Abstract
Cell plasticity has been found to play a critical role in tumor progression and therapy resistance. However, our understanding of the characteristics and markers of plastic cellular states during cancer cell lineage transition remains limited. In this study, multi-omics analyses show that prostate cancer cells undergo an intermediate state marked by Zeb1 expression with epithelial-mesenchymal transition (EMT), stemness, and neuroendocrine features during the development of neuroendocrine prostate cancer (NEPC). Organoid-formation assays and in vivo lineage tracing experiments demonstrate that Zeb1+ epithelioid cells are putative cells of origin for NEPC. Mechanistically, Zeb1 transcriptionally regulates the expression of several key glycolytic enzymes, thereby predisposing tumor cells to utilize glycolysis for energy metabolism. During this process, lactate accumulation-mediated histone lactylation enhances chromatin accessibility and cellular plasticity including induction of neuro-gene expression, which promotes NEPC development. Collectively, Zeb1-driven metabolic rewiring enables the epigenetic reprogramming of prostate cancer cells to license the adeno-to-neuroendocrine lineage transition.

Subject terms: Tumour biomarkers, Urogenital diseases
Introduction
Cell lineage plasticity is a major cause of therapeutic resistance in various cancer types [1]. With the increasing clinical use of second generation anti-androgen receptor (AR) drugs such as enzalutamide and abiraterone, the proportion of neuroendocrine prostate cancer (NEPC) has also significantly increased [2]. Currently available human single-cell RNA sequencing data including DNA copy number variation (CNV) analysis [3], combined with in vivo lineage tracing in mice, provide evidence supporting the origin of treatment-induced NEPC from prostate adenocarcinoma [4, 5]. The luminal epithelial cell state and neuroendocrine cell state represent the two ends of the cell differentiation continuum. An intermediate cell state with plasticity is a possible explanation for the transition from adenocarcinoma to NEPC [4, 6, 7]. However, this plastic state lacks clear molecular markers and its cellular characteristics are largely unknown. Identification and characterization of these cells in a plastic state is beneficial for the development of new treatment for NEPC.
Epithelial-mesenchymal plasticity (EMP) refers to the dynamic transition between epithelial and mesenchymal cell states, including the process of epithelial-mesenchymal transition (EMT) and mesenchymal-epithelial transition (MET) [8, 9], which has been shown to play an important role in cell fate determination such as differentiation of specific cell types in embryonic development [10] and reprogramming of induced pluripotent stem cells (iPSCs) [11, 12]. It has also been reported that tumor cells develop resistance to chemotherapy and immunotherapy in an EMT-dependent manner [13–19]. Therefore, we asked whether EMP is also involved in the cell lineage transition of prostate adenocarcinoma to NEPC.
Interestingly, recent studies revealed that metabolic reprogramming occurs during EMP dynamics. Altered energy metabolism in cancer cells has been shown to induce distinct epigenetic programs that are likely to contribute to tumorigenesis [20, 21]. Our recent study reported that downregulation of NUMB/NUMBL in prostate cancer (PCa) induces metabolic-epigenetic reprogramming leading to adenocarcinoma-to-neuroendocrine lineage transition [22]. Whether EMP induces metabolic changes and contributes to epigenetic alterations in NEPC development remains to be explored.
In this study, we report that Zeb1 marks a population of cells with plasticity, and that Zeb1-mediated changes in energy metabolism remodel chromatin accessibility, which in turn triggers neuroendocrine prostate cancer development.
Results
Zeb1 is dynamically expressed during NEPC development in the TRAMP mouse model
We previously found that Zeb1, an important EMT-regulating molecule, marks a population of basal stem cells and that Zeb1-expressing epithelial cells can be detected in mouse and human prostate tumor samples [23]. Therefore, we asked the influence of oncogenic transformation on the Zeb1 expression profile in the prostate epithelium and the possible connection of Zeb1 expression to the development of NEPC. For this purpose, we used a Zeb1/tdTomato mouse line in which the coding sequence of a tdTomato fluorescent reporter was fused to the last exon of Zeb1 through a P2A element [23] and crossed it with the TRAMP PCa mouse model [24]. The 8-week-old Zeb1/tdTomato; TRAMP mice began to develop initial hyperplasia in the prostate. Mild to severe prostatic intraepithelial neoplasia (PIN) developed at the age of 12 weeks (Fig. 1A, B). Adenocarcinoma was observed by 5 months of age in the Zeb1/tdTomato; TRAMP model (Fig. 1A, B). Foci of NEPC marked by SYP were observed in Zeb1/tdTomato; TRAMP mice at ≥7 months of age (Fig. 1A, B, F). In the control Zeb1/tdTomato reporter mice, Zeb1-expressing tdTomato+ cells were present only within Lineage− CD49f+ Sca-1+ basal cells, but not in Lineage− CD49f+ Sca-1− luminal cells (Fig. S1). In sharp contrast, Zeb1-expressing tdTomato+ cells could be detected in luminal cells of Zeb1/tdTomato; TRAMP mice (Fig. 1C, D). Specifically, Zeb1 expression in the prostate luminal cell compartment was first found in disorganized hyperplastic glands of TRAMP mice (Fig. 1C, D). In PIN, characterized by intraluminal papillary protrusions and intraductal invasion, the number of Zeb1+ luminal cells was further increased. Interestingly, Zeb1 expression gradually decreased with tumor progression to NEPC (Fig. 1C, D). Co-staining with tdTomato and the luminal cell marker CK18 or the neuroendocrine marker SYP in the prostate frozen sections from Zeb1/tdTomato; TRAMP mice supported the results of the flow cytometry analysis (Fig. 1E, F). In general, Zeb1 showed a dynamic expression profile that first increased and then decreased during the course of NEPC.
Fig. 1. Zeb1 is dynamically expressed during the course of NEPC development.
A Image of prostates or prostate tumors of Zeb1/tdTomato; TRAMP mice at 4 weeks, 8 weeks, 12 weeks, 5 months and 7 months of age. Scale bars = 1 cm. B H&E staining showing typical histology of prostates or prostate tumors in Zeb1/tdTomato; TRAMP mice at different ages. Scale bars = 2 mm (top panel), scale bars = 50 μm (bottom panel). C, D Proportion (C) and statistics (D) of tdTomato+ (Zeb1+) cells in the Lineage− CD49f+ Sca-1− luminal cells of prostates or prostate tumors of Zeb1/tdTomato; TRAMP mice at different ages by flow cytometry (n = 4 mice) (Two-tailed Student’ s t-test was used for the statistical analysis: *P < 0.05; **P < 0.01; ***P < 0.001. Data are presented as mean ± SEM). E, F Immunofluorescent staining images of tdTomato and CK18 (E) or SYP (F) in the prostates or prostate tumors of Zeb1/tdTomato; TRAMP mice at different ages. Representative images are presented. Scale bars = 50 μm.
Single-cell sequencing data from human and mouse tissues support the dynamic changes in Zeb1 expression during NEPC development
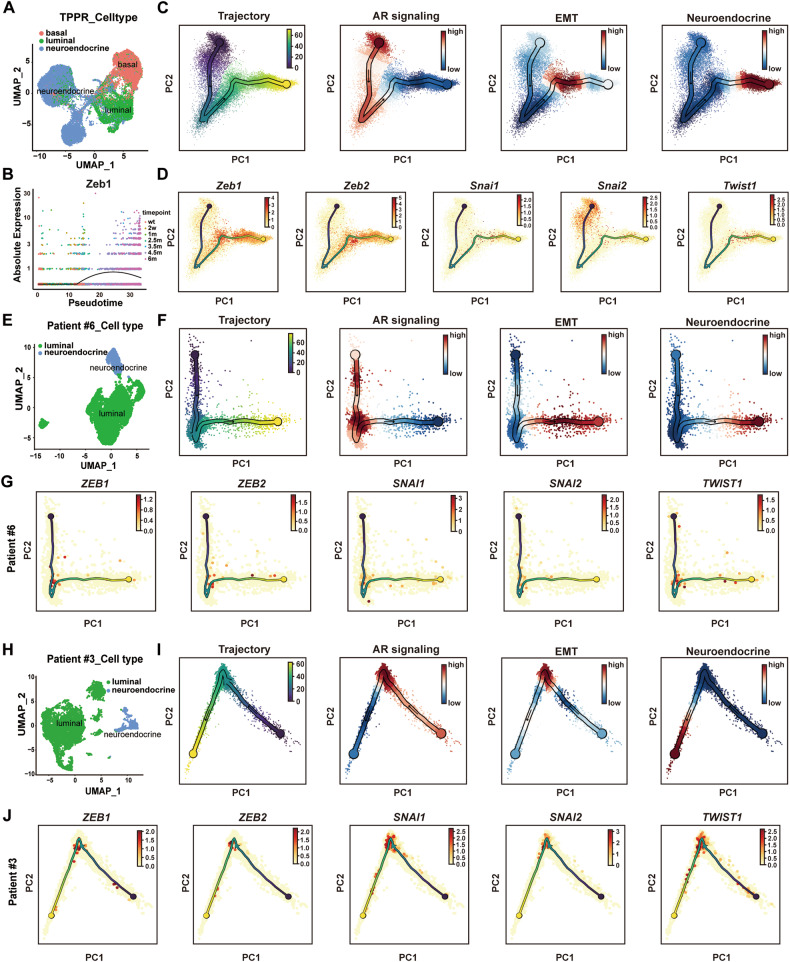
Ming Han et al. recently performed single-cell RNA sequencing using an inducible NEPC mouse model (TPPR) of Pten, Trp53 and Rb1 deletion driven by the luminal cell-specific inducible Tmprss2CreERT2 and reported cellular plasticity and molecular dynamics during NEPC development [25]. Inspired from their data, we examined the expression pattern of Zeb1 in the Pten/Trp53/Rb1-deficient NEPC animal models. We extracted all epithelial cells, including basal, luminal, and neuroendocrine cells for analysis (Fig. 2A and Fig. S2A–D). The number of neuroendocrine cells was increased as NEPC developed in Pten/Trp53/Rb1-deficient mice (Fig. S2A). We performed pseudotime analysis on all epithelial cells using Monocle 2 [26] (Fig. 2B) and scFates [27] (Fig. 2C, D). We found that Zeb1 began to be expressed in the prostate epithelium of mice at 1–2.5 months after induction of the Pten/Trp53/Rb1 knockout, then its expression increased and peaked at 4.5 months (Fig. 2B). From 4.5 months to 6 months after Pten/Trp53/Rb1 genes deletion, the expression of Zeb1 gradually decreased when the tumor was differentiated into NEPC (Fig. 2B). The results of this analysis were consistent with our observation of Zeb1 expression dynamics during NEPC development in TRAMP mice. Using scFates, we conducted a collective pseudotime analysis for gene sets. The results indicated that AR signaling pathway genes showed a decreasing trend in the process of TPPR-induced NEPC (Fig. 2C). The high expression of NEPC signature genes at the ends in the pseudotime analysis well reproduced the course of TPPR-induced NEPC (Fig. 2C). The expression profiles of EMT lineage genes showed a trend of first increasing and then decreasing in the pseudotime analysis (Fig. 2C). Specifically, the expression trend of Zeb1, Zeb2, Snai1, and Twist1 first increased and then decreased during the development of TPPR-induced NEPC, while Snai2 was highly expressed in the early stage in the pseudotime analysis, and also showed an expression trend that first increased and then decreased in the middle and late stages (Fig. 2D). We used UMAP to examine the expression of Snai2 and found that its expression levels were relatively higher in non-malignant basal cells (Fig. S2E), which is consistent with the stemness maintenance function of Snai2 in basal cells [28–30]. These results indicate that epithelial cells undergo a period of acquiring EMT characteristics during NEPC development.
Fig. 2. Single-cell sequencing data reveal dynamic changes of Zeb1 expression during human and mouse NEPC development.
A UMAP visualization of epithelial cell profiles in TPPR mouse scRNA-seq. Data were obtained from Han et al. [25] (OMIX: OMIX001928). B Zeb1 expression levels in pseudotime analysis of TPPR mice scRNA-seq [25] using Monocle 2. C, D Trajectory inference using scFates to analyze gene expression dynamics in prostate epithelial cells of TPPR mice [25]. Pseudotime analysis of gene sets of AR signaling, EMT and neuroendocrine signatures (C) and expression of specific EMT genes (D). E UMAP visualization of epithelial cell profiles from human PCa patient #6. Data were obtained from Baijun Dong et al.[3] (GEO: GSE137829). F, G Trajectory inference using scFates to analyze gene expression dynamics in PCa epithelial cells of patient #6 [3]. Pseudotime analysis of gene sets of AR signaling, EMT and neuroendocrine signatures (F) and expression of specific EMT genes (G). H UMAP visualization of epithelial cell profiles from human PCa patient #3. Data were obtained from Wang et al. [31] (GSA-Human: HRA002145). I, J Trajectory inference using scFates to analyze gene expression dynamics in PCa epithelial cells of patient #3 [31]. Pseudotime analysis of gene sets of AR signaling, EMT and neuroendocrine signatures (I) and expression of specific EMT genes (J).
Next, we performed a similar analysis on our [3] and Ziwei Wang et al.’s [31] previously reported single-cell sequencing data from human PCa. We extracted epithelial cells with DNA copy number variations from PCa patients with neuroendocrine differentiation, which included a subset of neuroendocrine cells expressing SYP and CHGA, as well as luminal cells with high levels of KRT8 and KRT18 expression (Fig. 2E, H, and Fig. S2F–I). In the pseudotime analysis of patient #6 [3] and patient #3 [31], which had the combination of adenocarcinoma and NEPC features, NEPC signature genes were highly expressed in the terminal phase, and the AR signaling pathway genes showed a downward trend in the process (Fig. 2F, I), which well recapitulated the development of NEPC in clinical patients. Consistent with the analysis results in mice, EMT lineage genes including ZEB1, ZEB2, SNAI1, SNAI2 and TWIST1 were relatively highly expressed at the middle stage of pseudotime analysis (Fig. 2G, J), suggesting that epithelial cells may acquire a state with EMT characteristics during the formation of NEPCs.
Zeb1+ epithelioid cells exhibit a more accessible chromatin with enrichment of neuroactive molecule expression
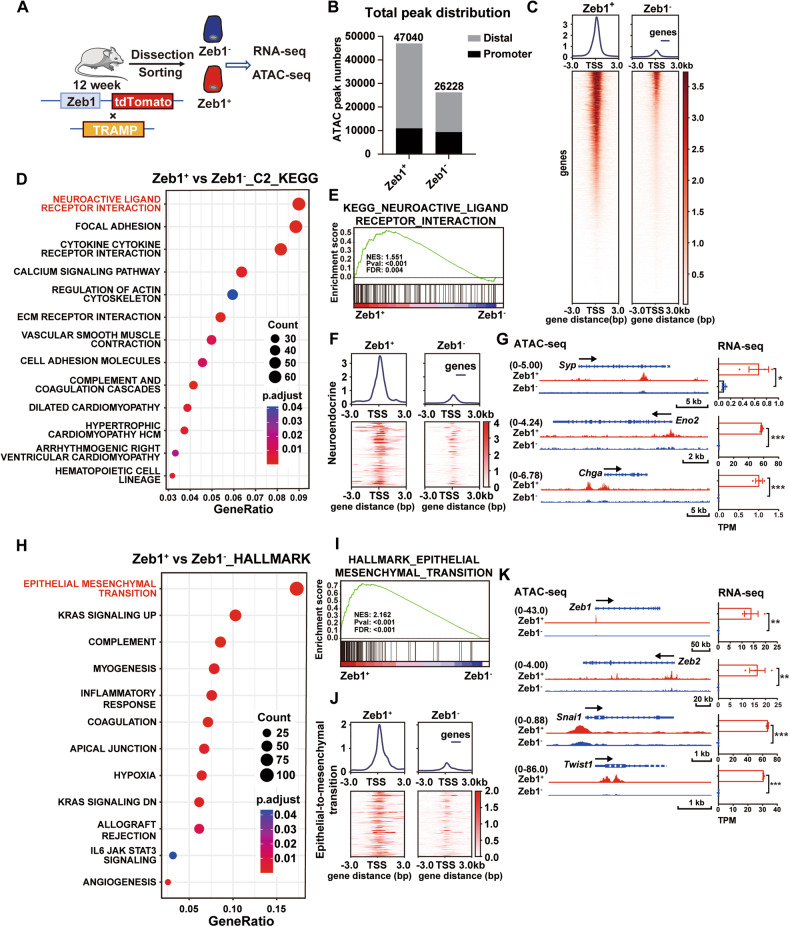
To explore the role of Zeb1+ epithelioid cells in the development of NEPC, we performed assays for transposase-accessible chromatin using sequencing (ATAC-seq) and RNA sequencing (RNA-seq) using biological replicates of flow cytometry-sorted Lineage− CD49f+ tdTomato− (Zeb1−) and Lineage− CD49f+ tdTomato+ (Zeb1+) cells (Fig. 3A). Analysis of ATAC-seq profiles revealed that Zeb1+ epithelioid cells possessed more open chromatin regions than the Zeb1− counterpart (Fig. 3B, C). Of note, Kyoto Encyclopedia of Genes and Genomes (KEGG) analysis (Fig. 3D) and Gene Set Enrichment Analysis (GSEA) (Fig. 3E) of RNA-seq showed that neuroactive ligand receptor interaction was the most upregulated gene set in Zeb1+ epithelioid cells compared to the Zeb1− counterpart. In addition, GSEA results showed that the neuroendocrine gene signature and neurotransmitter transport were enriched in Zeb1+ epithelioid cells (Fig. S3A and S3B). We then examined the transcript levels of known neuroendocrine lineage markers in Zeb1− and Zeb1+ epithelioid cells, and found that Syp, Eno2, and Chga were more abundantly expressed in Zeb1+ epithelioid cells (Fig. 3G). Consistent with the gene expression data, the ATAC-seq results showed that Zeb1+ epithelioid cells exhibited more open chromatin features of neuroendocrine lineage genes (Fig. 3F, G).
Fig. 3. Zeb1+ epithelioid cells exhibit a more accessible chromatin with an expression characteristic of neuroactive and EMT program.
A Experimental strategy for epigenetic and transcriptional profiling of flow cytometry-sorted Lineage− CD49f+ tdTomato− (Zeb1−) and Lineage− CD49f+ tdTomato+ (Zeb1+) cells from prostates of Zeb1/tdTomato; TRAMP mice around 12 weeks of age. B Total number of ATAC-seq peaks in Zeb1− and Zeb1+ epithelioid cells, separated into promoter (<±3 kb transcription start site [TSS]) and distal regions (>±3 kb TSS). C Heatmaps depicting chromatin accessibility based on ATAC-seq peaks from Zeb1− and Zeb1+ epithelioid cells. D Gene sets enriched in Zeb1+ epithelioid cells compared to the Zeb1− counterpart in KEGG enrichment analysis of RNA-seq. E GSEA analysis of RNA-seq of Zeb1+ epithelioid cells versus the Zeb1− counterpart in neuroactive ligand receptor interaction. F Heatmap depicting chromatin accessibility based on peaks of neuroendocrine gene set in ATAC-seq of Zeb1+ epithelioid cells and the Zeb1− counterpart. G ATAC-seq and RNA-seq of neuroendocrine indicator genes in Zeb1+ epithelioid cells and the Zeb1− counterpart. H Gene sets enriched in Zeb1+ epithelioid cells compared to the Zeb1− counterpart in Hallmark enrichment analysis of RNA-seq. I GSEA analysis of RNA-seq of Zeb1+ epithelioid cells versus the Zeb1− counterpart in epithelial-mesenchymal transition. J Heatmap depicting chromatin accessibility based on peaks of EMT gene set in ATAC-seq of Zeb1+ epithelioid cells and the Zeb1− counterpart. K ATAC-seq and RNA-seq of EMT lineage genes in Zeb1+ epithelioid cells and the Zeb1− counterpart (n = 2 mice for the ATAC-seq, n = 3 mice for the RNA-seq. Two-tailed Student’ s t-test was used for the statistical analysis: *P < 0.05; **P < 0.01; ***P < 0.001. Data are presented as mean ± SEM).
Zeb1+ epithelioid cells display cellular plasticity with EMT characteristics
In the RNA-seq data of Zeb1+ epithelioid cells versus the Zeb1− counterpart, epithelial-mesenchymal transition ranked top in the enrichment analysis and GSEA of hallmark gene sets (Fig. 3H, I). Compared to the Zeb1− counterpart, the chromatin accessibility of EMT genes and expression levels of Zeb2, Snai1 and Twist1 genes were higher in Zeb1+ epithelioid cells (Fig. 3J, K). GSEA results showed that stemness-related gene signaling pathways and pluripotent stem cell pathway were also enriched in Zeb1+ epithelioid cells (Fig. S3C). Compared to the Zeb1− counterpart, the expression of Mycn and Ascl1, which have been reported to drive NEPC, were also significantly upregulated in Zeb1+ epithelioid cells (Fig. S3D). In addition, AR signaling and expression of luminal lineage genes were significantly lower in Zeb1+ epithelioid cells than in the Zeb1− counterpart (Fig. S3E). These data prompted us to hypothesize that Zeb1+ epithelioid cells may play an important role in the development of NEPC.
Zeb1+ PCa epithelioid cells can generate organoids with neuroendocrine properties
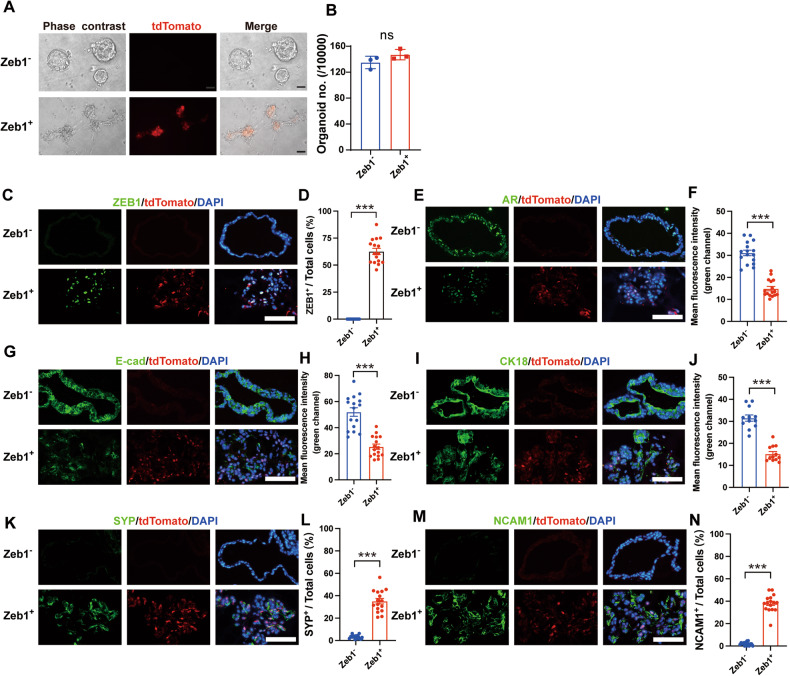
To provide experimental evidence that Zeb1+ epithelioid cells are essential for the development of NEPC in vitro, we performed organoid-formation culture experiments to examine their acquisition capability of neuroendocrine properties using flow cytometry-sorted Lineage− CD49f+ tdTomato− (Zeb1−) and Lineage− CD49f+ tdTomato+ (Zeb1+) cells. Although there was no significant difference in the organoid-formation ability of Zeb1− versus Zeb1+ PCa epithelioid cells (Fig. 4A, B), strikingly, we observed significant differences between the two in the morphological appearance of the formed organoids (Fig. 4A). Organoids derived from Zeb1− PCa cells exhibited cystic spherical shapes, while organoids generated from Zeb1+ PCa cells displayed irregular shapes and formed protrusions outside the exterior organoid border (Fig. 4A). Immunostaining analysis of frozen sections of organoids showed that tdTomato was able to faithfully reflect the expression of ZEB1 (Fig. 4C). tdTomato− (Zeb1−) cells did not give rise to tdTomato+ (Zeb1+) cells when cultured in vitro (Fig. 4C, D). In contrast, both tdTomato+ (Zeb1+) and tdTomato− (Zeb1−) cells could be detected in the organoids derived from Zeb1+ epithelioid cells (Fig. 4C, D). AR was expressed in organoids formed by both Zeb1− and Zeb1+ epithelioid cells, but at a relatively lower level in organoids formed by Zeb1+ epithelioid cells (Fig. 4E, F). We also examined the expression of AR signaling pathway genes in both organoids. The qRT-PCR results showed that the expression levels of Ar, Nkx3-1, and Tmprss2 were lower in organoids derived from Zeb1+ epithelioid cells than in organoids derived from the Zeb1− counterpart (Fig. S4A). Furthermore, the intensity of E-Cadherin and CK18 expression was lower in organoids generated from Zeb1+ epithelioid cells (Fig. 4G–J), and the mRNA levels of the luminal signature genes Krt8 and Krt18 were also lower in organoids formed by Zeb1+ epithelioid cells (Fig. S4B), which is consistent with the previously well-characterized function of Zeb1 in suppressing the epithelial phenotype [32, 33]. Notably, expression of the neuroendocrine lineage makers SYP and NCAM1 could be detected in organoids derived from Zeb1+ epithelioid cells, but was very rare in organoids derived from Zeb1− PCa cells (Fig. 4K–N). The qRT-PCR results showed that the expression levels of neuroendocrine signature genes Syp, Eno2, Chga and Ncam1 were also significantly higher in organoids derived from Zeb1+ epithelioid cells than in organoids derived from the Zeb1− counterpart (Fig. S4C). These results demonstrated that Zeb1+ epithelioid cells could generate organoids with neuroendocrine properties. Therefore, Zeb1+ epithelioid cells may act as the cellular origin of NEPC.
Fig. 4. Zeb1+ epithelioid cells can generate organoids with neuroendocrine characteristics.
A Representative brightfield (phase contrast) and fluorescence images of organoids derived from flow cytometry-sorted Lineage− CD49f+ tdTomato− (Zeb1−) and Lineage− CD49f+ tdTomato+ (Zeb1+) cells from prostates of Zeb1/tdTomato; TRAMP mice around 12 weeks of age. The passage number is P1. Scale bars = 50 μm. B Quantification of the number of organoids derived from Zeb1− and Zeb1+ epithelioid cells. 10,000 Zeb1− or Zeb1+ epithelioid cells sorted by flow cytometry were seeded in a low-adsorption 96-well plate, cultured in organoid culture medium for 6 days, and then image acquisition and organoids counting were performed. Organoids larger than 50 μm in diameter were counted. The passage number is P1 (n = 3 replicates). C, E, G, I, K, M Immunofluorescent staining images of tdTomato with ZEB1 (C), AR (E), E-cad (G), CK18 (I), SYP (K), or NCAM1 (M) in frozen sections of organoids derived from Zeb1− and Zeb1+ epithelioid cells. Representative images are presented. The passage number is P2. Scale bars = 50 μm. D, L, N Proportion of ZEB1+ cells (D), SYP+ cells (L), or NCAM1+ cells (N) in frozen sections of organoids derived from Zeb1− and Zeb1+ epithelioid cells. 16 organoids (over 1200 cells) from each group were analyzed. F, H, J Fluorescence intensity of AR (F), E-cad cells (H), or CK18 cells (J) in frozen sections of organoids derived from Zeb1− and Zeb1+ epithelioid cells. 16 organoids from each group were analyzed. (Two-tailed Student’ s t-test was used for the statistical analysis: ns, not significant; *P < 0.05; **P < 0.01; ***P < 0.001. Data are presented as mean ± SEM).
Lineage tracing indicates that Zeb1+ epithelioid cells are the cells of origin for NEPC
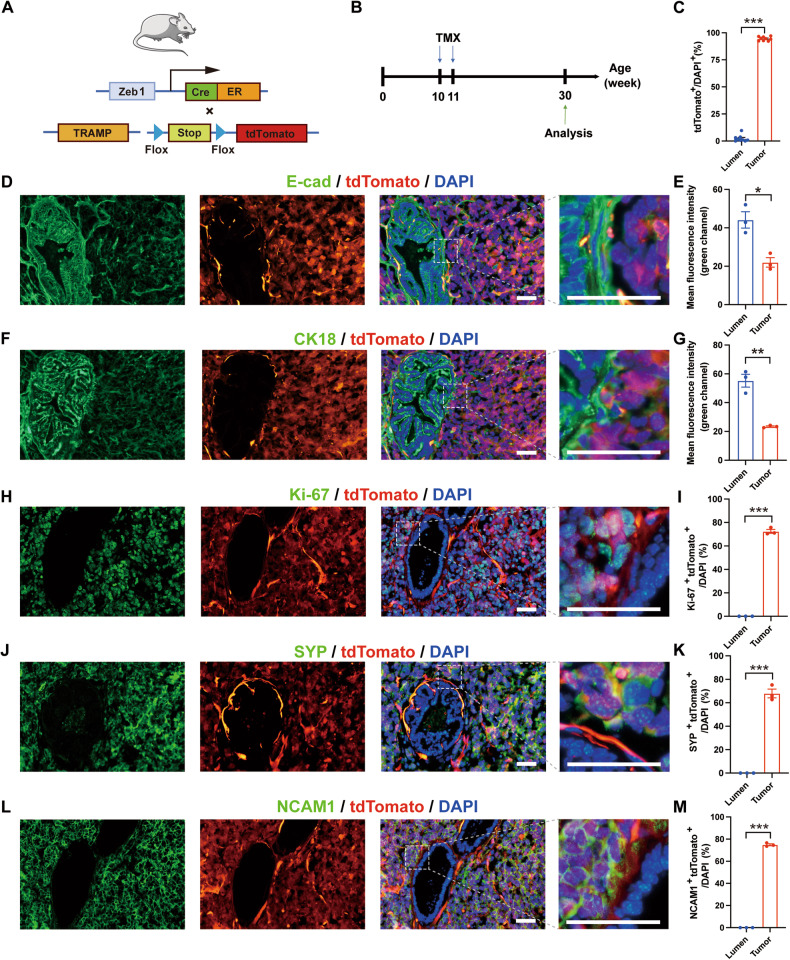
To further determine the role of Zeb1+ epithelioid cells in NEPC development in vivo, we crossed the Zeb1-CreERT2; Rosa-CAG-LSL-tdTomato [23] mouse line with TRAMP mice to track Zeb1+ epithelioid cells during NEPC development (Fig. 5A). As described above, a small number of Zeb1+ luminal cells were identified in the prostates of 8-12-week-old TRAMP mice with PIN (Fig. 1C, D). At this stage, we did not observe evident NEPC foci. Therefore, we chose to administer tamoxifen starting at week 10 to induce tdTomato expression in the progeny cells of Zeb1+ cells. Zeb1-CreERT2; tdTomato; TRAMP mice were then euthanized at ≥7 months of age to obtain prostate tumor tissues (Fig. 5B). Immunofluorescence staining for SYP and NCAM1 revealed that NEPC differentiation was detected in 57.1% (12/21) of the tumors in TRAMP mice. Among these 12 mice, we detected co-staining of SYP and tdTomato in the tumor area in prostate tumors of 9 mice (75%) (Fig. S5). We noticed that in prostate tumors grown in TRAMP mice, there were morphologically normal glandular areas and malignant tumor areas where cells were disorderly arranged. We counted the expression of tdTomato in these two regions, and the results showed that the expression ratio of tdTomato in the tumor areas was much higher than that in the normal glandular regions (Fig. 5C). As shown in Fig. 5D, F, the epithelial cell marker E-cad and luminal cell marker CK18 were expressed in both the morphologically normal lumen regions and the tumor areas. However, the expression intensity of E-cad and CK18 in the normal lumen regions was higher than that in the tumor regions (Fig. 5E, G). Interestingly, co-staining of tdTomato and E-cad or CK18 was mostly observed in the tumor areas, but was rare in the normal lumen regions (Figs. 5D, F). Approximately 70% of tdTomato+ cells in the tumor area were labeled by Ki-67, which indicated that these tumor areas were in a highly proliferative state; while co-staining of Ki-67 and tdTomato was not detectable in the normal glandular area (Figs. 5H, I). In these Ki-67+ highly proliferative tumorous cells, tdTomato co-stained with SYP or NCAM1 in 60–80% of cells (Fig. 5J–M), indicating that these SYP+ or NCAM1+ neuroendocrine cells were the progeny of Zeb1+ epithelioid cells. These lineage tracing results provide in vivo evidence that Zeb1+ epithelioid cells are the cell of origin for NEPC in TRAMP mice.
Fig. 5. Lineage tracing indicates that Zeb1+ epithelioid cells are the cellular origin of NEPC.
A Strategy for tracing tdTomato expression in Zeb1 progeny cells in vivo. B Illustration of protocols to track the fate of Zeb1+ cells during prostate tumorigenesis. C Ratios of tdTomato+ cells in the normal lumen and tumor areas of cryosections of mouse prostate tumors in the Zeb1-creERT2; tdTomato; TRAMP mice lineage tracing experiment (n = 9 mice). D, F, H, J, L Immunofluorescent staining of tdTomato with E-cad (D), CK18 (F), Ki-67 (H), SYP (J), or NCAM1 (L) in cryosections of mouse prostate tumors in the lineage tracing experiment. Representative images are presented. Scale bar = 50 μm. E, G Fluorescence intensity of E-cad (E) and CK18 (G) in the normal lumen and tumor areas of cryosections of mouse prostate tumors in the lineage tracing experiment (n = 3 mice). I, K, M Proportion of Ki-67+ tdTomato+ cells (I), SYP+ tdTomato+ cells (K), or NCAM1+ tdTomato+ (M) in the normal lumen and tumor areas of frozen sections of mouse prostate tumors in lineage tracing experiments (n = 3 mice).
Zeb1 promotes glycolysis and lactate accumulation in PCa
Our previous study reported that NEPCs tend to preferentially utilize aerobic glycolysis for energy metabolism [22]. Interestingly, gene enrichment analysis for the hallmark metabolic pathways showed that glycolysis and adipogenesis ranked top in Zeb1+ epithelioid cells (Fig. 6A). Although other metabolic pathways also showed a trend of change, we did not observe significant differences in these pathways between Zeb1− and Zeb1+ epithelioid cells. Enrichment analysis of RNA-seq (Fig. 6B) and ATAC-seq (Fig. 6C) showed that the glycolytic signaling pathway was highly enriched in prostate Zeb1+ epithelioid cells compared to the Zeb1− counterpart. We investigated the expression of key enzymes including Hk2, Pfkp, and lactate dehydrogenase (Ldha) in glucose metabolism (Fig. 6D) in Zeb1− and Zeb1+ epithelioid cells using qRT-PCR. Compared to the Zeb1− counterpart, the RNA levels of Hk2 and Pfkp were significantly increased in Zeb1+ epithelioid cells (Fig. 6E). The expression level of Ldha, which catalyzes the reaction that generates lactate from pyruvate, was higher in Zeb1+ epithelioid cells than in the Zeb1− counterpart (Fig. 6E).
Fig. 6. Zeb1 promotes glycolysis and accumulation of lactate in PCa.
A Rank of hallmark metabolic pathways in flow cytometry-sorted Lineage− CD49f+ tdTomato− (Zeb1−) and Lineage− CD49f+ tdTomato+ (Zeb1+) cells from prostates of Zeb1/tdTomato; TRAMP mice around 12 weeks of age based on normalized enrichment score. B, C GSEA analysis of RNA-seq (B) and ATAC-seq (C) of Zeb1+ epithelioid cells versus the Zeb1− counterpart in the hallmark glycolysis gene set. D Schematic diagram of the glucose metabolic pathway. E qRT-PCR analysis of Zeb1, Hk1, Hk2, Pfkp, Pkm, Gapdh, Ldha and Pdha1 mRNA levels in Zeb1− and Zeb1+ epithelioid cells. Gene expression was normalized to the expression of Actb (n = 3 replicates). F, G Protein levels of ZEB1, HK1, HK2, PFKP, PKM1/2, GAPDH, LDHA and PDHA1 in control and Zeb1-overexpressing LNCaP cells (F), and in scramble and Zeb1-knockdown TRAMP-C1 cells (G) were determined by immunoblotting. H Genome views of ZEB1 enrichment at the HK2, PFKP, LDHA genes in MDA-MB231 cells from analysis of published ChIP-seq data [34]. ZEB1 peaks are depicted in red. I ChIP-q-PCR analysis showing enrichment levels of ZEB1 at the HK2, PFKP and LDHA promoters in Zeb1-overexpressing LNCaP cells (n = 3 replicates). J Lactate levels in Zeb1− and Zeb1+ epithelioid cells in flow cytometry-sorted 4-month-old Zeb1/tdTomato; TRAMP mice (n = 3 replicates). K Lactate levels in control and Zeb1-overexpressing LNCaP cells (n = 3 replicates) (Two-tailed Student’ s t-test was used for the statistical analysis: ns not significant; *P < 0.05; **P < 0.01; ***P < 0.001. Data are presented as mean ± SEM).
Next, we transfected LNCaP, a prostate adenocarcinoma cell line, with Zeb1-overexpressing lentivirus. As shown in Fig. 6F, Zeb1 overexpression significantly upregulated the protein levels of HK2, PFKP and LDHA in the Zeb1-overexpressing LNCaP cells (Fig. 6F). Zeb1 knockdown in the NEPC-like cell line TRAMP-C1 resulted in decreased expression of key glycolytic enzymes HK2, PFKP and LDHA, further illustrating the positive regulatory effect of Zeb1 on HK2, PFKP and LDHA (Fig. 6G). In addition, qRT-PCR results showed that Zeb1 knockdown upregulated the expression of AR signaling genes (Nkx3.1, Tmprss2) and luminal genes (Krt8, Krt18), and downregulated the expression of EMT signature genes (Zeb2, Snai2), neuroendocrine genes (Eno2, Ncam1) and key glycolytic enzyme genes (Hk2, Pfkp, Ldha) (Fig. S6). These results indicate that Zeb1 regulates the metabolic pattern and affects the lineage plasticity of PCa cells. Analysis of previously published ChIP-seq data [34] revealed enrichment of ZEB1 at the promoter regions of HK2, PFKP and LDHA in the human breast cancer cell line MDA-MB231 (Fig. 6H). ChIP-qPCR in the Zeb1-overexpressing LNCaP cell line showed that ZEB1 could bind to the promoters of HK2, PFKP and LDHA to regulate their transcription in PCa (Fig. 6I). To test whether the increase in glycolytic enzymes and LDHA could lead to increased production of lactate, we evaluated lactate levels in flow cytometry-sorted Zeb1− and Zeb1+ epithelioid cells using a lactate detection kit. As expected, Zeb1+ epithelioid cells produced more lactate (Fig. 6J). In addition, we found that lactate was also accumulated in Zeb1-overexpressing LNCaP cells (Fig. 6K). Collectively, these results suggest that Zeb1 induces PCa cells to preferentially utilize aerobic glycolysis for energy metabolism by promoting the expression of key glycolytic enzymes HK2, PFKP and LDHA and leads to the accumulation of lactate.
Lactate accumulation in Zeb1+ epithelioid cells enhances histone lactylation to confer cell plasticity
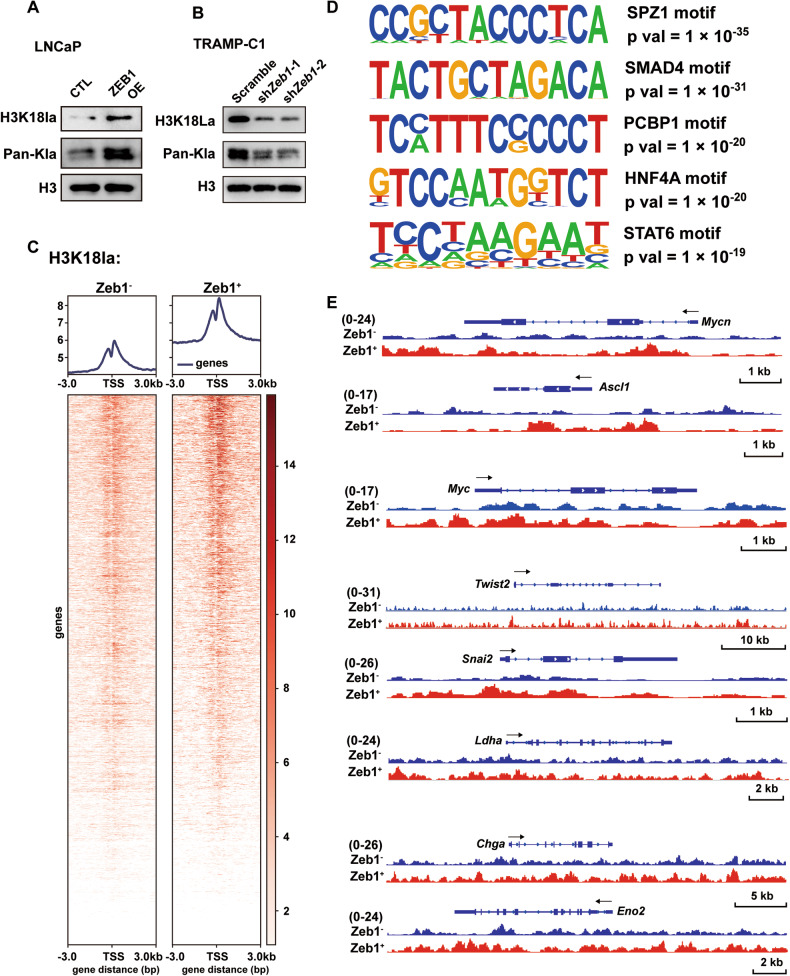
Recent studies have reported that histone lactylation, a specific epigenetic modification, is modulated by the level of cellular lactate content [35–37]. We found that Zeb1 overexpression promoted pan-lysine lactylation (Pan-Kla) and H3K18 lactylation (H3K18la) in LNCaP cells (Fig. 7A), while knockdown of Zeb1 in TRAMP-C1 significantly reduced the levels of Pan-Kla and H3K18la (Fig. 7B). To characterize the impact of altered histone lactylation on gene transcription, we performed CUT & Tag with anti-H3K18la (Fig. 7C) and anti-Pan Kla (Fig. S7A) antibodies using flow cytometry-sorted Lineage− CD49f+ tdTomato− (Zeb1−) and Lineage− CD49f+ tdTomato+ (Zeb1+) cells. As shown in Fig. 7C and Fig. S7A, the H3K18la signals and Pan-Kla signals around the TSS were significantly increased in Zeb1+ epithelioid cells.
Fig. 7. Lactate accumulation in Zeb1+ epithelioid cells enhances histone lactylation conferring lineage plasticity in PCa.
A, B Immunoblotting of Pan-Kla and H3K18 lactylation levels in control and Zeb1-overexpressing LNCaP cells (A), and in scramble and Zeb1-knockdown TRAMP-C1 cells (B). C Tag density pileups of H3K18la peaks in flow cytometry-sorted Lineage− CD49f+ tdTomato− (Zeb1−) and Lineage− CD49f+ tdTomato+ (Zeb1+) cells from prostates of Zeb1/tdTomato; TRAMP mice around 4 months of age. Two replicates were used for the CUT & Tag assay (n = 2 mice). D Motif analysis of H3K18la signal peaks in Zeb1+ epithelioid cells compared to the Zeb1− counterpart. SPZ1, SMAD4, PCBP1, HNF4A and STAT6 were matched to known motifs. E Genome views of H3K18la tag density at NEPC driver genes (Mycn and Ascl1), pluripotent gene (Myc), EMT genes (Twist2, Snai2), glycolysis-related gene (Ldha) and NE markers (Chga and Eno2) in Zeb1− and Zeb1+ epithelioid cells.
To further understand how the histone lactylation modifies gene transcriptional profile, which probably drives cell fate change, we performed motif analysis of H3K18la and Pan-Kla signal peaks. As shown in Figs. 7D and S7B, motifs of transcription factors that have been reported to be actively involved in neuro-genesis (SMAD4, STAT6 and CTCF), EMT (SPZ1 and SMAD4), stemness (ZSCAN4 and TGIF2), and glycolysis (PCBP1, HNF4A, NFY and CTCF) were enriched in Zeb1+ epithelioid cells. The genomic view showed that the Pan-Kla and H3K18la signals were upregulated in the TSS and gene body region of Mycn and Ascl1, which have been reported as transcription factors promoting neuroendocrine differentiation in PCa. In addition, Pan Kla and H3K18la levels of the pluripotency factor Myc, the EMT transcription factors Twist2 and Snai2, the key enzyme Ldha for the conversion of pyruvate to lactate, and the neuroendocrine lineage genes Chga and Eno2 were higher in Zeb1+ epithelioid cells (Figs. 7E and S7C). Collectively, the above data suggest that lactate accumulation in Zeb1+ epithelioid cells leads to a significant increase in histone lactylation in PCa, which in turn increases cellular chromatin accessibility and enhances cellular plasticity to induce neuroendocrine prostate carcinogenesis.
Discussion
Our study identifies that Zeb1 can serve as a marker of the intermediate cell state with cellular plasticity in the adeno-to-neuroendocrine lineage transition of prostate cancer. Zeb1+ epithelioid cells display a highly open chromatin, a prerequisite for cell plasticity, and possess EMT, stemness and neuroendocrine characteristics. In vitro organoid-formation assays and in vivo lineage tracing experiments indicate that Zeb1+ epithelioid cells are able to generate SYP+, NCAM1+ NEPC in TRAMP mice. Mechanistically, Zeb1 upregulates the expression of key enzymes of glycolysis, leading to a preferential utilization of aerobic glycolysis for cellular energy metabolism, and an accumulation of lactate in this process. Increased lactate incurs a high level of histone lactylation, thereby enhancing the chromatin accessibility and cellular lineage plasticity, which promotes the development of NEPC.
Clinically, the incidence of primary NEPC is rare, and in most cases, it occurs after resistance to androgen deprivation therapy has developed [4]. Previous studies have reported that adenocarcinomas that are resistant to androgen-targeted agents undergo dedifferentiation and then transition to a neuroendocrine state [4, 5, 7]. In this study, we demonstrate that Zeb1, an EMT transcription factor, can serve as a molecular marker of an intermediate cell state with plasticity. Trajectory analysis of single-cell RNA sequencing data from mouse and human NEPCs shows that the AR signaling decreased at the onset of NEPC, and then EMT lineage genes, including Zeb1, began to be expressed. Previous studies reported that androgen deprivation induced EMT in prostate tumors [38, 39], and that AR inhibition mediated the upregulation of Zeb1 [38]. This may provide a plausible explanation for the elevation of Zeb1 during the occurrence of NEPC. EMT is very critical for the induction of plasticity [40, 41]. We propose that PCa cells turn on the cell plasticity switch after acquiring EMT characteristics. Tumor cells then undergo a transition to a neuroendocrine lineage. Along with the neuroendocrine differentiation, the plasticity of tumor cells decreases and EMT characteristics are gradually lost.
In our lineage tracing experiments using the Zeb1-CreERT2; Rosa-CAG-LSL-tdTomato; TRAMP mice, NEPC foci are labeled with tdTomato in 75% of mice examined, suggesting that Zeb1+ epithelioid cells are the cells of origin of NEPC. The ratio of tdTomato-labeled NEPC is probably a reflection of a suboptimal induction efficiency of Zeb1 promoter-driven Cre-ERT2. Nevertheless, we cannot rule out the possibility that the generation of some NEPCs may not necessarily go through the EMT state.
Our study supports that metabolic dysregulation and epigenetic alterations are key drivers of cell fate change in cancer. Histone lactylation mediated by the accumulation of the glycolytic metabolite lactate increases cellular plasticity and alters cell fate. Previous studies have demonstrated that Zeb1 can directly bind to P300 [42], which is a lactyltransferase [36]. Therefore, in addition to the role of promoting glycolysis to increase lactate production and histone lactylation, it is possible that Zeb1 interacts with the histone lactylation writer P300 to further participate in histone lactylation-governed transcriptional regulation. It has been reported that Twist1-mediated EMT leads to epigenetic reprogramming, resulting in activating H3K4me3/repressive H3K27me3 bivalent chromatin formation [43, 44]. In breast cancer cells, bivalent chromatin states were also found on the Zeb1 promoter, which facilitates rapid switching of cell states in response to different cellular signals [45]. The dynamic expression of Zeb1 during the course of NEPC in our study may also be related to the bivalent chromatin state on Zeb1 promoter. Therefore, different epigenetic modifications are probably orchestrated to promote the development of NEPC.
Therapy resistance caused by the increased plasticity of tumor cells is one of the big challenges that need to be overcome in the current clinical treatment of PCa [1, 2, 7]. We demonstrate that Zeb1-driven propensity for glycolytic energy metabolism in PCa cells increases histone lactylation levels, and that this metabolic-epigenetic reprogramming is involved in the regulation of tumor cell plasticity and the induction of NEPC. Restraint of the metabolic-epigenetic cascade may offer significant potential for preventing resistance to targeted therapies in lineage-plastic tumors.
Materials and methods
Detailed “Materials and methods” are provided in the supplementary materials and methods.
Supplementary information
Acknowledgements
The study was supported by funds from the National Key R&D Program of China (2022YFA1302704 and 2023YFC1404101), the National Natural Science Foundation of China U23A20454, NSFC82372873, NSFC32022021, and NSFC81872406, Shanghai Pilot Program for Basic Research-Shanghai Jiao Tong University (21TQ1400225), the Program of Shanghai Academic/Technology Research Leader (21XD1422300), the Shanghai Municipal Education Commission–Gaofeng Clinical Medicine Grant Support (20181706), the Innovative research team of high-level local universities in Shanghai, 111 project (B21024), Peak Disciplines (Type IV) of Institutions of Higher Learning in Shanghai, the KC Wong foundation.
Author contributions
HHZ and WQG conceived the study; DW performed the experiments; GD, XC, JW, and NW supported data analysis; KL, NJ, YS, YL, and XX assisted in animal experiments; HZ and PX helped in construction of plasmids; YH and YZ participated in immunostaining and imaging; CC, WB, KZ, and PZ helped in experiments design. HHZ, WQG, and DW interpreted the data and wrote the manuscript.
Data availability
RNA-seq, ATAC-seq and CUT & Tag data in this study have been deposited to the National Genomics Data Center, China National Center for Bioinformation with Bioproject number PRJCA020095.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Deng Wang, Genyu Du.
Contributor Information
Wei-Qiang Gao, Email: gao.weiqiang@sjtu.edu.cn.
Helen He Zhu, Email: zhuhecrane@shsmu.edu.cn.
Supplementary information
The online version contains supplementary material available at 10.1038/s41418-024-01295-5.
References
- 1.Quintanal-Villalonga A, Chan JM, Yu HA, Pe’er D, Sawyers CL, Sen T, et al. Lineage plasticity in cancer: a shared pathway of therapeutic resistance. Nat Rev Clin Oncol. 2020;17:360–71. doi: 10.1038/s41571-020-0340-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Davies AH, Beltran H, Zoubeidi A. Cellular plasticity and the neuroendocrine phenotype in prostate cancer. Nat Rev Urol. 2018;15:271–86. doi: 10.1038/nrurol.2018.22. [DOI] [PubMed] [Google Scholar]
- 3.Dong B, Miao J, Wang Y, Luo W, Ji Z, Lai H, et al. Single-cell analysis supports a luminal-neuroendocrine transdifferentiation in human prostate cancer. Commun Biol. 2020;3:778. doi: 10.1038/s42003-020-01476-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Soundararajan R, Paranjape AN, Maity S, Aparicio A, Mani SA. EMT, stemness and tumor plasticity in aggressive variant neuroendocrine prostate cancers. Biochim Biophys Acta Rev Cancer. 2018;1870:229–38. doi: 10.1016/j.bbcan.2018.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zou M, Toivanen R, Mitrofanova A, Floch N, Hayati S, Sun Y, et al. Transdifferentiation as a mechanism of treatment resistance in a mouse model of castration-resistant prostate cancer. Cancer Discov. 2017;7:736–49. doi: 10.1158/2159-8290.CD-16-1174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Santoni M, Conti A, Burattini L, Berardi R, Scarpelli M, Cheng L, et al. Neuroendocrine differentiation in prostate cancer: novel morphological insights and future therapeutic perspectives. Biochim Biophys Acta. 2014;1846:630–7. doi: 10.1016/j.bbcan.2014.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Beltran H, Prandi D, Mosquera JM, Benelli M, Puca L, Cyrta J, et al. Divergent clonal evolution of castration-resistant neuroendocrine prostate cancer. Nat Med. 2016;22:298–305. doi: 10.1038/nm.4045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bakir B, Chiarella AM, Pitarresi JR, Rustgi AK. EMT, MET, plasticity, and tumor metastasis. Trends cell Biol. 2020;30:764–76. doi: 10.1016/j.tcb.2020.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lu W, Kang Y. Epithelial-mesenchymal plasticity in cancer progression and metastasis. Dev Cell. 2019;49:361–74. doi: 10.1016/j.devcel.2019.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thiery JP, Acloque H, Huang RY, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell. 2009;139:871–90. doi: 10.1016/j.cell.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 11.Li R, Liang J, Ni S, Zhou T, Qing X, Li H, et al. A mesenchymal-to-epithelial transition initiates and is required for the nuclear reprogramming of mouse fibroblasts. Cell Stem Cell. 2010;7:51–63. doi: 10.1016/j.stem.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Sun H, Qi J, Wang L, He S, Liu J, et al. Sequential introduction of reprogramming factors reveals a time-sensitive requirement for individual factors and a sequential EMT-MET mechanism for optimal reprogramming. Nat Cell Biol. 2013;15:829–38. doi: 10.1038/ncb2765. [DOI] [PubMed] [Google Scholar]
- 13.Boumahdi S, de Sauvage FJ. The great escape: tumour cell plasticity in resistance to targeted therapy. Nat Rev Drug Discov. 2020;19:39–56. doi: 10.1038/s41573-019-0044-1. [DOI] [PubMed] [Google Scholar]
- 14.Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527:525–30. doi: 10.1038/nature16064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Noman MZ, Janji B, Abdou A, Hasmim M, Terry S, Tan TZ, et al. The immune checkpoint ligand PD-L1 is upregulated in EMT-activated human breast cancer cells by a mechanism involving ZEB-1 and miR-200. Oncoimmunology. 2017;6:e1263412. doi: 10.1080/2162402X.2016.1263412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kurrey NK, Jalgaonkar SP, Joglekar AV, Ghanate AD, Chaskar PD, Doiphode RY, et al. Snail and slug mediate radioresistance and chemoresistance by antagonizing p53-mediated apoptosis and acquiring a stem-like phenotype in ovarian cancer cells. Stem Cells. 2009;27:2059–68. doi: 10.1002/stem.154. [DOI] [PubMed] [Google Scholar]
- 17.Fischer KR, Durrans A, Lee S, Sheng J, Li F, Wong ST, et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527:472–6. doi: 10.1038/nature15748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dongre A, Rashidian M, Reinhardt F, Bagnato A, Keckesova Z, Ploegh HL, et al. Epithelial-to-mesenchymal transition contributes to immunosuppression in breast carcinomas. Cancer Res. 2017;77:3982–9. doi: 10.1158/0008-5472.CAN-16-3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arumugam T, Ramachandran V, Fournier KF, Wang H, Marquis L, Abbruzzese JL, et al. Epithelial to mesenchymal transition contributes to drug resistance in pancreatic cancer. Cancer Res. 2009;69:5820–8. doi: 10.1158/0008-5472.CAN-08-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thakur C, Chen F. Connections between metabolism and epigenetics in cancers. Semin Cancer Biol. 2019;57:52–8. doi: 10.1016/j.semcancer.2019.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai Z, Ramesh V, Locasale JW. The evolving metabolic landscape of chromatin biology and epigenetics. Nat Rev Genet. 2020;21:737–53. doi: 10.1038/s41576-020-0270-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He Y, Ji Z, Gong Y, Fan L, Xu P, Chen X, et al. Numb/Parkin-directed mitochondrial fitness governs cancer cell fate via metabolic regulation of histone lactylation. Cell Rep. 2023;42:112033. doi: 10.1016/j.celrep.2023.112033. [DOI] [PubMed] [Google Scholar]
- 23.Wang X, Xu H, Cheng C, Ji Z, Zhao H, Sheng Y, et al. Identification of a Zeb1 expressing basal stem cell subpopulation in the prostate. Nat Commun. 2020;11:706. doi: 10.1038/s41467-020-14296-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Greenberg NM, DeMayo F, Finegold MJ, Medina D, Tilley WD, Aspinall JO, et al. Prostate cancer in a transgenic mouse. Proc Natl Acad Sci USA. 1995;92:3439–43. doi: 10.1073/pnas.92.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Han M, Li F, Zhang Y, Dai P, He J, Li Y, et al. FOXA2 drives lineage plasticity and KIT pathway activation in neuroendocrine prostate cancer. Cancer Cell. 2022;40:1306–23 e8. doi: 10.1016/j.ccell.2022.10.011. [DOI] [PubMed] [Google Scholar]
- 26.Qiu X, Mao Q, Tang Y, Wang L, Chawla R, Pliner HA, et al. Reversed graph embedding resolves complex single-cell trajectories. Nat Methods. 2017;14:979–82. doi: 10.1038/nmeth.4402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Faure L, Soldatov R, Kharchenko PV, Adameyko I. scFates: a scalable python package for advanced pseudotime and bifurcation analysis from single-cell data. Bioinformatics. 2023;39:btac746. doi: 10.1093/bioinformatics/btac746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Guo W, Keckesova Z, Donaher JL, Shibue T, Tischler V, Reinhardt F, et al. Slug and Sox9 cooperatively determine the mammary stem cell state. Cell. 2012;148:1015–28. doi: 10.1016/j.cell.2012.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mazzu YZ, Liao Y, Nandakumar S, Sjostrom M, Jehane LE, Ghale R, et al. Dynamic expression of SNAI2 in prostate cancer predicts tumor progression and drug sensitivity. Mol Oncol. 2022;16:2451–69. doi: 10.1002/1878-0261.13140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kahounova Z, Remsik J, Fedr R, Bouchal J, Mickova A, Slabakova E, et al. Slug-expressing mouse prostate epithelial cells have increased stem cell potential. Stem Cell Res. 2020;46:101844. doi: 10.1016/j.scr.2020.101844. [DOI] [PubMed] [Google Scholar]
- 31.Wang Z, Wang T, Hong D, Dong B, Wang Y, Huang H, et al. Single-cell transcriptional regulation and genetic evolution of neuroendocrine prostate cancer. iScience. 2022;25:104576. doi: 10.1016/j.isci.2022.104576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aigner K, Dampier B, Descovich L, Mikula M, Sultan A, Schreiber M, et al. The transcription factor ZEB1 (deltaEF1) promotes tumour cell dedifferentiation by repressing master regulators of epithelial polarity. Oncogene. 2007;26:6979–88. doi: 10.1038/sj.onc.1210508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vannier C, Mock K, Brabletz T, Driever W. Zeb1 regulates E-cadherin and Epcam (epithelial cell adhesion molecule) expression to control cell behavior in early zebrafish development. J Biol Chem. 2013;288:18643–59. doi: 10.1074/jbc.M113.467787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feldker N, Ferrazzi F, Schuhwerk H, Widholz SA, Guenther K, Frisch I, et al. Genome-wide cooperation of EMT transcription factor ZEB1 with YAP and AP-1 in breast cancer. EMBO J. 2020;39:e103209. doi: 10.15252/embj.2019103209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Izzo LT, Wellen KE. Histone lactylation links metabolism and gene regulation. Nature. 2019;574:492–3. doi: 10.1038/d41586-019-03122-1. [DOI] [PubMed] [Google Scholar]
- 36.Zhang D, Tang Z, Huang H, Zhou G, Cui C, Weng Y, et al. Metabolic regulation of gene expression by histone lactylation. Nature. 2019;574:575–80. doi: 10.1038/s41586-019-1678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li L, Chen K, Wang T, Wu Y, Xing G, Chen M, et al. Glis1 facilitates induction of pluripotency via an epigenome-metabolome-epigenome signalling cascade. Nat Metab. 2020;2:882–92. doi: 10.1038/s42255-020-0267-9. [DOI] [PubMed] [Google Scholar]
- 38.Sun Y, Wang BE, Leong KG, Yue P, Li L, Jhunjhunwala S, et al. Androgen deprivation causes epithelial-mesenchymal transition in the prostate: implications for androgen-deprivation therapy. Cancer Res. 2012;72:527–36. doi: 10.1158/0008-5472.CAN-11-3004. [DOI] [PubMed] [Google Scholar]
- 39.Miao L, Yang L, Li R, Rodrigues DN, Crespo M, Hsieh JT, et al. Disrupting androgen receptor signaling induces snail-mediated epithelial-mesenchymal plasticity in prostate cancer. Cancer Res. 2017;77:3101–12. doi: 10.1158/0008-5472.CAN-16-2169. [DOI] [PubMed] [Google Scholar]
- 40.Wilson MM, Weinberg RA, Lees JA, Guen VJ. Emerging mechanisms by which EMT programs control stemness. Trends Cancer. 2020;6:775–80. doi: 10.1016/j.trecan.2020.03.011. [DOI] [PubMed] [Google Scholar]
- 41.Yang J, Antin P, Berx G, Blanpain C, Brabletz T, Bronner M, et al. Guidelines and definitions for research on epithelial-mesenchymal transition. Nat Rev Mol Cell Biol. 2020;21:341–52. doi: 10.1038/s41580-020-0237-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Postigo AA, Depp JL, Taylor JJ, Kroll KL. Regulation of Smad signaling through a differential recruitment of coactivators and corepressors by ZEB proteins. EMBO J. 2003;22:2453–62. doi: 10.1093/emboj/cdg226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malouf GG, Taube JH, Lu Y, Roysarkar T, Panjarian S, Estecio MR, et al. Architecture of epigenetic reprogramming following Twist1-mediated epithelial-mesenchymal transition. Genome Biol. 2013;14:R144. doi: 10.1186/gb-2013-14-12-r144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Taube JH, Sphyris N, Johnson KS, Reisenauer KN, Nesbit TA, Joseph R, et al. The H3K27me3-demethylase KDM6A is suppressed in breast cancer stem-like cells, and enables the resolution of bivalency during the mesenchymal-epithelial transition. Oncotarget. 2017;8:65548–65. doi: 10.18632/oncotarget.19214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaffer CL, Marjanovic ND, Lee T, Bell G, Kleer CG, Reinhardt F, et al. Poised chromatin at the ZEB1 promoter enables breast cancer cell plasticity and enhances tumorigenicity. Cell. 2013;154:61–74. doi: 10.1016/j.cell.2013.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
RNA-seq, ATAC-seq and CUT & Tag data in this study have been deposited to the National Genomics Data Center, China National Center for Bioinformation with Bioproject number PRJCA020095.