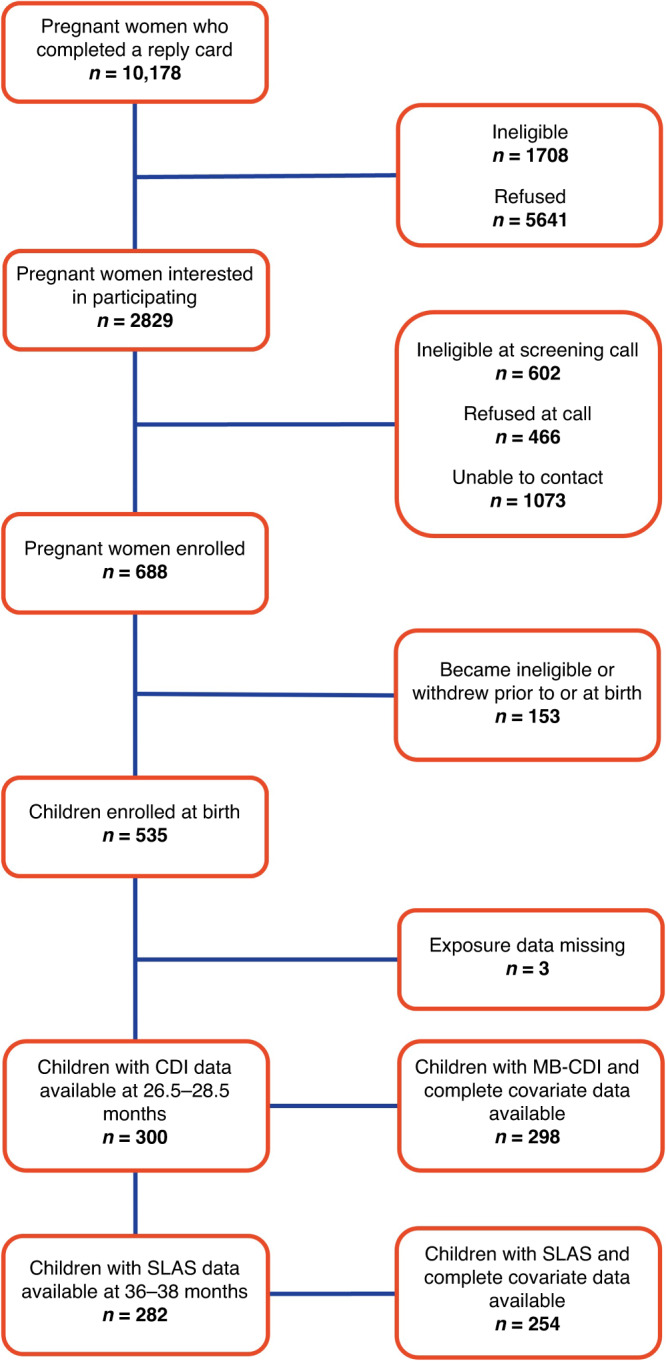
Flowchart of recruitment and retention to IKIDS study visits at 26.5–28.5 and 36–38 months as of January 2023.
Table 2.
Child demographics for all children enrolled in IKIDS with exposure data available (n = 532), those who provided language data at 26.5–28.5 months (CDI; n = 254), and those who provided language data at 36–38 months (SLAS; n = 254) by whether they reported taking acetaminophen during pregnancy or not.
| Child Characteristics | Participants with an infant enrolled in study & exposure data available | Participants who completed the CDI when their child was 26.5–28.5 months | Participants who completed the SLAS when their child was 36–38 months | |||||
|---|---|---|---|---|---|---|---|---|
| Participant did not take acetaminophen during pregnancy | Participant took acetaminophen during pregnancy | Participant did not take acetaminophen during pregnancy | Participant took acetaminophen during pregnancy | Participant did not take acetaminophen during pregnancy | Participant took acetaminophen during pregnancy | |||
| (n = 155) | (n = 377) | (n = 90) | (n = 208) | (n = 80) | (n = 174) | |||
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |||
| Child sex | ||||||||
| Male | 76 (49.0) | 181 (48.0) | 41 (45.6) | 110 (52.9) | 41 (51.3) | 82 (47.1) | ||
| Female | 79 (51.0) | 196 (52.0) | 49 (54.4) | 98 (47.1) | 39 (48.7) | 92 (52.9) | ||
| Child race & ethnicity | ||||||||
| White, Non-Hispanic | 107 (69.0) | 296 (78.5) | 65 (72.2) | 171 (82.2) | 61 (76.3) | 146 (83.9) | ||
| Other | 48 (31.0) | 79 (21.0) | 25 (27.8) | 37 (17.8) | 19 (23.7) | 27 (15.5) | ||
| Unknown/Missing | 0 (0.0) | 2 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.6) | ||
| Delivery type | ||||||||
| Vaginal | 106 (68.4) | 259 (68.7) | 64 (71.1) | 151 (75.6) | 60 (75.0) | 122 (70.1) | ||
| Cesarean section | 37 (23.9) | 102 (27.1) | 20 (22.2) | 53 (25.5) | 15 (18.8) | 47 (27.0) | ||
| Unknown/Missing | 12 (7.7) | 16 (4.2) | 6 (6.7) | 4 (1.9) | 5 (6.2) | 5 (2.9) | ||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | |||
| Child gestational age at birth (weeks) | 39.37 (1.57) | 39.27 (1.45) | 39.54 (1.32) | 39.32 (1.56) | 39.58 (1.33) | 39.32 (1.59) | ||
| Child weight at birth (kg) | 3.46 (0.45)a | 3.49 (0.42)b | 3.49 (0.44)d | 3.48 (0.43)e | 3.54 (0.47)f | 3.47 (0.41)q | ||
aChild weight at birth missing for n = 64.
bChild weight at birth missing for n = 27.
cChild weight at birth missing for n = 1.
dChild weight at birth missing for n = 19.
eChild weight at birth missing for n = 31.
fChild weight at birth missing for n = 16.
gChild weight at birth missing for n = 24.
Acetaminophen use during pregnancy
In IKIDS, 70.9% of participants used a medication containing acetaminophen at least once during pregnancy which is higher than reported in previous studies.1,4–6 Few participants took an analgesic other than acetaminophen during pregnancy (Supplementary Table S3). During the first trimester, 58.6% reported use, and fewer participants reported use in subsequent trimesters. Of the participants reporting any use of medications containing acetaminophen, most reported using acetaminophen itself rather than other medications containing acetaminophen, and most took acetaminophen for pain (Table 3). There was no difference in acetaminophen use between the subsets with CDI or SLAS data available and the full sample of children enrolled in IKIDS during the first, second, or third trimesters. Each subset took more acetaminophen throughout pregnancy than the full sample (p = 0.01; Supplementary Fig. S2), although the only significant difference was between the full cohort and the subset with SLAS data available (p = 0.007; Supplementary Table S4).
Table 3.
Maternal report of use of medications containing acetaminophen during pregnancy in the IKIDS cohort.
| Participants with an infant enrolled in study & exposure data available | Participants who completed the CDI when their child was 26.5-28.5 months | |||||||
|---|---|---|---|---|---|---|---|---|
| (n = 532) | (n = 298) | |||||||
| 1st Trimester | 2nd Trimester | 3rd Trimester | Entire Pregnancy | 1st Trimester | 2nd Trimester | 3rd Trimester | Entire Pregnancy | |
| N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | N (%) | |
| None | 220 (41.4) | 249 (46.8) | 338 (63.5) | 155 (29.1) | 129 (43.3) | 148 (49.7) | 197 (66.1) | 90 (30.2) |
| Any | 312 (58.6) | 283 (53.2) | 194 (36.5) | 377 (70.9) | 169 (56.7) | 150 (50.3) | 101 (33.9) | 208 (69.8) |
| Mean (SD) number of times taken | 17.9 (39.7) | 22.0 (44.1) | 15.1 (25.6) | 39.1 (75.1) | 14.5 (26.8) | 20.1 (44.9) | 13.2 (21.6) | 32.7 (66.0) |
| Median (IQR) number of times taken | 5.0 (14.0) | 7.5 (15.0) | 5.0 (12.0) | 14.0 (31.8) | 6.0 (13.5) | 7.0 (13.8) | 4.8 (11.3) | 12.4 (29.6) |
| Range number of times taken | 0.5–314.0 | 0.5–350.0 | 0.5–153.0 | 1.0–641.0 | 1.0–248.5 | 0.5–339.0 | 0.5–119.5 | 1.0–550.5 |
| Formulation | ||||||||
| Paracetamol alone | 258 (82.7) | 229 (80.9) | 158 (81.4) | 287 (76.1) | 140 (82.8) | 121 (80.7) | 83 (82.2) | 158 (76.0) |
| Paracetamol in combination | 25 (8.0) | 19 (6.7) | 17 (8.8) | 26 (6.9) | 16 (9.5) | 15 (10.0) | 10 (9.9) | 19 (9.1) |
| with other active ingredients | ||||||||
| Multiple formulations | 29 (9.3) | 35 (12.4) | 19 (9.8) | 64 (17.0) | 13 (7.7) | 14 (9.3) | 8 (7.9) | 31 (14.9) |
| Indication | ||||||||
| Pain | 214 (68.6) | 202 (71.4) | 134 (69.1) | 235 (62.3) | 110 (65.1) | 102 (68.0) | 68 (67.3) | 118 (56.7) |
| Other | 39 (12.5) | 31 (11.0) | 26 (13.4) | 54 (14.3) | 23 (13.6) | 20 (13.3) | 14 (13.9) | 36 (17.3) |
| Multiple reasons given | 27 (8.7) | 28 (9.9) | 19 (9.8) | 61 (16.2) | 16 (9.5) | 12 (8.0) | 10 (9.9) | 36 (17.3) |
| Missing | 32 (10.3) | 22 (7.8) | 15 (7.7) | 27 (7.2) | 20 (11.8) | 16 (10.7) | 9 (8.9) | 18 (8.7) |
| Participants who completed the SLAS when their child was 36–38 months | ||||
|---|---|---|---|---|
| (n = 254) | ||||
| 1st Trimester | 2nd Trimester | 3rd Trimester | Entire Pregnancy | |
| N (%) | N (%) | N (%) | N (%) | |
| None | 111 (43.7) | 123 (48.4) | 169 (66.5) | 80 (31.5) |
| Any | 143 (56.3) | 131 (51.6) | 85 (33.5) | 174 (68.5) |
| Mean (SD) number of times taken | 13.2 (27.1) | 21.9 (47.6) | 14.7 (24.2) | 34.5 (70.2) |
| Median (IQR) number of times taken | 4.5 (11.0) | 8.0 (14.5) | 5.0 (12.0) | 13.0 (29.0) |
| Range number of times taken | 1.0–248.5 | 0.5–339.0 | 0.5–119.5 | 1.0–550.5 |
| Formulation | ||||
| Acetaminophen alone | 118 (82.5) | 105 (80.2) | 69 (81.2) | 133 (76.4) |
| Acetaminophen in combination | 15 (10.5) | 13 (9.9) | 9 (10.6) | 16 (9.2) |
| with other active ingredients | ||||
| Multiple formulations | 10 (7.0) | 13 (9.9) | 7 (8.2) | 25 (14.4) |
| Indication | ||||
| Pain | 92 (64.3) | 86 (65.6) | 56 (65.9) | 96 (55.2) |
| Other | 22 (15.4) | 18 (13.7) | 12 (14.1) | 31 (17.8) |
| Multiple reasons given | 10 (7.0) | 12 (9.2) | 7 (8.2) | 29 (16.7) |
| Missing | 19 (13.3) | 15 (11.5) | 10 (11.8) | 18 (10.3) |
SD standard deviation, IQR interquartile range.
Language development
As of January 2023, 298 children had CDI outcome data (147 males, 151 females). Most children were 26.5–28.5 months of age with an average age of 27.42 (±1.08) months at the time of assessment. Data for 41 children were collected outside this age range. Not all participants completed all three outcome measures. As expected, females were more advanced in language development than males at this age (vocabulary p < 0.0001, M3L p = 0.0003, complexity p = 0.003, Supplementary Fig. S3, Table S5). Most children who had SLAS data available (n = 254; 133 females, 121 males) were 36–38 months at the time of assessment, with an average age of 37.52 (±1.00) months and data for 30 children collected outside this assessment window. Females had higher scores than males, although the sex difference was not as pronounced at this age (p = 0.07, Supplementary Fig. S4, Table S5).
Associations with CDI outcomes at 26.5–28.5 months
Generally, an increase in the number of times acetaminophen was taken during pregnancy was associated with lower vocabulary size, M3L, and complexity at 26.5–28.5 months of age (Fig. 2, Table 4, Supplementary Figs. S5–6). Associations of acetaminophen use during pregnancy with these outcomes did not differ by child sex. Per unit increase in acetaminophen use (i.e., report of number of times taking acetaminophen) during the second and third trimester, children showed a decrease in vocabulary size of 0.58 (95% CI: −1.13, −0.04) and of 1.83 words (95% CI: −3.13, −0.54), respectively. There was also an association of the number of times acetaminophen was taken across the entire pregnancy with smaller vocabularies (β = −0.35 words, 95% CI: −0.67, 0.03). Similar findings were observed for associations between acetaminophen use and M3L. An increase in acetaminophen use during the second or third trimester was associated with a small decrease in M3L (β = −0.007 morphemes, 95% CI: −0.01, 0.001; β = −0.02 morphemes, 95% CI: −0.04, 0.003). There were no associations of acetaminophen use during the first trimester with any outcomes, and complexity scores were not related to acetaminophen use during pregnancy. Associations were largely unchanged in sensitivity analyses (Supplementary Table S6).
Fig. 2. Associations of prenatal acetaminophen exposure and language development outcomes on the CDIa.
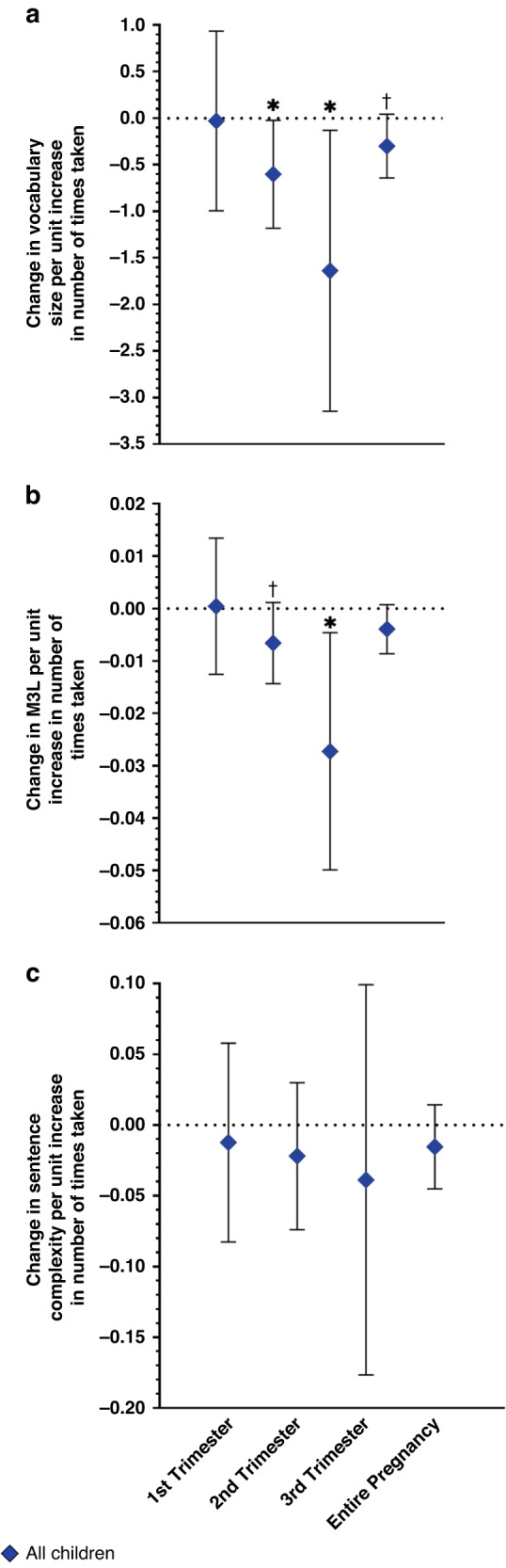
Associations of prenatal acetaminophen exposure and (a) change in vocabulary count (number of words produced), (b) mean length of three longest utterances (number of morphemes produced, M3L), and (c) complexity (number of more complex sentences produced) measured using the CDI at 26.5–28.5 months. More acetaminophen use during the second and third trimesters, and throughout pregnancy, was associated with a decrease in vocabulary size. Increased acetaminophen use in the second and third trimesters was associated with a decrease in M3L. Prenatal acetaminophen exposure was not related to complexity scores. aModels were adjusted for child sex, age at assessment, maternal parity (nulliparous vs. ≥1), maternal education (<bachelor’s degree vs. ≥bachelor’s degree), and mean stress and depression scores during pregnancy. †p < 0.10, *p < 0.05
Table 4.
Multivariable linear regression analyses of the relation (β estimate and 95% confidence interval) of prenatal acetaminophen exposure with language outcomes on the MB-CDI at 2 years.
| Outcome | Stratified by Sex | |||
|---|---|---|---|---|
| Unadjusteda | Exposure | Main Effect | Females | Males |
| Vocabulary | 1st Trimester | −0.44 (−1.31, 0.43) | −0.66 (−2.18, 0.85) | −0.33 (−1.39, 0.73) |
| 2nd Trimester | *−0.69 (−1.24, −0.14) | −0.83 (−1.57, −0.09) | −0.51 (−1.34, 0.31) | |
| 3rd Trimester | **−1.95 (−3.26, −0.65) | −1.89 (−3.59, −0.19) | −2.05 (−4.09, 0.003) | |
| Entire Pregnancy | *−0.41 (−0.74, −0.09) | −0.50 (−0.94, −0.05) | −0.33 (−0.79, 0.13) | |
| Mean Length of Utterance | 1st Trimester | −0.005 (−0.02, 0.01) | −0.004 (−0.03, 0.02) | −0.01 (−0.02, 0.01) |
| 2nd Trimester | †−0.007 (−0.01, 0.001) | −0.01 (−0.02, 0.002) | −0.005 (−0.02, 0.01) | |
| 3rd Trimester | *−0.02 (−0.04, −0.0004) | −0.02 (−0.05, 0.001) | −0.02 (−0.05, 0.02) | |
| Entire Pregnancy | †−0.004 (−0.01, 0.001) | −0.005 (−0.01, 0.001) | −0.003 (−0.01, 0.004) | |
| Complexity | 1st Trimester | −0.04 (−0.11, 0.02) | −0.05 (−0.16, 0.07) | −0.04 (−0.12, 0.03) |
| 2nd Trimester | −0.02 (−0.07, 0.02) | −0.03 (−0.11, 0.06) | −0.02 (−0.08, 0.04) | |
| 3rd Trimester | −0.04 (−0.15, 0.07) | −0.01 (−0.16, 0.14) | −0.08 (−0.26, 0.09) | |
| Entire Pregnancy | −0.02 (−0.04, 0.01) | −0.02 (−0.06, 0.03) | −0.02 (−0.05, 0.01) | |
| Adjustedb | ||||
| Vocabulary | 1st Trimester | −0.28 (−1.14, 0.58) | −0.48 (−1.97, 1.02) | −0.18 (−1.23, 0.87) |
| 2nd Trimester | *−0.58 (−1.13, −0.04) | −0.78 (−1.52, −0.05) | −0.33 (−1.16, 0.50) | |
| 3rd Trimester | **−1.83 (−3.13, −0.54) | −1.78 (−3.46, −0.11) | −1.91 (−3.93, 0.11) | |
| Entire Pregnancy | *−0.35 (−0.67, −0.03) | −0.45 (−0.90, −0.01) | −0.24 (−0.70, 0.22) | |
| Mean Length of Utterance | 1st Trimester | −0.002 (−0.01, 0.01) | −0.002 (−0.02, 0.02) | −0.003 (−0.02, 0.01) |
| 2nd Trimester | †−0.01 (−0.01, 0.001) | −0.01 (−0.02, 0.001) | −0.003 (−0.02, 0.01) | |
| 3rd Trimester | *−0.02 (−0.04, −0.003) | −0.02 (−0.05, −0.001) | −0.02 (−0.06, 0.01) | |
| Entire Pregnancy | −0.004 (−0.01, 0.001) | −0.01 (−0.01, 0.001) | −0.002 (−0.01, 0.005) | |
| Complexity | 1st Trimester | −0.03 (−0.10, 0.03) | −0.04 (−0.15, 0.07) | −0.03 (−0.10, 0.05) |
| 2nd Trimester | −0.02 (−0.07, 0.03) | −0.02 (−0.10, 0.06) | −0.01 (−0.07, 0.05) | |
| 3rd Trimester | −0.04 (−0.15, 0.08) | −0.0003 (−0.15, 0.15) | −0.08 (−0.26, 0.09) | |
| Entire Pregnancy | −0.01 (−0.04, 0.01) | −0.01 (−0.06, 0.03) | −0.01 (−0.05, 0.02) | |
aUnadjusted models were adjusted for child sex and age.
bAll models were adjusted for child sex and age at assessment, maternal parity, maternal education, mean perceived stress during pregnancy, and mean depression during pregnancy.
†p < 0.10; *p < 0.05; **p < 0.01.
In this cohort, 80 children (27%) had vocabulary scores ≤25th percentile, 44 (15%) had M3L scores ≤25th percentile, and 32 (11%) had complexity scores ≤25th percentile for their sex and age. Results of the logistic regression analyses were more mixed, but most showed that an increase in the number of times acetaminophen was taken during pregnancy was associated with an elevated odds of children having vocabulary, M3L, and complexity scores ≤25th percentile (Fig. 3, Table 5). In stratified analyses, increasing acetaminophen use was associated with moderately elevated odds of both M3L (OR = 1.07, 95% CI: 1.01, 1.09) and complexity (OR = 1.07, 95% CI: 1.01, 1. 13) scores ≤25th percentile specifically in male children. Associations were generally unchanged in sensitivity analyses (Supplementary Table S7).
Fig. 3. Odds of CDI scores ≤25th percentile in relation to prenatal acetaminophen exposurea.
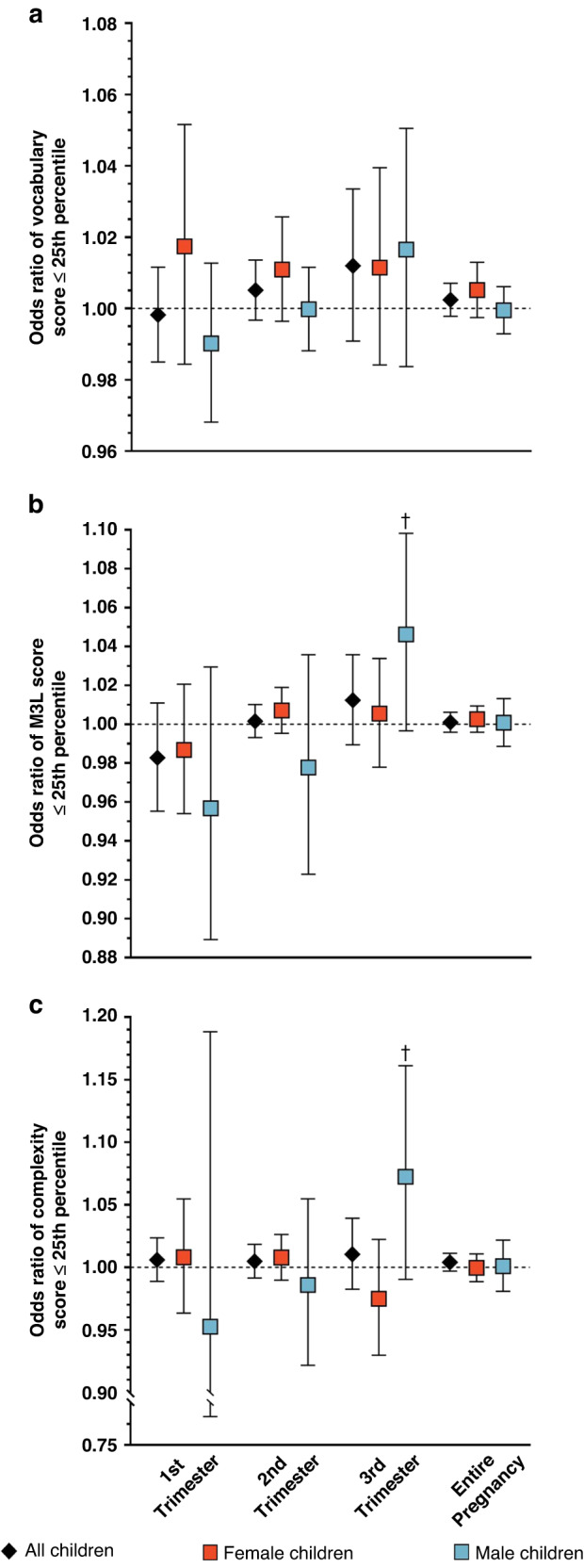
Odds ratios and 95% confidence intervals for the relationship of prenatal acetaminophen exposure and (a) vocabulary size, (b) mean length of three longest utterances (M3L), and (c) complexity ≤25th percentile measured using the CDI at 26.5–28.5 months. The number of times acetaminophen was taken was not related to the odds of children having vocabulary scores ≤25th percentile. However, the number of times acetaminophen was taken during the third trimester was associated with slightly increased odds of male children having M3L and complexity scores ≤25th percentile. aModels were adjusted for maternal parity (nulliparous vs. ≥1), maternal education (<bachelor’s degree vs. ≥bachelor’s degree), and mean stress and depression scores during pregnancy. †p < 0.10.
Table 5.
Odds ratios (odds, 95% CI) of children with prenatal acetaminophen exposure having a percentile score ≤25th percentile adjusted for child sex and age.
| Outcome | Stratified by Sex | |||
|---|---|---|---|---|
| Unadjusted | Exposure | Main Effect | Females | Males |
| Vocabulary | 1st Trimester | 1.003 (0.99, 1.01) | †1.02 (0.99, 1.04) | 0.99 (0.98, 1.01) |
| 2nd Trimester | 1.01 (0.99, 1.01) | 1.01 (0.99, 1.02) | 1.004 (0.99, 1.01) | |
| 3rd Trimester | †1.02 (0.99, 1.03) | 1.02 (0.99, 1.04) | 1.01 (0.99, 1.04) | |
| Entire Pregnancy | 1.003 (0.99, 1.01) | †1.01 (0.99, 1.01) | 1.001 (0.99, 1.01) | |
| Mean Length of Utterance | 1st Trimester | 0.996 (0.98, 1.01) | 0.99 (0.97, 1.02) | 0.99 (0.96, 1.02) |
| 2nd Trimester | 1.004 (0.99, 1.01) | 1.01 (0.99, 1.02) | 0.99 (0.98, 1.02) | |
| 3rd Trimester | †1.02 (0.99, 1.04) | 1.01 (0.99, 1.03) | †1.03 (0.99, 1.07) | |
| Entire Pregnancy | 1.002 (0.99, 1.01) | 1.003 (0.99, 1.01) | 1.001 (0.99, 1.01) | |
| Complexity | 1st Trimester | 1.003 (0.99, 1.02) | 1.01 (0.99, 1.03) | 0.93 (0.77, 1.12) |
| 2nd Trimester | 1.001 (0.99, 1.01) | 1.01 (0.99, 1.02) | 0.99 (0.92, 1.06) | |
| 3rd Trimester | 1.02 (0.99, 1.04) | 1.003 (0.97, 1.03) | *1.04 (1.01, 1.09) | |
| Entire Pregnancy | 1.002 (0.99, 1.01) | 1.004 (0.99, 1.01) | 1.00 (0.99, 1.01) | |
| Adjusteda | ||||
| Vocabulary | 1st Trimester | 1.002 (0.99, 1.01) | 1.02 (0.99, 1.04) | 0.99 (0.98, 1.01) |
| 2nd Trimester | 1.01 (0.99, 1.01) | 1.01 (0.99, 1.02) | 1.003 (0.99, 1.01) | |
| 3rd Trimester | †1.02 (0.99, 1.03) | 1.02 (0.99, 1.04) | 1.01 (0.99, 1.04) | |
| Entire Pregnancy | 1.003 (0.99, 1.01) | 1.01 (0.99, 1.01) | 1.001 (0.99, 1.01) | |
| Mean Length of Utterance | 1st Trimester | 0.99 (0.98, 1.01) | 0.99 (0.97, 1.02) | 0.99 (0.96, 1.03) |
| 2nd Trimester | 1.003 (0.99, 1.01) | 1.004 (0.99, 1.01) | 0.99 (0.98, 1.02) | |
| 3rd Trimester | †1.02 (0.997, 1.04) | 1.01 (0.98, 1.03) | *1.05 (1.01, 1.09) | |
| Entire Pregnancy | 1.002 (0.99, 1.01) | 1.002 (0.99, 1.01) | 1.003 (0.99, 1.01) | |
| Complexity | 1st Trimester | 1.01 (0.99, 1.02) | 1.01 (0.99, 1.03) | 0.95 (0.78, 1.15) |
| 2nd Trimester | 1.003 (0.99, 1.02) | 1.003 (0.99, 1.02) | 0.99 (0.93, 1.05) | |
| 3rd Trimester | 1.02 (0.99, 1.04) | 1.003 (0.97, 1.04) | *1.07 (1.01, 1.13) | |
| Entire Pregnancy | 1.003 (0.99, 1.01) | 1.003 (0.99, 1.01) | 1.004 (0.99, 1.02) | |
aAll models were adjusted for maternal parity, maternal education, mean perceived stress during pregnancy, and mean depression during pregnancy.
†p < 0.10; *p < 0.05; **p < 0.01.
Associations with total SLAS score at 36–38 months
An increase in acetaminophen use during pregnancy was generally associated with lower total SLAS scores at 36–38 months of age, and associations mostly did not differ by child sex (Fig. 4, Table 6, Supplementary Fig. S7). An increase in acetaminophen use during the second (β = −0.04, 95% CI: −0.09, 0.007) and third trimesters (β = −0.15, 95% CI: −0.26, −0.04), as well as across the entire pregnancy (β = −0.03, 95% CI: −0.06, −0.001) was associated with a decrease in SLAS scores. The sex-by-acetaminophen use interaction was p < 0.10 only for third trimester exposure, with males having significantly lower SLAS scores (β = −0.27, 95% CI: −0.43, −0.11; Supplementary Fig. S8) compared to females (β = −0.04, 95% CI: −0.20, 0.11). Associations were not substantially altered in sensitivity analyses (Supplementary Table S8).
Fig. 4. Associations of prenatal acetaminophen exposure and SLAS total score at 36–38 months by trimester of exposurea.
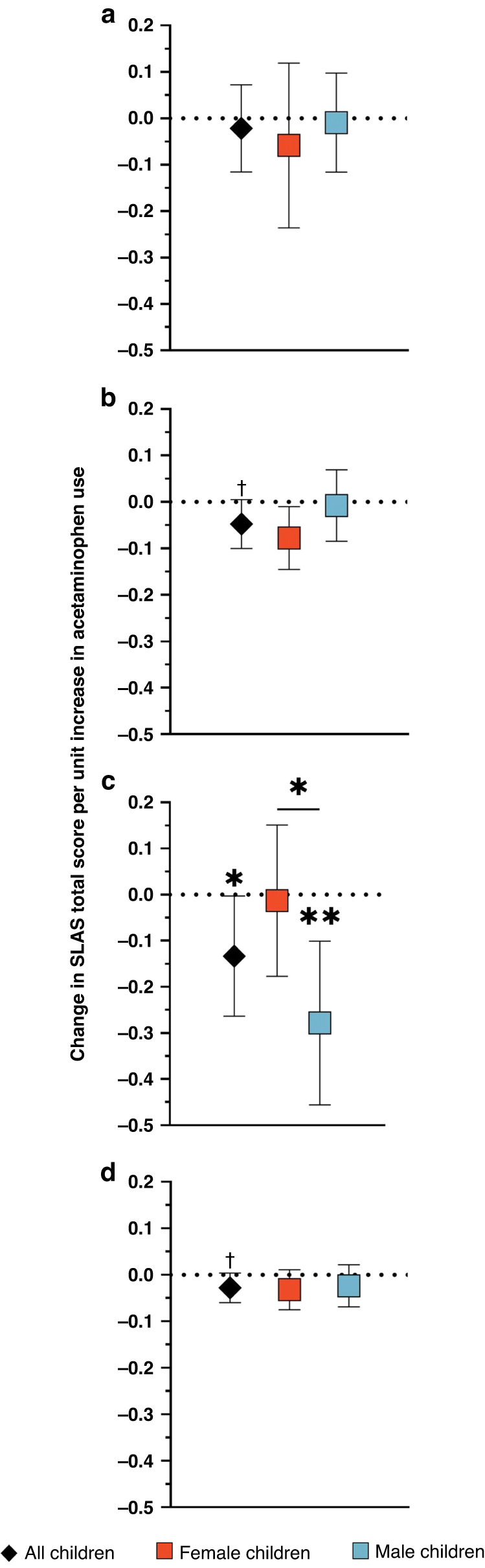
More acetaminophen use during (a) the first trimester was not related to SLAS scores. More frequent use during the (b) second and (c) third trimesters, and (d) throughout pregnancy, was associated with lower SLAS total scores in all children. More acetaminophen use during third trimester was also related to a decrease in total SLAS scores in male children specifically. aModels were adjusted for child sex and mean stress and depression scores during pregnancy. †p < 0.10, *p < 0.05, **p < 0.01.
Table 6.
Multivariable linear regression analyses of the relation (β estimate and 95% confidence interval) of prenatal acetaminophen exposure with language outcomes on the SLAS at 3 years.
| Unadjusteda | Stratified by Sex | ||
|---|---|---|---|
| Exposure | Main Effect | Females | Males |
| 1st Trimester | †−0.07 (−0.16, 0.01) | −0.12 (−0.27, 0.03) | −0.05 (−0.15, 0.06) |
| 2nd Trimester | *−0.06 (−0.11, −0.01) | −0.09 (−0.15, −0.02) | −0.03 (−0.10, 0.05) |
| 3rd Trimester | **−0.18 (−0.29, −0.06) | ‡−0.08 (−0.24, 0.08) | **−0.29 (−0.45, −0.12) |
| Entire Pregnancy | **−0.04 (−0.07, −0.01) | −0.05 (−0.09, −0.01) | −0.04 (−0.08, 0.01) |
| Adjustedb | |||
| 1st Trimester | −0.04 (−0.13, 0.04) | −0.11 (−0.25, 0.04) | −0.01 (−0.11, 0.09) |
| 2nd Trimester | †−0.04 (−0.09, 0.01) | −0.07 (−0.14, −0.01) | −0.002 (−0.08, 0.07) |
| 3rd Trimester | **−0.15 (−0.26, −0.04) | §−0.04 (−0.198, 0.11) | **−0.27 (−0.43, −0.11) |
| Entire Pregnancy | *−0.03 (−0.06, −0.001) | −0.04 (−0.08, 0.001) | −0.02 (−0.07, 0.02) |
aUnadjusted models were adjusted for child sex and age.
bAll models were adjusted for child sex, maternal education, mean perceived stress during pregnancy, and mean depression during pregnancy.
Interaction p-values: ‡p < 0.10, §p < 0.05.
*p < 0.05; **p < 0.01.
Discussion
Generally, more acetaminophen use during pregnancy was associated with modest decreases in early childhood language outcomes in this largely white, non-Hispanic, well-educated, and high-income cohort. Sex-specific associations were observed between increased acetaminophen use late in pregnancy and language outcomes in male children. Increased frequency of acetaminophen use during the second and third trimester were associated with slightly smaller vocabularies and shorter M3L when children were 26.5–28.5 months. An increase in the total number of times acetaminophen was taken throughout pregnancy was also related to slightly shorter M3L. Additionally, increased use during the third trimester was related to elevated odds of male children having M3L and complexity scores ≤25th percentile. Finally, increases in the number of times acetaminophen was taken during the second and third trimesters were related to small decreases in total SLAS scores in male children.
These results align with previous research examining the relationship between prenatal acetaminophen exposure and communication or language outcomes. Using samples of the same cohort, two studies found that acetaminophen exposure during pregnancy was associated with poorer communication skills at 3 years.4,49 A third observed a similar association earlier in children 18 months of age;31 however, all three studies used the ASQ. While there is substantial evidence that the ASQ has high specificity, reliability, and validity,57–60 the communication section has lower specificity, sensitivity, and accuracy than the CDI,61,62 and does not evaluate language development as extensively or in as much detail as the CDI. Only one previous study observed sex-specific effects, but, unlike this study, they found greater language delays in female children according to a routine nurse evaluation at 30 months.48 This difference may be related to the timing of exposure as that study only examined acetaminophen exposure during the first trimester, and the associations with greater acetaminophen use observed here for male children were only found during the third trimester. This suggests that additional research examining the timing of prenatal acetaminophen use in relation to child sex is necessary to better understand the role trimester of use plays in sex-specific associations with poorer language outcomes.
These results also suggest that the second and third trimesters may be windows of neurodevelopment particularly sensitive to disruption of language development by prenatal acetaminophen exposure. Few previous studies have evaluated whether timing of acetaminophen use during pregnancy played a role, but similarly, Brandlistuen et al. (2013)4 observed an association of more days of acetaminophen exposure during the third trimester with poorer communication skills in 3-year-old children. Several structures important in auditory pathways in the brain rapidly develop during the second trimester of fetal development, which could be why acetaminophen use during this period was associated with measures of language development.63–65 In particular, the tympanic membrane,66 middle ear,67,68 and much of the inner ear are rapidly developing during the second trimester,65,69–71 at which time, fetuses also begin responding to sound.71–73 Axons of brainstem auditory neurons also mature during the second trimester,65,74,75 followed by myelination, which begins towards the end of the second trimester.65,76,77 At the beginning of the third trimester, axonal conduction time along the auditory nerve rapidly develops, at which time hearing onset is reliably observed in healthy fetuses, and conduction time in brainstem pathways begins to mature.65,74,77 During the second trimester, the cortex develops from a thin-walled vesicle lined with densely packed immature neurons into a structure more similar to a mature cortex with distinct cortical layers.65,74 As cortical neurons continue to develop during the third trimester, the temporal lobe forms into a distinct structure with the primary auditory cortex contained within Heschl’s gyrus and the secondary auditory cortex, which includes Wernicke’s area, in the superior temporal gyrus.65,78 All of this lays the groundwork for further development and maturation necessary for language processing and acquisition. Recent evidence suggests that the analgesic effect of acetaminophen occurs via the endocannabinoid system, which plays an important role in multiple aspects of neurodevelopment, including cell differentiation, migration, and synaptogenesis.79 Thus, use of acetaminophen during the second or third trimester may interfere with the development of auditory structures and pathways via the endocannabinoid system.
Because of the way the acetaminophen use was ascertained, previous studies have not been able to examine associations by trimester, whereas participants in the current study reported acetaminophen use six times across pregnancy, allowing for evaluation by trimester of use, and a reduced risk of inaccuracies in reporting. There were clear associations of increased frequency of acetaminophen use with poorer language outcomes despite the small sample size of the current study. However, given the importance of acetaminophen as an analgesic, these findings should be interpreted cautiously until they are replicated in a larger and more diverse sample. The changes in language scores were also small with the largest being a decrease in vocabulary size by almost 2 words per number of times acetaminophen was taken during the third trimester. This suggests that if a pregnant person took acetaminophen thirteen times (or approximately once per week) during the third trimester, their child may express approximately 26 fewer words than other children of the same age.51 Similarly, the child may produce 0.33 fewer morphemes, and if the child was male, they would have 65% greater odds of having a M3L score ≤25th percentile and a 91% greater odds of having a complexity score ≤25th percentile. For the same scenario, children could have a nearly 2-point decrease in total SLAS scores, or a 3.6-point decrease if the child was male. While the estimates are small, the majority of participants in this cohort are of higher socioeconomic status, and individuals of lower socioeconomic status may show larger deficits.80,81 Further, even small effects measured in a sample may have a substantial impact at the population level, particularly when the exposure is common.82
While the CDI asks caregivers to evaluate the child’s language objectively, the SLAS asks parents to rate their child’s language skills relative to other children of the same age. Despite differences in the assessment measures used in this study, increased use of acetaminophen during pregnancy was associated with lower scores on both measures. Previous studies have demonstrated that parents are fairly accurate in their assessment of their child’s language abilities,50,83,84 and the initial studies introducing the SLAS also found that parental and Speech Language Pathologist ratings of a child’s language abilities using the SLAS were highly correlated with one another.51 Additionally, the SLAS has since been selected as a recommended protocol by the PhenX (consensus measures for Phenotypes and eXposures) Toolkit,85 a database of measures for a variety of developmental domains put together by expert review panels intended to facilitate research across studies, because it is short, easy to use and interpret, and is both well-established and validated.86
Strengths and limitations
This study has several strengths. First, it utilizes data from an ongoing prospective birth cohort study which continues to follow these children. Second, this study ascertained acetaminophen use multiple times during pregnancy, reducing the risk of memory inaccuracies, and allowing for consideration of acetaminophen use by trimester. Results were also robust to several sensitivity analyses, suggesting these associations may not be driven by extreme values for the number of times acetaminophen was taken. Another strength is the use of multiple measures of language development at different ages. This study is the first to our knowledge to utilize the CDI or SLAS to evaluate associations of acetaminophen use during pregnancy with language development in children. CDI scores at 2 years have been shown to be highly correlated with SLAS scores later in early childhood, and both measures have also been shown to be consistent with children’s performance on other standardized language assessments.53,54
This study also has limitations. Many analyses were conducted without correction for multiple comparisons. In epidemiology, it has been argued that the focus should be on looking for trends in results to better-inform future research,87 which was the approach taken here. Second, this cohort is relatively homogenous, limiting the generalizability of the findings. As is the case with cohort studies, this study is also subject to loss to follow-up, with participants remaining in the study tending to be white, non-Hispanic, well-educated with a high annual household income, and have lower EPDS and PSS scores. In this analysis, only 56% of participants with an infant enrolled at birth contributed CDI data, and while not all children enrolled had yet reached the 36-38 month assessment, only 48% contributed SLAS data. There is also a risk of dependent error due to the mother reporting both the exposure and, in almost all cases, outcomes. While there were multiple times of self-report of medication use during pregnancy, it is likely these reports were not entirely accurate due to some inaccuracies in memory. Additionally, dosage information was not collected and thus could not be assessed in this study. Unfortunately, whether participants experienced the indications (such as pain) but did not take acetaminophen or another analgesic was not available, and therefore could not be investigated. As such, the indications participants provided for taking acetaminophen cannot be ruled out as potentially contributing to the lower scores observed in this study. To our knowledge, the relationship of pain during pregnancy with neurodevelopment has not yet been investigated, but inflammation is commonly associated with pain as well as infections. Both inflammation and infections during pregnancy have been repeatedly linked to Autism Spectrum Disorder (ASD), a condition in which language is often impaired.88 However, one study found that diagnosed infection during pregnancy was not associated with ASD but treatment for infection was, and the authors note that the relationship of prenatal infection, treatment, and ASD could not be disentangled.89 Although a few studies have attempted to utilize biomarkers of acetaminophen exposure in maternal urine, maternal plasma, umbilical cord blood, or meconium, these have their own limitations.6,40,48,90,91 One promising, yet un-investigated, biomarker is shed teeth in which acetaminophen use during infancy can be measured.92 This should be explored as a biomarker of prenatal acetaminophen exposure in the future.83 Despite these limitations, the results of this study suggest that the impact of acetaminophen use during pregnancy on child neurodevelopment should be further investigated.
Importance of findings
Acetaminophen is the most common drug ingredient on the market, and one of very few medications considered safe for use throughout pregnancy.1 However, there is a growing body of literature indicating that its use during pregnancy may be related to poorer neurodevelopmental outcomes. As recently concluded in a systematic review, the idea that it is safe for use has been taken for granted for many years without any demonstration of its safety regarding neurodevelopment.8 This study provides additional evidence that more acetaminophen use during pregnancy is related to poorer language development93 and highlights the need for further investigation of the potential mechanisms through which prenatal acetaminophen exposure may impact neurodevelopment, as well as further investigation using larger and more diverse cohort studies to establish whether there is strong causal evidence for this association.
Supplementary information
Acknowledgements
Thank you to Dr. Brian Monson for his valuable feedback on the discussion, and to all IKIDS participants and their families and IKIDS staff for their continued dedication to this study.
Author contributions
IKIDS was initiated in 2013 by senior investigator S.S. at the University of Illinois in Urbana-Champaign with funding from the Children’s Environmental Health and Disease Prevention Research Center in the United States and continues to be funded by the National Institutes of Health Environmental Influences on Child Health Outcomes (ECHO) Program. M.L.W., P.C., S.N., P.A.H., and S.L.S. contributed to the ideas and design of the study. Under the supervision of S.L.S., M.L.W. was involved in data collection and preparation and led the data analysis, interpretation of results, and writing of the initial and revised drafts of the manuscript. S.N. aided in the data preparation, and P.C. helped with data analysis. P.A.H. and S.N. provided feedback on the data analysis and interpretation of the results. P.C., S.N., P.A.H., and S.L.S. helped revise the manuscript.
Funding
This work was supported by the Children’s Environmental Health and Disease Prevention Research Center funded by the National Institute of Environmental Health Sciences (grant number P01 ES022848) and the U.S. Environmental Protection Agency (grant number RD83543401), the National Institutes of Health Institutional Training Grant Predoctoral Traineeship in Endocrine, Developmental & Reproductive Toxicology (grant number T32 ES007326), and the National Institutes of Health Environmental Influences on Child Health Outcomes (ECHO) Program (grant number OD023272). Open access funding provided by Northeastern University Library.
Data availability
The data used in these analyses cannot be shared publicly to ensure the anonymity of participants. Underlying code is available upon request.
Competing interests
The authors declare no competing interests.
Ethics approval
University of Illinois at Urbana-Champaign Institutional Review Board (IRB) protocol code 09498.
Informed consent
Written informed consent was obtained from participants during pregnancy and at each time of assessment (when children were 26.5–28.5 and 36–38 months old).
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
The online version contains supplementary material available at 10.1038/s41390-023-02924-4.
References
- 1.Servey J, Chang J. Over-the-counter medications in pregnancy. Am. Fam. Physician. 2014;90:548–555. [PubMed] [Google Scholar]
- 2.ACOG Response to Consensus Statement on Paracetamol Use During Pregnancy. The American College of Obstetrics and Gynecologists (2021).
- 3.Bisson DL, Newell SD, Laxton C, Royal College of Obstetricians and Gynaecologists Antenatal and Postnatal Analgesia. Scientific Impact Paper No. 59. BJOG. 2019;126:e115–e124. doi: 10.1111/1471-0528.15510. [DOI] [PubMed] [Google Scholar]
- 4.Brandlistuen RE, Ystrom E, Nulman I, Koren G, Nordeng H. Prenatal paracetamol exposure and child neurodevelopment: a sibling-controlled cohort study. Int J. Epidemiol. 2013;42:1702–1713. doi: 10.1093/ije/dyt183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Thorpe PG, et al. Medications in the first trimester of pregnancy: most common exposures and critical gaps in understanding fetal risk. Pharmacoepidemiol Drug Saf. 2013;22:1013–1018. doi: 10.1002/pds.3495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ji, Y. et al. Maternal biomarkers of acetaminophen use and offspring attention deficit hyperactivity disorder. Brain Sci.8, (2018). [DOI] [PMC free article] [PubMed]
- 7.Black RA, Hill DA. Over-the-counter medications in pregnancy. Am. Fam. Physician. 2003;67:2517–2524. [PubMed] [Google Scholar]
- 8.Cendejas-Hernandez, J. et al. Paracetamol (acetaminophen) use in infants and children was never shown to be safe for neurodevelopment: a systematic review with citation tracking. Eur. J. Pediatr.10.1007/S00431-022-04407-W (2022). [DOI] [PMC free article] [PubMed]
- 9.Thiele K, Kessler T, Arck P, Erhardt A, Tiegs G. Acetaminophen and pregnancy: short- and long-term consequences for mother and child. J. Reprod. Immunol. 2013;97:128–139. doi: 10.1016/j.jri.2012.10.014. [DOI] [PubMed] [Google Scholar]
- 10.Nitsche JF, et al. Transplacental passage of acetaminophen in term pregnancy. Am. J. Perinatol. 2017;34:541–543. doi: 10.1055/s-0036-1593845. [DOI] [PubMed] [Google Scholar]
- 11.Levy G, Garrettson LK, Soda DM. Letter: evidence of placental transfer of acetaminophen. Pediatrics. 1975;55:895. doi: 10.1542/peds.55.6.895. [DOI] [PubMed] [Google Scholar]
- 12.Smarr MM, et al. Comparison of fetal growth by maternal prenatal acetaminophen use. Pediatr. Res. 2019;86:261–268. doi: 10.1038/s41390-019-0379-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Castro CT, et al. Effect of Acetaminophen use during pregnancy on adverse pregnancy outcomes: a systematic review and meta-analysis. Expert Opin. Drug Saf. 2022;21:241–251. doi: 10.1080/14740338.2022.2020246. [DOI] [PubMed] [Google Scholar]
- 14.de Castro, C. T., Pereira, M. & dos Santos, D. B. Association between paracetamol use during pregnancy and perinatal outcomes: prospective NISAMI cohort. PLoS One17, (2022). [DOI] [PMC free article] [PubMed]
- 15.Rebordosa C, Kogevinas M, Bech BH, Sørensen HT, Olsen J. Use of acetaminophen during pregnancy and risk of adverse pregnancy outcomes. Int J. Epidemiol. 2009;38:706–714. doi: 10.1093/ije/dyp151. [DOI] [PubMed] [Google Scholar]
- 16.Labba, N. A. et al. Paracetamol perturbs neuronal arborization and disrupts the cytoskeletal proteins SPTBN1 and TUBB3 in both human and chicken in vitro models. Toxicol. Appl. Pharmacol.449, (2022). [DOI] [PubMed]
- 17.Herrington, J. A. et al. Elevated ghrelin alters the behavioral effects of perinatal acetaminophen exposure in rats. Dev. Psychobiol.64, (2022). [DOI] [PubMed]
- 18.Baker, B. H. et al. Sex-specific neurobehavioral and prefrontal cortex gene expression alterations following developmental acetaminophen exposure in mice. Neurobiol. Dis.177, (2022). [DOI] [PMC free article] [PubMed]
- 19.Khan, F. Y. et al. A systematic review of the link between autism spectrum disorder and acetaminophen: a mystery to resolve. Cureus14, (2022). [DOI] [PMC free article] [PubMed]
- 20.Patel, R., Sushko, K., van den Anker, J. & Samiee‐zafarghandy, S. Long-term safety of prenatal and neonatal exposure to paracetamol: a systematic review. Int. J. Environ. Res. Public Health19, (2022). [DOI] [PMC free article] [PubMed]
- 21.Kwok, J., Luedecke, E., Hall, H. A., Murray, A. L. & Auyeung, B. Analgesic drug use in pregnancy and neurodevelopment outcomes: an umbrella review. Neurosci. Biobehav. Rev.136, (2022). [DOI] [PubMed]
- 22.Patel, E. et al. The safety of pediatric use of paracetamol (acetaminophen): a narrative review of direct and indirect evidence. Minerva Pediatr.74, (2022). [DOI] [PubMed]
- 23.Duesman, S. J. The effects of maternal acetaminophen exposure on the development of ADHD-like behaviors in rat offspring. (Western Illinois University, 2015).
- 24.Blecharz-Klin K, et al. Paracetamol—the outcome on neurotransmission and spatial learning in rats. Behav. Brain Res. 2013;253:157–164. doi: 10.1016/j.bbr.2013.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Blecharz-Klin K, et al. Effect of prenatal and early life paracetamol exposure on the level of neurotransmitters in rats—focus on the spinal cord. Int. J. Dev. Neurosci. 2015;47:133–139. doi: 10.1016/j.ijdevneu.2015.09.002. [DOI] [PubMed] [Google Scholar]
- 26.Blecharz-Klin K, et al. Developmental exposure to paracetamol causes biochemical alterations in medulla oblongata. Environ. Toxicol. Pharm. 2015;40:369–374. doi: 10.1016/j.etap.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Blecharz-Klin K, et al. Early paracetamol exposure decreases brain-derived neurotrophic factor (BDNF) in striatum and affects social behaviour and exploration in rats. Pharm. Biochem Behav. 2018;168:25–32. doi: 10.1016/j.pbb.2018.03.004. [DOI] [PubMed] [Google Scholar]
- 28.Blecharz-Klin K, et al. Cerebellar level of neurotransmitters in rats exposed to paracetamol during development. Pharmacol. Rep. 2016;68:1159–1164. doi: 10.1016/j.pharep.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Blecharz-Klin K, et al. Paracetamol−effect of early exposure on neurotransmission, spatial memory and motor performance in rats. Behav. Brain Res. 2017;323:162–171. doi: 10.1016/j.bbr.2017.01.051. [DOI] [PubMed] [Google Scholar]
- 30.Philippot G, Gordh T, Fredriksson A, Viberg H. Adult neurobehavioral alterations in male and female mice following developmental exposure to paracetamol (acetaminophen): characterization of a critical period. J. Appl. Toxicol. 2017;37:1174–1181. doi: 10.1002/jat.3473. [DOI] [PubMed] [Google Scholar]
- 31.Vlenterie R, et al. Neurodevelopmental problems at 18 months among children exposed to paracetamol in utero: a propensity score matched cohort study. Int. J. Epidemiol. 2016;45:1998–2008. doi: 10.1093/ije/dyw192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cendejas-Hernandez, J. et al. Paracetamol (Acetaminophen) use in infants and children was never shown to be safe for neurodevelopment: a systematic review. Preprints (Basel) 2020110173 10.20944/PREPRINTS202011.0173.V1 (2020). [DOI] [PMC free article] [PubMed]
- 33.Liew Z, Ritz B, Rebordosa C, Lee P-C, Olsen J. Acetaminophen use during pregnancy, behavioral problems, and hyperkinetic disorders. JAMA Pediatr. 2014;168:313. doi: 10.1001/jamapediatrics.2013.4914. [DOI] [PubMed] [Google Scholar]
- 34.Thompson JMD, et al. Associations between acetaminophen use during pregnancy and ADHD symptoms measured at ages 7 and 11 years. PLoS One. 2014;9:e108210. doi: 10.1371/journal.pone.0108210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Avella-Garcia CB, et al. Acetaminophen use in pregnancy and neurodevelopment: attention function and autism spectrum symptoms. Int. J. Epidemiol. 2016;45:dyw115. doi: 10.1093/ije/dyw115. [DOI] [PubMed] [Google Scholar]
- 36.Liew Z, Bach CC, Asarnow RF, Ritz B, Olsen J. Paracetamol use during pregnancy and attention and executive function in offspring at age 5 years. Int. J. Epidemiol. 2016;45:dyw296. doi: 10.1093/ije/dyw296. [DOI] [PubMed] [Google Scholar]
- 37.Ystrom E, et al. Prenatal exposure to acetaminophen and risk of ADHD. Pediatrics. 2017;140:20163840. doi: 10.1542/peds.2016-3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Golding, J. et al. Associations between paracetamol (acetaminophen) intake between 18 and 32 weeks gestation and neurocognitive outcomes in the child: a longitudinal cohort study. Paediatr. Perinat. Epidemiol. 1–10. 10.1111/ppe.12582 (2019). [DOI] [PMC free article] [PubMed]
- 39.Liew Z, et al. Use of negative control exposure analysis to evaluate confounding: an example of acetaminophen exposure and attention-deficit/hyperactivity disorder in Nurses’ Health Study II. Am. J. Epidemiol. 2019;188:768–775. doi: 10.1093/aje/kwy288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ji Y, et al. Association of cord plasma biomarkers of in utero acetaminophen exposure with risk of attention-deficit/hyperactivity disorder and autism spectrum disorder in childhood. JAMA Psychiatry. 2020;77:180–189. doi: 10.1001/jamapsychiatry.2019.3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sznajder, K. K., Teti, D. M. & Kjerulff, K. H. Maternal use of acetaminophen during pregnancy and neurobehavioral problems in offspring at 3 years: a prospective cohort study. PLoS One17, (2022). [DOI] [PMC free article] [PubMed]
- 42.Stergiakouli E, Thapar A, Smith GD, Davey Smith G, Smith GD. Association of acetaminophen use during pregnancy with behavioral problems in childhood: evidence against confounding. JAMA Pediatr. 2016;170:964–970. doi: 10.1001/jamapediatrics.2016.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tovo-Rodrigues L, et al. Is intrauterine exposure to acetaminophen associated with emotional and hyperactivity problems during childhood? Findings from the 2004 Pelotas Birth Cohort. BMC Psychiatry. 2018;18:368. doi: 10.1186/s12888-018-1942-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Inoue K, et al. Behavioral problems at age 11 years after prenatal and postnatal exposure to acetaminophen: parent-reported and self-reported outcomes. Am. J. Epidemiol. 2020;190:1009–1020. doi: 10.1093/aje/kwaa257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Trønnes JN, Wood M, Lupattelli A, Ystrom E, Nordeng H. Prenatal paracetamol exposure and neurodevelopmental outcomes in preschool-aged children. Paediatr. Perinat. Epidemiol. 2020;34:247–256. doi: 10.1111/ppe.12568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zubrick SR, Taylor CL, Rice ML. Late language emergence at 24 months: an epidemiological study of prevalence, predictors, and covariates. J. Speech Lang. Hearing Res. 2007;50:1562–1592. doi: 10.1044/1092-4388(2007/106). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nelson BB, et al. Predictors of poor school readiness in children without developmental delay at age 2. Pediatrics. 2016;138:e20154477. doi: 10.1542/peds.2015-4477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bornehag C-G, et al. Prenatal exposure to acetaminophen and children’s language development at 30 months. Eur. Psychiatry. 2018;51:98–103. doi: 10.1016/j.eurpsy.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 49.Skovlund E, Handal M, Selmer R, Brandlistuen RE, Skurtveit S. Language competence and communication skills in 3‐year‐old children after prenatal exposure to analgesic opioids. Pharmacoepidemiol. Drug Saf. 2017;26:625–634. doi: 10.1002/pds.4170. [DOI] [PubMed] [Google Scholar]
- 50.Fenson, L. et al. MacArthur-Bates Communicative Development Inventories User’s Guide and Technical Manual. (Paul H. Brookes Publishing Company, 2007).
- 51.Hadley PA, Rice ML. Parental judgments of preschoolers’ speech and language development: a resource for assessment and IEP planning. Semin Speech Lang. 1993;14:278. doi: 10.1055/s-2008-1064177. [DOI] [Google Scholar]
- 52.Centers for Disease Control and Prevention, U. S. D. of H. and H. S. National Report on Human Exposure to Environmental Chemicals. https://www.cdc.gov/exposurereport/index.html (2022).
- 53.Ratner NB, Silverman S. Parental perceptions of children’s communicative development at stuttering onset. J. Speech Lang. Hearing Res. 2000;43:1252–1263. doi: 10.1044/jslhr.4305.1252. [DOI] [PubMed] [Google Scholar]
- 54.Newman R, et al. Infants’ early ability to segment the conversational speech signal predicts later language development: a retrospective analysis. Psychol. Assoc. 2006;42:643–655. doi: 10.1037/0012-1649.42.4.643. [DOI] [PubMed] [Google Scholar]
- 55.Eriksson M, et al. Differences between girls and boys in emerging language skills: evidence from 10 language communities. Br. J. Dev. Psychol. 2012;30:326–343. doi: 10.1111/j.2044-835X.2011.02042.x. [DOI] [PubMed] [Google Scholar]
- 56.Venkatesh KK, et al. Trends in opioid and psychotropic prescription in pregnancy in the united states from 2001 to 2015 in a privately insured population: a cross-sectional study. Ann. Intern Med. 2020;173:S19–S28. doi: 10.7326/M19-3249. [DOI] [PubMed] [Google Scholar]
- 57.Lindsay NM, Healy GN, Colditz PB, Lingwood BE. Use of the ages and stages questionnaire to predict outcome after hypoxic-ischaemic encephalopathy in the neonate. J. Paediatr. Child Health. 2008;44:590–595. doi: 10.1111/j.1440-1754.2008.01388.x. [DOI] [PubMed] [Google Scholar]
- 58.Charafeddine L, et al. The psychometric properties of the ages and stages questionnaires-3 in Arabic: cross-sectional observational study. Early Hum. Dev. 2019;136:33–38. doi: 10.1016/j.earlhumdev.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 59.Velikonja T, et al. The psychometric properties of the ages & stages questionnaires for ages 2-2.5: a systematic review. Child Care Health Dev. 2017;43:1–17. doi: 10.1111/cch.12397. [DOI] [PubMed] [Google Scholar]
- 60.Mendonça B, Sargent B, Fetters L. Cross-cultural validity of standardized motor development screening and assessment tools: a systematic review. Dev. Med. Child Neurol. 2016;58:1213–1222. doi: 10.1111/dmcn.13263. [DOI] [PubMed] [Google Scholar]
- 61.Kim SW, Kim JY, Lee SY, Jeon HR. The comparison of M-B CDI-K short form and K-ASQ as screening test for language development. Ann. Rehabil. Med. 2016;40:1108–1113. doi: 10.5535/arm.2016.40.6.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Berkman, N. D. et al. Screening for Speech and Language Delays and Disorders in Children Age 5 Years or Younger. A Systematic Review for the U.S. Preventive Services Task Force.https://www-ncbi-nlm-nih-gov.ezproxy.neu.edu/books/NBK305674/ (2015).
- 63.Hickok G. The functional neuroanatomy of language. Phys. Life Rev. 2009;6:121. doi: 10.1016/j.plrev.2009.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Friederici AD. The brain basis of language processing: from structure to function. Physiol. Rev. 2011;91:1357–1392. doi: 10.1152/physrev.00006.2011. [DOI] [PubMed] [Google Scholar]
- 65.Moore JK, Linthicum FHJ. The human auditory system: a timeline of development. Int. J. Audio. 2007;46:460–478. doi: 10.1080/14992020701383019. [DOI] [PubMed] [Google Scholar]
- 66.Nishimura, Y. & Kumoi, T. The embryologic development of the human external auditory meatus. 112, 496–503. 10.3109/00016489209137431 (2009). [DOI] [PubMed]
- 67.Whyte J, et al. Development of the dynamic structure (force lines) of the middle ear ossicles in human foetuses. Histol. Histopathol. 2008;23:1049–1060. doi: 10.14670/HH-23.1049. [DOI] [PubMed] [Google Scholar]
- 68.Swarts JD, Rood SR, Doyle WJ. Fetal development of the auditory tube and paratubal musculature - PubMed. Cleft Palate J. 1986;23:289–311. [PubMed] [Google Scholar]
- 69.Lavigne-Rebillard M, Pujol R. Development of the auditory hair cell surface in human fetuses. A scanning electron microscopy study. Anat. Embryol. (Berl.) 1986;174:369–377. doi: 10.1007/BF00698787. [DOI] [PubMed] [Google Scholar]
- 70.Jeffery N, Spoor F. Prenatal growth and development of the modern human labyrinth. J. Anat. 2004;204:71–92. doi: 10.1111/j.1469-7580.2004.00250.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Igarashi Y, Ishii T. Embryonic development of the human organ of Corti: Electron microscopic study. Int. J. Pediatr. Otorhinolaryngol. 1980;2:51–62. doi: 10.1016/0165-5876(80)90028-2. [DOI] [PubMed] [Google Scholar]
- 72.Hepper PG, Shahidullah BS. Development of fetal hearing. Arch. Dis. Child Fetal Neonatal Ed. 1994;71:F81. doi: 10.1136/fn.71.2.F81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jutras, B., Lagacé, J. & Koravand, A. The development of auditory functions. in Handbook of Clinical Neurology (eds. Gallagher, A., Bulteau, C., Cohen, D. & Michaud, J. L.) vol. 173 143–155 (Elsevier, 2020). [DOI] [PubMed]
- 74.Moore JK. Maturation of human auditory cortex: implications for speech perception. Ann. Otol. Rhinol. Laryngol. Suppl. 2002;189:7–10. doi: 10.1177/00034894021110S502. [DOI] [PubMed] [Google Scholar]
- 75.Moore JK, Guan YL, Shi SR. Axogenesis in the human fetal auditory system, demonstrated by neurofilament immunohistochemistry. Anat. Embryol. (Berl.) 1997;195:15–30. doi: 10.1007/s004290050021. [DOI] [PubMed] [Google Scholar]
- 76.Moore JK, Perazzo LM, Braun A. Time course of axonal myelination in the human brainstem auditory pathway. Hear Res. 1995;87:21–31. doi: 10.1016/0378-5955(95)00073-D. [DOI] [PubMed] [Google Scholar]
- 77.Kisilevsky BS, Davies GAL. Auditory processing deficits in growth restricted fetuses affect later language development. Med. Hypotheses. 2007;68:620–628. doi: 10.1016/j.mehy.2006.08.004. [DOI] [PubMed] [Google Scholar]
- 78.The Auditory Cortex. in Neuroscience (eds. Purves, D. et al.) (Sinauer Associates, 2001).
- 79.Bauer AZ, Kriebel D, Herbert MR, Bornehag C-G, Swan SH. Prenatal paracetamol exposure and child neurodevelopment: a review. Horm. Behav. 2018;101:125–147. doi: 10.1016/j.yhbeh.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 80.Lee J. Size matters: early vocabulary as a predictor of language and literacy competence. Appl. Psycholinguist. 2011;32:69–92. doi: 10.1017/S0142716410000299. [DOI] [Google Scholar]
- 81.Fernald A, Marchman VA, Weisleder A. SES differences in language processing skill and vocabulary are evident at 18 months. Dev. Sci. 2013;16:234–248. doi: 10.1111/desc.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Bellinger DC. Interpretation of small effect sizes in occupational and environmental neurotoxicology: individual versus population risk. Neurotoxicology. 2007;28:245–251. doi: 10.1016/j.neuro.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 83.Reznick JS, Schwartz BB. When is an assessment an intervention? Parent perception of infant intentionality and language. J. Am. Acad. Child Adolesc. Psychiatry. 2001;40:11–17. doi: 10.1097/00004583-200101000-00011. [DOI] [PubMed] [Google Scholar]
- 84.Fenson, L. et al. MacArthur Communicative Development Inventories User’s Guide and Technical Manual. (Paul H. Brookes Publishing Company, 1992).
- 85.Morton CC, et al. Tools for standardized data collection: speech, language, and hearing measurement protocols in the PhenX Toolkit. Ann. Hum. Genet. 2022;86:45–51. doi: 10.1111/ahg.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.PheX Toolkit Protocol - Early Childhood Speech and Language Assessment - Speech and Language Assessment Scale. PhenX Toolkithttps://www.phenxtoolkit.org/protocols/view/200302?origin=search (2023).
- 87.Rothman KJ. No adjustments are needed for multiple comparisons. Epidemiology. 1990;1:43–46. doi: 10.1097/00001648-199001000-00010. [DOI] [PubMed] [Google Scholar]
- 88.Gevezova, M., Sarafian, V., Anderson, G. & Maes, M. Inflammation and Mitochondrial Dysfunction in Autism Spectrum Disorder. CNS & Neurological Disorders - Drug Targets19 (2020). [DOI] [PubMed]
- 89.Hisle-Gorman E, et al. Prenatal, perinatal, and neonatal risk factors of autism spectrum disorder. Pediatr. Res. 2018;84:190–198. doi: 10.1038/pr.2018.23. [DOI] [PubMed] [Google Scholar]
- 90.Laue HE, et al. Association between meconium acetaminophen and childhood neurocognitive development in GESTE, a Canadian cohort study. Toxicol. Sci. 2019;167:138–144. doi: 10.1093/toxsci/kfy222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Baker BH, et al. Association of prenatal acetaminophen exposure measured in meconium with risk of attention-deficit/hyperactivity disorder mediated by frontoparietal network brain connectivity. JAMA Pediatr. 2020;174:1073–1081. doi: 10.1001/jamapediatrics.2020.3080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Camann DE, et al. Acetaminophen, pesticide, and diethylhexyl phthalate metabolites, anandamide, and fatty acids in deciduous molars: potential biomarkers of perinatal exposure. J. Expo. Sci. Environ. Epidemiol. 2012;23:190–196. doi: 10.1038/jes.2012.71. [DOI] [PubMed] [Google Scholar]
- 93.Bauer AZ, et al. Paracetamol use during pregnancy — a call for precautionary action. Nat. Rev. Endocrinol. 2021;17:757–766. doi: 10.1038/s41574-021-00553-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data used in these analyses cannot be shared publicly to ensure the anonymity of participants. Underlying code is available upon request.


