Abstract
Purpose of Review
We summarized research on the bidirectional association between intake of ultra-processed food (UPF) and sleep.
Recent Findings
Sleep contributes to cardiometabolic health in part via food intake patterns. Restricting sleep increases intakes of high-carbohydrate/high-fat foods, a profile representative of UPF.
Summary
This systematic review covers the association of UPF intake, as an exposure or an outcome, and sleep. UPF was defined as NOVA Group 4. MEDLINE and EMBASE were searched through April 2023 for epidemiological studies with general-population adult samples. Fifteen studies met the inclusion criteria; all were cross-sectional, published between 2016 and 2023, with samples from Brazil (n = 8), Spain (n = 2), Italy (n = 1), the UK (n = 1), Paraguay (n = 1), Iran (n = 1) and China (n = 1). Thirteen studies examined UPF intake as the exposure whereas two tested UPF intake as the outcome. UPF intakes were determined using food frequency questionnaires (73%) or 24-h recalls (27%). Two studies assessed sleep via accelerometry; the remaining studies relied on self-reports of sleep quality, duration, anxiety-induced insomnia, and napping, with 60% using a single question. The average methodological quality across the studies was deemed “fair”. Six of the 13 studies that examined UPF consumption as the exposure revealed inverse associations with sleep outcomes in adjusted (n = 5) or bivariate (n = 1) analyses. Both studies addressing UPF consumption as the outcome and sleep as the exposure showed significant inverse associations. Evidence for UPF-sleep associations is accumulating, although sleep assessment limitations are apparent. This review can provide impetus for research using comprehensive and validated sleep measures and nudge policymakers towards refining dietary guidelines worldwide.
Keywords: General population, NOVA classification, Observational studies, Review, Sleep quality, Ultra-processed food
Introduction
Dietary nutrient composition is a known contributor to chronic health and disease [1, 2]. All major health organizations recommend higher intakes of fruits and vegetables, whole grains and legumes, all of which have proven health benefits, and lower intakes of animal protein and fat to reduce the risk of cardiovascular diseases (CVD), type 2 diabetes (T2D), obesity, and cancer [1, 3-5]. A dietary profile comprised of more complex carbohydrates and unsaturated fats and less refined carbohydrates and saturated fats has been associated with reduced incidence of CVD, T2D, and mortality [6-8]. Beyond the nutritive components of diet, more recently, the degree of food processing has come under scrutiny as a potential contributor to cardiometabolic risk [9].
Globalization of the food systems and technological progress have led to a high prevalence of consumption of industrially manufactured products [10•, 11, 12], referred to as ultra-processed foods and beverages (UPF), across age, sex, ethnicity, and socioeconomic strata [13]. A product is considered UPF if it has been produced by means of extensive physical and chemical modifications of its basic ingredients, includes substantial amounts of sugar, fat, or salt, a low amount of dietary fiber, and contains colorants, flavorings, or other additives [14]. Such products are ready-to-eat, palatable, attractively packaged, easy to locate on supermarket shelves, well marketed, affordable for consumers and profitable for manufacturers [15]. Although the potential health risks associated with UPF intake were first evoked in 2009 [9], a review of representative studies published since 2016 revealed that UPF consumption contributes up to 25.4% of daily caloric intake in Brazil, 31.1% in France, 56.8% in the UK, and 57.9% in the US [16••]. Cross-sectional and prospective studies almost consistently link UPF consumption with an increased risk of T2D, metabolic syndrome, overweight, obesity, dyslipidemia, eating disorders, irritable bowel syndrome, Crohn’s disease/ulcerative colitis, non-alcoholic fatty liver disease, depressive symptoms, CVD, hypertension, asthma/wheezing, frailty, cancer, and mortality [13, 16••, 17-21].
Sleep has recently been noted as contributing to cardiometabolic health [22••] and studies show that sleep restriction leads to increased energy intake, particularly from snacks, high-carbohydrate, and high-fat foods [23], a profile representative of UPF. Conversely, additional sleep was shown to be associated with a significant decrease in overall appetite and desire for sweet and salty foods [24]. Interestingly, diet quality has also emerged as a potential contributor to sleep quality [25••]. A recent literature review noted an association between a higher intake of processed, free-sugar rich and high glycemic index foods and worse sleep outcomes, whereas a high-quality diet (i.e., Mediterranean diet, antioxidant-rich food, low inflammatory potential) was associated with better sleep quality [26•]. Despite these associations, none of the existing literature reviews focused on multiple chronic outcomes has examined the associations of UPF consumption with sleep. To our knowledge, only one meta-analysis of cross-sectional studies carried out among children, adolescents and adults has synthesized the evidence for the association between consumption of any type of processed food (e.g., fast food, processed meat, instant noodles, salty snacks, confectionary, soft drinks, energy drinks, etc.) and sleep quality/duration, reporting significant results across all age groups [27].
Therefore, the objective of the present systematic review was to synthesize and evaluate the available epidemiological evidence regarding the bidirectional association between UPF intake, using a uniform measure (NOVA, described below), and sleep parameters among adults.
Methods
This review covers observational epidemiological studies assessing the association of UPF intake, as an exposure or as an outcome, and sleep. UPF intake was defined using the 4-level NOVA classification [15]. Accounting for the extent and purpose of processing, NOVA assigns unprocessed and minimally processed food that does not contain added substances to Group 1 (e.g., fruit, eggs, plain yogurt); processed culinary ingredients to Group 2 (e.g., butter, honey, vinegar); processed food containing few ingredients to Group 3 (e.g., cheese, fresh bread, canned fish); and UPF containing multiple ingredients including substances not commonly used in culinary preparations to Group 4 (e.g., ice-cream, hot dogs, instant soups, carbonated drinks, energy bars) [15].
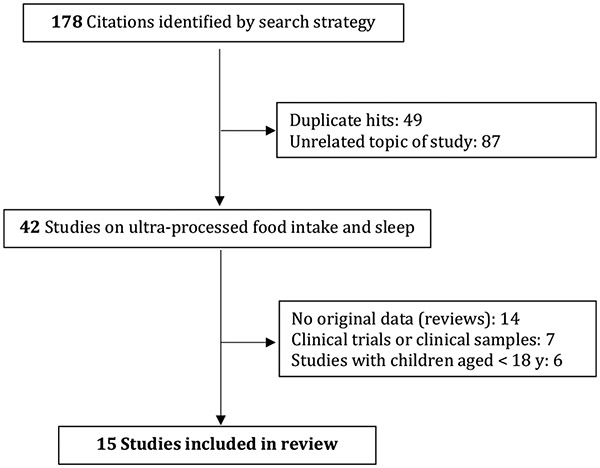
We searched MEDLINE/PubMed and EMBASE using the following selection criteria: publication in any language through April 2023; cross-sectional or prospective design; general population or non-clinical sample of adults aged ≥18 y. Studies based on mixed adolescent-adult samples were retained if participants aged ≥18 y constituted ≥10% of the sample. The diet-related terms “processed food”, “NOVA”, and “ultra-processed food” were crossed with each of the following sleep-related keywords: sleep, insomnia, sleep apnea, sleep disorders, sleep quality, sleep duration, napping, sleep variability, circadian rhythm, daytime sleepiness, social jetlag, hypersomnolence, sleep-related breathing disorders, sleep-wake disorders, sleep disturbances, and parasomnia. The following MeSH terms were also applied: sleep; sleep deprivation; sleep initiation and maintenance disorders; sleep apnea syndromes; sleep apnea, obstructive; sleep wake disorders; sleep disorders, circadian rhythm; sleep quality; dyssomnias. The reference lists of pertinent articles were manually searched. From a total of 178 hits, we excluded duplicates, conference proceedings, studies that fell outside the scope of the review, those conducted in children or in clinical samples, review/position papers, and clinical trials, thus retaining 15 studies for this review (Fig. 1). Using a standardized extraction form, we obtained the following information from each of the studies: reference (author, year); study population (N, mean age, age range; country); dietary data collection tool; sleep assessment method and sleep variable studied; statistical analysis method and adjustment for covariates; main results.
Fig. 1.
Study selection flowchart
The methodological quality of each of the 15 studies was evaluated by two independent reviewers (VAA and JPJ) using the National Institutes of Health (NIH) Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies, which resulted in a 3-level quality score (“good”=3.0, “fair”=2.0, or “poor”=1.0) [28]. The quality score is computed on the basis of 14 items that take into consideration the following aspects of the work: research question, study population, eligibility criteria, sample size justification, exposure assessed prior to outcome measurement, sufficient timeframe, different levels of exposure of interest, exposure measures, repeated exposure assessment, outcome measures, blinding of outcome assessors, follow-up rate, and statistical analyses [28].
The present research was based on previously published and publicly available data; as such it was exempt from an ethics committee review. This systematic review, which was not registered, also serves as a study protocol (e.g., Methods section).
Results
Characteristics of the Selected Studies
All 15 studies included in this systematic review were cross-sectional and published in English between 2016 and 2023 (Table 1). Most of the research was from Brazil (8 studies), with other studies conducted in Spain (2 studies), Italy (1 study), the UK (n = 1), Paraguay (n = 1), Iran (n = 1), and China (1 study). Thirteen of the studies (87%) examined UPF consumption as the exposure, while 2 (13%) investigated UPF consumption as the outcome. Three of the identified studies used mixed adolescent-adult samples: de Oliveira et al. reported that participants aged 17-19 y constituted 40.7% of the sample [29]; Werneck et al. reported that 30.4% of the participants were older than 14 y [30]; Ruggiero et al. presented findings from separate analyses in children/adolescents (ages 5-19 y) and adults (ages 20-97 y) [31]; only the latter are included in this review. Four studies reported baseline UPF-sleep association in the context of longitudinal analyses of unrelated outcomes; 2 studies were conducted during the COVID-19 pandemic.
Table 1.
Cross-sectional evidence for the bidirectional association between consumption of ultra-processed food (UPF, as defined by NOVA) and sleep parameters among adults
| Reference | Study sample | Dietary data & UPF quantity |
Sleep data & sleep parameter |
Statistical model | Main results | Comment | Quality rating [28] |
|---|---|---|---|---|---|---|---|
| UPF intake as exposure | |||||||
| Da Silva et al. [32] | N=964; 18-19 y; Brazil | FFQ; Brazilian Food Composition Table; Nutritional Composition Table of Foods Consumed in Brazil; average daily % calories from UPF 57.6±13.3; 38.6% of sample consumed UPF |
Accelerometry-assessed average sleep duration over 4-7 nights | Linear regression adjusted for age, gender, skin color, BMI, education, economic class, smoking, alcohol use, illicit drug use, occupation, screen time, physical activity, anxiety, depressive symptoms | NS association between UPF intake and sleep duration (β= 0.00003; 95% CI: −0.004 - 0.005; p=0.99) | Multiple sampling strategies (subsample of [35]) | QR=2.5 |
| De Oliveira et al. [29] | N=432; 14-19 y; Brazil | FFQ; median UPF intake: 726.8 and 582.9 Kcal/d in those with adequate and inadequate sleep, respectively | 1 question about average sleep duration; adequate if 8-10 h sleep/d | Mann-Whitney test | NS association between UPF intake and sleep duration; low un-processed or minimally processed food intake associated with inadequate sleep duration (p<0.05) | Random selection of adolescents in one city | QR=1.5 |
| Hajmir et al. [38] | N=278 overweight or obese women; 18-56 y; mean age 36.5 y; Iran | FFQ; median UPF intake: 415.6 (unclear if g or Kcal per day or per week) | PSQI: no cutoff cited for good/poor sleep quality | ANCOVA, logistic regression adjusted for age, BMI, education, occupation, economic and marital status, energy intake, physical activity | Women with “higher NOVA classification system” had poorer sleep quality in fully adjusted analysis (OR=2.51; 95% CI 1.23-5.14; p=0.012) | Multistage random sampling of health center outpatients; UPF does not refer only to Group 4 NOVA | QR=2.0 |
| Kramer Fiala Machado et al. [33] | N=2,738; age 22 y; Brazil | FFQ; UPF (average daily % of energy contribution) consumed by 31.0% of men and 35.5% of women | Accelerometry-assessed sleep onset & offset, total sleep time & variability, sleep efficiency over 3-7 nights; PSQI – continuous scale; Epworth’s excessive daytime sleepiness – continuous scale | Poisson regression adjusted for wealth index, skin color, education, occupation, shift work, having children < 2 y | Among men, shorter & poorer sleepers had highest % of individuals in highest tertile of UPF intake (35.9%; 95% CI: 31.3-40.5); among women, late & poor-quality sleepers had higher % of individuals in highest tertile of UPF intake (43.0%; 95% CI: 37.7-48.2) compared to healthy sleepers | Recruitment from 1993 Pelotas Birth Cohort; multidimensional sleep clusters | QR=3.0 |
| Li et al. [40] | N=12,451; age >20 y; China | 3 consecutive 24-h dietary recalls; mean UPF intake 41.5 g/d; in short sleepers (<6 h/d): 7.8% and 8.6% had no UPF intake and UPF intake ≥50 g/d, respectively | 1 question about sleep duration recorded as <6, 6-9 and >9 h/d | Chi-squared tests | NS association between UPF intake and sleep duration (p=0.81) | Baseline component of longitudinal analyses of obesity; China Nutrition and Health Survey | QR=1.5 |
| Li et al. [41] | N=72,083; age ≥55 y; mean age 61.6 y; UK | ≥2 non-consecutive 24-h dietary assessments (Oxford WebQ); Q cutoffs of %UPF: 11.9, 17.1, 23.6 in men; 11.2, 16.0, 21.8 in women |
1 question about sleep duration “About how many hours sleep do you get in every 24 hours (include naps)?” (h/d) |
Simple one-way ANOVA | NS association between UPF intake and sleep duration (p=0.61) | Baseline component of longitudinal analysis of dementia; UK Biobank cohort | QR=2.0 |
| Mendonça et al. [42] | N=8,451; mean age= 37.6±11.0 y; Spain | Semi-quantitative FFQ; mean UPF servings/d range: 1.5 (Q1) - 6.1 (Q4) | 1 question about ‘siesta sleep’ (h/d) | ANOVA adjusted for sex and age | NS association between UPF intake and taking a nap (p=0.12) | Baseline component of longitudinal analysis of obesity; SUN cohort | QR=2.5 |
| Mendonça et al. [43] | N=14,790; mean age 36.3 y; Spain | Semi-quantitative FFQ; mean UPF servings/d range: 2.1 (T1) - 5.0 (T3) | 1 question about ‘siesta sleep’ (h/d) | Simple linear regression | NS association between UPF intake and taking a nap (p=0.05) | Baseline component of longitudinal analysis of hypertension; SUN cohort | QR=2.5 |
| Menezes-Júnior et al. [36] | N=1,762; ≥18 y; Brazil | FFQ (UPF quantity not reported) | PSQI: good sleep quality score ≤5; poor sleep quality score >5 | Logistic regression adjusted for age, sex, marital status, income, anxiety symptoms | Highest frequency of UPF intake & lowest frequency of fresh or minimally processed foods intake associated with increased risk of poor sleep quality (OR=2.44; 95% CI: 1.32-4.50) | COVID-19 context; probability sampling in 2 cities | QR=3.0 |
| Noll et al. [44] | N=225; post-menopausal women age ≥40 y; Brazil | 3 non-consecutive 24-h dietary recalls; mean UPF intake: 459.5 and 524.2 Kcal/d in those with better and worse quality of life (including sleep), respectively |
Women’s Health Questionnaire (2 items about sleep); insomnia from Kupperman-Blatt Menopausal Index | Poisson regression adjusted for age, marital status, income, early/late menopause | NS association between UPF intake & sleep disorders (PR=0.92; 95% CI 0.75-1.13) | Non-probability convenience outpatient sample; sleep problems assessed as 1 component of menopausal symptoms | QR=3.0 |
| Rodriguez et al. [39] | N=273; 18-60 y; mean age 36.5±13.2 y; Paraguay | NOVA-based FFQ; among participants with insufficient sleep, 46.5% and 30.5% had moderate and excessive processed and UPF intake, respectively | 1 question about hours of sleep (≥9 h optimal; <8 h insufficient) | Chi-squared tests | Moderate & excessive processed food & UPF intake associated with sleep duration (p=0.033) | COVID-19 context; non-probability sample; processed food & UPF modelled together | QR=1.0 |
| Sousa et al. [35] | N= 2,499; 18-19 y; Brazil | FFQ; mean UPF energy contribution: 34.9% and 36.4% in those with good and poor sleep quality, respectively | PSQI: good sleep quality score ≤4; poor sleep quality score >4 | Poisson regression adjusted for age, socio-economic class, education, screen time, parental marital status | Highest quartile of UPF intake associated with increased risk of poor sleep quality (PR=1.14; 95% CI: 1.03-1.27) | Multiple sampling strategies (includes sample of [32]) | QR=2.5 |
| Werneck et al. [30] | N=99,791; 11-19 y; Brazil | NOVA-based questionnaire, past 7 days; UPF intake 7 times/week: 36.3% and 43.5% of boys and girls, respectively | 1 question about anxiety-induced insomnia/sleep disturbance over past 12 months, 5 response options from ‘never’ to ‘very frequently’ | Logistic regression adjusted for age, ethnicity, physical activity, food insecurity, city type, region of residence | Daily UPF consumption associated with higher odds of anxiety-induced sleep disturbance (males: OR=1.48, 95% CI 1.30-1.70; females: OR = 1.46, 95% CI 1.34-1.60) | Nationally representative Adolescent School-Based Health Survey; associations mediated by loneliness & eating while watching TV | QR=2.0 |
| UPF intake as outcome | |||||||
| Mattar et al. [45] | N=2,826; 20-59 y; Brazil | Semi-quantitative FFQ; mean UPF energy contribution (%) range: 12.4 (Q1) - 39.5 (Q4); in short sleepers (≤6 h/d): 31.8% and 36.4% in Q1 and Q4, respectively | 1 question about average sleep duration (h/d) over past 12 months | Path analysis including sex, age, income, employment, time on computer, meals/d, fried food intake | Sleep duration inversely & directly associated with UPF intake (std coeff= −0.05; p< 0.01) | Cohort of Universities of Minas Gerais | QR=1.5 |
| Ruggiero et al. [31] | N=8,569; 20–97 y; mean age 56.9 ±14.6; Italy | 1 day 24-h dietary recall; European Food Propensity Questionnaire; mean UPF energy contribution: 17.3%; mean UPF intake: 154.8 g/d | 1 question about general sleep quality, response options: restful vs restless | Multiple linear regression adjusted for age, sex, BMI, education, geographical area, place of residence, sports activity, occupation, marital status, energy intake, smoking, prevalent cancer, cardiovascular disease, hypertension, diabetes, hyperlipidemia | Lower sleep quality associated with increased UPF intake (beta=2.34; 95% CI: 1.45-3.23) | Italian Nutrition and Health Survey; nationwide sample of N=9,078 aged 5-97 y | QR=1.5 |
BMI body mass index, CI confidence interval, FFQ food frequency questionnaire, NS non-significant, OR odds ratio, PR prevalence ratio, PSQI Pittsburgh Sleep Quality Index, Q quartile, QR study quality rating ranging from “poor”=1.0 to “good”=3.0, T tertile, UPF ultra-processed food and beverages
Next, substantial heterogeneity was noted among the subjective sleep indices used (i.e., sleep quality, average sleep duration, anxiety-induced insomnia, daytime napping, excessive daytime sleepiness), with two-thirds of the studies (60%) relying on a single question to evaluate sleep. In turn, two studies relied on accelerometry to assess sleep duration [32] and multidimensional sleep parameters including sleep onset and offset, total sleep time and variability, and sleep efficiency [33]. Four studies assessed sleep quality using the Pittsburgh Sleep Quality Index (PSQI) [34], however, the definition of poor sleep quality differed: a global score >4 [35], a global score >5 [36], a continuous variable without a cutoff [37], or a dichotomous variable for which the cutoff was not indicated [38]. In turn, diet was examined using food frequency questionnaires (FFQ) in 9 studies, NOVA-specific questionnaires in 2 studies, and 24-h recalls in 4 studies. Given the substantial methodological heterogeneity, a meta-analysis was deemed unfeasible.
Association Between UPF Consumption (exposure) and Sleep Indices (outcomes)
Among the 13 studies that investigated UPF as the exposure and sleep as an outcome, 6 studies reported significant associations [30, 33, 35, 36, 38, 39] while the rest reported non-significant associations [29, 32, 40-44]. There was marked heterogeneity in the statistical analyses used. Of the 6 studies reporting statistically significant findings, 5 employed multivariable analyses with notable variability in the covariates included in the models. The research by Menezes-Junior et al. [36] observed that during the COVID-19 pandemic, the highest frequency of UPF consumption and the lowest frequency of fresh or minimally processed food consumption were associated with increased risk of poor sleep quality (PSQI >5) in logistic regression models adjusted for age, sex, marital status, income, and anxiety symptoms (OR=2.44; 95% CI: 1.32-4.50). That study used probability sampling in two Brazilian cities, including 1,762 adults aged ≥18 y [36]. Rodriguez et al. [39], who also conducted their research during the COVID-19 pandemic, performed chi-squared tests and reported that among participants with insufficient sleep (defined as <8 h), 46.5% and 30.5% had moderate and excessive intake of processed food and UPF, respectively. In that study, NOVA Groups 3 and 4 were analyzed together [39].
Using a sample of 2,499 Brazilian young adults aged 18-19 y, Sousa et al. [35] observed that the highest quartile of UPF consumption was associated with increased risk of poor sleep quality (PSQI >4). The authors used Poisson regression models adjusted for age, socio-economic class, education, screen time, and marital status of the parents (PR=1.14; 95% CI: 1.03-1.27) [35]. Interestingly, a subsample of participants from the same cohort were also studied by da Silva et al. [32] who reported non-significant findings regarding the link between UPF intake and accelerometry-assessed sleep duration. Next, in a mixed adolescent-adult sample of 99,791 participants from the nationally representative Adolescent School-Based Health Survey in Brazil, Werneck et al. [30] observed that daily UPF consumption was associated with higher odds of anxiety-induced sleep disturbances (males: OR=1.48, 95% CI 1.30-1.70; females: OR 1.46, 95% CI 1.34-1.60) in logistic regression models adjusted for age, ethnicity, physical activity, food insecurity, type of city, and region of residence. In that study, the outcome was a self-report of “insomnia due to worries or concerns” [30].
The study by Kramer Fiala Machado et al. [33] relied on both objective and subjective sleep measures and reported sex-specific associations according to sleep clusters from analyses adjusted for wealth index, skin color, education, current occupation, shift work, and having children < 2 y of age. Specifically, the highest percentage of individuals in the highest tertile of UPF intake was found among men who were shorter and poorer sleepers (adjusted prevalence =35.9%; 95% CI: 31.3-40.5); among women, late and poor-quality sleepers presented a higher percentage of individuals in the highest tertile of UPF intake (adjusted prevalence =43.0%; 95% CI: 37.7-48.2) compared to healthy sleepers [33]. In turn, the study by Hajmir et al. [38] reported that overweight and obese women with “higher NOVA classification system” had poorer sleep quality in models adjusted for age, BMI, occupation, economic status, education, marital status, energy intake, and physical activity (OR=2.51; 95% CI: 1.23-5.14). However, these authors modelled the entire 4-level NOVA system on a continuous scale without distinguishing UPF [38].
Among the 7 studies reporting null associations between UPF consumption and sleep, 4 used bivariate analyses (Mann-Whitney test, Chi-squared tests, simple linear regression, one-way ANOVA) [29, 40, 41, 43], 1 study used ANOVA adjusted for sex and age [42], 1 study used multivariable linear regression models adjusted for gender, age, skin color, education, economic class, occupation, alcohol use, smoking, screen time, physical activity, illicit drug use, anxiety, depressive symptoms, lean and fat mass [32], and 1 study used Poisson regression adjusted for age, marital status, income, and timing of menopause [44]. Whereas a more detailed interpretation is provided in the Discussion, it should be noted that the null bivariate and age- and sex-adjusted associations came from baseline descriptive analyses of the China Nutrition and Health Survey [40], the Spanish SUN cohort [42, 43], and the UK Biobank [41]. These studies pertained to longitudinal investigations of incident events and sleep was not a primary outcome. Likewise, non-significant results with UPF were observed by de Oliveira et al. studying 432 adolescents and young adults from one Brazilian city, using bivariate models (a significant association emerged between low intake of unprocessed/minimally processed food (NOVA Group 1) and inadequate sleep duration) [29] and by Noll et al. in a convenience outpatient sample of 225 women aged ≥40 y, using adjusted Poisson regression and various menopausal symptoms, including sleep problems, as outcomes (PR=0.92; 95% CI 0.75-1.13) [44].
Association Between Sleep (Exposure) and UPF Consumption (Outcome)
Both studies investigating sleep as an exposure and UPF as an outcome reported significant associations. In a sample of 2,826 adults aged 20-59 y participating in the Brazilian Cohort of Universities of Minas Gerais, Mattar et al. [45] observed that the average sleep duration over the past 12 mo was inversely and directly associated with UPF consumption (standardized coefficient= −0.05; p< 0.01). Their findings were based on a path analysis including sex, age, income, employment, time spent on a computer, number of meals/d, and fried food intake [45]. In turn, Ruggiero et al. [31] reported that lower sleep quality defined as self-reported restless sleep, was associated with increased UPF intake (beta=2.34; 95% CI: 1.45-3.23) in a sample of 8,569 adults aged 20–97 y participating in the Italian Nutrition and Health Survey. Their linear regression model was adjusted for age, sex, BMI, education, geographical area, place of residence, sports activity, occupation, marital status, energy intake, smoking, prevalent CVD, hypertension, T2D, hyperlipidemia, and cancer [31].
Study Quality Evaluation
Using the NIH Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies [28,] the methodological quality of each of the 15 studies was evaluated by two independent reviewers and the consensual quality ratings are presented in Table 1. The rating took into consideration only the UPF and sleep aspects of each study, regardless of any other primary or secondary exposures or outcomes investigated. Thus, the quality evaluation performed as part of this systematic review does not fully reflect the overall methodological quality of each study. The average rating across the studies was 2.13 points (“fair”), with the rating ranging between “poor”=1.0 point and “good”=3.0 points. The ratings did not vary by the presence or absence of statistically significant findings. All studies had clearly specified study samples and all but one study had a clearly stated research question. In turn, only three studies provided a sample size justification or a statistical power description. Given the cross-sectional design of all studies included in the review, several methodological aspects, such as assessment of the exposure preceding the outcome, availability of repeated measures, and loss to follow-up were not applicable. Finally, none of the studies reported whether the outcome assessors were blinded to the exposure status of the participants.
Discussion
This systematic review of observational research in adults revealed that the association between UPF consumption and sleep has been the subject of several cross-sectional studies but has not been investigated prospectively. More than half of the reviewed studies (53%) were carried out in Brazil where the NOVA classification originated and where it has been the subject of a substantial body of research. In fact, Brazil is among more than a dozen countries that have addressed food processing in their national dietary guidelines [46].
Among the 13 studies that examined UPF consumption as the exposure and sleep as an outcome, 7 reported null findings and 6 observed significant associations. The latter reported that increased UPF consumption among adults was inversely associated with sleep problems, with the risk estimates ranging from 1.14 to 2.51 [30, 35, 36, 38]. The associations were consistent across sex [30, 33] and are congruent with findings among adolescents [47]. Of note, significant inverse associations were reported by the two studies conducted during the COVID-19 pandemic [36, 39] which has been independently associated with reduced sleep quality [48] and increased UPF consumption, especially during the lockdown periods [49].
Mechanistically, the impact of diet on sleep has been attributed to neuroendocrine regulation (e.g., serotonin, orexin, noradrenaline, histamine) and neuro-inflammatory processes that alter brain functionality via the gut-brain axis [26]. Indeed, the correct functioning of the sleep-wake cycle is promoted by melatonin, which is exclusively synthesized from dietary tryptophan, via serotonin [25]. Various dietary sources of tryptophan, serotonin, and melatonin, such as dairy, fish, fruit, and vegetables have been shown to have sleep-promoting effects [25, 50]. These foods are generally categorized as Groups 1 or 3 in the NOVA classification system, falling outside of the UPF category, Group 4. Moreover, reviews of the epidemiological and mechanistic evidence have highlighted the central role of the intestinal microbiota in connecting UPF and health status via alterations in the composition and function of the microbiota which are involved in food digestion, metabolism, and maturation of host immunity [13, 16].
The significant association of UPF consumption with risk of sleep problems is consistent with the large body of scientific evidence regarding the deleterious impact of UPF on a wide range of physical and mental health outcomes [13, 16••. 17-21]. To our knowledge, only one literature review with a meta-analysis has addressed the link between UPF and sleep [27]. These authors reviewed 15 cross-sectional studies carried out among children/adolescents (n = 8) and adults (n = 7), and modeling intake of any type of processed food as the exposure. Only two of the studies included in that review relied on NOVA for the assessment of UPF, with the other 13 studies using a variety of food groups (e.g., fast food, junk food, salty snacks, confectionary, soft drinks, etc.). The authors reported stronger effect sizes among the studies that used NOVA compared to those that used other processed food classifications. In addition, among adults, there were null findings for short sleep duration and statistically significant results for poor sleep quality [27].
Among the 7 studies that reported null findings in the present review, 1 was focused on menopausal symptoms, including sleep problems among 225 women aged ≥40 y [44]. A recent literature review evoked specific etiologic aspects of postmenopausal sleep difficulties, which might result from decreased estrogen and melatonin levels, vasomotor and psychological disturbances, and weight gain [51]. Next, 4 studies with non-significant findings pertained to baseline descriptions of cohorts used for the prospective investigation of chronic diseases [40-43]. For example, two of the studies, one regarding the incidence of obesity (n = 8,451) [42] and the other regarding the incidence of hypertension (n = 14,790) [43], were conducted within the Spanish SUN cohort by the same research team. In both studies, the sleep variable was a self-report of taking a nap (h/d), which did not vary significantly by the level of UPF consumption. It is possible that UPF intake differentially impacts daytime versus nighttime sleep parameters. Moreover, studies of napping included a single question related to this behavior and did not capture the intentionality of the nap. Future research ought to assess total sleep duration more thoroughly, including daytime and nighttime sleep, before conclusions could be drawn. Whereas De Oliveira et al. [29] did not find a significant association between UPF intake and sleep duration, assessed with a single question, they reported a significant correlation between low consumption of unprocessed or minimally processed food and inadequate sleep duration using a mixed adolescent-adult sample of 432 participants. Specifically, the median intake of such food among participants with adequate (defined as 8-10 h sleep/d) and inadequate sleep duration was 996.2 and 729.9 Kcal/d, respectively.
It is interesting to note that 5 of the 6 studies showing significant associations between UPF consumption and sleep assessed sleep quality whereas 6 of the 7 studies that reported null findings examined sleep duration (with 2 studies measuring solely napping duration from a single question). Thus, it is possible that diet quality is a more important modulator of sleep quality rather than sleep quantity. Future studies should extend beyond single questions of sleep duration and quality to truly capture overall sleep health, which includes concepts related to regularity, satisfaction, alertness, timing, efficiency, and duration [52]. In the present review, only one study [33] employed a comprehensive assessment of sleep with objective and subjective measures such as accelerometry-assessed sleep onset and offset, total sleep time and variability, sleep efficiency, along with PSQI and Epworth’s excessive daytime sleepiness [53]. In turn, Noll et al. [44] employed a composite sleep index based on both the Women's Health Questionnaire, where 2 of the 37 items pertain to sleep (insomnia underscored by difficulty falling asleep, early waking and restlessness) [54], and the Kupperman-Blatt Menopausal Index where insomnia is one of 11 types of menopausal symptoms that are all grouped together [55].
Two of the 15 studies included in this review investigated sleep as the exposure and UPF consumption as the outcome of interest. Both studies reported significant inverse associations between sleep duration [45] or quality [31] and UPF consumption in adjusted analyses. This is in line with the large body of observational and experimental evidence regarding the impact of sleep on dietary choices and diet quality and quantity [25]. For example, poor sleep quality has been associated with higher intakes of energy, sugar and fat, and lower intakes of fruit, vegetables, and whole grains [56-58]. Such dietary behaviors have been explained by a combination of factors, including an increased opportunity to eat due to added wake time, altered time of intake (i.e., late evening), changes in hormonal regulation, in reward valuation, and in taste sensitivity, as well as a potential homeostatic compensation effect for nocturnal energy deficit, and increased susceptibility to food stimuli [25, 26, 59]. The current review adds to the available knowledge by expanding the range of dietary outcomes influenced by sleep disturbances. Mechanistically, an increased hedonic drive for foods might explain shifts toward poor diet quality following periods of inadequate sleep [25]. Indeed, sleep restriction has been shown to result in increased hunger, appetite, and energy intake [60]. In turn, UPF exposure has been positively associated with appetitive drive and hedonic valence [61].
This review revealed substantial heterogeneity in the reporting of UPF intake, which included number of servings per day or per week, grams per day, Kcal per day, and percentage contribution of UPF to mean daily energy intake. Thus, differences among the reviewed studies might be partly due to different UPF amounts consumed within each NOVA category. This methodological heterogeneity might also help explain the differences between the studies showing significant associations and those reporting null findings. Dietary data for the studies came from various sources, including standard or semi-quantitative FFQ (9 studies), a NOVA-specific questionnaire (2 studies), and 24-h recalls (4 studies). It has been suggested that the use of multiple 24-h dietary records is preferrable over FFQ for UPF estimation [16]. In the present review, however, only three studies relied on more than one 24-h recall [40, 41, 44], finding no association between UPF consumption and menopause-related insomnia symptoms or sleep duration. Methodological heterogeneity was also observed in the self-reported sleep measures, which included average sleep duration, sleep quality, afternoon napping, and anxiety-induced insomnia/sleep disturbance, with more than half of these studies relying on a single question for the assessment of sleep. Finally, in the case of anxiety-induced sleep problems, the measure pertained to “insomnia due to worries or concerns” rather than an actual anxiety assessment or diagnosis [30].
Evidence regarding the bidirectional association between diet and sleep has been evaluated and summarized in several systematic reviews. For example, Sutanto et al. [62] reviewed observational and intervention studies regarding the role of the dietary macronutrient composition in sleep duration and reported inconclusive findings without a dose-dependent association. These authors concluded that the macronutrient profile alone may not play a strong role in sleep [62]. Next, evidence for the role of specific food groups, micro- and macro-nutrients, and dietary patterns in sleep quality among children, adolescents, and adults was recently reviewed by Godos et al. [26]. Whereas the methodological quality of the included studies was not high according to the NIH quality assessment measure, the authors were able to conclude that for some aspects of the diet (e.g., protein, carbohydrate content), the type and quality might be more important than the quantity of intake [26•].
An increased dietary share of UPF has been associated in a direct, dose-response manner with the dietary content of free/added sugars, saturated fat, trans fat, sodium, and energy density, whereas an inverse dose-response association has been found with protein, fiber, potassium, vitamins A, C, D, and E, calcium, zinc, magnesium, phosphorus [63•] and water intake [64]. Higher UPF intakes have been inversely associated with intake of fruit, vegetables, legumes, and seafood [65] which are all sources of sleep-promoting compounds. Indeed, prospective research has shown that individuals adhering to nutrient-dense and fiber-rich diets, such as the Mediterranean diet, have better sleep health [66] and lower risk of insomnia [67]. In contrast, higher dietary glycemic index and glycemic load, underscored by an increased intake of added sugars, starch, and refined grains, have been suggested as independent risk factors for insomnia incidence [68]. Likewise, pooled fully adjusted analyses of three large US cohorts demonstrated that a higher dietary inflammatory potential was associated with a significantly greater risk of obstructive sleep apnea [69].
The present systematic review is subject to some limitations. It cannot provide any evidence of causal effects as it included only cross-sectional research. It was based on published observational studies, none of which employed non-linear modelling. Another important limitation pertains to the inconsistent adjustment for potential confounders of the UPF-sleep association. The review identified notable methodological weaknesses related to the assessment of diet and sleep. It is recommended that future research in this domain employ validated tools and established criteria for the assessment of sleep, such as the DSM-5 criteria for chronic insomnia [70], the Berlin questionnaire for obstructive sleep apnea [71], Epworth Sleepiness Scale [53] (used in only one of the reviewed studies), and the PSQI [34] (used in less than a third of the reviewed studies). Objective measures of sleep (also used in only one of the reviewed studies) including duration, wake after sleep onset, and sleep efficiency are likewise needed. In addition, future epidemiological research conducted outside of Brazil could augment the generalizability of the overall evidence.
Conclusion
To our knowledge, this systematic review is the first to synthesize and evaluate the available epidemiological evidence regarding the bidirectional association between UPF intake and sleep parameters in adults. An important strength of the review was the use of a uniform measure of UPF (i.e., Group 4 of the NOVA classification system), which can facilitate the comparison of findings across studies and across countries. The NOVA classification system has been recognized by the Food and Agriculture Organization of the United Nations and by the Pan American Health Organization as a valid tool for nutrition and public health research and policy development [15, 63].
The findings from this review are well positioned to inform sleep-focused primary and secondary prevention programs and serve as impetus for future epidemiological and experimental research evaluating whether improvements in sleep could have a favorable effect on diet and vice versa.
Acknowledgments
This research was funded in part by the National Heart, Lung, and Blood Institute Award # R35HL155670. J.P.-J. was funded by the Spanish Ministry of Universities (mobility program for senior researchers) and by the Fulbright Commission.
Abbreviations
- BMI
Body mass index
- CI
Confidence interval
- CVD
Cardiovascular diseases
- FFQ
Food Frequency Questionnaire
- NIH
National Institutes of Health
- OR
Odds ratio
- PR
Prevalence ratio
- PSQI
Pittsburgh Sleep Quality Index
- T2D
Type 2 diabetes
- UPF
Ultra-processed food (and beverages)
Footnotes
Conflict of Interests The authors have no relevant financial or non-financial interests to disclose.
Human and Animal Rights and Informed Consent This article does not contain any studies with human or animal subjects performed by any of the authors.
References
Papers of particular interest, published recently, have been highlighted as:
• Of importance
•• Of major importance
- 1.World Health Organization (WHO). Diet, nutrition and the prevention of chronic diseases: report of a joint WHO/FAO expert consultation. Geneva: WHO. 2003. [Google Scholar]
- 2.American Heart Association (AHA). Top things to know: Life’s Essential Eight. Updating and enhancing the American Heart Association’s cardiovascular health construct. Dallas: AHA. 2022. [Google Scholar]
- 3.World Health Organization (WHO). Healthy diet. Geneva: WHO. 2020. [Google Scholar]
- 4.American Diabetes Association (ADA). Eating well. What superstar foods are good for diabetes? Arlington: ADA. 2022. [Google Scholar]
- 5.International Agency for Research on Cancer (IARC). 12 ways to reduce your cancer risk. In: Diet: European code against cancer. Lyon: IARC. 2016. [Google Scholar]
- 6.Yu E, Malik VS, Hu FB. Cardiovascular disease prevention by diet modification: JACC health promotion series. J Am Coll Cardiol. 2018;72:914–26. 10.1016/j.jacc.2018.02.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mozaffarian D. Dietary and policy priorities for cardiovascular disease, diabetes, and obesity: a comprehensive review. Circulation. 2016;133:187–225. 10.1161/CIRCULATIONAHA.115.018585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.GBD Diet Collaborators. Health effects of dietary risks in 195 countries, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2019;393:1958–72. 10.1016/S0140-6736(19)30041-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Monteiro CA. Nutrition and health. The issue is not food, nor nutrients, so much as processing. Public Health Nutr. 2009;12:729–31. 10.1017/S1368980009005291. [DOI] [PubMed] [Google Scholar]
- 10.•. Baker P, Machado P, Santos T, Sievert K, Backholer K, Hadjikakou M, et al. Ultra-processed foods and the nutrition transition: global, regional and national trends, food systems transformations and political economy drivers. Obes Rev. 2020;21:e13126. 10.1111/obr.13126. This review concludes that as countries become wealthier, higher volumes and a wider variety of ultra-processed foods are sold. The authors link these trends with advances in technology, industrialization of the food systems, and globalization, highlighting the growing market and political activities of transnational food corporations and the nutrition policies that do not adequately reflect these new contexts.
- 11.Koiwai K, Takemi Y, Hayashi F, Ogata H, Matsumoto S, Ozawa K, et al. Consumption of ultra-processed foods decreases the quality of the overall diet of middle-aged Japanese adults. Public Health Nutr. 2019;22:2999–3008. 10.1017/S1368980019001514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahmed A, Lazo DPL, Alatinga KA, Gasparatos A. From Ampesie to French fries: systematising the characteristics, drivers and impacts of diet change in rapidly urbanising Accra. Sustain Sci 2022:1–25. 10.1007/s11625-022-01195-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dicken SJ, Batterham RL. Ultra-processed food: a global problem requiring a global solution. Lancet Diabetes Endocrinol. 2022;10:691–4. 10.1016/S2213-8587(22)00248-0. [DOI] [PubMed] [Google Scholar]
- 14.Monteiro CA, Cannon G, Levy RB, Moubarac JC, Louzada ML, Rauber F, et al. Ultra-processed foods: what they are and how to identify them. Public Health Nutr. 2019;22:936–41. 10.1017/S1368980018003762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Monteiro CA, Cannon G, Levy R, Moubarac J-C, Jaime P, Martins AP, et al. NOVA. The star shines bright. World Nutrition. 2016;7:28–38. [Google Scholar]
- 16.••. Srour B, Kordahi MC, Bonazzi E, Deschasaux-Tanguy M, Touvier M, Chassaing B. Ultra-processed foods and human health: from epidemiological evidence to mechanistic insights. Lancet Gastroenterol Hepatol. 2022;7:1128–40. 10.1016/S2468-1253(22)00169-8. This is a comprehensive overview of the epidemiological evidence for a role of ultra-processed food intake in the etiology of numerous chronic diseases. The review also details several mechanistic pathways that have been advanced as regards causality of the associations.
- 17.Harb AA, Shechter A, Koch PA, St-Onge MP. Ultra-processed foods and the development of obesity in adults. Eur J Clin Nutr. 2022. 10.1038/s41430-022-01225-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pagliai G, Dinu M, Madarena MP, Bonaccio M, Iacoviello L, Sofi F. Consumption of ultra-processed foods and health status: a systematic review and meta-analysis. Br J Nutr. 2021;125:308–18. 10.1017/S0007114520002688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Figueiredo N, Kose J, Srour B, Julia C, Kesse-Guyot E, Peneau S, et al. Ultra-processed food intake and eating disorders: cross-sectional associations among French adults. J Behav Addict. 2022;11:588–99. 10.1556/2006.2022.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen X, Zhang Z, Yang H, Qiu P, Wang H, Wang F, et al. Consumption of ultra-processed foods and health outcomes: a systematic review of epidemiological studies. Nutr J. 2020;19:86. 10.1186/s12937-020-00604-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lane MM, Davis JA, Beattie S, Gomez-Donoso C, Loughman A, O'Neil A, et al. Ultraprocessed food and chronic noncommunicable diseases: a systematic review and meta-analysis of 43 observational studies. Obes Rev. 2021;22:e13146. 10.1111/obr.13146. [DOI] [PubMed] [Google Scholar]
- 22.••. Lloyd-Jones DM, Allen NB, Anderson CAM, Black T, Brewer LC, Foraker RE, et al. Life's Essential 8: updating and enhancing the American Heart Association's construct of cardiovascular health: a Presidential Advisory from the American Heart Association. Circulation. 2022;146:e18–e43. 10.1161/CIR.0000000000001078. This advisory from the American Heart Association acknowledges, for the first time, the role of sleep in predicting cardiovascular disease, and includes sleep duration in its metrics to evaluate cardiovascular health.
- 23.St-Onge MP. Sleep-obesity relation: underlying mechanisms and consequences for treatment. Obes Rev. 2017;18(S1):1:34–9. 10.1111/obr.12499. [DOI] [PubMed] [Google Scholar]
- 24.Tasali E, Chapotot F, Wroblewski K, Schoeller D. The effects of extended bedtimes on sleep duration and food desire in overweight young adults: a home-based intervention. Appetite. 2014;80:220–4. 10.1016/j.appet.2014.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.••. Zuraikat FM, Wood RA, Barragan R, St-Onge MP. Sleep and diet: mounting evidence of a cyclical relationship. Annu Rev Nutr. 2021;41:309–32. 10.1146/annurev-nutr-120420-021719. This review highlights the bi-directional relations between diet and sleep, emphasizing the role of healthful dietary patterns for sleep health.
- 26.•. Godos J, Grosso G, Castellano S, Galvano F, Caraci F, Ferri R. Association between diet and sleep quality: a systematic review. Sleep Med Rev. 2021;57:101430. 10.1016/j.smrv.2021.101430. This systematic review of 29 mostly cross-sectional studies concluded that increased consumption of processed food and food containing relatively large amounts of added sugars was associated with worse sleep outcomes whereas consumption of healthy foods was associated with better sleep quality.
- 27.Delpino FM, Figueiredo LM, Flores TR, Silveira EA, Silva Dos Santos F, Werneck AO, et al. Intake of ultra-processed foods and sleep-related outcomes: a systematic review and meta-analysis. Nutrition. 2023;106:111908. 10.1016/j.nut.2022.111908. [DOI] [PubMed] [Google Scholar]
- 28.National Institutes of Health (NIH). Quality assessment tool for observational cohort and cross-sectional studies. Bethesda: NIH, Nat Heart, Lung, Blood Inst; 2021. [Google Scholar]
- 29.de Oliveira IDR, Maciel NMS, da Costa BT, Soares ADN, Gomes JMG. Association between abdominal obesity, screen time and sleep in adolescents. J Pediatr (Rio J). 2023;99:45–52. 10.1016/j.jped.2022.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Werneck AO, Hoare E, Silva DR. Do TV viewing and frequency of ultra-processed food consumption share mediators in relation to adolescent anxiety-induced sleep disturbance? Public Health Nutr. 2021;24:5491–7. 10.1017/S1368980021000379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruggiero E, Esposito S, Costanzo S, Di Castelnuovo A, Cerletti C, Donati MB, et al. Ultra-processed food consumption and its correlates among Italian children, adolescents and adults from the Italian Nutrition & Health Survey (INHES) cohort study. Public Health Nutr. 2021;24:6258–71. 10.1017/S1368980021002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.da Silva EC, Carneiro JR, de Almeida Fonseca Viola PC, Confortin SC, da Silva AAM. Association of food intake with sleep durations in adolescents from a capital city in Northeastern Brazil. Nutrients. 2022;14. 10.3390/nu14235180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kramer Fiala Machado A, Wendt A, Menezes AMB, Goncalves H, Wehrmeister FC. Sleep clusters and modifiable risk behaviors for noncommunicable diseases in young adults: data from a birth cohort in Brazil. Sleep Health. 2023. 10.1016/j.sleh.2023.01.004. [DOI] [PubMed] [Google Scholar]
- 34.Buysse DJ, Reynolds CF 3rd, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 35.Sousa RDS, Braganca M, Oliveira BR, Coelho C, Silva A. Association between the degree of processing of consumed foods and sleep quality in adolescents. Nutrients. 2020;12. 10.3390/nu12020462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Menezes-Junior LAA, Andrade ACS, Coletro HN, Mendonca RD, Menezes MC, Machado-Coelho GLL, et al. Food consumption according to the level of processing and sleep quality during the COVID-19 pandemic. Clin Nutr ESPEN. 2022;49:348–56. 10.1016/j.clnesp.2022.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Machado PP, Steele EM, Levy RB, Sui Z, Rangan A, Woods J, et al. Ultra-processed foods and recommended intake levels of nutrients linked to non-communicable diseases in Australia: evidence from a nationally representative cross-sectional study. BMJ Open. 2019;9:e029544. 10.1136/bmjopen-2019-029544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hajmir MM, Shiraseb F, Ebrahimi S, Noori S, Ghaffarian-Ensaf R, Mirzaei K. Interaction between ultra-processed food intake and genetic risk score on mental health and sleep quality. Eat Weight Disord. 2022;27:3609–25. 10.1007/s40519-022-01501-8. [DOI] [PubMed] [Google Scholar]
- 39.Rodriguez ALB, Amarilla NJD, Rodriguez MMT, Martinez BEN, Meza-Miranda ER. Processed and ultra-processed foods consumption in adults and its relationship with quality of life and quality of sleep. Revista De Nutricao-Brazil J Nutr. 2022;35:e220173. 10.1590/1678-9865202235e220173. [DOI] [Google Scholar]
- 40.Li M, Shi Z. Ultra-processed food consumption associated with overweight/obesity among Chinese adults - results from China Health and Nutrition Survey 1997-2011. Nutrients. 2021;13. 10.3390/nu13082796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li H, Li S, Yang H, Zhang Y, Zhang S, Ma Y, et al. Association of ultraprocessed food consumption with risk of dementia: a prospective cohort study. Neurology. 2022;99:e1056–66. 10.1212/WNL.0000000000200871. [DOI] [PubMed] [Google Scholar]
- 42.Mendonca RD, Pimenta AM, Gea A, de la Fuente-Arrillaga C, Martinez-Gonzalez MA, Lopes AC, et al. Ultra-processed food consumption and risk of overweight and obesity: the University of Navarra Follow-Up (SUN) cohort study. Am J Clin Nutr. 2016;104:1433–40. 10.3945/ajcn.116.135004. [DOI] [PubMed] [Google Scholar]
- 43.Mendonca RD, Lopes AC, Pimenta AM, Gea A, Martinez-Gonzalez MA, Bes-Rastrollo M. Ultra-processed food consumption and the incidence of hypertension in a Mediterranean cohort: the Seguimiento Universidad de Navarra Project. Am J Hypertens. 2017;30:358–66. 10.1093/ajh/hpw137. [DOI] [PubMed] [Google Scholar]
- 44.Noll P, Noll M, Zangirolami-Raimundo J, Baracat EC, Louzada M, Soares Junior JM, et al. Life habits of postmenopausal women: association of menopause symptom intensity and food consumption by degree of food processing. Maturitas. 2022;156:1–11. 10.1016/j.maturitas.2021.10.015. [DOI] [PubMed] [Google Scholar]
- 45.Mattar JB, Domingos ALG, Hermsdorff HHM, Juvanhol LL, de Oliveira FLP, Pimenta AM, et al. Ultra-processed food consumption and dietary, lifestyle and social determinants: a path analysis in Brazilian graduates (CUME project). Public Health Nutr. 2022;25:1–9. 10.1017/S1368980022002087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quinn M, Jordan H, Lacy-Nichols J. Upstream and downstream explanations of the harms of ultra-processed foods in national dietary guidelines. Public Health Nutr. 2021;24:5426–35. 10.1017/S1368980021003505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lane KE, Davies IG, Darabi Z, Ghayour-Mobarhan M, Khayyatzadeh SS, Mazidi M. The association between ultra-processed foods, quality of life and insomnia among adolescent girls in Northeastern Iran. Int J Environ Res Public Health. 2022;19. 10.3390/ijerph19106338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Casagrande M, Favieri F, Tambelli R, Forte G. The enemy who sealed the world: effects quarantine due to the COVID-19 on sleep quality, anxiety, and psychological distress in the Italian population. Sleep Med. 2020;75:12–20. 10.1016/j.sleep.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhutani S, Cooper JA, vanDellen MR. Self-reported changes in energy balance behaviors during COVID-19-related home confinement: a cross-sectional study. Am J Health Behav. 2021;45:756–70. 10.5993/AJHB.45.4.14. [DOI] [PubMed] [Google Scholar]
- 50.St-Onge MP, Mikic A, Pietrolungo CE. Effects of diet on sleep quality. Adv Nutr. 2016;7:938–49. 10.3945/an.116.012336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Laudisio D, Barrea L, Pugliese G, Aprano S, Castellucci B, Savastano S, et al. A practical nutritional guide for the management of sleep disturbances in menopause. Int J Food Sci Nutr. 2021;72:432–46. 10.1080/09637486.2020.1851658. [DOI] [PubMed] [Google Scholar]
- 52.Ravyts SG, Dzierzewski JM, Perez E, Donovan EK, Dautovich ND. Sleep health as measured by RU-SATED: a psychometric evaluation. Behav Sleep Med. 2021;19:48–56. 10.1080/15402002.2019.1701474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Johns MW. Reliability and factor analysis of the Epworth Sleepiness Scale. Sleep. 1992;15:376–81. 10.1093/sleep/15.4.376. [DOI] [PubMed] [Google Scholar]
- 54.Hunter M. The women’s health questionnaire: a measure of mid-aged women’s perceptions of their emotional and physical health. Psychol Health. 1992;7:45–54. 10.1080/08870449208404294. [DOI] [Google Scholar]
- 55.Kupperman HS, Blatt MH, Wiesbader H, Filler W. Comparative clinical evaluation of estrogenic preparations by the menopausal and amenorrheal indices. J Clin Endocrinol Metab. 1953;13:688–703. 10.1210/jcem-13-6-688. [DOI] [PubMed] [Google Scholar]
- 56.Katagiri R, Asakura K, Kobayashi S, Suga H, Sasaki S. Low intake of vegetables, high intake of confectionary, and unhealthy eating habits are associated with poor sleep quality among middle-aged female Japanese workers. J Occup Health. 2014;56:359–68. 10.1539/joh.14-0051-oa. [DOI] [PubMed] [Google Scholar]
- 57.Mossavar-Rahmani Y, Weng J, Wang R, Shaw PA, Jung M, Sotres-Alvarez D, et al. Actigraphic sleep measures and diet quality in the Hispanic Community Health Study/Study of Latinos Sueno ancillary study. J Sleep Res. 2017;26:739–46. 10.1111/jsr.12513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zuraikat FM, Makarem N, Liao M, St-Onge MP, Aggarwal B. Measures of poor sleep quality are associated with higher energy intake and poor diet quality in a diverse sample of women from the Go Red for Women strategically focused research network. J Am Heart Assoc. 2020;9:e014587. 10.1161/JAHA.119.014587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.St-Onge MP, McReynolds A, Trivedi ZB, Roberts AL, Sy M, Hirsch J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am J Clin Nutr. 2012;95:818–24. 10.3945/ajcn.111.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Broussard JL, Klein S. Insufficient sleep and obesity: cause or consequence. Obesity (Silver Spring). 2022;30:1914–6. 10.1002/oby.23539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.David IA, Krutman L, Fernandez-Santaella MC, Andrade JR, Andrade EB, Oliveira L, et al. Appetitive drives for ultra-processed food products and the ability of text warnings to counteract consumption predispositions. Public Health Nutr. 2018;21:543–57. 10.1017/S1368980017003263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sutanto CN, Wang MX, Tan D, Kim JE. Association of sleep quality and macronutrient distribution: a systematic review and meta-regression. Nutrients. 2020;12. 10.3390/nu12010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.•. Monteiro CA, Cannon G, Lawrence M, Costa Louzada ML, Pereira Machado P. Ultra-processed foods, diet quality, and health using the NOVA classification system. Rome: Food Agric Organization United Nations. 2019b. This monograph presents the definition and characteristics of the 4-level NOVA classification system which is the most widely used system in the scientific literature on ultra-processed foods.
- 64.Dicken SJ, Batterham RL. The role of diet quality in mediating the association between ultra-processed food intake, obesity and health-related outcomes: a review of prospective cohort studies. Nutrients. 2021;14. 10.3390/nu14010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Baraldi LG, Steele EM, Louzada MLC, Monteiro CA. Associations between ultraprocessed food consumption and total water intake in the US population. J Acad Nutr Diet. 2021;121:1695–703. 10.1016/j.jand.2021.02.011. [DOI] [PubMed] [Google Scholar]
- 66.Zuraikat FM, Makarem N, St-Onge MP, Xi H, Akkapeddi A, Aggarwal B. A Mediterranean dietary pattern predicts better sleep quality in US women from the American Heart Association Go Red for Women strategically focused research network. Nutrients. 2020;12. 10.3390/nu12092830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Castro-Diehl C, Wood AC, Redline S, Reid M, Johnson DA, Maras JE, et al. Mediterranean diet pattern and sleep duration and insomnia symptoms in the Multi-Ethnic Study of Atherosclerosis. Sleep. 2018;41. 10.1093/sleep/zsy158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gangwisch JE, Hale L, St-Onge MP, Choi L, LeBlanc ES, Malaspina D, et al. High glycemic index and glycemic load diets as risk factors for insomnia: analyses from the Women’s Health Initiative. Am J Clin Nutr. 2020;111:429–39. 10.1093/ajcn/nqz275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu Y, Tabung FK, Stampfer MJ, Redline S, Huang T. Overall diet quality and proinflammatory diet in relation to risk of obstructive sleep apnea in 3 prospective US cohorts. Am J Clin Nutr. 2022;116:1738–47. 10.1093/ajcn/nqac257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.American Psychiatric Association (APA). Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Washington, DC: APA. 2013. [Google Scholar]
- 71.Netzer NC, Stoohs RA, Netzer CM, Clark K, Strohl KP. Using the Berlin Questionnaire to identify patients at risk for the sleep apnea syndrome. Ann Intern Med. 1999;131:485–91. 10.7326/0003-4819-131-7-199910050-00002. [DOI] [PubMed] [Google Scholar]