Abstract
A pathogenetic hallmark of retroviral neurodegeneration is the affinity of neurovirulent retroviruses for microglia cells, while degenerating neurons are excluded from retroviral infections. Microglia isolated ex vivo from rats peripherally infected with a neurovirulent retrovirus released abundant mature type C virions; however, infectivity associated with microglia was very low. In microglia, viral transcription was unaffected but envelope proteins were insufficiently cleaved into mature viral proteins and were not detected on the microglia cell surface. These microglia-specific defects in envelope protein translocation and processing not only may have prevented formation of infectious virus particles but also may have caused further cellular defects in microglia with the consequence of indirect neuronal damage. It is conceivable that similar events play a role in neuro-AIDS.
A key observation for retrovirus-associated neurodegeneration is the infection of microglia cells (3, 5, 7, 22), while degenerating neurons do not express retroviral gene products (1, 3, 5–7, 17, 18, 22, 39). This finding has led to the hypothesis that, as a consequence of microglia infection, toxic metabolites or viral gene products released from the infected cells may play an important pathogenic role in the development of central nervous system (CNS) disease. Yet none of these hypotheses have been unequivocally proven, and little is known about the virus-microglia interaction in infected brain tissue. Microglia cells are ubiquitous within the CNS parenchyma and comprise 5 to 20% of all glia cells (19). Under pathophysiological conditions, microglia cells transform from a resting to an activated state and might act as immune effectors, as phagocytes, and/or as a source of potentially cytotoxic substances, like reactive nitrogen and oxygen intermediates and tumor necrosis factor alpha. Since microglia cells have been found to be activated in retrovirally infected brains (30, 37), neurons might have been damaged by toxic metabolites produced by infected microglia cells. This hypothesis is supported by a number of in vivo (e.g., references 36 and 38) and in vitro (e.g., references 11 and 33) studies.
Important viral determinants of neurovirulence have been identified as residing within the retroviral envelope genes (12, 24, 27). Studies of chimeric neurovirulent murine leukemia viruses (MuLVs) (reviewed in reference 26) revealed that receptor-binding domains within viral envelope proteins of MuLV help direct neurovirulent retroviruses to certain subpopulations of microglia cells. Moreover, it has been shown previously that retroviral envelope proteins are toxic for brain cells in vitro (8) and in vivo (16, 35, 42). Apparently, in vivo toxicity of envelope proteins is observed only if the viral proteins are processed by endogenous brain cells, and not if the proteins are synthesized by cells transplanted into mouse brains (23). These data suggest that interactions of retroviral envelope proteins with cellular receptor molecules on brain cells do not instantly induce neurodegenerative alterations. Our working hypothesis rather advocates that a later step in the retroviral replication cycle initiates disturbances of brain functions. We addressed this question by investigating the replication cycle of the neurovirulent retrovirus NT40 within microglia cells isolated from rat brain tissue.
MATERIALS AND METHODS
Animal model and titration of infectivity.
Neonatal intraperitoneal inoculation of Fisher rats with 5 × 104 focus-forming units (FFU) of MuLV NT40 led to neurological disease within 25 to 45 days postinfection (5). Infectivity of cells and cell culture supernatants was determined by means of a focal immunoassay (4) using monoclonal antibody 48 (29).
Isolation, flow cytometry, and immunoblot analyses of microglia and peritoneal macrophages.
Purification, subsequent immunostaining, immunoblotting, and cytofluorometric analyses of microglia and of peritoneal macrophages were performed as described in detail before (5, 14, 25). Briefly, brains were removed from perfused animals, stripped of meninges, enzymatically digested, and subjected to density gradient centrifugation. Microglia cells were collected from the 1.077/1.066-g/cm3 interface and analyzed further.
To test for possible changes of infectivity due to the purification procedure, e.g., shear forces, we subjected peritoneal macrophages from infected rats to the microglia isolation scheme, both with and without prior digestion with collagenase and DNase. Productive infection of macrophages was determined by an infectious center assay. To examine whether factors possibly secreted by microglia might have reduced infectivity associated with these cells, we added 100 μl of NT40 virus stock containing log 4.7 FFU to 105 microglia cells isolated from uninfected and infected rats, respectively, and incubated the cells at 37°C for 45 min. Supernatants were titrated for infectivity.
Electron microscopy.
After purification, cells were washed with phosphate-buffered saline, fixed in 2.5% glutaraldehyde–phosphate-buffered saline, and gently collected after low-speed centrifugation. After staining with 2% osmium tetroxide, cells were embedded in Epon 812 resin, sectioned ultrathinly, and impregnated with 0.6% uranyl acetate–0.5% lead citrate.
Analyses of viral transcripts.
mRNA was isolated from cells and tissues, employing oligo(dT)25 Dynabeads following the recommendations of the manufacturer (Dynal). After Northern blotting, blots were probed with a viral envelope-specific 32P-labeled probe (14) as well as with a 32P-labeled probe for a housekeeping gene, rat glyceraldehyde-3-phosphate dehydrogenase.
RESULTS
Abundant defective viral particles budding from microglia.
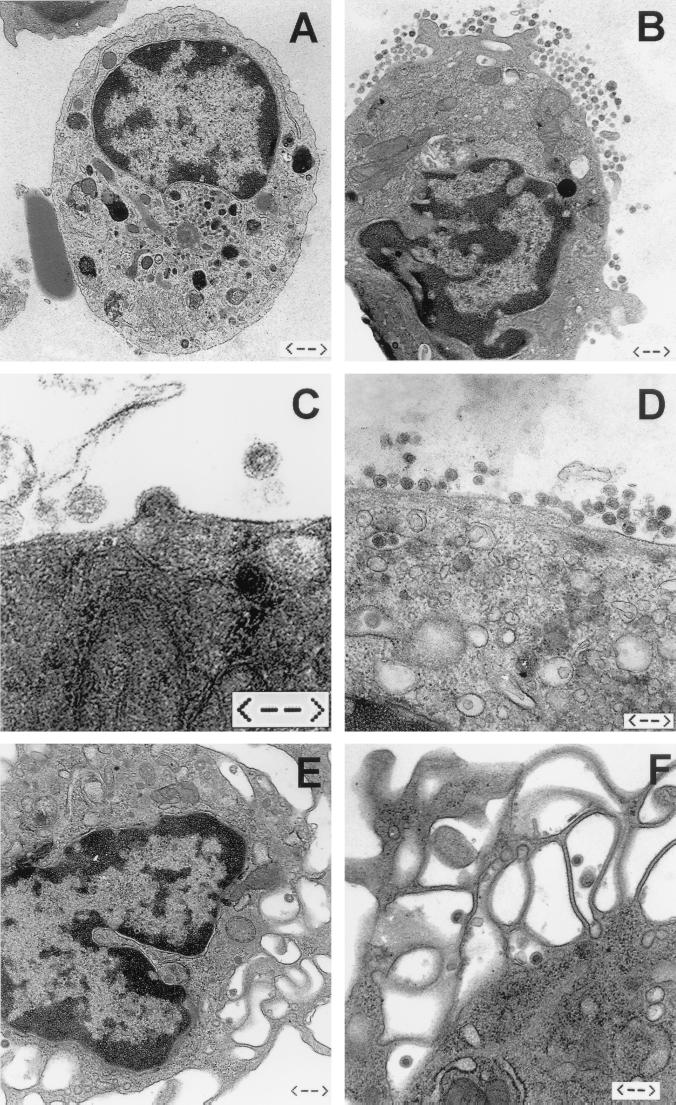
Like other neurovirulent retroviruses (1, 3, 7) MuLV-NT40 infects microglia cells in vivo (5). However, previous (5) and current (Table 1) results demonstrate that there is only very limited infectivity associated with microglia cells isolated ex vivo from clinically ill rats (MGexvi), both in the supernatants of cultivated MGexvi and in MGexvi directly seeded as infectious centers (Table 1; see also reference 5). This is in contrast to the relatively high numbers of microglia cells expressing viral proteins and RNA. In an attempt to resolve this discrepancy, we examined on the ultrastructural level whether retroviral particles were assembled at all in MGexvi. By means of thin-section electron microscopy, both virus assembly and abundant cell-released virus particles could easily be detected in association with MGexvi (Fig. 1B to D). We roughly estimated that—depending on the particular preparation—5 to 25% of MGexvi produced particles. Budding (Fig. 1C) and cell-released (Fig. 1D) particles were often found concentrated in certain regions of the cell, occasionally in a polar fashion. Cell-released particles were often present in similar amounts both extracellularly and in cytoplasmic vacuoles of the same cell. The free virus particles were enveloped and revealed round to ovoid, occasionally also slightly angular, shapes with diameters ranging between 100 and 140 nm. The appearance of viral cores differed. While most viruses contained a centrally located polygonal core, typical of mature type C particles, another fraction of particles showed electron-lucent centers surrounded by the ribonucleoprotein shell typical of immature virions (9, 10). The presence of the ribonucleoprotein shell in released particles points to an immature state of the virion and can be explained by a slow or ineffective maturation process (15, 41). Taken together, all the viral structures observed are consistent with the presence of typical type C mammalian retroviruses. All preparations from noninfected rats were devoid of retroviral particles, both MGexvi (Fig. 1A) and cells from other organs.
TABLE 1.
Retroviral infectivity associated with microglia and macrophages isolated ex vivo
| Type of value | Macrophagesa | Microgliaa | Microglia-SNb |
|---|---|---|---|
| Infectivity titer | 4.6 ± 0.2 | 1.7 ± 0.3 | 1.7 ± 0.3 |
| No. of rats | 23 | 8 | 16 |
Titers are expressed as log FFU per 106 cells ± SE. Macrophages and microglia cells were isolated from neonatally infected adult rats.
Titers are expressed as log FFU per milliliter of supernatant (SN) ± SE from 106 microglia cells cultured for 24 to 48 h.
FIG. 1.
Ultrastructural analyses of microglia and macrophages isolated ex vivo. Viral particles were never found in or around microglia cells isolated from noninfected rats (A) (bar = 1.1 μm). Abundant retroviral virions were associated with microglia cells (B to D) (bars = 0.6, 0.25, and 0.4 μm, respectively) and—although fewer in numbers—with peritoneal macrophages (E and F) (bars = 0.6 and 0.4 μm, respectively) from infected rats. Budding of retroviruses from the cell membrane of microglia could be detected frequently (C), while intracellular particles were rarely found in microglia (D) and peritoneal macrophages (F).
Low levels of infectivity associated with microglia.
To test whether infectivity of retroviruses produced by MGexvi might have been destroyed by the purification procedure used to isolate MGexvi, e.g., by shear forces (40), infected peritoneal macrophages (Table 1; see also reference 5) were subjected to the purification scheme. In contrast to infected microglia cells, infected peritoneal macrophages isolated ex vivo do release high amounts of infectivity into culture supernatants and score positive for productive infection in infectious center assays (5). No loss of infectivity was observed when infected macrophages were subjected to the method used to purify MGexvi (Table 2). From these results, we assume that this procedure would neither be harmful for any infectivity produced by MGexvi too, excluding the possibility of artificial reduction of any infectivity associated with MGexvi.
TABLE 2.
Influence of the purification procedure used to isolate microglia cells on retroviral infectivity
| Type of value | Macrophages | Macrophages with gradient centrifugationa | Macrophages with enzymatic digestion and gradient centrifugationb |
|---|---|---|---|
| Infectivity titerc | 4.6 ± 0.2 | 4.5 ± 0.1 | 4.3 ± 0.1 |
| No. of rats | 6 | 6 | 3 |
Peritoneal macrophages were subjected to the microglia cell purification scheme without prior enzymatic digestion.
Peritoneal macrophages were subjected to the microglia cell purification scheme with prior enzymatic digestion.
Titers are expressed as log FFU per 106 cells ± SE.
Production and/or scoring of infectious virus might have been hampered by cytotoxic factors (32, 34) potentially secreted by MGexvi. To control these possibilities, we added and incubated (37°C, 45 min) 5 × 104 FFU of NT40 virus with purified microglia and macrophages, respectively. We did not find any reduction of exogenously added infectivity, nor did we observe cytotoxic effects on the indicator cells, 3T3, after determining infectivity (Table 3). These results indicate that there is no mechanism(s) initiated by MGexvi and/or factors secreted from these cells that would reduce retroviral infectivity.
TABLE 3.
Influence of cytotoxic factors on retroviral infectivity produced by microglia cells
| Type of value | NT40a | Microglia plus NT40b | NT40/microglia plus NT40c | Microgliad |
|---|---|---|---|---|
| Infectivity titere | 3.9 ± 0.4 | 3.9 ± 0.7 | 3.8 ± 0.6 | 1.2 ± 0.5 |
| No. of rats | 2 | 2 | 2 | 2 |
NT40 virus stock (100 μl) containing log 4.7 FFU was subjected to incubation at 37°C for 45 min, and titers were determined.
Microglia cells (105) isolated from uninfected rats were incubated with 100 μl of NT40 virus stock containing log 4.7 FFU and kept at 37°C for 45 min.
Microglia cells (105) isolated from NT40-infected rats were incubated with 100 μl of NT40 virus stock containing log 4.7 FFU and kept at 37°C for 45 min.
Microglia cells (105) isolated from NT40-infected rats were incubated at 37°C for 45 min.
Titers of supernatants are expressed as log FFU per milliliter ± SE.
To investigate whether intracellular budding of retroviral virions (21) may have resulted in substantial amounts of infectious viral particles trapped inside MGexvi, we snap-froze (on dry ice) and thawed MGexvi. Infectivity (log 1.6 ± 0.6 [standard error (SE)] FFU/106 MGexvi; n = 4) did not increase after freezing-thawing (log 0.6 ± 0.3 [SE] FFU/106 MGexvi; n = 4), indicating that there were at least no functional viral particles inside MGexvi. Altogether, our data clearly point to defective retroviral replication in microglia, rather than to a mechanism(s) reducing any infectivity associated with MGexvi.
No defect of viral transcription in microglia.
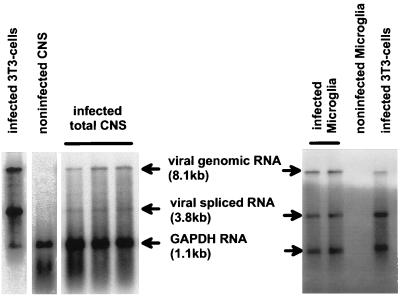
Initial defects of the retroviral replication cycle could have occurred during transcription of the retroviral genome (20). Therefore, mRNA from MGexvi was subjected to Northern blotting. In comparison to the viral mRNA patterns of total brains from rats and 3T3 cells infected with NT40, viral transcripts isolated from MGexvi exhibited similar molecular weights, representing viral full-length genomic and spliced RNAs (Fig. 2). Thus, considering the molecular weight and number of viral mRNA species, viral transcription appeared not to be altered in rat microglia.
FIG. 2.
Expression of viral transcripts in microglia isolated ex vivo. mRNA was isolated from microglia and total CNS from rats and 3T3 cells, respectively, subjected to Northern blotting, and probed with a virus-specific envelope and a glyceraldehyde-3-phosphate dehydrogenase (GAPDH) probe. Note that no obvious differences in the viral transcriptional patterns were detectable.
Defective processing of retroviral envelope proteins in microglia.
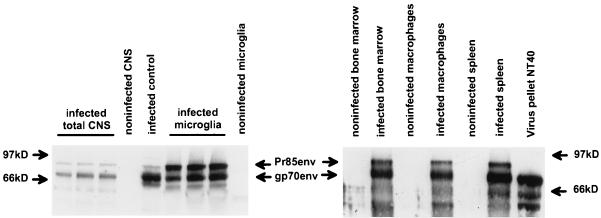
According to previous reports, processing of retroviral envelope proteins of molecular descendants of neurovirulent ecotropic viruses is sometimes altered (6, 21, 31). In MGexvi as well as in other cells (Fig. 3), we found envelope surface protein gp70 and its uncleaved precursor, Pr85. However, in MGexvi the uncleaved precursor protein, Pr85, persisted in high amounts throughout the early course of infection and cleavage of the envelope precursor protein, Pr85, into gp70 and p15E was not performed to completion (Fig. 3). This observation contrasted with the fate of Pr85 in other cells, e.g., peritoneal macrophages, in which the relative amounts of gp70 were greater than those of Pr85 (Fig. 3). Our results show that transcription and translation of the retroviral genome appeared to be normal in MGexvi; however, processing of the envelope precursor protein, Pr85, was not completed in MGexvi, compared to other cells.
FIG. 3.
Expression of viral envelope proteins. Proteins from microglia, total CNS, bone marrow, peritoneal macrophages, and spleen from rats and 3T3 cells, respectively, were subjected to immunoblotting and probed with a virus-specific anti-envelope antibody. In microglia, higher amounts of the uncleaved precursor protein, Pr85, than of the cleaved outer membrane portion, gp70, of the viral envelope protein were found. In control cells (3T3) and in total rat brain as well as in all other rat organs, mainly gp70 was found.
Envelope proteins are expressed intracellularly but not on the surface of microglia.
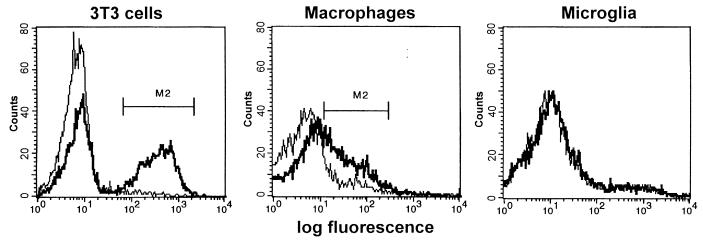
We previously demonstrated by flow cytometry that 10 to 20% of MGexvi expressed viral envelope proteins (5). While the proportion of MGexvi expressing viral envelope proteins—as determined by fluorescence-activated cell sorting analyses (5)—coincides with the number of MGexvi that produce viral particles shown here, it was surprising that in the face of abundant viral particles on many MGexvi, cell surface staining of envelope proteins was not successful and intracellular immunostaining had to be applied (5). We repeated the cell surface staining of viral envelope proteins using biotinylated monoclonal antibody 48 (29), recognizing NT40 envelope proteins. While surface staining of viral envelope proteins could be demonstrated on cells from the peritoneal cavity, i.e., macrophages, MGexvi was negative for viral envelope staining (Fig. 4). This indicates that transport of envelope proteins to the cell surface of microglia is altered in vivo.
FIG. 4.
Surface expression of viral envelope proteins on microglia isolated ex vivo. Microglia and peritoneal macrophages isolated from rats and 3T3 cells were stained with monoclonal antibody 48 (thick line), specific for MuLV-NT40 envelope proteins, or with an irrelevant antibody of the same type (thin line). Surface expression of viral envelope proteins was detected in peritoneal macrophages isolated from NT40-infected rats and in 3T3 cells infected with NT40 but not in microglia isolated from infected rats.
DISCUSSION
The two major findings of this paper are (i) that microglia isolated ex vivo from rats neonatally inoculated with neurovirulent MuLV-NT40 released abundant retroviral particles and (ii) that the vast majority of these virions, however, were not infectious. Furthermore, we demonstrated that the defect in the retroviral replication cycle in microglia was associated with altered processing of the viral envelope proteins.
The pathogeneses of various retroviral encephalopathies—including those caused by human immunodeficiency virus and simian immunodeficiency virus—depend on retroviral infection of microglia cells. While several in vitro studies of interactions of neurovirulent retroviruses with monocytic cells like fetal or neonatal microglia exist, investigations of microglia isolated from juvenile or adult individuals are rare (14, 33). Here, we present electron microscopic evidence that microglia cells isolated from neonatally infected, adult rats are a frequent target for neurovirulent MuLV-NT40 in vivo and that infected microglia gave rise to high numbers of progeny virions. This result was not anticipated, since retroviral infectivity associated with infected microglia is known to be very low (5, 21). Our finding was also surprising in the face of ultrastructural results for neonatal microglia cells infected in vitro with neurovirulent MuLV-FrCasE (21) as well as for astrocytes infected in vitro with neurovirulent MuLV-TS-1Mo (31): in both in vitro studies, retroviral particles were detected only occasionally and located primarily intracellularly. The remarkable differences in the locations and in the numbers of retroviral particles associated with glial cells are probably due to the experimental procedure, i.e., whether glial cells were infected in vitro or in vivo.
We found the morphology of the retroviral virions associated with microglia to be that of typical type C particles (9, 10). Some of those virions had an electron-dense center, indicating that cleavage of Gag polyprotein and rearrangement of the cleavage products had occurred during assembly. This step is performed by the viral protease, and it shows that not only Gag but also Pol-Pro polyprotein, the latter in a functional conformation, was incorporated into virus particles.
Fluorescence-activated cell sorting analysis revealed that retroviral envelope proteins were not expressed on the surface of microglia cells, in contrast to other cells like peritoneal macrophages. As incorporation of MuLV envelope proteins into viral particles does not occur in the absence of cell surface expression of retroviral envelope proteins, retroviral particles released from rat microglia were probably devoid of envelope proteins and thus not capable of initiating the retroviral replication cycle by binding to their cellular receptor. Similarly, virions released from microglia infected in vitro with neurovirulent MuLV did not contain envelope proteins in sufficient amounts and were not infectious (21).
According to our data and those of others (21, 31), the reason for the defect in the packaging of envelope proteins into retroviral particles appears to be a failure to cleave the envelope precursor protein into outer surface and transmembrane proteins. Whether the processing defect is based on the functional absence of the cellular protease, a furin-type proprotein convertase (2), or is rather due to an abnormal translocation of envelope precursor protein (31), or both, remains to be resolved. Comparison with other cells and tissues (e.g., bone marrow and spleen [Fig. 3]) revealed that the (relative) inability to cleave envelope precursor proteins was restricted to microglia cells. It is noteworthy that persistence of Borna disease virus in the CNS might result from insufficient proteolytic cleavage of envelope proteins usually performed by a furin-like protease (28). This indicates that this type of protease might functionally be on a low level within the CNS. In favor of an aberrant intracellular translocation of the envelope precursor protein and thus a deprivation of proteolytic processing is the observation that the envelope precursor protein accumulates around the nucleus rather than being exported to Golgi vesicles in astrocytes infected with neurovirulent MuLV in vitro (31). The principle of aberrant protein translocation might be of a more general relevance for the pathogenesis of infectious neurodegenerative diseases, since the process of protein translocation appears to be significant for the induction of neurodegeneration found after scrapie-like disease as well (13).
Experiments employing genetically modified neural transplants, i.e., cells secreting retroviral envelope proteins (23), as well as transgenic mouse models (16, 35, 42) revealed that the presence of envelope proteins within brain tissue is not sufficient (alone) to induce spongiform neurodegeneration. Rather, these studies indicated that retroviral envelope proteins need to be synthesized by endogenous brain cells in order to initiate brain damage. Lynch et al. presented evidence that, for all brain cells infection of microglia alone is sufficient to induce neurodegeneration (23). Our results demonstrate that while substantial amounts of all primary viral gene products were synthesized within microglia in vivo, processing of envelope proteins was defective. As a consequence, microglia accumulated retroviral envelope proteins intracellularly and shed vast numbers of viral particles that were mostly devoid of retroviral envelope proteins. Which of these pathways—aberrant accumulation of retroviral envelope proteins within microglia or neuronal and glial overflow with envelope-defective retroviral particles—finally leads to neuronal degeneration demands further investigation. It will also be important to find out whether these observations can be made for other retroviral infections of the CNS, including neuro-AIDS.
ACKNOWLEDGMENTS
We thank R. Martini and S. Sopper for helpful discussions and critical reading of the manuscript.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (Cz 56/1-2) and from the Bundesministerium für Bildung, Wissenschaft, Forschung und Technologie (II-068-88); the Wilhelm Sander-Stiftung; and the Max-Planck-Forschungspreis (V.T.).
REFERENCES
- 1.Baszler T V, Zachary J F. Murine retroviral-induced spongiform neuronal degeneration parallels resident microglia cell infection. Lab Investig. 1990;63:612–623. [PubMed] [Google Scholar]
- 2.Bedgood R M, Stallcup M R. A novel intermediate in processing of murine leukemia virus envelope glycoproteins. J Biol Chem. 1992;267:7060–7065. [PubMed] [Google Scholar]
- 3.Brinkmann R, Schwinn A, Narayan O, Zink C, Kreth H, Roggendorf W, Dörries R, Schwender S, Imrich H, ter Meulen V. Human immunodeficiency virus in microglia: correlation between cells infected in the brain and cells cultured from infectious brain tissue. Ann Neurol. 1992;31:361–365. doi: 10.1002/ana.410310403. [DOI] [PubMed] [Google Scholar]
- 4.Czub M, Czub S, McAtee F J, Portis J L. Age-dependent resistance to murine retrovirus-induced spongiform neurodegeneration results from central nervous system-specific restriction of virus replication. J Virol. 1991;65:2539–2544. doi: 10.1128/jvi.65.5.2539-2544.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Czub M, Czub S, Rappold M, Mazgareanu S, Schwender S, Demuth M, Hein A, Dörries R. Murine leukemia virus induced neurodegeneration of rats: enhancement of neuropathogenicity correlates with enhanced viral tropism for macrophages, microglia, and brain vascular cells. Virology. 1995;214:239–244. doi: 10.1006/viro.1995.0027. [DOI] [PubMed] [Google Scholar]
- 6.Czub S, Lynch W P, Czub M, Portis J L. Kinetic analysis of spongiform neurodegenerative disease induced by a highly virulent murine retrovirus. Lab Investig. 1994;70:711–723. [PubMed] [Google Scholar]
- 7.Czub S, Müller J G, Czub M, Müller-Hermelink H-K. Nature and sequence of simian immunodeficiency virus-induced central nervous system lesions: a kinetic study. Acta Neuropathol. 1996;92:487–498. doi: 10.1007/s004010050551. [DOI] [PubMed] [Google Scholar]
- 8.Dawson V L, Dawson T M, Uhl G R, Snyder S H. Human immunodeficiency virus type 1 coat protein neurotoxicity mediated by nitric oxide in primary cortical cultures. Proc Natl Acad Sci USA. 1993;90:3256–3259. doi: 10.1073/pnas.90.8.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank H, Schwarz H, Graf T, Schäfer W. Properties of mouse leukemia viruses. XV. Electron microscopic studies on the organization of Friend leukemia virus and other mammalian C-type viruses. Z Naturforsch. 1978;33c:124–138. doi: 10.1515/znc-1978-1-224. [DOI] [PubMed] [Google Scholar]
- 10.Gelderblom H R, Özel M, Gheysen D, Reupke H, Winkel T, Herz U, Grund C, Pauli G. Morphogenesis and fine structure of lentiviruses. In: Schellekens H, Horzinek M C, editors. Animal models in AIDS. Amsterdam, The Netherlands: Elsevier Science Publishers B.V.; 1990. pp. 1–26. [Google Scholar]
- 11.Giulian D, Vaca K, Noonan C A. Secretion of neurotoxins by mononuclear phagocytes infected with HIV-1. Science. 1990;250:1593–1596. doi: 10.1126/science.2148832. [DOI] [PubMed] [Google Scholar]
- 12.Hasenkrug K J, Robertson S J, Portis J L, McAtee F, Nishio J, Chesebro B. Two separate envelope regions influence induction of brain disease by a polytropic murine retrovirus (FMCF98) J Virol. 1996;70:4825–4828. doi: 10.1128/jvi.70.7.4825-4828.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hegde R S, Mastrianni J A, Scott M R, DeFea K A, Tremblay P, Torchia M, DeArmond S J, Prusiner S B, Lingappa V R. A transmembrane form of the prion protein in neurodegenerative disease. Science. 1998;279:827–834. doi: 10.1126/science.279.5352.827. [DOI] [PubMed] [Google Scholar]
- 14.Hein A, Czub S, Xiao L X, Schwender S, Dörries R, Czub M. Effects of adoptive immune transfers on murine leukemia virus-infection of rats. Virology. 1995;211:408–417. doi: 10.1006/viro.1995.1423. [DOI] [PubMed] [Google Scholar]
- 15.Katoh I, Yoshinaka Y, Rein A, Shibuya M, Odaka T, Oroszlan S. Murine leukemia virus maturation: protease region required for conversion from “immature” to “mature” core form and for virus infectivity. Virology. 1985;145:280–292. doi: 10.1016/0042-6822(85)90161-8. [DOI] [PubMed] [Google Scholar]
- 16.Kay D G, Gravel C, Pothier F, Laperriere A, Robitaile Y, Jolicoeur P. Neurological disease induced in transgenic mice expressing the env gene of the Cas-Br-E murine retrovirus. Proc Natl Acad Sci USA. 1993;90:4538–4542. doi: 10.1073/pnas.90.10.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kay D G, Gravel C, Robitaille Y, Jolicoeur P. Retrovirus-induced spongiform myeloencephalopathy in mice: regional distribution of infected target cells and neuronal loss occurring in the absence of viral expression in neurons. Proc Natl Acad Sci USA. 1991;88:1281–1285. doi: 10.1073/pnas.88.4.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koenig S, Gendelman H E, Orenstein J M, DalCanto M C, Pezeshkpour G H, Yungblut M, Janotta F, Aksamit A, Martin M A, Fauci A S. Detection of AIDS virus in macrophages in brain tissues from AIDS patients with encephalopathy. Science. 1986;233:1089–1093. doi: 10.1126/science.3016903. [DOI] [PubMed] [Google Scholar]
- 19.Lawson L J, Perry V H, Dri P, Gordon S. Heterogeneity in the distribution and morphology of microglia in the normal, adult mouse brain. Neuroscience. 1991;39:151–170. doi: 10.1016/0306-4522(90)90229-w. [DOI] [PubMed] [Google Scholar]
- 20.Lund A H, Duch M, Pedersen F S. Transcriptional silencing of retroviral vectors. J Biomed Sci. 1996;3:365–378. doi: 10.1007/BF02258042. [DOI] [PubMed] [Google Scholar]
- 21.Lynch W P, Brown W J, Spangrude G J, Portis J L. Microglia infection by a neurovirulent murine retrovirus results in defective processing of envelope protein and intracellular budding of virus particles. J Virol. 1994;68:3401–3409. doi: 10.1128/jvi.68.5.3401-3409.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynch W P, Czub S, McAttee F J, Hayes S F, Portis J L. Murine retrovirus-induced spongiform encephalopathy: productive infection of microglia and cerebellar neurons in accelerated CNS disease. Neuron. 1991;7:365–379. doi: 10.1016/0896-6273(91)90289-c. [DOI] [PubMed] [Google Scholar]
- 23.Lynch W P, Snyder E Y, Qualtiere L, Portis J L, Sharpe A H. Late virus replication events in microglia are required for neurovirulent retrovirus-induced spongiform neurodegeneration: evidence from neural progenitor-derived chimeric mouse brains. J Virol. 1996;70:8896–8907. doi: 10.1128/jvi.70.12.8896-8907.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Masuda M, Hoffman P M, Ruscetti S K. Viral determinants that control the neuropathogenicity of PVC-211 murine leukemia virus in vivo determine brain capillary endothelial cell tropism of the virus in vitro. J Virol. 1993;67:4580–4587. doi: 10.1128/jvi.67.8.4580-4587.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazgareanu S, Müller J G, Czub S, Schimmer S, Bredt M, Czub M. Suppression of rat bone marrow cells by Friend murine leukemia virus envelope-proteins. Virology. 1998;242:357–365. doi: 10.1006/viro.1997.8998. [DOI] [PubMed] [Google Scholar]
- 26.Portis J L, Lynch W P. Dissecting the determinants of neuropathogenesis of the murine oncornaviruses. Virology. 1998;247:127–136. doi: 10.1006/viro.1998.9240. [DOI] [PubMed] [Google Scholar]
- 27.Power C, McArthur J C, Nath A, Wehrly K, Mayne M, Nishio J, Langelier T, Johnson R T, Chesebro B. Neuronal death induced by brain-derived human immunodeficiency virus type 1 envelope genes differs between demented and nondemented AIDS patients. J Virol. 1998;72:9045–9053. doi: 10.1128/jvi.72.11.9045-9053.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richt J A, Fürbringer T, Koch A, Pfeuffer I, Herden C, Bause-Niedrig I, Garten W. Processing of the Borna disease virus glycoprotein gp94 by the subtilisin-like endoprotease furin. J Virol. 1998;72:4528–4533. doi: 10.1128/jvi.72.5.4528-4533.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robertson M N, Miyazawa M, Mori S, Caughey B, Evans L, Hayes S F, Chesebro B. Production of monoclonal antibodies reactive with a denatured form of the Friend murine leukemia virus gp70 envelope protein: use in a focal infectivity assay, immunohistochemical studies, electron microscopy and Western blotting. J Virol Methods. 1991;34:255–271. doi: 10.1016/0166-0934(91)90105-9. [DOI] [PubMed] [Google Scholar]
- 30.Robertson S J, Hasenkrug K J, Chesebro B, Portis J. Neurologic disease induced by polytropic murine retroviruses: neurovirulence determined by efficiency of spread to microglia cells. J Virol. 1997;71:5287–5294. doi: 10.1128/jvi.71.7.5287-5294.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shikova E, Lin Y C, Saha K, Brooks B R, Wong P K Y. Correlation of specific virus-astrocyte interactions and cytopathic effects induced by ts1, a neurovirulent mutant of Moloney murine leukemia virus. J Virol. 1993;67:1137–1147. doi: 10.1128/jvi.67.3.1137-1147.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith T A, Davis E, Carpenter S. Endotoxin treatment of equine infectious anaemia virus-infected horse macrophage cultures decreases production of infectious virus. J Gen Virol. 1998;79:747–755. doi: 10.1099/0022-1317-79-4-747. [DOI] [PubMed] [Google Scholar]
- 33.Sopper S, Demuth M, Stahl-Hennig C, Hunsmann G, Plesker R, Coulibaly C, Czub S, Ceska M, Koutsilieri E, Riederer P, Brinkmann R, Katz M, ter Meulen V. The effect of simian immunodeficiency virus infection in vitro and in vivo on the cytokine production of isolated microglia and peripheral macrophages from rhesus monkey. Virology. 1996;220:320–329. doi: 10.1006/viro.1996.0320. [DOI] [PubMed] [Google Scholar]
- 34.Taylor M D, Korth M J, Katze M G. Interferon treatment inhibits the replication of simian immunodeficiency virus at an early stage: evidence for a block between attachment and reverse transcription. Virology. 1998;241:156–162. doi: 10.1006/viro.1997.8964. [DOI] [PubMed] [Google Scholar]
- 35.Toggas S M, Masliah E, Rockenstein E M, Rall G F, Abraham C R, Mucke L. Central nervous system damage produced by expression of the HIV-1 coat protein gp120 in transgenic mice. Nature. 1994;367:188–193. doi: 10.1038/367188a0. [DOI] [PubMed] [Google Scholar]
- 36.Tyor W R, Glass J D, Griffin J W, Becker P S, McArthur J C, Bezman L, Griffin D E. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992;31:349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- 37.Weis S, Neuhaus B, Mehraein P. Activation of microglia in HIV-1 infected brains is not dependent on the presence of HIV-1 antigens. Neuroreport. 1994;5:1514–1516. doi: 10.1097/00001756-199407000-00026. [DOI] [PubMed] [Google Scholar]
- 38.Wesselingh S L, Griffin D E. Local cytokine responses during acute and chronic viral infections of the central nervous system. Semin Virol. 1994;5:457–463. [Google Scholar]
- 39.Wiley C A, Schrier R D, Nelson J A, Lampert P W, Oldstone M B A. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci USA. 1986;83:7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Witter R, Frank H, Moennig V, Hunsmann G, Lange J, Schäfer W. Properties of mouse leukemia viruses. Virology. 1973;54:330–345. doi: 10.1016/0042-6822(73)90147-5. [DOI] [PubMed] [Google Scholar]
- 41.Yoshinaka Y, Luftig R B. Murine leukemia virus morphogenesis: cleavage of P70 in vitro can be accompanied by a shift from a concentrically coiled internal strand (“immature”) to a collapsed (“mature”) form of the virus core. Proc Natl Acad Sci USA. 1977;74:3446–3450. doi: 10.1073/pnas.74.8.3446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Yu Y E, Choe W, Zhang W, Stoica G, Wong P K Y. Development of pathological lesions in the central nervous system of transgenic mice expressing the env gene of ts1 Moloney murine leukemia virus in the absence of the viral gag and pol genes and viral replication. J Neurovirol. 1997;3:274–282. doi: 10.3109/13550289709029468. [DOI] [PubMed] [Google Scholar]