Abstract
Exposure to high, marginally lethal doses or higher of ionizing radiation, either intentional or accidental, results in injury to various organs. Currently, there is only a limited number of safe and effective radiation countermeasures approved by US Food and Drug Administration for such injuries. These approved agents are effective for only the hematopoietic component of the acute radiation syndrome and must be administered only after the exposure event: currently, there is no FDA-approved agent that can be used prophylactically. The nutraceutical, gamma-tocotrienol (GT3) has been found to be a promising radioprotector of such exposure-related injuries, especially those of a hematopoietic nature, when tested in either rodents or nonhuman primates. We investigated the nature of injuries and the possible protective effects of GT3 within select organ systems/tissues caused by both non-lethal level (4.0 Gy), as well as potentially lethal level (5.8 Gy) of ionizing radiation, delivered as total-body or partial-body exposure. Results indicated that the most severe, dose-dependent injuries occurred within those organ systems with strong self-renewing capacities (e.g., the lymphohematopoietic and gastrointestinal systems), while in other tissues (e.g., liver, kidney, lung) endowed with less self-renewal, the pathologies noted tended to be less pronounced and less dependent on the level of exposure dose or on the applied exposure regimen. The prophylactic use of the test nutraceutical, GT3, appeared to limit the extent of irradiation-associated pathology within blood forming tissues and, to some extent, within the small intestine of the gastrointestinal tract. No distinct, global pattern of bodily protection was noted with the agent’s use, although a hint of a possible radioprotective benefit was suggested not only by a lessening of apparent injury within select organ systems, but also by way of noting the lack of early onset of moribundity within select GT3-treated animals.
Keywords: Gamma-radiation, Histopathology, Linear accelerator, Nonhuman primates, Partial-body irradiation, Radiation injury, Total-body irradiation
Subject terms: Cell death, Drug development
Introduction
Recent global events and related military actions by select countries have resulted in ever increasing risks of radiological/nuclear devices being deployed. This dire prospect is further accentuated by increases in terrorist activity worldwide in concert with the dissemination of nuclear materials1,2. In sum, these situational risks serve to increase the possibility that individuals, military and civilians alike, might be exposed unwantedly to relatively high levels of ionizing radiation (IR) and, in turn, impart serious adverse health effects. The ‘radiological devices’ being referred to would certainly include tactical nuclear weapons, but also the use of radiological dispersal devices (RDD), dirty bombs or improvised nuclear devices (IND): all of these IR-emitting devices can lead to serious, life-threatening bodily injuries such as the ‘acute radiation syndrome’ (ARS)3–6. Such intense IR-related exposures often result in different types of multi-organ injuries requiring arrays of diagnostic tools and therapeutic interventions. Currently, there are only a limited number of agents approved by United States Food and Drug Administration (US FDA) for use as medical countermeasures (MCMs) for treatment of these unwanted exposures7–9. These FDA-approved MCMs include the following agents: Neupogen, Neulasta, Stimufend, Udenyca, Ziextenzo, and Leukine for treatment of acute IR-associated neutropenia and Nplate to in treat thrombocytopenia10–20.
Radiation MCM development following the US FDA Animal Rule requires well defined and validated animal models for radiation injury21–25. Various types of radiation exposures including total- and partial-body irradiation (TBI, PBI), whole thorax lung irradiation (WTLI) or abdominal irradiation have been used to induce various types and levels of IR-related injuries upon which a given medicinal can be tested for its therapeutic effect. Further, these animal models of IR injury have been used not only for MCM discovery and development, but also for the identification and validation of select surrogate biomarkers of drug safety and efficacy26.
In general, there are significant gaps in our knowledge relative to how these animal models respond, in turn, how the test medicinal responds, relative to the wide array of possible exposure conditions and to different types/qualities of exposing IR (e.g., gamma, X-ray, neutron, proton, or mixed field). In addition, the previously referred to ‘multi-organ’-type IR injuries requires further research in terms of the animal model being applied in MCM development.
The large nonhuman primate (NHP) animal model is a highly applicable and extraordinarily useful surrogate for studying various sub-syndromes of ARS, including the ‘delayed effects of acute radiation exposure’ (DEARE). These animals bear close resemblance to humans in terms of general anatomy and underlying commonality of basic physiology processes and genetic structure and composition (i.e., shared homology)27–29. It is well-known that there is > 95% DNA sequence homology between Macaques and humans30. Further, the NHP model of radiation injury closely resembles the clinical, and pathological response characteristics of radiation exposure-related injuries in humans. Also, similar supportive care requirements for ARS in NHPs and humans makes it easy to compare the dose effect relationships. In addition, the extended life span of NHPs is an additional attribute making this animal model quite valuable.
A member of vitamin E family, Gamma-tocotrienol (GT3, a tocol), has been extensively evaluated for its radioprotective efficacy in both murine and NHP models in several laboratories31,32. This agent has been selected by the Armed Forces Radiobiology Research Institute for advanced development out of eight available tocol family members as potential radiation MCM candidates32–34. Studies from the laboratories of several investigators have demonstrated that GT3 has significant levels of radioprotective efficacy for both hematopoietic and gastrointestinal ARS (H-ARS and GI-ARS) when investigated using murine models of ARS31,32,35,36. GT3 has also been shown to induce high levels of the reparative cytokine/growth factor, granulocyte colony-stimulating factor (G-GSF) and to indirectly mobilize progenitors. Furthermore, GT3 has been shown to enhance the radioprotectivness of low doses of amifostine, another important radioprotective agent to demonstrate polypharmacy approach35,37–39. These findings prompted us to initiate its advanced development using large animal model of NHPs34,40–43. Furthermore, we have also conducted studies using various omics platforms to identify its biomarkers which will be helpful to obtain regulatory approval following US FDA Animal Rule44–51. With additional investigation, this agent may prove to be highly useful and safe for prophylaxis against potentially ARS-related injuries. It is also important to note that within the context of the NHP model, single parenterally administered dose of GT3 without any supportive care is equivalent to multiple doses of either the already FDA-approved recombinant-based MCMs, Neupogen or to a, sustained-release formulation, Neulasta, in terms of improving hematopoietic recovery13,17,34. GT3 was administered once subcutaneously (sc) 24 h prior to irradiation with 6.5 Gy (~ LD50/60) or 5.8 Gy (~ LD30/60) gamma-radiation. In light of these observations, it is not unreasonable to suggest that GT3 might prove to be a safe and effective radioprotector for use in humans, particularly for military personnel and first responders.
In this study, we have used NHPs as a translational animal model system to investigate the histopathology of various organs from animals exposed to total- and partial-body exposure doses of 4 and 5.8 Gy. Cobalt-60 gamma-radiation and linear accelerator-derived photon radiation were used for TBI and PBI, respectively. Further, we have tested and report here the selective IR-injury mitigating effects of GT3, a component of vitamin E family and a promising MCM under advanced development. Our results demonstrate that GT3 provides varying degrees of protection to various organ systems/tissue types to two levels of radiation injury induced by either TBI or PBI.
Materials and methods
Experimental design and animals
In order to assess the mechanism of radioprotection by a potentially useful radiation MCM, GT3, this study was conducted using NHPs that were exposed to either a relatively low, non-lethal level (4.0 Gy), or a moderately lethal (5.8 Gy) dose. The irradiation procedure was conducted using two different sources, cobalt-60 gamma ray or a linear accelerator (LINAC)-derived photon for total- (TBI) or partial-body irradiation (PBI), respectively44,50.
Briefly, 31 healthy rhesus macaques (Macaca mulatta) 15 males and 16 females, were utilized for this study. The animals weighed from 4.0 to 8.0 kg and were 2.5–7 years of age. Randomization of the animals was accomplished by stratification using gender and body weight increments during the initial quarantine period and were then assigned to either TBI or PBI groups (16 and 15 animals assigned to the TBI and PBI groups, respectively). The grouped animals were then subsequently divided into subgroups that received either the test agent, GT3, or as a control, the agent’s vehicle alone. Out of the 16 animals within the TBI group, 8 were exposed to 4 Gy and the remaining 8 animals were exposed to 5.8 Gy. Similar grouping and subgrouping and treatments were applied to animals destined for PBI exposures. The entire study lasted for thirty days post-irradiation and all surviving animals were euthanized for tissue collection and other analysis52,53.
| 4 Gy | 5.8 Gy | ||||||
|---|---|---|---|---|---|---|---|
| TBI | PBI | TBI | PBI | ||||
| Vehicle | GT3 | Vehicle | GT3 | Vehicle | GT3 | Vehicle | GT3 |
| Animal assignment in various treatment groups | |||||||
| 4 | 4 | 4 | 4 | 4 | 4 | 3 | 4 |
This animal study was conducted in a facility fully accredited by the AAALAC International. Additional details on the animals’ housing have been described earlier34,54. The study design and procedures received approvals from the Institutional Animal Care and Use Committee (BIOQUAL Inc. Rockville, MD and Armed Forces Radiobiology Research Institute, Uniformed Services University of the Health Sciences, Protocol #18-060) and the Department of Defense Animal Care and Use Review Office (ACURO) on 8th March 2019. The guidelines and recommendations in the Guide for the Care and Use of Laboratory Animals was followed strictly for conducting all procedures55. This study is reported in accordance with ARRIVE (Animal Research: Reporting of In Vivo Experiments) guidelines.
Drug preparation and administration
GT3 and its vehicle were procured from Callion Pharma (Jonesborough, TN, USA). An olive oil formulation was used as the vehicle. GT3 in the vehicle was supplied as an injectable suspension at a concentration of 50 mg/ml. The quantity of GT3 and vehicle for each NHP was based on individual animal weight. Each animal received 37.5 mg/kg of GT3 or an equivalent volume of vehicle alone via sc injections. Details of these drug/vehicle administrations have been reported earlier43. In brief, drug and vehicle were administered at the dorsal scapular area (between the shoulder blades) 24 h prior to irradiation. The area surrounding the injection site was shaved at least 48 h before the administration of drug, so the site could be easily observed for any adverse skin reactions such as rash/eruption, inflammation, irritation or abscess formation following GT3 or vehicle administration. Immediately prior to drug injection, the site was wiped with 70% isopropyl rubbing alcohol and allowed to air dry; the drug was administered sc using a 3 ml disposable luer-lock syringe with 25-gauge 5/8-inch needle. At each injection site, injection volume was ~ 2–3 ml.
Irradiation
Both TBI and PBI were used in this study. TBI: The High-Level Cobalt Facility (HLCF) used for TBI is a 35-foot square room with an internal height of 26 feet. The exposure room provides sufficient shielding for up to 400,000 curies of cobalt-60. The cobalt-60 pellets are encased in stainless steel pins that are approximately 18" long and 3/8" in diameter. The number of pins used in an irradiation depends on the required dose rate. The HLCF is categorized as a wet-storage panoramic dry irradiator, suggesting that the sources are stored underwater and raised out of the water on elevators to accomplish irradiations. There are two elevators, one on either side of a central irradiation location, allowing for both unilateral or bilateral irradiations. Typically, irradiations are performed bilateral to give the best dose uniformity through the samples. The elevators can be positioned at varying distances from the objects to achieve the required dose rate. The procedure for conducting TBI has been reported in detail previously34. In brief, animals were fasted for at least 12 h prior to IR exposure in order to reduce the risk of radiation-induced emesis. Two NHPs were placed on the exposure platform facing away from each other and irradiated with midline doses of either 4.0 or 5.8 Gy at a dose rate of 0.6 Gy/min from both sides of the animal’s abdominal core (bilateral, simultaneous exposure). The abdominal width of each animal was measured with digital calipers. The radiation dosimetry details have been provided earlier54. During the exposure, the mildly sedated and conscious animals were observed via in-room monitors. After the procedure, animals were maintained in a staging area, until they were sufficiently alert to allow for a return to their cage, where full recovery was completed. PBI: Similar to TBI, the PBI procedure has been described earlier40. In brief, food was withheld from the animal for about 12 h before the procedure. In contrast to TBI, the animals receiving PBI were fully sedated, as the use of a restraining device was not possible. Animals were singularly irradiated at the dose-rate of 1.3 Gy/min using a 4 MV photon beam from an Elekta Infinity clinical LINAC. Prior to the exposures, both the IR dose and animal’s ID were verified by the attending dosimetrist of the LINAC facility. Select vital signs (e.g., heart rates and blood oxygen levels) of the irradiated animals were monitored using SurgiVet Advisor tech Vital Signs Monitor (Smiths Medical, Dublin, OH, USA). In order to achieve a ~ 5% sparing of bone marrow, the hind limbs, fibula, tibia, and feet were excluded from the radiation field. To accurately deliver the dose to the animal at the location of the desired target (abdominal core), the anterior/posterior (AP) measurements of the animal’s abdomen were accomplished with digital calipers. Near the field edge at which the knees were positioned, approximately 1 cm of the spared limb was in the penumbra region (80% to 20% dose of the field center). The rest of the spared limb received absorbed doses below 20% of the field center.
Supportive care
After any procedures, NHPs were monitored at least twice daily for signs of complications. The type of supportive care provided was based on CBC and cage side observations56. The primary antibiotic used was enrofloxacin (Baytril Bayer HealthCare LLC, Shawnee Mission, KS). The dose administered was 5 + 0.25 mg/kg intramuscularly (im) or sc twice a day (BID); or 10 + 0.5 mg/kg administered im or intravenous (iv) once daily (QD). Additional supportive care measures included rehydration fluids, alternate antibiotics, antipyretics, antidiarrheal agents, analgesics, antiemetics, and nutritional support. Details of supportive care is described in detail earlier53.
Clinical assessments
General behavioral observations were recorded daily for each animal under test. This included noting any/all changes in urination/defecation patterns, along with periodic measures of body weights, heart rates, and blood pressures. Conventional hematological assessments were also conduced. Details of the blood drawing procedures and related CBC analyses were previously reported43. Standard CBC analyses were performed using a Bayer Advia 120 cell counter (Siemens, Malvern, PA, USA).
Euthanasia
Moribundity was used as a surrogate endpoint for mortality. In select cases, euthanasia was carried out prior to the study’s designated duration of 30 days post-exposure in order to reduce undue pain and suffering for animals (2 animals at 21 & 22 d following 5.8 Gy radiation exposures were found to be moribund). When an animal reached a state of moribundity, the animal was immediately euthanized. Euthanasia was conducted in line with the directives of the approved IACUC protocol, the Guide for the Care and Use of Laboratory Animals (8th edition), and the American Veterinary Medical Association (AVMA) Guidelines for the Euthanasia of Animals (2020 Edition), with the details of the parameters and guidelines previously described55,56. The moribund animals were given pentobarbital sodium iv (Virbac AH Inc., Fort Worth, TX) using either saphenous or cephalic veins, needle size 20–25 gauge (100 mg/kg, 1–5 ml). Prior to pentobarbital sodium administration, animals were sedated using ketamine hydrochloride injection (Mylan Institutional LLC, Rockford, IL) (5–15 mg/kg, im). Intra-cardiac administration was performed if unable to administer pentobarbital sodium through peripheral veins. The animals were deeply anesthetized by Isoflurane (Baxter Healthcare Corporation, Deerfield, IL) (1–5%) with oxygen at 1–4 L per minute via mask before administering the intra-cardiac injection. The animals were euthanized only under the guidance of a staff veterinarian or a trained technician in consultation with the veterinarian. After pentobarbital sodium administration, the animals were examined by assessing the heart auscultation and pulse to confirm death.
Necropsy
Following euthanasia, tissue samples were harvested under the direction of a board-certified veterinary pathologist and all observed gross abnormalities were recorded during the procedure. The harvested tissues, including sternum, spleen, duodenum, jejunum, ileum, colon, liver, kidney, and lung, were loaded into histology cassettes and subsequently placed in containers and fixed in 10% buffered zinc formalin. Collected and fixed tissues were embedded, processed and sectioned using standard histological procedures as described earlier53.
Histopathology
Zinc-buffered formalin fixed NHP tissues were subsequently processed for paraffin embedding, sectioning, and hematoxylin and eosin (H&E) staining of prepared slides (via Histoserv, Inc, Germantown, MD, USA)57. The histological scoring and morphometric analyses were conducted by a board-certified veterinary pathologist. The scoring system, i.e., the grading system, employed was based on a point system of noted pathology within the observed tissue; namely, 0 = none, 1 = minimal, 2 = mild, 3 = moderate, 4 = marked, and 5 = severe. Digital images of tissue sections were obtained with a Ziess Axioscan slide scanner and Zeiss Zen software (Carl Ziess Meditech, Inc., Dublin, CA, USA). An Olympus IX73 microscope (Olympus, Center Valley, PA) was used for 40× magnification. Histological and morphometric analyses were conducted under blinded conditions related to given treatment groups.
Statistical analysis
Statistical analysis of CBC and vital signs data was accomplished using statistical software IBM SPSS Statistics (version 28.0.1.1, https://www.ibm.com/products/spss-statistics). All error bars signify standard deviations. For CBC data, One-Way ANOVA tests were performed between GT3-treated groups and their respective vehicle groups (i.e., PBI GT3 vs. PBI Vehicle, and TBI GT3 vs. TBI Vehicle), vehicle-treated groups (i.e., PBI Vehicle vs. TBI Vehicle), and GT3-treated groups (i.e., PBI GT3 vs. TBI GT3). P-values < 0.05 were considered statistically significant for all tests performed.
Ethics statement
All procedures were approved by the Institutional Animal Care and Use Committee Armed Forces Radiobiology Research Institute/Uniformed Services University of the Health Sciences and BIOQUAL Inc., Rockville, and Department of Defense Animal Care and Use Review Office (ACURO). This study was carried out in strict accordance with the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health.
Results
Clinical assessments
General observations. General clinical/behavioral/pathological observations were made on all test animals throughout the study period (i.e., − 8 days to 30 days). With the exception of the two ‘vehicle control’ animals receiving 5.8 Gy TBI and that became moribund prior to the scheduled end of the test period, the most frequent behavioral change that manifest acute radiation illness was a ‘back-hunching’ response seen at the higher, sublethal dose tested (5.8 Gy). Primary metrics of clinical status are described with major findings listed. In brief, body weight (Table 1), body temperature (Table 2), heart rate (Table 3), blood pressure (Table 4), gross pathology (Table 5), and histopathology (Table 6) of all irradiated animals are listed. In aggregate, these data indicate varying, but mostly modest degrees of change in clinical status following IR-exposure. By contrast however, the clinical condition of several animals changed rather markedly during the initial post-exposure period.
Table 1.
Body weights of NHPs subjected to 4 or 5.8 Gy TBI or PBI and treated with GT3 or vehicle.
| Radiation dose | Exposure level | Treatment | Age (years) | (N size), Average % Change ± SD | ||
|---|---|---|---|---|---|---|
| Total | Males | Females | ||||
| 4 Gy | PBI | Vehicle | 3.6 ± 0.5 | (4) − 0.20 ± 1.86 | (2) − 0.09 ± 1.44 | (2) − 0.31 ± 2.87 |
| GT3 | 3.8 ± 0.1 | (4) 2.22 ± 6.00 | (2) − 0.58 ± 1.86 | (2) 5.02 ± 8.54 | ||
| TBI | Vehicle | 4.0 ± 0.6 | (4) − 2.59 ± 2.59 | (2) − 2.52 ± 2.43 | (2) − 2.66 ± 3.77 | |
| GT3 | 4.0 ± 0.7 | (4) 0.48 ± 5.73 | (2) 4.75 ± 1.64 | (2) 3.79 ± 4.78 | ||
| 5.8 Gy | PBI | Vehicle | 3.6 ± 0.2 | (3) 0.30 ± 3.08 | (1) 1.11 | (2) − 0.10 ± 4.24 |
| GT3 | 3.4 ± 0.6 | (4) 1.04 ± 7.00 | (2) − 2.79 ± 2.84 | (2) 4.87 ± 8.95 | ||
| TBI | Vehicle | 3.6 ± 0.2 | (4) − 5.25 ± 7.46 | (2) − 3.57 ± 3.34 | (2) − 6.93 ± 12.01 | |
| GT3 | 3.6 ± 0.5 | (4) − 1.93 ± 2.84 | (2) − 2.36 ± 4.59 | (2) − 1.51 ± 1.54 | ||
± , standard deviation.
Table 2.
Temperatures of NHPs subjected to 4 or 5.8 Gy TBI or PBI and treated with GT3 or vehicle.
| Radiation dose | Exposure level | Treatment | Average temperature (Celsius) ± SD recorded post-irradiation (d) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| − 8 | 2 | 7 | 14 | 16 | 18 | 20 | 22 | 24 | 26 | 28 | 30 | |||
| 4 Gy | PBI | Vehicle | 38.38 ± 0.60 | 38.11 ± 0.67 | 38.82 ± 0.50 | 38.63 ± 0.51 | 39.00 ± 0.32 | 38.99 ± 0.66 | 39.04 ± 0.53 | 39.29 ± 0.52 | 39.24 ± 0.41 | 39.41 ± 0.74 | 38.96 ± 0.37 | 39.04 ± 0.90 |
| GT3 | 38.79 ± 0.23 | 38.88 ± 0.27 | 39.49 ± 0.41 | 39.38 ± 0.29 | 39.39 ± 0.72 | 39.60 ± 0.39 | 39.33 ± 0.56 | 39.56 ± 0.45 | 39.39 ± 0.69 | 39.07 ± 1.02 | 39.11 ± 1.00 | 39.79 ± 0.64 | ||
| TBI | Vehicle | 38.96 ± 1.19 | 39.13 ± 0.60 | 38.54 ± 0.73 | 38.85 ± 0.45 | 38.47 ± 0.84 | 38.28 ± 1.18 | 39.11 ± 0.16 | 38.95 ± 0.33 | 38.48 ± 1.01 | 38.88 ± 0.67 | 39.15 ± 0.90 | 38.82 ± 0.61 | |
| GT3 | 39.14 ± 0.52 | 38.88 ± 0.31 | 38.95 ± 0.44 | 38.85 ± 0.09 | 38.69 ± 0.81 | 38.64 ± 0.88 | 39.18 ± 0.34 | 39.18 ± 0.67 | 38.81 ± 0.91 | 39.01 ± 0.62 | 38.91 ± 0.88 | 39.17 ± 0.15 | ||
| 5.8 Gy | PBI | Vehicle | 38.78 ± 0.31 | 37.08 ± 1.15 | 37.02 ± 0.12 | 37.44 ± 0.43 | 39.48 ± 0.51 | 39.43 ± 0.32 | 39.26 ± 0.57 | 39.45 ± 0.48 | 40.13 ± 0.54 | 40.74 ± 0.19 | 40.04 ± 0.75 | 38.77 ± 0.54 |
| GT3 | 39.03 ± 0.24 | 37.69 ± 1.02 | 38.52 ± 0.37 | 37.63 ± 0.63 | 39.19 ± 0.79 | 39.35 ± 0.56 | 39.31 ± 0.39 | 39.49 ± 0.58 | 39.92 ± 0.65 | 40.15 ± 0.62 | 40.12 ± 0.67 | 38.71 ± 0.66 | ||
| TBI | Vehicle | 37.96 ± 0.52 | 38.67 ± 0.32 | 38.45 ± 0.55 | 38.42 ± 0.72 | 39.47 ± 0.44 | 39.74 ± 0.43 | 39.56 ± 0.87 | 40.28 ± 0.31 | 40.36 ± 0.59 | 40.73 ± 1.18 | 39.92 ± 1.61 | 38.06 ± 0.47 | |
| GT3 | 38.85 ± 0.89 | 37.79 ± 0.64 | 38.64 ± 0.34 | 38.39 ± 0.89 | 39.47 ± 0.41 | 39.49 ± 0.92 | 39.37 ± 0.42 | 39.79 ± 0.41 | 40.13 ± 0.70 | 40.03 ± 1.15 | 40.00 ± 0.70 | 38.71 ± 0.60 | ||
d, day; ± , standard deviation.
Table 3.
Heart rates of NHPs subjected to 4 or 5.8 Gy TBI or PBI and treated with GT3 or vehicle.
| Radiation Dose | Exposure Level | Treatment | Average Heart Rate (BPM) recorded post-irradiation (d) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| − 8 | 16 | 18 | 20 | 22 | 24 | 26 | 28 | |||
| 4 Gy | PBI | Vehicle | 249 ± 13 | 221 ± 25 | 236 ± 25 | 222 ± 53 | 189 ± 34 | 188 ± 43 | 213 ± 46 | 190 ± 36 |
| GT3 | 225 ± 31 | 207 ± 17 | 228 ± 23 | 214 ± 22 | 196 ± 44 | 176 ± 49 | 224 ± 69 | 190 ± 34 | ||
| TBI | Vehicle | ND | 180 ± 97 | 193 ± 0 | 161 ± 59 | 217 ± 40 | 187 ± 71 | 219 ± 35 | 195 ± 57 | |
| GT3 | ND | 254 ± 24 | 236 ± 12 | 205 ± 41 | 229 ± 30 | 235 ± 23 | 224 ± 30 | 211 ± 11 | ||
| 5.8 Gy | PBI | Vehicle | 245 ± 16 | 203 ± 8 | 238 ± 19 | 220 ± 14 | 197 ± 2 | 217 ± 26 | 205 ± 5 | 209 ± 12 |
| GT3 | 224 ± 12 | 225 ± 20 | 235 ± 10 | 203 ± 12 | 209 ± 9 | 206 ± 15 | 217 ± 18 | 210 ± 15 | ||
| TBI | Vehicle | ND | 234 ± 35 | 232 ± 50 | 224 ± 27 | 162 ± 45 | 197 ± 11 | 194 ± 57 | 213 ± 101 | |
| GT3 | ND | 221 ± 19 | 234 ± 31 | 171 ± 50 | 204 ± 39 | 214 ± 33 | 209 ± 22 | 181 ± 5 | ||
d, day; ND, no data; ± , standard deviation.
Table 4.
Blood pressures of NHPs subjected to 4 or 5.8 Gy TBI or PBI and treated with GT3 or vehicle.
| Radiation dose | Exposure level | Treatment | Average blood pressure recorded post-irradiation (d) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| − 8 | 16 | 18 | 20 | 22 | 24 | 26 | 28 | |||
| 4 Gy | PBI | Vehicle | 143/87 ± 26/27 | 132/92 ± 20/11 | 149/82 ± 22/24 | 142/91 ± 19/8 | 145/98 ± 21/18 | 153/108 ± 20/11 | 152/89 ± 30/18 | 125/89 ± 12/12 |
| GT3 | 148/94 ± 28/21 | 133/78 ± 24/35 | 135/77 ± 28/13 | 133/85 ± 18/9 | 129/84 ± 34/29 | 140/80 ± 16/17 | 150/80 ± 24/5 | 132/82 ± 5/13 | ||
| TBI | Vehicle | ND | 134/90 ± 7/11 | 99/70 ± 16/13 | 111/80 ± 62/49 | 116/73 ± 42/46 | 162/110 ± 11/6 | 122/55 ± 30/11 | 131/59 ± 13/13 | |
| GT3 | ND | 114/80 ± 35/28 | 118/84 ± 18/5 | 125/88 ± 11/5 | 124/72 ± 32/1 | 145/78 ± 28/11 | 129/85 ± 11/14 | 121/88 ± 7/1 | ||
| 5.8 Gy | PBI | Vehicle | 133/86 ± 8/7 | 140/80 ± 9/14 | 151/91 ± 21/30 | 134/79 ± 23/21 | 127/88 ± 11/9 | 126/78 ± 12/4 | 119/74 ± 7/4 | 126/67 ± 10/1 |
| GT3 | 134/69 ± 19/8 | 129/78 ± 4/16 | 136/82 ± 11/17 | 128/92 ± 18/23 | 130/86 ± 12/6 | 137/97 ± 27/27 | 129/68 ± 17/18 | 135/71 ± 27/18 | ||
| TBI | Vehicle | ND | 107/78 ± 23/20 | 98/53 ± 32/15 | 84/49 ± 10/13 | 116/91 ± 31/41 | 124/61 ± 11/18 | 113/82 ± 6/7 | 106/65 ± 1/5 | |
| GT3 | ND | 114/72 ± 19/14 | 95/66 ± 35/34 | 133/85 ± 5/17 | 127/96 ± 6/8 | 120/77 ± 20/20 | 104/62 ± 33/13 | 125/80 ± 25/30 | ||
d, day; ND, no data; ± , standard deviation.
Table 5.
Gross pathological findings of NHP tissues from animals exposed to 4 or 5.8 Gy TBI or PBI and treated with GT3 or vehicle.
| Radiation dose | Exposure level | Treatment | Euthanasia (d) | NHP# | Sex | Age (years) | Gross pathological findings |
|---|---|---|---|---|---|---|---|
| 4 Gy | PBI | Vehicle | 30 | 1603102 | F | 3.9 | Kidneys appear pale, mild hemorrhage on both lungs. No other gross abnormalities were noted. BCS 2.5/5 (thin) |
| 1707119 | M | 3.8 | Minute petechiae noted in GI tract, some hemorrhage noted in both lungs. BCS 3/5 (ideal) | ||||
| 1704305 | M | 2.8 | Mild to moderate hemorrhage noted in abdominal cavity. BCS 3/5 (ideal) | ||||
| 1603026 | F | 3.9 | Approximately 5% dehydrated, shriveled spleen, pale kidneys. No other gross abnormalities noted. BCS 2.5/5 (lean) | ||||
| GT3 | 1603079 | M | 3.9 | Overall, animal was unremarkable. Few petechiae noted in few locations along the GI tract. BCS 3/5 (ideal) | |||
| 1704119 | M | 3.8 | In general, animal was unremarkable. BCS 3/5) ideal | ||||
| 1606088 | F | 3.6 | Petechiae noted on the distal portion of bladder. BCS 3/5 (ideal) | ||||
| 1603168 | F | 3.9 | Animal appears dehydrated, mild hemorrhage noted on the ileo-cecal junction. BCS 1.5/5 (thin) | ||||
| TBI | Vehicle | 30 | RA2695 | M | 4.7 | Overall, animal appeared unremarkable, few to moderate petechiae noted in the stomach and duodenum. BCS 3/5 (ideal) | |
| 1606069 | M | 3.6 | Moderate pallor at the lung margins, few petechiae noted on both lobes with moderate focal hemorrhage and blotched serrations across the surface of the spleen accompanied with ulcerative-like lesions. Splenic exhaustion noted from gross presentation, approximately 7% dehydrated. BCS 3/5 (ideal) | ||||
| RA3228 | F | 4.1 | Apparent dehydrations presented with pale kidneys, shriveled spleen, petechiae/hemorrhage in jejunum and ileum, and bloated colon. BCS 2.5/5 (lean) | ||||
| 1608010 | F | 3.5 | Severe, diffuse, hemorrhagic pneumonitis with adhesions noted in lungs. Moderate cardiomegaly noted with significant hemorrhage on the proximal section of the heart. Less than 5% dehydrated, pale mucous membranes, and intussusceptions along the GI tract. BCS 2.5/5 (lean) | ||||
| GT3 | RA3275 | M | 3.9 | Slight dehydration presented with pale spleen, kidneys, and liver. Moderate petechiae noted in small and large intestines. BCS 2.5/5 (lean) | |||
| 1606093 | M | 3.6 | Moderate pallor at the lung margins with few hemorrhages noted on the distal lobes of both lungs and moderate focal hemorrhage noted on and across the surface of the spleen accompanied with ulcerative-like lesions. Splenic exhaustion noted from gross with shriveled appearance. Approximately 7% dehydrated with pale mucous membranes. BCS 3/5 (ideal) | ||||
| RA2770 | F | 5.1 | Mild to moderate swelling of right antebrachium region and hand of unknown origin. No other gross abnormalities were noted. BCS 2.5/5 (lean) | ||||
| 1607012 | F | 3.5 | Mild pallor at the margins of the lungs. Approximately 5–7% dehydrated with pale mucous membranes. BCS 3/5 (ideal) | ||||
| 5.8 Gy | PBI | Vehicle | 30 | 1608017 | M | 3.5 | Cardiac lesion of unknown cause presented with hemorrhages and petechiae noted on the surface and inside if the heart, enlarged mediastinal lymph nodes with several fat deposits. Moderately distended urinary bladder with few petechiae noted on its surface. BCS 2.5/5 (slightly thin) |
| 1608018 | F | 3.5 | Interventricular septum hypertrophy, swelling on the right side of the heart with moderate petechiae noted on the surface of the heart, pale kidneys, intussusceptions in the GI tract. No other remarkable gross findings. BCS 2/5 (thin) | ||||
| 1604188 | F | 3.8 | Moderate dehydration presented with mild pale margins of the lung lobes, few adhesions noted on the upper and middle lobes of right lung, petechiae on both lungs. Mild hemorrhage throughout the small intestines with few intussusceptions. Approximately 7% dehydrated with pale mucus membranes and pale kidneys. BCS 2.5/5 (lean) | ||||
| GT3 | 1608007 | M | 3.5 | Roughened, mottled edges of the spleen with shriveled appearance, less than 5% dehydrated with pale mucus membranes. BCS 2.5/5 (lean) | |||
| 1708011 | M | 2.5 | Moderate generalized hemorrhagic enteritis, few petechiae/hemorrhages noted in small and large intestines no other remarkable gross findings. 5–7% dehydrated with pale mucus membranes. BCS 2/5 (thin) | ||||
| 1603104 | F | 3.9 | Enlarged right ventricle with roughened heart surface, swollen mediastinal lymph nodes. No other gross abnormalities noted. BCS 2/5 (thin) | ||||
| 1608006 | F | 3.5 | Enlarged spleen with no other remarkable gross findings. 5–7% dehydrated with pale mucus membranes. BCS 3/5 | ||||
| TBI | Vehicle | 30 | 1608039 | M | 3.5 | Moderate focal hemorrhagic lesions towards margins of the lungs, some adhesions, petechiae, and hemorrhages noted in both lungs. Globoid heart with increased pericardial effusion, roughened heart surface with moderate hemorrhages noted on the surface of the heart. Moderate focal hemorrhage of the left renal cortex and on parietal surface beneath the capsule, both kidneys appear pale. BCS 3/5 (lean) | |
| 1603015 | M | 3.9 | Pallor noted at the margins of the lung lobes, mild pallor of the liver, inflamed gall bladder with greenish grainy bile fluid. Some petechiae and hemorrhages noted in small and large intestines No other remarkable gross findings. Approximately 7% dehydrated. BCS 3/5 (lean) | ||||
| 22 | 1607030 | F | 3.5 | Marked pallor of mucus membranes suggestive of dehydration, generalized pallor noted throughout lungs with multifocal hemorrhagic necrosis and moderate petechiae noted in both lungs. Severe pericardial edema and pallor of the heart tissue, with some serrations noted on the cardiac surface. Enlarged mesenteric lymph nodes with petechiae in small and large intestines, and enlarged left kidney with focal area of hemorrhagic necrosis at the cortex. Both kidneys appear pale. Severe hemorrhagic lesion and intussusception at the ileo-cecal junction, several petechiae along the jejunal surface. Approximately 10–12% dehydrated. BCS 1.5/5 (very thin) | |||
| 21 | 1608016 | F | 3.5 | Generalized pallor throughout lungs, with both lungs presented with petechiae, hemorrhages, and some adhesions in few of the lobes. Serrated surface of the heart presented with moderate hemorrhagic carditis at the left ventricle, moderate pericardial edema, multifocal petechial hemorrhage on the surface of the heart, and some blood clots on the inside of the heart. Moderate pallor of the left kidney with both kidneys looking pale, multifocal necrotic lesions of the spleen with a shriveled appearance. GI Tract presented with lesions, hemorrhages, and petechiae, with intussusceptions noted in the small intestines. Less than 5% dehydrated with marked pallor of mucous membranes. BCS 2.5/5 (thin) | |||
| GT3 | 30 | 1703269 | M | 2.9 | No remarkable gross findings. Mild to moderate petechiae noted in GI tract. Approximately 7% dehydrated with pale mucous membranes. BCS 3/5 (normal) | ||
| 1607029 | M | 3.5 | Diffuse hemorrhagic congestion of the lungs, more prevalent throughout left lung field, with both lungs presented with some petechiae and adhesions. Stomach slightly distended with some bloating noted in the large intestine. Approximately 7% dehydrated with pale mucous membranes. BCS 2.5/5 (lean) | ||||
| 1603138 | F | 3.9 | Diffuse petechial hemorrhage of the lungs with pale margins of the lobes, with mild to moderate petechiae in both lungs. Golf ball sized ovarian mass discovered at necropsy, no history of clinical signs relative to this finding. Moderate petechiae noted in bladder. Approximately 7% dehydrated with pale mucous membranes. BCS 3/5 (ideal) | ||||
| 1603030 | F | 3.9 | Mild pale margins of the lung lobes, mild hemorrhage throughout small intestine with few intussusceptions noted in small and large intestines. Approximately 7% dehydrated with pale mucous membranes. BCS 2.5/5 (lean) |
The body condition score (BCS) is a subjective method of gaging body fat and muscle by palpation. All scores were provided by the veterinarian at the time of necropsy. The scale used both whole and half units, an optimal body condition would be scored a 3.0. Lower scores represent emaciated to lean conditions (1.0 to 2.0), and higher values (4.0 to 5.0) indicate excessive body fat.
Table 6.
Histopathological scoring of various organs of healthy animals in addition to TBI and PBI animals treated with either GT3 or vehicle.
| Organs | Average histopathology scores | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Vehicle | GT3 | Healthy (n = 3) | |||||||
| 4 Gy | 5.8 Gy | 4 Gy | 5.8 Gy | ||||||
| TBI (n = 4) | PBI (n = 4) | TBI (n = 4) | PBI (n = 3) | TBI (n = 4) | PBI (n = 4) | TBI (n = 4) | PBI (n = 4) | ||
| Lung | |||||||||
| Alveolar septal degeneration | 2 ± 1 | 1 ± 0 | 2 ± 1 | 1 ± 1 | 1 ± 1 | 2 ± 1 | 1 ± 1 | 2 ± 1 | 1 ± 1 |
| Alveolar edema | 0 ± 1 | 0 ± 0 | 1 ± 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 1 ± 1 | 0 ± 0 | 1 ± 2 |
| Septal cellularity | 0 ± 0 | 0 ± 0 | 1 ± 2 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Kidney | |||||||||
| Tubular degeneration | 3 ± 1 | 2 ± 0 | 3 ± 0 | 3 ± 0 | 2 ± 1 | 2 ± 1 | 3 ± 1 | 3 ± 1 | 3 ± 1 |
| Tubular regeneration | 0 ± 0 | 0 ± 0 | 0 ± 1 | 0 ± 0 | 0 ± 0 | 0 ± 1 | 0 ± 0 | 0 ± 0 | 1 ± 1 |
| Cellular infiltrates | 0 ± 0 | 0 ± 1 | 0 ± 0 | 1 ± 1 | 0 ± 1 | 0 ± 1 | 1 ± 1 | 1 ± 1 | 0 ± 0 |
| Sternum | |||||||||
| Cellular depletion | 0 ± 1 | 1 ± 1 | 2 ± 2 | 1 ± 1 | 1 ± 1 | 3 ± 1 | 1 ± 1 | 2 ± 1 | 1 ± 1 |
| Spleen | |||||||||
| White pulp depletion | 3 ± 1 | 2 ± 1 | 4 ± 0 | 3 ± 1 | 3 ± 2 | 2 ± 1 | 3 ± 0 | 2 ± 1 | 1 ± 1 |
| Duodenum | |||||||||
| Villi/crypt ratio | 4 | 3 ± 1 | 2 ± 0 | 3 ± 1 | 4 | 3 ± 1 | 3 ± 1 | 3 ± 0 | 4 ± 1 |
| Inflammatory cell infiltrates | 2 ± 1 | 1 ± 1 | 1 ± 1 | 2 ± 1 | 2 ± 1 | 1 ± 1 | 2 ± 1 | 2 ± 1 | 0 ± 0 |
| Crypt dilation | 1 ± 1 | 0 ± 0 | 0 ± 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 1 ± 1 | 0 ± 0 | 0 ± 0 |
| Villi fusion | 2 ± 1 | 2 ± 1 | 2 ± 1 | 1 ± 0 | 1 ± 1 | 1 ± 0 | 1 ± 1 | 1 ± 1 | 1 ± 1 |
| Villi loss | 3 ± 0 | 2 ± 1 | 3 ± 1 | 2 ± 1 | 3 ± 1 | 3 ± 1 | 2 ± 1 | 2 ± 1 | 0 ± 0 |
| Jejunum | |||||||||
| Villi/crypt ratio | 4 ± 2 | 5 ± 1 | 4 ± 1 | 4 ± 2 | 4 ± 1 | 4 ± 1 | 4 ± 1 | 4 ± 1 | 4 ± 1 |
| Inflammatory cell infiltrates | 0 ± 1 | 1 ± 1 | 0 ± 0 | 1 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 1 |
| Crypt dilation | 0 ± 0 | 0 ± 0 | 1 ± 1 | 0 ± 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Villi fusion | 1 ± 1 | 1 ± 1 | 2 ± 0 | 1 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 1 | 1 ± 0 |
| Villi loss | 2 ± 1 | 2 ± 1 | 3 ± 1 | 2 ± 1 | 2 ± 1 | 1 ± 1 | 2 ± 0 | 3 ± 1 | 1 ± 1 |
| Ileum | |||||||||
| Villi/crypt ratio | 5 ± 1 | 4 ± 1 | 4 ± 1 | 5 ± 1 | 4 ± 1 | 4 ± 0 | 4 ± 1 | 4 ± 1 | 5 |
| Inflammatory cell infiltrates | 0 ± 1 | 1 ± 1 | 0 ± 1 | 1 ± 1 | 0 ± 0 | 1 ± 1 | 1 ± 1 | 0 ± 1 | 0 ± 0 |
| Crypt dilation | 0 ± 0 | 0 ± 0 | 0 ± 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Villi fusion | 2 ± 1 | 2 ± 0 | 2 ± 1 | 1 ± 1 | 2 ± 1 | 2 ± 1 | 1 ± 1 | 2 ± 1 | 1 ± 1 |
| Villi loss | 3 ± 1 | 2 ± 1 | 3 ± 1 | 3 ± 0 | 3 ± 1 | 3 ± 1 | 3 ± 1 | 2 ± 1 | 2 ± 1 |
| Large intestine | |||||||||
| Inflammatory cell infiltrates | 2 ± 1 | 1 ± 0 | 2 ± 1 | 2 ± 0 | 1 ± 1 | 2 ± 1 | 2 ± 1 | 2 ± 1 | 1 ± 0 |
| Crypt dilation | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 1 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 | 0 ± 0 |
| Crypt loss | 3 ± 1 | 3 ± 1 | 2 ± 1 | 2 ± 1 | 2 ± 1 | 2 ± 2 | 2 ± 1 | 2 ± 1 | 3 ± 1 |
| Liver | |||||||||
| Inflammatory cell infiltrates | 0 ± 1 | 0 ± 0 | 1 ± 1 | 0 ± 0 | 0 ± 1 | 0 ± 0 | 0 ± 1 | 0 ± 1 | 0 ± 0 |
| Cystic degeneration | 1 ± 1 | 2 ± 1 | 2 ± 1 | 2 ± 1 | 1 ± 1 | 2 ± 1 | 2 ± 1 | 2 ± 1 | 1 ± 0 |
± , standard deviation; PBI, partial-body irradiation; TBI, total-body irradiation.
Body weight. Significant shifts in body weights (± 10%) were not noted generally in animals subjected to the different IR exposure levels (4 or 5.8 Gy), or to the two different exposure protocols (TBI or PBI), or the types of treatments (vehicle or GT3). The two exceptions to the later were noted however and both cases were observed at the higher level of exposure (5.8 Gy): the first animal, pretreated with GT3 and subjected to PBI, exhibited a weight gain of ~ 11% at 30 d post-exposure (i.e., at the time of euthanasia), while the second animal, pretreated with the control ‘vehicle’ alone and exposed TBI, showed significant weight loss (~ 15%) at 21 d post exposure. This animal was deemed moribund and in turn euthanized. The average body weight of all animals under test prior to IR exposure was 5.24 kg (SD = 0.994), whereas the average weight at the time of euthanasia was 5.252 (SD = 0.934) (Table 1, Supplementary Table 1). Body weights of the GT3 and vehicle-treated animals over the test period did not demonstrate significant difference.
Temperature. No significant shifts in body temperatures were recorded for the entire cohort or various subsets test animals subjected to the various doses and regimens of IR exposure or to the two different pretreatments. The average body temperature of all animals under test prior to IR exposure was 38.7 C (SD = 0.68), whereas the average body temperature at the time of euthanasia was 39.0 C (SD = 0.68) (Table 2, Supplementary Table 2).
Heart rate and blood pressure. ‘Heart rate’ along with ‘Blood pressure’ measurements were monitored throughout the entire test period. For both these clinical assessments, no clear patterns were noted relative to the type of IR exposure, level of those exposures, or the type of medicinal being tested. The average heart rate of 15 (out of 31) animals under test prior to IR exposure was 235 beats/min (SD = 0.21), whereas the average heart rate at the time of euthanasia was 198 beats/min (SD = 0.33) for 25 of the 31 animals under test (Table 3, Supplementary Table 3). The average blood pressure measurements (systolic/diastolic readings) of animals under test under test prior to IR exposure (for 15/31 animals monitored) were 140/84 (SDs = 21/19), whereas those average readings at the time of euthanasia were 126/76 (SD2 = 16/17) (for 25/31 animals) (Table 4, Supplementary Table 4).
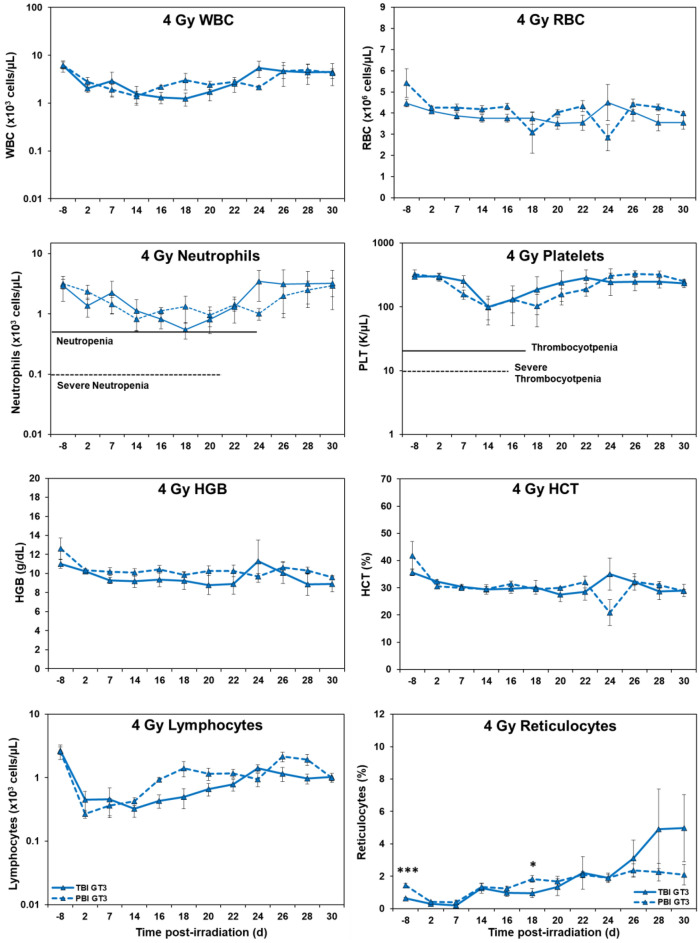
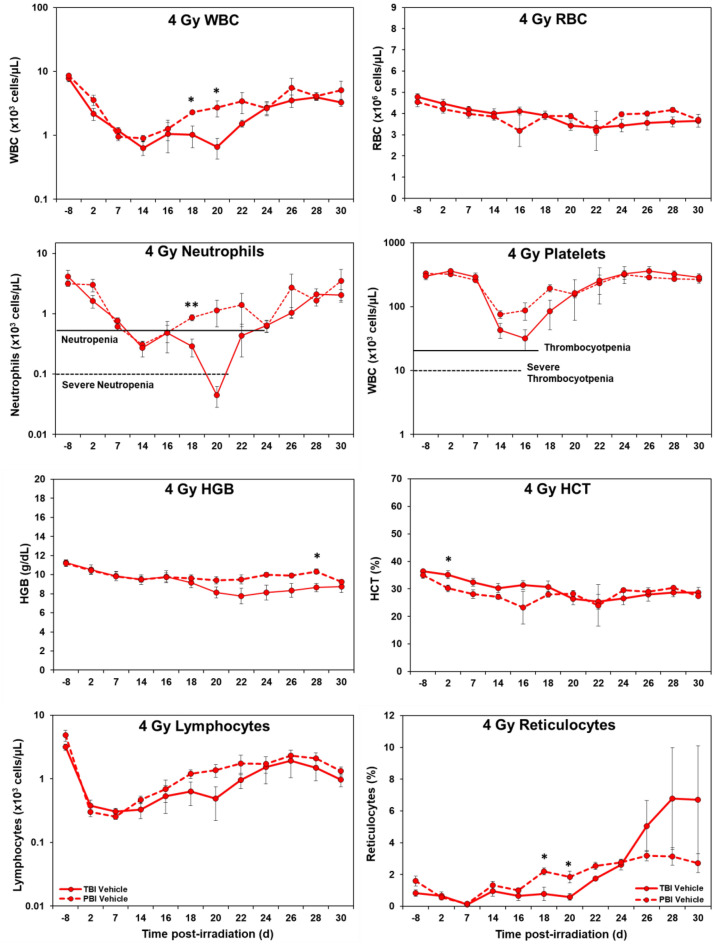
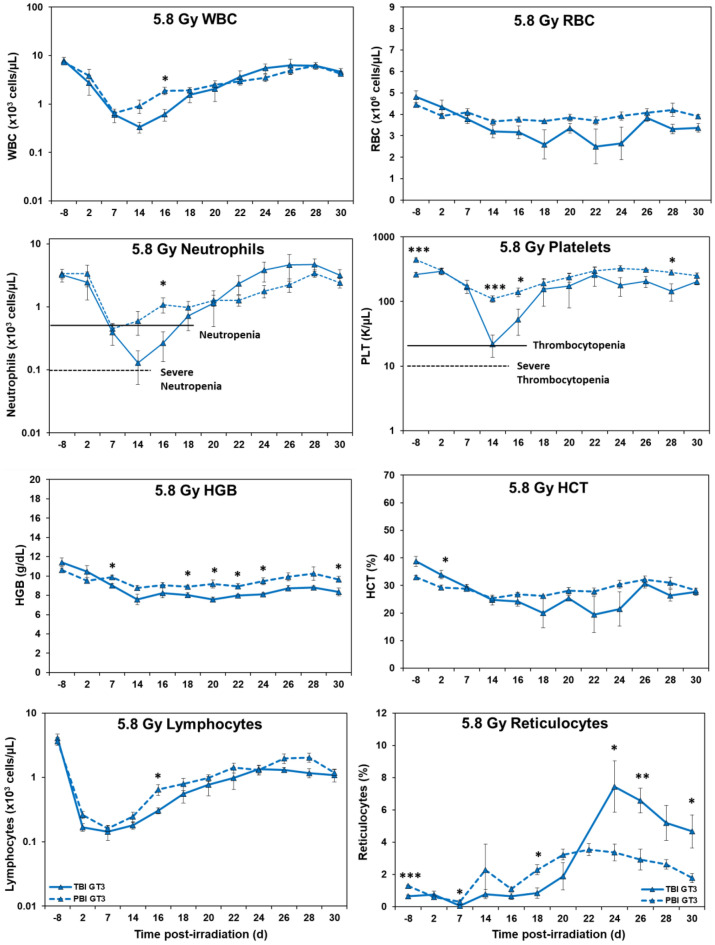
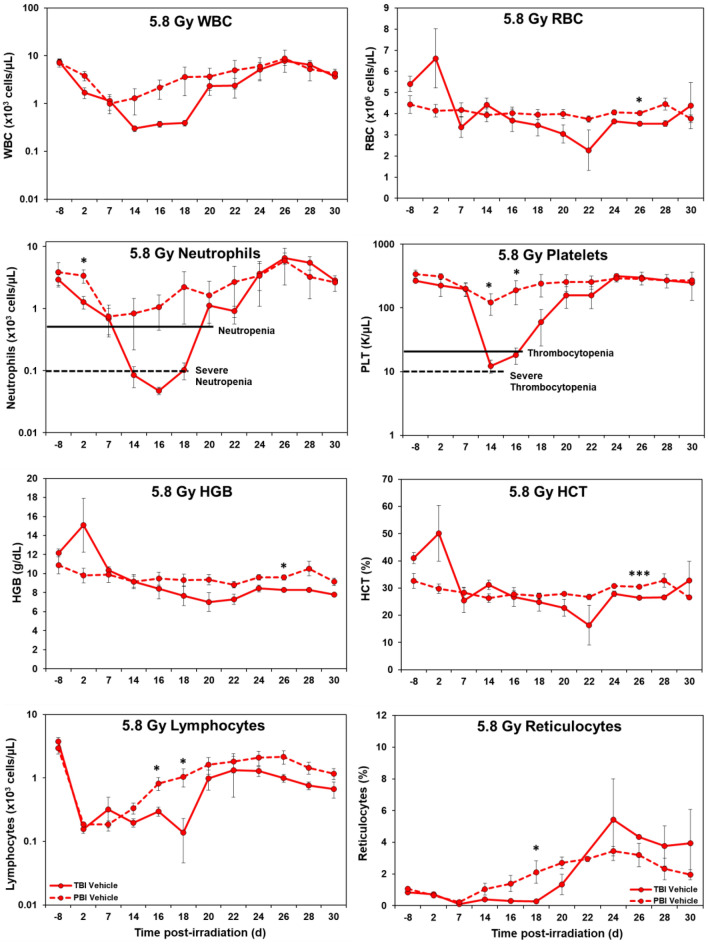
Hematology. Marked differences were noted in the temporal patterns of peripheral blood responses of the test animals under the two IR exposure/dose regimens, as well as those animals receiving either the control vehicle treatment or the test agent, GT3 (Figs. 1, 2, 3, and 4). In general, the extent and duration of acute cytopenia (e.g., leukopenia, neutropenia and thrombocytopenia) were exaggerated and prolonged not only by the higher radiation exposure level (5.8 Gy), but also by the lack of use of the prospective, injury-mitigating GT3. The time-dependent recovery from radiation exposure-induced cytopenia tended to exhibit reciprocal patterns relative to radiation dose, treatment applied, and the extent of bodily exposure (i.e., TBI vs. PBI). Of note were the less pronounced changes in other erythroid-related blood counts/parameters (RBCs, HGB, HCT, etc.) following lower, non-lethal exposures of 4 Gy as compared to those observed at the higher exposure levels of 5.8 Gy.
Figure 1.
Effects of 4 Gy total-body and partial-body irradiation on GT3-treated NHPs. The data for each time point is presented as the mean for each group. There was a total of 8 NHPs; 4 in the TBI group and 4 in the PBI group. Analysis of variance was performed with SPSS to detect significant differences between TBI and PBI groups. The asterisk corresponds to a statistical significance of p < 0.05.
Figure 2.
Effects of 4 Gy total-body and partial-body irradiation on vehicle-treated NHPs. The data for each time point is presented as the mean for each group. There was a total of 8 NHPs; 4 in the TBI group and 4 in the PBI group. Analysis of variance was performed with SPSS to detect significant differences between TBI and PBI groups. The number of asterisks corresponds to the level of statistical significance as follows: *p < 0.05, **p < 0.01.
Figure 3.
Effects of 5.8 Gy total-body and partial-body irradiation on GT3-treated NHPs. The data for each time point is presented as the mean for each group. There was a total of 8 NHPs; 4 in the TBI group and 4 in the PBI group. Analysis of variance was performed with SPSS to detect significant differences between TBI and PBI groups. The number of asterisks corresponds to the level of statistical significance as follows: *p < 0.05, **p < 0.01, ***p < 0.001.
Figure 4.
Effects of 5.8 Gy total-body and partial-body irradiation on vehicle-treated NHPs. The data for each time point is presented as the mean for each group. There was a total of 7 NHPs; 4 in the TBI group and 3 in the PBI group. Analysis of variance was performed with SPSS to detect significant differences between TBI and PBI groups. The number of asterisks corresponds to the level of statistical significance as follows: *p < 0.05, ***p < 0.001.
Pathological assessments
Gross pathological observations. General pathological observations of all euthanized animals at the time of necropsy were recorded and listed in Table 5. In general, the ‘body-condition score’ (BCS) tended to be lower, albeit marginally lower, in those animals exposed to the higher dose of IR (5.8 Gy). Further, lower (from normal) BCS appeared related not only to the IR regimen applied (TBI vs. PBI), but also whether the animals were treated with test agent, GT3 or solely its vehicle (Table 5). Further, as per the BCS data, the type/frequency of gross organ/tissue pathology noted during necropsy seemed to not only be related to the IR protocol and IR dose applied, but also whether the animals were pretreated with GT3 or its vehicle (Table 5).
Organ system/tissue pathology
Lymphohematopoietic system/sternum. Direct, histopathologic observations of the sternum generally revealed a degree of hypoplasia within the marrow space of the sternum tissues of all the IR exposed animals. In general, hypoplasia was more evident and pronounced within tissues of animals exposed at the higher dose level of 5.8 Gy (relative to the lower dose level of 4 Gy). By comparison, the use of PBI (by contrast to TBI) appeared to lessen somewhat the severity of marrow hypoplasia, as did the test GT3 pretreatment (Fig. 5).
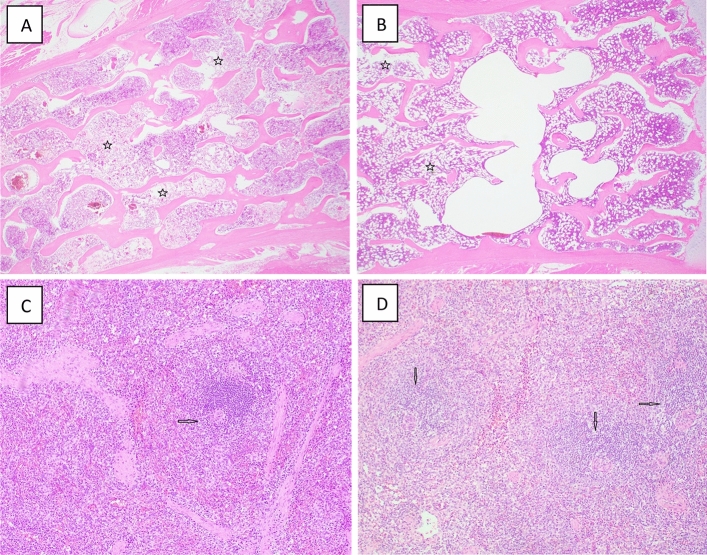
Figure 5.
Representative photomicrographs of lymphohematopoietic organs collected from NHPs exposed to 4 or 5.8 Gy TBI. (A) 1607030F TBI 5.8 Gy, Sternum, 200x, regional areas of moderate cellular losses (stars). (B) 4.0 Gy TBI, Sternum, multiple areas of mild cellular losses (stars). (C) 1608016F 5.8 Gy TBI Veh, Spleen, 100x, rare white pulp and markedly depleted of cellular components arrow). (D) 1603138F 5.8 Gy TBI GT3, Spleen, 100x, multiple white pulps are moderately depleted of cellular components (arrows).
Lymphohematopoietic system/spleen. Histopathologic examination of splenic tissues of all the IR exposed animals showed varying degrees of cellular depletion of the white pulp, with greater losses noted in those animals exposed TBI at the higher doses (5.8 Gy vs. 4 Gy) (Table 6). In general, pretreatments with test medicinal, GT3, appeared to lessen somewhat this depletion- effect.
Gastrointestinal system/small intestine/duodenum. The intestinal duodenal tissues of all animals in all groups showed varying degrees of tissue pathology, as evidenced by the noted loss of villi, villus fusion, decline in the villus to crypt cell ratio, as well as the presence of inflammatory cellular infiltrates (Fig. 6). In general, the later changes were marginally greater following TBI when compared to PBI and were somewhat lessened with GT3 pretreatments (Table 6).
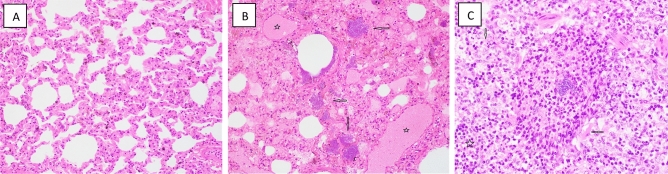
Figure 6.
Representative photomicrographs of digestive organs collected from NHPs exposed to 4 Gy TBI or PBI. (A) 1606069 4.0 Gy TBI Veh, Duodenum, 100x, moderate loss and fusion of villi (arrows) and mild inflammatory cellular infiltrates (stars). (B) 1603168F 4.0 Gy PBI GT3, Duodenum 100x, mild loss and fusion of villi (arrows) and mild inflammatory cellular infiltrates (stars). (C) 1707119M 4.0 Gy PBI Veh, Liver 40x, multiple cystic dilation centered on portal area (stars).
Gastrointestinal system/small intestine/jejunum. The extent of pathologic changes noted within the jejunum was limited and not as obvious as those seen within the duodenum. Nevertheless, loss of villi, coupled with villus fusion and declining villi/crypt radios were indeed noted following both the high and the low levels of TBI and PBI exposures (4 Gy & 5.8 Gy). With GT3 pretreatments, the extent of these changes appeared somewhat less, but variable and unremarkable (Table 6).
Gastrointestinal system/small intestine/ileum. The histopathologic changes noted within the ileum were consistent with those changes noted within the duodenum and the jejunum. Substantial losses of villi, coupled with the extent of villus fusion and declining villi/crypt radios were noted following both the high and the low levels of TBI (Table 6). The latter dose-dependent changes were generally noted under PBI as well, with the exceptions being the marginal decline in villus fusion along with slight rise in the villus/crypt cell ratio following the higher exposure (5.8 Gy) (Table 6). With GT3 pretreatments (compared to vehicle alone pretreatments), the extent of these changes appeared somewhat less, but not markedly so (Table 6).
Gastrointestinal system/large intestine/colon. Clear and consistent differences in the histologic patterns of pathology within the sampled colonic tissues were not seen based on the loss or dilation of intestinal crypt cells, relative to the extent or IR exposure. Similarly, pretreatments with the test agent, GT3, had little noticeable effect on these colonic structures (Table 6).
Digestive system/liver. Pathologic lesions within liver were evident for the irradiated animals, but these lesions (e.g., cystic degeneration, inflammatory infiltrates) were generally unremarkable and lacked definite association with the IR regimen or the type of pretreatment (Fig. 6 and Table 6).
Urinary system/kidney. Regardless of the dose or regimen of radiation exposure, or the pretreatment applied, all kidney sections demonstrated some degree of tubular degeneration, characterized by variably dilated lumen lined with attenuated tubular epithelial cells. However, these test variables (i.e., dose and regiment of exposure, type of pretreatment) appeared to have only marginal impact on the changes noted, with the exception of the apparent lessening of tubular epithelial degeneration following GT3 pretreatments (Fig. 7 and Table 6).
Figure 7.
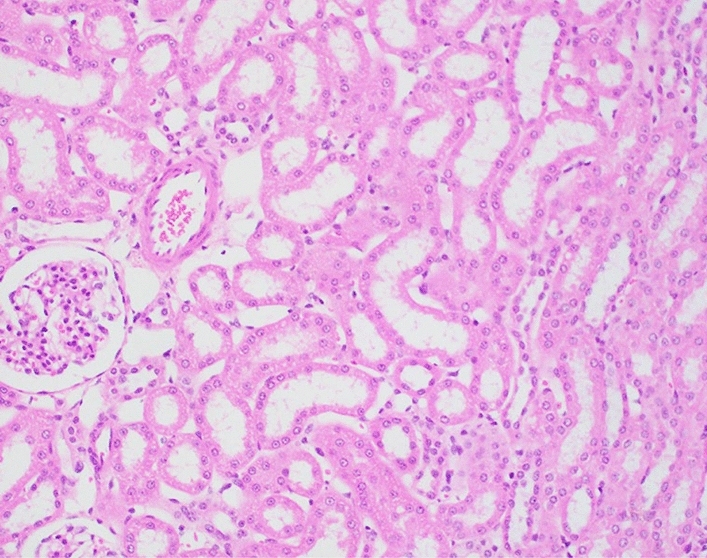
Representative photomicrograph of a kidney collected from an NHP exposed to 5.8 Gy TBI. 1608039M, 200x, area of tubular epithelial attenuation with moderately dilated lumen.
Respiratory system/pulmonary tissue/lung. There were slight but discernable histopathologic differences in alveolar septal degeneration within lung sections of 4.0 and 5.8 Gy TBI vehicle-treated animals compared to the GT3-treated animals (Table 6). The degree of septal degeneration was marginally greater for the vehicle-treated groups compared to the GT3-treated NHPs. These differences appeared significant and might well have been associated with GT3 pretreatments.
Within select animals, most notably those within the 5.8 Gy TBI cohort, septal regions of the lung had moderately high cellular infiltrates comprised mainly of lymphocytes and histocytes within their tissues (Fig. 8). Of note, GT3-pretreated animals did not exhibit such cellular infiltrates. Further, two of the vehicle-pretreated animals exhibited locally extensive bronchointerstitial pneumonia, although in one of these animals there was minimal involvement of adjacent alveolar septal area. No septal cellular infiltration was observed in the PBI groups.
Figure 8.
Representative photomicrographs of respiratory organs collected from NHPs. (A) RA3228F 4 Gy TBI, Lung, 200x, diffusely and moderately degenerated septum. (B) RA1251M Control, Lung, 400x, multiple large bacterial colonies (arrows) and medium vessels congested (stars). (C) 1607030F 5.8 Gy TBI, Lung, 400x, foci of lymphocytes (stars) and histiocytes (arrows).
Discussion
This study focused primarily on the histopathological nature of IR-associated injuries induced at two levels of IR-exposure, namely a relatively low, non-lethal level (4 Gy) and a higher, level (5.8 Gy) that would elicit ~ 30% lethality (within ~ 60 days) (Figs. 5, 6, 7, and 8). Two types of exposure regimens were also employed, namely a ‘total body-type’ exposure (TBI) and a ‘partial body-type’ exposure (PBI), with the later regimen designed to partially limit the exposure to ~ 95% of the full dose to the widely distributed blood-forming tissues of the body (or conversely, a sparing of ~ 5% of the marrow tissue from IR). Recently in a related reported ‘side-arm’ study, we demonstrated that the GT3 mediated hematopoietic recovery in these total- or partial-body exposed NHPs42,43. In this study, flow cytometry was used to immunophenotype the bone marrow (BM) lymphoid cell populations, while select, hematopoietic progenitor populations (HSCs, CFUs) were assessed periodically over a 30-day post-exposure period using standard assays. Significant changes in the frequencies of B and T-cell subsets were noted, along with changes in the self-renewing capacity of HSCs. Importantly, GT3 accelerated the recovery in CD34+ cells, increased HSC function as shown by improved recovery of CFU-granulocyte macrophages (CFU-GM) and burst-forming units erythroid (B-FUE), and aided the recovery of circulating neutrophils and platelets. In yet other related ‘side-arm’ analyses, we evaluated metabolomic and lipidomic profiles in multiple tissues (jejunum, lung, kidney, and spleen) of these tocotrienol-treated and irradiated animals58. Results indicated that IR-induced metabolic changes were partially mitigated by GT3-prophylaxis, with lung and splenic tissues being most responsive. Further, these analyses suggest that a prophylactic administration of GT3 might provide a simple solution in combating this type of metabolically-based radiation injury.
In general, and not surprisingly, the types and severity of injuries noted within the various organs/tissues examined varied with some exhibiting little change (e.g., liver) while others were more extensively altered (e.g., bone marrow). This is consistent with the well-recognized, well-documented radiobiological principle, namely that organ systems that are functionally dependent on high cell replacement rates tend to be more radiosensitive than those organ systems with lower rates. The lymphohematopoietic system represents the ‘archetype’ of a ‘high cell turnover’ organ system that functionally depends on amplifying small numbers of primitive, immature progenitors and their lineage-determined progeny through multiple rounds of reproduction, followed by differentiation and maturing of those amplified cells into fully functional blood cells (e.g., granulocytes, erythrocytes, etc.). It is these progenitor subpopulations that serve to not only to drive self-renewal of the tissue/organ system, but also is the primary determinant of its sensitivity to acute IR. The marked histopathology noted within the marrow sternum specimens of the irradiated animals clearly manifests this tissue’s relatively high radiosensitivity, as does the loss of white pulp within the splenic tissues. Further, these pathologies appear more severe following TBI (relative to the PBI regimen) at the higher of the two exposure levels applied (5.8 Gy vs. 4 Gy). Of particular note, the severity of these pathologies seemed to have lessened by the GT3 nutraceutical pretreatments. Results of the periodic clinical monitoring of these irradiated animals, especially in terms of the changing blood parameters, tend to support overall these pathologic trends.
In a similar fashion, the integrity and function of gastrointestinal system (GIS) is dependent on a comparable system of progenitor-dependent, self-renewing epithelia that lines outer mucosal walls of the intestine. Like the hematopoietic system, moderate doses of IR tend to disrupt the function of this cell renewing system, resulting in gastrointestinal injury that manifests both structurally and functionally, as exemplified by the histopathology of villus and crypt cell structures within the various segments of the small intestine. Again, like the lymphohematopoietic tissues, the GIS response to acute irradiation was modified not only by the IR dose, but also by the exposure regimen and by the applied nutraceutical pretreatment.
In contrast to the pathologic responses of these two robustly, self-renewing tissues (lymphohematopoietic and gastrointestinal tissues) and the modification of those responses relative to radiation dose, type of exposure regimen, or nutraceutical prophylaxes, the remaining tissues examined, namely liver, kidney and lung, with significantly lower levels of self-renewal, appeared less responsive in terms of the severity of the pathologies observed. This is not to say that these tissues were unresponsive to IR, for indeed they clearly harbored IR-associated lesions, but that these lesions appeared not to be acute or life-threatening in the short term. Through previous work59–61, it is well recognized that seemingly innocuous IR lesions within weakly, self-renewing tissues such as the kidney or the liver, can progress with time into pathologies that are more severe and more lethal. Such pathologies are often characterized or labeled as being ‘delayed or late-arising’ by nature and again, by contrast, can arise either stochastically (e.g., IR induced cancers) or deterministically (e.g., tissue fibrosis).
As mentioned earlier, part of the study attempted to examine the possible ‘pathology-sparing effect’ of the PBI-type IR exposures and it was found that not all of the tissues examined were spared (or affected) equally or in the same manner. For example, sternal bone marrow, splenic white pulp, duodenal intestine, and lung exhibited a lessening of pathology induced at the higher exposure level (5.8 Gy) under PBI as compared to the same dose delivered TBI. By contrast, the kidneys of animals subjected to PBI or TBI exhibited little difference in terms of the extent of tubular degeneration.
We used high level cobalt-60 facility for TBI. Cobalt facility has a panoramic field. Even if one tries to protect any part of the body, one cannot provide full protection. Thus, for PBI, LINAC is used because LINAC provides a collimated beam and the irradiation field is limited. Therefore, one can place in the field only part of the body one is interested in. Since same dose of radiation in total-body and partial-body with 5% bone marrow sparing will have different lethality, use of two different sources was not a major issue.
Finally, a major goal of the study was to evaluate the capacity of a nutraceutical, gamma tocotrienol or GT3, when used prophylactically to suppress or to limit IR-associated pathologies. Although the radioprotective effect of GT3 was somewhat less than we had expected, we did observe an apparent lessening of pathology within select tissues/organ systems when the GT3 was administered. The improved blood and marrow pictures of the GT3 treated animals, relative to the untreated control animals, tend to support our working hypothesis; namely that this agent, GT3, has radioprotective properties when used under defined exposure conditions. It should also be noted that the only two acutely irradiated animals in vehicle treated group became moribund prior to scheduled euthanasia date. This number likely would have been higher others not a sizable fraction of the animals not been pretreated with GT3. Admittedly, the number of animals here are too small to preclude a definitive conclusion on the agent’s capacity to reduce IR induced lethality: without doubt, additional studies are needed and are currently in the planning stage.
Supplementary Information
Acknowledgements
The authors would like to thank the staff of the Radiation Science Department, Armed Forces Radiobiology Research Institute, for dosimetry and radiation exposure to the animals.
Disclaimer
The opinions or assertions contained herein are the private views of the authors and are not necessarily those of the Uniformed Services University of the Health Sciences, or the Department of Defense.
Author contributions
Conceptualization: V.K.S., T.M.S.; In vivo Investigation: A.D.C., O.O.F., S.Y.W.; Methodology: O.O.F., S.Y.W., S.H.L.; Supervision: V.K.S.; Data acquisition: S.Y.W., O.O.F., A.D.C., S.A.P.; Data curation: S.H.L.; O.O.F., S.Y.W., A.D.C., S.H.L., TMS; Formal analysis: T.M.S., S.H.L., A.D.C., S.A.P., Writing original draft: O.O.F., S.Y.W., V.K.S., A.D.C., S.H.L., L.A.L., M.H.-J., T.M.S.; Writing—review & Editing: V.K.S., S.H.L., A.D.C., L.A.L., M.H.-J.; Funding Acquisition: V.K.S., M.H.-J. All authors have read and approved the final submitted manuscript.
Funding
The authors gratefully acknowledge the research support from the Congressionally Directed Medical Research Programs (W81XWH-15-C-0117, JW140032), the Armed Forces Radiobiology Research Institute/Uniformed Services University of the Health Sciences (AFR-12080, AFR-12848), and Defense Health Agency (13266) to VKS.
Data availability
All relevant data are within the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-024-64102-8.
References
- 1.Bushberg JT, Kroger LA, Hartman MB, Leidholdt EM, Jr, Miller KL, Derlet R, Wraa C. Nuclear/radiological terrorism: Emergency department management of radiation casualties. J. Emerg. Med. 2007;32:71–85. doi: 10.1016/j.jemermed.2006.05.034. [DOI] [PubMed] [Google Scholar]
- 2.Gale RP. Medical and policy considerations for nuclear and radiation accidents, incidents and terrorism. Curr. Opin. Hematol. 2017;24:496–501. doi: 10.1097/MOH.0000000000000380. [DOI] [PubMed] [Google Scholar]
- 3.Gale RP, Armitage JO. Are we prepared for nuclear terrorism? N. Engl. J. Med. 2018;378:1246–1254. doi: 10.1056/NEJMsr1714289. [DOI] [PubMed] [Google Scholar]
- 4.Andersson KG, Mikkelsen T, Astrup P, Thykier-Nielsen S, Jacobsen LH, Schou-Jensen L, Hoe SC, Nielsen SP. Estimation of health hazards resulting from a radiological terrorist attack in a city. Radiat. Prot. Dosim. 2008;131:297–307. doi: 10.1093/rpd/ncn173. [DOI] [PubMed] [Google Scholar]
- 5.Hasegawa A, Tanigawa K, Ohtsuru A, Yabe H, Maeda M, Shigemura J, Ohira T, Tominaga T, Akashi M, Hirohashi N, et al. Health effects of radiation and other health problems in the aftermath of nuclear accidents, with an emphasis on Fukushima. Lancet. 2015;386:479–488. doi: 10.1016/S0140-6736(15)61106-0. [DOI] [PubMed] [Google Scholar]
- 6.Huff LA, Olabisi AO, Singh VK. Global health security: Radiation countermeasure for acute radiation syndrome. J. Radiat. Cancer Res. 2018;9:1–3. doi: 10.4103/jrcr.jrcr_43_17. [DOI] [Google Scholar]
- 7.U.S. Food and Drug Administration. Animal Rule Approvals. Available online: https://www.fda.gov/drugs/nda-and-bla-approvals/animal-rule-approvals (accessed on December 5).
- 8.Singh VK, Seed TM. Pharmacological management of ionizing radiation injuries: Current and prospective agents and targeted organ systems. Expert Opin. Pharmacother. 2020;21:317–337. doi: 10.1080/14656566.2019.1702968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh VK, Seed TM. Repurposing pharmaceuticals previously approved by regulatory agencies to medically counter injuries arising either early or late following radiation exposure. Front. Pharmacol. 2021;12:624844. doi: 10.3389/fphar.2021.624844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farese AM, MacVittie TJ. Filgrastim for the treatment of hematopoietic acute radiation syndrome. Drugs Today (Barc.) 2015;51:537–548. doi: 10.1358/dot.2015.51.9.2386730. [DOI] [PubMed] [Google Scholar]
- 11.Singh VK, Seed TM. An update on sargramostim for treatment of acute radiation syndrome. Drugs Today (Barc.) 2018;54:679–693. doi: 10.1358/dot.2018.54.11.2899370. [DOI] [PubMed] [Google Scholar]
- 12.Singh VK, Seed TM. Radiation countermeasures for hematopoietic acute radiation syndrome: Growth factors, cytokines and beyond. Int. J. Radiat. Biol. 2021;97:1526–1547. doi: 10.1080/09553002.2021.1969054. [DOI] [PubMed] [Google Scholar]
- 13.Hankey KG, Farese AM, Blaauw EC, Gibbs AM, Smith CP, Katz BP, Tong Y, Prado KL, MacVittie TJ. Pegfilgrastim improves survival of lethally irradiated nonhuman primates. Radiat. Res. 2015;183:643–655. doi: 10.1667/RR13940.1. [DOI] [PubMed] [Google Scholar]
- 14.Clayton NP, Khan-Malek RC, Dangler CA, Zhang D, Ascah A, Gains M, Gardner B, Mockbee C, Keutzer JM, McManus J, Authier S. Sargramostim (rhu GM-CSF) improves survival of non-human primates with severe bone marrow suppression after acute, high-dose, whole-body irradiation. Radiat. Res. 2021;195:191–199. doi: 10.1667/RADE-20-00131.1. [DOI] [PubMed] [Google Scholar]
- 15.Zhong Y, Pouliot M, Downey AM, Mockbee C, Roychowdhury D, Wierzbicki W, Authier S. Efficacy of delayed administration of sargramostim up to 120 hours post exposure in a nonhuman primate total body radiation model. Int. J. Radiat. Biol. 2021;97:S100–S116. doi: 10.1080/09553002.2019.1673499. [DOI] [PubMed] [Google Scholar]
- 16.Singh VK, Seed TM. An update on romiplostim for treatment of acute radiation syndrome. Drugs Today (Barc.) 2022;58:133–145. doi: 10.1358/dot.2022.58.3.3367994. [DOI] [PubMed] [Google Scholar]
- 17.Farese AM, Cohen MV, Katz BP, Smith CP, Gibbs A, Cohen DM, MacVittie TJ. Filgrastim improves survival in lethally irradiated nonhuman primates. Radiat. Res. 2013;179:89–100. doi: 10.1667/RR3049.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lazarus HM, McManus J, Gale RP. Sargramostim in acute radiation syndrome. Expert Opin. Biol. Ther. 2022;22:1345–1352. doi: 10.1080/14712598.2022.2143261. [DOI] [PubMed] [Google Scholar]
- 19.Bunin DI, Javitz HS, Gahagen J, Bakke J, Lane JH, Andrews DA, Chang PY. Survival and hematologic benefits of romiplostim after acute radiation exposure supported FDA approval under the animal rule. Int. J. Radiat. Oncol. Biol. Phys. 2023;117:705–717. doi: 10.1016/j.ijrobp.2023.05.008. [DOI] [PubMed] [Google Scholar]
- 20.U.S. Food and Drug Administration. Radiological and Nuclear Emergency Preparedness Information from FDA. Available online: https://www.fda.gov/emergency-preparedness-and-response/mcm-issues/radiological-and-nuclear-emergency-preparedness-information-fda (accessed on November 2).
- 21.Singh VK, Newman VL, Berg AN, MacVittie TJ. Animal models for acute radiation syndrome drug discovery. Expert Opin. Drug Discov. 2015;10:497–517. doi: 10.1517/17460441.2015.1023290. [DOI] [PubMed] [Google Scholar]
- 22.U.S. Food and Drug Administration. Guidance Document: Product Development Under the Animal Rule. Available online: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm399217.pdf (accessed on October 20).
- 23.Williams JP, Brown SL, Georges GE, Hauer-Jensen M, Hill RP, Huser AK, Kirsch DG, MacVittie TJ, Mason KA, Medhora MM, et al. Animal models for medical countermeasures to radiation exposure. Radiat. Res. 2010;173:557–578. doi: 10.1667/RR1880.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams JP, Jackson IL, Shah JR, Czarniecki CW, Maidment BW, DiCarlo AL. Animal models and medical countermeasures development for radiation-induced lung damage: Report from an NIAID Workshop. Radiat. Res. 2012;177:e0025–0039. doi: 10.1667/RROL04.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Augustine AD, Gondre-Lewis T, McBride W, Miller L, Pellmar TC, Rockwell S. Animal models for radiation injury, protection and therapy. Radiat. Res. 2005;164:100–109. doi: 10.1667/RR3388. [DOI] [PubMed] [Google Scholar]
- 26.MacVittie TJ, Farese AM. Defining the concomitant multiple organ injury within the ARS and DEARE in an animal model research platform. Health Phys. 2020;119:519–526. doi: 10.1097/HP.0000000000001327. [DOI] [PubMed] [Google Scholar]
- 27.Singh VK, Olabisi AO. Nonhuman primates as models for the discovery and development of radiation countermeasures. Expert Opin. Drug Discov. 2017;12:695–709. doi: 10.1080/17460441.2017.1323863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Uno Y, Uehara S, Yamazaki H. Utility of non-human primates in drug development: Comparison of non-human primate and human drug-metabolizing cytochrome P450 enzymes. Biochem. Pharmacol. 2016;121:1–7. doi: 10.1016/j.bcp.2016.06.008. [DOI] [PubMed] [Google Scholar]
- 29.VandeBerg JL, Williams-Blangero S. Advantages and limitations of nonhuman primates as animal models in genetic research on complex diseases. J. Med. Primatol. 1997;26:113–119. doi: 10.1111/j.1600-0684.1997.tb00042.x. [DOI] [PubMed] [Google Scholar]
- 30.Dillman JF, Phillips CS. Comparison of non-human primate and human whole blood tissue gene expression profiles. Toxicol. Sci. 2005;87:306–314. doi: 10.1093/toxsci/kfi243. [DOI] [PubMed] [Google Scholar]
- 31.Singh VK, Seed TM. Development of gamma-tocotrienol as a radiation medical countermeasure for the acute radiation syndrome: Current status and future perspectives. Expert Opin. Investig. Drugs. 2023;32:25–35. doi: 10.1080/13543784.2023.2169127. [DOI] [PubMed] [Google Scholar]
- 32.Singh VK, Hauer-Jensen M. Gamma-tocotrienol as a promising countermeasure for acute radiation syndrome: Current status. Int. J. Mol. Sci. 2016;17:e663. doi: 10.3390/ijms17050663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Singh VK, Beattie LA, Seed TM. Vitamin E: Tocopherols and tocotrienols as potential radiation countermeasures. J. Radiat. Res. 2013;54:973–988. doi: 10.1093/jrr/rrt048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Singh VK, Kulkarni S, Fatanmi OO, Wise SY, Newman VL, Romaine PL, Hendrickson H, Gulani J, Ghosh SP, Kumar KS, Hauer-Jensen M. Radioprotective efficacy of gamma-tocotrienol in nonhuman primates. Radiat. Res. 2016;185:285–298. doi: 10.1667/RR14127.1. [DOI] [PubMed] [Google Scholar]
- 35.Kulkarni S, Singh PK, Ghosh SP, Posarac A, Singh VK. Granulocyte colony-stimulating factor antibody abrogates radioprotective efficacy of gamma-tocotrienol, a promising radiation countermeasure. Cytokine. 2013;62:278–285. doi: 10.1016/j.cyto.2013.03.009. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh SP, Kulkarni S, Hieber K, Toles R, Romanyukha L, Kao TC, Hauer-Jensen M, Kumar KS. Gamma-tocotrienol, a tocol antioxidant as a potent radioprotector. Int. J. Radiat. Biol. 2009;85:598–606. doi: 10.1080/09553000902985128. [DOI] [PubMed] [Google Scholar]
- 37.Singh VK, Fatanmi OO, Verma A, Newman VL, Wise SY, Romaine PL, Berg AN. Progenitor cell mobilization by gamma-tocotrienol: A promising radiation countermeasure. Health Phys. 2016;111:85–92. doi: 10.1097/HP.0000000000000458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Singh VK, Fatanmi OO, Wise SY, Newman VL, Romaine PL, Seed TM. The potentiation of the radioprotective efficacy of two medical countermeasures, gamma-tocotrienol and amifostine, by a combination prophylactic modality. Radiat. Prot. Dosim. 2016;172:302–310. doi: 10.1093/rpd/ncw223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Singh VK, Wise SY, Fatanmi OO, Scott J, Romaine PL, Newman VL, Verma A, Elliott TB, Seed TM. Progenitors mobilized by gamma-tocotrienol as an effective radiation countermeasure. PLoS One. 2014;9:e114078. doi: 10.1371/journal.pone.0114078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Garg S, Garg TK, Miousse IR, Wise SY, Fatanmi OO, Savenka AV, Basnakian AG, Singh VK, Hauer-Jensen M. Effects of gamma-tocotrienol on partial-body irradiation-induced intestinal injury in a nonhuman primate model. Antioxidants. 2022;11:1895. doi: 10.3390/antiox11101895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Garg S, Garg TK, Wise SY, Fatanmi OO, Miousse IR, Savenka AV, Basnakian AG, Singh VK, Hauer-Jensen M. Effects of gamma-tocotrienol on intestinal injury in a GI-specific acute radiation syndrome model in nonhuman primate. Int. J. Mol. Sci. 2022;23:4643. doi: 10.3390/ijms23094643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Garg TK, Garg S, Miousse IR, Wise SY, Carpenter AD, Fatanmi OO, van Rhee F, Singh VK, Hauer-Jensen M. Gamma-tocotrienol modulates total-body irradiation-induced hematopoietic injury in a nonhuman primate model. Int. J. Mol. Sci. 2022;23:16170. doi: 10.3390/ijms232416170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Garg TK, Garg S, Miousse IR, Wise SY, Carpenter AD, Fatanmi OO, van Rhee F, Singh VK, Hauer-Jensen M. Modulation of hematopoietic injury by a promising radioprotector, gamma-tocotrienol, in rhesus macaques exposed to partial-body radiation. Radiat. Res. 2024;201:55–70. doi: 10.1667/RADE-23-00075.2. [DOI] [PubMed] [Google Scholar]
- 44.Vellichirammal NN, Sethi S, Pandey S, Singh J, Wise SY, Carpenter AD, Fatanmi OO, Guda C, Singh VK. Lung transcriptome of nonhuman primates exposed to total- and partial-body irradiation. Mol. Ther. Nucleic Acids. 2022;29:584–598. doi: 10.1016/j.omtn.2022.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fendler W, Malachowska B, Meghani K, Konstantinopoulos PA, Guha C, Singh VK, Chowdhury D. Evolutionarily conserved serum microRNAs predict radiation-induced fatality in nonhuman primates. Sci. Transl. Med. 2017;9:eaal2408. doi: 10.1126/scitranslmed.aal2408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pannkuk EL, Laiakis EC, Fornace AJ, Jr, Fatanmi OO, Singh VK. A metabolomic serum signature from nonhuman primates treated with a radiation countermeasure, gamma-tocotrienol, and exposed to ionizing radiation. Health Phys. 2018;115:3–11. doi: 10.1097/HP.0000000000000776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cheema AK, Hinzman CP, Mehta KY, Hanlon BK, Garcia M, Fatanmi OO, Singh VK. Plasma derived exosomal biomarkers of exposure to ionizing radiation in nonhuman primates. Int. J. Mol. Sci. 2018;19:3427. doi: 10.3390/ijms19113427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rosen E, Fatanmi OO, Wise SY, Rao VA, Singh VK. Tocol prophylaxis for total-body irradiation: A proteomic analysis in murine model. Health Phys. 2020;119:12–20. doi: 10.1097/HP.0000000000001221. [DOI] [PubMed] [Google Scholar]
- 49.Rosen E, Fatanmi OO, Wise SY, Rao VA, Singh VK. Gamma-tocotrienol, a radiation countermeasure, reverses proteomic changes in serum following total-body gamma irradiation in mice. Sci. Rep. 2022;12:3387. doi: 10.1038/s41598-022-07266-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vellichirammal NN, Sethi S, Avuthu N, Wise SY, Carpenter AD, Fatanmi OO, Guda C, Singh VK. Transcriptome profile changes in the jejunum of nonhuman primates exposed to supralethal dose of total- or partial-body radiation. BMC Genom. 2023;24:274. doi: 10.1186/s12864-023-09385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cheema AK, Mehta KY, Fatanmi OO, Wise SY, Hinzman CP, Wolff J, Singh VK. A Metabolomic and lipidomic serum signature from nonhuman primates administered with a promising radiation countermeasure, gamma-tocotrienol. Int. J. Mol. Sci. 2018;19:79. doi: 10.3390/ijms19010079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Singh VK, Carpenter AD, Janocha BL, Petrus SA, Fatanmi OO, Wise SY, Seed TM. Radiosensitivity of rhesus nonhuman primates: Consideration of sex, supportive care, body weight and age at time of exposure. Expert Opin. Drug Discov. 2023;18:797–814. doi: 10.1080/17460441.2023.2205123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Singh VK, Fatanmi OO, Wise SY, Carpenter AD, Olsen CH. Determination of lethality curve for cobalt-60 gamma-radiation source in rhesus macaques using subject-based supportive care. Radiat. Res. 2022;198:599–614. doi: 10.1667/RADE-22-00101.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li Y, Singh J, Varghese R, Zhang Y, Fatanmi OO, Cheema AK, Singh VK. Transcriptome of rhesus macaque (Macaca mulatta) exposed to total-body irradiation. Sci. Rep. 2021;11:6295. doi: 10.1038/s41598-021-85669-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.National Research Council of the National Academy of Sciences . Guide for the Care and Use of Laboratory Animals. 8. National Academies Press; 2011. [PubMed] [Google Scholar]
- 56.Farese AM, Cohen MV, Katz BP, Smith CP, Jackson W, Cohen DM, MacVittie TJ. A nonhuman primate model of the hematopoietic acute radiation syndrome plus medical management. Health Phys. 2012;103:367–382. doi: 10.1097/HP.0b013e31825f75a7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Singh VK, Serebrenik AA, Fatanmi OO, Wise SY, Carpenter AD, Janocha BL, Kaytor MD. The radioprotectant, BIO 300, protects the lungs from total-body irradiation injury in C57L/J mice. Radiat. Res. 2023;199:294–300. doi: 10.1667/RADE-22-00142.1. [DOI] [PubMed] [Google Scholar]
- 58.Carpenter, A. D. et al. Metabolomic profiles in tissues of nonhuman primates exposed to total- or partial-body radiation. Radiat. Res.10.1667/RADE-23-00091.1 (2024). [DOI] [PubMed]
- 59.Singh VK, Wise SY, Fatanmi OO, Petrus SA, Carpenter AD, Lee SH, Hauer-Jensen M, Seed TM. Histopathological studies of nonhuman primates exposed to supralethal doses of total- or partial-body radiation: Influence of a medical countermeasure, gamma-tocotrienol. Sci. Rep. 2024;14:5757. doi: 10.1038/s41598-024-56135-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Parker GA, Cohen EP, Li N, Takayama K, Farese AM, MacVittie TJ. Radiation nephropathy in a nonhuman primate model of partial-body irradiation with minimal bone marrow sparing-part 2: Histopathology, mediators, and mechanisms. Health Phys. 2019;116:409–425. doi: 10.1097/HP.0000000000000935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Parker GA, Li N, Takayama K, Farese AM, MacVittie TJ. Lung and heart injury in a nonhuman primate model of partial-body irradiation with minimal bone marrow sparing: Histopathological evidence of lung and heart injury. Health Phys. 2019;116:383–400. doi: 10.1097/HP.0000000000000936. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All relevant data are within the manuscript.