Abstract
Ligand‐protected heterometallic nanoclusters in contrast to homo‐metal counterparts show more broad applications due to the synergistic effect of hetero‐metals but their controllable syntheses remain a challenge. Among heterometallic nanoclusters, monovalent Ag‐Cu compounds are rarely explored due to much difference of Ag(I) and Cu(I) such as atom radius, coordination habits, and redox potential. Encouraged by copper‐catalyzed alkyne‐azide cycloaddition (CuAAC) reaction, comproportionation reaction of Cu(II)X2 and Cu(0) in the presence of (PhC≡CAg)n complex and molybdate generated a core‐shell peanut‐shaped 66‐nuclear Ag(I)‐Cu(I) heterometallic nanocluster, [(Mo4O16)2@Cu12Ag54(PhC≡C)50] (referred to as Ag54Cu12 ). The structure and composition of Ag‐Cu heterometallic nanocluster are fully characterized. X‐ray single crystal diffraction reveals that Ag54Cu12 has a peanut‐shaped silver(I)/copper(I) heterometallic nanocage protected by fifty phenylacetylene ligands in µ 3–modes and encapsulated two mutually twisted tetramolybdates. Heterometallic nanocage contains a 54‐Ag‐atom outer ellipsoid silver cage decorated by 12 copper inside wall. Nanosized Ag54Cu12 is a n‐type narrow‐band‐gap semiconductor with a good photocurrent response. Preliminary experiments demonstrates that Ag54Cu12 itself and activated carbon supported Ag54Cu12/C are effective catalysts for 1,3‐dipole cycloaddition between alkynes and azides at ambient conditions. The work provides not only a new synthetic route toward Ag(I)‐Cu(I) nanoclusters but also an important heterometallic intermediate in CuAAC catalytic reaction.
Keywords: 1,3‐dipole cycloadditions; CuAAC reaction; Heterometallic nanoclusters; Intermediate
A 66‐nuclear all‐alkynyl protected (Mo4O16)2@Cu12Ag54 (Ag54Cu12 ) nanocluster is isolated by copper‐catalyzed alkyne‐azide cycloaddition (CuAAC) reaction by using silver‐phenylacetylene precursor during CuAAC reaction without azides. Ag54Cu12 has a peanut‐shaped Ag(I)/Cu(I) nanocage protected by fifty PhC≡C− ligands in µ 3–modes and encapsulates two mutually twisted Mo4O16 8−. Ag54Cu12 itself and Ag54Cu12/C are effective catalysts for 1,3‐dipole cycloaddition reaction at ambient conditions.
1. Introduction
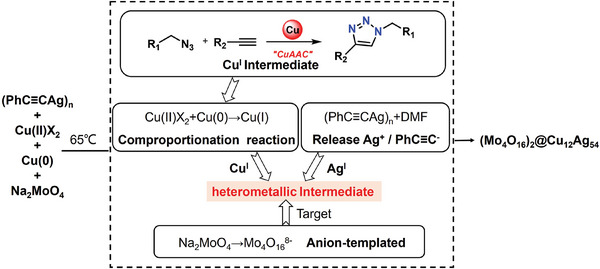
High‐nuclearity ligand‐protected heterometallic nanoclusters are more attractive than homo‐metal nanoclusters for potential applications such as catalysis, photoluminescence, electrochemistry, and other fields, which is due to the synergistic effect of hetero‐metal nanoclusters on physicochemical properties.[ 1 ] The key to tuning physicochemical behavior of heterometallic clusters is the preparation of nanoclusters with controllable composition/doping sites.[ 2 ] Generally, heteroatoms that can be dope into silver nanoclusters are noble metals with similar radius in the periodic table.[ 3 ] Conventional doping methods are based on co‐reduction, metal exchange, metal deposition of atoms/ions [(also known as anti‐galvanic reaction (AGR)], or a combination of these strategies.[ 4 ] These strategies are less effective in controlling reduction of double metal salts and formation of heteroatomic nanoclusters. A search reveals that the availability of heteronuclear Ag(I)‐Cu(I) alkynyl clusters via Cu doping are rare.[ 5 ] As far as dopants of Cu element are concerned, the standard potential of Cu2+/Cu+ couple is as low as 159 mV, which indicates that the +1 oxidation state of copper is susceptible to be oxidized to much more stable +2 state. Furthermore, the aggregative nature of Cu(I) alkynyl causes a larger challenge in isolation of discrete Ag(I)‐Cu(I) alkynyl nanoclusters. To solve this puzzle, the comproportionation reaction based copper‐catalyzed alkyne‐azide cycloaddition (CuAAC) reaction could provide a route toward stable homogeneous high‐nuclearity monovalent copper alkynyl clusters.[ 6 ] The origin of Cu(I) intermediate is generated by Cu(II) with various Cu(0) sources such as wire, turnings, powder and nanoparticles in CuAAC reaction. This reaction could overcome potential difference to produce monovalent copper,[ 7 ] which in combination with a variety of alkynyl precursors give metal nanoclusters with tunable composition, structure, and properties.[ 8 ] In spite of success in homogeneous copper, the implementation of the experiments remains a challenge in heterogeneous Cu/Ag nanoclusters(.
On the other hand, Cu(I) alkynyl complexes have been extensively studied as key intermediates in copper‐catalyzed transformations of alkynes as well as in click chemistry synthesis of 1,2,3‐triazole.[ 9 ] The widely utilized CuAAC involves generation of CuI‐alkynyl species of various nuclearities.[ 10 ] Reported examples include Cu33 and Cu62,[ 11 ] and Cu20 [ 12 ] nanocluster.[ 13 ] Apart from these homogeneous intermediates, there are only sporadic reports on heterometallic intermediates involving the click reaction. However, understanding of interaction of Cu(I) and hetero‐metals in heterogeneous CuAAC catalytic systems is not well clear. Recently, Zhu et al captured three crucial Au4Cu4−π‐alkyne intermediates and discussed an abnormal mechanism in CuAAC reaction, which is different from comproportionation reaction to dehydrogenate.[ 14 ] Therefore, the revealing of Cu(I) and Ag(I) interaction in heterometallic Cu/Ag clusters is of significance in click chemistry of CuAAC reaction. It is still an open question whether heterometallic intermediates can be achieved by comproportionation reaction and alkyne precursor. Inspired by the widely used methodology for Cu‐ethyne nanoclusters, we would like to explore the similar story in Ag‐ethyne cousins.
In order to generate new heterometallic nanoclusters and intermediates in CuAAC reaction, a series of Ag‐Cu‐alkyne products in the system need to be obtained by alkyne ligands. Our strategy is to creatively use silver‐phenylacetylene precursor instead of alkyne or copper‐alkyne ligand during CuAAC reaction without azides. Silver‐phenylacetylene precursor provides alkyne source for CuAAC intermediate because alkynyl as a π‐acid ligand can bind to d10 ions of Au, Ag, Cu via σ‐π modes and form various metal‐carbon interactions.[ 15 ] As such, the pronounced interactions of alkynyl in a variety of coordination modes may affect the physicochemical properties of metal clusters due to metal atom kernel and metal‐alkyne interactions.[ 16 ] This allows the atomic‐level understanding of structure‐property correlations, which in turn favours the targeted preparation of metal nanoclusters.[ 17 ] In addition, the slow release of Ag+ from silver‐phenylacetylene in a weakly reducing DMF can bind Cu+ in situ.[ 18 ] As in Scheme 1 , we envisage that the polymolydate anion template will target Ag and Cu metal centres to aggregate in a core‐shell structure, resulting in the formation of Ag‐Cu clusters.
Scheme 1.
The synthesis route of Ag54Cu12 nanocluster.
Herein, we present a 66‐nuclear all‐alkynyl protected peanut‐shaped silver(I)/copper(I) heterometallic nanocluster, [(Mo4O16)2@Cu12Ag54(PhC≡C)50] (Ag54Cu12), which was synthesized by using sliver‐alkyne complex and protective alkyne ligands in the presence of molybdate. The two tetramolybdates [Mo4O16]8− mutually twisted ca. 24° were encapsulated by Cu12Ag54 cage to form a core‐shell peanut‐shaped (Mo4O16)2@Cu12Ag54 nanocluster. The surface PhC≡C− ligands are ligated Ag and Cu in five types coordination modes to stabilize the nanocluster. The structure and composition of Ag54Cu12 were determined by high‐resolution electrospray ionization mass spectrometry (ESI‐TOF‐MS), X‐ray diffraction (XRD), energy‐dispersive X‐ray spectroscopy (EDS), Fourier‐transform infrared (FT‐IR) spectroscopy, and X‐ray photoelectron spectroscopy (XPS). Furthermore, a solid‐state UV–vis absorption spectrum of Ag54Cu12 showed a narrow‐bandgap semiconductor with a good photocurrent response. Ag54Cu12 itself and Ag54Cu12 loaded with actived carbon as catalysts were applied in [3+2] cycloaddition reactions between alkynes and azides at ambient conditions.
2. Results and Discussion
Single X‐ray diffraction analysis indicated Ag54Cu12 nanocluster crystalized in triclinic P1 space group.[ 19 ] The asymmetry unit contains a whole Ag54Cu12 cluster (Figure 1a). The shape of the 66‐nucleus cluster resembles a peanut with Cu12Ag54 ellipsoidal cage as peanut shell and two Mo4O16 8− groups as peanut seeds (Figure 1b). Notably, the nuclearity of Ag54Cu12 is comparable with those of the previously reported monovalent Ag‐Cu heterometallic nanocluster, namely [Ag74‐xCuxO12(PhC≡C)50]27 [ 18a ] and [Ag40.13Cu13.87S19(tBuS)20(tBuSO3)12].[ 20 ] The overall structure contains 54 Ag atoms, 12 Cu atoms, 50 PhC≡C− ligands, and two Mo4O16 units (Figure 1a). The Cu12Ag54 cage consists of an Ag shell on the periphery and Cu embedded in the inner shell, which generates Ag‐Cu heterometallic interactions (Figure 1c,d). The shell of peanut‐shaped Cu12Ag54 consists of silver triangles and tetragons with short Ag···Ag interactions of 2.84–3.41 Å (Figure 1e), whose surface is covered by 50 PhC≡C− groups in µ 3–η 1:η 1:η 1, µ 3–η 1:η 1:η 2, µ 3–η 1:η 2:η 2, and µ 3–η 2:η 2:η 2 coordination modes with Ag‐C distances of 2.01(2)−2.704(16) Å and Cu‐C distances of 1.815(18)–1.888(13) Å. Inner wall twelve Cu atoms coordinate to carbon atoms of PhC≡C− and oxygen atoms of Mo4O16 8− group, which are interacted with Ag atoms of shell with short Cu···Ag distances of 2.734(3)−3.056(3). Considering the arrangement of copper atoms, twelve Cu atoms form a Cu12 motif comprised of pentagons and triangles (Figure 1f). The 3D packing modes of Ag54Cu12 nanoclusters in b and c directions are shown in Figure S1 (Supporting Information).
Figure 1.
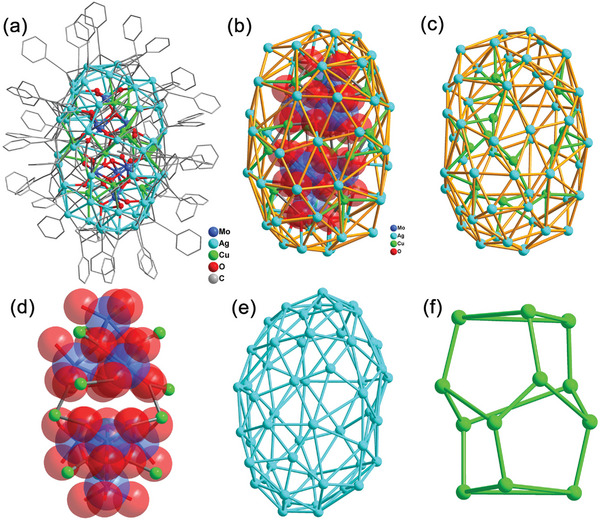
a) Full structure of Ag54Cu12 nanocluster. b) The core‐shell peanut shaped of Ag54Cu12 . c) The Cu12Ag54 ellipsoid cage as peanut shell. d) The two tetrabolydates serve as seeds for the nanocluster. e) Ag54 shell. f) Cu12 motif.
The peanut kernel consists of two independent Mo4O16 8− tetramolybdates as seeds (Figure S3a,b, Supporting Information), which show similar cubane‐like structures with T d symmetry, with Mo─O bond distances ranging from 1.705(10) to 2.288(19) Å.[ 21 ] The bond valence for Mo ions by valence sum (BVS) calculations (Table S2, Supporting Information) demonstrated +6. The [Mo4O16]8− anion was self‐assembled in situ from molybdate during the solvothermal process. Surprisingly, in contrast to the orientation of Mo4O16 8− groups in our recently reported [Cu3Mo8O32]10−,[ 22 ] the two Mo4O16 8− groups in Ag54Cu12 are not overlapped but staggered by ≈24 degrees (Figure S3c, Supporting Information).
Compared to reported metal nanoclusters encapsulating multiple polyoxometallates (POMs), the twisted configuration of two unconnected [Mo4O16]8‐ units is unique (reports listing these high nuclear clusters are in Table S3, Supporting Information). The unconnected arrangement of two POMs allows more oxygen sites to be exposed, which is beneficial for enhancing the template effect. Careful check revealed that two [Mo4O16]8− units are bonded to 12 Cu atoms through 6 µ 2‐O and 24 terminal oxygen atoms via weak Cu···O bonds, and to 34 Ag atoms via 20 terminal oxygen atoms. The 20 Ag atoms uncoordinated to POMs were found to be embedded into Ag54 shell by Ag─Cu, Ag─C, and Ag─Ag interactions. The Cu12Ag54 shell was further consolidated by all alkyne ligands.
To investigate the solution behavior and composition, Ag54Cu12 cluster was monitored utilizing electrospray ionization‐time of flight‐mass spectrometry (ESI‐TOF‐MS) in the mass‐to‐charge ratio (m/z) range of 1000–20000. As shown in Figure 2 , Ag54Cu12 was dissolved in a mixed solvent of methanol and dichloromethane. In positive ion mode, peaks with two valence states were observed in the m/z ranges of 3400–3500 and 4200–4400, respectively. The main peak b3 at the highest abundance m/z = 4379.85 can be attributed to [(Mo4O16)2@Cu12Ag54(PhC≡C)50(CH3OH)(CH3OH2)3]3+ (cal. 4379.91), which was considered to be the Ag54Cu12 molecular ion peak with three protonated MeOH molecules.
Figure 2.
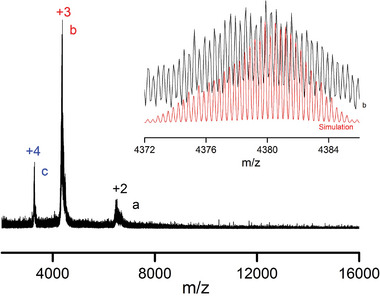
ESI‐TOF‐MS spectra of Ag54Cu12 .
The composition of hetero‐metal cluster was supported by PXRD and FI‐IR spectra (Figures S4 and S5, Supporting Information, Experimental Section). XPS spectra shown the presence of Ag, Cu, Mo, C, and O elements, which was consistent with the results of EDS‐mapping (Figures S6 and S7a, Supporting Information). High‐resolution spectra clearly illustrated the valences of metal ions of Ag54Cu12 . As shown in Figure S7b (Supporting Information), XPS data of the Ag 3d5/2 and Ag 3d3/2 binding energies are 368.26 and 374.24 eV, respectively, confirming that Ag atoms in the cluster are positively charged.[ 23 ] The Mo binding energy at 232.9 and 235.2 eV in Figure S7c (Supporting Information) can be attributed to Mo6+ 3d5/2 and 3d3/2 spin‐splitting slits.[ 24 ] Figure S7d (Supporting Information) shows XPS peaks of monovalent Cu(I). The Cu LMM Auger chemical shift also showed monovalent CuI state (Figure S9, Supporting Information).
The solid‐state UV absorption spectra of Ag54Cu12 show a broadband absorption in the wavelength range 300–400 nm, which is attributed to the π→π* transition due to appearance of similar band in (PhC≡CAg)n precursor. The optical bandgap of Ag54Cu12 was determined by Tauc equation to be 1.98 eV, which is narrower than that of 2.79 eV in precursor (PhC≡CAg)n (Figure 3c). This suggests that Ag54Cu12 has potential as a narrow‐band‐gap semiconductor.[ 18 , 25 ] The photoelectrochemical behavior of Ag54Cu12 was tested in a typical three‐electrode system in a 0.2 m Na2SO4 aqueous solution. Compared with (PhC≡CAg)n, an obvious photocurrent response was detected upon on‐off cycling irradiation, indicating a better electron and hole separation efficiency of Ag54Cu12 . The photocurrent density could reach up to 0.11 µA cm−2, which remained nearly constant with increased test times, indicating high photophysical stability of Ag54Cu12 . Considering the board absorption semiconductor property of Ag54Cu12 , Mott‐Schottky (M‐S) measurements were performed at frequencies of 300, 500 and 1000 Hz in darkness (Figure 3d). The positive slope of the C−2‐E plot confirms that Ag54Cu12 is an n‐type semiconductor.[ 26 ] The flat band potential (EFB) was determined by the intersection to be ≈−1.3 V versus Ag/AgCl, corresponding to a potential of −0.68 V versus NHE. It is expected that Based on the previous reports, the conduction band edge of semiconductor should be ≈0.10 V more negative than the EFB. Therefore, it can be estimated that the conduction band (LUMO) of Ag54Cu12 is approximately −1.2 V versus NHE. The valence band (HOMO) of Ag54Cu12 is estimated to be 0.68 V versus NHE.
Figure 3.
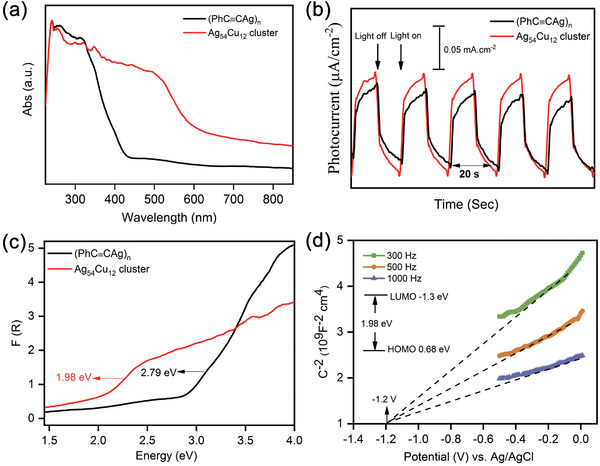
a) Solid‐state UV–vis absorption spectra and b)photocurrent responses under UV irradiation of (PhC≡CAg)n and Ag54Cu12 . c) Kubelka‐Munk function versus energy (eV) and Tauc plots of (PhC≡CAg)n and Ag54Cu12 . d) Mott‐Schottky plot of Ag54Cu12 in a 0.2 m Na2SO4 aqueous solution.
To understand the role of electron orbitals around the Fermi level, the total and partial densities of states (DOSs) of Ag54Cu12 were calculated. As is shown Figure 4a, the top of the valence band is mainly composed of metal Ag 4d, Cu 3d, and a few Mo 4d states. The bottom of the conduction band is dominated by metal Mo 4d, a few Ag 4d and Cu 3d, proving that the optical properties of Ag54Cu12 are determined by Ag, Cu and [Mo4O16]8− anion template. The HOMO‐LUMO bandgap difference is calculated to be 1.383 eV, in well agreement the experimental value. The lowest empty orbital of Ag54Cu12 is primarily located in the Mo 4d orbital on [Mo4O16]8−, while the highest occupied orbital is mainly concentrated in the Mo 4d and C 2p of PhC≡C− (Figure 4b). This indicates that [Mo4O16]8− anion has a significant effect on the optical properties of Ag54Cu12 . Several high lying occupied orbitals, such as HOMO‐1, HOMO‐2, HOMO‐3, and HOMO‐4, are almost entirely contributed by orbitals of peripheral ligand C≡C bonds (Figure S9, Supporting Information). According to Frontier molecular orbitals theory, LUMO is logically considered electrophilic, which may partly explain the fact that the outer O atoms of the non‐coordinated/exposed portion of Mo4O16 8− unit are less likely to further coordinate with the Ag atoms.
Figure 4.
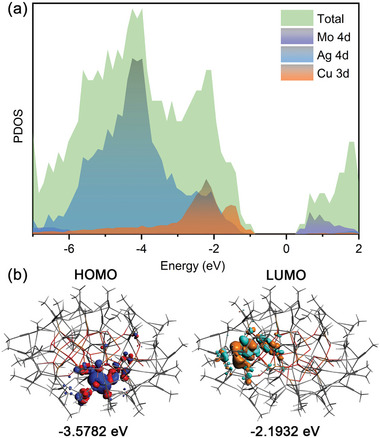
a) Total and partial DOS of Ag54Cu12 cluster. b) Frontier molecular orbitals: HOMO and LUMO.
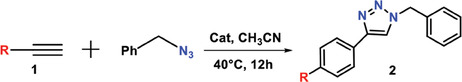
Copper‐catalyzed [3+2] Huisgen cycloadditions of terminal alkynes and organic azides (CuAAC) were the cornerstone of Meldal and Sharpless research, most of which involved homometallic Cu‐based materials.[ 27 , 28 ] To investigate and develop heterometallic 1,3‐dipole cycloaddition reaction catalysis, Ag54Cu12 was implemented by using phenylacetylene and benzyl azide at 40 °C as the model reaction. This reaction exhibits completely regioselectivity and is a powerful method for the rapid assembly of 1,4‐disubstituted‐1,2,3‐triazoles. The solution of Ag54Cu12 cluster was gradually added to a suspension of activated carbon/titanium dioxide in ethanol, and resulted mixture was stirred and centrifuged to give C/TiO2 supported nanocluster catalysts. Transmission electron microscopy (TEM) illustrated that all particles of as‐synthesized products were less than 2 nm in size, indicating good dispersion of the clusters loaded on the activated carbon (Figure S11, Supporting Information).
First, the influence of different solvents on the reaction was investigated. A relatively higher yield was realized in CH3CN (99%), compared to CH3OH solvent (Table 1 , entries 1–5). In addition, the effect of supports and loading capacity was also investigated under the same reaction conditions, and a high isolated yield of 99% was realized (Table 1, entry 1–2 and 4–5). The unsupported Ag54Cu12 NCs gave a lower isolated yield of 88% (Table 1, entry 6). Importantly, the control experiments showed that Ag54Cu12 significantly contributes to the cycloaddition process (Table 1, entries 7–11).
Table 1.
Cycloaddition of phenylacetylene and benzyl azide.

| ||||||
|---|---|---|---|---|---|---|
| Entry a) | Catalyst | Cu Loading [mmol, %] b) | Solvent | Time | Yield [%] c) | TOF |
| 1 | Ag54Cu12/C | 0.23 | CH3CN | 12 h | 80% | 28.98 |
| 2 | Ag54Cu12/C | 0.46 | CH3CN | 12 h | 99% | 17.93 |
| 3 | Ag54Cu12/C | 0.23 | CH3OH | 12 h | 45% | 16.30 |
| 4 | Ag54Cu12/TiO2 | 0.23 | CH3CN | 12 h | 13% | 4.71 |
| 5 | Ag54Cu12/TiO2 | 0.46 | CH3CN | 12 h | 70% | 12.68 |
| 6 | Ag54Cu12 | 2.3 | CH3CN | 12 h | 88% | 3.19 |
| 7 | C | — | CH3CN | 24 h | 0 | — |
| 8 | TiO2 | — | CH3CN | 24 h | 0 | — |
| 9 | AgSO3CF3 | — | CH3CN | 24 h | 0 | — |
| 10 | Na2MoO4 | — | CH3CN | 24 h | 0 | — |
| 11 | None | — | CH3CN | 24 h | 0 | — |
Reaction conditions: phenylacetylene (0.22 mmol), benzyl azide (0.2 mmol), acetonitrile (1 mL), 40 °C.
Catalyst loading defined as mmol% Cu‐based Ag54Cu12 cluster.
Isolated yield.
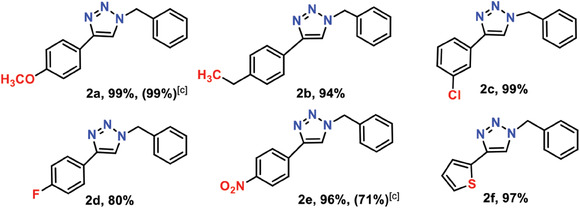
Based on optimized standard conditions, the generality of cycloaddition was explored for various aryl‐terminated acetylenes and the corresponding results were summarized in Table 2 . Good yields (90%–94%) were obtained for substituted aryl alkynes with both electron‐donating (–OCH3 and –CH3) and electron‐withdrawing (–F, –Cl, and –NO2) substituents (Table 2, entries 1–6). These results demonstrated the important interaction between Ag54Cu12 and activated carbon substrate.
Table 2.
Scope of benzyl azide with different alkynes.
Reaction conditions: phenylacetylene (0.22 mmol), benzyl azide (0.2 mmol), acetonitrile (1 mL), Ag54Cu12/C (Cu loading 0.46% mmol), 40 °C.
Isolated yield.
Reaction conditions: phenylacetylene (0.22 mmol), benzyl azide (0.2 mmol), acetonitrile (1 mL), Ag54Cu12 (Cu loading 2.3% mmol), 40 °C.
Interestingly, some 1,2,3‐triazole products were crystallized from reaction environment (Tables 1, entry 6 and 2, entries 7–8). The single crystal data of products were collected and solved (Figure S12a and Tables S5 and S6, Supporting Information), directly confirming their corresponding identity. Both Ag54Cu12 and Ag54Cu12/C as catalysts exhibited excellent cycle stability after six experiments (Figure S12b, Supporting Information). These results demonstrated that Ag54Cu12 NCs could be a high‐ performance molecular catalysts for CuAAC.
3. Conclusion
In conclusion, we have isolated an all‐alkyne protected silver(I)/copper(I) heterometallic nanocluster, [(Mo4O16)2@Cu12Ag54(PhC≡C)50] (Ag54Cu12 ), which was synthesized by the reduction of Cu (II) salt and copper powder in presence with (PhC≡CAg)n and Na2MoO4 under solvothermal method. Two mutually twisted [Mo4O16]8− anions were encapsulated by Cu12Ag54 cage to form a core‐shell peanut‐shaped (Mo4O16)2@Cu12Ag54 nanocluster. The surface PhC≡C− ligands are ligated to Ag and Cu in σ‐π modes to stabilize the nanocluster. XRD, XPS, and ESI‐TOF‐MS certify the structure and composition of Ag54Cu12 . The n‐type narrow‐band‐gap material was confirmed by solid state UV–vis spectra and DFT calculation. Furthermore, Ag54Cu12 itself and Ag54Cu12/C are good catalysts for 1,3‐dipole cycloaddition between alkynes and azides at ambient conditions. Different from conventional methods for heterometallic clusters, the comproportionation reaction in the work could provide an effective method in controlling reduction of double metal salts and formation of heteroatomic high‐nuclearity monovalent copper alkynyl clusters.
Conflict of Interest
The authors declare no conflict of interest.
Supporting information
Supporting Information
Supporting Information
Acknowledgements
This work was financially supported by the Natural Science Foundation of China (91961201).
Gao J.‐P., Zhang F.‐Q., Zhang X.‐M., A 66‐Nuclear All‐Alkynyl Protected Peanut‐Shaped Silver(I)/Copper(I) Heterometallic Nanocluster: Intermediate in Copper‐Catalyzed Alkyne‐Azide Cycloaddition. Adv. Sci. 2024, 11, 2400377. 10.1002/advs.202400377
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.a) Zhang M.‐M., Dong X.‐Y., Wang Y.‐J., Zang S.‐Q., Mak T. C. W., Coord. Chem. Rev. 2022, 453, 214315; [Google Scholar]; b) Sharma S., Chakrahari K. K., Saillard J. Y., Liu C. W., Acc. Chem. Res. 2018, 51, 2475; [DOI] [PubMed] [Google Scholar]; c) Kawawaki T., Imai Y., Suzuki D., Kato S., Kobayashi I., Suzuki T., Kaneko R., Hossain S., Negishi Y., Chem.‐Eur. J. 2020, 26, 16150; [DOI] [PubMed] [Google Scholar]; d) Ma X.‐H., Si Y., Luo L.‐L., Wang Z.‐Y., Zang S.‐Q., Mak T. C. W., ACS Nano 2022, 16, 5507; [DOI] [PubMed] [Google Scholar]; e) Hossain S., Niihori Y., Nair L. V., Kumar B., Kurashige W., Negishi Y., Acc. Chem. Res. 2018, 51, 3114. [DOI] [PubMed] [Google Scholar]
- 2.a) Chai J., Lv Y., Yang S., Song Y., Zan X., Li Q., Yu H., Wu M., Zhu M., J. Phys. Chem. C 2017, 121, 21665; [Google Scholar]; b) Wang S., Song Y., Jin S., Liu X., Zhang J., Pei Y., Meng X., Chen M., Li P., Zhu M., J. Am. Chem. Soc. 2015, 137, 4018. [DOI] [PubMed] [Google Scholar]
- 3.a) Zou X., Li Y., Jin S., Kang X., Wei X., Wang S., Meng X., Zhu M., J. Phys. Chem. Lett. 2020, 11, 2272; [DOI] [PubMed] [Google Scholar]; b) Yang J., Pang R., Song D., Li M.‐B., Nanoscale Adv. 2021, 3, 2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Wang S., Li Q., Kang X., Zhu M., Acc. Chem. Res. 2018, 51, 2784; [DOI] [PubMed] [Google Scholar]; b) Negishi Y., Iwai T., Ide M., Chem. Commun. 2010, 46, 4713; [DOI] [PubMed] [Google Scholar]; c) Takano S., Ito S., Tsukuda T., J. Am. Chem. Soc. 2019, 141, 15994. [DOI] [PubMed] [Google Scholar]
- 5. Anumula R., Xiao P., Cui C., Wu H., Cui G., Fang W.‐H., Luo Z., Yao J., Nanoscale 2020, 12, 7864. [DOI] [PubMed] [Google Scholar]
- 6.a) Zhuo H.‐Y., Su H.‐F., Cao Z.‐Z., Liu W., Wang S.‐A., Feng L., Zhuang G.‐L., Lin S.‐C., Kurmoo M., Tung C.‐H., Sun D., Zheng L.‐S., Chem.‐Eur. J. 2016, 22, 17619; [DOI] [PubMed] [Google Scholar]; b) Zhang L. L.‐M., Mak T. C. W., Angew. Chem., Int. Ed. 2017, 56, 16228. [DOI] [PubMed] [Google Scholar]
- 7.a) Gan Z., Xia N., Wu Z., Acc. Chem. Res. 2018, 51, 2774; [DOI] [PubMed] [Google Scholar]; b) Liu X., Astruc D., Adv. Mater. 2017, 29, 1605305; [DOI] [PubMed] [Google Scholar]; c) Han B.‐L., Wang Z., Gupta R. K., Feng L., Wang S., Kurmoo M., Gao Z.‐Y., Schein S., Tung C.‐H., Sun D., ACS Nano 2021, 15, 8733. [DOI] [PubMed] [Google Scholar]
- 8.a) Zhu M., Wang P., Yan N., Chai X., He L., Zhao Y., Xia N., Yao C., Li J., Deng H., Zhu Y., Pei Y., Wu Z., Angew. Chem., Int. Ed. 2018, 57, 4500; [DOI] [PubMed] [Google Scholar]; b) da Silva A. G. M., Rodrigues T. S., Haigh S. J., Camargo P. H. C., Chem. Commun. 2017, 53, 7135; [DOI] [PubMed] [Google Scholar]; c) Li M.‐B., Tian S.‐K., Wu Z., Jin R., Chem. Commun. 2015, 51, 4433. [DOI] [PubMed] [Google Scholar]
- 9.a) Liang L., Astruc D., Coord. Chem. Rev. 2011, 255, 2933; [Google Scholar]; b) Worrell B. T., Malik J. A., Fokin V. V., Science 2013, 340, 457; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Buckley B. R., Dann S. E., Harris D. P., Heaney H., Stubbs E. C., Chem. Commun. 2010, 46, 2274; [DOI] [PubMed] [Google Scholar]; d) Singh R., Singh G., George N., Singh G., Devi A., Singh H., Kaur G., Singh J., RSC Adv. 2023, 13, 32399; [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Singh G., Singh R., George N., Singh G., Sushma G. K., Kaur G., Singh H., Singh J., J. Photochem. Photobiol. A 2023, 441, 114741; [Google Scholar]; f) George N., Singh G., Singh R., Singh G., Priyanka H. S., Kaur G., Singh J., J. Mol. Struct. 2023, 1288, 135666. [Google Scholar]
- 10.a) Hein J. E., Fokin V. V., Chem. Soc. Rev. 2010, 39, 1302; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Tiwari V. K., Mishra B. B., Mishra K. B., Mishra N., Singh A. S., Chen X., Chem. Rev. 2016, 116, 3086. [DOI] [PubMed] [Google Scholar]
- 11. Zhang L. M., Mak T. C., J. Am. Chem. Soc. 2016, 138, 2909. [DOI] [PubMed] [Google Scholar]
- 12. Cook A. W., Jones Z. R., Wu G., Scott S. L., Hayton T. W., J. Am. Chem. Soc. 2018, 140, 394. [DOI] [PubMed] [Google Scholar]
- 13.a) Buschbeck R., Low P. J., Lang H., Coord. Chem. Rev. 2011, 255, 241; [Google Scholar]; b) Lang H., Jakob A., Milde B., Organometallics 2012, 31, 7661; [Google Scholar]; c) Mingos D. M. P., Vilar R., Rais D., J. Organomet. Chem. 2002, 641, 126. [Google Scholar]
- 14. Fang Y., Bao K., Zhang P., Sheng H., Yun Y., Hu S. X., Astruc D., Zhu M., J. Am. Chem. Soc. 2021, 143, 1768. [DOI] [PubMed] [Google Scholar]
- 15. Wan X.‐K., Yuan S.‐F., Tang Q., Jiang D.‐E., Wang Q.‐M., Angew. Chem. 2015, 127, 6075. [Google Scholar]
- 16. Connell T. U., Sandanayake S., Khairallah G. N., White J. M., O'Hair R. A. J., Donnelly P. S., Williams S. J., Dalton Trans. 2013, 42, 4903. [DOI] [PubMed] [Google Scholar]
- 17. Wei X., Chu K., Adsetts J. R., Li H., Kang X., Ding Z., Zhu M., J. Am. Chem. Soc. 2022, 144, 20421. [DOI] [PubMed] [Google Scholar]
- 18.a) Yang Y., Jia T., Han Y.‐Z., Nan Z.‐A., Yuan S.‐F., Yang F.‐L., Sun D., Angew. Chem., Int. Ed. 2019, 58, 12280; [DOI] [PubMed] [Google Scholar]; b) Wang Z., Gupta R. K., Luo G.‐G., Sun D., Chem. Rec. 2020, 20, 389; [DOI] [PubMed] [Google Scholar]; c) Su Y.‐M., Ji B.‐Q., Wang Z., Zhang S.‐S., Feng L., Gao Z.‐Y., Li Y.‐W., Tung C.‐H., Sun D., Zheng L.‐S., Sci. China Chem. 2021, 64, 1482. [Google Scholar]
- 19. Deposition numbers 2243094 contain the supplementary crystallographic data for this paper. These data are provided free of charge by the joint Cambridge Crystallographic Data Centre and Fachinformationszentrum Karlsruhe Access Structures service.
- 20. Yang S., Chai J., Chong H., Song Y., Yu H., Zhu M., Chem. Commun. 2018, 54, 4314. [DOI] [PubMed] [Google Scholar]
- 21. Borel M. M., Chardon J., Leclaire A., Grandin A., Raveau B., J. Solid State Chem. 1994, 112, 317. [Google Scholar]
- 22. Gao J.‐P., Qi Z., Zhang F.‐Q., Zhang X.‐M., Nanoscale 2022, 14, 4469. [DOI] [PubMed] [Google Scholar]
- 23.a) Huang R.‐W., Xu Q.‐Q., Lu H.‐L., Guo X.‐K., Zang S.‐Q., Gao G.‐G., Tang M.‐S., Mak T. C. W., Nanoscale 2015, 7, 7151; [DOI] [PubMed] [Google Scholar]; b) Shi W.‐Q., Guan Z.‐J., Li J.‐J., Han X.‐S., Wang Q.‐M., Chem. Sci. 2022, 13, 5148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lin L., Yang Z. K., Jiang Y.‐F., Xu A.‐W., ACS Catal. 2016, 6, 4449. [Google Scholar]
- 25. Wan X.‐K., Xu W. W., Yuan S.‐F., Gao Y., Zeng X.‐C., Wang Q.‐M., Angew. Chem. Int. Ed. 2015, 54, 9683. [DOI] [PubMed] [Google Scholar]
- 26.a) Wang Z., Li L., Feng L., Gao Z.‐Y., Tung C.‐H., Zheng L.‐S., Sun D., Angew. Chem. Int. Ed. 2022, 61, 202200823; [DOI] [PubMed] [Google Scholar]; b) Saha S., Das G., Thote J., Banerjee R., J. Am. Chem. Soc. 2014, 136, 14845. [DOI] [PubMed] [Google Scholar]
- 27.a) Tornøe C. W., Christensen C., Meldal M., J. Org. Chem. 2002, 67, 3057; [DOI] [PubMed] [Google Scholar]; b) Rostovtsev V. V., Green L. G., Fokin V. V., Sharpless K. B., Angew. Chem., Int. Ed. 2002, 41, 2596. [DOI] [PubMed] [Google Scholar]
- 28.a) Meldal M., Tornøe C. W., Chem. Rev. 2008, 108, 2952; [DOI] [PubMed] [Google Scholar]; b) Blastik Z. E., Voltrová S., Matoušek V., Jurásek B., Manley D. W., Klepetářová B., Beier P., Angew. Chem. Int. Ed. 2017, 56, 346. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.