Abstract
Background
Epithelial sinonasal cancers (SNC) are rare tumours with recognized associations with known/suspected occupational carcinogens (wood/leather dust, nickel/chromium compounds and formaldehyde). In Italy, a national SNC registry organized as a network of regional registries was established by law in 2008.
Aims
To describe SNC time trends, occupational exposures and geographical distribution in Lombardy, North-West Italy, based on population registry data (2008–20).
Methods
The Lombardy SNC Registry records epithelial SNCs using various sources. Interviews to collect occupational history are performed using a standardized questionnaire. Using several standard populations, we calculated yearly crude and age-standardized rates (ASRs per 100,000 person-years). Standardized incidence ratios (SIR) at municipality level were calculated, and Bayesian models were fitted to produce smoothed SIR maps.
Results
We recorded 827 cases (553 men, 274 women). Crude (world standardized) ASRs were 0.9 (0.4) in men and 0.4 (0.2) in women, with no time trends. Interviews were obtained for 485 (88%) men and 223 (81%) women. Among men, 217 (45%) had been exposed to occupational carcinogens (wood/leather dust: 150/65 cases, 31%/13%), while only 36 women (16%) were exposed. Among 201 men with adenocarcinoma, exposure to wood/leather dust occurred in 103/50 cases (75%/50%). Areas with elevated SIRs associated with leather dust were found in the Western areas. Exposure to wood dust was more widespread.
Conclusions
This study found a high frequency of occupational exposures (wood and leather dust), particularly in men with SNC. Employment in shoe industries clustered in the Western part, while work in furniture industries was less spatially structured.
Sinonasal cancers (SNC) are rare tumours with recognized or suspected associations with several occupational carcinogens. We investigated SNC cases using population-based registry data in a large (10 million people) Italian region. We found high frequencies of exposure to wood and leather dust, particularly in men. We identified areas with high incidence associated with exposure to leather dust in shoe factories, while more widespread were SNC excesses associated with wood dust in furniture manufacturing.
Key learning points.
What is already known about this subject:
Sinonasal cancers are associated with exposure to several occupational agents, including wood and leather dust, nickel, hexavalent chromium compounds and formaldehyde.
In Italy, a national sinonasal cancer registry was officially established by law in 2008 to monitor sinonasal cancer incidence and evaluate occupational exposure.
What this study adds:
We identified sinonasal cancer excesses in a few municipalities concentrated in the Western areas, mostly associated with exposure to leather dust in shoe factories. Excesses associated with wood dust in carpentries and joineries or in furniture manufacturing were more diffusely distributed in many municipalities.
We confirmed that the main carcinogens for sinonasal cancers are wood and leather dust (particularly in men with adenocarcinoma), while few individuals have been exposed to nickel and chromium compounds or formaldehyde.
Sinonasal cancer incidence did not decrease over time: this might suggest that preventive measures to reduce wood and leather dust in work environments were not sufficient or that their effectiveness is not evident yet due to the long latency time.
What impact this may have on practice or policy:
Implementing population-based registries allows complete and accurate identification of sinonasal cancer occurrence and their association with occupational exposures.
This allows the recognition of occupational diseases and compensation of affected subjects.
Our results suggest there is a need to control exposure to wood and leather dust in selected industries.
INTRODUCTION
Sinonasal cancers (SNCs) are rare malignant tumours representing around 0.2% of all cancers and 3%–5% of cancers of the upper respiratory tract [1]. They include cancers of the nasal cavity (the majority) and paranasal sinuses (mostly the maxillary sinus) [2]. SNCs include histologically different groups [3], squamous cell carcinoma (SCC) and adenocarcinoma (mainly intestinal-type adenocarcinoma, ITAC) being the most common subtypes. Incidence is around 1 case per 100 000 person-years, and 5-year overall survival range is between 30% and 50%. SNCs arise more often in older adults and affect men almost twice more frequently than women [2]. Because of the anatomic location, SNCs can produce significant morbidity resulting from the involvement of nearby structures (orbits, olfactory nerves, facial nerves and intracranial space) [4].
The low absolute occurrence in the general population contrasts with the high relative risks (RRs) for specific exposures at workplaces; the occupation-attributable fraction is estimated to be in the range of 20%–46% [5,6]. The International Agency for Research on Cancer identified several agents with sufficient evidence of carcinogenicity in humans for SNCs, including wood and leather dust, nickel compounds and tobacco smoking. Other exposure circumstances or agents with limited evidence in humans for SNCs include carpentry and joinery, working in textile manufacturing industry, and hexavalent chromium compounds and formaldehyde [7]. The association of SNC adenocarcinoma with exposure to wood and leather dust is particularly strong [8].
The well-defined relationship with exposure to specific occupational carcinogens provides strong support for developing a specific epidemiological surveillance of SNC incidence. In Italy, it is mandatory since 2008, by means of a national SNC registry (Registro Nazionale Tumori Naso-Sinusali, ReNaTuNS) established at the National Institute for Insurance Against Accidents at Work (Istituto Nazionale per l’Assicurazione contro gli Infortuni sul Lavoro: INAIL). Currently, the registry covers more than 85% of the national population. It is dedicated to collect clinical information of SNC cases, to identify those of occupational origin (for compensation purposes and identifying areas and workplaces at increased risk), and estimate incidence rates [9]. ReNaTuNS is organized as a network of local registries officially named Regional Operating Centers (Centri Operativi Regionali: COR), which search for SNC cases in their health care centres and assess previous exposure to occupational carcinogenic agents through interviews.
The objective of this study is to describe SNC incidence in the Lombardy Region, North-West Italy, one of the most industrialized Italian Regions and the most populated (about 10 million people out of 60 million Italians; Figure 1, available as Supplementary data at Occupational Medicine Online). Using data from the Lombardy SNC registry (2008–20), in this paper, we report time trends, occupational exposures to carcinogens and geographical distribution.
METHODS
The Lombardy SNC Registry has been operating since 2008 [10]. It identifies and collects information on all SNC cases occurring among subjects residing in the region at the time of first diagnosis. Reporting SNC cases to the registry is compulsory by law (law 81/2008). Primary sources of information on SNC cases are the departments of diagnosis and treatment of SNC in the regional hospitals (more than 100), particularly pathology, otolaryngology, maxillofacial surgery and radiotherapy. The completeness of reporting is checked by periodic linkage with databases of pathology departments and hospital admissions (also in hospitals outside Lombardy). Auxiliary sources include mortality and occupational disease compensation data. Diagnosis of SNC is verified by the Registry based on complete clinical information and finally evaluated as certain or probable according to ReNaTuNS guidelines [9,11].
More specifically, the Registry includes all newly diagnosed primary malignant epithelial cancers of the nasal cavity (code C30.0 of the International Classification of Diseases, Tenth Revision, ICD-10) and paranasal sinuses (ICD-10 codes from C31.0 to C31.9). Histological types are coded according to the International Classification of Diseases for Oncology, third edition (ICD-O-3) and grouped according to the World Health Organization (WHO) classification [3].
For confirmed cases, trained interviewers administered a standardized questionnaire to patients or their next of kin to collect lifetime occupational history and assess occupational and extra-occupational exposure to agents with an established or suspected association with SNC.
Since reporting SNC cases to the registry is compulsory by law (law 81/2008), ethics approval and consent for participation are not required.
For this study, we included all cases diagnosed in 2008–20 (years in which case collection was completed). For evaluating incidence time trends, we calculated yearly crude and age-standardized rates (ASR, per 100 000 person-years) and 90% confidence intervals (CIs) [12] using different standard populations (Lombardy 2008, Italy 2001, Europe 2013 and world Segi’s populations) [13]. Rates by province of residence were calculated using as standard the Italian population 2001. We used chi-squared tests to analyse categorical variables across gender, tumour morphology and tobacco smoking. Age distribution across gender was evaluated using Wilcoxon rank-sum (Mann–Whitney) test. These analyses were performed with Stata 18 [14]. In interpreting results, we followed the current recommendations of important international scientific associations: we did not stick to the usual statistical threshold of 0.05 and provided 90% CIs [12,15].
We used internal indirect standardization to analyse spatial patterns of disease at a small area (municipality) level [16]. Overall reference rates of Lombardy, 2008–20, specific by gender and age (18 classes: 0–4, … , 85+), were used to compute the expected number of cases for each municipality. This is common in spatial analysis to make the spatial pattern of the disease interpretable: the regional mean RR is set to 1 and each municipality has a risk which could be higher or lower. Let area counts of disease Yi (i = 1, … , 1546) follow a Poisson distribution with mean Eiθi, where Ei is the expected number of cases under indirect standardization and θi is the RR. The maximum-likelihood estimator of θi is the standardized incidence ratio (SIRi = Yi/Ei). The precision of the SIR is proportional to Ei. To describe the geographical pattern of disease risk at municipality level, we first mapped SIRs.
Then, we used two hierarchical Bayesian models to smooth SIRs, the Poisson-Gamma (PG) [17] and the Besag-York-Mollié (BYM) [18]. These models account for overdispersion and stabilize RR estimates. With sparse geographical data, there will be large sampling variability: municipalities with small populations and hence few expected cases (for rare tumours like SNC even well below 1) may have extremely unstable SIRs ranging from 0 (when there are no observed cases) to extremely large values. Bayesian models allow efficient filtering out of sampling variability and for systematic patterns to emerge. Indeed, the Bayesian estimator of RR can be viewed as a weighted average between the maximum-likelihood estimator—the SIR in our case—and a suitable prior guess. The PG model assumes as prior guess the overall risk among all the observations: since we used internal indirect standardization the overall risk for the entire Lombardy Region would be 1. The BYM model beyond the overall regional risk additionally includes as prior guess for each municipality also the average risk among adjacent municipalities.
Specification of appropriate prior distributions on model parameters is required in Bayesian inference. In the PG model, a Gamma(k,ν) prior distribution is assumed for θi and an exponential distribution for the hyperparameters k and ν. Through this model, posterior RR estimates of θi are shrunken towards the general mean [17].
In the BYM formulation, a random effect log-linear model is assumed for the RR: log(θi) = ui + vi. The term ui, called heterogeneity random term, captures the unstructured spatial variability, while vi, called clustering random term, captures the spatially structured overdispersion. The a priori distribution for ui is assumed to be Normal (0, λu), λu representing the precision. The random term vi is the structured spatial variability component, assumed a priori to follow an Intrinsic Conditional Autoregressive (ICAR) model. In simpler terms, denoting Si as the set of areas adjacent to the ith one, we assume that the distribution of vi|vj ∈ Si follows a Normal(</mathgraphic>, λvni) distribution, with being the mean of the terms of areas adjacent to the ith one, and λvni representing the precision, which is dependent on ni, the number of areas in Si. The standard first-order binary weighting scheme assigns a weight equal to 1 if i, j are adjacent (or 0 if not). The hyper-prior distributions of the precision parameters λv and λu are assumed to be Gamma (0.5, 0.0005). The BYM model shrinks the RR estimates toward the local (i.e. the adjacent municipalities) and the general (i.e. the Lombardy Region) mean through these two random terms [18]. For all the models described, the marginal posterior distributions of the parameters of interest were approximated by Monte Carlo Markov chain (MCMC) methods. We used WinBUGS software for MCMC analyses [19].
Finally, we selected municipalities with a PG posterior probability of RR > 1 greater than 70% in one or both genders, and reviewed the occupational information collected at interviews of residents in those municipalities. We chose to report PG posterior probabilities because in the trade-off between noise and bias the PG model is less biased than BYM model [20].
RESULTS
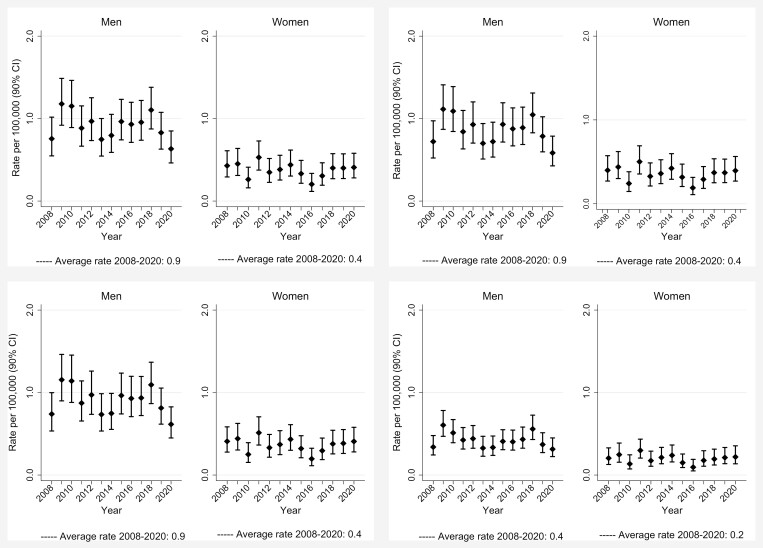
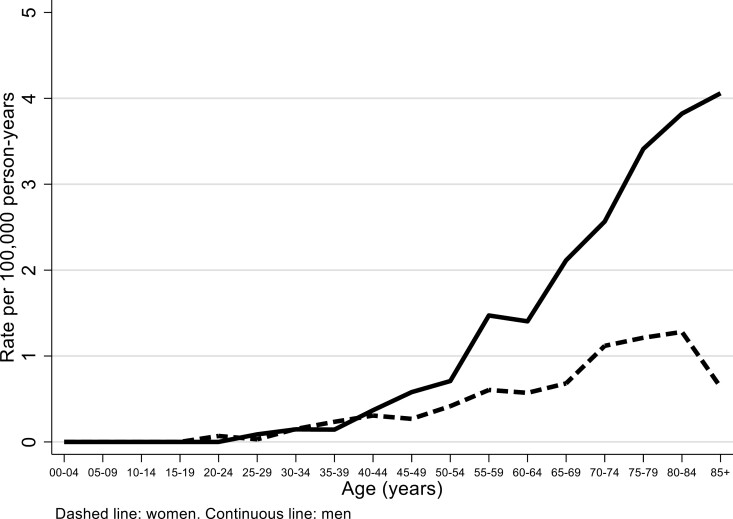
In the period 2008–20, we recorded 827 SNC cases, 553 in men (42.5 per year on average, crude rate 0.9 per 100 000) and 274 in women (21.1 per year, crude rate 0.4) (Table 1). Overall regional ASRs using Lombardy, Italy and European standard population were identical to crude rates, while world ASRs were about half (Figure 1); 90% CIs for the overall rates (2008–20) coincided or were at most ±0.1 the rate estimate. There were no monotonic trends of incidence rates over the years. No clear trends were evident for number of cases, either overall or for different morphological types (Figures 2 and 3, available as Supplementary data at Occupational Medicine Online). Incidence increased with age, especially in men (Figure 2).
Table 1.
Number of cases, person-years (PY), crude rates and age-standardized rates (ASR, per 100,000 person-years, age 0–99 years) of sinonasal cancers (SNC) by gender and year of diagnosis, Lombardy SNC Registry, 2008–20
| Rates | |||||||
|---|---|---|---|---|---|---|---|
| Gender/year | Cases | Person-years | Crude | ASR Lombardy | ASR Italy | ASR Europe | ASR World |
| Men | |||||||
| 2008 | 32 | 4 711 487 | 0.7 | 0.8 | 0.7 | 0.7 | 0.3 |
| 2009 | 52 | 4 762 370 | 1.1 | 1.2 | 1.1 | 1.2 | 0.6 |
| 2010 | 49 | 4 802 363 | 1.0 | 1.2 | 1.1 | 1.1 | 0.5 |
| 2011 | 40 | 4 844 524 | 0.8 | 0.9 | 0.8 | 0.9 | 0.4 |
| 2012 | 42 | 4 711 292 | 0.9 | 1.0 | 0.9 | 1.0 | 0.4 |
| 2013 | 33 | 4 764 897 | 0.7 | 0.7 | 0.7 | 0.7 | 0.3 |
| 2014 | 36 | 4 866 278 | 0.7 | 0.8 | 0.7 | 0.7 | 0.3 |
| 2015 | 46 | 4 881 615 | 0.9 | 1.0 | 0.9 | 1.0 | 0.4 |
| 2016 | 44 | 4 886 543 | 0.9 | 0.9 | 0.9 | 0.9 | 0.4 |
| 2017 | 47 | 4 894 363 | 1.0 | 1.0 | 0.9 | 0.9 | 0.4 |
| 2018 | 57 | 4 907 685 | 1.2 | 1.1 | 1.0 | 1.1 | 0.6 |
| 2019 | 42 | 4 863 558 | 0.9 | 0.8 | 0.8 | 0.8 | 0.4 |
| 2020 | 33 | 4 875 282 | 0.7 | 0.6 | 0.6 | 0.6 | 0.3 |
| 2000–20 | 553 | 62 772 257 | 0.9 | 0.9 | 0.9 | 0.9 | 0.4 |
| Women | |||||||
| 2008 | 23 | 4 930 919 | 0.5 | 0.4 | 0.4 | 0.4 | 0.2 |
| 2009 | 24 | 4 980 306 | 0.5 | 0.5 | 0.4 | 0.4 | 0.2 |
| 2010 | 15 | 5 023 778 | 0.3 | 0.3 | 0.2 | 0.3 | 0.1 |
| 2011 | 28 | 5 073 190 | 0.6 | 0.5 | 0.5 | 0.5 | 0.3 |
| 2012 | 19 | 4 989 589 | 0.4 | 0.3 | 0.3 | 0.3 | 0.2 |
| 2013 | 21 | 5 029 628 | 0.4 | 0.4 | 0.4 | 0.4 | 0.2 |
| 2014 | 25 | 5 107 119 | 0.5 | 0.4 | 0.4 | 0.4 | 0.2 |
| 2015 | 19 | 5 121 000 | 0.4 | 0.3 | 0.3 | 0.3 | 0.1 |
| 2016 | 12 | 5 121 806 | 0.2 | 0.2 | 0.2 | 0.2 | 0.1 |
| 2017 | 17 | 5 124 803 | 0.3 | 0.3 | 0.3 | 0.3 | 0.2 |
| 2018 | 23 | 5 128 573 | 0.4 | 0.4 | 0.4 | 0.4 | 0.2 |
| 2019 | 23 | 5 072 170 | 0.5 | 0.4 | 0.4 | 0.4 | 0.2 |
| 2020 | 25 | 5 079 760 | 0.5 | 0.4 | 0.4 | 0.4 | 0.2 |
| 2008–20 | 274 | 65 782 641 | 0.4 | 0.4 | 0.4 | 0.4 | 0.2 |
ASR Lombardy: standard Lombardy population 2008.
ASR Italy: standard Italian population 2001.
ASR Europe: standard European population 2013.
ASR World: standard world (Segi’s) population.
Figure 1.
Age-standardized rates (ASR, per 100 000 person-years, age 0–99 years) of sinonasal cancers (SNC) by gender and year of diagnosis, Lombardy SNC Registry, Italy, 2008–20. Top left panels: Standard Lombardy 2008: top right panels: standard Italy 2001; bottom left panels: standard Europe 2013; bottom right panels: standard world.
Figure 2.
Age-specific sinonasal cancer (SNC) rates (per 100 000 person-years) by gender, Lombardy SNC Registry, 2008–20.
Median ages at diagnosis were quite similar in men and women (Table 2). More than one-third of tumours originate from the nasal cavity. Origin from maxillary sinus was more frequent in women (26% versus 17%), while ethmoidal tumours were more frequent in men (13% versus 8%). SNCs involving multiple sites were about 25% in both genders. Most tumours were SCC and variants. Adenocarcinomas were more frequent in men (214, 39% versus 78, 28% in women): of these, ITAC were 135 (24%) in men and 18 (7%) in women. In patients with histologically verified cancers of nasal cavities (n = 312) or maxillary sinus (n = 163), frequencies of SCC and variants were 190 (61%) and 106 (65%), respectively. Conversely, out of 91 histologically verified cancers of the ethmoidal sinus, 52 (57%) were adenocarcinomas. In 217 patients with SNC involving multiple sites, 98 (45%) were adenocarcinomas. Interviews were obtained for 88% of men and 82% of women, mainly from SNC patients. Among 485 interviewed men, 217 (45%) had been ever exposed to carcinogens at workplace, mostly wood dust (including two women exposed to cork dust) and leather dust, while exposed women were much less (36 out of 223, 16%). Median length of exposure was 28 years in men and 12 in women, while median time since first exposure (‘latency’) was 56 years in men and 52 in women. Ever smokers were 66% among males and 47% in women. Smokers were more frequent in the 371 subjects with SCC and variants (261, 70%) than in 283 with adenocarcinoma (166, 59%) and in 109 with other morphologies (68, 62%).
Table 2.
Characteristics of subjects with sinonasal cancer (SNC) by gender, Lombardy Region SNC Registry, Italy, 2008–20
| Men | Women | P valuea | |
|---|---|---|---|
| n (%) | n (%) | ||
| All subjects | 553 (100) | 274 (100) | |
| Age (median, min-max) | 69.0 (25.7–93.1) | 68.3 (21.1–99.7) | 0.18 |
| Cancer site (ICD-10 code) | |||
| Nasal cavity (C30.0) | 215 (39) | 99 (36) | 0.005 |
| Maxillary sinus (C31.0) | 95 (17) | 72 (26) | |
| Ethmoidal sinus (C31.1) | 73 (13) | 21 (8) | |
| Frontal sinus (C31.2) | 2 (0) | 3 (1) | |
| Sphenoidal sinus (C31.3) | 13 (2) | 11 (4) | |
| Multiple sites (C31.8) | 155 (28) | 68 (25) | |
| Cancer morphology (WHO 2017) | |||
| Squamous cell carcinoma and variants | 255 (46) | 145 (53) | 0.03 |
| Adenocarcinoma | 214 (39) | 78 (28) | |
| Neuroendocrine carcinoma | 19 (3) | 7 (3) | |
| Other | 55 (10) | 32 (12) | |
| Malignant tumour | 2 (0) | 2 (1) | |
| Unknown | 8 (1) | 10 (4) | |
| Interview | |||
| Patient | 352 (64) | 159 (58) | 0.05 |
| Relative | 133 (24) | 64 (23) | |
| Not performed | 68 (12) | 51 (19) | |
| Occupational exposureb | |||
| Never exposed | 268 (55) | 187 (84) | |
| Ever exposed (any agent) | 217 (45) | 36 (16) | <0.001 |
| Wood dust | 150 (31) | 13 (6) | <0.001 |
| Leather dust | 65 (13) | 20 (9) | 0.09 |
| Nickel compounds | 9 (2) | 3 (1) | 0.63 |
| Chromium compounds | 18 (4) | 3 (1) | 0.09 |
| Formaldehyde | 3 (1) | 1 (0) | 0.78 |
| Cigarette smoking | |||
| Never | 154 (28) | 122 (45) | <0.001 |
| Former | 207 (37) | 55 (20) | |
| Current | 162 (29) | 74 (27) | |
| Unknown | 30 (5) | 23 (8) | |
ICD-10, International Classification of Diseases, 10th Edition.
aFrom chi-squared test, except for age (Wilcoxon rank-sum test).
bPercentages calculated taking as denominators interviewed subjects (485 men, 223 women).
In men, most (151, 75%) of the 201 patients with adenocarcinoma had been occupationally exposed to carcinogens, mainly to wood (103, 51%) or leather (50, 25%) dust (Table 3). Exposure proportions were lower in patients with SCC or other morphologies. In women, 16 (23%) of the 69 patients with adenocarcinoma had been occupationally exposed to carcinogens, to leather (11, 16%) or wood (6, 9%) dust. Exposure proportions were lower in patients with SCC or other morphologies.
Table 3.
Occupational exposure to carcinogenic agents among subjects with sinonasal cancer (SNC)a, by gender and tumour morphology, Lombardy Region SNC Registry, Italy, 2008–20
| SCC | Adenocarcinoma | Otherb | P valuec | |
|---|---|---|---|---|
| Occupational exposure | n (%) | n (%) | n (%) | |
| Men | 220 (100) | 201 (100) | 60 (100) | |
| Never exposed | 176 (80) | 50 (25) | 40 (67) | |
| Ever exposed (any agent) | 44 (20) | 151 (75) | 20 (33) | <0.001 |
| Ever exposed to: | ||||
| Wood dust | 31 (14) | 103 (51) | 15 (25) | <0.001 |
| Leather dust | 8 (4) | 50 (25) | 6 (10) | <0.001 |
| Nickel compounds | 3 (1) | 3 (1) | 3 (5) | 0.16 |
| Chromium compounds | 7 (3) | 7 (3) | 2 (3) | 0.98 |
| Formaldehyde | 1 (0) | 2 (1) | 0 (0) | 0.63 |
| Women | 117 (100) | 69 (100) | 32 (100) | |
| Never exposed | 102 (87) | 53 (77) | 27 (84) | |
| Ever exposed (any agent) | 15 (13) | 16 (23) | 5 (16) | 0.18 |
| Ever exposed to: | ||||
| Wood dust | 6 (5) | 6 (9) | 1 (3) | 0.47 |
| Leather dust | 6 (5) | 11 (16) | 3 (9) | 0.05 |
| Nickel compounds | 1 (1) | 0 (0) | 2 (6) | 0.03 |
| Chromium compounds | 2 (2) | 0 (0) | 1 (3) | 0.41 |
| Formaldehyde | 1 (1) | 0 (0) | 0 (0) | 0.65 |
A subject may have been exposed to more than one agent in his/her occupational history. SCC, squamous cell carcinoma and variants.
aIncluded: 699 subjects (481 men, 218 women) with interview and morphology.
bIncluded: neuroendocrine carcinoma, other morphology, malignant tumour.
cFrom chi-squared test.
The highest SNC incidence rates were in the Provinces of Pavia (men) and Sondrio (both genders) (Figure 4, available as Supplementary data at Occupational Medicine Online).
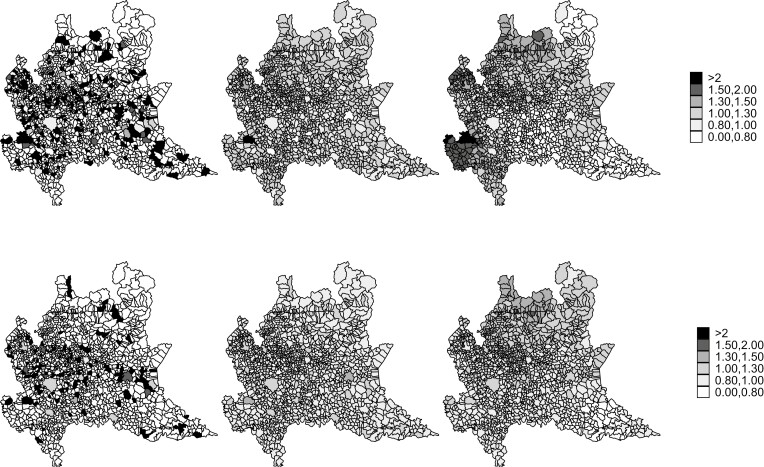
Maps of SIRs by municipality show no clear patterns due to high random variability (Figure 3, left panels). The shrinking effect of Bayesian estimators was evident: Bayesian posterior RRs of areas with few expected counts and extreme SIR were regressed to the mean in both PG (Figure 3, middle panels) and BYM (Figure 3, right panels) models. In men, smoothed RR maps show three areas with higher RRs: South-West (Province of Pavia), North-West (Province of Varese) and North (Province of Sondrio), and a central area covering different Provinces. In women, the geographical pattern of risk is less clear, although a central area in various Provinces appears to be at high RRs (Figure 3, bottom panels). These results are consistent with the distribution of posterior probabilities of RR > 1 (Figure 5, available as Supplementary data at Occupational Medicine Online), that is, the probability of a given municipality to be in excess compared to the regional mean of 1.
Figure 3.
Maps of standardized incidence ratios (SIR) and posterior relative risks (RR) of sinonasal cancers (SNC) by gender and municipality of residence at diagnosis, Lombardy SNC Registry, Italy, 2008–20. Top panels: men; bottom panels: women; left panels: SIR; middle panels: posterior RR from the Poisson-Gamma (PG) models; right panels: posterior RR from the Besag, York, and Mollié (BYM) models.
We identified 34 municipalities with a PG posterior probability of RR > 1 greater than 70% in one or both genders (Table 1, with comments, available as Supplementary data at Occupational Medicine Online).
DISCUSSION
In this study, we found in a highly populated and industrialized Italian Region that SNC incidence was higher in men, and has been rather constant over time. Among SNC cases, there was a high prevalence of occupational exposure, mainly in men, mostly to wood and leather dust and in subjects with SNC adenocarcinoma. Geographical analyses at municipality level showed spatial clustering in areas with a high prevalence of shoe and leather industries involving exposure to leather dust, mainly in Vigevano (Province of Pavia), in the Province of Varese, and in Parabiago (Province of Milan). In contrast, exposure to wood dust in carpentries, joineries and furniture industries was more diffuse across several Provinces.
This study had several strengths. First, the recording of SNC cases by the Lombardy SNC Registry is virtually complete because it is based (beyond reporting of occupational cases, which is largely incomplete despite being required by law) on periodical active search of potential SNC cases in various archives, most importantly hospital admission and pathology databases, but also mortality and occupational disease compensation records. Second, diagnosis is verified by carefully evaluating all available clinical documentation (paper clinical records are obtained by the hospitals). Third, a high proportion of patients or their next of kin were interviewed to collect lifetime occupational histories. Fourth, we applied Bayesian smoothing techniques to examine geographical patterns of SNC occurrence. Fifth, this study was based on more than 800 cases, the largest SNC series in Italy [21].
The major weakness is the fact that some data exploited to complete SNC collection (e.g. hospital admission databases) become available several months after case occurrence. This leads to delays in identifying cases, with two consequences: first, contact with subjects may happen when they are severely ill, so that interview can only be administered to the next of kin (with possibly lower information accuracy) or cannot be performed at all; second, timely completion of all the necessary activities cannot be achieved (this is the reason why we are only able to report information through the year 2020).
Our results align with data from the Italian SNC Registry: in the period 2010–14, overall ASRs (per 100 000) were 0.65 in men and 0.26 in women. Lombardy was one with the highest rates (men: 0.81; women: 0.41). Occupational exposure was found in 63% of SNCs (73% in men, 35% in women) in the period 2000–16, mainly in subjects with adenocarcinoma (88% of subjects with a defined exposure) [21]. The most frequent agents were wood and leather dust. This result was also confirmed in a subsequent study of the Italian SNC Registry exploring jobs where an association with such carcinogens is unusual [22].
The data from the Italian Association of Cancer Registries (AIRTUM; period of diagnosis 2000–10) reported incidence rates for epithelial SNCs comparable to our study (0.67 per 100 000 in men versus 0.34 in women and an overall rate of 0.50) [23]. Data from 94 European cancer registries (2000–2007) reported a similar estimate of 0.45 per 100 000 [24].
The present study found that nasal cavity tumours make up over one-third of cancers, and the most frequent morphology was SCC. The nasal cavity involved similar percentages in both genders, while the maxillary sinus was more represented in females and the ethmoid sinus in males. This proportion of genders by localization was comparable to other studies [25,26]. Similar findings were reported in a US study where nasal cavity accounted for 46% of tumours and SCC comprised 51% of patients [27]. A high proportion of male cases (around 60%) was reported in two US studies [27,28] and in a study in Taiwan, where males had higher annual incidence than females (2.3 versus 1.0) [29]. A Danish study on 1720 SNC patients found a higher proportion of male cases (63%) attributable to occupational exposure to wood and leather dust [30]. That study also found a stable trend of SNC incidence over time (1980–2014).
The absence of decreasing SNC trends in our study might suggest that preventive measures to reduce wood and leather dust in work environments were not sufficient or that their effectiveness is not evident yet due to the long latency time. There are examples of success in SNC prevention. In a region near London where chairs were produced using beech wood an unusual occurrence of adenocarcinoma of the ethmoidal sinuses was first reported in 1968. After the introduction of dust extraction measures a steep reduction of cases was noted in the late 1980s, and no cases were diagnosed from 2010 on [31].
Tobacco smoking is a recognized risk factor for SNC [7,32]. Information on smoking habits is collected by the Italian SNC Registry [21]. The availability of smoking data as a potential confounder would be important if analytical studies (e.g. case–control studies) are implemented.
In conclusion, our study shows that a population-based SNC registry allows a complete and accurate collection of SNC cases and provides a clear picture of their association with occupational exposures with the collection of lifetime histories through interviews. This is important for compensation for the affected subjects. Moreover, our results suggest there is an opportunity to control exposure to wood and leather dust in selected industries.
Supplementary Material
ACKNOWLEDGEMENTS
We wish to thank the personnel of the Departments and Units of Pathology, Otorhinolaryngology, and Occupational Health of Lombardy Region hospitals; and the Occupational Health Services of the Local Health Units of the Lombardy Region.
Contributor Information
D Consonni, Occupational Health Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan 20122, Italy.
G Stoppa, Unit of Biostatistics, Epidemiology and Public Health, DCTVPH, University of Padova, Padua 35131, Italy.
A Binazzi, Department of Occupational and Environmental Medicine, Epidemiology and Hygiene, National Institute for Insurance against Accidents at Work (INAIL), Rome 00143, Italy.
B Dallari, Occupational Health Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan 20122, Italy.
S Stella, Occupational Health Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan 20122, Italy.
S Rugarli, Occupational Health Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan 20122, Italy.
C Trobbiani, School of Occupational Health, University of Milan, Milan 20122, Italy.
A Biggeri, Unit of Biostatistics, Epidemiology and Public Health, DCTVPH, University of Padova, Padua 35131, Italy.
D Catelan, Unit of Biostatistics, Epidemiology and Public Health, DCTVPH, University of Padova, Padua 35131, Italy.
C Mensi, Occupational Health Unit, Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan 20122, Italy.
FUNDING
This work was supported by the Italian National Institute for Insurance against Accidents at Work (INAIL: Istituto Nazionale per l’Assicurazione contro gli Infortuni sul Lavoro) within the Project ‘Sviluppo della rete di sorveglianza epidemiologica dei tumori naso-sinusali attraverso il rafforzamento del registro nazionale (ReNaTuNS) per la prevenzione della malattia’ (development of the epidemiological surveillance of sinonasal cancer in Italy) in a collaboration agreement with Fondazione IRCCS Ca’ Granda Ospedale Maggiore Policlinico, Milan, Italy [Grant PB-0162].
COMPETING INTERESTS
None declared.
REFERENCES
- 1. Lund VJ, Stammberger H, Nicolai P.. European position paper on endoscopic management of tumours of the nose, paranasal sinuses, and skull base. Rhinol Suppl 2010;1:1–143. [PubMed] [Google Scholar]
- 2. Turner JH, Reh DD.. Incidence, and survival in patients with sinonasal cancer: a historical analysis of population-based data. Head Neck 2012;34:877–885. [DOI] [PubMed] [Google Scholar]
- 3. El-Naggar AK, Chan JKC, Grandis JR, Takata T, Slootweg PJ.. WHO Classification of Head and Neck Tumours. Vol. 9, 4th edn. Lyon: IARC WHO Classification of Tumors, 2017; 1–348. [Google Scholar]
- 4. Taylor MA, Saba NF.. Cancer of the paranasal sinuses. Hematol Oncol Clin North Am 2021;35:949–962. [DOI] [PubMed] [Google Scholar]
- 5. Rushton L, Hutchings SJ, Fortunato L. et al. Occupational cancer burden in Great Britain. Br J Cancer 2012;107:S3–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Slack R, Young C, Rushton L; British Occupational Cancer Burden Study Group. Occupational cancer in Britain. Nasopharynx and sinonasal cancers. Br J Cancer 2012;107:S49–S55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. IARC. List of Classifications by Cancer Sites with Sufficient or Limited Evidence in Humans; IARC Monographs. Vol. 1–133. Lyon: International Agency for Research on Cancer (IARC); 2023. https://monographs.iarc.who.int/agents-classified-by-the-iarc/ (accessed December 1, 2023). [Google Scholar]
- 8. IARC. Arsenic, Metals, Fibres, and Dust. Volume 100 C. A Review of Human Carcinogens. Lyon: IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, 2012. [PMC free article] [PubMed] [Google Scholar]
- 9. Binazzi A, Corfiati M, Di Marzio D. et al. Sinonasal cancer in the Italian national surveillance system: Epidemiology, occupation, and public health implications. Am J Ind Med 2018;61:239–250. [DOI] [PubMed] [Google Scholar]
- 10. Mensi C, Consonni D, Sieno C, De Matteis S, Riboldi L, Bertazzi PA.. Sinonasal cancer and occupational exposure in a population-based registry. Int J Otolaryngol 2013;2013:672621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Binazzi A, Miligi L, Giovannetti. et al. ReNaTuNS. Sorveglianza Epidemiologica dei Tumori Naso-Sinusali. Manuale Operativo. Milan: INAIL Press; 2020. (Italian) https://www.inail.it/cs/internet/comunicazione/pubblicazioni/catalogo-generale/pubbl-renatuns-sorv-epid-tumori-naso-sinusali-manuale.html [Google Scholar]
- 12. Sterne JAC, Davey Smith G.. Sifting the evidence-what’s wrong with significance tests? BMJ (Clin Res Ed.) 2001;322:226–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Consonni D, Coviello V, Buzzoni C, Mensi C.. A command to calculate age-standardized rates with efficient interval estimation. Stata J 2012;12:688–701. [Google Scholar]
- 14. StataCorp. 2023. Stata 18. Statistical software. College Station, TX: StataCorp LLC. [Google Scholar]
- 15. Wasserstein RL, Schirm AL, Lazar NA.. Moving to a world beyond ‘p < 0.05’. Am Stat 2019;73:1–19. [Google Scholar]
- 16. Breslow NE, Day NE.. Indirect standardization, and multiplicative models for rates, with reference to the age adjustment of cancer incidence and relative frequency data. J Chronic Dis 1975;28:289–303. [DOI] [PubMed] [Google Scholar]
- 17. Clayton D, Kaldor J.. Empirical Bayes estimates of age-standardized relative risks for use in disease mapping. Biometrics 1987;43:671–681. [PubMed] [Google Scholar]
- 18. Besag J, York J, Mollié A.. Bayesian image restoration, with two applications in spatial statistics. Ann Inst Stat Math 1991;43:1–20. [Google Scholar]
- 19. Lunn DJ, Thomas A, Best N, Spiegelhalter D.. WinBUGS—a Bayesian modelling framework: concepts, structure, and extensibility. Stat Comput 2000;10:325–337. [Google Scholar]
- 20. Lawson AB, Biggeri AB, Boehning D. et al. Disease mapping models: an empirical evaluation. Disease Mapping Collaborative Group. Stat Med 2000;19:2217–2241. [DOI] [PubMed] [Google Scholar]
- 21. Binazzi A, Corfiati M, Di Marzio D. et al. Sinonasal cancer in the Italian national surveillance system: epidemiology, occupation, and public health implications. Am J Ind Med 2018;61:239–250. [DOI] [PubMed] [Google Scholar]
- 22. Binazzi A, Mensi C, Miligi L, Di Marzio D, Zajacova J, Galli P, Camagni A, Calisti R, Balestri A, Murano S, Piro S, d'Errico A, Bonzini M, Massacesi S, Sorasio D, Marinaccio A, On Behalf Of ReNaTuNS Working Group. Exposures to IARC carcinogenic agents in work settings not traditionally associated with sinonasal cancer risk: the experience of the Italian National Sinonasal Cancer Registry. Int J Environ Res Public Health 2021;18:12593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. AIRTUM Working Group. Italian cancer figures—report 2015. The burden of rare cancers in Italy. Epidemiol Prev 2016;40:1–120. [DOI] [PubMed] [Google Scholar]
- 24. Gatta G, Capocaccia R, Botta L. et al.; RARECAREnet working group. Burden and centralised treatment in Europe of rare tumours: results of RARECAREnet—a population-based study. Lancet Oncol 2017;18:1022–1039. [DOI] [PubMed] [Google Scholar]
- 25. Elliot A, Jangard M, Marklund L. et al. Sinonasal malignancies in Sweden 1960-2010: a nationwide study of the Swedish population. Rhinology 2015;53:75–80. [DOI] [PubMed] [Google Scholar]
- 26. Youlden DR, Cramb SM, Peters S. et al. International comparisons of the incidence and mortality of sinonasal cancer. Cancer Epidemiol 2013;37:770–779. [DOI] [PubMed] [Google Scholar]
- 27. Gore MR. Survival in sinonasal and middle ear malignancies: a population-based study using the SEER 1973-2015 database. BMC Ear Nose Throat Disorders 2018;18:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dutta R, Dubal PM, Svider PF, Liu JK, Baredes S, Eloy JA.. Sinonasal malignancies: a population-based analysis of site-specific incidence and survival. Laryngoscope 2015;125:2491–2497. [DOI] [PubMed] [Google Scholar]
- 29. Yang TH, Xirasagar S, Cheng YF, Chen CS, Chang WP, Lin HC.. Trends in the incidence of head and neck cancer: a nationwide population-based study. Oral Oncol 2023;140:106391. [DOI] [PubMed] [Google Scholar]
- 30. Sjöstedt S, Jensen DH, Jakobsen KK. et al. Incidence and survival in sinonasal carcinoma: a Danish population-based, nationwide study from 1980 to 2014. Acta Oncol 2018;57:1152–1158. [DOI] [PubMed] [Google Scholar]
- 31. Capper JWR. Fifty years of woodworkers’ nasal adenocarcinoma in High Wycombe. J Laryngol Otol 2022;136:45–48. [DOI] [PubMed] [Google Scholar]
- 32. Greiser EM, Greiser KH, Ahrens W. et al. Risk factors for nasal malignancies in German men: the South-German Nasal cancer study. BMC Cancer 2012;12:506. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.