Abstract
Persistent hepatitis C virus (HCV) infection is a major cause of chronic liver dysfunction in humans and is epidemiologically closely associated with the development of human hepatocellular carcinoma. Among HCV components, core protein has been reported to be implicated in cell growth regulation both in vitro and in vivo, although mechanisms explaining those effects are still unclear. In the present study, we identified that members of the 14-3-3 protein family associate with HCV core protein. 14-3-3 protein bound to HCV core protein in a phosphoserine-dependent manner. Introduction of HCV core protein caused a substantial increase in Raf-1 kinase activity in HepG2 cells and in a yeast genetic assay. Furthermore, the HCV core–14-3-3 interaction was essential for Raf-1 kinase activation by HCV core protein. These results suggest that HCV core protein may represent a novel type of Raf-1 kinase-activating protein through its interaction with 14-3-3 protein and may contribute to hepatocyte growth regulation.
The molecular cloning of the hepatitis C virus (HCV) genome established that persistent HCV infection is the most important mediator for non-A, non-B chronic liver disease (2, 7). A significant number of primary liver cancer (hepatocellular carcinoma [HCC]) cases in humans has a high positive correlation with HCV infection (40, 44). Hepatitis C virus proteins are composed of putative structural and nonstructural proteins, encoded by a single open reading frame from approximately 9,500 bases of RNA genome. Among the processed HCV polyprotein, the core protein of 191 amino acids is a central component of virion and is necessary for nucleocapsid formation. Antibodies to HCV core protein are frequently detected in patients with chronic active hepatitis C (6). Besides, HCV core protein is thought to regulate the expression of various genes in vitro (19, 36, 37) and to be implicated in Fas-mediated apoptotis both in vitro and in vivo (13, 39). Overexpression of HCV core protein resulted in transformation of rat embryonic fibroblasts to the tumorigenic phenotype (4, 35). More interestingly, constitutive expression of HCV core protein induced HCC in transgenic mice; the expression level of HCV core protein in the liver in these mice was similar to that in patients with chronic hepatitis C (31). Thus, evidence that HCV core protein may contribute to mammalian cell growth regulation has now accumulated, although detailed molecular mechanisms explaining these effects remain unknown.
Identification of the cellular targets for virus protein is a potential approach to better understanding the pathogenesis of the virus. Several HCV core-binding proteins such as apolipoprotein AII (3), cytoplasmic tails of lymphotoxin-β receptor (5, 27), tumor necrosis factor receptor (52), heterogeneous nuclear ribonucleoprotein K (15), and cellular putative RNA helicases (25, 50) have been reported. Nonetheless, the functional significance of these interactions with HCV core protein has not yet been fully defined in the context of the mitogenic and/or oncogenic effect of HCV core protein. We reasoned that additional HCV core-binding proteins that would further elucidate mitogenic pathways in hepatocytes expressing HCV core protein might exist. To identify the additional HCV core-binding protein(s), we performed a yeast two-hybrid screen by using the interaction trap system (9) with the HCV core protein fused to the DNA-binding protein LexA (termed LexA-Core) as a bait. One group of positive interactors was identified as an epsilon isoform of 14-3-3 protein (14-3-3ɛ). The 14-3-3 protein family is known to associate with components of several signal transduction pathways, including the Raf-1 kinase cascade. We also demonstrated that HCV core protein activated the Raf-1 kinase through the HCV core–14-3-3 interaction, suggesting that HCV core protein may play an important role in regulating hepatocyte growth, senescence, and differentiation through its interaction with 14-3-3 protein.
MATERIALS AND METHODS
Yeast two-hybrid system.
The cDNAs encoding the various lengths of HCV core protein were generated by PCR amplification using a plasmid pSC11 containing genotype 1b of HCV core cDNA (32) (gift from A. Nishizono) as a template and primers incorporating appropriate restriction sites. The bait plasmid (pLexA-Core) was made by insertion of cDNA encoding the 128 amino acids of HCV core protein (without C-terminal 63 amino acids to ensure translocation to the nucleus) into pEG202 (obtained from Clontech) (9). A human liver cDNA library cloned into pJG4-5, lacZ reporter plasmid pSH18-34, and yeast reporter strain EGY48 (9) were obtained from Clontech. The two-hybrid screen and interaction assays were performed essentially as described previously (9) in the presence of 2% galactose and 80 mg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside (X-Gal) per liter. Introduction of nucleotide changes in HCV core cDNA, corresponding to the residue mutations serine-53 to alanine (S53A) and/or serine-56 to alanine (S56A) within HCV core protein, were carried out with the Gene Editor in vitro site-directed mutagenesis system (Promega). The PCR-generated human Ha-Ras, Raf-1, p53, lamin C, and deletion mutants of 14-3-3 cDNAs were cloned into pJG4-5. All the PCR products were sequenced.
GST pull-down experiments.
The glutathione S-transferase (GST) fusion constructs (termed pYEX-Core and pGEX-Core) were made by inserting cDNA encoding the genotype 1b of wild-type HCV core protein (without C-terminal 18 amino acids for efficient recombinant protein production) into pYEX-4T-1 yeast expression vector (Clontech) or pGEX-4T-1 bacterial expression vector (Amersham Pharmacia Biotech). The bacterial expression vector pGEX-Core (S53A) was made by introducing the S53A mutation in pGEX-Core as described above. GST-HCV core fusion proteins [yeast-produced GST-Core, bacterially produced GST-Core, and GST-Core (S53A)] and yeast or bacterially produced GST proteins were expressed in Saccharomyces cerevisiae DY150 (Clontech) induced by 0.5 mM copper sulfate for 2 h at 30°C or in Escherichia coli BL21 (Stratagene) induced by 0.1 mM isopropyl-1-thio-β-d-galactopyranoside (IPTG) for 3 h at 25°C. Cells were resuspended in lysis buffer (1% Triton X-100 in phosphate-buffered saline) and sonicated on ice. The bacterially produced GST-Core and GST-Core (S53A) proteins were treated with or without recombinant protein kinase A (PKA; Promega) or protein kinase C (PKC; Promega) essentially as described previously (41). The soluble GST proteins were immobilized on glutathione 4B-Sepharose (Amersham Pharmacia Biotech) and washed three times with ice-cold lysis buffer.
For in vitro translation, PCR-generated 14-3-3 cDNAs were cloned into pGEM-7Zf(+) (Promega). [35S]Methionine-labeled 14-3-3 proteins were translated in vitro by using the TNT reticulocyte lysate system (Promega). Five microliters of 35S-labeled 14-3-3 proteins was incubated with immobilized GST proteins (2 μg of GST-Core, 2 μg of GST-Core (S53A), and 4 μg of GST protein) in 0.1 ml of binding buffer (125 mM NaCl, 50 mM Tris-HCl [pH 7.4], 1 mM EDTA, 10% [vol/vol] glycerol, 0.5% [vol/vol] Nonidet P-40, 5 μg of aprotinin per ml, 1 mM phenylmethylsulfonyl fluoride) for 2 h at 4°C, washed five times with binding buffer, and then analyzed by 15% sodium dodecyl sulfate (SDS)–polyacrylamide gel electrophoresis (PAGE) and with a Fujix BAS2000 image analyzer (Fuji Photo Film).
Coprecipitation assay.
The plasmid pCAHA-14-3-3ɛ was made by insertion of human 14-3-3ɛ cDNA into the mammalian expression vector pCAHA, a derivative of pCAGGS (33) (gift from J. Miyazaki) with a hemagglutinin (HA) epitope tag at the 5′ end of the polylinker.
HepG2 cells were purchased from the American Type Culture Collection (ATCC) and maintained in Earle's minimal essential-nonessential amino acid (MEM-NEAA) medium (GIBCO BRL) supplemented with 10% fetal bovine serum. The HepΔNCTH cell is a HepG2 cell stably expressing HCV core protein (genotype 1b) under the control of the elongation factor 1α promoter (43) (gift from T. Wakita and K. Tokushige). The plasmid p/3EFproΔNCTH is a mammalian expression vector carrying the wild-type HCV core gene (genotype 1b) driven by the elongation factor 1α promoter (43) (gift from T. Wakita and K. Tokushige). The p/3EFproΔNCTH (S53A) vector was made by introducing an S53A mutation into p/3EFproΔNCTH as described above. The HepΔNCTH (S53A) cell, a HepG2 cell stably expressing mutant HCV core protein, was established as described previously (43) by transfection with the p/3EFproΔNCTH (S53A) vector by using Tfx-20 (Promega) and by selection in MEM-NEAA medium supplemented with 10% fetal bovine serum and G418 (Geneticin [Sigma]; final concentration, 1 mg/ml).
In 60-mm-diameter culture dishes, 5 × 105 HepΔNCTH cells and HepΔNCTH (S53A) cells were transiently transfected with 5 μg of pCAHA-14-3-3ɛ or pCAHA empty vector by using Tfx-20. Immunoprecipitation and Western blot analysis were performed as described previously (48) with anti-HCV core monoclonal antibody C7-50A (43) (gift from T. Wakita) or anti-HA monoclonal antibody 12CA5 (Boehringer Mannheim), and probed proteins were detected by chemiluminescence (ECL kit; Amersham Pharmacia Biotech).
In vitro-coupled kinase assay.
Cell extracts (500 μg) from serum-deprived HepG2 cells, HepΔNCTH cells, HepΔNCTH (S53A) cells, or TPA (12-O-tetradecanoyl phorbol 13-acetate)-treated (at 100 nmol/ml for 30 min) HepG2 cells were immunoprecipitated by using anti-Raf-1 polyclonal antibody C-12 (Santa Cruz Biotechnology). Prior to immunoprecipitation using anti-Raf-1 antibody, one aliquot of cell extract from HepΔNCTH cells was subjected to preabsorption of HCV core protein by using 4 μg of C7-50A antibody and protein A+G agarose (Oncogene Research) for 1 h at 4°C. The washed immunoprecipitates were assayed for Raf-1 kinase activity in a two-stage incubation as described before (23) with recombinant MEK-1 (Santa Cruz Biotechnology) and kinase-inactive recombinant ERK-2 (New England Biolabs). The reaction was terminated by the addition of SDS-PAGE sample buffer, separated on a 10% SDS–PAGE gel, and transferred to an Immobilon-P membrane (Millipore). 32P incorporation into ERK-2 was quantified with a BAS2000 image analyzer. The amounts of immunoprecipitated Raf-1 were detected by Western blot analysis.
Mammalian Raf-1 activation assay in yeast.
PCR-generated cDNAs [from full-length HCV core, HCV core (S53A), HCV core (1-128), human 14-3-3ɛ, and wild-type human Ha-Ras proteins] were cloned into pVT102-L (gift from K. Yano) carrying an ADH1 promoter and a leu2-d nutritional marker (46). The S. cerevisiae strain SY1984RP, a SY1984 (MATα ste11Δ pep4Δ his3Δ FUS1::HIS3 ura3 trp1 can1) strain expressing mammalian Raf-1 and yeast Ste7P368 (16) (gift from K. Matsumoto), was transformed with these pVT102-L derivatives by electroporation. Raf-1-dependent activation of the FUS1::HIS3 reporter gene was monitored by growth on a histidine-deficient synthetic complete medium (SC) plate for 3 days with incubation at 30°C.
RESULTS
Screening for proteins that interact with HCV core protein.
A plasmid library representing the normal human liver was screened with LexA-Core bait in a yeast reporter strain. Of 106 independent clones screened, 95 clones with a Leu+ LacZ+ phenotype were obtained. The representative plasmids from each group of positives were retransformed back into yeast to confirm their correct phenotype. Finally, of nine true positives obtained, two independent clones were identified as the portion of cDNA encoding 14-3-3ɛ protein. The remaining seven true positives were identified as follows: two independent clones encoding the portion of myotonic dystrophy kinase-related Cdc42-binding kinase α (24) and five unknown clones. Since the biochemical functions of the 14-3-3 protein family have been well characterized, we decided to concentrate upon the analysis of the physical and functional interactions of HCV core protein and 14-3-3 proteins in the present study. In the two-hybrid system, HCV core protein specifically bound to 14-3-3ɛ protein but not to Ha-Ras, Raf-1, p53, or lamin C (data not shown).
Determination of essential domains for HCV core protein–14-3-3 protein interaction.
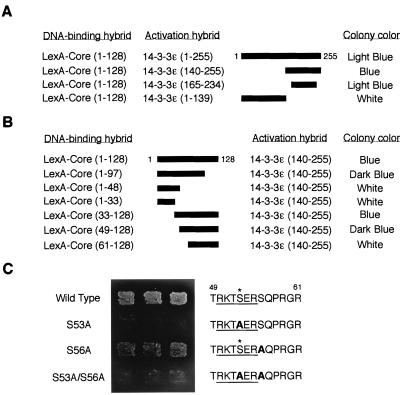
To determine the essential domains for HCV core–14-3-3 interaction, we performed two-hybrid analysis using deletion mutants. We mapped the HCV core-binding domain on 14-3-3 protein to amino acids 165 to 234 (Fig. 1A).
FIG. 1.
Interaction of HCV core protein with 14-3-3 protein in the yeast two-hybrid system. (A) Determination of binding domain within 14-3-3 protein. Various activation hybrid mutants were expressed in the yeast strain EGY48 with LexA-Core protein (amino acids 1 to 128). A schematic of these 14-3-3 deletion mutants is shown with thick bars. Protein interaction was monitored by activation of the lacZ reporter gene (determined by colony color; blue colony represents a positive interaction) on an SC plate containing 2% galactose and 80 mg of X-Gal per liter with 3 days of incubation at 30°C. (B) Determination of binding domain within HCV core protein. LexA fusion proteins containing the indicated sequences of HCV core protein were expressed with an activation domain hybrid of 14-3-3 protein, 14-3-3ɛ (amino acids 140 to 255), in the yeast strain EGY48. Interactions were monitored as described for panel A. (C) HCV core protein interacts with 14-3-3 protein in a phosphoserine-dependent manner in yeast. LexA fusion proteins containing wild-type and S53A, S56A, and S53A/S56A mutants of HCV core protein (amino acids 1 to 128) were expressed with 14-3-3ɛ, an activation hybrid of 14-3-3 protein (amino acids 140 to 255), in the yeast strain EGY48, and the phosphoserine-dependent interaction was monitored by activation of leucine reporter gene and by colony color on a leucine-deficient SC plate containing galactose and X-Gal for 3 days. The putative 14-3-3 binding motifs (underlined) within HCV core proteins are indicated on the right. Substituted alanine residues are indicated by boldface type. Asterisks indicate phosphorylation sites for PKA and PKC.
In the next step, we mapped the 14-3-3 binding domain on HCV core protein to amino acids 49 to 97 (Fig. 1B). We found that one motif, RKTpSER (residues 50 to 55) (pS, phosphoserine), whose serine residue at position 53 (serine-53) is likely phosphorylated by PKA and/or PKC (41), was embedded in this region (Fig. 1C). Interestingly, this motif was similar to the mode 1 14-3-3 protein binding motif [R-(S/Ar)-(+/Ar)-pS-(L/E/A/M)-P] (where Ar indicates an aromatic residue and “+” indicates a basic residue) (49). Furthermore, this RKTpSER motif was highly conserved among HCV strains (data not shown).
HCV core protein interacts with 14-3-3 protein in a phosphoserine-dependent manner in yeast.
Since 14-3-3 protein has been demonstrated to be a specific phosphoserine-binding protein (49), we tested whether serine-53 within residues 50 to 55 (RKTpSER) of HCV core protein was critical for their interaction. The introduction of a mutation of serine-53 to alanine (S53A) but not serine-56 to alanine (S56A) within HCV core protein abolished its binding to 14-3-3 protein (Fig. 1C), suggesting that HCV core protein might interact with 14-3-3 protein in a phosphoserine-dependent manner.
HCV core protein interacts with several 14-3-3 protein isoforms in vitro.
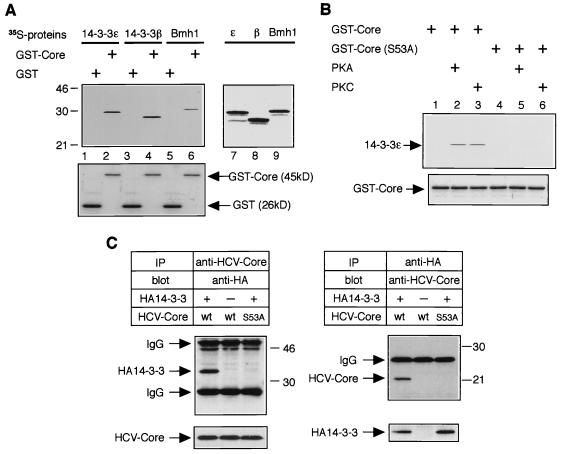
In the yeast two-hybrid system, we mapped HCV core-binding domain on 14-3-3 protein to its C-terminal 70 amino acids (Fig. 1A). This minimal binding domain is highly conserved among all 14-3-3 protein species (1). To extend the interaction of HCV core protein to other 14-3-3 isoforms, we performed in vitro GST pull-down experiments. The budding yeast-produced HCV core protein fused to GST protein (GST-Core), but not GST alone, was able to bind directly to several species of 14-3-3 protein (human 14-3-3ɛ, rat 14-3-3β, and S. cerevisiae Bmh1) (Fig. 2A, top panel, lanes 2, 4, and 6).
FIG. 2.
Interaction of HCV core protein with 14-3-3 protein in vitro and in mammalian cells. (A) HCV core protein interacts with several 14-3-3 protein isoforms in vitro. Three isoforms of 35S-labeled 14-3-3 proteins were subjected to a GST pull-down experiment using budding yeast-produced GST-Core protein (top panel, lanes 2, 4, and 6) and GST alone (top panel, lanes 1, 3, and 5). Positions of molecular weight standards (in kilodaltons) are shown at the left. Input of 35S-labeled 14-3-3 proteins (14-3-3ɛ, 14-3-3β, and Bmh1) are shown in lanes 7, 8, and 9, respectively. Coomassie blue-stained GST and GST-Core from the same gel are aligned to show protein content (bottom panel, lanes 1 to 6). (B) Phosphorylation of serine-53 by PKA or PKC is essential for HCV core–14-3-3 interaction in vitro. Bacterially produced GST-Core proteins (wild type and the S53A mutant) were treated with or without recombinant PKA or recombinant PKC and subjected to GST pull-down experiments using 35S-labeled 14-3-3ɛ protein. (C) HCV core protein interacts with 14-3-3 protein in mammalian cells. HepΔNCTH cells expressing wild-type (wt) HCV core protein and HepΔNCTH cells expressing the S53A mutant of HCV core protein (S53A) were transiently transfected with pCAHA-14-3-3ɛ [HA14-3-3(+)] or pCAHA empty vector [HA14-3-3(−)]. After 48 h, cell lysate was subjected to immunoprecipitation (IP) and Western blot (blot) with the antibodies indicated above the upper panels. The presence of HCV core protein and HA14-3-3 protein in extracts was verified by reprobing the same membranes with antibodies for immunoprecipitation (bottom lanes). Positions of molecular mass standards (in kilodaltons) are shown at the right.
Phosphorylation of serine-53 by PKA or PKC is essential for HCV core–14-3-3 interaction.
In our preliminary experiments, bacterially produced GST-Core protein was not able to bind to 14-3-3 proteins in vitro (data not shown), suggesting that HCV core protein might be phosphorylated on serine-53 by a PKA or PKC homolog in budding yeast. To test whether phosphorylation of serine-53 by PKA or PKC was essential for HCV core–14-3-3 interaction, further GST pull-down experiments were performed with bacterially produced wild-type GST-Core protein and its mutant GST-Core (S53A) protein. Prior to the binding reaction, bacterially produced GST-Core proteins were treated with recombinant PKA or PKC. The wild-type GST-Core protein treated with recombinant PKA or PKC was able to bind to 14-3-3 protein (Fig. 2B, lanes 2 and 3 of top panel), although untreated wild-type GST-Core protein was not (Fig. 2B, lane 1). In contrast, GST-Core (S53A) protein was not able to bind to 14-3-3 protein regardless of PKA or PKC treatments (Fig. 2B, lanes 4, 5, and 6). These results suggest that phosphorylation of serine-53 by PKA or PKC was essential for HCV core–14-3-3 interaction.
HCV core protein interacts with 14-3-3 protein in mammalian cells.
To confirm that association of HCV core protein and 14-3-3 protein can occur in mammalian cells, we performed coprecipitation assays using HepΔNCTH cells (43), the human hepatoma line HepG2 cells stably expressing wild-type HCV core protein, and HepΔNCTH (S53A) cells stably expressing the S53A mutant of HCV core protein. HepΔNCTH cells and HepΔNCTH (S53A) cells were transiently transfected with an expression plasmid producing an HA-tagged 14-3-3 protein (HA14-3-3); immunoprecipitates were then subjected to Western blot analysis. As shown in Fig. 2C, HCV core protein could specifically bind to HA14-3-3 protein, and this interaction was confirmed reciprocally. However, the S53A mutant of HCV core protein was not able to bind to HA14-3-3 protein (Fig. 2C).
HCV core protein activates the Raf-1 kinase in HepG2 cells.
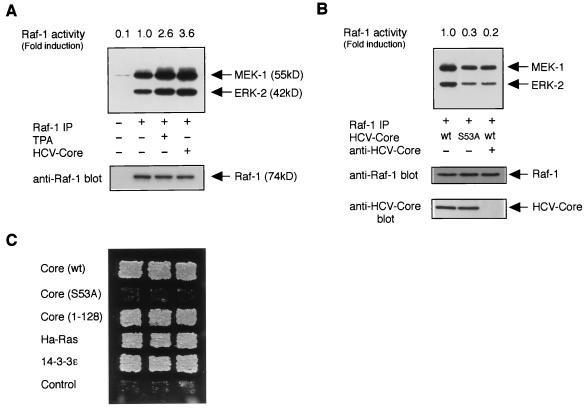
The 14-3-3 protein family has been reported to associate with several signaling proteins in eukaryotes (1, 29), including protein kinase Raf-1, a central component of the mitogen-activated protein (MAP) kinase pathway in mammalian cells (14, 21). We therefore tested whether HCV core protein modulated Raf-1 kinase activity through its interaction with 14-3-3 protein. In coupled kinase assay in vitro, an anti-Raf-1 immunoprecipitate prepared from HepΔNCTH cells (expressing wild-type HCV core protein) efficiently phosphorylated recombinant MEK-1, which in turn phosphorylated kinase-inactive recombinant ERK-2 (3.6-fold) as compared to those analyzed with parental HepG2 cells or TPA-treated HepG2 cells (2.6-fold) (Fig. 3A). However, an anti-Raf-1 immunoprecipitate prepared from HepΔNCTH (S53A) cells (expressing the S53A mutant of HCV core protein) phosphorylated lesser amounts of MEK-1 and ERK-2 (0.3-fold 32P incorporation) (Fig. 3B, middle lane) as compared to that analyzed with HepΔNCTH cells (Fig. 3B, left lane). These results suggest that the binding of HCV core protein to 14-3-3 protein was essential for Raf-1 kinase activation. In addition, preabsorption of HCV core protein by anti-HCV core antibody reduced the kinase activity of Raf-1 to a basal level (Fig. 3B, right lane), suggesting that activation of Raf-1 kinase in HepΔNCTH cells was observed specifically in the presence of wild-type HCV core protein.
FIG. 3.
HCV core protein activates the kinase Raf-1. (A) An in vitro-coupled kinase assay for Raf-1 was performed with extracts from HepG2 (untreated or treated with TPA) or HepΔNCTH [HCV core (+)] cells. Phosphorylated MEK-1 and ERK-2 are indicated (top panel, arrows). The levels of immunoprecipitated Raf-1 are shown (bottom panel, arrows). Relative Raf-1 activities (fold induction; the 32P incorporation into ERK-2) shown are the means for duplicate determination and are representative of three experiments. (B) Extracts from HepΔNCTH cells expressing wild-type HCV core protein (wt) and HepΔNCTH (S53A) cells expressing the S53A mutant of HCV core protein (S53A) were subjected to an in vitro-coupled kinase assay. (Top panel) In the right lane, HCV core protein in cell extract was preabsorbed by using anti-HCV core antibody. (Bottom panel) The presence of HCV core protein in precleared lysates was verified by Western blot analysis. (C) Mammalian Raf-1 activation assay in yeast. Yeast strain SY1984RP cells were transformed with plasmids expressing wild-type [Core (wt)] or S53A mutant [Core (S53A)] of full-length HCV core protein, truncated HCV core protein (Core 1-128), Ha-Ras, 14-3-3ɛ, and pVT102-L empty vector (Control). Activation of Raf-1 was monitored by growth on a histidine-deficient SC plate with 3 days of incubation at 30°C.
HCV core protein activates the mammalian Raf-1 kinase in budding yeast.
To verify these phenomena by an alternative approach, we employed the yeast genetic assay that was designed for detecting the activation of mammalian Raf-1 kinase. This in vivo system is composed of S. cerevisiae SY1984RP, a SY1984 (MATα ste11Δ pep4Δ his3Δ FUS1::HIS3 ura3 trp1 can1) strain expressing mammalian Raf-1 and Ste7P368, a gain-of-function mutant of yeast MAP kinase kinase Ste7 (16). Introduction of a component that functions in Raf-1 activation enhances Raf-1 activity, which in turn activates a yeast mating pheromone-induced MAP kinase pathway, including a mating pathway-responsive reporter gene (FUS1::HIS3), and eventually allows SY1984RP cells to grow without histidine (16). As expected, introduction of full-length wild-type HCV core protein could activate Raf-1 in this system, as well as known positive controls (Ha-Ras or mammalian 14-3-3) (Fig. 3C). The C-terminally truncated version of HCV core protein (Core 1-128), which showed no preferential submembranous accumulation unlike the full-length HCV core protein (3), also could activate the kinase activity of Raf-1. However, mutant full-length HCV core protein (S53A), which was incapable of binding to 14-3-3 protein, could not activate Raf-1 in this system (Fig. 3C).
DISCUSSION
In the present study, 14-3-3 proteins were identified as interacting with HCV core protein. On the basis of studies of the association of 14-3-3 protein with a large number of proteins, it has been suggested that 14-3-3 protein may play an organizational role in mitogenic signal transduction. In terms of the association between viral protein and 14-3-3 protein, the middle tumor antigen (MT) of murine polyomavirus has been described previously (34). The MT interacts with 14-3-3 protein in NIH 3T3 cells and activates ADP ribosylation, suggesting that regulation of 14-3-3 protein function by MT may contribute to cell proliferation, including neoplasia (34). In this context, interaction of HCV core protein with 14-3-3 proteins may play some physiological role in growth regulation of human hepatocytes. Recently, several studies demonstrated that development of HCCs might be associated with activation of the Ras/Raf/MAP kinase pathway in humans (17) and rodents (18, 28). Raf-1 kinase activity was increased in mouse liver tumors about fourfold, in comparison to that in normal liver tissue (18). Ito et al. (17) argued that 1.1- to 3.1-fold enhanced activation of MAP kinase (ERK-1 and ERK-2) in human HCCs, as compared with that in adjacent noncancerous lesions, might contribute to the development and progression of HCCs. It is striking that HCV core protein was able to activate Raf-1 kinase activity (about 3.6-fold) in HepG2 cells and downstream effector molecules of Raf-1 (e.g., ERKs) in HepG2 cells and NIH 3T3 cells (10). It was demonstrated that constitutive expression of HCV core protein in MCF-7 cells resulted in a high basal activity of MAP kinase kinase, as determined by immunodetection of hyperphosphorylated ERK-1 and ERK-2 (42). This data supports our findings that constitutive expression of HCV core protein might be involved in the activation of the Ras/Raf/MAP kinase pathway in mammalian cells. Importantly, as has been reported, HCV core protein transforms mammalian cells, including hepatocytes, in vitro (4, 35) and in vivo (31). Taken together, we suppose that HCV core protein may play a key role in HCV-mediated human liver disease, including the development and progression of HCCs, through its activation of the MAP kinase cascade. Although the intrahepatic expression level of HCV core protein may vary in cases of chronic hepatitis C or HCV-related HCCs, it is also likely that HCV core protein acts in concert with other factors, such as loss of tumor suppressors or genomic instability associated with chronic active hepatitis (11, 12).
Like HCV core protein, hepatitis B virus X protein (HBx) can also increase MAP kinase activity (8, 20) through the activation of the Src family of tyrosine kinases and/or effectors of Ras (20), although the direct cytoplasmic target for HBx involved in this phenomenon is still unknown. Here we have clearly demonstrated that HCV core–14-3-3 interaction was essential for Raf-1 activation in cells expressing HCV core protein (Fig. 3B and C). In contrast, Bad, a distant Bcl-2 family member that selectively dimerized with Bcl-XL and Bcl-2 but not with itself, was able to interact with 14-3-3 protein but could not activate Raf-1 kinase in vitro or in SY1984RP cells (51).
Since the HCV core protein homodimerized or multimerized (26), we propose the possibility that HCV core protein might work as a bridging molecule. Although we can only speculate as to how the HCV core–14-3-3 interaction promotes activation of Raf-1 kinase, HCV core protein might form a ternary complex with 14-3-3 or Raf-1, thereby creating a Raf-Raf homo-oligomer with homodimerization or multimerization of HCV core protein or stabilizing an active conformation of Raf-1 (30, 45). In this regard, HCV core protein might be a novel type of Raf-1-activating protein that uses different mechanism than known Raf-1-activating protein Ras (22) and Bcl-2 interacting protein Bag-1 (47).
We also propose another possibility, as follows: when Raf-1 is maintained in an inactive state by the binding of 14-3-3 dimer to a phosphorylated Ser-259, HCV core protein might displace several portions of the 14-3-3 dimer from phosphorylated Ser-259 instead of Ras-GTP (which displaces 14-3-3 from phosphorylated Ser-259 within Raf-1) (30, 38).
In conclusion, we identified that HCV core protein is able to interact with 14-3-3 protein and to activate the kinase Raf-1. This model may incorporate most of the available biochemical evidence concerning the mitogenic function of HCV core protein and may provide new insight in understanding the molecular mechanism of hepatocyte growth regulation and, at least in part, development of human HCC mediated by chronic HCV infection. The precise step in the Raf-1 activation pathway mediated by HCV core protein is currently under investigation.
ACKNOWLEDGMENTS
We thank T. Wakita and K. Tokushige for HepΔNCTH, plasmid p/3EFproΔNCTH, and C7-50A antibody, K. Matsumoto for the SY1984RP yeast strain, K. Yano for plasmid pVT102-L, A. Nishizono for HCV core cDNA, and J. Miyazaki for plasmid pCAGGS. We also thank H. Sugano and T. Kitagawa for valuable comments, Y. Hirayama for excellent technical assistance, and K. Orimoto, T. Kobayashi, J. T. Woitach, and M. R. Jensen for helpful discussion and critical reading of the manuscript.
H.A. was supported by a research fellowship from the Japan Society for the Promotion of Science.
REFERENCES
- 1.Aitken A. 14-3-3 proteins on the MAP. Trends Cell Biol. 1996;6:341–347. doi: 10.1016/0962-8924(96)10029-5. [DOI] [PubMed] [Google Scholar]
- 2.Alter H J, Purcell R H, Shih J W, Melpolder J C, Houghton M, Choo Q-L, Kuo G. Detection of antibody to hepatitis C virus in prospectively followed transfusion recipients with acute and chronic non-A, non-B hepatitis. N Engl J Med. 1989;321:1494–1500. doi: 10.1056/NEJM198911303212202. [DOI] [PubMed] [Google Scholar]
- 3.Barba G, Harper F, Harada T, Kohara M, Goulinet S, Matsuura Y, Eder G, Schaff Z, Chapman M J, Miyamura T, Brechot C. Hepatitis C protein shows a cytoplasmic localization and associates to cellular lipid storage droplets. Proc Natl Acad Sci USA. 1997;94:1200–1205. doi: 10.1073/pnas.94.4.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chang J, Yang S H, Cho Y G, Hwang S B, Hahn Y S, Sung Y C. Hepatitis C virus core from two different genotypes has an oncogenic potential but is not sufficient for transforming primary rat embryo fibroblasts in cooperation with the H-ras oncogene. J Virol. 1998;72:3060–3065. doi: 10.1128/jvi.72.4.3060-3065.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen C-M, You L-R, Hwang L-H, Lee Y-H W. Direct interaction of hepatitis C virus core protein with the cellular lymphotoxin-β receptor modulates the signal pathway of the lymphotoxin-β receptor. J Virol. 1997;71:9417–9426. doi: 10.1128/jvi.71.12.9417-9426.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chiba J, Ohba H, Matsuura Y, Watanabe Y, Katayama T, Kikuchi S, Saito I, Miyamura T. Sero diagnosis of hepatitis C virus (HCV) infection with an HCV core protein molecularly expressed by a recombinant baculovirus. Proc Natl Acad Sci USA. 1991;88:4641–4645. doi: 10.1073/pnas.88.11.4641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choo Q-L, Kuo G, Weiner A J, Overby L R, Bradley D W, Houghton M. Isolation of a cDNA clone derived from a blood borne non-A, non-B viral hepatitis genome. Science. 1989;244:359–362. doi: 10.1126/science.2523562. [DOI] [PubMed] [Google Scholar]
- 8.Doria M, Klein N, Lucito R, Schneider R J. The hepatitis B virus HBx protein is a dual specificity cytoplasmic activator of Ras and nuclear activator of transcription factor. EMBO J. 1995;14:4747–4757. doi: 10.1002/j.1460-2075.1995.tb00156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gyuris J, Golemis E, Chertkov H, Brent R. Cdi1, a human G1 and S phase protein phosphatase that associates with Cdk2. Cell. 1993;75:791–803. doi: 10.1016/0092-8674(93)90498-f. [DOI] [PubMed] [Google Scholar]
- 10.Hayashi, J., and H. Aoki. Unpublished data.
- 11.Hino O, Kajino K. Hepatitis virus-related hepatocarcinogenesis. Intervirology. 1994;37:133–135. doi: 10.1159/000150368. [DOI] [PubMed] [Google Scholar]
- 12.Hino O, Tabata S, Hotta Y. Evidence for increased in vitro recombination with insertion of human hepatitis B virus DNA. Proc Natl Acad Sci USA. 1991;88:9248–9252. doi: 10.1073/pnas.88.20.9248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hiramatsu N, Hayashi N, Katayama K, Mochizuki K, Kawanishi Y, Kasahara A, Fusamoto H, Kamada T. Immunohistochemical detection of Fas antigen in liver tissue of patients with chronic hepatitis C. Hepatology. 1994;19:1354–1359. [PubMed] [Google Scholar]
- 14.Howe L R, Leevers S J, Gomez N, Nakielny S, Cohen P, Marshall C J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992;71:335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- 15.Hsieh T-Y, Matsumoto M, Chou H-C, Schneider R, Hwang S B, Lee A S, Lai M M C. Hepatitis C virus core protein interacts with heterogeneous nuclear ribonucleoprotein K. J Biol Chem. 1998;273:17651–17659. doi: 10.1074/jbc.273.28.17651. [DOI] [PubMed] [Google Scholar]
- 16.Irie K, Gotoh Y, Yashar B M, Errede B, Nishida E, Matsumoto K. Stimulatory effects of yeast and mammalian 14-3-3 proteins on the Raf protein kinase. Science. 1994;265:1716–1719. doi: 10.1126/science.8085159. [DOI] [PubMed] [Google Scholar]
- 17.Ito Y, Sasaki Y, Horimoto M, Wada S, Tanaka Y, Kasahara A, Ueki T, Hirano T, Yamamoto H, Fujimoto J, Okamoto E, Hayashi N, Hori M. Activation of mitogen-activated protein kinases/extracellular signal-regulated kinases in human hepatocellular carcinoma. Hepatology. 1998;27:951–958. doi: 10.1002/hep.510270409. [DOI] [PubMed] [Google Scholar]
- 18.Kalkuhl A, Troppmair J, Buchmann A, Stinchcombe S, Buenemann C L, Rapp U R, Kaestner K, Schwarz M. p21Ras downstream effectors are increased in activity or expression in mouse liver tumors but do not differ between ras-mutated and ras-wild type lesions. Hepatology. 1998;27:1081–1088. doi: 10.1002/hep.510270425. [DOI] [PubMed] [Google Scholar]
- 19.Kim D W, Suzuki R, Harada T, Saito I, Miyamura T. Trans-suppression of gene expression by hepatitis C viral core protein. Jpn J Med Sci Biol. 1994;47:221–220. doi: 10.7883/yoken1952.47.211. [DOI] [PubMed] [Google Scholar]
- 20.Klein N P, Schneider R J. Activation of Src family kinases by hepatitis B virus HBx protein and coupled signaling to Ras. Mol Cell Biol. 1997;17:6427–6436. doi: 10.1128/mcb.17.11.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kyriakis J M, App H, Zhang X F, Banerjee P, Brautigan D L, Rapp U R, Avruch J. Raf-1 activates MAP kinase-kinase. Nature. 1992;358:417–421. doi: 10.1038/358417a0. [DOI] [PubMed] [Google Scholar]
- 22.Leevers S J, Paterson H F, Marshall C J. Requirement for Ras in Raf activation is overcome by targeting Raf to the plasma membrane. Nature. 1994;369:411–414. doi: 10.1038/369411a0. [DOI] [PubMed] [Google Scholar]
- 23.Luo Z, Tzivion G, Belshaw P J, Vavvas D, Marshall M, Avruch J. Oligomerization activates c-Raf-1 through a Ras-dependent mechanism. Nature. 1996;383:181–185. doi: 10.1038/383181a0. [DOI] [PubMed] [Google Scholar]
- 24.Leung T, Chen X Q, Tan I, Manser E, Lim L. Myotonic dystrophy kinase-related Cdc42-binding kinase acts as a Cdc42 effector in promoting cytoskeletal reorganization. Mol Cell Biol. 1998;18:130–140. doi: 10.1128/mcb.18.1.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mamiya N, Worman H J. Hepatitis C virus core protein binds to a DEAD box helicase. J Biol Chem. 1999;274:15751–15756. doi: 10.1074/jbc.274.22.15751. [DOI] [PubMed] [Google Scholar]
- 26.Matsumoto M, Hwang S B, Jeng K S, Zhu N, Lai M M. Homotypic interaction and multimerization of hepatitis C virus core protein. Virology. 1996;218:43–51. doi: 10.1006/viro.1996.0164. [DOI] [PubMed] [Google Scholar]
- 27.Matsumoto M, Hsieh T-Y, Zhu N, VanArsdale T, Hwang S B, Jeng K-S, Gorbalenya A E, Lo S-Y, Ou J-H, Ware C F, Lai M M C. Hepatitis C virus core protein interacts with the cytoplasmic tail of lymphotoxin-β receptor. J Virol. 1997;71:1301–1309. doi: 10.1128/jvi.71.2.1301-1309.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McKillop I H, Schmidt C M, Cahill P A, Sitzmann J V. Altered expression of mitogen-activated protein kinases in a rat model of experimental hepatocellular carcinoma. Hepatology. 1997;26:1484–1491. doi: 10.1002/hep.510260615. [DOI] [PubMed] [Google Scholar]
- 29.Morrison D. 14-3-3: modulaters of signaling proteins? Science. 1994;266:56–57. doi: 10.1126/science.7939645. [DOI] [PubMed] [Google Scholar]
- 30.Morrison D K, Cutler R E., Jr The complexity of Raf-1 protein. Curr Opin Cell Biol. 1997;9:174–179. doi: 10.1016/s0955-0674(97)80060-9. [DOI] [PubMed] [Google Scholar]
- 31.Moriya K, Fujie H, Shintani Y, Yotsuyanagi H, Tsutsumi T, Ishibashi K, Matsuura Y, Kimura S, Miyamura T, Koike K. The core protein of hepatitis C virus induces hepatocellular carcinoma in transgenic mice. Nat Med. 1998;4:1065–1067. doi: 10.1038/2053. [DOI] [PubMed] [Google Scholar]
- 32.Nishizono A, Hiraga M, Mifune K, Terao H, Fujioka T, Nasu M, Goto T, Misumi J, Moriyama M, Arakawa Y, Hayashi N, Esumi M, Shikata T. Correlation of serum antibody titers against hepatitis C virus core protein with clinical features by Western blot (immunoblot) analysis using a recombinant vaccinia virus expression system. J Clin Microbiol. 1993;31:1173–1178. doi: 10.1128/jcm.31.5.1173-1178.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Niwa H, Yamamura K, Miyazaki J. Efficient selection for high-expression transfectants with a novel eukaryotic vector. Gene. 1991;108:93–99. doi: 10.1016/0378-1119(91)90434-d. [DOI] [PubMed] [Google Scholar]
- 34.Pallas D C, Fu H, Haehnel L C, Weller W, Collier R J, Roberts T M. Association of polyomavirus middle tumor antigen with 14-3-3 proteins. Science. 1994;265:535–537. doi: 10.1126/science.8036498. [DOI] [PubMed] [Google Scholar]
- 35.Ray R B, Lagging L M, Meyer K, Ray R. Hepatitis C core protein cooperates with ras and transforms primary rat embryo fibroblasts to tumorigenic phenotype. J Virol. 1996;70:4438–4443. doi: 10.1128/jvi.70.7.4438-4443.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ray R B, Lagging L M, Meyer K, Steele R, Ray R. Transcriptional regulation of cellular and viral promoters by hepatitis C virus core protein. Virus Res. 1995;37:209–220. doi: 10.1016/0168-1702(95)00034-n. [DOI] [PubMed] [Google Scholar]
- 37.Ray R B, Steele R, Meyer K, Ray R. Transcriptional repression of p53 promoter by hepatitis C virus core protein. J Biol Chem. 1997;272:10983–10986. doi: 10.1074/jbc.272.17.10983. [DOI] [PubMed] [Google Scholar]
- 38.Rommel C, Radziwill G, Lovric J, Noeldeke J, Heinnicke T, Jones D, Aitken A, Moelling K. Activated Ras displaces 14-3-3 protein from the amino terminus of c-Raf-1. Oncogene. 1996;12:609–619. [PubMed] [Google Scholar]
- 39.Ruggieri A, Harada T, Matsuura Y, Miyamura T. Sensitization to Fas-mediated apoptosis by hepatitis C virus core protein. Virology. 1997;229:68–76. doi: 10.1006/viro.1996.8420. [DOI] [PubMed] [Google Scholar]
- 40.Saito I, Miyamura T, Ohbayashi A, Harada H, Katayama T, Kikuchi S, Watanabe Y, Koi S, Onji M, Ohta Y, Choo Q-L, Houghton M, Kuo G. Hepatitis C virus infection is associated with the development of hepatocellular carcinoma. Proc Natl Acad Sci USA. 1990;87:6547–6549. doi: 10.1073/pnas.87.17.6547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shih C-M, Chen C-M, Chen S-Y, Lee Y-H W. Modulation of the trans-suppression activity of hepatitis C virus core protein by phosphorylation. J Virol. 1995;69:1160–1171. doi: 10.1128/jvi.69.2.1160-1171.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shrivastava A, Manna S K, Ray R, Aggarwal B B. Ectopic expression of hepatitis C virus core protein differentially regulates nuclear transcription factors. J Virol. 1998;72:9722–9728. doi: 10.1128/jvi.72.12.9722-9728.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tokushige K, Moradopour D, Wakita T, Geissler M, Hayashi N, Wands J R. Comparison between cytomegalovirus promoter and elongation factor-1α promoter driven constructs in the establish of cell lines expressing hepatitis C virus core protein. J Virol Methods. 1997;64:73–80. doi: 10.1016/s0166-0934(96)02143-x. [DOI] [PubMed] [Google Scholar]
- 44.Tsukuma H, Hiyama T, Tanaka S, Nakao M, Yabuuchi T, Kitamura T, Nakanishi K, Fujimoto I, Inoue A, Yamazaki H, Kawashima T. Risk factors for hepatocellular carcinoma among patients with chronic liver disease. N Engl J Med. 1993;328:1797–1801. doi: 10.1056/NEJM199306243282501. [DOI] [PubMed] [Google Scholar]
- 45.Tzivion G, Luo Z, Avruch J. A dimeric 14-3-3 protein is an essential cofactor for Raf kinase activity. Nature. 1998;394:88–92. doi: 10.1038/27938. [DOI] [PubMed] [Google Scholar]
- 46.Vernet T, Dignard D, Thomas D Y. A family of yeast expression vectors containing the phage f1 intergenic region. Gene. 1987;52:225–233. doi: 10.1016/0378-1119(87)90049-7. [DOI] [PubMed] [Google Scholar]
- 47.Wang H-G, Takayama S, Rapp U R, Reed J C. Bcl-2 interacting protein, BAG-1, binds to and activates the kinase Raf-1. Proc Natl Acad Sci USA. 1996;93:7063–7068. doi: 10.1073/pnas.93.14.7063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Winston L A, Hunter T. JAK2, Ras, and Raf are required for activation of extracellular signal-regulated kinase/mitogen-activated protein kinase by growth hormone. J Biol Chem. 1995;270:30837–30840. doi: 10.1074/jbc.270.52.30837. [DOI] [PubMed] [Google Scholar]
- 49.Yaffe M B, Rittinger K, Volinia S, Caron P R, Aitken A, Leffers H, Gamblin S J, Smerdon S J, Cantley L C. The structural basis for 14-3-3: phosphopeptide binding specificity. Cell. 1997;91:961–971. doi: 10.1016/s0092-8674(00)80487-0. [DOI] [PubMed] [Google Scholar]
- 50.You L-R, Chen C-M, Yeh T-S, Tsai T-Y, Mai R-T, Lin C-H, Lee Y-H W. Hepatitis C virus core protein interacts with cellular putative RNA helicase. J Virol. 1999;73:2841–2853. doi: 10.1128/jvi.73.4.2841-2853.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zha J, Harada H, Yang E, Jockel J, Korsmeyer S J. Serine phosphorylation of death agonist BAD in response to survival factor results in binding to 14-3-3 not BCL-X. Cell. 1996;87:619–628. doi: 10.1016/s0092-8674(00)81382-3. [DOI] [PubMed] [Google Scholar]
- 52.Zhu N, Khoshnan A, Schneider R, Matsumoto M, Dennert G, Ware C, Lai M M C. Hepatitis C virus core protein binds to the cytoplasmic tail of tumor necrosis factor (TNF) receptor 1 and enhances TNF-induced apoptosis. J Virol. 1998;72:3691–3697. doi: 10.1128/jvi.72.5.3691-3697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]