Abstract
Cervical cancer stands as a prevalent gynaecologic malignancy affecting women globally, often linked to persistent human papillomavirus infection. Biomarkers associated with cervical cancer, including VEGF-A, VEGF-B, VEGF-C, VEGF-D, and VEGF-E, show upregulation and are linked to angiogenesis and lymphangiogenesis. This research aims to employ in-silico methods to target tyrosine kinase receptor proteins—VEGFR-1, VEGFR-2, and VEGFR-3, and identify novel inhibitors for Vascular Endothelial Growth Factors receptors (VEGFRs). A comprehensive literary study was conducted which identified 26 established inhibitors for VEGFR-1, VEGFR-2, and VEGFR-3 receptor proteins. Compounds with high-affinity scores, including PubChem ID—25102847, 369976, and 208908 were chosen from pre-existing compounds for creating Deep Learning-based models. RD-Kit, a Deep learning algorithm, was used to generate 43 million compounds for VEGFR-1, VEGFR-2, and VEGFR-3 targets. Molecular docking studies were conducted on the top 10 molecules for each target to validate the receptor-ligand binding affinity. The results of Molecular Docking indicated that PubChem IDs—71465,645 and 11152946 exhibited strong affinity, designating them as the most efficient molecules. To further investigate their potential, a Molecular Dynamics Simulation was performed to assess conformational stability, and a pharmacophore analysis was also conducted for indoctrinating interactions.
Keywords: VEGFR inhibitors, Machine-learning, Deep learning, Molecular docking, Molecular dynamics simulation, R programming, Python, ADMET studies
Subject terms: Biotechnology, Cancer, Cell biology, Computational biology and bioinformatics, Drug discovery, Structural biology, Systems biology, Mathematics and computing
Introduction
Cervical cancer, a widespread gynaecologic malignancy affecting women globally, comprises predominantly squamous cell carcinoma (80%) and adenocarcinoma (15%). Human papillomavirus (HPV) infection is the primary cause. Population-based studies highlight the vulnerability of the 35–44 age group. Early detection significantly enhances survival rates, with metastasis to lung, bone, liver, and brain tissues if untreated for an extended period1–4. Traditional treatments involve surgical excision, radiation, and chemotherapy, but complexities pose challenges5. Developing accessible medicines and screening measures for all stages of cervical cancer is crucial. The initiation of cervical cancer involves migratory-linked vascular permeability, proliferation, and survival of surrounding endothelial cells in angiogenesis and lymphangiogenesis.
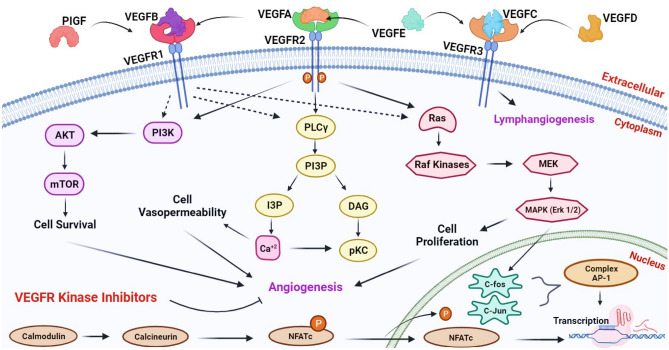
In cervical cancer, biomarkers of the vascular endothelial growth factor family exhibit upregulation. The VEGFR proteins—VEGFR-1, VEGFR-2, and VEGFR-3—interact with VEGF proteins (VEGF-A, VEGF-B, VEGF-C, VEGF-D, and VEGF-E). These VEGFR proteins, classified as tyrosine kinase receptors (RTK), initiate autophosphorylation and dimerization upon ligand binding, activating the RTK’s cytoplasmic tyrosine kinase domain. This activation leads to transcriptional regulation of various genes, triggering a signalling cascade that induces vascular permeability (increased plasma protein leakage) and supports angiogenesis and lymphangiogenesis6–8 (Fig. 1).
Figure 1.
Comprehensive Illustration of the VEGFR-VEGF Signaling Pathway Mechanism inCancer Initiation and Progression.
VEGFR-1 and VEGFR-2, situated on vascular endothelial cells, drive angiogenesis, while VEGFR-3, located on lymphatic endothelial cells, participates in lymph angiogenesis. Elevated expression levels of VEGFR-1/2 are linked to cancer development and poorer overall survival. VEGFR-1 mediates distant metastasis by binding to VEGF-A, VEGF-B, and PIGF9,10. VEGFR-2 binds to multiple VEGF variants-A/B/C/D/E, activating signalling pathways that enhance tumor growth through increased cellular proliferation. Soluble forms of VEGFR-2 and VEGF-A are elevated in the blood of cervical cancer patients11.VEGFR-3 interacts with partially processed VEGF-C, VEGF-D, and VEGF-E, whose overexpression is associated with ascites development and tumor cell spread to nearby lymph nodes, facilitating cancer progression12. Higher circulating levels of VEGF-D in pre-invasive cervical carcinomas indicate a predilection for the lymphangiogenic pathway13. Chemotherapy targets tumor cells directly, causing significant damage. However, antiangiogenic drugs, which also address stromal components, exhibit a more favourable response than chemotherapy14. Immunotherapy using receptor antibodies, short peptides, and small molecule inhibitors has emerged as a promising therapeutic and anti-angiogenic strategy. Bevacizumab, a multi-target antibody blocking VEGF receptors, is frequently used alone or in combination with other therapies for treating cervical cancer14.
The significance of small molecular compounds has grown due to high manufacturing and maintenance costs, coupled with limited optimization potential for antibody-based therapy. Small molecular compounds, functioning as tyrosine kinase inhibitors, offer a targeted approach in cancer, competing for ligand binding sites with proangiogenic factor ligands. This technique presents a focused strategy for blocking angiogenesis in both early and late-stage cervical malignancies, minimizing off-target effects in clinical oncology.Higher VEGF expression levels significantly impact the prognosis of cervical cancer patients in stages IB and IIA. Moreover, the oncoproteins E5 and E6 of HPV stimulate substantial VEGF expression, underscoring their role in cervical cancer development15–17.
In the present investigation, a comprehensive review of the literature was carried out in which 26 potent established inhibitors (Brivanib, Sunitinib, Pazopanib, etc.) were gleaned against all the VEGFR targets towards cervical cancer. Several experimental studies utilizing conventional medicinal chemistry techniques have been carried out by researchers on established compounds such as Pazopanib, Brivanib, and Sunitinib for cervical cancer. According to their research, these medications have a high prevalence of side effects, which has led to a decrease in the use of these compounds18–23. The process of finding and creating novel pharmaceuticals can be speed up and improved with the help of Artificial Intelligence (AI), especially Machine Learning and Deep Learning, which are subcategories of AI. Though it is still in its early stages, artificial intelligence is already having a profound impact on the process of finding and developing novel targeted anti-cancer therapeutics. By utilizing Deep learning and Machine learning, scientists have discovered novel compounds that can treat various human diseases like cancer24–30. Machine learning-based drug discoveries that target all of the VEGFRs has not been extensively studied so far. Therefore the current investigation aims to leverage Machine Learning, Deep Learning, Molecular Docking, Molecular Dynamics Simulation and ADMET studies to identify a novel compound having high affinity, and less toxicity targeting VEGFR1, VEGFR2, and VEGFR3 for the treatment of cervical cancer.
Methodology
Established inhibitors of VEGFR
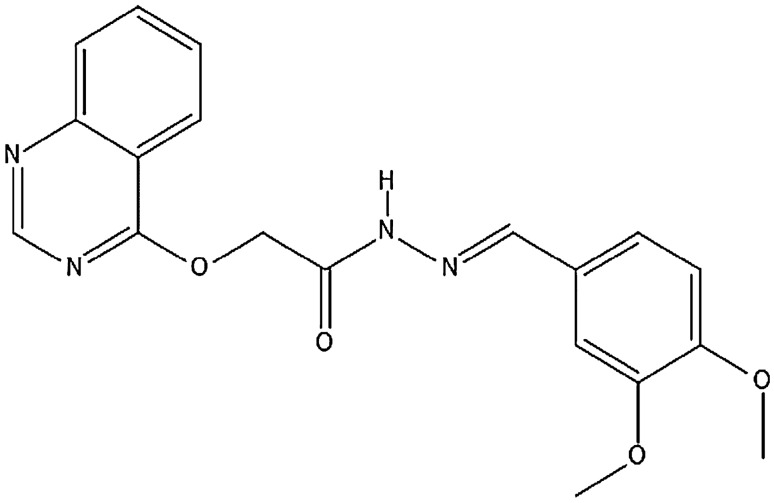
Supplementary Table 1 has provided a comprehensive overview of the chemical characteristics of validated inhibitors targeting VEGFR proteins, along with their selectivity towards the respective VEGFR isoform31–55. This data was meticulously curated through literature mining and extensive surveys of published research publications. In Fig. 2, a structural representation of a benzyl quinone derivative has been illustrated, which was generated using Marvin sketch software56.
Figure 2.
(E)-N′-(3,4-dimethoxybenzylidene)-2-(quinazolin-4-yloxy) acetohydrazide.
Ligand and Protein preparation
The co-crystallized structures of the target proteins VEGFR1, VEGFR2, and VEGFR3 were retrieved from the PDB database57,58 with the corresponding PDB IDs: 3HNG59, 1Y6A60, and 4BSJ61. These structures were stored in the PDB file format and have been summarized in Table 1. The construction of the target binding sites commenced with the identification of suitable cavities, guided by criteria outlined in Table 1, including chain type, volume, and radius62–65. The molecular structures of all 26 established inhibitors were sourced from NCBI’s PubChem chemical database (refer to Supplementary Table 1)18–23,31–55. Additionally, using Marvin sketch software, the compound ‘(E)-N’-(3,4-dimethoxybenzylidene)-2-(quinazolin-4-yloxy) acetohydrazide,’ which was not available in the PubChem database, was meticulously depicted and saved in 3D SDF file format. Subsequently, all inhibitors in .sdf format underwent further refinement in both 2D and 3D using the Marvin-sketch program before being introduced into the docking assay66–75.
Table 1.
VEGFR isoforms and their binding cavity details utilized in the present study.
| Isoform | GENE-ID | RefnSeq | UniProt ID | PDB-ID | Chain Type | Radius (Å) | Binding Site Volume (Å3) |
|---|---|---|---|---|---|---|---|
| VEGFR-1 | 2321 | NP_002010.2 | P17948 | 3HNG | A | 25 | 87.55 |
| VEGFR-2 | 3791 | NP_002244.1 | P35968 | 1Y6A | A | 25 | 22.01 |
| VEGFR-3 | 2324 | NP_891555.2 | P35916 | 4BSJ | A | 25 | 183.8 |
Molecular docking
The Molegro Virtual Docker software, serving as the docking platform, utilized high-scoring functions including Piece-wise Linear Potential (PLP), heuristic search functions, and the MolDock scoring function for energy reduction and compound scoring76–82. In the docking process, key parameters included a maximum population size of 50, a maximum iteration of 1,500, and a grid resolution of 0.383–86. All known ligands were systematically docked with the prepared target cavities87–89. Results were obtained based on parameters like ligand evaluation, internal electrostatic score (ES), internal hydrogen bond, internal angles, sp2-sp2 torsion, and energy minimization. The post-docking process involved hydrogen bond optimization and energy minimization using MolDock Simplex Evolution, with a maximum of 300 steps and a neighbour distance factor of 1.00. The final step included energy minimization of the ligand-receptor complex via Nelder Mead Simplex Minimization90–93. The resulting output file was saved in the designated directory as an .xls file. The re-rank score emerged as the primary parameter for prioritizing compounds, guiding the selection of the top 10 hits for each VEGFR receptor. Machine learning-based compound models were subsequently generated based on the compound with the lowest re-rank score29,30.
Deep learning-based de-novo designing of novel compounds
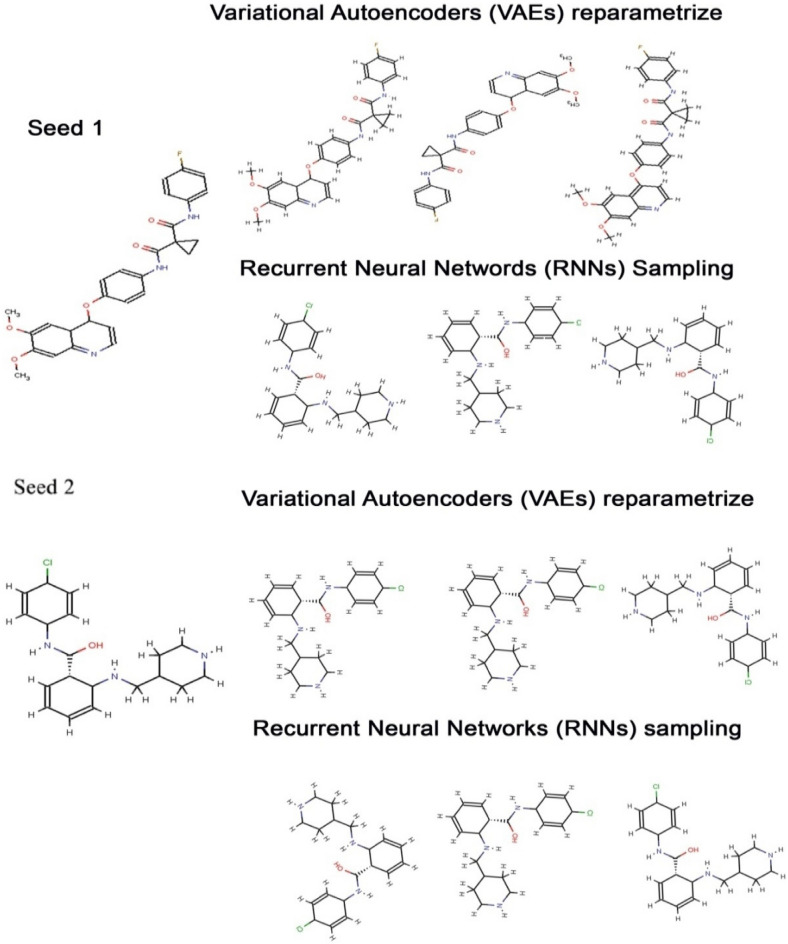
Shape-based compounds targeting VEGFR1, VEGFR2, and VEGFR3 were constructed using deep learning algorithms29,30. The chemical structures were developed using SMILES strings and corresponding chemical spatial data, and Convolutional Neural Networks (CNN) were employed for encoding structural and shape variations94–96. Compounds were trained based on H-bond donor, H-bond Acceptor, Molecular weight, and LogP values, adhering to Lipinski’s Rule of Five97. The PubChem database facilitated the extraction of compounds for constructing training and test datasets. The ligand extracted from the complex structure of each targets (VEGFR1, VEGFR2 and VEGFR3) and the best compound obtained from molecular docking of each targets (VEGFR1, VEGFR2 and VEGFR3) were considered as seed molecules for Deep Learning. Seed compounds, N-(4-Chlorophenyl)-2-((pyridin-4-ylmethyl)amino)benzamide (from the 3D structure of VEGFR1—PDB ID: 3HNG) and PubChem ID: 25102847 served as seed 1 and seed 2, respectively, for generating shape-based models. Similarly, for VEGFR2 (PDB ID: 1Y6A), the compound 2-anilino-5-aryl-oxazole inhibitor (from the co-crystallized structure) and PubChem ID: 369976 were chosen as seed 1 and seed 2. For VEGFR3 (PDB ID: 4BSJ), AXITINIB (AG-013736 or (N-Methyl-2-(3-((E)-2-pyridin-2-yl- vinyl)-1H-indazol-6-ylsulfanyl)-benzamide) and PubChem ID: 208908 were considered as seed 1 and seed 2, respectively (Fig. 3). Molecular docking results guided the selection of effectively established compounds for seed compounds, contributing to the generation of shape-based models29,30.
Figure 3.
The De-novo shape-based compounds generated for VEGFR1 by VAE and RNN sampling using seed 1 and seed 2 as reference molecules.
In Python 3.10, the RDKit98 Machine Learning module was employed to create shape-based 3D conformers for individual compounds derived from the CNN-based shape variation autoencoder. Molecular mechanics force fields (MMFF94s) were then applied to the models using Python 3.10. To compute the Van der Waals radius of each compound, the HTMD miniconda module was utilized, by applying the relevant formulae98:
where rvdw, represent the particular Van der Waals radius of each atom of the compounds. The total binary cross entropies present in the Conditional Variational Autoencoder (CVAE) in the Autoencoder was calculated using the Kullback–Leibler divergence algorithm98. Canonical SMILES of each compound were used as input for training compounds99,100.
where pi ∈ P243×5andyj ∈ Y243×5 indicated voxel arrays and the model-generated ground-truth respectively, and £KL for sample j was defined as101,102:
where σ and μ were the outputs for the variational autoencoder (VAE) encoder and J represented the dimensionality of the latent vector. The captioning network minimized multiclass log lossas illustrated below103,104:
where "N" denoted the number of different samples in a batch and "M" represented the length of the protein sequence of the VEGFR gene. The training was carried out in batches using 410 trained samples. Variational Autoencoder (VAE) and recurrent neural networks (RNN) sampling were utilized for constructing fathomable de-novo shape-based structures concerning seed 2 chemicals (Fig. 4)104–108.To ensure the convergence of the captioning network, the shape-based Variational Autoencoder (VAE) and shape captioning structures underwent extensive training. This process yielded 20 million, 15 million, and 8 million compounds targeted towards VEGFR1, VEGFR2, and VEGFR3, respectively. The learning rate of the captioning network was halved after every 25,000 iterations. Subsequently, the top 10 chemically tailored compounds, containing valuable information, were selected for the simulation investigation through molecular docking and molecular dynamics [Supplementary Data 1]. The names and PubChem IDs of these top 10 compounds were determined by conducting a structure-based similarity search against the PubChem database.
Figure 4.
Workflow used to generate shape- based features for de-novo designing of novel compound.
Generation of novel compounds
To formulate novel compounds, the generated chemical models underwent a comprehensive analysis using two sets of trained compound libraries: Recurrent Neural Networks and autoencoders reparameterizations102,109–112. From the pools of 20 million, 15 million, and 8 million shape-based generated compounds targeting VEGFR1, VEGFR2, and VEGFR3, respectively, the top 10 compounds were selected for the construction of novel compounds. These chosen 10 compounds underwent further assessment for drug-likeness and physicochemical characterization. Subsequently, leveraging the tunable homology within the seed compounds, 10 de-novo compounds were filtered and subjected for further molecular docking studies. The high-affinity compound derived from molecular docking of each target(VEGFR1, VEGFR2 and VEGFR3) were then further utilized for Molecular Dynamics Simulation (MDS)113–116.
Molecular dynamics simulation
To analyse the stability and conformational dynamics of atoms in the compound, a molecular dynamics simulation was conducted on three isoforms of VEGFR in conjunction with the best-established compound and the top machine learning-based compound. The simulation duration was set to 100 ns using the Desmond module in Schrodinger, which operated based on the Newtonian dynamic equation113–116. Initially, the TIP3P model and OPLS5 force field were employed, and the system was neutralized with NaCl ions before being placed in a cubic simple point charge (SPC) water box. A two-step energy minimization at 50,000 ps was conducted until completion117–119. The simulation ran for 100 ns at an ambient temperature of 1.013 bar with a temperature set at 310 K109,120. To ensure high-quality results, the simulation was performed three times, generating around 1000 frames per simulation. The findings provided insights into thermodynamic aspects, including root mean square fluctuations (RMSF) and root mean square deviation (RMSD), unveiling structural and functional characteristics of the protein–ligand complex109,120.
Pharmacophore studies
Amino acid residues participating in crucial hydrogen bond interactions were identified through the Molegro Virtual Docker. Various forms of chemical bond interactions, including hydrogen bonds, Vander Waals interactions, ionic bonds, covalent bonds, and water interactions within different pairs of receptor-inhibitor complexes, were comprehensively analyzed using the Discovery Studio 3.5 Visualize program121–125.
ADMET studies and Boiled-egg plot
The AdmetSAR server was utilized for predicting the chemical profiles of small molecules with anticipated drug-like properties, encompassing aspects of absorption, digestion, metabolism, excretion, and toxicity126. These predictions offer insights into the behavior and efficacy of these molecules during clinical trials. ADMET studies play a crucial role in forecasting a drug’s potential success based on pharmacokinetic features, including bioactivity and toxicity127–129. For each VEGFR isomer, R Studio was employed to create a graphical interface for data visualization, facilitating a comparison between machine learning-built compounds and the best-established compounds. The Boiled Egg plot, a statistical visualization generated using the SwissADME web tool, was employed to assess the blood–brain barrier and gastrointestinal permeability130. This plot considered factors such as molecular weight, total polar surface area, MLogP value, gastrointestinal absorption, and blood–brain barrier characteristics130. The Boiled Egg plot was divided into three regions: yellow or yolk (indicating a higher probability of Blood–Brain Barrier penetration), white (indicating a good chance of intestinal absorption), and grey (indicating a low probability of intestinal absorption, characterizing a compound as non-absorptive and non-penetrative). After docking with each VEGFR isoform, based on a lower re-rank score for generating the Boiled Egg plot, two best-established compounds and two best machine learning-based compounds were identified. Each drug was separately evaluated for gastrointestinal absorption and blood–brain barrier characteristics131–137.
Drug—drug comparison
The machine learning-based compound with the lowest re-rank score was sourced from the PubChem compound database. The protein structure was cleaned by reconfiguring all constraints, cavities, and ligands, resulting in a pristine protein structure saved in SDF format138–140. The best-established inhibitor was also cleaned and imported in .sdf format for a drug-drug comparison study. The comparison between the best-established molecule and the best machine learning-based compound with VEGFR isomers considered criteria such as relative hydrogen bond interactions, steric energy, and a lower re-rank score141–144.
Results and discussion
Protein and ligand preparation
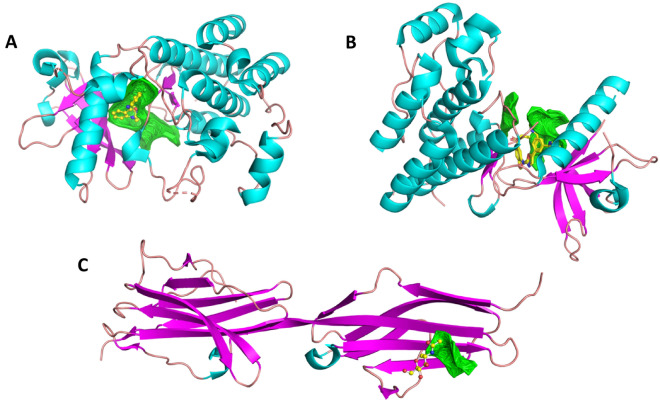
VEGFR-1 (PDB ID: 3HNG) is a 15.2 kDa dual-chained heterotetrametric structure with 2299 atoms, 2374 bonds, 17 helices, and 7 beta-strands (Fig. 5A). VEGFR-2 (PDB ID: 1Y6A) is an 86.57 kDa double-chained protein, with about 2099 atoms, 2217 bonds, fourteen helices, and 7 beta strands (Fig. 5B). VEGFR-3 (PDB ID: 4BSJ) is a 13.59 kDapentachained protein, featuring 213 groups, 1673 atoms, 1751 chemical interactions, 2 helices, and 19 beta strands (Fig. 5C). For the molecular docking study, the first cavity of all receptors was selected, with VEGFR-1’s having a volume of 87.55, VEGFR-2’s with a volume of 22.016, and VEGFR-3’s with a volume of 183.8. The radius for all three receptors was maintained at 25 Å.
Figure 5.
Representation of Protein 3D structure of VEGFR’s obtained from PDB—(A). VEGFR-1 (PDBID: 3HNG); (B). VEGFR-2 (PDBID: 1Y6A); (C). VEGFR-1 (PDBID: 4BSK); in which, each color represents as helix (Cyan), sheet (pink), loop (dark tints), and co-crystalized compound as yellow with elements color, as well as binding pocket in green.
Molecular docking studies
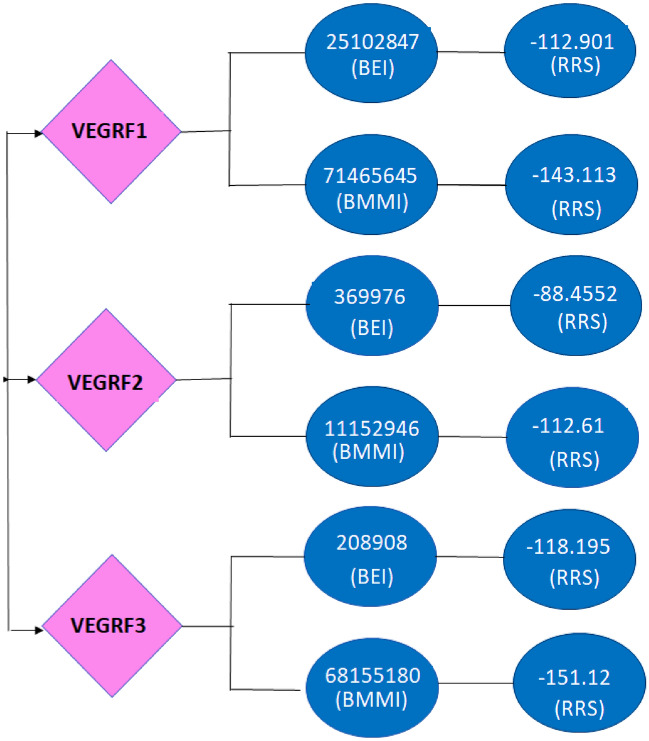
The Molegro Virtual Software’s Docking Wizard loaded all inhibitors into the created target cavity for molecular docking. The top 10 compounds with the lowest re-rank and MolDock Score, along with their molecular weights, were extracted and presented in tabular format for all three VEGFR isoforms. Notably, Compound Cabozantinib (22) with PubChem ID: 25102847 against the VEGFR-1 receptor (Table 2), Compound TNP-470(23–24) with PubChem ID: 36997 against the VEGFR-2 receptor (Table 3), and Lapatinib (25) with PubChem ID: 208908 against the VEGFR-3 receptor (Table 4) were selected for the construction of Machine Learning Models.
Table 2.
Molecular docking: established inhibitors against VEGFR-1.
| PubChem ID | Name | MolDock score (KJ/mol) | Re-rank score (KJ/mol) | MW (g/mol) |
|---|---|---|---|---|
| [00] 25102847 | Cabozantinib | − 155.779 | − 112.901 | 501.506 |
| [01] 25102847 | Cabozantinib | − 141.584 | − 107.22 | 501.506 |
| [04] 208908 | Lapatinib | − 133.312 | − 103.862 | 581.058 |
| [01] 10297043 | AEE-788 | − 139.967 | − 102.39 | 440.583 |
| [01] 208908 | Lapatinib | − 136.122 | − 101.227 | 581.058 |
| [01] 11234052 | Brivanib | − 128.031 | − 100.245 | 370.378 |
| [00] 6450551 | Axitinib | − 122.085 | − 99.9181 | 386.47 |
| [02] 9933475 | Cediranib | − 130.775 | − 98.8826 | 450.505 |
| [04] 10297043 | AEE-788 | − 128.374 | − 98.5575 | 440.583 |
| [03] 25017411 | Anlotinib | − 126.322 | − 98.0086 | 407.437 |
| [00] 442126 | Decursin | − 122.708 | − 97.7696 | 328.359 |
Table 3.
Molecular docking: established inhibitors against VEGFR-2.
| PubChem ID | Name | MolDock score (KJ/mol) | Re-rank score (KJ/mol) | MW (g/mol) |
|---|---|---|---|---|
| [00] 369976 | Tnp-470 | − 116.686 | − 88.4552 | 401.882 |
| [00] 208908 | Lapatinib | − 131.941 | − 88.4185 | 581.058 |
| [01] 369976 | Tnp-470 | − 111.987 | − 87.8381 | 401.882 |
| [02] 369976 | Tnp-470 | − 114.702 | − 87.0651 | 401.882 |
| [01] 25017411 | Anlotinib | − 114.843 | − 85.8629 | 407.437 |
| [00] 25102847 | Cabozantinib | − 115.648 | − 85.6008 | 501.506 |
| [01] 208908 | Lapatinib | − 137.694 | − 85.1272 | 581.058 |
| [03] 25102847 | Cabozantinib | − 106.648 | − 84.6492 | 501.506 |
| [04] 25017411 | Anlotinib | − 119.836 | − 83.8888 | 407.437 |
| [01] 11234052 | Brivanib | − 116.095 | − 83.869 | 370.378 |
| [01] 25102847 | Cabozantinib | − 111.291 | − 81.7752 | 501.506 |
Table 4.
Molecular docking: Established inhibitors against VEGFR-3.
| PubChem ID | Name | MolDock score (KJ/mol) | Re-rank score (KJ/mol) | MW (g/mol) |
|---|---|---|---|---|
| [02] 208908 | Lapatinib | − 158.144 | − 118.195 | 581.058 |
| [00] 9911830 | Tivozanib | − 144.231 | − 113.369 | 454.863 |
| [03] 208908 | Lapatinib | − 152.213 | − 109.955 | 581.058 |
| [01] 25102847 | Cabozantinib | − 133.435 | − 108.037 | 501.506 |
| [01] 17536 | Oxypeucedanin hydrate | − 134.001 | − 106.623 | 304.295 |
| [00] 6398883 | AAL-993 | − 129.671 | − 106.112 | 371.356 |
| [01] 10297043 | AEE-788 | − 124.179 | − 105.301 | 440.583 |
| [04] 208908 | Lapatinib | − 148.129 | − 104.772 | 581.058 |
| [04] 5329102 | Sunitinib | − 136.513 | − 103.711 | 398.474 |
| [02] 6398883 | AAL-993 | − 121.182 | − 101.047 | 371.356 |
| [02] 25017411 | Anlotinib | − 129.917 | − 100.759 | 407.437 |
Molecular docking of top 10 compounds obtained from machine learning
The top 10 compounds were chosen for Molecular Docking from a pool of 43 million compounds generated by Machine Learning models, following filtration based on drug-likeness and physicochemical characteristics. With the lowest re-rank scores, compounds 71465645 (Table 5), 11152946 (Table 6), and 1115294 (Table 7) emerged as the top candidates. Compound 71465645 (26) exhibits 4 Hydrogen Bond Donors (HBD), 10 Hydrogen Bond Acceptors (HBA), and a Topological Polar Surface Area of 148.2 in a 606.6 Da compound. Compound 11152946 (27), a 371.9 Da compound, features an HBD: HBA count of 1:5 and a Topological Polar Surface Area of 80.52. Compound 68155180 (28) possesses a Topological Polar Surface Area of 127.2, a molecular weight of 596.1 Da, along with three HBD and ten HBA. The re-rank scores for these three compounds are lower than the best-established inhibitors for the targeted VEGFR isoforms, suggesting their potential as superior drug candidates (Fig. 6). To validate this hypothesis, a drug-drug comparison analysis was conducted.
Table 5.
VEGFR-1: Molecular Docking of Top 10 compounds obtained from Machine Learning.
| Compound ID | Ligand | MolDock score (KJ/mol) | Re-rank score (KJ/mol) | MW (g/mol) |
|---|---|---|---|---|
| [00] 71465645 | 71465645 | − 172.118 | − 143.113 | 606.573 |
| [00] 42642645 | 42642645 | − 168.825 | − 128.832 | 632.700 |
| [00] 71576419 | 71576419 | − 164.778 | − 126.609 | 588.600 |
| [00] 11594543 | 11594543 | − 162.423 | − 125.364 | 296.320 |
| [00] 56604907 | 56604907 | − 156.805 | − 125.303 | 528.500 |
| [00] 11317348 | 11317348 | − 161.427 | − 122.725 | 469.400 |
| [02] 86269669 | 86269669 | − 154.597 | − 122.698 | 474.400 |
| [01] 86269462 | 86269462 | − 156.966 | − 122.625 | 597.600 |
| [02] 78325042 | 78325042 | − 154.085 | − 122.197 | 517.500 |
| [01] 57810164 | 57810164 | − 155.67 | − 121.197 | 408.400 |
| [01] 46189868 | 46189868 | − 149.907 | − 120.952 | 828.600 |
Table 6.
VEGFR-2: Molecular Docking of Top 10 compounds obtained from Machine Learning.
| Compound ID | Ligand | MolDock score (KJ/mol) | Re-rank score (KJ/mol) | MW |
|---|---|---|---|---|
| [01] 11152946 | 11152946 | − 115.968 | − 112.61 | 371.856 |
| [00] 125367 | 125367 | − 117.208 | − 101.185 | 325.400 |
| [02] 9930932 | 9930932 | − 113.044 | − 101.011 | 401.900 |
| [00] 5326425 | 5326425 | − 117.093 | − 100.301 | 403.900 |
| [00] 60791 | 60791 | − 117.015 | − 99.101 | 401.900 |
| [04] 45482966 | 45482966 | − 110.481 | − 99.100 | 381.500 |
| [00] 45482954 | 45482954 | − 116.648 | − 98.562 | 410.500 |
| [02] 44385040 | 44385040 | − 111.353 | − 96.231 | 387.900 |
| [01] 44343813 | 44343813 | − 115.426 | − 96.117 | 401.900 |
| [00] 14942882 | 14942882 | − 113.483 | − 95.917 | 401.900 |
| [01] 163806427 | 163806427 | − 110.475 | − 93.564 | 483.800 |
| [00] 163508288 | 163508288 | − 104.696 | − 81.739 | 508.400 |
Table 7.
VEGFR-3: Molecular Docking of Top 10 compounds obtained from Machine Learning.
| Name | Ligand | MolDockScore(KJ/mol) | Re-rank Score(KJ/mol) | MW |
|---|---|---|---|---|
| [00] 68155180 | 68155180 | − 198.138 | − 151.12 | 596.100 |
| [00] 9941095 | 9941095 | − 181.263 | − 148.112 | 925.500 |
| [00] 91798457 | 91798457 | − 279.923 | − 146.013 | 1868.900 |
| [02] 11679357 | 11679357 | − 278.288 | − 143.102 | 753.300 |
| [00] 208909 | 208909 | − 241.055 | − 142.001 | 915.400 |
| [00] 16219404 | 16219404 | − 189.778 | − 141.500 | 671.000 |
| [01] 139061731 | 139061731 | − 99.451 | − 140.911 | 925.500 |
| [00] 11181296 | 11181296 | − 112.116 | − 138.186 | 473.900 |
| [01] 5329480 | 5329480 | − 218.191 | − 136.109 | 598.100 |
| [00] 118753054 | 118753054 | − 171.121 | − 132.876 | 597.100 |
Figure 6.
Re-rank Score Comparison between Established versus Machine Learning (ML) model compounds. BEI = Best established inhibitor; BMLI = Best ML model inhibitor; RRS = Re-rank score.
Molecular dynamics simulation
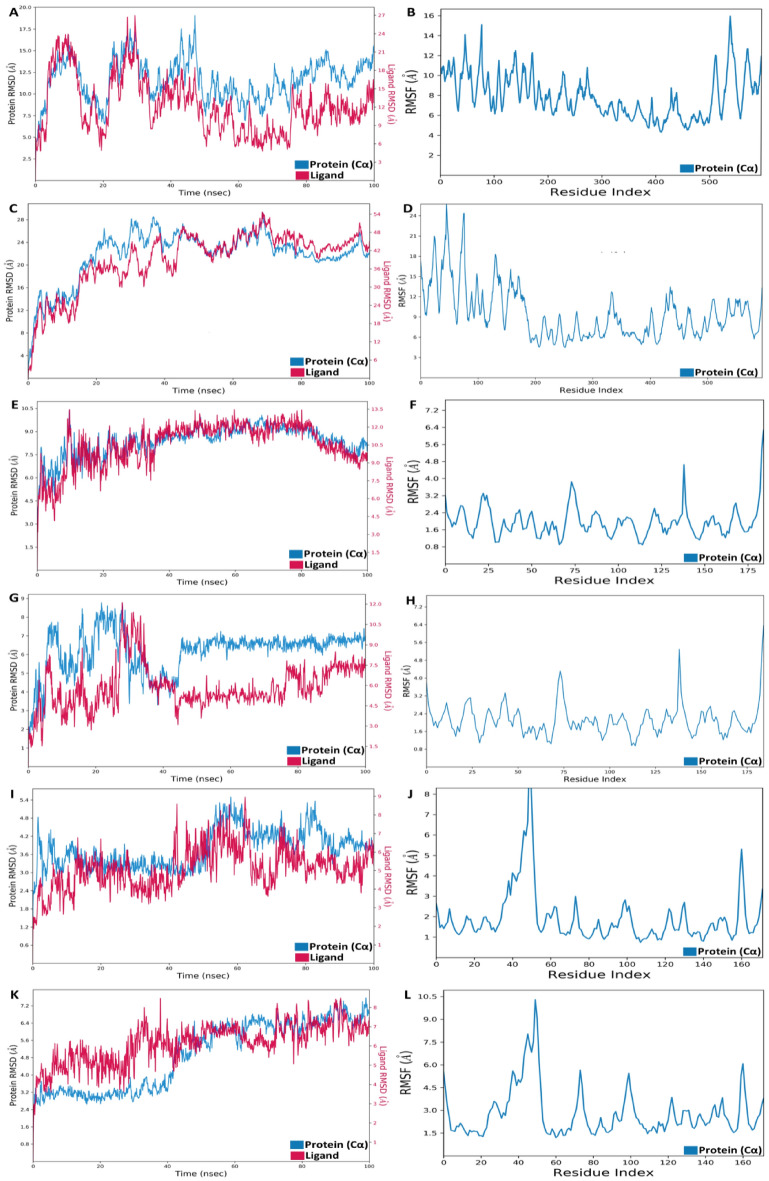
Following the docking of the protein–ligand complex, a dynamic simulation was conducted over 100 ns, assessing the complex’s thermodynamic stability through the examination of Root Mean Square Fluctuations (RMSF) and Root Mean Square Deviation (RMSD) values. The RMSD data offered insights into the intermolecular distances between the protein and ligand molecules, providing a measure of the overall stability of the complex throughout the simulation. Meanwhile, RMSF values illustrated the fluctuations of individual protein residues during the entire 100 ns simulation, shedding light on the dynamic behavior of the complex at the residue level. In the VEGFR-1:25102847 complex, as depicted in Fig. 7A & B, the Root Mean Square Deviation (RMSD) value for the protein exhibited a range of 6.5–17 Å, with an average RMSD of 13 Å in the last 50 ns of the simulation in the presence of the ligand. Meanwhile, the flexibility of residues within the protein showed a fluctuation range of 4.2–15.6 Å. Despite occasional conformational changes, the RMSD value for the VEGFR-1:71465645 complex was higher, albeit with less fluctuation. The most substantial fluctuation observed was 28 Å for the protein and 54 Å for the ligand, likely attributed to highly flexible residues within the range of 5–26 Å, as illustrated in Fig. 7C & D.In contrast, the RMSD values in the VEGFR-2:369976 complex remained relatively stable in the presence of the ligand, fluctuating within the range of 4.5–10 Å with an average of 9 Å for the ligand, while the protein exhibited fluctuations within the range of 7.5–12 Å with an average of 12 Å over the 100-ns simulation. Notably, the residual flexibility in this complex displayed the lowest trend, ranging from 0.9 to 5.9 Å, as illustrated in Fig. 7E & F. Furthermore, the RMSD values of both the protein and the ligand in the VEGFR-2:11152946 complex demonstrated high fluctuations during the initial 45 ns, ranging from 2 to 8.8 Å for the protein and 2–12 Å for the ligand. However, in the last 55 ns, the average RMSD value for the protein stabilized at 6.5 Å, possibly attributed to less flexible residues within the range of 0.8 to 5.6 Å, as depicted in Fig. 7G & H. Furthermore, the protein RMSD value for the VEGFR-3:208908 complexes exhibited fluctuations in the range of 2.4–7.2 Å, averaging 3.6 Å for the initial 50 s and 4.8 Å for the subsequent fluctuating period of 60 ns. These variations could be attributed to occasional peak fluctuations in the residues, as illustrated within the range of 1.5–9.0 Å, as shown in Fig. 7I & J. In contrast, the VEGFR-3:68155180 complex demonstrated RMSD stability within 3.3 Å for the first 40 ns, followed by an increase, indicating flexibility in amino-acid residues that escalated up to 10 Å, as depicted in Fig. 7K & L.
Figure 7.
Molecular dynamics Root Mean Square Deviation (RMSD) and Root Mean Square Fluctuation (RMSF) analyses of VEGFR-1, VEGFR-2, and VEGFR-3 complexes with both the best-established compounds and Machine Learning-based generated compounds. The VEGFR-1 complexes are represented with PubChem ID: 25102847 for the best-established compound (A & B) and PubChem ID: 71465645 for the best ML Model compound (C & D). Similarly, the VEGFR-2 complexes are illustrated with PubChem ID: 369976 for the best-established compound (E & F) and PubChem ID: 11152946 for the best ML Model compound (G & H). Lastly, the VEGFR-3 complexes are depicted with PubChem ID: 208908 for the best-established compound (I & J) and PubChem ID: 68155180 for the best ML Model compound (K & L).
Simulated complex interaction profile
The interaction between the protein and ligand was scrutinized through a histogram and heatmap, illustrating the interaction fraction and types of interactions, including hydrogen bonding, hydrophobic bonds, ionic bonds, and water bridges. In the VEGFR-1:25102847 complex, numerous residues engage in hydrophobic interactions, with one demonstrating ionic bonding. Notably, residues such as TRY 105 and VAL 67 exhibit robust interactions with the ligand, forming hydrophobic bonds with the ligand’s benzene ring, amine, and keto group [Supplementary Data II Fig. I]. These interactions are attributed to the hydrophobic nature of the compound. In contrast, the VEGFR-1:71465645 complex showcases a prevalence of hydrogen bonding over hydrophobic bonding. Nevertheless, residues such as TRY 105 and VAL 67 continue to play a significant role, forming strong hydrogen bonds with the ligand and emphasizing the robust interaction between the ligand and the protein molecule [Supplementary Data II Fig. II].
In the MD simulation analysis of the best-established molecule, (PubChem CID: 369976) with VEGFR-2, hydrogen bond interactions emerged as the predominant chemical interactions. SER 153 and ILE 154, involved in hydrogen bonding and water bridge formation, were the key contributors, donating their side chains to the ligand’s keto-oxygen. Additionally, LEU 157 made substantial hydrophobic interactions. Specifically, SER 153 formed two bonds, comprising one hydrogen bond and another with a water molecule contact, with interaction occupancies of 54 percent and 45 percent, respectively. ILE 154 interfaced with one of SER 153’s interacting oxygen with 46 percent occupancy. On average, five amino acid residues engaged with the ligand, illustrating the multifaceted nature of the interactions [Supplementary Data II Fig. III]. Conversely, the intermolecular interaction between the VEGFR-2 receptor complexed with its best ML Model compound (PubChem CID: 11152946) was characterized as primarily hydrophobic, accompanied by a substantial number of hydrogen bonding events facilitated by water bridges. Notably, TYR 214 and ILE 212 formed a robust water bridge, while interacting hydrophobically with TRP 179 and LEU 157. Hydrogen bonding interactions involved CYS 150 and LEU 151. The interaction occupancy of ILE 212 with the water molecule was notable at 36 percent. On average, the residue made six interactions with the lead compound, highlighting the intricate interplay of hydrophobic and hydrogen bonding interactions in this complex [Supplementary Data II Fig. IV].
In the MD simulations of VEGFR-3 with the best-established compound (PubChem CID: 208908), TYR 109 emerged as a key regulator of protein–ligand interaction, prominently involved in hydrophobic contacts and water bridge formation. Other amino acid residues with notable interaction frequencies included ALA 64, contributing to hydrogen bonds, water bridges, and hydrophobic interactions; ILE 49 and LEU 96, involved in hydrophobic interactions; LEU 97, engaged in hydrogen bonds; and PRO 91, facilitating hydrogen bonds and water bridges in the latter half of the simulation period. On average, an amino acid residue made approximately 5 contacts with the ligand, as illustrated in Supplementary Data II Fig. V. In comparison, the best ML Model compound for VEGFR-3:68155180 exhibited a 1.4-fold increase in interaction frequency with TYR 109 compared to the best-established compound (208908). Additionally, the ML Model compound demonstrated heightened interactions with TYR 109, TRP 61, ASN 104, TRP 59, LEU 96, LEU 47, and VAL 130. TYR 109, through side chain donation, exhibited a 43 percent interaction occupancy with the ligand’s molecule, while ASN 104 and TRP 59 demonstrated 40 percent and 63 percent interaction occupancy, respectively, primarily through the creation of robust hydrophobic interactions with the ligand’s benzene ring. Moreover, the average number of amino acid residues coming into contact with the ML Model compound increased to eight [as shown in Supplementary Data II Fig. VI].
Examination of ligand properties during simulation
The characteristics of ligands in association with VEGFR compounds, including both the best-established and ML model compounds, were examined through a comprehensive analysis of Molecular Surface Area (MolSA), equivalent to the Van der Waals surface area; Solvent Accessible Surface Area (SASA), representing the surface area accessible to water molecules; and Polar Surface Area (PSA), denoting the surface area accessible for polar bond interactions. Additionally, parameters such as Root Mean Square Deviation (RMSD) and Radius of Gyration (rGyr) were evaluated to gauge the atomic moment of the compounds.
The RMSD values for the VEGFR-1: 25102847 complexes exhibited a range of 1–3 Å until 30 ns, gradually stabilizing around an average value of approximately 2.7 Å. In contrast, the rGyr graph indicated minimal variation, maintaining an average value of about 6.2 Å, ranging from 5.7 to 6.6 Å. Over the initial 30 ns, the molecule’s surface area displayed fluctuations, subsequently stabilizing around 464 Å2. The average surface area exposed to solvent settled at 210 Å2, while the polar surface area fluctuated between 100 to 120 Å2, with a mean of 110 Å2 [Supplementary Data III Fig. A]. For the VEGFR-1:71465645 complex, the RMSD value was comparatively lower, averaging 1.4 Å, while the rGyr graph indicated a mean value of 6.5 Å. The molecular surface area ranged from 540 to 550 Å2, with a mean value of 530 Å2, providing increased interaction contact compared to the previous complex structure. Despite a polar surface area of about 225 Å2, the solvent surface area exhibited variability, ranging from 200 to 400 Å2 [Supplementary Data III Fig. B].
In contrast to the best-established complex VEGFR-2:369976, the ligand properties associated with the VEGFR-2 receptor exhibited a notable decrease in the best ML Model compound complex VEGFR-2:11152946 [Supplementary Data III Fig. C and Fig. D]. The VEGFR2:369976 complex displayed an average RMSD of 1.8 Å, which was higher than the 0.6 Å observed in the VEGFR-2:11152946 complex. For the VEGFR-2:369976 complex, the values for rGyr, MolSA, SASA, and PSA ranged from 4 to 4.75 Å, 360–384 Å2, and 180–360 Å2, respectively. Conversely, in the VEGFR-2:11152946 complex, these values were within the ranges of 3.6–4.4 Å, 315–360 Å2, and 100–130 Å2, respectively. Notably, there was a discernible fluctuation trend in all parameters in the VEGFR-2:11152946 complex, spanning from 30 ns to roughly 42 ns, attributed to protein–ligand interactions [Supplementary Data II Fig. IV].
Whereas, in the VEGFR-3: 208908 complexes, the RMSD value of the ligand ranged from 1.5 to 3 Å, with a mean of 2.4 Å. The moment of inertia was concentrated in the range of 5–7.2 Å, with a mean of 6.13 Å. The Molecular Surface Area (MolSA) was around 520 Å2, and the surface interface for solvent averaged between 300 and 400 Å2, while the polar surface area was approximately 127 Å2 [Supplementary Data III Fig. E]. Conversely, in the VEGFR3: 68155180 complexes, a larger RMSD value was observed, with the rGyr value ranging from 5.6 to 8 Å, stabilizing in the second half of the simulation time. The molecular surface area was approximately 530 Å2 larger than the previous complex, yet the accessible surface area showed reduced variability, averaging between 90 and 250 Å2. The polar surface area exhibited inconsistency across the simulation period, with an average of around 170–180 Å2 [Supplementary Data III Fig. F]. These dynamic assessments of ligand behavior in complex with VEGFR-3 provide valuable insights into the structural stability and interaction profiles of these complexes.
Drug—drug comparative studies
The drug-drug comparison involves assessing the efficiency of an ML Model compound in comparison to an established compound for a specific inhibitor. This evaluation is based on the disparity in values across various parameters detailed in [Supplementary Table II]. Individual re-rank scores are assigned to key parameters such as Total Energy, Protein–Ligand Interactions, Steric (by PLP), and Torsional Strain, measured in kJ/mol. These scores play a crucial role in determining the stability of the interaction. For VEGFR-1, the ML Model compound consistently outperforms the best-established compound, as reflected in lower re-rank scores across all parameters. Notably, the Protein–Ligand interactions and Steric (by PLP) parameters exhibit more negative values for the best-established inhibitor of VEGFR-2, indicating a potentially superior interaction. In the case of VEGFR-3, the ML Model inhibitor demonstrates a higher Torsional Strain compared to the best-established compound. However, the Total Energy re-rank score is consistently more negative for the ML Model inhibitors across all three VEGFR receptors, suggesting enhanced efficiency. Overall, the ML Model compounds exhibit favorable re-rank scores, particularly in total Energy, indicating their potential as more efficient inhibitors across the VEGFR receptors.
Pharmacophore interactive studies
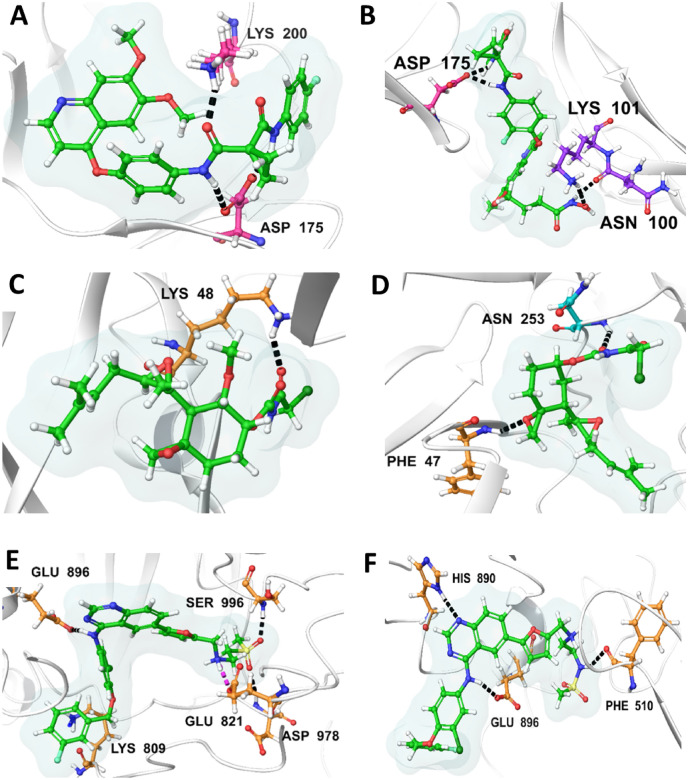
Hydrogen bond interactions play a crucial role in contributing to the stability of protein–ligand complexes. Examining the interactions in VEGFR-1, the best-established compound (ID: 25102847) forms two hydrogen bonds with Lys200 and Asp175 residues, as illustrated in (Fig. 8A). In contrast, the best ML model compound (ID: 71465645) establishes three hydrogen bonds with Asn100, Lys101, and Asp175, as depicted in (Fig. 8B). Moving to VEGFR-2, the best-established compound (ID: 369976) engages in a single hydrogen bond interaction with Lys48, as shown in (Fig. 8C). Conversely, the ML model compound (ID: 11152946) establishes two hydrogen bonds with Phe47 and Asn253, as illustrated in (Fig. 8D). In the case of VEGFR-3, the established inhibitor (ID: 208908) forms five hydrogen bonds with residues Lys809, Glu821, Glu896, Asp978, and Ser996, as seen in (Fig. 8E). On the other hand, the best ML model compound (ID: 68155180) engages in three hydrogen bond interactions with residues Phe510, His890, and Glu896, as depicted in (Fig. 8F).
Figure 8.
Illustrating the 3D interaction profiles of the most effective established and machine-learning (ML) model compounds against VEGFRs: (A) The most effective established compound PubChem ID: 25102847, shows H-bond interactions with VEGFR-1 [Pink—Y Chain Residues, Green—Ligand], (B) The most effective Machine Learning model compound PubChem ID: 71465645, shows H-bond interactions with VEGFR-1 [Pink—Y Chain & Purple—V Chain Residues, Green—Ligand]; (C) The most effective established compound PubChem ID: 369976, shows H-bond interactions with VEGFR-2 [Golden—A Chain Residues, Green—Ligand], (D) The most effective machine learning model compound PubChem ID: 11152946, shows H-bond interactions with VEGFR-2 [Golden—A Chain & Cyan—V Chain Residues, Green—Ligand]; (E) The most effective established compound PubChem CID: 208908, shows H-bond interactions with VEGFR-3 [Golden—A Chain Residues, Green—Ligand], (F) The most effective machine learning model compound PubChem CID: 68155180 shows H-bond interactions with VEGFR-3[Golden—A Chain Residues, Green—Ligand].
Supplementary Data IV represents the surface image active site cleft of bound VEGFR Receptor with different inhibitors which are used to understand the chemical interactions happening between two protein entities in a complex. Compound ID 25102847 established 7 electrostatic interactions and 12 Van der Wall interactions [Supplementary Data IV Fig. I:A] while 71465645 established 11 electrostatic interactions and 13 Van der Wall [Supplementary Data IV Fig. I:B]. 369976 established 9 electrostatic interactions and 10 Van der Wall interactions [Supplementary Data IV Fig. I:C] while 11152946 established 7 electrostatic interactions and 11 Van der Wall [Supplementary Data IV Fig. I:D]. 208908 established 13 electrostatic interactions and 11 Van der Wall interactions [Supplementary Data IV Fig. I:E] while 68155180 established 11 electrostatic interactions and 15 van der Wall [Supplementary Data IV Fig. I:F].
ADMET studies
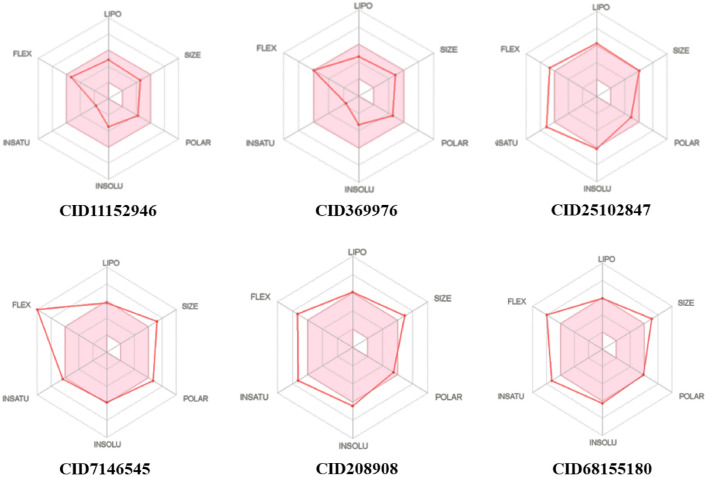
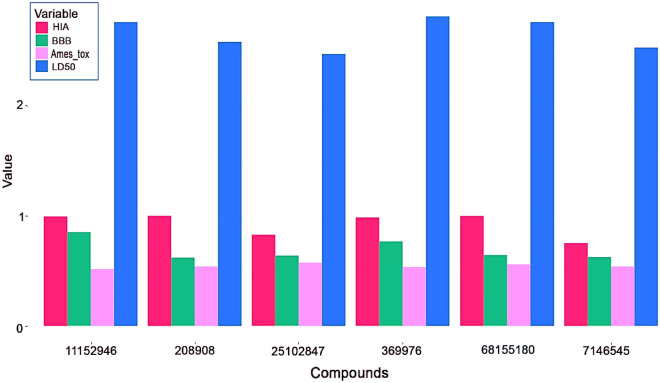
ADMET analysis played a pivotal role in assessing the viability of the best machine learning model compound for its potential in blocking VEGFR receptors. This analysis, integral in drug development, serves to predict a medicine’s clinical success, thereby minimizing the likelihood of drug failure. Predictions encompassing carcinogenicity, toxicity, blood–brain barrier penetration, and human intestinal barrier permeation were crucial in evaluating the drug’s suitability for clinical trials, particularly for screening inhibitors of VEGFR 1, VEGFR2, and VEGFR 3, as detailed in Supplementary Table III. The bioavailability radar, generated by SwissADME software, provided a comprehensive overview of the best-established compounds and machine learning compounds inhibiting the target receptor proteins VEGFR 1, VEGFR2, and VEGFR 3 (Fig. 9). The pink region in the radar represents the optimal range of values. Notably, all compounds demonstrated the ability to penetrate the blood–brain barrier (BBB-) but not the small intestine (HIA +). Furthermore, negative results in carcinogenic and hazardous tests suggest their potential utility as VEGFR inhibitors. These findings collectively underscore the promising characteristics of the machine learning model compound in its journey towards clinical application.
Figure 9.
Bioavailability radar related to physicochemical properties of two of each best compound from established and machine learning compounddocked result.
Figure 10 represents the outcomes of drug-likeness properties, physicochemical characteristics, and pharmacokinetics for both the top established compounds and machine learning model compounds. To facilitate the interpretation of parameters such as Human Intestinal Absorption (HIA), Blood–Brain Barrier (BBB) permeability, Ames’s toxicity, and Rat LD50, a barplot was generated using the ggplot2145,146 package in the R programming language147. This visual representation offers a concise and comparative overview of these critical properties for the established and machine learning model compounds, aiding in the assessment of their overall suitability and safety profiles for further consideration in drug development.
Figure 10.
Comparative analysis of HIA, BBB, AMES toxicity, LD50 values of established compounds against machine learning compounds.
Boiled EGG Plot analysis
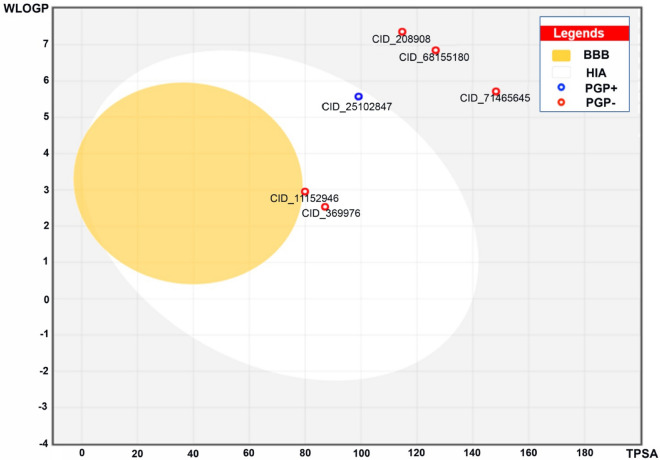
The boiled egg plot is employed to assess the efficacy and capability of small compounds in traversing the gastrointestinal and blood–brain barriers, with these properties determined by the molecules’ polarity and lipophilicity, as outlined in Table 8. Physicochemical spaces are categorized into three regions: yellow, white, and grey. Molecules residing in the yellow area have the highest likelihood of crossing the blood–brain barrier, while the white region, encompassing compounds with PubChem IDs 11152946, 25102847, and 369976, signifies compounds capable of passing through the gastrointestinal barrier. Notably, compounds 208908, 68155180, and 71465645 are situated in the grey area, indicating diminished absorption across both the gastrointestinal and blood–brain barriers. Specifically, the VEGFR-2 receptor machine learning compound 11152946 exhibits a reduced Blood–Brain Barrier (BBB) crossing index and enhanced gastrointestinal perseverance. The grey region also implies reduced penetration of the gastrointestinal and blood–brain barriers for machine learning model compounds VEGFR-1:71465645 and VEGFR-3:68155180. VEGFR-1 and VEGFR-2 Bioavailability Equivalent Indices (BEI) fall within the white region, whereas VEGFR-3 BEI is positioned in the grey region (Fig. 11).
Table 8.
Best established docked compounds and bestML compounds used for Boiled Egg plot.
| PubChem ID | MW (g/mol) | TPSA(Å2) | MLOGP | GI absorption | BBB permeant |
|---|---|---|---|---|---|
| 11152946 | 371.9 | 80.5 | 1.75 | High | No |
| 369976 | 401.9 | 89.7 | 1.18 | High | No |
| 25102847 | 501.5 | 98.8 | 2.68 | High | No |
| 71465645 | 606.6 | 148.11 | 2.46 | Low | No |
| 208908 | 581.1 | 115 | 3.44 | Low | No |
| 68155180 | 596.1 | 126.76 | 2.67 | Low | No |
Figure 11.
Boiled Egg Plot of six most effective machine learning compounds and established compounds.
Discussion
The rising red queen race of AI and DL algorithms have emerged as chief parameters for combatting myriad neoplasms, especially in the drug designing, and discovery field. These AI models aid in random screening of various compounds, and predict bioactivities of known and unknown biological compounds. In the current investigation, a thorough literary survey was conducted in which 26 potent established inhibitors, such as Brivanib, Sunitinib, Pazopanib, etc. were gleaned against all the selected VEGFR (1–3) genes. These established inhibitors were then subjected to Molecular Docking studies, in which the best-established inhibitors depending upon re-rank score were gleaned; VEGFR-1—Cabozantinib (PubChem ID: 25102847), VEGFR-2—Tnp-470(PubChem ID: 369976), VEGFR-3—Lapatinib (PubChem ID: 208908). The data from the top hits of the Molecular Docking studies were then fed as training datasets and Deep Learning algorithms were employed to generate millions of chemically tailored compounds exhibiting profound chemical and novel characteristics.
The top selected novel compounds against VEGFR-1—PubChem ID: 71465645, VEGFR-2—PubChem ID: 11152946, and VEGFR-3 –PubChem ID: 68155180 were confirmed via Molecular Docking and Deep Learning algorithms. These novel compounds were further subjected to comparative analysis with the established inhibitors, testing their drug-likeness, physiochemical characterization, and Molecular Dynamic Simulation studies, which revealed that the Deep Learning generated novel compounds, exhibited augmented affinity scores, and better physiochemical characteristics than the best-established inhibitors. Moreover, pharmacophore and ADMET analysis also indoctrinated the lower toxicity, and higher vitality levels of the new drugs, suggesting their suitability as a prime therapeutic agent for treating cervical cancer.
Despite the numerous advantages of these DL and AI models, there are countable limitations that hinder the ability of these models to govern the absolute predictions, such as sensitivity to noisy data, over-fitting the training data sets, and limited training data availability which ultimately leads to challenges in its predictions and validations, etc. But with the advent of technology, numerous Machine Learning (ML), DeepLearning (DL), Artificial Intelligence (AI) models, and algorithms are being devised daily, to circumscribe these limitations and restrictions.
Conclusion
Cervical cancer, often associated with human papillomavirus infection, disrupts the tyrosine-kinase receptor pathway and gene signaling cascade, impacting VEGFR-1, VEGFR-2, and VEGFR-3 receptor proteins. In our study, we identified 26 established inhibitors for VEGFR proteins through an extensive literature search. Subsequently, molecular docking of these inhibitors led to the discovery of a machine learning-derived compound with PubChem IDs: 71465645 (VEGFR-1), 11152946 (VEGFR-2), and 68155180 (VEGFR-3) exhibiting inhibitory effects on respective VEGF receptors. ADMET analysis and a boiled egg-plot were employed to assess drug similarity features, revealing substantial bioavailability for the machine learning compound with PubChem ID: 11152946 compared to PubChem IDs: 71465645 and 68155180. A 100 ns molecular dynamics simulation confirmed the conformational stability of the compound-receptor complex, demonstrating that hydrophobic interactions and hydrogen bonding predominantly couple the machine learning-based compound with the protein receptor. Notably, compounds with PubChem IDs: 71465645, 11152946 and 68155180 exhibited significant inhibitory effects on VEGFR-1, VEGFR-2, and VEGFR-3, respectively. These findings underscore the potential of these compounds for further exploration and validation through in-vitro research, particularly for understanding their pharmacodynamic characteristics and clinical pharmacokinetics in cervical cancer treatment.
Supplementary Information
Acknowledgements
AN, AB, AK, KS, UP, and SKS thankfully acknowledge the MHRD-RUSA Phase 2.0 under [Grant sanctioned wide letter no. F.24-51/ 2014-U, Policy (TN Multi-Gen), Department of Education, Government of India, dated 09.10.2018]; Tamil Nadu State Council for Higher Education (TANSCHE) under [grant sanctioned wide letter no. AU: S.O. (P&D): TANSCHE Projects: 117/2021, dated 31.03.2021, File No. RGP/2019-20/ALU/HECP-0048 dated 27.04.2021]; Department of Biotechnology, Ministry of Science and Technology, New Delhi, under Grant/Award [No. BT/PR40154/BTIS/137/ 34/2021, dated 07.03.2022]; DST-PURSE 2nd Phase Program [Order No. SR/PURSE Phase 2/38 (G dated 21.02.2017) & DST-FIST (SR/FST/LSI—667/2016)]; DBT-NNP Project, New Delhi, under Grant/Award [No. BT/PR40156/BTIS/54/2023 dated 06.02.2023] for providing financial assistance, infrastructure facilities in the lab. AB thanks DBT-NNP for the Fellowship.
Author contributions
A.N. involved in conceptualization, investigation, methodology, project administration, supervision, writing—review & editing. M.A., I.J., M.Y., A.B. and I.C. was involved in Molecular docking, Molecular Dynamics Simulation, Writing—review & editing. A.K., A.S. and K.S. were contributed to Inhibitors collection, Data Curation, Formal analysis, Validation, and Visualization. M.A. and I.J., were involved in Molecular dynamic simulation. A.K., A.S., K.S., A.P. and U.P. were also involved in Molecular Docking, ADMET analysis, R Programming analysis, and Writing—review & editing. F.J.B.M.J., A.N., and S.K.S. contributed to the investigation, supervision, writing—review & editing.
Data availability
The chemical structures and inhibitors data that support the findings of this study are available in Figshare with the identifier 10.6084/m9.figshare.24078207.v1.
Code availability
The code used for running this study, model fitting, and plotting is available on a GitHub repository at https://github.com/AnurajNayarisseri/Vegfr_Cervical_Cancer.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Anuraj Nayarisseri, Email: anuraj@eminentbio.com.
Sanjeev Kumar Singh, Email: skysanjeev@gmail.com.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-63762-w.
References
- 1.https://www.cancer.net/cancer-types/cervical-cancer/statistics.
- 2.https://www.who.int/health-topics/cervical-cancer#tab=tab_1
- 3.https://www.nccc-online.org/hpvcervical-cancer/cervical-cancer-overview/
- 4.Ceci C, Atzori MG, Lacal PM, Graziani G. Role of VEGFs/VEGFR-1 Signaling and its inhibition in modulating tumor invasion: Experimental evidence in different metastatic cancer models. Int. J. Mol. Sci. 2020;21(4):1388. doi: 10.3390/ijms21041388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Willmott LJ, Monk BJ. Cervical cancer therapy: current, future and anti-angiogensis targeted treatment. Expert Rev. Anticancer Ther. 2009;9(7):895–903. doi: 10.1586/era.09.58. [DOI] [PubMed] [Google Scholar]
- 6.Cheng WF, Chen CA, Lee CN, Wei LH, Hsieh FJ, Hsieh CY. Vascular endothelial growth factor and prognosis of cervical carcinoma. Obstet. Gynecol. 2000;96(5):721–726. doi: 10.1016/s0029-7844(00)01025-5. [DOI] [PubMed] [Google Scholar]
- 7.del Campo JM, Prat A, Gil-Moreno A, Pérez J, Parera M. Update on novel therapeutic agents for cervical cancer. Gynecol. Oncol. 2008;110(3):S72–S76. doi: 10.1016/j.ygyno.2008.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Tomao F, Papa A, Rossi L, Zaccarelli E, Caruso D, Zoratto F, Panici PB, Tomao S. Angiogenesis and antiangiogenic agents in cervical cancer. OncoTargets ther. 2014;7:2237. doi: 10.2147/OTT.S68286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dang, Y. Z., Zhang, Y., Li, J. P., Hu, J., Li, W. W., Li, P., Wei, L.C. & Shi, M. High VEGFR-1/2 expression levels are predictors of poor survival in patients with cervical cancer. Medicine, 96(1) (2017). [DOI] [PMC free article] [PubMed]
- 10.Yoshida K, Suzuki S, Sakata J, Utsumi F, Niimi K, Yoshikawa N, Nishino K, Shibata K, Kikkawa F, Kajiyama H. The upregulated expression of vascular endothelial growth factor in surgically treated patients with recurrent/radioresistant cervical cancer of the uterus. Oncol. Lett. 2018;16(1):515–521. doi: 10.3892/ol.2018.8610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sawada M, Oishi T, Komatsu H, Sato S, Chikumi J, Nonaka M, Kudoh A, Osaku D, Harada T. Serum vascular endothelial growth factor A and vascular endothelial growth factor receptor 2 as prognostic biomarkers for uterine cervical cancer. Int. J. Clin. Oncol. 2019;24(12):1612–1619. doi: 10.1007/s10147-019-01495-x. [DOI] [PubMed] [Google Scholar]
- 12.Shi X, Xi L, Weng D, Chen G, Song X, Wu P, Wang B, Wei J, Wang S, Zhou J, Ma D. Clinico pathological significance of VEGF-C, VEGFR-3 and cyclooxygenase-2 in early-stage cervical cancer. Int. J. Biomed. Sci. IJBS. 2008;4(1):58. doi: 10.59566/IJBS.2008.4058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kuemmel S, Thomas A, Landt S, Fuger A, Schmid P, Kriner M, Blohmer JU, Sehouli J, Schaller G, Lichtenegger W, Koeninger A. Circulating vascular endothelial growth factors and their soluble receptors in pre-invasive, invasive and recurrent cervical cancer. Anticancer Res. 2009;29(2):641–645. [PubMed] [Google Scholar]
- 14.Jain RK, Duda DG, Clark JW, Loeffler JS. Lessons from phase III clinical trials on anti-VEGF therapy for cancer. Nat. Clin. Pract. Oncol. 2006;3(1):24–40. doi: 10.1038/ncponc0403. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Juhnn YS, Kang S, Park SW, Sung MW, Bang YJ, Song YS. Human papillomavirus 16 E5 up-regulates the expression of vascular endothelial growth factor through the activation of epidermal growth factor receptor, MEK/ERK1, 2 and PI3K/Akt. Cell. Mol. Life Sci. CMLS. 2006;63(7–8):930–938. doi: 10.1007/s00018-005-5561-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Narisawa-Saito M, Kiyono T. Basic mechanisms of high-risk human papillomavirus-induced carcinogenesis: Roles of E6 and E7 proteins. Cancer Sci. 2007;98(10):1505–1511. doi: 10.1111/j.1349-7006.2007.00546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukherjee S, Abdalla M, Yadav M, Madhavi M, Bhrdwaj A, Khandelwal R, Prajapati L, Panicker A, Chaudhary A, Albrakati A, Hussain T. Structure-based virtual screening, molecular docking, and molecular dynamics simulation of VEGF inhibitors for the clinical treatment of ovarian cancer. J. Mol. Model. 2022;28(4):1–21. doi: 10.1007/s00894-022-05081-3. [DOI] [PubMed] [Google Scholar]
- 18.Chan JK, Deng W, Higgins RV, Tewari KS, Bonebrake AJ, Hicks M, Aghajanian C. A phase II evaluation of brivanib in the treatment of persistent or recurrent carcinoma of the cervix: An NRG Oncology/Gynecologic Oncology Group study. Gynecol. Oncol. 2017;146(3):554–559. doi: 10.1016/j.ygyno.2017.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vici P, Mariani L, Pizzuti L, Sergi D, Di Lauro L, Vizza E, Venuti A. Emerging biological treatments for uterine cervical carcinoma. J. Cancer. 2014;5(2):86. doi: 10.7150/jca.7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Monk BJ, Mas Lopez L, Zarba JJ, Oaknin A, Tarpin C, Termrungruanglert W, Pandite LN. Phase II, open-label study of pazopanib or lapatinib monotherapy compared with pazopanib plus lapatinib combination therapy in patients with advanced and recurrent cervical cancer. J. Clin. Oncol. 2010;28(22):3562–3569. doi: 10.1200/JCO.2009.26.9571. [DOI] [PubMed] [Google Scholar]
- 21.Symonds RP, Gourley C, Davidson S, Carty K, McCartney E, Rai D, Paul J. Cediranib combined with carboplatin and paclitaxel in patients with metastatic or recurrent cervical cancer (CIRCCa): A randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol. 2015;16(15):1515–1524. doi: 10.1016/S1470-2045(15)00220-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhou JG, Zhou NJ, Zhang Q, Feng YY, Zhou H. Apatinib for patients with advanced or recurrent cervical cancer: Study protocol for an open-label randomized controlled trial. Trials. 2018;19(1):500. doi: 10.1186/s13063-018-2858-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mackay HJ, Tinker A, Winquist E, Thomas G, Swenerton K, Oza A, Eisenhauer EA. A phase II study of sunitinib in patients with locally advanced or metastatic cervical carcinoma: NCIC CTG trial IND. 184. Gynecol. Oncol. 2010;116(2):163–167. doi: 10.1016/j.ygyno.2009.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Askr H, Elgeldawi E, Aboul Ella H, Elshaier YA, Gomaa MM, Hassanien AE. Deep learning in drug discovery: An integrative review and future challenges. Artif. Intell. Rev. 2023;56(7):5975–6037. doi: 10.1007/s10462-022-10306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mehmood A, Nawab S, Jia G, Kaushik AC, Wei DQ. Supervised screening of Tecovirimat-like compounds as potential inhibitors for the monkeypox virus E8L protein. J. Biomol. Struct. Dyn. 2023 doi: 10.1080/07391102.2023.2245042. [DOI] [PubMed] [Google Scholar]
- 26.Mehmood A, Kaushik AC, Wang Q, Li CD, Wei DQ. Bringing structural implications and deep learning-based drug identification for KRAS mutants. J. Chem. Inf. Model. 2021;61(2):571–586. doi: 10.1021/acs.jcim.0c00488. [DOI] [PubMed] [Google Scholar]
- 27.Mehmood A, Nawab S, Jin Y, Kaushik AC, Wei DQ. Mutational impacts on the N and C terminal domains of the MUC5B protein: A transcriptomics and structural biology study. ACS Omega. 2023;8(4):3726–3735. doi: 10.1021/acsomega.2c04871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mehmood A, Nawab S, Jin Y, Hassan H, Kaushik AC, Wei DQ. Ranking breast cancer drugs and biomarkers identification using machine learning and pharmacogenomics. ACS Pharm. Transl. Sci. 2023;6(3):399–409. doi: 10.1021/acsptsci.2c00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nayarisseri A, Khandelwal R, Tanwar P, Madhavi M, Sharma D, Thakur G, Speck-Planche A, Singh SK. Artificial intelligence, big data and machine learning approaches in precision medicine & drug discovery. Curr. Drug Targ. 2021;22(6):631–655. doi: 10.2174/18735592MTEzsMDMnz. [DOI] [PubMed] [Google Scholar]
- 30.Nayarisseri A, Khandelwal R, Madhavi M, Selvaraj C, Panwar U, Sharma K, Hussain T, Singh SK. Shape-based machine learning models for the potential novel COVID-19 protease inhibitors assisted by molecular dynamics simulation. Curr. Top. Med. Chem. 2020;20(24):2146–2167. doi: 10.2174/1568026620666200704135327. [DOI] [PubMed] [Google Scholar]
- 31.Zhu J, Song C, Zheng Z, Xia L, Chen Y, Ke G, Wu X. Anlotinib in Chinese patients with recurrent advanced cervical cancer: A prospective single-arm, open-label phase II trial. Front. Oncol. 2021;11:720343. doi: 10.3389/fonc.2021.720343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Traxler P, Allegrini PR, Brandt R, Brueggen J, Cozens R, Fabbro D, Caravatti G. AEE788: a dual family epidermal growth factor receptor/ErbB2 and vascular endothelial growth factor receptor tyrosine kinase inhibitor with antitumor and antiangiogenic activity. Cancer Res. 2004;64(14):4931–4941. doi: 10.1158/0008-5472.CAN-03-3681. [DOI] [PubMed] [Google Scholar]
- 33.Zhang RR, Wang H, Hui N, Zhang P. Enhanced antitumor effect of axitinib synergistic interaction with AG490 via VEGFR2/JAK2/STAT3 signaling mediated epithelial-mesenchymal transition in cervical cancer in vitro. Asian Biomed. 2013;7(1):39–49. [Google Scholar]
- 34.Lu L, Zhao TT, Liu TB, Sun WX, Xu C, Li DD, Zhu HL. Synthesis, molecular modeling and biological evaluation of 4-alkoxyquinazoline derivatives as novel inhibitors of VEGFR-2. Chem. Pharm. Bull. 2016;64(11):1570–1575. doi: 10.1248/cpb.c16-00386. [DOI] [PubMed] [Google Scholar]
- 35.Bernard B, Fest T, Prétet JL, Mougin C. Staurosporine-induced apoptosis of HPV positive and negative human cervical cancer cells from different points in the cell cycle. Cell Death Differ. 2001;8(3):234–244. doi: 10.1038/sj.cdd.4400796. [DOI] [PubMed] [Google Scholar]
- 36.Fabbro D, Buchdunger E, Wood J, Mestan J, Hofmann F, Ferrari S, Meyer T. Inhibitors of protein kinases: CGP 41251, a protein kinase inhibitor with potential as an anticancer agent. Pharm. Ther. 1999;82(2–3):293–301. doi: 10.1016/S0163-7258(99)00005-4. [DOI] [PubMed] [Google Scholar]
- 37.Shehzad A, Parveen S, Qureshi M, Subhan F, Lee YS. Decursin and decursinol angelate: Molecular mechanism and therapeutic potential in inflammatory diseases. Inflamm. Res. 2018;67(3):209–218. doi: 10.1007/s00011-017-1114-7. [DOI] [PubMed] [Google Scholar]
- 38.Zhu ML, Li JC, Wang L, Zhong X, Zhang YW, Tan RZ, Wang L. Decursin inhibits the growth of HeLa cervical cancer cells through PI3K/Akt signaling. J. Asian Nat. Prod. Res. 2020;23(6):1–12. doi: 10.1080/10286020.2020.1821669. [DOI] [PubMed] [Google Scholar]
- 39.Jung MH, Lee SH, Ahn EM, Lee YM. Decursin and decursinol angelate inhibit VEGF-induced angiogenesis via suppression of the VEGFR-2-signaling pathway. Carcinogenesis. 2009;30(4):655–661. doi: 10.1093/carcin/bgp039. [DOI] [PubMed] [Google Scholar]
- 40.Zhang J, Li L, Jiang C, Xing C, Kim SH, Lu J. Anti-cancer and other bioactivities of Korean Angelica gigas Nakai (AGN) and its major pyranocoumarin compounds. Anti-Cancer Agents Med. Chem. Former. Curr. Med. Chem. Anti-Cancer Agents. 2012;12(10):1239–1254. doi: 10.2174/187152012803833071. [DOI] [PubMed] [Google Scholar]
- 41.Batran RZ, Dawood DH, El-Seginy SA, Ali MM, Maher TJ, Gugnani KS, Rondon-Ortiz AN. New coumarin derivatives as anti-breast and anti-cervical cancer agents targeting VEGFR-2 and p38α MAPK. Archiv der Pharm. 2017;350(9):1700064. doi: 10.1002/ardp.201700064. [DOI] [PubMed] [Google Scholar]
- 42.Lee DH, Lee J, Jeon J, Kim KJ, Yun JH, Jeong HS, Cho CH. Oleanolic acids inhibit vascular endothelial growth factor receptor 2 signaling in endothelial cells: Implication for anti-angiogenic therapy. Mol. cells. 2018;41(8):771. doi: 10.14348/molcells.2018.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Edathara PM, Chintalapally S, Makani VKK, Pant C, Yerramsetty S, Rao DM, Bhadra MP. Inhibitory role of oleanolic acid and esculetin in hela cells involve multiple signaling pathways. Gene. 2020;771:145370. doi: 10.1016/j.gene.2020.145370. [DOI] [PubMed] [Google Scholar]
- 44.Park SL, Won SY, Song JH, Lee SY, Kim WJ, Moon SK. Esculetin inhibits VEGF-induced angiogenesis both in vitro and in vivo. Am. J. Chin. Med. 2016;44(01):61–76. doi: 10.1142/S0192415X1650004X. [DOI] [PubMed] [Google Scholar]
- 45.Kumar A, Sunita P, Jha S, Pattanayak SP. Daphnetin inhibits TNF-α and VEGF-induced angiogenesis through inhibition of the IKK s/IκBα/NF-κB, Src/FAK/ERK 1/2 and Akt signalling pathways. Clin. Exp. Pharm. Physiol. 2016;43(10):939–950. doi: 10.1111/1440-1681.12608. [DOI] [PubMed] [Google Scholar]
- 46.Dar MY, Ara T, Akbar S. A new prenylated coumarin from Daphne oleoides and its cytotoxic activity. Chem. Nat. Compd. 2019;55(1):5–7. doi: 10.1007/s10600-019-02603-z. [DOI] [Google Scholar]
- 47.Zahri S, Razavi SM, Moatamed Z. Antioxidant activity and cytotoxic effect of aviprin and aviprin-3 ″-O-d-glucopyranoside on LNCaP and HeLa cell lines. Nat. Prod. Res. 2012;26(6):540–547. doi: 10.1080/14786419.2010.529442. [DOI] [PubMed] [Google Scholar]
- 48.Satchi-Fainaro R, Mamluk R, Wang L, Short SM, Nagy JA, Feng D, Folkman J. Inhibition of vessel permeability by TNP-470 and its polymer conjugate, caplostatin. Cancer cell. 2005;7(3):251–261. doi: 10.1016/j.ccr.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 49.Kudelka AP, Levy T, Verschraegen CF, Edwards CL, Piamsomboon S, Termrungruanglert W, Kavanagh JJ. A phase I study of TNP-470 administered to patients with advanced squamous cell cancer of the cervix. Clin. Cancer Res. 1997;3(9):1501–1505. [PubMed] [Google Scholar]
- 50.https://newdrugapprovals.org/tag/nintedanib/
- 51.Han M, Sun D. Rational creation and systematic analysis of cervical cancer kinase–inhibitor binding profile. J. Comput. Aided Mol. Des. 2019;33(7):689–698. doi: 10.1007/s10822-019-00211-1. [DOI] [PubMed] [Google Scholar]
- 52.Ban HS, Uno M, Nakamura H. Suppression of hypoxia-induced HIF-1α accumulation by VEGFR inhibitors: Different profiles of AAL993 versus SU5416 and KRN633. Cancer Lett. 2010;296(1):17–26. doi: 10.1016/j.canlet.2010.03.010. [DOI] [PubMed] [Google Scholar]
- 53.Kuo GH, Prouty C, Wang A, Emanuel S, DeAngelis A, Zhang Y, Murray WV. Synthesis and structure—Activity relationships of pyrazine-pyridine biheteroaryls as novel, potent, and selective vascular endothelial growth factor receptor-2 inhibitors. J. Med. Chem. 2005;48(15):4892–4909. doi: 10.1021/jm058205b. [DOI] [PubMed] [Google Scholar]
- 54.Costa DCS, Forezi LS, Cardoso MFC, Ribeiro RCB, Pinto AC, Ferreira VF, da Silva FDC. A Compendium of tyrosine-kinase Inhibitors: Powerful and efficient drugs against cancer. Rev. Virtual Quim. 2017;9(3):974–1064. doi: 10.21577/1984-6835.20170063. [DOI] [Google Scholar]
- 55.https://clinicaltrials.gov/ct2/show/NCT04230954
- 56.Csizmadia, P. MarvinSketch and MarvinView: Molecule applets for the World Wide Web (1999).
- 57.Berman HM. The protein data bank: A historical perspective. Acta Crystallogr. Sect. A. 2008;A64(1):88–95. doi: 10.1107/S0108767307035623. [DOI] [PubMed] [Google Scholar]
- 58.Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE. The protein data bank. Nucleic Acids Res. 2000;28(1):235–242. doi: 10.1093/nar/28.1.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tresaugues, L., Roos, A., Arrowsmith, C., Berglund, H., Bountra, C., Collins, R., Edwards, A.M., Flodin, S., Flores, A., Graslund, S. & Hammarstrom, M. Crystal structure of VEGFR1 in complex with N-(4-Chlorophenyl)-2-((pyridin-4-ylmethyl) amino) benzamide. The RCSB PDB. (2013)
- 60.Harris PA, Cheung M, Hunter RN, Brown ML, Veal JM, Nolte RT, Wang L, Liu W, Crosby RM, Johnson JH, Epperly AH. Discovery and evaluation of 2-anilino-5-aryloxazoles as a novel class of VEGFR2 kinase inhibitors. J. Med. Chem. 2005;48(5):1610–1619. doi: 10.1021/jm049538w. [DOI] [PubMed] [Google Scholar]
- 61.Leppänen VM, Tvorogov D, Kisko K, Prota AE, Jeltsch M, Anisimov A, Markovic-Mueller S, Stuttfeld E, Goldie KN, Ballmer-Hofer K, Alitalo K. Structural and mechanistic insights into VEGF receptor 3 ligand binding and activation. Proc. Nat. Acad. Sci. 2013;110(32):12960–12965. doi: 10.1073/pnas.1301415110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kim S, Chen J, Cheng T, Gindulyte A, He J, He S, Li Q, Shoemaker BA, Thiessen PA, Yu B, Zaslavsky L. PubChem in 2021: New data content and improved web interfaces. Nucleic acids Res. 2021;49(D1):D1388–95. doi: 10.1093/nar/gkaa971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Reddy KK, Singh SK, Dessalew N, Tripathi SK, Selvaraj C. Pharmacophore modelling and atom-based 3D-QSAR studies on N-methyl pyrimidones as HIV-1 integrase inhibitors. J. Enzyme Inhib. Med. Chem. 2012;27(3):339–347. doi: 10.3109/14756366.2011.590803. [DOI] [PubMed] [Google Scholar]
- 64.Bandaru S, Alvala M, Nayarisseri A, Sharda S, Goud H, Mundluru HP, Singh SK. Molecular dynamic simulations reveal suboptimal binding of salbutamol in T164I variant of β2 adrenergic receptor. PloS one. 2017;12(10):e0186666. doi: 10.1371/journal.pone.0186666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chinnasamy S, Selvaraj G, Selvaraj C, Kaushik AC, Kaliamurthi S, Khan A, Wei DQ. Combining in silico and in vitro approaches to identification of potent inhibitor against phospholipase A2 (PLA2) Int. J. Biol. Macromol. 2020;144:53–66. doi: 10.1016/j.ijbiomac.2019.12.091. [DOI] [PubMed] [Google Scholar]
- 66.Shukla P, Khandelwal R, Sharma D, Dhar A, Nayarisseri A, Singh SK. Virtual screening of IL-6 inhibitors for idiopathic arthritis. Bioinformation. 2019;15(2):121. doi: 10.6026/97320630015121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dunna NR, Bandaru S, Raj Akare U, Rajadhyax S, Ravi Gutlapalli V, Yadav M, Nayarisseri A. Multiclass comparative virtual screening to identify novel Hsp90 inhibitors: A therapeutic breast cancer drug target. Curr. Topics Med. Chem. 2015;15(1):57–64. doi: 10.2174/1568026615666150112113627. [DOI] [PubMed] [Google Scholar]
- 68.Vuree S, Dunna NR, Khan IA, Alharbi KK, Vishnupriya S, Soni D, Shah P, Chandok H, Yadav M, Nayarisseri A. Pharmacogenomics of drug resistance in breast cancer resistance protein (BCRP) and its mutated variants. J. Pharm. Res. 2013;6(7):791–798. [Google Scholar]
- 69.Nayarisseri A, Moghni SM, Yadav M, Kharate J, Sharma P, Chandok KH, Shah KP. In silico investigations on HSP90 and its inhibition for the therapeutic prevention of breast cancer. J. Pharm. Res. 2013;7(2):150–156. [Google Scholar]
- 70.Grover A, Katiyar SP, Singh SK, Dubey VK, Sundar D. A leishmaniasis study: Structure-based screening and molecular dynamics mechanistic analysis for discovering potent inhibitors of spermidine synthase. BiochimBiophys Acta. 2012;1824(12):1476–1483. doi: 10.1016/j.bbapap.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 71.Reddy KK, Singh SK, Tripathi SK, Selvaraj C. Identification of potential HIV-1 integrase strand transfer inhibitors: In silico virtual screening and QM/MM docking studies. SAR QSAR Environ. Res. 2013;24(7):581–595. doi: 10.1080/1062936X.2013.772919. [DOI] [PubMed] [Google Scholar]
- 72.Patidar K, Deshmukh A, Bandaru S, Lakkaraju C, Girdhar A, Vr G, Banerjee T, Nayarisseri A, Singh SK. Virtual screening approaches in identification of bioactive compounds akin to Delphinidin as potential HER2 inhibitors for the treatment of breast cancer. Asian Pac. J. Cancer Prev. 2016;17(4):2291–2295. doi: 10.7314/APJCP.2016.17.4.2291. [DOI] [PubMed] [Google Scholar]
- 73.Praseetha S, Bandaru S, Nayarisseri A, Sureshkumar S. Pharmacological analysis of vorinostat analogues as potential anti-tumor agents targeting human histone deacetylases: An epigenetic treatment stratagem for cancers. Asian Pac. J. Cancer Prev. 2016;17(3):1571–1576. doi: 10.7314/APJCP.2016.17.3.1571. [DOI] [PubMed] [Google Scholar]
- 74.Khandekar N, Singh S, Shukla R, Tirumalaraju S, Bandaru S, Banerjee T, Nayarisseri A. Structural basis for the in vitro known acyl-depsipeptide 2 (ADEP2) inhibition to Clp 2 protease from Mycobacterium tuberculosis. Bioinformation. 2016;12(3):92. doi: 10.6026/97320630012092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Gudala S, Khan U, Kanungo N, Bandaru S, Hussain T, Parihar MS, Nayarisseri A, Mundluru HP. Identification and pharmacological analysis of high efficacy small molecule inhibitors of EGF-EGFR interactions in clinical treatment of non-small cell lung carcinoma: A computational approach. Asian Pac. J. Cancer Prev. 2016;16(18):8191–8196. doi: 10.7314/APJCP.2015.16.18.8191. [DOI] [PubMed] [Google Scholar]
- 76.Gutlapalli VR, Sykam A, Nayarisseri A, Suneetha S, Suneetha LM. Insights from the predicted epitope similarity between Mycobacterium tuberculosis virulent factors and its human homologs. Bioinformation. 2015;11(12):517. doi: 10.6026/97320630011517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kelotra S, Jain M, Kelotra A, Jain I, Bandaru S, Nayarisseri A, Bidwai A. An in silico appraisal to identify high affinity anti-apoptotic synthetic tetrapeptide inhibitors targeting the mammalian caspase 3 enzyme. Asian Pac. J. Cancer Prev. 2015;15(23):10137–10142. doi: 10.7314/APJCP.2014.15.23.10137. [DOI] [PubMed] [Google Scholar]
- 78.Bandaru S, Tiwari G, Akka J, Kumar Marri V, Alvala M, Ravi Gutlapalli V, Nayarisseri A, Prasad Mundluru H. Identification of high affinity bioactive Salbutamol conformer directed against mutated (Thr164Ile) beta 2 adrenergic receptor. Curr. Top. Med. Chem. 2015;15(1):50–56. doi: 10.2174/1568026615666150112113040. [DOI] [PubMed] [Google Scholar]
- 79.Tabassum A, Rajeshwari T, Soni N, Raju DSB, Yadav M, Nayarisseri A, Jahan P. Structural characterization and mutational assessment of podocin—A novel drug target to nephrotic syndrome—An in silico approach. Interdiscip. Sci. Comput. Life Sci. 2014;6(1):32–39. doi: 10.1007/s12539-014-0190-4. [DOI] [PubMed] [Google Scholar]
- 80.Nayarisseri A, Yadav M, Wishard R. Computational evaluation of new homologous down regulators of translationally controlled tumor protein (TCTP) targeted for tumor reversion. Interdiscip. Sci. Comput. Life Sci. 2013;5(4):274–279. doi: 10.1007/s12539-013-0183-8. [DOI] [PubMed] [Google Scholar]
- 81.Udhwani T, Mukherjee S, Sharma K, Sweta J, Khandekar N, Nayarisseri A, Singh SK. Design of PD-L1 inhibitors for lung cancer. Bioinformation. 2019;15(2):139. doi: 10.6026/97320630015139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gokhale P, Chauhan APS, Arora A, Khandekar N, Nayarisseri A, Singh SK. FLT3 inhibitor design using molecular docking based virtual screening for acute myeloid leukemia. Bioinformation. 2019;15(2):104. doi: 10.6026/97320630015104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sinha K, Majhi M, Thakur G, Patidar K, Sweta J, Hussain T, Nayarisseri A, Singh SK. Computer-aided drug designing for the identification of high-affinity small molecule targeting cd20 for the clinical treatment of chronic lymphocytic leukemia (CLL) Curr. Top. Med. Chem. 2018;18(29):2527–2542. doi: 10.2174/1568026619666181210150044. [DOI] [PubMed] [Google Scholar]
- 84.Nayarisseri A, Hood EA. Advancement in microbial cheminformatics. Curr. Top. Med. Chem.stry. 2018;18(29):2459–2461. doi: 10.2174/1568026619666181120121528. [DOI] [PubMed] [Google Scholar]
- 85.Chandrakar B, Jain A, Roy S, Gutlapalli VR, Saraf S, Suppahia A, Verma A, Tiwari A, Yadav M, Nayarisseri A. Molecular modeling of Acetyl-CoA carboxylase (ACC) from Jatropha curcas and virtual screening for identification of inhibitors. J. Pharm. Res. 2013;6(9):913–918. [Google Scholar]
- 86.Nayarisseri A, Singh SK. Functional inhibition of VEGF and EGFR suppressors in cancer treatment. Curr. Top. Med. Chem. 2019;19(3):178–179. doi: 10.2174/156802661903190328155731. [DOI] [PubMed] [Google Scholar]
- 87.Patidar K, Panwar U, Vuree S, Sweta J, Sandhu MK, Nayarisseri A, Singh SK. An in silico approach to identify high affinity small molecule targeting m-TOR inhibitors for the clinical treatment of breast cancer. Asian Pac. J. Cancer Prev. APJCP. 2019;20(4):1229. doi: 10.31557/APJCP.2019.20.4.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rao DM, Nayarisseri A, Yadav M, Patel D. Comparative modeling of methylentetrahydrofolate reductase (MTHFR) enzyme and its mutational assessment: in silico approach. Int J. Bioinform. Res. 2010;2:5–9. doi: 10.9735/0975-3087.2.1.5-9. [DOI] [Google Scholar]
- 89.Ali MA, Vuree S, Goud H, Hussain T, Nayarisseri A, Singh SK. Identification of high-affinity small molecules targeting gamma secretase for the treatment of Alzheimer’s disease. Curr. Top. Med. Chem. 2019;19(13):1173–1187. doi: 10.2174/1568026619666190617155326. [DOI] [PubMed] [Google Scholar]
- 90.Sweta J, Khandelwal R, Srinitha S, Pancholi R, Adhikary R, Ali MA, Singh SK. Identification of high-affinity small molecule targeting IDH2 for the clinical treatment of acute myeloid leukemia. Asian Pac. J. Cancer Prev. APJCP. 2019;20(8):2287. doi: 10.31557/APJCP.2019.20.8.2287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Limaye A, Sweta J, Madhavi M, Mudgal U, Mukherjee S, Sharma S, Hussain T, Nayarisseri A, Singh SK. In silico insights on gd2: A potential target for pediatric neuroblastoma. Curr. Top. Med. Chem. 2019;19(30):2766–2781. doi: 10.2174/1568026619666191112115333. [DOI] [PubMed] [Google Scholar]
- 92.Nayarisseri A. Prospects of utilizing computational techniques for the treatment of human diseases. Curr. Top. Med. Chem. 2019;19(13):1071–1074. doi: 10.2174/156802661913190827102426. [DOI] [PubMed] [Google Scholar]
- 93.Nayarisseri A. Experimental and computational approaches to improve binding affinity in chemical biology and drug discovery. Curr. Top. Med. Chem. 2020;20(19):1651–1660. doi: 10.2174/156802662019200701164759. [DOI] [PubMed] [Google Scholar]
- 94.Gawehn E, Hiss JA, Schneider G. Deep learning in drug discovery. Mol. Inform. 2016;35(1):3–14. doi: 10.1002/minf.201501008. [DOI] [PubMed] [Google Scholar]
- 95.Cheirdaris DG. Artificial neural networks in computer-aided drug design: An overview of recent advances. GeNeDis. 2020;2018:115–125. doi: 10.1007/978-3-030-32622-7_10. [DOI] [PubMed] [Google Scholar]
- 96.Torng W, Altman RB. Graph convolutional neural networks for predicting drug-target interactions. J. Chem. Inform. Model. 2019;59(10):4131–4149. doi: 10.1021/acs.jcim.9b00628. [DOI] [PubMed] [Google Scholar]
- 97.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Deliv. Rev. 2001;46(1–3):3–26. doi: 10.1016/S0169-409X(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 98.Hien LTK, Gillis N. Algorithms for nonnegative matrix factorization with the Kullback–Leibler divergence. J. Sci. Comput. 2021;87(3):93. doi: 10.1007/s10915-021-01504-0. [DOI] [Google Scholar]
- 99.Yu J, Xu T, Rong Y, Huang J, He R. Structure-aware conditional variational auto-encoder for constrained molecule optimization. Pattern Recognit. 2022;126:108581. doi: 10.1016/j.patcog.2022.108581. [DOI] [Google Scholar]
- 100.Overhoff B, Falls Z, Mangione W, Samudrala R. A deep-learning proteomic-scale approach for drug design. Pharmaceuticals. 2021;14(12):1277. doi: 10.3390/ph14121277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Xiong J, Xiong Z, Chen K, Jiang H, Zheng M. Graph neural networks for automated de novo drug design. Drug Discov. Today. 2021;26(6):1382–1393. doi: 10.1016/j.drudis.2021.02.011. [DOI] [PubMed] [Google Scholar]
- 102.Polykovskiy D, Zhebrak A, Vetrov D, Ivanenkov Y, Aladinskiy V, Mamoshina P, Bozdaganyan M, Aliper A, Zhavoronkov A, Kadurin A. Entangled conditional adversarial autoencoder for de novo drug discovery. Mol. Pharm. 2018;15(10):4398–4405. doi: 10.1021/acs.molpharmaceut.8b00839. [DOI] [PubMed] [Google Scholar]
- 103.Oliveira AF, Da Silva JL, Quiles MG. Molecular property prediction and molecular design using a supervised grammar variational autoencoder. J. Chem. Inform. Model. 2022;62(4):817–828. doi: 10.1021/acs.jcim.1c01573. [DOI] [PubMed] [Google Scholar]
- 104.Ye, Q., Zhang, X., & Lin, X. De Novo Drug Design via Multi-Label Learning and Adversarial Autoencoder. In 2021 IEEE International Conference on Bioinformatics and Biomedicine (BIBM) (pp. 3456–3463). IEEE. (2021, December)
- 105.Liu X, IJzerman AP, van Westen GJ. Computational approaches for de novo drug design: Past, present, and future. In: Cartwright H, editor. Artificial neural networks, methods in molecular biology. Humana; 2021. pp. 139–165. [DOI] [PubMed] [Google Scholar]
- 106.Martinelli DD. Generative machine learning for de novo drug discovery: A systematic review. Comput. Biol. Med. 2022;145:105403. doi: 10.1016/j.compbiomed.2022.105403. [DOI] [PubMed] [Google Scholar]
- 107.Kumar R, Sharma A, Alexiou A, Ashraf GM. Artificial intelligence in de novo drug design: Are we still there? Curr. Topics Med. Chem. 2022;22(30):2483–2492. doi: 10.2174/1568026623666221017143244. [DOI] [PubMed] [Google Scholar]
- 108.Zeng X, Wang F, Luo Y, Kang SG, Tang J, Lightstone FC, Fang EF, Cornell W, Nussinov R, Cheng F. Deep generative molecular design reshapes drug discovery. Cell Rep. Med. 2022 doi: 10.1016/j.xcrm.2022.100794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tripathi SK, Selvaraj C, Singh SK, Reddy KK. Molecular docking, QPLD, and ADME prediction studies on HIV-1 integrase leads. Med. Chem. Res. 2012;21:4239–4251. doi: 10.1007/s00044-011-9940-6. [DOI] [Google Scholar]
- 110.Xu Y, Li X, Yao H, Lin K. Neural networks in drug discovery: Current insights from medicinal chemists. Future Med. Chem. 2019;11(14):1669–1672. doi: 10.4155/fmc-2019-0118. [DOI] [PubMed] [Google Scholar]
- 111.Bongini P, Bianchini M, Scarselli F. Molecular generative graph neural networks for drug discovery. Neurocomputing. 2021;450:242–252. doi: 10.1016/j.neucom.2021.04.039. [DOI] [Google Scholar]
- 112.Wenzel J, Matter H, Schmidt F. Predictive multitask deep neural network models for ADME-Tox properties: Learning from large data sets. J. Chem. Inform. Model. 2019;59(3):1253–1268. doi: 10.1021/acs.jcim.8b00785. [DOI] [PubMed] [Google Scholar]
- 113.Panwar U, Chandra I, Selvaraj C, Singh SK. Current computational approaches for the development of anti-HIV inhibitors: An overview. Curr. Pharm. Des. 2019;25(31):3390–3405. doi: 10.2174/1381612825666190911160244. [DOI] [PubMed] [Google Scholar]
- 114.Panwar U, Singh SK. In silico virtual screening of potent inhibitor to hamper the interaction between HIV-1 integrase and LEDGF/p75 interaction using E-pharmacophore modeling, molecular docking, and dynamics simulations. Comput. Biol. Chem. 2021;93:107509. doi: 10.1016/j.compbiolchem.2021.107509. [DOI] [PubMed] [Google Scholar]
- 115.Panwar U, Singh SK. Identification of novel pancreatic lipase inhibitors using in silico studies. Endocr. Metab. Immune Disord. Drug Targets (Former. Curr. Drug Targets Immune, Endocr. Metab. Disord.) 2019;19(4):449–457. doi: 10.2174/1871530319666181128100903. [DOI] [PubMed] [Google Scholar]
- 116.Panwar U, Singh SK. Structure-based virtual screening toward the discovery of novel inhibitors for impeding the protein-protein interaction between HIV-1 integrase and human lens epithelium-derived growth factor (LEDGF/p75) J. Biomol. Struct. Dyn. 2018;36(12):3199–3217. doi: 10.1080/07391102.2017.1384400. [DOI] [PubMed] [Google Scholar]
- 117.Reddy KK, Singh P, Singh SK. Blocking the interaction between HIV-1 integrase and human LEDGF/p75: Mutational studies, virtual screening and molecular dynamics simulations. Mol. BioSyst. 2014;10(3):526–536. doi: 10.1039/c3mb70418a. [DOI] [PubMed] [Google Scholar]
- 118.Reddy KK, Singh SK, Tripathi SK, Selvaraj C, Suryanarayanan V. Shape and pharmacophore-based virtual screening to identify potential cytochrome P450 sterol 14α-demethylase inhibitors. J. Recept. Signal Transduct. 2013;33(4):234–243. doi: 10.3109/10799893.2013.789912. [DOI] [PubMed] [Google Scholar]
- 119.Vijayalakshmi P, Selvaraj C, Singh SK, Nisha J, Saipriya K, Daisy P. Exploration of the binding of DNA binding ligands to Staphylococcal DNA through QM/MM docking and molecular dynamics simulation. J. Biomol. Struct. Dyn. 2013;31(6):561–571. doi: 10.1080/07391102.2012.706080. [DOI] [PubMed] [Google Scholar]
- 120.Selvaraj C, Panwar U, Dinesh DC, Boura E, Singh P, Dubey VK, Singh SK. Microsecond MD simulation and multiple-conformation virtual screening to identify potential anti-COVID-19 inhibitors against SARS-CoV-2 main protease. Front. Chem. 2021;8:595273. doi: 10.3389/fchem.2020.595273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sharda S, Khandelwal R, Adhikary R, Sharma D, Majhi M, Hussain T, Nayarisseri A, Singh SK. A computer-aided drug designing for pharmacological inhibition of mutant ALK for the treatment of non-small cell lung cancer. Curr. Top. Med. Chem. 2019;19(13):1129–1144. doi: 10.2174/1568026619666190521084941. [DOI] [PubMed] [Google Scholar]
- 122.Yadav M, Khandelwal R, Mudgal U, Srinitha S, Khandekar N, Nayarisseri A, Vuree S, Singh SK. Identification of potent VEGF inhibitors for the clinical treatment of glioblastoma, a virtual screening approach. Asian Pac. J. Cancer Prev. APJCP. 2019;20(9):2681. doi: 10.31557/APJCP.2019.20.9.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nayarisseri A. Most promising compounds for treating COVID-19 and recent trends in antimicrobial & antifungal agents. Curr. Top. Med. Chem. 2020;20(24):2119–2125. doi: 10.2174/156802662023201001094634. [DOI] [PubMed] [Google Scholar]
- 124.Adhikary R, Khandelwal R, Hussain T, Nayarisseri A, Singh SK. Structural insights into the molecular design of ROS1 inhibitor for the treatment of non-small cell lung cancer (NSCLC) Curr. Comput. Aided Drug Des. 2021;17(3):387–401. doi: 10.2174/1573409916666200504105249. [DOI] [PubMed] [Google Scholar]
- 125.Qureshi S, Khandelwal R, Madhavi M, Khurana N, Gupta N, Choudhary SK, Suresh RA, Hazarika L, Srija CD, Sharma K, Hindala MR. A multi-target drug designing for BTK, MMP9, proteasome and TAK1 for the clinical treatment of mantle cell lymphoma. Curr. Top. Med. Chem. 2021;21(9):790–818. doi: 10.2174/1568026621666210119112336. [DOI] [PubMed] [Google Scholar]
- 126.Yang H, Lou C, Sun L, Li J, Cai Y, Wang Z, Tang Y. admetSAR 2.0: Web-service for prediction and optimization of chemical ADMET properties. Bioinformatics. 2019;35(6):1067–1069. doi: 10.1093/bioinformatics/bty707. [DOI] [PubMed] [Google Scholar]
- 127.Majhi M, Ali MA, Limaye A, Sinha K, Bairagi P, Chouksey M, Shukla R, Kanwar N, Hussain T, Nayarisseri A, Singh SK. An in silico investigation of potential EGFR inhibitors for the clinical treatment of colorectal cancer. Curr. Top. Med. Chem. 2018;18(27):2355–2366. doi: 10.2174/1568026619666181129144107. [DOI] [PubMed] [Google Scholar]
- 128.Natchimuthu V, Abdalla M, Yadav M, Chopra I, Bhrdwaj A, Sharma K, Ravi S, Ravikumar K, Alzahrani KJ, Hussain T, Nayarisseri A. Synthesis, crystal structure, hirshfeld surface analysis, molecular docking and molecular dynamics studies of novel olanzapinium 2, 5-dihydroxybenzoate as potential and active antipsychotic compound. J. Exp. Nanosci. 2022;17(1):247–273. doi: 10.1080/17458080.2022.2063278. [DOI] [Google Scholar]
- 129.Mendonça-Junior FJB, Scotti MT, Muratov EN, Scotti L, Nayarisseri A. Natural bioactive products with antioxidant properties useful in neurodegenerative diseases 2020. Oxid. Med. Cell. Longev. 2021 doi: 10.1155/2021/62623164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Daina A, Michielin O, Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017;7(1):42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sharda S, Sarmandal P, Cherukommu S, Dindhoria K, Yadav M, Bandaru S, Sharma A, Sakhi A, Vyas T, Hussain T, Nayarisseri A. A virtual screening approach for the identification of high affinity small molecules targeting BCR-ABL1 inhibitors for the treatment of chronic myeloid leukemia. Curr. Top. Med. Chem. 2017;17(26):2989–2996. doi: 10.2174/1568026617666170821124512. [DOI] [PubMed] [Google Scholar]
- 132.Sharma K, Panwar U, Madhavi M, Joshi I, Chopra I, Soni L, Khan A, Bhrdwaj A, Parihar AS, Mohan VP, Prajapati L, Sharma R, Agrawal S, Hussain T, Nayarisseri A, Singh SK. Unveiling the ESR1 conformational stability and screening potent inhibitors for breast cancer treatment. Med. Chem. 2023 doi: 10.2174/0115734064256978231024062937. [DOI] [PubMed] [Google Scholar]
- 133.Maia MDS, Mendonça-Junior FJB, Rodrigues GCS, Silva ASD, Oliveira NIPD, Silva PRD, Scotti L. Virtual screening of different subclasses of lignans with anticancer potential and based on genetic profile. Molecules. 2023;28(16):6011. doi: 10.3390/molecules28166011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Bhrdwaj A, Abdalla M, Pande A, Madhavi M, Chopra I, Soni L, Vijayakumar N, Panwar U, Khan M, Prajapati L, Gujrati D. Structure-based virtual screening, molecular docking, molecular dynamics simulation of EGFR for the clinical treatment of glioblastoma. App. Biochem. Biotechnol. 2023;195(8):1–26. doi: 10.1007/s12010-023-04430-z. [DOI] [PubMed] [Google Scholar]
- 135.Yadav M, Abdalla M, Madhavi M, Chopra I, Bhrdwaj A, Soni L, Shaheen U, Prajapati L, Sharma M, Sikarwar MS, Albogami S. Structure-based virtual screening, molecular docking, molecular dynamics simulation and pharmacokinetic modelling of cyclooxygenase-2 (COX-2) inhibitor for the clinical treatment of colorectal cancer. Mol. Simul. 2022;48(12):1–21. doi: 10.1080/08927022.2022.2068799. [DOI] [Google Scholar]
- 136.Basak SC, Nayarisseri A, González-Díaz H, Bonchev D. Editorial (thematic issue: Chemoinformatics models for pharmaceutical design, part 1) Curr. Pharm. Des. 2016;22(33):5041–5042. doi: 10.2174/138161282233161109224932. [DOI] [PubMed] [Google Scholar]
- 137.Basak SC, Nayarisseri A, González-Díaz H, Bonchev D. Editorial (thematic issue: Chemoinformatics models for pharmaceutical design, part 2) Curr. Pharm. Des. 2016;22(34):5177–5178. doi: 10.2174/138161282234161110222751. [DOI] [PubMed] [Google Scholar]
- 138.Mendonça-Junior FJ, Scotti MT, Nayarisseri A, Zondegoumba EN, Scotti L. Natural bioactive products with antioxidant properties useful in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2019 doi: 10.1155/2019/7151780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Shameer K, Nayarisseri A, Duran FXR, González-Díaz H. Improving neuropharmacology using big data, machine learning and computational algorithms. Curr. Neuropharmacol. 2017;15(8):1058. doi: 10.2174/1570159X1508171114113425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Joshi I, Bhrdwaj A, Khandelwal R, Pande A, Agarwal A, Srija CD, Suresh RA, Mohan M, Hazarika L, Thakur G, Hussain T. Big data analytics in chemoinformatics and bioinformatics. Elsevier; 2023. Artificial intelligence, big data and machine learning approaches in genome-wide SNP-based prediction for precision medicine and drug discovery; pp. 333–357. [Google Scholar]
- 141.Prasoona RK, Jyoti A, Mukesh Y, Nishant S, Anuraj NS, Shobha J. Optimization of gaussian kernel function in support vector machine aided QSAR studies of C-aryl glucoside SGLT2 inhibitors. Interdiscip. Sci. Comput. Life Sci. 2013;5:45–52. doi: 10.1007/s12539-013-0156-y. [DOI] [PubMed] [Google Scholar]
- 142.Khuntwal K, Yadav M, Nayarisseri A, Joshi S, Sharma D, Suhane S. Credential role of van der Waal volumes and atomic masses in modeling hepatitis C virus NS5B polymerase inhibition by Tetrahydrobenzo-thiophenes using SVM and MLR aided QSAR studies. Curr. Bioinform. 2013;8(4):465–471. doi: 10.2174/1574893611308040008. [DOI] [Google Scholar]
- 143.Sharma N, Ethiraj KR, Yadav M, Nayarisseri SA, Chaurasiya M, Naik Vankudavath R, Rajender RK. Identification of LOGP values and electronegativities as structural insights to model inhibitory activity of HIV-1 capsid inhibitors-a SVM and MLR aided QSAR studies. Curr. Top. Med. Chem. 2012;12(16):1763–74. doi: 10.2174/1568026611209061763. [DOI] [PubMed] [Google Scholar]
- 144.Marunnan SM, Pulikkal BP, Jabamalairaj A, Bandaru S, Yadav M, Nayarisseri A, Doss VA. Development of MLR and SVM aided QSAR models to identify common SAR of GABA uptake herbal inhibitors used in the treatment of Schizophrenia. Curr. Neuropharmacol. 2017;15(8):1085–1092. doi: 10.2174/1567201814666161205131745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wickham H, Wickham H. ggplot2: Elegant graphics for data analysis. Springer International Publishing; 2016. Getting Started with ggplot2; pp. 11–31. [Google Scholar]
- 146.Wickham, H. An introduction to ggplot: An implementation of the grammar of graphics in R. Statistics, 1–8 (2006).
- 147.Tippmann S. Programming tools: Adventures with R. Nature. 2015;517(7532):109–110. doi: 10.1038/517109a. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The chemical structures and inhibitors data that support the findings of this study are available in Figshare with the identifier 10.6084/m9.figshare.24078207.v1.
The code used for running this study, model fitting, and plotting is available on a GitHub repository at https://github.com/AnurajNayarisseri/Vegfr_Cervical_Cancer.