Abstract
Gamma-Glutamyl Transferase (γGT) is a key transferase involved in the transpeptidation of functional gamma-glutamyl groups to various receptor moieties. It performs important roles in antioxidant defence mechanisms, particularly glutathione recycling, xenobiotic metabolism, but analogously may also have a pro-oxidant role.
γ GT is very sensitive for diagnosis of liver injury, although it has poor specificity for particular aetiologies. It has been used to reflect temporal changes as a form of monitoring depending on aetiology.
Given its cellular role in antioxidant function, it has been investigated as a surrogate biomarker of oxidative stress. It has also been found to be a predictor of mortality across a spectra of non-hepatic disease pathologies, from metabolic and cardiovascular risk to chronic kidney disease and neoplasia. Similarly, it also remains of interest to the insurance industry given an apparent ability to predict mortality, in addition to an historical interest from law enforcement as a marker of chronic alcohol ingestion.
Here, we review some of the unique characteristics of this important enzyme, previously considered as a mere specific marker of liver dysfunction, but now with clear extra-hepatic implications and novel applications and utility.
Keywords: gamma glutamyl transferase (γGT), Glutathione, Glutamyl Cycle, Cholestasis, prognostication
Introduction
Gamma- Glutamyl Transferase (γGT) is a key transferase involved in the transpeptidation of functional gamma-glutamyl groups to receptor molecules. Biologically, γGT is very sensitive for diagnosis of liver injury, although enzyme induction can lead to loss of specificity for damage and it has poor specificity for particular aetiologies. More recent evidence has suggested that it also plays an important role in antioxidant defence and xenobiotic metabolism, as well as offering tantalising associations across a continuum of disease states including cardiovascular disease and cancer. In this mini review we explore the structure and function of γGT, some of the historic applications of γGT, as well as its evolution beyond a simple marker of cholestasis. We have summarised some of the key aspects of γGT’s unique physiology in table 1, but we are keen to suggest readers may wish to access a number of additional insightful recent reviews on the topic. 1,2
TABLE 1.
Physiological associations of γGT in disease
| Organ/Tissue System | Physiological associations of γGT |
|---|---|
| Cardiovascular | • Evidence of co-localisation of γGT with typical foam cells and CD68+ macrophages, in addition to LDL in atherosclerotic deposits. • Increased incidence of congestive cardiac failure in those with higher serum values of γGT. • May be reflective of adaptative sequelae post-myocardial infarct |
| Lung | • Incompletely understood • γGT produced by type II pneumocytes and secreted into epithelial lining fluids (ELF); which may have a pathogenic effect. • Increased levels of ELF γGT are found in cystic fibrosis (CF) and likely reflect secretion from neutrophils within chronically inflamed airways. |
| Neoplasia | • Biologically plausible that γGT expressive tumours would have survival advantage in mitigating levels of oxidative stress in high cellular turnover state • γGT may protect certain cell lines from pro-oxidant chemotherapeutic regimes |
| Neurological | • Animal models of inflammatory CNS disease states implicate γGT and GSH pathways • γGT appears to have pro-oxidant signature, whereby blockade appears to ameliorate inflammatory effect. |
| Skeletal | • May behave as a damage associated molecular pattern (DAMP), inducing RANKL and causing osteoclastogenesis |
| Renal | • Ischaemia/perfusion deficit to renal tubules results in increased γGT activity and reduced GSH concentrations. • Injurious effect of YGT-related cell toxicity likely related to membrane lipid peroxidation. |
γGT biology
Gamma glutamyl transferase (γGT) is a glycosylated microsomal enzyme that catalyses the transfer of a gamma-glutamyl group to acceptor peptides and L-amino acids, particularly cysteine. .3,4 γGT is relatively ubiquitously expressed in human tissue and cell types, except within myocytes, and with some variability in expression profiles between cell types. It is most abundant on the luminal surfaces of cells with secretory or absorptive properties but can also be found on the basolateral surfaces of renal epithelial cells.5. Alternate gamma-glutamyl targets include γGT1 substrates, including Glutathione di-sulfide (GSSH), leukotriene C4 (LTC4)6 and S-nitroso-glutathione7 and GSH-xenobiotic adducts. As such, γGT plays a prominent role in homeostasis of glutathione production and recycling, inflammatory and nitric oxide signalling, as well as oxidative stress amelioration.
γGT and Glutathione
Glutathione exists as a tripeptide, γ-L-glutamyl-L-cysteinylglycine, present in all mammalian tissues, with the highest intracellular concentrations found in the liver.8 Here, as the most abundant non-protein thiol, it is responsible for oxidative stress mitigation. It exists in a thiol-reduced state (GSH), as the most prominent form (>98% of total), and as a disulfide-oxidised (GSSG) variant. 9 Within eukaryotic cells, GSH resides principally in the cytosol (80–85%), with 10–15% in the mitochondria, whilst the endoplasmic reticulum has a minute reservoir of GSH. GSH principally is derived from hepatocytes, with most being excreted into plasma and bile. 10 GSH is unique in its structure whereby the gamma-carboxyl portion of glutamate links to cysteine, rather than traditional α-carboxyl dipeptide formation. It is this unique characteristic that means this bond can only be hydrolysed by γGT.
Another fundamental aspect of GSH homeostasis relates to cysteine cycling, which is required for de-novo intracellular GSH synthesis, a process enzymatically facilitated by γGT. γGT also coordinates the transfer of the gamma-glutamyl moiety of glutathione S-compounded to mercapturic acid, thereby releasing cysteinyl-glycine.11 Importantly, cysteinyl glycine may interact with free iron species, thereby inducing the Fenton reaction and consequent superoxide production, a potent ROS, which may have pathogenic role in a number of human diseases. 12
γGT Induction
It has been consistently reported that serum γGT increases in patients taking anticonvulsant drugs. A number of older anticonvulsant therapies are well recognised as enzyme -inducing moieties including phenytoin and phenobarbitone, which are again associated with deranged serum γGT levels. There appears to be a synergistic effect on modulation of alcohol and ingestion of phenytoin in incrementing serum γGT levels. In another study by Herzberg13, 40% of patients exposed to commonly used medications including α-methyldopa, quinidine, digoxin, diazepam and furosemide demonstrated a sustained rise in γGT which suggests induction of hepatic microsomal enzymes by these commonly prescribed compounds. Evidence extrapolated from some studies demonstrated that serum γGT incremented following a reduction in hepatic glutathione secondary to drug induction.14 This would indirectly support the supposition that γGT mediates hepatic glutathione homeostasis. Interestingly, phenobarbital (a potent enzyme inducer) paradoxically increases both γGT and glutathione. However, a trial combination of cysteine and phenobarbital resulted in a nonsignificant decrease in γGT relative to control. This infers that γGT undergoes induction when enzyme-inducing drugs decimate hepatic glutathione levels; γGT induction may therefore subsequently result in higher than normal glutathione as a consequence. As a corollary, if glutathione does not fall, γGT is not induced, which provides important insight into how this might be exploited translationally.
γ-Glutamyl Cycle
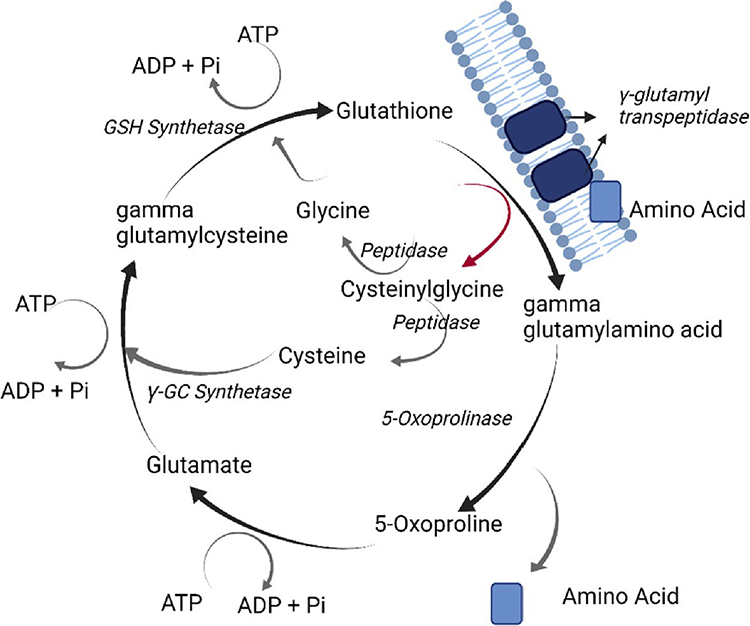
The γ-glutamyl cycle comprises of six enzymatically catalysed reactions that maintain glutathione homeostasis, which is summarised in figure 1. Within the gamma-glutamyl cycle, inherited genetic defects have been described in four enzymes: γ-glu-cysH Synthetase (γ-GC synthetase), GSH synthetase, 5-oxoprolinase and γ-glutamyl transpeptidase.15 Impaired GSH-synthetase and γ-GC synthetase functionality causes relative GSH deficiency. GSH synthetase deficiency is characterised by excess production of 5-oxoproline, which is manifested biologically as metabolic acidosis, 5-oxoprolinuria, haemolytic anaemia and neuromuscular disorders. 1716 In patients with isolated γ-GC synthetase deficiency, haemolytic anaemia predominates, with or without hepatosplenomegaly. Importantly, isolated erythrocytic GSH-synthetase deficiency results in haemolytic anaemia but not 5-oxoprolinuria. Treatment is principally focused on acidosis correction, high concentrate supplementation of vitamins C and E and avoidance of precipitating haemolytic crises. 15
Fig 1:
Overview of gamma glutamyl cycle
γGT as a liver function test
γGT has been adopted as a liver function test since the 1960s. A seminal paper by Szczeklik17 demonstrated mean values in differing aetiologies of liver disease, highlighting variation in serum γGT over time, and contrasting with other enzymatic trends including aldolase, phosphohexose isomerase and aspartate and alanine transferases. γGT demonstrates high sensitivity for liver damage, being abnormal in majority of patients with liver disease irrespective of pathogenesis, however, extremes of upper limits are most commonly seen patients with cholestasis. Conversely, there is an inherent lack of specificity in γGT, especially given other contributing diseases and conditions including pancreatitis, diabetes mellitus, obesity, excessive alcohol, enzyme-inducing drugs.14 An interesting observation in patients with chronic active hepatitis, was that γGT tended to normalisation in remission and in those undergoing treatment, however, this never reached statistical significance, but may provide a useful insight into treatment response.18
Importantly, while the normal reference range for γGT remains relatively consistent across age, there is inherent male – female variation in serum concentrations. Additionally, there are a number of demographic, physiological and inducible factors which affect γGT so there is heterogeneity in defining the reference interval. The standard reference thresholds for γGT in adult humans as recommended by the International Federation of Clinical Chemistry and Laboratory Medicine (IFCC) are presented in table 2.19
Table 2:
Proposed IFCC γGT standard ranges
| Gender | Upper reference limit* | 90% confidence intervals |
|---|---|---|
|
| ||
| Women | 38 (U/l) | (37–39 U/l) |
| Men | 55 (U/l) | (53–58 U/l) |
There have been incident case reports of isolated high serum gamma-glutamyl transferase levels in the absence of other predisposing factors within an extended Italian familial series. This condition has an autosomal dominant mode of inheritance and does not confer any disease specific risks.20
Conversely, there is a spectrum of related cholestatic conditions which manifest as low – normal γGT, collectively termed low gamma-GT familial intrahepatic cholestasis, with disease severity which may range from mild to severe.21 These forms of inherited cholestatic disease appear to be inherited in an autosomal recessive manner. There are a number of other disease variants within this continuum, however, it beyond the scope of this article to cover in more detail.
Diagnostic utility in liver disease
After perfecting the methodology for measuring serum γGT,22 Orlowski evaluated the diagnostic potential of γGT in ~100 conditions felt likely to alter serum levels.23 In a mixed cohort, patients with alcohol-related liver disease (ArLD), viral hepatitis, carcinomatosis and malabsorption were compared with those with no hepatic or renal pathology. γGT was markedly elevated in hepatobiliary disease as well as ArLD and carcinomatosis, whilst transaminases and alkaline phosphate remained within normal limits.24 The authors concluded that serum γGT may therefore be useful in the clinical assessment of ArLD and primary and secondary forms of hepatic neoplasia.
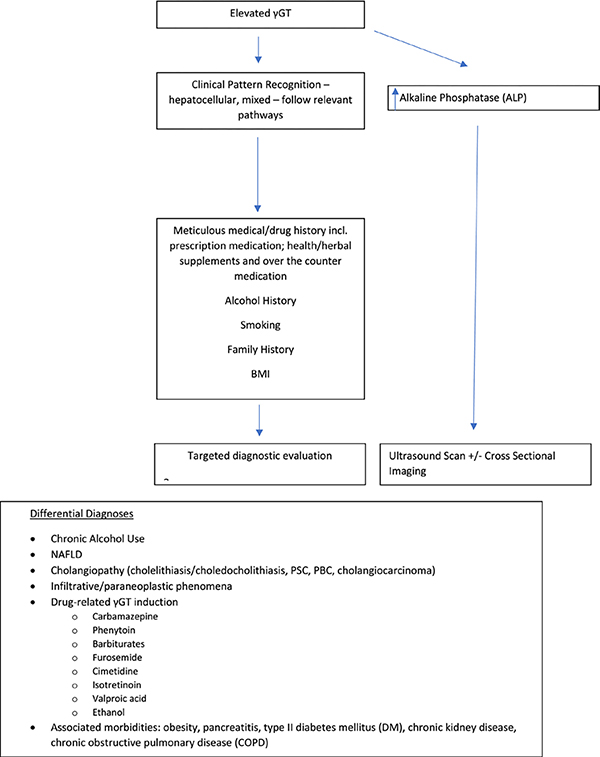
Whilst γGT is relatively ubiquitously expressed across tissues as previously described, its principle utility has been as part of the extended panel of liver function tests, including as a composite of a number of non-invasive algorithms of liver fibrosis assessment.25 It is well established as a very sensitive marker of hepatobiliary disease, with lower specificity than aspartate aminotransferase (AST) and alanine aminotransferase (ALT) for diagnosis of liver disease26, however, it is more sensitive than ALT and AST in anicteric, non-alcohol related parenchymal disease. Despite the introduction of a standardised measurement methodology for γGT, there have not been more contemporaneous studies to determine if new diagnostic reference thresholds should be derived. A broad clinical approach to determining the cause of an elevated γGT is provided in figure 2.
Fig 2:
Suggested approach to elevated γGT
Alcohol-related Liver Disease
In 1976, the Blennerhassett Committee recommended the identification of high-risk individuals among those persons convicted of driving under the influence of alcohol.27 There was increasing interest in prospectively identifying high-risk offenders and supporting assessment by clinicians from the Department of Transport in determining evidence of alcohol dependence. There was widespread enthusiasm in the clinical utility of γGT as a prospective biomarker of problem alcohol ingestion. Given its enzymatic activity is not considerably raised following a single bout of drinking, it was seen to represent an obvious candidate.28
This premise was applied across a number of cohort studies in Tayside, Scotland. A quarter of drivers convicted of drink driving had abnormal γGT activity at time of arrest. Dunbar and colleagues surmised that overall γGT activity demonstrated that blood ethanol level in isolation is poorly predictive of wider-alcohol related issues among drunk drivers (other than those associated with driving under the influence). Serial γGT levels also suggested that up to a 1/3 of these persons may be problem drinkers.29 They did, however, note a strong association between raised yGT activity and accidents in older offenders. Among young drivers the traditional association between blood alcohol concentration and accidents was strongly associated. Focusing solely on measured blood alcohol concentration may therefore disguise a relation between chronic, rather than acute, alcohol abuse and accidents.30 Whilst γGT proved very sensitive for chronic alcohol use, it’s specificity was relatively poor, with 17% of persons having abnormal levels in the context of no identifiable cause. Despite its relative sensitivity, these limitations continue to limit γGT as a reliable and definitive biomarker of alcohol related disease. Given the historical nature of these studies, there would likely be significant interest in revisiting the wider applicability of γGT in chronic alcohol use, especially where it might be combined with novel serum and urine alcohol metabolites.
Cholestasis
γGT has been reported as a marker of biliary disease, in part owing to its association with alkaline phosphatase, another indirect biochemical marker of cholestasis. This association was demonstrated by Whitfield and colleagues31 in a large cohort of patients with liver disease and healthy controls. Average serum activities of γGT and alkaline phosphatase (ALP) were three to six times greater in biliary disease compared to parenchymal aetiologies. In mixed parenchymal and biliary diseases, the ratios of mean γGT to ALP were approximately equivalent. Within this cohort, they also noted that γGT elevations often resolved corresponding to overall liver recovery, whether primary biliary or hepatocyte in origin.
Similar findings were subsequently reported by Cushieri and Baker18, whereby patients with extrahepatic biliary obstruction had significant elevations of γGT and ALP, which resolved on removal of the offending lesion. They noted an appreciable lag time in reduction of γGT, particularly in cases of significant jaundice pre-procedure.
General Prognostication
Beyond the interest in using γGT to identify those at risk of alcohol-related vehicle accidents, there is increasing curiosity around the utility of γGT in risk stratification relating to cardiovascular disease and malignancy. Clearly, this is of significant interest to clinicians and healthcare providers, however, additionally, it is of enormous interest to the insurance and life assurance industries.
Evidence from a number of epidemiological studies suggest that increased serum levels of γGT are associated with increased cardiovascular risk, including addition of the various components of the metabolic syndrome (metS). Similarly, novel cardiovascular predictive determinants including hsCRP, fibrinogen and F2-isoprostanes also demonstrate strong correlation with γGT. 32
There has been significant dissonance regarding the mortality effect seen in those with increased γGT in cardiovascular disease. Parallel epidemiological studies were insufficient to delineate whether serum γGT simply reflected increased cardiovascular disease risk determinants, or, whether γGT had prognostic propensity beyond these individual risk strata. 32 The Framingham heart study was developed from the Framingham Offspring Study in 1971. Within this cohort elevated γGT was associated with fatal and non-fatal incident cardiovascular events and predicted development of the metS. This association was apparent even on correcting for additional variables including CRP, and additional risk factors when temporally modelled as covariates including fasting glucose and the other individual components of the metS.
Interestingly, moderate to high levels of serum γGT (50th-90th percentiles) has been demonstrated to significantly associate with the incidence of heart failure in a Finnish cohort.33 Similarly, γGT appears to have a mechanistic role in reversing the down-regulation of K+ channels in the myocardium post myocardial infarct and further scavenging ROS, which may aid cardiac remodelling.
Another area of increased interest is individual cancer risk stratification. Whilst there are relative predictors for cardiovascular disease (metabolic syndrome, hyperlipidaemia, diabetes), cancer risk profiling usually relies on clinical or family history and is generally less robust by underwriting standards. A number of clinical studies have attempted to model for cancer risk using laboratory markers and physical attributes, however, they often only demonstrate univariate risk factors. A large study by Palmier and Lanzrath34 utilising 1.25 million insurance applicants who were matched to claims records, demonstrated that γGT positively correlated with cancer mortality across most modelled ranges, albeit the apparent risk appeared to plateau at higher values. Alkaline phosphatase levels and relative risk appear to positively correlate with γGT in this cohort
Conclusion
γGT is an enzyme of many parts, vital to maintaining oxidative stress and signalling homeostasis. Once a mainstay of the liver function test panel, which in many areas it was discontinued as a cost-saving measure. It is now making a resurgence as a sensitive gateway test to early detection of chronic liver disease and as a prognostic marker for cardiovascular disease. These novel insights clearly represent exciting new avenues of research for this figurative “old dog”.
Key Points.
An important enzyme for transpeptidation
Sensitive biomarker for many liver pathologies
Not specific for any individual liver disease or of direct liver injury
γ-GT increases in response to oxidative stress and glutathione depletion
Indirect biomarker for cardiovascular and metabolic risk
Funding
The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors relating to this work. PNB has received lecture honoraria from Takeda. JFD has received research grants and lecture honoraria from MSD, Abbvie, and Gilead. EBT receives funding from the National Institutes of Health through the NIDDK (1K23DK117055). EBT has served as a consultant to Novartis, Kaleido, Axcella, and Allergan, has served on advisory boards for Mallinckrodt, Rebiotix, and Bausch Health, and has received unrestricted research grants from Gilead and Valeant.
List of Abbreviations:
- γGT
gamma glutamyl transferase
- GSSH
glutathione disulfide
- LTC4
leukotriene C4
- GSH
glutathione
- γ-GC synthetase
γ-glu-cysH synthetase
- ROS
reactive oxygen species
- ArLD
alcohol-related liver disease
- AST
aspartate transaminase
- ALT
alanine transferase
- ALP
alkaline phosphatase
- MetS
metabolic syndrome
- hsCRP
high-sensitivity c-reactive protein
Footnotes
Competing Interests The authors have no competing interests relating to this work
Patient consent for publication Not required.
Provenance and peer review Commissioned, externally peer reviewed.
Data sharing is not applicable to this article as no new data were created or analysed in this study.
References
- 1.Koenig G, Seneff S. Gamma-Glutamyltransferase: A Predictive Biomarker of Cellular Antioxidant Inadequacy and Disease Risk. Disease Markers [Internet] 2015. [cited 2021 Aug 25];2015. Available from: /pmc/articles/PMC4620378/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Corti A, Belcastro E, Dominici S, Maellaro E, Pompella A. The dark side of gamma-glutamyltransferase (GGT): Pathogenic effects of an ‘antioxidant’ enzyme. Free Radical Biology and Medicine 2020;160:807–19. [DOI] [PubMed] [Google Scholar]
- 3.Bibas M, Zampa G, Procopio A, Guaitolini R. High Serum Gamma-Glutamyltransferase Concentrations in a Family. New England Journal of Medicine [Internet] 1994. [cited 2021 Jun 24];330(25):1832–3. Available from: https://www.nejm.org/doi/full/10.1056/NEJM199406233302518 [DOI] [PubMed] [Google Scholar]
- 4.Dalle-Donne I, Rossi R, Giustarini D, Colombo R, Milzani A. S-glutathionylation in protein redox regulation [Internet]. Free Radical Biology and Medicine. 2007. [cited 2021 Jun 24];43(6):883–98. Available from: https://pubmed.ncbi.nlm.nih.gov/17697933/ [DOI] [PubMed] [Google Scholar]
- 5.Lieberman MW, Barrios R, Carter BZ, et al. γ-Glutamyl transpeptidase: What does the organization and expression of a multipromoter gene tell us about its functions? [Internet]. American Journal of Pathology. 1995. [cited 2021 Jun 24];147(5):1175–85. Available from: /pmc/articles/PMC1869519/?report=abstract [PMC free article] [PubMed] [Google Scholar]
- 6.Mayatepek E, Okun JG, Meissner T, et al. Synthesis and metabolism of leukotrienes in γ-glutamyl transpeptidase deficiency. Journal of Lipid Research 2004;45(5):900–4. [DOI] [PubMed] [Google Scholar]
- 7.Angeli V, Tacito A, Paolicchi A, et al. A kinetic study of gamma-glutamyltransferase (GGT)-mediated S-nitrosoglutathione catabolism. Archives of Biochemistry and Biophysics 2009;481(2):191–6. [DOI] [PubMed] [Google Scholar]
- 8.Lu SC. Glutathione synthesis. Biochimica et Biophysica Acta (BBA) - General Subjects 2013;1830(5):3143–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaplowitz N, Aw Y, Ookhtens M. THE REGULATION OF HEPATIC GLUTATHIONE l. Ann Rev Pharmacol Toxicol [Internet] 1985. [cited 2021 Jul 19];25:715–59. Available from: www.annualreviews.org [DOI] [PubMed] [Google Scholar]
- 10.Ookhtens M, Kaplowitz N. Role of the Liver in Interorgan Homeostasis of Glutathione and Cyst(e)ine. Seminars in Liver Disease [Internet] 2008. [cited 2021 Jul 29];18(04):313–29. Available from: http://www.thieme-connect.com/products/ejournals/html/10.1055/s-2007-1007167 [DOI] [PubMed] [Google Scholar]
- 11.H Z, HJ F, J C. Gamma-glutamyl transpeptidase in glutathione biosynthesis. Methods in enzymology [Internet] 2005. [cited 2021 Jul 28];401:468–83. Available from: https://pubmed.ncbi.nlm.nih.gov/16399403/ [DOI] [PubMed] [Google Scholar]
- 12.Koenig G, Seneff S. Gamma-Glutamyltransferase: A Predictive Biomarker of Cellular Antioxidant Inadequacy and Disease Risk. Disease Markers [Internet] 2015. [cited 2021 Jul 28];2015. Available from: /pmc/articles/PMC4620378/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.M H, B F, MH W. Hepatic microsomal enzyme induction and its evaluation in a clinical laboratory. Israel Journal of Medical Sciences [Internet] 1977. [cited 2021 Aug 6];13(5):471–6. Available from: https://europepmc.org/article/med/17578 [PubMed] [Google Scholar]
- 14.JB W. Gamma glutamyl transferase. Critical reviews in clinical laboratory sciences [Internet] 2001. [cited 2021 Aug 6];38(4):263–355. Available from: https://pubmed.ncbi.nlm.nih.gov/11563810/ [DOI] [PubMed] [Google Scholar]
- 15.Ristoff E, Larsson A. Patients with genetic defects in the γ-glutamyl cycle. Chemico-Biological Interactions [Internet] 1998. [cited 2021 Jul 8];111–112:113–21. Available from: https://pubmed.ncbi.nlm.nih.gov/9679548/ [DOI] [PubMed] [Google Scholar]
- 16.Beutler E, Moroose R, Kramer L, Gelbart T, Forman L. Gamma-Glutamylcysteine Synthetase Deficiency and Hemolytic Anemia. Blood 1990;75(1):271–3. [PubMed] [Google Scholar]
- 17.Szczeklik E, Orlowski M, Szewczuk A. Serum γ-Glutamyl Transpeptidase Activity in Liver Disease. Gastroenterology 1961;41(4):353–9. [Google Scholar]
- 18.Cuschieri A, Baker PR. Gamma-Glutamyl-Transpeptidase in Hepato-Biliary Disease— Value as an Enzymatic Liver Function Test. British Journal of Experimental Pathology [Internet] 1974. [cited 2021 Aug 18];55(2):110. Available from: /pmc/articles/PMC2072525/?report=abstract [PMC free article] [PubMed] [Google Scholar]
- 19.G S, R B, F C, et al. IFCC primary reference procedures for the measurement of catalytic activity concentrations of enzymes at 37 degrees C. International Federation of Clinical Chemistry and Laboratory Medicine. Part 5. Reference procedure for the measurement of catalytic concentration of aspartate aminotransferase. Clinical chemistry and laboratory medicine [Internet] 2002. [cited 2021 Nov 1];40(7):725–33. Available from: https://pubmed.ncbi.nlm.nih.gov/12241022/ [DOI] [PubMed] [Google Scholar]
- 20.M B, G Z, A P, R G. High serum gamma-glutamyltransferase concentrations in a family. The New England journal of medicine [Internet] 1994. [cited 2021 Jul 9];330(25):1832–3. Available from: https://pubmed.ncbi.nlm.nih.gov/7910666/ [DOI] [PubMed] [Google Scholar]
- 21.R T, S S. BSEP: function and role in progressive familial intrahepatic cholestasis. Seminars in liver disease [Internet] 2001. [cited 2021 Aug 12];21(4):545–50. Available from: https://pubmed.ncbi.nlm.nih.gov/11745042/ [DOI] [PubMed] [Google Scholar]
- 22.M O, A S. Colorimetric determination of gamma-glutamyl transpeptidase activity in human serum and tissues with synthetic substrates. Acta Biochimica Polonica [Internet] 1961. [cited 2021 Jul 12];8:189–200. Available from: https://europepmc.org/article/med/13731220 [PubMed] [Google Scholar]
- 23.M O. THE ROLE OF GAMMA-GLUTAMYL TRANSPEPTIDASE IN THE INTERNAL DISEASES CLINIC. Archivum immunologiae et therapiae experimentalis [Internet] 1963. [cited 2021 Jul 12];11:1–61. Available from: https://pubmed.ncbi.nlm.nih.gov/14062968/ [PubMed] [Google Scholar]
- 24.M Z, G D. Serum gamma-glutamyl transpeptidase as a diagnostic aid. Lancet (London, England) [Internet] 1970. [cited 2021 Jul 14];2(7676):748–50. Available from: https://pubmed.ncbi.nlm.nih.gov/4195980/ [DOI] [PubMed] [Google Scholar]
- 25.Dillon JF, Miller MH. Gamma glutamyl transferase ‘To be or not to be’ a liver function test?: 10.1177/0004563216659887 [Internet] 2016. [cited 2021 Aug 18];53(6):629–31. Available from: https://journals.sagepub.com/doi/full/10.1177/0004563216659887 [DOI] [PubMed] [Google Scholar]
- 26.Pratt DS, Kaplan MM. Evaluation of Abnormal Liver-Enzyme Results in Asymptomatic Patients. 10.1056/NEJM200004273421707 [Internet] 2009. [cited 2021 Aug 18];342(17):1266–71. Available from: https://www.nejm.org/doi/10.1056/NEJM200004273421707 [DOI] [PubMed] [Google Scholar]
- 27.Departmental Committee. Drinking drivers and the law. British Medical Journal, London [Internet] 1976. [cited 2021 Jul 8];Available from: http://www.bmj.com/
- 28.PM C, LJ K, S Z. Drivers, binge drinking, and gamma-glutamyltranspeptidase. British medical journal (Clinical research ed) [Internet] 1982. [cited 2021 Jul 8];285(6355):1656–7. Available from: https://pubmed.ncbi.nlm.nih.gov/6128053/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Problem drinking among drunk drivers. British Medical Journal (Clinical research ed) [Internet] 1983. [cited 2021 Jul 7];286(6374):1319. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1547610/ [PMC free article] [PubMed] [Google Scholar]
- 30.Dunbar JA, Ogston SA, Ritchie A, Devgun MS, Hagart J, Martin BT. Are problem drinkers dangerous drivers? An investigation of arrest for drinking and driving, serum gamma glutamyltranspeptidase activities, blood alcohol concentrations, and road traffic accidents: the Tayside Safe Driving Project. British Medical Journal (Clinical research ed) [Internet] 1985. [cited 2021 Jul 7];290(6471):827. Available from: /pmc/articles/PMC1418594/?report=abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whitfield JB, Pounder RE, Neale G, Moss DW. Serum γ-glutamyl transpeptidase activity in liver disease. Gut [Internet] 1972. [cited 2021 Aug 18];13(9):702–8. Available from: https://gut.bmj.com/content/13/9/702 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.DS L, JC E, SJ R, et al. Gamma glutamyl transferase and metabolic syndrome, cardiovascular disease, and mortality risk: the Framingham Heart Study. Arteriosclerosis, thrombosis, and vascular biology [Internet] 2007. [cited 2021 Aug 2];27(1):127–33. Available from: https://pubmed.ncbi.nlm.nih.gov/17095717/ [DOI] [PubMed] [Google Scholar]
- 33.Wang Y, Tuomilehto J, Jousilahti P, et al. Serum γ-glutamyltransferase and the risk of heart failure in men and women in Finland. Heart [Internet] 2013. [cited 2021 Aug 5];99(3):163–7. Available from: https://heart.bmj.com/content/99/3/163 [DOI] [PubMed] [Google Scholar]
- 34.J P, BJ L. Laboratory and biometric predictors of cancer-related mortality in an insured population. Journal of Insurance Medicine (New York, NY) [Internet] 2012. [cited 2021 Aug 2];43(3):162–8. Available from: https://europepmc.org/article/med/23451617 [PubMed] [Google Scholar]