Abstract
Introduction
Precise prediction of hospital stay duration is essential for maximizing resource utilization during surgery. Existing lumbar spinal stenosis (LSS) surgery prediction models lack accuracy and generalizability. Machine learning can improve accuracy by considering preoperative factors. This study aimed to develop and validate a machine learning-based model for estimating hospital stay duration following decompression surgery for LSS.
Methods
Data from 848 patients who underwent decompression surgery for LSS at three hospitals were examined. Twelve prediction models, using 79 preoperative variables, were developed for postoperative hospital stay estimation. The top five models were chosen. Fourteen models predicted prolonged hospital stay (≥14 days), and the most accurate model was chosen. Models were validated using a randomly divided training sample (70%) and testing cohort (30%).
Results
The top five models showed moderate linear correlations (0.576-0.624) between predicted and measured values in the testing sample. The ensemble of these models had moderate prediction accuracy for final length of stay (linear correlation 0.626, absolute mean error 2.26 days, standard deviation 3.45 days). The c5.0 decision tree model was the top predictor for prolonged hospital stay, with accuracies of 89.63% (training) and 87.2% (testing). Key predictors for longer stay included JOABPEQ social life domain, facility, history of vertebral fracture, diagnosis, and Visual Analogue Scale (VAS) of low back pain.
Conclusions
A machine learning-based model was developed to predict postoperative hospital stay after LSS decompression surgery, using data from multiple hospital settings. Numerical prediction of length of stay was not very accurate, although favorable prediction of prolonged stay was accomplished using preoperative factors. The JOABPEQ social life domain score was the most important predictor.
Keywords: Degenerative lumbar spinal stenosis, hospital stay, predictive model, machine learning, surgery
Introduction
Lumbar spinal stenosis (LSS) is a prominent spinal ailment usually resulting in symptoms including lower back discomfort, leg pain, and tingling feelings1). Often, surgery becomes the sought-after recourse when traditional methods prove inadequate2-7). However, the subsequent hospital stay duration postsurgery tends to vary. This variability can elevate medical expenses and affect patient well-being. Therefore, identifying variables associated with postsurgical length of stay (LOS) is essential for medical practitioners to properly schedule and maximize recovery times8-15).
Frequent spinal procedures are conducted to address various conditions like LSS, disc issues, and spinal injuries, aiming to alleviate discomfort, reinstate physical functionality, and uplift the patient's quality of life1-4). However, these procedures are not devoid of risks; they can lead to complications such as infections or postoperative pain4,6-8,16). Hospital durations following these surgeries might vary, sometimes extending for weeks, resulting in increased healthcare costs, possible work absences, and patient worries8-15). Consequently, accurately estimating postoperative stay becomes essential for resource management, patient satisfaction, and fiscal prudence12-16).
Numerous studies have attempted to develop predictive models for hospital stay after spinal procedures, examining a myriad of demographic, clinical, and radiographic factors that may affect LOS for patients8-15). Such models empower healthcare teams to identify patients with potential for prolonged hospitalization, facilitating timely interventions. Furthermore, these frameworks help in predicting the costs associated with these procedures13-16).
Historical data suggests that elements like age, gender, surgical specifics, existing health conditions, and radiographic details play a role in influencing LOS postspinal surgeries8-15). Factors such as socioeconomic status and social support networks also weigh in on hospitalization duration17-20).
With this backdrop, our research endeavors to formulate robust and dependable models that predict LOS after LSS procedures test these structures across diverse patient demographics and elucidate factors influencing extended stays. The ultimate goal is to equip healthcare professionals with practical insights for improved patient care, efficient resource utilization, and cost-effectiveness.
Materials and Methods
Patient enrollment
We conducted a retrospective analysis of data entered prospectively into three different databases. This study was approved by the institutional review board of the hospitals. We used a prospective database from an academic hospital, tertiary center, and private hospital to analyze data from 848 consecutive patients who were surgically treated for LSS by posterior decompression alone (laminectomy) between April 2012 and March 2019 and who had 2 years of follow-up data. All data were gathered prospectively and examined retrospectively. This database was established previously and used for the previous study (Supplemental Table 1)20).
Inclusion and exclusion criteria
This study included adult patients (age ≥40 years) diagnosed with primary LSS or lumbar degenerative spondylolisthesis (DS, Meyerding grade 1) with clinical symptoms such as sciatic pain, neurological claudication, or leg numbness and unsuccessful conservative treatment for at least 3 months or recurrent symptoms. We chose laminectomy only when the patient did not have obvious spinal instability on flexion-extension radiographs before surgery. Patients were excluded if they were followed up for fewer than 2 years following surgery or had an incomplete dataset.
Candidate predictors
A comprehensive set of sociodemographic, clinical, lifestyle, and surgical factors were selected as candidate predictors, including both nonmodifiable and modifiable variables. All possible predictors were chosen a priori based on established clinical significance. The candidate predictors included 72 preoperative variables and 5 operative variables. Variables were included in the study if they had less than 10% missing data by using the MissForest multiple imputation approach.
Sociodemographic factors
Sociodemographic predictors included age, sex, blood type, and current working status.
Clinical factors
The two categories of baseline patient-reported outcomes (PROs), including the visual analog scale (VAS) scores for lower back pain, buttock-leg discomfort, and leg numbness and Japanese Orthopedic Association Back Pain Evaluation Questionnaire (JOABPEQ) scores, were included (Supplemental Table 1)21,22). The JOABPEQ is a PRO instrument developed by the JOA for the assessment of lower back pain and lumbar spinal disease, and the JOABPEQ has been validated in several countries, including the United States, Korea, Thailand, China, and Iran23-25).
Furthermore, the numbers and types of comorbidities were gathered. Patient frailty was calculated using the modified frailty index-5 (mFI-5)26). Patients were divided into robust (mFI-5=0), prefrail (mFI-5=1), or frail (mFI-5 ≥2). The duration of symptoms was assessed by self-report at the initial visit.
Lifestyle factors
Body mass index (BMI, kg/m2) was computed from height and weight measured at hospitalization. Current smoking status and smoking history were also assessed by self-report at hospitalization.
Operative factors
The diagnosis, number of intervertebral levels involved, and history of spine surgery were collected as operative factors, and the duration of procedure, amount of predicted blood loss, and intra- and perioperative problems were also collected as operative factor.
Missing data
Among the 961 eligible patients, 848 patients who completed JOABPEQ were included (retrieval rate of 88.2%).
Model development
Predictive model of length of hospital stay
To create a numerical predictive model, the patient samples were randomly divided into training and testing cohorts at a 7:3 ratio. This is a common procedure for randomly selecting data from a common dataset for training and testing27).
We chose 12 applicable machine learning algorithms from IBM SPSS modeler program for the prediction of length of hospital stay (generalized linear regression model [GLM], generalized linear mixed model [GLMM], linear regression [LR], linear support vector machines [LSVM], single-layer artificial neural networks, random trees, linear-AS, tree AS, extreme gradient boosting linear [XGBoost Linear], XGBoost Tree, chi-squared automatic interaction detection [CHIAD], and classification and regression tree [C&R tree])27).
Each model was used to predict values for its corresponding outcome using the testing dataset, and those predicted values were compared with the true observed values. Calibration, which is one component of predictive performance, was evaluated using the correlation coefficient with relative error when choosing the top 5 models among 12 predictive models. The ensemble of the top 5 accurate models was used for the final prediction of length of hospital stay after surgery. Linear correlation, mean absolute error, median difference, and root mean squared log error between the predictive values and the observed values were used to evaluate the predictive probability of the final models27).
Predictive model of prolonged hospital stay
For this discriminant prediction model, the binary target variable was prolonged hospital stay versus no prolonged hospital stay. Prolonged hospital stay was defined as hospitalization for more than 14 days after surgery based on the average length of hospital stay following decompression surgery for lumbar degenerative diseases in the 2022 national database and the distribution of the LOS after surgery in the current study cohort28).
Thirteen applicable machine learning algorithms from the IBM SPSS modeler program were selected for the prediction of prolonged hospital stay, including the c5.0 decision-making tree, logistic regression model, decision list, Bayesian network, discriminant, LSVM, random trees, tree AS, XGBoost tree, CHAID, Quest, C&R tree, and neural net27). An ensemble of decision-making trees was created using the c5.0 algorithm with ten distinct bootstrapped models. The final overall predictions from the models were combined and chosen by voting, with random selection for tied votes27). The accuracy and area under the receiver operating characteristic curve (AUC) were computed to assess each of the predictive models.
Statistical analysis
Data were analyzed with Statistical Package for the Social Sciences software (SPSS statistics version 29.0, IBM Corp., Armonk, NY). All predictive modeling was performed using SPSS modeler (SPSS modeler subscription version 1.0, IBM Corp., Armonk, NY). A P value less than .05 was considered statistically significant. Sample size and power calculations were performed using appropriate software (G*Power 3.1)27).
Results
Length of hospital stay after surgery in LSS
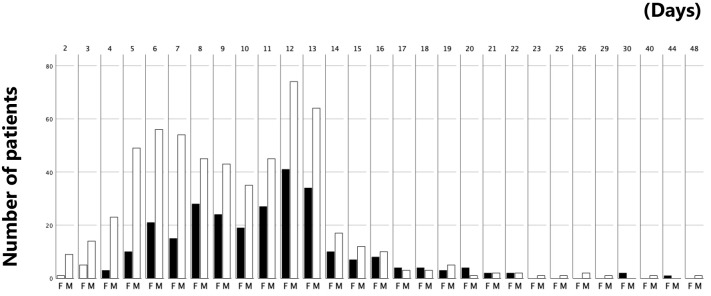
In this study, 848 patients who had undergone decompression surgery for LSS and lumbar DS at university hospital, tertiary hospital, and private hospital for 2 years were included, with an average age of 71±9 years and 68% male. LSS was found in 91% of the cases, and the average number of intervertebral spaces was 1.8±0.8. The mean operation time was 68±37 minutes, and the mean blood loss was 48±112 mL. The average length of hospital stay after surgery was 10.06±4.58 days, with a range of 2-50 days and a median of 10 days. Among the patient cohort, 742 patients (87.5%) were discharged within 14 days, while 106 patients (12.5%) stayed more than 14 days after surgery (Fig. 1). The mean LOS for patients discharged within 14 days was 8.92±3.02 days, whereas the mean LOS for those who stayed more than 14 days was 17.81±5.67 days, with a mean difference of 8.84±0.56 days [7.79-9.98].
Figure 1.
Distribution of the length of hospital stay after surgery stratified by gender.
F: female patient, M: male patient
87.5% (n=769) of the patients discharged within 14 days after surgery.
Additionally, comparisons among three different hospital setting found no significant difference in age and gender across an academic hospital, a tertiary center, and a private institution. However, BMI was significantly lower in the tertiary center compared to the academic hospital (p=0.01), with no difference when compared to the private institution. Patients at the academic hospital had a higher number of comorbidities (p=0.01), and there were notable variations in job status and smoking habits across all institutions (p=0.01). The academic hospital reported a considerably shorter LOS compared to the tertiary and private institutions (p=0.01), despite comparable operative times and EBL across the academic and tertiary centers (Supplemental Table 2).
Predictive model of length of hospital stay
The linear correlation between predicted and measured values in the testing cohort for the top five predictive models created was moderate (.576-.624, Table 1), and the predictive probability of the final model for postoperative LOS by the ensemble of top 5 models was also moderate (linear correlation: .626, absolute mean error: 2.26 days, standard deviation: 3.45 days; Fig. 2, Table 2). Among the testing cohort, the predicted values were within 2 days of the actual values in 62% of cases (155/250 cases).
Table 1.
Correlation of Observed and Predictive Values of the Length of Hospital Stay after Surgery for LSS.
| Predictive model | Correlation | No. of fields used |
|---|---|---|
| XG boost linear | 0.589 | 71 |
| Linear SVM | 0.576 | 71 |
| C&RT | 0.578 | 24 |
| ALM | 0.624 | 13 |
Figure 2.
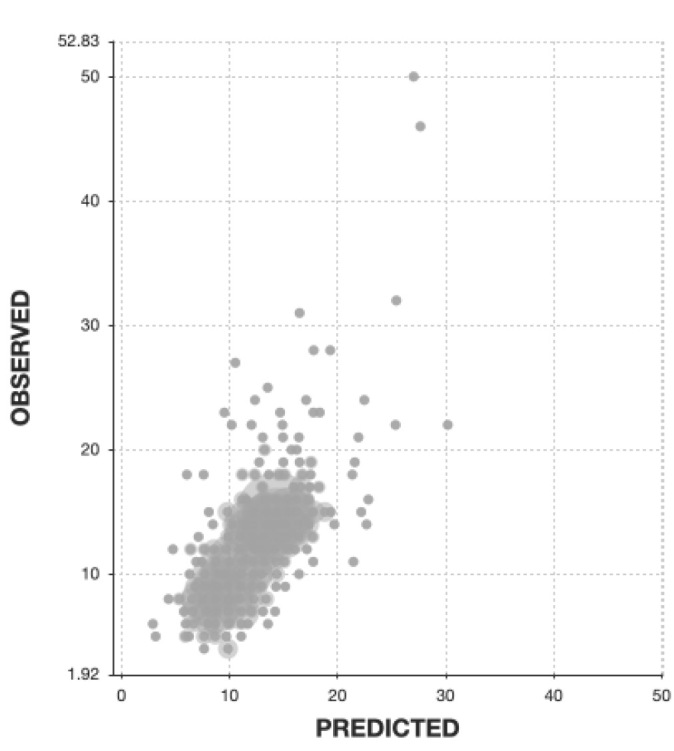
Scatter plot of observed vs. predicted values for prediction of length of stay after decompression surgery for LSS by the final predictive model.
The linear correlation between predicted and measured values in the testing cohort for the top five predictive models developed was moderate (r=.576–.624).
Table 2.
Accuracy of Final Predictive Model for Length of Hospital Stay after Surgery for LSS.
| Training sample | Testing sample | |
|---|---|---|
| Mean error | 0.295 | 0.033 |
| Mean absolute error | 1.927 | 2.24 |
| Standard deviation | 2.946 | 3.455 |
| Linear correlation | 0.786 | 0.623 |
| Occurrences | 598 | 250 |
Predictive model of prolonged hospital stay
The JOABPEQ social life domain score was the most important predictor of hospital stay (importance .43), followed by institution (.22), history of vertebral fracture (.14), diagnosis (.10), and VAS of low back pain (.08; Fig. 3). Patients with prolonged hospital stay following surgery had substantially lower baseline JOABPEQ social life domain score compared to those discharged within 14 days (40.36±20.95 vs. 29.31±22.36 days, p<.001, mean difference 11.05±2.19 [6.74-15.36 days]). The c5.0 decision-making tree was the most accurate model for predicting prolonged hospital stay, with a predictive accuracy of 89.63% in the training cohort and 87.2% in the testing cohort.
Figure 3.
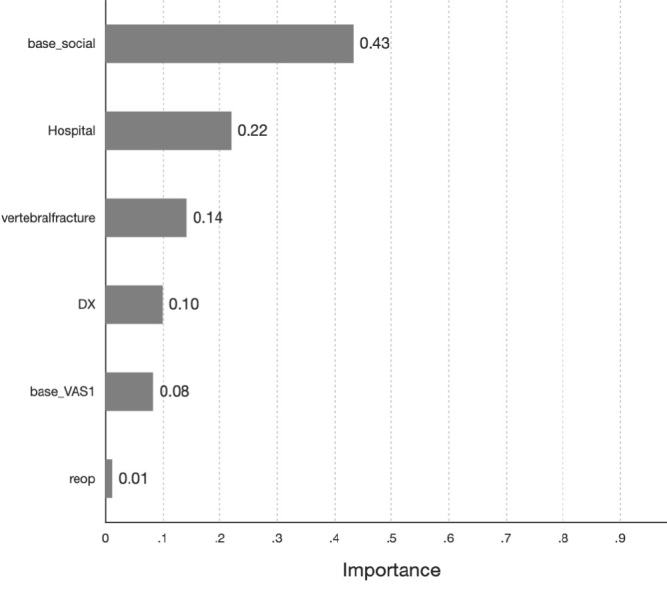
The top six most important predictors for prediction of prolonged hospital stay after decompression surgery.
The baseline JOABPEQ social life domain score was the most important predictor for prolonged hospital stay following decompression surgery for LSS.
Discussion
The current study aimed to develop a predictive framework to determine the length of hospital stay subsequent to posterior decompression surgery among LSS patients, using a wide range of sociodemographic, clinical, lifestyle, and surgical factors. This comprehensive approach collated data from a sequence of 848 patients who chose laminectomy as a treatment between April 2012 and March 2019, followed by a rigorous two-year postoperative monitoring. Of the 79 probable determinants, our analytical strategy was formed by integrating 12 different machine learning techniques to improve prediction accuracy.
In the present study, the XGBoost Linear algorithm was found to have the best performance in predicting length of hospital stay. The linear correlation between predicted and measured values in the testing cohort for the top five predictive models developed was moderate, ranging from 0.576 to 0.624. The predictive probability of the final model for postoperative LOS by the ensemble of top 5 models was also moderate, with a linear correlation of 0.626, an absolute mean error of 2.26 days with 62% of the predicted values being within 2 days of the actual values, and a standard deviation of 3.45 days. However, it is crucial to remember that although the predicted values were within 2 days of the actual values in 62% of cases, the remaining 38% of predictions were more than 2 days off, demonstrating the model's poor accuracy in predicting length of hospital stay in a clinical setting.
Despite the model's limitations, the predictive model established in the present study successfully predicted prolonged hospital stay after decompression surgery for LSS, with a predictive accuracy of 89.63% in the training cohort and 87.2% in the testing cohort. The JOABPEQ social life domain score was identified as the most significant predictor. Therefore, while caution should be exercised when using the predictive model to predict the length of hospital stay, it may still be useful in identifying patients at high risk for prolonged hospitalization and providing tailored postoperative care.
Our research demonstrates that a multifaceted array of sociodemographic, clinical, lifestyle, and surgical factors are intricately linked with the LOS for patients having laminectomy for LSS. In line with prior studies, we identified older age, retirement status, higher BMI, current smoking, and an array of comorbidities as factors contributing to longer LOS9-12,15-18). Notably, our results echo the recent research highlighting the vital role of social determinants of health in postoperative recovery16-18). Consistent with the findings of Holbert SE et al.29,30), our model recognized the importance of social support structures, such as marital status and financial stability, in influencing LOS. The association of these factors with shorter or longer stays emphasizes the possibility for routine screening of social determinants to enable customized resource allocation and intervention measures, hence reducing the risk of extended hospitalization.
The predictive strength of the JOABPEQ social life domain score in our study particularly underscores the impact of work-related quality of life on recovery and LOS. This bolsters the claim by Rethorn ZD et al. that a comprehensive understanding of social determinants yields a more precise assessment of their impact on patient outcomes30). It also corroborates Lechman C et al.'s insights into the predictive value of psychosocial problems19). We note that our AI-based method provides improved predictive capabilities by supporting a wider range of variables and their intricate interactions. While conventional models provide valuable benchmarks, our machine learning model's capacity to discern subtle patterns within the data has demonstrated a notable improvement in predicting prolonged LOS. This ability is demonstrated by the excellent predictive accuracy for extended hospital stay in our study, consistent with the established relevance of social factors but leveraging computational power to improve predictions further. Clinical implications of these insights are profound. By identifying patients prone to endure prolonged hospitalizations, healthcare practitioners can proactively conduct targeted interventions. These may include social support services, tailored physical therapy regimens, and personalized discharge planning to reduce LOS and enhance patient satisfaction and outcomes.
The present study has several strengths, including the large sample size, use of prospectively collected data, and extensive list of possible predictors. However, there are also some limitations. First, the data for model development were derived from three distinct hospitals, encompassing an academic hospital, a tertiary center, and a private institution. While this variety offers a broad perspective, it may also limit the generalizability of the findings to other settings. Every hospital group presented distinct attributes that influenced the duration of stay. Second, our study did not include a comprehensive analysis of perioperative pain management protocols, such as intravenous morphine usage, which could have a significant impact on the length of hospital stay. We acknowledge this as a weakness in our analysis and propose that subsequent studies should consider these parameters to increase predictive accuracy. Third, patient preferences and expectations, which may influence the LOS, were not accounted for. It is critical to recognize that these arbitrary measures could impact recovery and discharge timing.
However, in this study, our objective was to construct a predictive model exclusively utilizing preoperative and surgical factors to estimate the LOS for patients undergoing LSS surgery. We purposefully concentrated on preoperative and surgical to provide surgeons with a tool to predict LOS even before the surgical intervention takes place. While patient preferences and expectations certainly play a role in postoperative recovery and could influence LOS, they were beyond the scope of our predictive framework due to our preoperative focus. This purposeful restriction was set to guarantee that the model could serve as a presurgical planning tool, enabling medical professionals to identify high-risk patients early on and manage resources more effectively.
Nevertheless, it is important to acknowledge that the exclusion of intraoperative and postoperative variables, including subjective patient experiences and postoperative rehabilitation as well as pain management, may limit the model's comprehensive applicability in practice. Future research is urged to build on our findings by incorporating a wider variety of parameters, including those related to the perioperative and postoperative periods, to further improve the predictive accuracy of LOS models.
Conclusion
We used machine learning to predict the LOS after decompression surgery for LSS using patient data from three different hospitals. The current investigation discovered that several sociodemographic, clinical, lifestyle, and surgical variables were connected to the length of hospital stay in patients with LSS who underwent laminectomy. Although numerical prediction of postoperative hospital stay was not sufficiently accurate, favorable prediction of prolonged hospital stay was achieved from preoperative variables. The results of this study have significant clinical implications for optimizing patient care and enhancing patient outcomes. Further research is needed to validate these findings and explore their potential applications in clinical practice.
Conflicts of Interest: The authors declare that there are no relevant conflicts of interest.
Author Contributions: M.Y. designed the study; T.Y. and T.I. performed the experiments and analyzed the data; S.S., Y.O, M.O., Y.T., O.T., and N.N. provided critical reagents; J.O., H.K., M.M., M.N., and K.W. supervised the experiments; T.Y. and M.Y. wrote the manuscript.
Ethical Approval: This study was approved by the institutional review board of Keio University Hospital (IRB approval number #20110142).
Informed Consent: All subjects consented and agreed with their inclusion. All methods were performed in accordance with the relevant guidelines and regulations.
Supplementary Material
Acknowledgement
We gratefully acknowledge Dr Takehiro Michikawa for his advice for statistical analyses.
References
- 1.Thomé C, Börm W, Meyer F. Degenerative lumbar spinal stenosis: current strategies in diagnosis and treatment. Dtsch Arztebl Int. 2008;105(20):373-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghogawala Z, Resnick DK, Glassman SD, et al. Randomized controlled trials for degenerative lumbar spondylolisthesis: which patients benefit from lumbar fusion? J Neurosurg Spine. 2017;26(2):260-6. [DOI] [PubMed] [Google Scholar]
- 3.Ghogawala Z, Dziura J, Butler WE, et al. Laminectomy plus fusion versus laminectomy alone for lumbar spondylolisthesis. N Engl J Med. 2016;374(15):1424-34. [DOI] [PubMed] [Google Scholar]
- 4.Försth P, Ólafsson G, Carlsson T, et al. A randomized, controlled trial of Fusion surgery for lumbar spinal stenosis. N Engl J Med. 2016;374(15):1413-23. [DOI] [PubMed] [Google Scholar]
- 5.Steiger F, Becker HJ, Standaert CJ, et al. Surgery in lumbar degenerative spondylolisthesis: indications, outcomes and complications. A systematic review. Eur Spine J. 2014;23(5):945-73. [DOI] [PubMed] [Google Scholar]
- 6.Weinstein JN, Tosteson TD, Lurie JD, et al. Surgical versus nonsurgical therapy for lumbar spinal stenosis. N Engl J Med. 2008;358(8):794-810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ghogawala Z, Benzel EC, Amin-Hanjani S, et al. Prospective outcomes evaluation after decompression with or without instrumented fusion for lumbar stenosis and degenerative Grade I spondylolisthesis. J Neurosurg Spine. 2004;1(3):267-72. [DOI] [PubMed] [Google Scholar]
- 8.Deyo RA, Mirza SK, Martin BI, et al. Trends, major medical complications, and charges associated with surgery for lumbar spinal stenosis in older adults. JAMA. 2010;303(13):1259-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pearson A, Blood E, Lurie J, et al. Degenerative spondylolisthesis versus spinal stenosis: does a slip matter? Comparison of baseline characteristics and outcomes (SPORT). Spine. 2010;35(3):298-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steiger F, Becker HJ, Standaert CJ, et al. Surgery in lumbar degenerative spondylolisthesis: indications, outcomes and complications. A systematic review. Eur Spine J. 2014;23(5):945-73. [DOI] [PubMed] [Google Scholar]
- 11.Kuo CC, Hess RM, Khan A, et al. Factors affecting postoperative length of stay in patients undergoing anterior lumbar interbody fusion. World Neurosurg. 2021;155:e538-47. [DOI] [PubMed] [Google Scholar]
- 12.Khazanchi R, Bajaj A, Shah RM, et al. Using machine learning and deep learning algorithms to predict postoperative outcomes following anterior cervical discectomy and fusion. Clin Spine Surg. 2023;36(3):143-9. [DOI] [PubMed] [Google Scholar]
- 13.Dial BL, Esposito VR, Danilkowicz R, et al. Factors associated with extended length of stay and 90-day readmission rates following ACDF. Global Spine J. 2020;10(3):252-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aiyer SN, Kumar A, Shetty AP, et al. Factors influencing postoperative urinary retention following elective posterior lumbar spine surgery: A prospective study. Asian Spine J. 2018;12(6):1100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Frisch NB, Wessell NM, Charters MA, et al. Predictors and complications of blood transfusion in total hip and knee arthroplasty. J Arthroplasty. 2014;29(9 Suppl):189-92. [DOI] [PubMed] [Google Scholar]
- 16.Gruskay JA, Fu M, Bohl DD, et al. Factors affecting length of stay after elective posterior lumbar spine surgery: a multivariate analysis. Spine J. 2015;15(6):1188-95. [DOI] [PubMed] [Google Scholar]
- 17.Bydon M, Abt NB, De la Garza-Ramos R, et al. Impact of age on short-term outcomes after lumbar fusion: An analysis of 1395 patients stratified by decade cohorts. Neurosurgery. 2015;77(3):347-53. [DOI] [PubMed] [Google Scholar]
- 18.Lubelski D, Ehresman J, Feghali J, et al. Prediction calculator for nonroutine discharge and length of stay after spine surgery. Spine J. 2020;20(7):1154-8. [DOI] [PubMed] [Google Scholar]
- 19.Lechman C, Duder S. Hospital length of stay: social work services as an important factor. Soc Work Health Care. 2009;48(5):495-504. [DOI] [PubMed] [Google Scholar]
- 20.Yagi M, Michikawa T, Yamamoto T, et al. Development and validation of machine learning-based predictive model for clinical outcome of decompression surgery for lumbar spinal canal stenosis. Spine J. 2022;22(11):1768-77. [DOI] [PubMed] [Google Scholar]
- 21.Fukui M, Chiba K, Kawakami M, et al. Japanese orthopaedic association back pain evaluation questionnaire: initial report. J Orthop Sci. 2007;12(5):443-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fukui M, Chiba K, Kawakami M, et al. Japanese orthopaedic association back pain evaluation questionnaire. Part 2. Verification of its reliability: The subcommittee on low back pain and cervical myelopathy evaluation of the clinical outcome committee of the Japanese orthopaedic association. J Orthop Sci. 2007;12(6):526-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cheung PWH, Wong CKH, Cheung JPY. Psychometric validation of the adapted Traditional Chinese version of the Japanese Orthopaedic Association Back Pain Evaluation Questionnaire (JOABPEQ). J Orthop Sci. 2018;23(5):750-7. [DOI] [PubMed] [Google Scholar]
- 24.Azimi P, Shahzadi S, Montazeri A. The Japanese orthopedic association back pain evaluation questionnaire (JOABPEQ) for low back disorders: a validation study from Iran. J Orthop Sci. 2012;17(5):521-5. [DOI] [PubMed] [Google Scholar]
- 25.Jung KS, Jung JH, Jang SH, et al. The reliability and validity of the Korean version of the Japanese orthopaedic association back pain evaluation questionnaire. J Phys Ther Sci. 2017;29(7):1250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chimukangara M, Helm MC, Frelich MJ, et al. A 5-item frailty index based on NSQIP data correlates with outcomes following paraesophageal hernia repair. Surg Endosc. 2017;31(6):2509-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Abbott D. Applied predictive analytics: principles and techniques for the professional data analyst. 1st ed. Indianapolis, IN: John Wiley & Sons, Inc.; 2014. [Google Scholar]
- 28.Handbook of health and welfare statistics 2020, Ministry of Health, Labour and Welfare. [Google Scholar]
- 29.Holbert SE, Andersen K, Stone D, et al. Social determinants of health influence early outcomes following lumbar spine surgery. Ochsner J. 2022;22(4):299-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rethorn ZD, Garcia AN, Cook CE, et al. Quantifying the collective influence of social determinants of health using conditional and cluster modeling. PLoS One. 2020;15(11):e0241868. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.