Abstract
ChimeriVax-JE is a live, attenuated recombinant virus prepared by replacing the genes encoding two structural proteins (prM and E) of yellow fever 17D virus with the corresponding genes of an attenuated strain of Japanese encephalitis virus (JE), SA14-14-2 (T. J. Chambers et al., J. Virol. 73:3095–3101, 1999). Since the prM and E proteins contain antigens conferring protective humoral and cellular immunity, the immune response to vaccination is directed principally at JE. The prM-E genome sequence of the ChimeriVax-JE in diploid fetal rhesus lung cells (FRhL, a substrate acceptable for human vaccines) was identical to that of JE SA14-14-2 vaccine and differed from sequences of virulent wild-type strains (SA14 and Nakayama) at six amino acid residues in the envelope gene (E107, E138, E176, E279, E315, and E439). ChimeriVax-JE was fully attenuated for weaned mice inoculated by the intracerebral (i.c.) route, whereas commercial yellow fever 17D vaccine (YF-Vax) caused lethal encephalitis with a 50% lethal dose of 1.67 log10 PFU. Groups of four rhesus monkeys were inoculated by the subcutaneous route with 2.0, 3.0, 4.0, and 5.0 log10 PFU of ChimeriVax-JE. All 16 monkeys developed low viremias (mean peak viremia, 1.7 to 2.1 log10 PFU/ml; mean duration, 1.8 to 2.3 days). Neutralizing antibodies appeared between days 6 and 10; by day 30, neutralizing antibody responses were similar across dose groups. Neutralizing antibody titers to the homologous (vaccine) strain were higher than to the heterologous wild-type JE strains. All immunized monkeys and sham-immunized controls were challenged i.c. on day 54 with 5.2 log10 PFU of wild-type JE. None of the immunized monkeys developed viremia or illness and had mild residual brain lesions, whereas controls developed viremia, clinical encephalitis, and severe histopathologic lesions. Immunized monkeys developed significant (≥4-fold) increases in serum and cerebrospinal fluid neutralizing antibodies after i.c. challenge. In a standardized test for neurovirulence, ChimeriVax-JE and YF-Vax were compared in groups of 10 monkeys inoculated i.c. and analyzed histopathologically on day 30. Lesion scores in brains and spinal cord were significantly higher for monkeys inoculated with YF-Vax. ChimeriVax-JE meets preclinical safety and efficacy requirements for a human vaccine; it appears safer than yellow fever 17D vaccine but has a similar profile of immunogenicity and protective efficacy.
Japanese encephalitis virus (JE), a mosquito-borne flavivirus, is endemic-epidemic throughout Asia. JE causes a devastating acute neurological illness with a case-fatality rate of approximately 35%. In developed countries such as Japan, Korea, and Taiwan, the disease incidence has been reduced to a low level over the past 30 years due principally to routine childhood immunization; however, virus transmission continues in the enzootic cycle (involving mosquitoes, birds, and pigs), mandating continuing human immunization. Unimmunized expatriates living in Asia, tourists, and military personnel are also at risk.
Inactivated and live, attenuated vaccines prepared from primary hamster kidney cell cultures are used exclusively in China, whereas formalin-inactivated mouse brain vaccine is used elsewhere. These vaccines have certain disadvantages, which have been reviewed recently (2, 32). A new, single-dose vaccine, manufactured in an acceptable cell culture substrate, inducing rapid onset and long-lasting immunity without the need for booster doses, and having a low incidence of adverse events would represent a marked improvement over existing products.
We have developed a JE vaccine candidate that is expected to meet these criteria. ChimeriVax-JE is a live, attenuated genetically engineered virus prepared by replacing the genes encoding two structural proteins (prM and E) of yellow fever virus (YF) 17D vaccine strain with the corresponding genes of an attenuated vaccine strain (SA14-14-2) of JE (3, 9). The prM and E proteins contain critical antigens conferring protective humoral and cellular immunity against JE (13).
Safety of the chimeric vaccine is ensured by deriving all genes from attenuated vaccine virus strains. The JE prM and E genes are from SA14-14-2, a live, attenuated JE vaccine strain licensed for use in China (32, 34). The remaining genes of the chimeric virus, including the capsid gene and all of the nonstructural (NS) genes responsible for intracellular replication, are derived from YF 17D, a live, attenuated vaccine strain used over the past 60 years with an excellent record of safety and effectiveness. The vaccine elicits rapid onset of immunity, which is extremely durable (probably lifelong), and the vaccine is licensed by national control authorities worldwide (18).
We previously reported preliminary results demonstrating preclinical activity of the ChimeriVax-JE vaccine in mice (9) and in a nonhuman primate model (20). In this study, we extended the observations in monkeys and determined the effect of vaccine dose on the immune response and protection against challenge. Safety was assessed by measuring viremia, clinical signs, and neuropathological lesions after intracerebral (i.c.) inoculation of the virus. The neutralizing antibody responses to graded, subcutaneous (s.c.) doses of vaccine were determined. Immunity was severely challenged by the administration of a large dose of virulent JE by the i.c. route to vaccinated monkeys and controls. The results demonstrated that ChimeriVax-JE vaccine administered over a range of doses from 2 to 5 log10 PFU was safe and elicited rapid onset of protective immunity.
MATERIALS AND METHODS
ChimeriVax-JE.
Genetic construction of the YF-JE chimera has been described by Chambers et al. (3). Briefly, the entire genome of YF 17D (17D-204 substrain; American Type Culture Collection) was cloned in two plasmids. Cloning sites were engineered to permit replacement of the entire pre-M and E coding sequences of JE SA14-14-2 for the corresponding sequences of YF 17D. Sites for posttranslational cleavage of the capsid and pre-M proteins and of the E and NS1 proteins were preserved. Restriction sites were incorporated in both the YF and JE sequences for in vitro ligation of full-length cDNA. Transcription to mRNA was performed using a commercial kit (AmpliScribe SP6 transcription kit; Epicentre Technologies, Madison, Wis.).
Preparation of virus for immunization.
Virus was prepared by transfecting full-length RNA transcripts into diploid fetal rhesus lung (FRhL) cells by electroporation and passaging the progeny virus in FRhL cells. Passage 5 (FRhL5) is the intended passage level for the vaccine to be administered to humans. For safety studies by i.c. inoculation, virus produced under Good Manufacturing Practices for clinical-grade products was used. In this case, full-length RNA transcripts were transfected by electroporation into FRhL cells from a cell bank at passage level 19 that had been fully qualified for vaccine production. The virus used for monkey safety tests, i.e., the master seed for subsequent production of vaccine lots for human testing, was at FRhL3. Viruses were stored in 50% fetal calf serum at −70°C.
YF 17D was obtained from Pasteur-Mérieux-Connaught, Swiftwater, Pa. The virus was lyophilized commercial vaccine (YF-Vax); it was rehydrated with diluent provided by the manufacturer and used without further passage.
JE IC-37 was derived from a full-length clone of the wild-type JE strain JaOArS982 (isolated from a mosquito pool, Osaka, Japan, 1982) as described by Sumiyoshi et al. (31). The virus was grown in Vero cells.
Nucleotide sequencing.
RNA was extracted from infected FRhL cell monolayers, reverse transcribed to DNA, and sequenced using Dye-Terminator dRhodamine fluorescent sequencing reaction mix (Perkin-Elmer/ABI) and a Genetic Analyzer (model 310; Perkin-Elmer/ABI). DNA sequences were analyzed with Sequencer 3.0 (GeneCodes) software.
Mouse neurovirulence.
ChimeriVax-JE FRhL5 and unpassaged YF-Vax were inoculated by the i.c. route into groups of eight female ICR mice 32 days of age (Taconic Farms, Germantown N.Y.). Mice were inoculated under isofluorane anesthesia with 30 μl of virus suspension containing 0.1, 1.0, 2.0, or 3.0 log10 PFU determined by back-titration of the inoculum. In a separate experiment, 10 female 4-week-old ICR mice were inoculated i.c. with ChimeriVax FRhL3 master seed (2.8 log10 PFU in 30 μl); five mice were inoculated with diluent, and five received undiluted YF-Vax (3.8 log10 PFU in 30 μl). Mice were monitored for illness and death for 21 days.
Subcutaneous immunization of rhesus monkeys.
All studies involving nonhuman primates were conducted in accordance with the USDA Animal Welfare Act (9 CFR parts 1 to 3) as described in the Guide for the Care and Use of Laboratory Animals (20b). Protocols were approved by the Animal Care and Use Committee of each institution undertaking the work.
Preclinical efficacy studies were performed in young adult, colony-reared rhesus monkeys (Macacca mulatta) at the Tulane Regional Primate Center. Sixteen monkeys, weighing 2.0 to 3.3 kg, were negative by hemagglutination inhibition test to YF, dengue virus, and St. Louis encephalitis virus antigens (Robert E. Shope, University of Texas Medical Branch, Galveston, unpublished data). The monkeys were randomized into groups of four animals each. Animals in each group were inoculated by the s.c. route in the deltoid region with 1.0 ml of ChimeriVax-JE FRhL5 containing 5.0, 4.0, 3.0, or 2.0 log10 PFU. Blood was collected under ketamine anesthesia immediately before immunization and then daily for 10 days to determine viremia and antibody responses. Subsequent blood samples for antibody tests were taken on days 15, 30, and 52.
On day 54 postimmunization, the 16 immunized monkeys plus 2 unimmunized controls were challenged by i.c. inoculation of 0.25 ml containing 5.2 log10 PFU of JE IC-37. For i.c. inoculation, monkeys were anesthetized, a small incision made over the right parietal lobe, a burr hole was drilled through which the inoculum was injected, and the incision was then sutured. The inoculum was frozen for back-titration. Animals were observed daily for clinical signs and bled (days 1 to 9 postchallenge) for viremia tests. Severely ill animals were euthanized and necropsied. Surviving animals were bled 18 and 33 days after challenge for antibody tests. Cerebrospinal fluids (CSF) were collected by cisternal atlanto-occipital puncture before challenge and 33 days after challenge.
Four additional unimmunized control animals were inoculated i.c. with JE IC-37 as part of a separate study to develop a model of lethal encephalitis (20).
Monkey safety test.
The monkey safety test was performed according to the U.S. Food and Drug Administration Good Laboratory Practice regulations (21 CFR, part 58) and also complied with regulatory guidelines of the European Community and Japan. The study was conducted according to the World Health Organization requirements for testing YF 17D vaccine for preclinical safety (33), modified to include determinations of clinical laboratory tests and microscopic examination of multiple tissues in addition to brain. The study was performed at Sierra Biomedical Inc., Sparks, Nev. Twenty rhesus monkeys (10 male and 10 female) weighing 2.1 to 5.7 kg were obtained from the Tulane Regional Primate Center. One group of 10 monkeys received a single inoculation of YF-Vax into the frontal lobe of the brain, and a second group of 10 monkeys received ChimeriVax-JE FRhL3 master seed. Each 0.25-ml inoculum contained 4.1 to 4.6 log10 PFU of YF-Vax (determined by back-titration, depending on titer of individual single-dose vials of the vaccine) or 3.8 log10 PFU of ChimeriVax-JE. Monkeys were evaluated for changes in clinical signs and body weight, and hematology and clinical chemistry determinations were performed preinoculation (day 1) and on days 2, 4, 6, and 31. The following tests were performed: serum sodium, potassium, chloride, carbon dioxide, total, direct and indirect bilirubin, alkaline phosphatase, lactate dehydrogenase, aspartate aminotransferase, alanine aminotransferase, gamma-glutamyltransferase, calcium, phosphorus, urea nitrogen, creatinine, uric acid, total protein, albumin, globulin, albumin/globulin ratio, glucose, cholesterol, and triglycerides. Hematological parameters tested at the same intervals included total white cell count and differential counts, red cell count, hemoglobin, hematocrit, red blood cell indices, platelet count, and platelet, white, and red cell morphology. Viremia levels were measured on days 2 to 10 after inoculation, and neutralizing antibodies were measured on day 31.
On day 31, animals were euthanized and a full necropsy was performed. Brains and spinal cords were examined and scored for pathological changes as described by Levenbook et al. (16) and incorporated into a standardized neurovirulence test for YF 17D vaccine (33). Tissue blocks of brain and spinal cord regions were fixed in formalin, dehydrated, and embedded in paraffin; 15-μm sections were cut and stained with gallocyanin. Neurovirulence was assessed by the presence and severity of lesions in various anatomical formations of the central nervous system. Severity was scored within each tissue block using the scale specified by the World Health Organization (33) and described in Table 5, footnote a. Structures involved in the pathologic process most often and with greatest severity were designated target areas, while structures discriminating between wild-type JE and ChimeriVax-JE were designated discriminator areas. As shown in a previous report (20), the substantia nigra constituted the target area and the corpus striatum, thalamus, and spinal cord (cervical and lumbar enlargements) constituted discriminator areas (for details, see Table 7). Statistical comparisons of mean neuropathological scores (for the target area, discriminator areas, and target-plus-discriminator areas) were performed by Student's t test. All neuropathological evaluations were done by a single, experienced investigator who was blinded to the treatment code.
TABLE 5.
Neuropathological evaluation of monkeys immunized with ChimeriVax-JE, challenged i.c. with wild-type JE (IC-37, 5.2 log10 PFU) on day 54 after immunization, and euthanized 30 days after challenge
| Monkey | Vaccine dose (log10 PFU) | Illness (day euthanized) | Individual and group mean mean histopathological scorea
|
||
|---|---|---|---|---|---|
| Target areab | Discriminator areasc | Target + discriminator areas | |||
| AI74 | 2.0 | Not ill (30) | 1.0 | 0.64 | 0.82 |
| AG72 | 2.0 | Not ill (30) | 0.0 | 0.00 | 0.00 |
| AG58 | 2.0 | Not ill (30) | 0.5 | 0.68 | 0.59 |
| AH32 | 2.0 | Not ill (30) | 0.5 | 0.59 | 0.55 |
| Group mean SD | 0.5 | 0.48 | 0.49 | ||
| 0.17 | 0.10 | 0.12 | |||
| AK91 | 3.0 | Not ill (30) | 1.5 | 1.53 | 1.52 |
| AI62 | 3.0 | Not ill (30) | 0.0 | 0.54 | 0.27 |
| AK38 | 3.0 | Not ill (30) | 0.5 | 0.82 | 0.66 |
| AP54 | 3.0 | Not ill (30) | 0.0 | 0.00 | 0.00 |
| Group mean | 0.5 | 0.72 | 0.61 | ||
| SD | 0.5 | 0.41 | 0.44 | ||
| AI67 | 4.0 | Not ill (30) | 0.5 | 0.90 | 0.70 |
| AI86 | 4.0 | Not ill (30) | 1.5 | 0.92 | 1.21 |
| AP03 | 4.0 | Not ill (30) | 0.5 | 0.50 | 0.50 |
| AP52 | 4.0 | Not ill (30) | 1.0 | 0.95 | 0.98 |
| Group mean | 0.88 | 0.82 | 0.85 | ||
| SD | 0.23 | 0.05 | 0.09 | ||
| AI65 | 5.0 | Not ill (30) | 1.0 | 0.89 | 0.95 |
| AH82 | 5.0 | Not ill (30) | 1.0 | 1.07 | 1.04 |
| AK13 | 5.0 | Not ill (30) | 2.0 | 0.68 | 1.34 |
| AN99 | 5.0 | Not ill (30) | 1.5 | 1.58 | 1.54 |
| Group mean | 1.38 | 1.06 | 1.22 | ||
| SD | 0.23 | 0.15 | 0.07 | ||
| AI63 | 0 (sham) | Encephalitis (11) | 2.0 | 2.13 | 2.07 |
| AH27 | 0 (sham) | Encephalitis (11) | 2.0 | 2.27 | 2.14 |
| 151d | 0 (unimmunized) | Encephalitis (10) | 1.5 | 2.95 | 2.23 |
| 178d | 0 (unimmunized) | Encephalitis (9) | 2.0 | 1.71 | 1.86 |
| 26d | 0 (unimmunized) | Encephalitis (10) | 1.5 | 2.69 | 2.10 |
| 146d | 0 (unimmunized) | Encephalitis (10) | 2.0 | 1.57 | 1.79 |
| Group mean | 1.8 | 2.22 | 2.03 | ||
| SD | 0.24 | 0.49 | 0.16 | ||
Grade 1, minimal; one to three small focal inflammatory infiltrates. A few neurons may be changed or lost. Grade 2, moderate; more extensive focal inflammatory infiltrates. Neuronal changes or loss affects not more than one-third of neurons. Grade 3, severe; neuronal changes or loss affecting 33 to 90% of neurons; moderate focal or diffuse inflammatory changes. Grade 4, overwhelming. More than 90% of neurons are changed or lost, with variable but frequently severe inflammatory infiltration
Substantia nigra.
Corpus striatum and thalamus, right and left side (nucleus caudatus, globus pallidus, putamen, nucleus ant./lat. thalami, N. lat. thalami; cervical and lumbar enlargements of the spinal cord (six levels).
Monkey inoculated i.c. with a similar dose (5.4 log10 PFU) and methodology but in a separate experiment; these animals were examined in a previous study (20), but the neuropathological assessment was repeated under code simultaneously with the other monkeys evaluated.
TABLE 7.
Neuropathological evaluation of monkeys inoculated i.c. with ChimeriVax-JE FRhL3 or YF-Vax and necropsied on day 30 postinoculation
| Test virus | Monkey | Gendera | Dose (log10 PFU/0.25 ml) | Clinical scoreb (maximum score/mean daily score) | Individual and group mean histopathological scorec
|
||
|---|---|---|---|---|---|---|---|
| Target aread | Discriminator arease | Target + discriminator areas | |||||
| YF-Vax | RT702M | M | 4.4 | 1/0 | 2.00 | 0.51 | 1.26 |
| RT758M | M | 4.6 | 1/0 | 0.25 | 0.01 | 0.13 | |
| RT653M | M | 4.4 | 1/0 | 2.00 | 0.39 | 1.20 | |
| RT776M | M | 4.6 | 3/1 | 2.00 | 1.29 | 1.65 | |
| RT621M | M | 4.6 | 3/2 | 1.00 | 0.46 | 0.73 | |
| RAH80F | F | 4.4 | 3/1 | 1.50 | 0.71 | 1.10 | |
| RALO2F | F | 4.4 | 1/1 | 2.00 | 0.80 | 1.40 | |
| RT698F | F | 4.1 | 3/1 | 1.50 | 0.64 | 1.07 | |
| RAI12F | F | 4.4 | 1/1 | 2.00 | 1.45 | 1.73 | |
| RP942F | F | 4.4 | 1/0 | 2.00 | 0.81 | 1.41 | |
| Mean SD | 4.4 | 1 | 1.63 | 0.71 | 1.17 | ||
| 0.1 | 1 | 0.59 | 0.42 | 0.47 | |||
| ChimeriVax-JE | RT452M | M | 3.8 | 1/0 | 0.50 | 0.08 | 0.29 |
| RR257M | M | 3.8 | 1/0 | 1.00 | 0.14 | 0.57 | |
| RT834M | M | 3.8 | 1/0 | 0.50 | 0.38 | 0.44 | |
| RT620M | M | 3.8 | 1/0 | 1.00 | 0.14 | 0.57 | |
| RT288M | M | 3.8 | 1/0 | 0.50 | 0.19 | 0.35 | |
| RAJ98F | F | 3.8 | 1/1 | 0.00 | 0.11 | 0.05 | |
| RAR08F | F | 3.8 | 1/0 | 0.00 | 0.13 | 0.07 | |
| RV481F | F | 3.8 | 1/0 | 0.00 | 0.06 | 0.03 | |
| RT841F | F | 3.8 | 1/0 | 0.50 | 0.05 | 0.28 | |
| RT392F | F | 3.8 | 1/0 | 0.50 | 0.07 | 0.29 | |
| Mean SD | 3.8 | 0 | 0.45 | 0.14 | 0.29 | ||
| 0 | 0.37 | 0.10 | 0.20 | ||||
| P valuef | 0.037/0.025 | <0.00001 | 0.0019 | 0.00014 | |||
M, male; F, female.
Clinical score: 0, no signs; 1, rough coat, not eating; 2, high-pitched voice, inactive, slow moving; 3, tremor, incoordination, shaky movements, limb weakness; 4, inability to stand, paralysis, moribund, or dead. The maximum score on any day and the mean score over the 30-day observation period are shown.
c–e See Table 5, footnotes b to d.
Student's t test, two-sided, heteroscedastic, comparing YF-Vax and ChimeriVax-JE.
Viremia determinations.
Virus titers in serum were determined by direct plaquing in Vero cells. Undiluted and serial 10-fold dilutions of serum in medium 199 or minimal essential medium containing 20% heat inactivated fetal calf serum were inoculated (0.1 ml) into duplicate wells of 12-well tissue culture plates containing monolayer cultures of Vero cells. After 1 h of adsorption at 37°C, wells were overlaid and stained for plaques using one of two methods. In one method, plates were overlaid with nutrient agarose, followed on day 4 with a second overlay containing neutral red; 24 h later, monolayers were fixed with formaldehyde and stained with crystal violet (see “Neutralization tests,” below). In a second method, plates were overlaid with methylcellulose, which was removed on day 6, and the cells fixed with formaldehyde and stained with crystal violet. The two methods were compared in multiple assays and shown to produce equivalent results.
Neutralization tests.
Neutralizing antibody titers were determined by plaque reduction in Vero cells, without the addition of complement. Sera or CSF were inactivated (56°C, 30 min); 125 μl of serial twofold dilutions of sample was mixed with an equal volume of virus suspension containing a nominal 120 PFU. The mixture was incubated (4°C, 18 h), and 0.1 ml was added to duplicate wells of Vero cell monolayers in 12-well plates. After 1.5 h of incubation at 37°C, the first overlay (0.6% agarose containing 3% fetal bovine serum, 1% glutamine, 1% HEPES, and antibiotics in medium 199) was added to the wells, and plates were returned to the incubator for 4 days, when a second agarose overlay containing 3% neutral red was added. Twenty-four hours later, monolayers were fixed with formaldehyde and stained with crystal violet. The antibody titer was the highest dilution of antibody reducing the number of plaques by 50% compared to the plaque titer in a mixture of virus with serum from the same monkey obtained prior to immunization.
Neutralizing antibody titers were measured to the homologous virus (ChimeriVax-JE), the challenge virus (IC-37), plus other selected wild-type JE strains representing the known genotypes of JE (4, 6), as described by Wills et al. (32a). The latter virus strains included P-20778 (human brain, Vellore, India, 1985), JKT-1724 (Culex tritaenorrhynchus mosquitoes, Java, Indonesia, 1979), KPO 439 (mosquito, Thailand, 1984), and Korea (C. tritaenorrhynchus, Kyong Kee Province, South Korea, 1991). All strains were at low passage and were grown in C6/36 cells or Vero cells.
RESULTS
Nucleotide sequence.
The nucleotide sequences of the prM and E genes of ChimeriVax-JE were compared to published sequences of JE SA14-14-2 and selected wild-type JE strains, including the parental virus from which SA14-14-2 was derived (SA14) and the virus used for challenge, JaOArS982 (Table 1). The sequence of ChimeriVax-JE FRhL5 was identical to that of a previously sequenced chimeric construct derived by transfection of Vero cells and passage in Vero and FRhL cells (9) and to the published sequence of JE SA14-14-2 vaccine passed in primary hamster kidney cells (1). An attenuated JE SA14-14-2 strain passed in primary hamster kidney and then in primary dog kidney cells had amino acid residues E177 and E264 identical to the parental SA14 strain, possibly indicating reversion during passage (23). Thus, attenuated strains, including ChimeriVax-JE, differed from virulent wild-type virus strains at a minimum of six amino acid residues (E107, E138, E176, E279, E315, and E439).
TABLE 1.
Comparison of amino acid differences in the E proteins of ChimeriVax-JE FRhL3 master seed and ChimeriVax-JE FRhL5 with published sequences of JE SA14-14-2 vaccine, the wild-type SA14 parent of the vaccine strain, and the wild-type JE strains Nakayama and JaOArS982a
| Virus (reference) | Amino acid in E protein at position:
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 107 | 138 | 167 | 176 | 177 | 227 | 243 | 244 | 264 | 279 | 315 | 439 | |
| ChimeriVax-JE FRhL5 | F | K | V | V | A | S | E | G | H | M | V | R |
| ChimeriVax-JE FRhL3 (9) | F | K | V | V | A | S | E | G | H | M | V | R |
| JE SA14-14-2b (24) | F | K | V | V | T | S | K | G | Q | M | V | R |
| JE SA14-14-2c (1) | F | K | V | V | A | S | E | G | H | M | V | R |
| JE SA14 (1, 24) | L | E | V | I | T | S | E | G | Q | K | A | K |
| JE SA14 (22, 23) | L | E | V | I | T | S | E | E | Q | K | A | K |
| JE Nakayama (9) | L | E | V | I | T | P | E | E | Q | K | A | K |
| JaOArS982 (31) | L | E | I | I | T | S | E | E | Q | K | A | K |
A full-length infectious clone (IC-37) of JaOArS982 was used to challenge monkeys in the protection study. Six residues that distinguish ChimeriVax-JE and SA14-14-2 vaccine from wild-type, virulent strains are shown in boldface.
Isolated from primary dog kidney.
Isolated from primary hamster kidney.
Mouse neurovirulence.
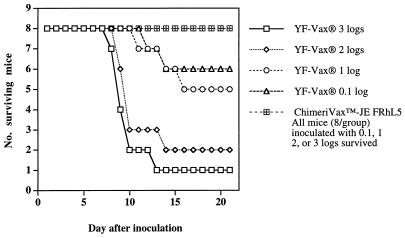
No mouse inoculated with graded doses of ChimeriVax-JE FRhL5 showed signs of illness or died, whereas YF-Vax caused lethal encephalitis (Fig. 1). The 50% lethal dose (LD50) of YF-Vax was 1.67 log10 PFU. In a second experiment, none of 10 mice inoculated with 2.8 log10 PFU of ChimeriVax-JE FRhL3 master seed or five diluent controls showed signs of illness or death, whereas all of five mice inoculated with 3.8 log10 PFU YF-Vax developed illness, and four died.
FIG. 1.
Neurovirulence of YF-Vax and ChimeriVax-JE FRhL5 for 4-week-old outbred ICR mice inoculated by the i.c. route with graded doses.
Viremia and neutralizing antibody response to s.c. immunization.
All 16 monkeys inoculated by the s.c. route with 2.0 to 5.0 log10 PFU of ChimeriVax-JE FRhL5 developed brief, low-level viremias (Table 2). The mean peak viremia (1.7 to 2.1 log10 PFU/ml) and mean duration (1.8 to 2.3 days) were nearly identical across the different dose groups. The onset of viremia was related to dose; onset in monkeys inoculated with 5.0 log10 PFU was on days 2 to 3, while onset in those inoculated with 2.0 log10 PFU was on day 4.
TABLE 2.
Viremia in rhesus monkeys after s.c. inoculation of graded doses of ChimeriVax-JE FRhL5
| Monkey | Dose (log10 PFU) | Viremia (log10 PFU/ml) by indicated day postimmunization
|
Mean peak titer ± SD | Mean duration (days) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | ||||
| AI74 | 2.0 | 0a | 0 | 0 | 2.0 | 0 | 1.3 | 0 | 0 | 0 | 0 | ||
| AG72 | 2.0 | 0 | 0 | 0 | 1.6 | 0 | 0 | 0 | 0 | 0 | 0 | 1.9 ± 0.2 | 2.3 |
| AG58 | 2.0 | 0 | 0 | 0 | 1.3 | 2.0 | 1.3 | 0 | 0 | 0 | 0 | ||
| AH32 | 2.0 | 0 | 0 | 0 | 1.6 | 1.3 | 2.1 | 0 | 0 | 0 | 0 | ||
| AK91 | 3.0 | 0 | 0 | 0 | 1.3 | 1.3 | 0 | 0 | 0 | 0 | 0 | ||
| AI62 | 3.0 | 0 | 0 | 1.3 | 0 | 1.8 | 1.3 | 0 | 0 | 0 | 0 | 1.8 ± 0.3 | 2.3 |
| AK38 | 3.0 | 0 | 0 | 2.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| AP54 | 3.0 | 0 | 0 | 1.3 | 2.0 | 1.6 | 0 | 0 | 0 | 0 | 0 | ||
| AI67 | 4.0 | 0 | 2.0 | 2.3 | 1.3 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| AI86 | 4.0 | 0 | 0 | 0 | 2.0 | 0 | 0 | 0 | 0 | 0 | 0 | 2.1 ± 0.2 | 2.0 |
| AP03 | 4.0 | 0 | 0 | 1.6 | 1.6 | 2.0 | 0 | 0 | 0 | 0 | 0 | ||
| AP52 | 4.0 | 0 | 2.0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| AI65 | 5.0 | 0 | 1.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1.7 ± 0.5 | 1.8 |
| AH82 | 5.0 | 0 | 1.3 | 2.0 | 2.0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| AK13 | 5.0 | 0 | 1.3 | 2.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
| AN99 | 5.0 | 0 | 0 | 1.3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | ||
0 = <1.3 log10 PFU/ml.
As expected based on the detection of viremia, all 16 immunized monkeys seroconverted. The kinetics of the neutralizing antibody response were dose dependent; monkeys receiving higher doses had earlier onset of immunity (Table 3). Monkeys inoculated with 4.0 or 5.0 log10 PFU had detectable neutralizing antibodies on day 6 or 7, while most monkeys receiving 2 or 3 log10 PFU developed antibodies on days 8 to 10. Antibody levels increased rapidly in all animals during the second week after inoculation, and high titers were attained by day 30 in all groups. Geometric mean titers on days 30 and 52 (prechallenge) were not statistically different across the dose groups by analysis of variance methods. The postchallenge antibody response is described below.
TABLE 3.
Reciprocal neutralizing antibody titers in rhesus monkeys immunized by the s.c. route with graded doses of ChimeriVax-JE FRhL5
| Monkey | Dose (log10 PFU) | Reciprocal neutralizing antibody titera
|
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Day postimmunization
|
Day postchallenge
|
|||||||||||
| 5 | 6 | 7 | 8 | 9 | 10 | 15 | 30 | 52 | 18 | 30 | ||
| AI74 | 2.0 | <2 | <2 | <2 | 2 | 2 | 8 | ≥160 | ≥1280 | 1,280 | ≥20,480 | ≥10,240 |
| AG72 | 2.0 | <2 | <2 | <2 | <2 | <2 | 2 | 40 | 640 | 2,560 | 5,120 | 2,560 |
| AG58 | 2.0 | <2 | <2 | <2 | 2 | <2 | 2 | 20 | 320 | 640 | ≥20,480 | ≥10,240 |
| AH32 | 2.0 | <2 | <2 | 2 | 2 | 2 | 8 | 160 | 1,280 | 1,280 | 5,120 | ≥10,240 |
| GMTb | 2 | 2 | 2 | 4 | 67 | 761 | 1,280 | 10,240 | 7,241 | |||
| AK91 | 3.0 | <2 | <2 | <2 | <2 | <2 | 2 | 80 | 160 | 640 | 10,240 | ≥20,480 |
| AI62 | 3.0 | <2 | <2 | <2 | 2 | 2 | 8 | ≥160 | 320 | 1,280 | 10,240 | ≥20,480 |
| AK38 | 3.0 | <2 | <2 | <2 | <2 | 2 | 8 | ≥160 | 640 | 1,280 | 5,120 | ≥10,240 |
| AP54 | 3.0 | <2 | <2 | <2 | 2 | 2 | 32 | 80 | 320 | 1,280 | 1,280 | 1,280 |
| GMT | 2 | 2 | 8 | 113 | 320 | 1,076 | 5,120 | 8,611 | ||||
| AI67 | 4.0 | <2 | 2 | 8 | 32 | 32 | 128 | 160 | 320 | 640 | 640 | 2,560 |
| AI86 | 4.0 | <2 | <2 | 2 | 2 | 8 | 32 | 160 | 320 | 640 | 5,120 | ≥10,240 |
| AP03 | 4.0 | <2 | <2 | 2 | 2 | 8 | 32 | 640 | 1,280 | 1,280 | 2,560 | ≥20,480 |
| AP52 | 4.0 | <2 | 2 | 2 | 2 | 8 | 32 | 80 | 320 | 1,280 | 1,280 | 2560 |
| GMT | 2 | 3 | 4 | 11 | 45 | 190 | 453 | 905 | 1,810 | 6,089 | ||
| AI65 | 5.0 | <2 | 2 | 8 | 8 | 32 | 128 | 160 | 320 | 320 | 5,120 | ≥10,240 |
| AH82 | 5.0 | <2 | 2 | 8 | 32 | ≥256 | ≥256 | 1,280 | 640 | 640 | 320 | 10,240 |
| AK13 | 5.0 | <2 | 8 | 128 | 128 | 32 | ≥256 | 640 | 1,280 | 2,560 | 2,560 | ≥10,240 |
| AN99 | 5.0 | <2 | 2 | 128 | 8 | ≥256 | ≥256 | 1,280 | 1,280 | 320 | 5,120 | ≥10,240 |
| GMT | 3 | 32 | 23 | 91 | 215 | 640 | 761 | 640 | 2,153 | 10,240 | ||
| AI63 | 0 (sham) | <20 | <20 | Dead | ||||||||
| AH27 | 0 (sham) | 20 | <20 | Dead | ||||||||
50% plaque reduction neutralization measured against homologous virus. Sera were tested for antibodies on days 1 to 4 after immunization and found negative (data not shown). Monkeys were challenged by the i.c. route with wild-type JE on day 54, and antibody levels were determined 18 and 30 days after challenge.
GMT, geometric mean titer (positive sera only). Where the endpoint was not determined, the lowest possible titer was used in the calculation (e.g., ≥160 taken as 160).
The four monkeys in the highest-dose group (5.0 log10 PFU) were tested for neutralizing antibodies to heterologous (wild-type) JE strains (Table 4). Antibody titers to the homologous (vaccine) strain were ≥4-fold higher than titers to the heterologous strains. Two monkeys with the lowest homologous antibody titers had no detectable antibodies (at a serum dilution of 1:20) against JE Korea, JKT-1724 (Indonesia), or P-20778 (India). However, all monkeys developed antibodies to the virus used for challenge (JaOArS892 and IC-37, derived from an infectious cDNA clone of JaOArS892) (31).
TABLE 4.
to homologous virus, challenge virus (IC-37), and heterologous wild-type virusesa
| Test sample | Neutralizing antibody titer by virus (substrate used to propagate virus)
|
||||||
|---|---|---|---|---|---|---|---|
| ChimeriVax-JE (Vero) | IC-37 (Vero) | JaOArS892 (C6/36) | JKT-1724 (C6/36) | Korea (C6/36) | Korea (Vero) | P-20778 (C6/36) | |
| Controls | |||||||
| Hyperimmune rabbit anti-JE NB690 | NTb | NT | NT | >160 | 320 | 640 | 320/160c |
| Positive control (human immunized with JE vaccine) | NT | NT | 640 | 20 | <20 | <20 | <20/<20 |
| Negative control (human antitetanus) | NT | NT | <20 | <20 | <20 | <20 | <20/<20 |
| Monkeys | |||||||
| AI65 | 320 | 20 | 20 | <20 | <20 | <20 | <20/<20 |
| AH82 | 640 | 80 | 160 | 40 | 160 | 80 | 20/<20 |
| AK13 | 2,560 | 160 | 160 | 40 | 80 | 80 | 20/<20 |
| AN99 | 320 | 20 | 20 | <20 | <20 | <20 | <20/<20 |
Virus strains: IC-37, progeny from infectious clone of JaOArS892; JaOArS892, Osaka, Japan, mosquito pool, 1982; JKT-1724, Java, Indonesia, C. tritaeniorrhynchus, 1979; Korea, Kyung Kee Province, C. tritaeniorrhynchus, 1991; P-20778, Vellore, India, human brain, 1985.
NT, not tested.
Replicate tests.
Protection against i.c. challenge.
All immunized monkeys and two sham-immunized controls were challenged i.c. on day 54 with 5.2 log10 PFU of IC-37 virus (determined by back-titration of the inoculum). None of the 16 immunized monkeys developed signs of illness, whereas both sham-immunized controls developed severe encephalitis and were euthanized on day 11 after inoculation. None of the immunized monkeys developed viremia during the 10-day postchallenge interval, whereas both controls developed low-level viremias (monkey AI63, days 1 to 3, peak titer of 1.7 log10 PFU/ml; monkey AH27, days 1 and 2, peak titer of 1.3 log10 PFU/ml).
Pathological examination of brains and spinal cords was conducted at the time of euthanasia of control monkeys and 30 days after challenge of protected, immunized monkeys. Tissues from four unimmunized monkeys inoculated i.c. with a similar dose of IC-37 (5.4 log10 PFU) during a previous experiment (20) were also evaluated pathologically (Table 5). Control monkeys that developed clinical signs of encephalitis had the most severe pathological changes. Although no uninoculated control monkeys were included in this test, most immunized monkeys challenged i.c. with wild-type JE had some residual histopathological changes 30 days after challenge, which were significantly (P < 0.0005, two-tailed t test) less than for the unimmunized controls. Interestingly, there was a relationship between vaccine dose and the severity of residual changes, with the monkeys given 2.0 log10 PFU of the vaccine having the lowest mean score (analysis of variance P = 0.0175). Because of variability in the lesion scores, trend (linear regression) analysis showed a weak correlation between dose and combined lesion score (r2, 0.34; standard error, 0.40).
To further examine the relationship between humoral immunity and protection, neutralizing antibody levels were measured in serum during the postchallenge period and in CSF before challenge and at sacrifice 30 days after challenge. Of 16 immunized monkeys, 13 developed significant (≥4-fold) increases in serum neutralizing antibody titers after i.c. challenge (Table 3), indicating that the challenge virus replicated in brain tissue and stimulated a booster immune response. Of the three monkeys that showed no increase in antibodies, two (AG72 [2 log10 PFU dose group] and AP54 [3 log10 PFU dose group]) represented the only monkeys that showed no pathological signs of subclinical encephalitis at necropsy (Table 5).
Only one monkey (AP03, 4 log10 PFU dose group) had detectable neutralizing antibodies in CSF (1:20 against the homologous virus) on day 52 (prechallenge), demonstrating the inaccessibility of the central nervous system to blood-borne antibodies. However, at necropsy, 30 days after challenge, most monkeys had high levels of antibody in the CSF (Table 6). The only exceptions were the two monkeys (AG72 and AP54) that had no pathological response and did not show a boost in serum antibody after challenge. Neither sham-immunized monkey had detectable antibodies in CSF at the time of illness and euthanasia.
TABLE 6.
Neutralizing antibodies in CSF of rhesus monkeys immunized with graded doses of ChimeriVax-JE vaccine, measured before and after i.c. challenge with wild-type JE
| Monkey | Vaccine dose (log10 PFU) | Neutralizing antibody titera on indicated day with respect to challenge
|
|
|---|---|---|---|
| −2 | 30 | ||
| AI74 | 2.0 | <2 | 2,560 |
| AG72 | 2.0 | <2 | <2 |
| AG58 | 2.0 | <2 | 5,120 |
| AH32 | 2.0 | <2 | 2,560 |
| AK91 | 3.0 | <2 | 5,120 |
| AI62 | 3.0 | <2 | 5,120 |
| AK38 | 3.0 | <2 | 2,560 |
| AP54 | 3.0 | <2 | <2 |
| AI67 | 4.0 | <2 | 1,280 |
| AI86 | 4.0 | <2 | 5,120 |
| AP03 | 4.0 | 20 | 2,560 |
| AP52 | 4.0 | <2 | 640 |
| AI65 | 5.0 | <2 | 5,120 |
| AH82 | 5.0 | <2 | 2,560 |
| AK13 | 5.0 | <2 | 5,120 |
| AN99 | 5.0 | <2 | 1,280 |
50% plaque reduction neutralization versus homologous virus (ChimeriVax-JE).
Monkey safety tests.
ChimeriVax-JE FRhL3 and YF-Vax were compared with respect to neurovirulence and other markers in a Good Laboratory Practice study in which each virus was inoculated by the i.c. route into 10 rhesus monkeys. None of the 10 monkeys inoculated with ChimeriVax-JE developed signs of clinical illness, whereas 4 of 10 (40%) of those inoculated with YF-Vax developed signs of central nervous system dysfunction (tremors) with onset on days 15 to 19 after inoculation, lasting 4 to 15 days. All animals survived, with clinical signs resolving spontaneously; none of the animals developed motor weakness or paralysis. Mean and maximum mean clinical scores were significantly higher in the YF-Vax group (Table 7). There were no changes in body weights, serum chemistry, or hematology tests attributable to either virus.
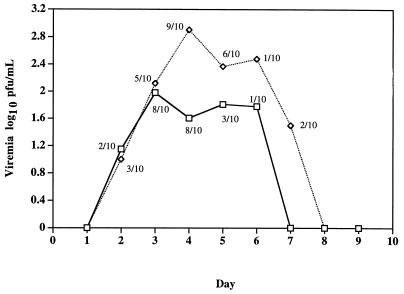
Viremia was detected in 8 of 10 monkeys inoculated i.c. with ChimeriVax-JE FRhL3 master seed and in 9 of 10 monkeys inoculated with YF-Vax (Fig. 2). ChimeriVax-JE viremias were lower than those produced by YF-Vax; the dose of YF-Vax administered was two to six times higher than that of ChimeriVax-JE, depending on the vial of YF-Vax used (Table 7). The viremia safety specifications for YF 17D vaccine (33) were met by both ChimeriVax-JE and YF-Vax; these specifications require that no monkey shall have a viremia exceeding 500 mouse (LD50)/0.03 ml or the equivalent in PFU and in no more than one case shall the viremia exceed 100 mouse LD50/0.03 ml. We determined that YF-Vax contained 1.67 log10 PFU per mouse LD50. The highest viremia in either test group was 2.18 log10 PFU (equivalent to 3.3 mouse LD50). All monkeys developed high levels of neutralizing antibodies on day 31 to the virus used for i.c. inoculation (data not shown).
FIG. 2.
Geometric mean viremia (viremic animals only) for rhesus monkeys inoculated by the i.c. route with ChimeriVax-JE master seed (□) or YF-Vax (◊). The proportion of animals of viremic animals on each day is shown.
Complete autopsies were performed on all animals, with microscopic examination of heart, kidney, spleen, adrenal glands, and liver. Histopathologic alterations included minor, focal mononuclear infiltrates in the liver, kidneys, and heart, as well as mineralized foci in the adrenals of some animals. These lesions were interpreted as incidental to the challenge viruses and are commonly observed in rhesus monkeys. One monkey inoculated with YF-Vax had amyloid protein deposits in the red pulp of the spleen, presumed to be a preexisting condition.
The principal histopathological findings were limited to the brain and spinal cords (Table 7). Lesion scores were significantly higher for monkeys inoculated with YF-Vax than for those given ChimeriVax-JE. The combined target-discriminator scores (±standard deviation) for YF-Vax and ChimeriVax-JE were 1.17 (±0.47) and 0.29 (±0.20), respectively (P = 0.00014, t test). Although the main symptom in monkeys with encephalitic signs was tremor, which may reflect lesions of the cerebellum, thalamic nuclei, or globus pallidus, there was clear relationship between clinical and histopathological score. The cerebellar cortex and/or nucleus dentatus and other cerebellar nuclei were evaluated histopathologically in all animals, and no lesions were found in these structures.
DISCUSSION
The chimeric virus constructed from the envelope (prM and E) genes of an attenuated JE vaccine strain (SA14-14-2) and the capsid and NS genes of YF 17D vaccine was significantly less neurovirulent for mice and rhesus monkeys than YF 17D vaccine and had a neurovirulence profile similar to that of the JE SA14-14-2 vaccine strain (32, 34). Chambers et al. (3) previously reported that a chimeric virus incorporating the prM and E genes of a wild-type (Nakayama) JE strain in the same YF 17D infectious clone had a neurovirulence profile in mice similar to that of YF 17D but was less virulent than JE Nakayama. Thus, both the prM-E and YF NS genes play a role in attenuation of ChimeriVax-JE. Ni and Barrett (21) found that attenuated JE variants bound less well to brain tissue than wild-type virus, presumably due to a mutation at amino acid residue 306 in domain III of the E protein. One amino acid in the SA14-14-2 sequence (E315 A→V) occurs in a contiguous region of domain III, and this portion of the envelope has been implicated in neurovirulence of several flaviviruses (reviewed in reference 25). However, it is unlikely that E315 determines attenuation of ChimeriVax-JE or SA14-14-2 vaccine, since some substrains of virulent parental SA14 virus have valine at this position (9, 20).
A total of six amino acid residues in the E gene distinguish JE SA14-14-2 from wild-type JE (Table 1). The contribution of these six mutations to the attenuated phenotype of JE SA14-14-2 and ChimeriVax-JE is under study in our laboratory (J. Arroyo et al., unpublished data); the results, to be published elsewhere, indicate that at least three of the six SA14-14-2-specific mutations are required for attenuation. Based on a comparison of the sequences of JE attenuated strains in the lineage of SA14-14-2 (23) and on the observation that a single site mutation at E138 attenuated wild-type JE (31), amino acids E107, E138, E176, E279, and E439 are implicated in neurovirulence. The demonstration that multiple reversions to the wild-type residues are required to restore neurovirulence and that a chimera with a fully wild-type prM-E sequence has neurovirulence similar to that of YF 17D vaccine (3) provides a large margin of safety. Moreover, the ChimeriVax-JE sequence and attenuated phenotype were stable across multiple passages in cell culture and mouse brain (9).
The safety of ChimeriVax-JE virus was rigorously studied in tests for neurotropism and viscerotropism in rhesus monkeys. Such testing is important because the chimeric virus encodes the E gene of a member of the neurotropic JE complex in the backbone of lymphotropic YF. The E gene contains determinants responsible for interaction with cell receptors, tropism, and virulence.
The histopathologic scoring methodology for neurotopism was developed by Levenbook et al. (16) and incorporated into biological standards for YF 17D vaccines (33). Since YF 17D has a long history of safe use in humans, we used a commercial vaccine (YF-Vax) as a reference against which to compare ChimeriVax-JE in the monkey neurovirulence test. The brain lesion scores in monkeys inoculated i.c. with ChimeriVax-JE were significantly lower than those in monkeys inoculated with YF-Vax (Table 7), indicating that the chimeric vaccine is likely to be even safer than YF 17D in humans. The dose of YF-Vax inoculated i.c. was slightly (two to six times) higher, but it is unlikely that this accounted for the difference in neurotropism. A previous study in which monkeys were inoculated with a dose of ChimeriVax-JE virus 250 times higher than the dose of YF-Vax also showed lower histopathological scores for ChimeriVax-JE (20).
Although over 300 million doses of YF 17D vaccine have been administered, only 21 cases of postvaccinal encephalitis to YF 17D vaccine have been reported in the literature, the majority in infants ≤4 months of age prior to restriction of use of 17D vaccine to infants over 9 months of age (18). The incidence of postvaccinal encephalitis in very young infants was estimated to be 0.5 to 4 per 1,000, whereas the risk of developing encephalitis in persons over 9 months of age is less than 1 in 8 million (18). No case of postvaccinal encephalitis has been reported with the use of JE SA14-14-2 vaccine (32). As noted above, the neurovirulence of JE SA14-14-2 virus resembles that of ChimeriVax-JE and is less than that of YF 17D.
Viscerotropism of ChimeriVax-JE was assessed by measurement of viremia after i.c. and s.c. inoculation, by clinical chemistry tests, and by histopathological examination of visceral organs of monkeys. Viremia levels were low after s.c. inoculation (Table 2) and were lower than those produced by YF 17D after i.c. injection (Fig. 2). No evidence for hepatic, renal, or myocardial or other organ dysfunction or pathology was found. Thus, no unexpected tissue tropism or altered pathogenesis was observed.
The most important aspect of this paper is the immunogenicity of ChimeriVax-JE when inoculated by the s.c. route. All monkeys given doses as low as 100 PFU developed low viremias and neutralizing antibodies and were solidly protected against challenge with wild-type JE. These results suggest that the ChimeriVax-JE vaccine will be as immunogenic as YF 17D vaccine, which elicits protective immunity when administered to monkeys at a similar dose (18). The kinetics of the neutralizing antibody response were dose dependent (Table 3). Monkeys inoculated with 4.0 or 5.0 log10 PFU of ChimeriVax-JE (a dose similar to that contained in YF 17D vaccines) seroconverted by days 6 to 7. This finding suggests that ChimeriVax-JE vaccine will elicit rapid immunity in humans. Studies of YF 17D vaccine in rhesus monkeys also demonstrated appearance of neutralizing antibodies on day 6 or 7, at which time the animals were completely refractory to challenge (29, 30).
The immunogenicity of ChimeriVax-JE virus compares favorably with that of YF 17D vaccine in rhesus monkeys. The dose of YF 17D vaccine conferring 90% protection against lethal YF challenge in rhesus monkeys is 200 mouse LD50 (estimated to be equivalent to 4.0 log10 PFU) and the 50% immunizing dose was 2 mouse LD50 (approximately 100 PFU) (reviewed in reference 18).
In our study, wild-type JE inoculation directly into the brain served as a hypervirulent challenge and severe test of effective immunity. The natural route of infection by mosquito bite resembles experimental s.c. challenge (which does not cause encephalitis [20a]), whereas experimental i.c. challenge with wild-type JE leads to uniformly lethal encephalitis (20). An alternative to i.c. challenge is intranasal (i.n.) inoculation of high virus doses, which also causes encephalitis (10, 26); the i.n. route presumably leads to direct viral spread to the brain via olfactory neurons and is thus simply a delayed form of i.c. inoculation (19). Both i.c. and i.n. challenge provide a reasonable approach to testing vaccine efficacy, since the incubation period of encephalitis is sufficiently long (8 to 12 days) to allow preexisting immunity to abrogate the infection. A vaccine capable of protecting nonhuman primates from i.c. or i.n. challenge will be even more effective against natural peripheral infection. In the immunized host, the virus inoculum injected by the infected mosquito during blood feeding would encounter neutralizing antibodies in extracellular transudate and lymph and would be inactivated before it could reach the brain or olfactory neurons. Since the inoculum is small, a low level of preexposure immunity is sufficient to protect against disease. The kinetics of JE neutralization in the presence of complement are extremely rapid. Virus that enters cells and escapes neutralization by antibody is eliminated by a second line of defense provided by cytotoxic T lymphocytes (CTLs). The E protein of JE (13) as well as NS proteins expressed by infected cells serve as targets for cell killing by CTLs. Since ChimeriVax-JE virus contains NS genes of YF 17D, anti-YF immunity (e.g., antibodies to YF 17D NS1 or CTLs directed against NS3) could modulate the immunogenicity of ChimeriVax-JE. This is an area of active study, but preliminary results in mice and monkeys have not demonstrated interference by YF immunity.
Protection against JE virus challenge is mediated principally by preformed neutralizing antibodies. In mice and monkeys, transfer of immune serum confers protection against challenge with JE (17, 35), and protection is directly proportional to the passive titer of neutralizing antibodies (17). Hosts immunized in advance of exposure also have immunological memory, by virtue of T and B cells with high-affinity antigen receptors. Subsequent infection with wild-type JE results in rapid anamnestic responses. Very small amounts of viral antigen (e.g., that contained in the mosquito saliva inoculum) are sufficient to initiate anamnestic immune responses. These memory responses to JE do not depend on replication of live virus (15); thus, even a small amount of virus injected by a mosquito and sterilized by preexisting antibody in the host can generate a boost in immunity.
Recall immunity after challenge plays a critical role in protection against JE (14). In our study, monkeys immunized with ChimeriVax-JE and challenged i.c. with wild-type JE had dramatic increases in serum neutralizing antibodies (Table 3) as well as the appearance of high neutralizing antibody titers in CSF (Table 6). The latter may have been the result of local antibody production or leakage of serum antibodies at sites of perivascular inflammation. Only 2 of 16 monkeys (AG72 and AP54) had no detectable CSF antibody response (Table 6). These animals also had no anamnestic serum antibody response to challenge (Table 3) and no evidence for residual histopathology (Table 5). We have no explanation for these observations, since the prechallenge antibody responses of these animals were not distinguishable from other monkeys. It is possible that the challenge inoculum was delivered differently (e.g., into a ventricle rather than into brain parenchyma) or that the CTL response, which was not measured, cleared infected cells at a very early stage of infection.
An important consideration for the development of new JE vaccines is antigenic variation of wild-type JE strains. All JE strains belong to a single virus serotype as defined by neutralization. Inactivated JE vaccine produced with virus strains (Nakayama and Beijing) representing a single genotype (genotype 1 [4]) protect against viruses representing the same genotype in Korea and India, as well as strains belonging to different genotypes in Thailand (4, 11) and Indonesia/Australia (4, 5, 27). Based on monoclonal antibody analysis, there is no correspondence between JE genotype and antigenic variation.
Since multiple neutralizing epitopes are distributed across all domains of the E protein of flaviviruses (25), it is likely that JE strains have evolved a degree of antigenic diversity but retain shared neutralizing epitopes representing critical functional determinants. The diversity of natural JE strains was reflected in the neutralizing antibody responses of monkeys immunized with ChimeriVax-JE expressing the E protein of a Chinese (genotype 1) virus; prechallenge neutralizing antibodies to heterologous strains were significantly lower than to the homologous vaccine and were undetectable (titer of <20) in some cases (Table 4). Postchallenge anamnestic responses to heterologous strains were not measured in this study and will be reported elsewhere; however, it is likely that these responses would be characterized by a marked broadening of the antibody response across all JE strains (7, 12, 26). Cross-protection between heterologous members of the JE antigenic complex has been repeatedly demonstrated (8). In the present study, ChimeriVax-JE vaccine produced from a Chinese virus strain (JE SA14-14-2) protected against challenge with a JE strain of Japanese origin; in the future, protection against challenge with JE strains occurring elsewhere in Asia (and having recognized genotypic differences) may be a useful component of the preclinical testing program. The heterotypic nature of the anamnestic neutralizing antibody response may in fact be the most critical determinant of protection against JE antigenic variants and precludes the requirement to include multiple strains in an effective vaccine.
ACKNOWLEDGMENTS
We are grateful to K. Eckels, R. Olson, and T. C. Tsakounis (Walter Reed Army Medical Research Institute, Silver Spring, Md.) and to Gwen Myers, Simon Delagrave, Keith Wells, Diane Silva, Michael Knauber, Robert Schrader, Jennifer Ingrassia, Glenn Drabik, Jason Gale, Peter Antoniuk, and Ron Marchesani (OraVax Inc.) for assistance in preclinical studies, production, and quality control of ChimeriVax-JE. We thank Michelle M. Mazzone, Glen Stevenson, Craig Schmidt, and Pat Lappin (Sierra BioMedical) for technical assistance in the monkey toxicological tests and tissue preparation. Farrukh Rizvi (Pasteur Mérieux Connaught) assisted in various aspects of the study and procured YF-Vax for control of the candidate vaccine.
REFERENCES
- 1.Aihara S, Rao C M, Yu Y X. Identification of mutations that occurred on the genome of Japanese encephalitis virus during the attenuation process. Virus Genes. 1991;5:95–109. doi: 10.1007/BF00571925. [DOI] [PubMed] [Google Scholar]
- 2.Chambers T J, Tsai T F, Pervikov Y, Monath T P. Vaccine development against dengue and Japanese encephalitis: report of a World Health Organization meeting. Vaccine. 1997;15:1494–1502. doi: 10.1016/s0264-410x(97)00195-3. [DOI] [PubMed] [Google Scholar]
- 3.Chambers T J, Nestorowicz A, Mason P W, Eckels K H, Rice C M. Yellow fever/Japanese encephalitis chimeric viruses: construction and biological properties. J Virol. 1999;73:3095–3101. doi: 10.1128/jvi.73.4.3095-3101.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chen W R, Tesh R B, Rico-Hesse R. Genetic variation of Japanese encephalitis virus in nature. J Gen Virol. 1990;71:2915–2920. doi: 10.1099/0022-1317-71-12-2915. [DOI] [PubMed] [Google Scholar]
- 5.Chen W R, Rico-Hesse R, Tesh R B. A new genotype of Japanese encephalitis from Indonesia. Am J Trop Med Hyg. 1992;47:61–69. doi: 10.4269/ajtmh.1992.47.61. [DOI] [PubMed] [Google Scholar]
- 6.Chung Y J, Nam J H, Ban S J, Cho H W. Antigenic and genetic analysis of Japanese encephalitis viruses isolated from Korea. Am J Trop Med Hyg. 1996;55:91–97. doi: 10.4269/ajtmh.1996.55.91. [DOI] [PubMed] [Google Scholar]
- 7.Edelman R, Nisalak A, Pariyanonda A, Udomsakdi S, Johnsen D O. Immunoglobulin response and viremia in dengue-vaccinated gibbons repeatedly challenged with Japanese encephalitis virus. Am J Epidemiol. 1973;97:208–218. doi: 10.1093/oxfordjournals.aje.a121501. [DOI] [PubMed] [Google Scholar]
- 8.Goverdhan M K, Kulkarni A B, Gupta A K, Tupe C D, Rodrigues J J. Two-way cross-protection between West Nile and Japanese encephalitis viruses in bonnet macaques. Acta Virol. 1992;36:277–283. [PubMed] [Google Scholar]
- 9.Guirakhoo F, Zhang Z, Chambers T J, Delagrave S, Arroyo J, Barrett A D T, Monath T P. Immunogenicity, genetic stability and protective efficacy of a recombinant, chimeric yellow fever-Japanese encephalitis virus (Chimerivax™-JE) as a live, attenuated vaccine candidate against Japanese encephalitis. Virology. 1999;257:363–372. doi: 10.1006/viro.1999.9695. [DOI] [PubMed] [Google Scholar]
- 10.Harrington D G, Hilmas D E, Elwell M R, Whitmire R E, Stephen E L. Intranasal infection of monkeys with Japanese encephalitis virus: clinical response and treatment with a nuclease-resistant derivative of poly(I)-poly(C) Am J Trop Med Hyg. 1977;26:1191–1198. doi: 10.4269/ajtmh.1977.26.1191. [DOI] [PubMed] [Google Scholar]
- 11.Hoke C H, Nisalak A, Sangawhipa N, Jatanasen S, Laorakapongse T, Innis B L, Kotchasenee S, Gingrich J B, Latendresse J, Fukai K, Burke D S. Protection against Japanese encephalitis by inactivated vaccines. N Engl J Med. 1988;319:608–614. doi: 10.1056/NEJM198809083191004. [DOI] [PubMed] [Google Scholar]
- 12.Kayser M, Klein H, Paasch I. Human antibody response to immunization with 17D yellow fever and inactivated TBE vaccine. J Med Virol. 1985;17:35–44. doi: 10.1002/jmv.1890170106. [DOI] [PubMed] [Google Scholar]
- 13.Konishi E, Yamaoka M, Khin-Sane-Win, Kurane I, Mason P W. Induction of protective immunity against Japanese encephalitis with a plasmid encoding Japanese encephalitis virus premembrane and envelope genes. J Virol. 1998;72:4925–4930. doi: 10.1128/jvi.72.6.4925-4930.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Konishi E, Yamaoka M, Kin-Sane-Win, Kurane I, Takada K, Mason P W. The anamnestic neutralizing antibody response is critical for protection of mice from challenge following vaccination with a plasmid encoding the Japanese encephalitis virus premembrane and envelope genes. J Virol. 1999;73:5527–5534. doi: 10.1128/jvi.73.7.5527-5534.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee H W, Scherer W F. The anamnestic antibody response to Japanese encephalitis virus in monkeys and its implications concerning naturally acquired immunity in man. J Immunol. 1961;86:151–164. [PubMed] [Google Scholar]
- 16.Levenbook I S, Pelleu L J, Elisberg B L. The monkey safety test for neurovirulence of yellow fever vaccines: the utility of quantitative clinical evaluation and historical examination. J Biol Stand. 1987;15:305–313. doi: 10.1016/s0092-1157(87)80003-3. [DOI] [PubMed] [Google Scholar]
- 17.Lubiniecki A S, Cypess R H, Hammon W M. Passive immunity for arbovirus infection. Am J Trop Med Hyg. 1973;22:535–542. doi: 10.4269/ajtmh.1973.22.535. [DOI] [PubMed] [Google Scholar]
- 18.Monath T P. Yellow fever. In: Plotkin S, Orenstein W, editors. Vaccines. W. B. Philadelphia, Pa: Saunders and Company; 1999. pp. 815–879. [Google Scholar]
- 19.Monath T P, Cropp C B, Harrison A K. Mode of entry of a neurotropic arbovirus into the central nervous system: reinvestigation of an old controversy. Lab Investig. 1983;48:399–410. [PubMed] [Google Scholar]
- 20.Monath T P, Soike K, Levenbook I, Zhang Z-X, Arroyo J, Delagrave S, Myers G, Barrett A D T, Shope R E, Chambers T J, Guirakhoo F. Recombinant, chimaeric live, attenuated vaccine (ChimeriVax™) incorporating the envelope genes of Japanese encephalitis (SA14-14-2) virus and the capsid and nonstructural genes of yellow fever (17D) virus is safe, immunogenic and protective in non-human primates. Vaccine. 1999;17:1869–1882. doi: 10.1016/s0264-410x(98)00487-3. [DOI] [PubMed] [Google Scholar]
- 20a.Morris J A, Connor J R, Smadel J E. Infection and immunity patterns in monkeys injected with viruses of Russian spring-summer and Japanese encephalitis. Am J Hyg. 1955;62:327–341. doi: 10.1093/oxfordjournals.aje.a119782. [DOI] [PubMed] [Google Scholar]
- 20b.National Institutes of Health. Guide for the care and use of laboratory animals, rev. ed. Department of Health and Human Services publication no. (NIH) 85-23. Bethesda, Md: National Institutes of Health; 1985. [Google Scholar]
- 21.Ni H, Barrett A D T. Attenuation of Japanese encephalitis virus by selection of its mouse brain membrane receptor preparation escape variants. Virology. 1998;241:30–36. doi: 10.1006/viro.1997.8956. [DOI] [PubMed] [Google Scholar]
- 22.Ni H, Burns N J, Chang G-J, Zhang M-J, Wills M R, Trent D W, Sanders P G, Barrett A D T. Comparison of nucleotide and deduced amino acid sequence of the 5′ non-coding region and structural protein genes of the wild-type Japanese encephalitis virus strain SA14 and its attenuated vaccine derivatives. J Gen Virol. 1994;75:1505–1510. doi: 10.1099/0022-1317-75-6-1505. [DOI] [PubMed] [Google Scholar]
- 23.Ni H, Chang G-J, Xie H, Trent D W, Barrett A D T. Molecular basis of attenuation of neurovirulence of wild-type Japanese encephalitis virus strain SA14. J Gen Virol. 1995;76:409–413. doi: 10.1099/0022-1317-76-2-409. [DOI] [PubMed] [Google Scholar]
- 24.Nitayaphan S, Grant J G, Chang G-J, Trent D W. Nucleotide sequence of virulent SA14 strain of Japanese encephalitis and its attenuated derivative. Virology. 1990;177:541–552. doi: 10.1016/0042-6822(90)90519-w. [DOI] [PubMed] [Google Scholar]
- 25.Rey F A, Heinz F X, Mandl C, Kunz C, Harrison S C. The envelope glycoprotein from tick-borne encephalitis virus at 2 A resolution. Science. 1995;375:291–195. doi: 10.1038/375291a0. [DOI] [PubMed] [Google Scholar]
- 26.Raengsakulrach B, Nisalak A, Gettayacamin M, Thirawuth V, Young G D, Myint K S A, Ferguson L M, Hoke C H, Jr, Innis B L, Vaughn D W. An intranasal challenge model for testing Japanese encephalitis vaccines in rhesus monkeys. Am J Trop Med Hyg. 1999;60:329–337. doi: 10.4269/ajtmh.1999.60.329. [DOI] [PubMed] [Google Scholar]
- 27.Ritchie S A, Phillips D, Broom A, Mackenzie J, Poidinger M, van den Hurk A. Isolation of Japanese encephalitis virus from Culex annulirostris in Australia. Am J Trop Med Hyg. 1997;56:80–84. doi: 10.4269/ajtmh.1997.56.80. [DOI] [PubMed] [Google Scholar]
- 28.Rozensweig E C, Babione R W, Wisseman C L., Jr Immunological studies with group B arthropod-borne viruses. Am J Trop Med Hyg. 1963;12:232–241. [PubMed] [Google Scholar]
- 29.Smithburn K C. Immunology of yellow fever. In: Smithburn K C, et al., editors. Yellow fever vaccination. Geneva, Switzerland: World Health Organization; 1956. pp. 11–27. [Google Scholar]
- 30.Smithburn K C, Mahaffy A F. Immunization against yellow fever. Am J Trop Med. 1945;45:217–226. [Google Scholar]
- 31.Sumiyoshi H, Tignor G H, Shope R E. Characterization of a highly attenuated Japanese encephalitis virus generated from molecularly cloned cDNA. J Infect Dis. 1995;171:1144–1151. doi: 10.1093/infdis/171.5.1144. [DOI] [PubMed] [Google Scholar]
- 32.Tsai T F, Chang G-J J, Yu Y-X. Japanese encephalitis vaccines. In: Plotkin S, Orenstein W, editors. Vaccines. W. B. Philadelphia, Pa: Saunders and Company; 1999. pp. 672–710. [Google Scholar]
- 32a.Wills M R, Sil B K, Lao T X, Barrett A D T. Antigenic characterization of the live attenuated Japanese encephalitis virus SA14-14-2: a comparison with isolates of virus covering a wide geographic area. Vaccine. 1992;10:861–872. doi: 10.1016/0264-410x(92)90051-k. [DOI] [PubMed] [Google Scholar]
- 33.World Health Organization. Requirements for yellow fever vaccine (Requirements for Biological Substances no. 3, revised 1995) WHO Tech Rep Ser. 1998;872:31–68. [PubMed] [Google Scholar]
- 34.Yu Y-X, Wu P F, Ao J, Liu L H, Lim H M. Selection of a better immunogenic and highly attenuated live vaccine virus strain of JE. I. Some biological characteristics of SA14-14-2 mutant. Chin J Microbiol Immunol. 1981;1:77–84. [Google Scholar]
- 35.Zhang M, Wang M, Jiang S, Ma W. Passive protection of mice, goats, and monkeys against Japanese encephalitis with monoclonal antibodies. J Med Virol. 1989;29:133–138. doi: 10.1002/jmv.1890290211. [DOI] [PubMed] [Google Scholar]